Simple Summary
This study enhanced the relative activity of glycosyltransferase UGTBL1 through engineering mutations near its loop regions. The engineered enzyme efficiently catalyzed the glycosylation of the lignin-derived compound p-hydroxybenzaldehyde (C7H6O2), enabling the environmentally benign synthesis of a Helicid analogue (p-hydroxybenzaldehyde β-glucoside (C13H16O7)). Accordingly, this work provided novel insights for lignin valorization. Under optimized reaction conditions (35 °C, pH 7.5, 200 mM glucose (C6H12O6)), an exceptional yield of 97.8% for p-hydroxybenzaldehyde β-glucoside was attained within 10 h using 2 mM p-hydroxybenzaldehyde substrate. Biotransformation of 3 mM p-hydroxybenzaldehyde (366.4 mg/L) afforded up to 2.7 mM of target β-glucoside product (767.5 mg/L). This research establishes a simpler, more economical, and sustainable enzymatic approach for the efficient synthesis of p-hydroxybenzaldehyde β-glucoside, offering significant potential benefits for pharmaceutical, cosmetic, or agricultural applications.
Abstract
Lignin, as one of the three primary components of renewable lignocellulosic biomass, can be converted into aromatic platform chemicals and holds significant potential for high-value applications. p-Hydroxybenzaldehyde is a compound derived from lignin. In this study, the mutant Δ60 of the glycosyltransferase UGTBL1 derived from Bacillus licheniformis was adopted to catalyze the glycosylation reaction of p-hydroxybenzaldehyde, producing a bioactive compound Helicid analogue (p-hydroxybenzaldehyde β-glucoside). Truncation mutations targeting loop regions may reduce local flexibility, thereby facilitating enhanced access of p-hydroxybenzaldehyde to the active site pocket and promoting relative activity. Under optimal conditions (35 °C, pH 7.5, and glucose 200 mM), a high yield of 97.8% for p-hydroxybenzaldehyde β-glucoside was achieved from 2 mM p-hydroxybenzaldehyde within 10 h. The conversion of 3 mM p-hydroxybenzaldehyde (366.4 mg/L) yielded up to 2.7 mM (767.5 mg/L) of p-hydroxybenzaldehyde β-glucoside within 48 h. According to the molecular docking results, the CDOCKER energy value of mutant Δ60 was lower than that of the wild-type, at −16.0 kcal/mol. To our knowledge, this is the first example of an efficient and environmentally sustainable approach for the synthesis of p-hydroxybenzaldehyde β-glucoside, providing a new insight for the valorization of lignin into valuable biobased chemicals.
1. Introduction
Currently, as industrial development progresses, fossil resources are becoming increasingly scarce. Consequently, the utilization of biomass as a substitute for fossil resources has emerged as a promising and sustainable approach. Among various biomass sources, lignocellulose, which is one of the most abundant renewable resources worldwide, showcases considerable potential for application in this field [1,2,3]. It is primarily composed of cellulose, hemicellulose, and lignin. To date, both cellulose and hemicellulose have been extensively researched and utilized and can be efficiently transformed into higher-value products. For instance, Wu et al. conducted enzymolysis of cellulose in the pretreated sugarcane bagasse to produce glucose, which was subsequently used in the fermentation process for the production of adipic acid, a key monomer in nylon synthesis [4]. Yang et al. successfully converted hemicellulose derived from Phyllostachys edulis into the platform chemical furfural and functional xylo-oligosaccharides using solid acid catalysis [5]. Lignin can be converted into bio-based aromatic platform chemicals containing benzene rings, such as vanillin [6], p-hydroxybenzaldehyde [7,8], and syringaldehyde [9]. Furthermore, they can be further converted into various high-value-added compounds. For example, vanillin can undergo transamination catalyzed by transaminase to synthesize vanillyl amine, an important intermediate in the production of capsaicin [10]. Nevertheless, the high-value-added utilization of lignin-derived compounds still requires further development. p-Hydroxybenzaldehyde, as one of the lignin derivatives, possesses a wide range of application potentials. For instance, the biologically active compound 2-arylthiazoline can be synthesized from p-hydroxybenzaldehyde via vanillyl alcohol oxidase-catalyzed transformation [11]. Novel AB3-type porphyrin derivatives can be synthesized via the condensation of p-bromobenzaldehyde and p-hydroxybenzaldehyde with pyrrole in propionic acid [12]. In order to achieve the high-value utilization of lignin derivatives, this study aims to synthesize p-hydroxybenzaldehyde β-glucoside, an analogue of Helicid, from p-hydroxybenzaldehyde.
Helicid is a natural compound isolated from the fruit of Helicia nilagirica Bedd, and it exhibits therapeutic effects in the treatment of headache, insomnia, and depression [13,14]. Although it can be obtained via extraction, this method is generally considered cumbersome and cost-prohibitive. Helicid and its analogues are typically synthesized using p-hydroxybenzaldehyde as the starting substrate. For example, Wen et al. chemically synthesized Helicid analogues and assessed their cholinesterase inhibitory activity [15]. He et al. evaluated the α-glucosidase inhibitory activity of p-hydroxybenzaldehyde β-glucoside [16]. p-Hydroxybenzaldehyde β-glucoside demonstrates superior tyrosinase inhibitory activity compared to p-hydroxybenzaldehyde and even the well-known inhibitor arbutin [17]. The conventional chemical synthesis method for the Helicid analogue, p-hydroxybenzaldehyde β-glucoside, typically involves the use of p-hydroxybenzaldehyde and bromo-tetraacetyl glucose as starting materials [17]. In this reaction, the hydroxyl groups of glucose must be protected to yield bromo-tetraacetyl glucose, which subsequently undergoes glycosylation with p-hydroxybenzaldehyde followed by deprotection. This synthetic process entails multiple steps and involves the use of environmentally hazardous catalysts and solvents.
Biocatalytic synthesis represents an efficient and environmentally sustainable strategy for the production of glycosides [18,19]. Glycosyltransferase exhibits stereoselectivity, mild reaction conditions, and environmental compatibility. In this study, the glycosyltransferase UGTBL1 was utilized to catalyze the glucosylation of p-hydroxybenzaldehyde for the synthesis of the Helicid analogue p-hydroxybenzaldehyde β-glucoside. To enhance its relative activity, truncation mutations were introduced to amino acid residues near the enzyme’s substrate binding pocket (Figure 1). Truncation of low-conservation loop regions may induce subtle conformational adjustments in proximal protein domains, thereby favorably modulating catalytic properties. Following systematic optimization of reaction parameters, an efficient synthetic method for p-hydroxybenzaldehyde β-glucoside was established based on whole-cell catalysis using the engineered glycosyltransferase mutant.
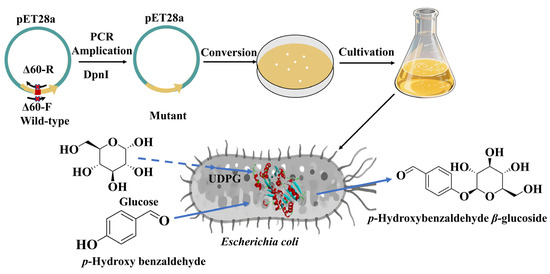
Figure 1.
Glucosylation of p-hydroxybenzaldehyde for the synthesis of the Helicid analogue.
2. Materials and Methods
2.1. Materials
The p-hydroxybenzaldehyde, potassium dihydrogen phosphate, and potassium hydrogen phosphate were obtained from Leyan (Shanghai, China). Glucose and sodium chloride were sourced from Heowns (Tianjin, China). p-Nitrophenol was purchased from the Sinopharm (Shanghai, China). Tryptone and yeast extract were acquired from Aladdin (Shanghai, China). UDPG and other reagents were obtained from Titan (Shanghai, China).
The UGTBL1 gene (GenBank: KP123426.1) was cloned into the plasmid pET28a with XhoI and EcoRI restriction sites and was maintained in our laboratory. Escherichia coli (E. coli) BL21(DE3) was used as the expression host. High-Fidelity PCR Master Mix and DpnI endonuclease were purchased from Adamas (Shanghai, China). Primers were synthesized by GENCEFE Biotech (Wuxi, China). The plasmid miniprep kit was obtained from Tsingke (Beijing, China).
2.2. Full-Plasmid PCR Mutagenesis of Glycosyltransferase
The wild-type glycosyltransferase UGTBL1 exhibits glycosylation activity toward p-hydroxybenzaldehyde, which can be further improved through mutagenesis. Based on the structural model of UGTBL1, amino acid residues 59–69 located in the N-terminal substrate binding pocket were selected as mutation targets. Specific primers were designed and synthesized according to the wild-type glycosyltransferase gene sequence (primer sequences are provided in Table S1 (see Supplementary File). Single-point deletions were introduced at each position from 59 (Thr) to 69 (Glu) to generate truncated mutants. The amino acid sequence is listed in Table S2 (see the Supplementary File) This region was selected for modification due to its proximity to the active center, low conservation level, and the presence of loops and α-helices. The 25 μL full-plasmid PCR reaction mixture consisted of 0.5 μL template plasmid, 1 μL forward primer, 1 μL reverse primer, 10 μL ddH2O, and 12.5 μL High-Fidelity PCR Master Mix. PCR amplification was performed under the following conditions: 30 cycles of denaturation at 95 °C for 30 s, annealing at 62 °C for 30 s, and extension at 72 °C for 4 min. Following amplification, the PCR products were treated with DpnI endonuclease to degrade the methylated parental DNA and subsequently transformed into E. coli BL21(DE3). Positive transformants were identified by colony PCR using T7 universal primers and further confirmed by DNA sequencing at GENCEFE Biotech.
2.3. Cultivation of Engineered Bacteria and Enzyme Expression
Engineered E. coli strains were inoculated onto LB agar plates supplemented with 50 μg/mL kanamycin and incubated overnight at 37 °C. A single colony was selected and transferred into 5 mL of LB liquid medium containing 50 μg/mL kanamycin, followed by incubation at 37 °C and 180 rpm overnight to generate the seed culture. The seed culture was then inoculated into fresh LB liquid medium (at a 5% v/v ratio) containing 50 μg/mL kanamycin and cultured under the same conditions until the OD600 reached 0.6–0.8. Protein expression was induced by the addition of 0.5 mM IPTG, and the culture was further incubated at 25 °C for 12–16 h. Following induction, the bacterial cells were harvested by centrifugation at 8000 rpm for 3 min.
2.4. Optimization of Whole-Cell Reaction Conditions
Whole-cell catalysis represents an environmentally benign alternative to traditional chemical catalysts [20]. The typical whole-cell catalytic reaction was conducted under the following conditions: 2 mM p-hydroxybenzaldehyde, whole cell (0.025 g/mL), 100 mM glucose, 360 μL buffer solution, pH 7.0, 30 °C, and 180 rpm. The reaction was terminated by adding an equal volume of methanol to the reaction mixture. Subsequently, the samples were centrifuged at 4300× g for 3 min, and the supernatant was filtered through a 0.22 μm membrane filter prior to HPLC analysis. To improve the efficiency of whole-cell catalysis mediated by the glycosyltransferase, the effects of reaction time, substrate concentration, glucose concentration from 0–350 mM, reaction temperature in the range of 25 to 50 °C, solution pH between 6.0 and 8.5, and Δ60 loading from 0.01–0.045 g/mL were systematically evaluated. The formula for calculating relative activity is given as below:
2.5. Homology Modeling and Molecular Docking Analysis
Homology modeling based on amino acid sequences was performed using the Swiss-Model server to predict the 3D structures of glycosyltransferase UGTBL1 wild-type and its mutant Δ60. The glycosyltransferase (PDB:7VLB) from Bacillus spizizenii was selected as the template for modeling UGTBL1 from Bacillus licheniformis, which shares a sequence identity of 56.80%. The 3D structure of p-hydroxybenzaldehyde was imported into Discovery Studio 2019 and subjected to molecular structure optimization. Molecular docking simulations between p-hydroxybenzaldehyde and both the wild-type and mutant Δ60 were performed using the CDOCKER module in Discovery Studio 2019 Client. The interactions between the enzyme and the substrate were analyzed in terms of intermolecular binding modes and CDOCKER interaction energies [21]. The settlement formula for binding energy is as below:
EnergyBinding = EnergyComplex − EnergyLigand − EnergyReceptor
2.6. HPLC and High-Resolution Mass Spectrometry Analysis Methods
The Thermo Fisher Vanquish Core HPLC system (Germering, Germany) with Athena C18-WP column (ANPEL Laboratory Technologies (Shanghai) Inc., Shanghai, China) (5 μm, 100 A, 4.6 × 250 mm) was adopted for the detection and analysis of p-hydroxybenzaldehyde and p-hydroxybenzaldehyde β-glucoside. Detection was carried out at 275 nm. The column temperature maintained at 30 °C, and the mobile phase was controlled at a flow rate of 1 mL/min. The mobile phase consisted of water (eluent A) and methanol (eluent B). The gradient elution program was as follows: 0–4 min, 10% B; 4–15 min, 10–50% B; 15–19 min, 50% B; 19–26 min, 50–100% B; 26–31 min, 100% B; 31–33 min, 100–10% B; 33–35 min, 10% B. HRMS data were acquired by analyzing samples using an Agilent 6230 TOF mass spectrometer(Agilent Technologies, Santa Clara, CA, USA).
3. Results and Discussion
3.1. Construction of the Mutant Library of Glycosyltransferase and Protein Expression
Using the plasmid encoding the wild-type glycosyltransferase as a template, the truncated target gene was successfully amplified via whole-plasmid PCR. Fan et al. achieved site-directed mutagenesis of a glycosyltransferase via whole-plasmid PCR, resulting in resveratrol with 87.7% regioselectivity [22]. By modifying the loop region, the channel can be altered, reducing the restraint on the substrate entering the enzyme’s interior and enhancing the enzymatic activity. The nucleic acid electrophoresis results of positive clones obtained through colony PCR are presented in Figure 2a, indicating that the DNA fragment size is approximately 1500 bp, which is consistent with the fragment size between the T7 primer pairs. SDS-PAGE analysis confirmed the successful expression of the mutant glycosyltransferase in E. coli BL21(DE3). As shown in Figure 2b, the molecular weight of the expressed protein is close to 49 kDa, and the expression of the mutant is comparable to that of the wild-type. Furthermore, the protein electrophoresis was carried out utilizing the supernatant derived from cell disruption (Figure S1, see Supplementary File).
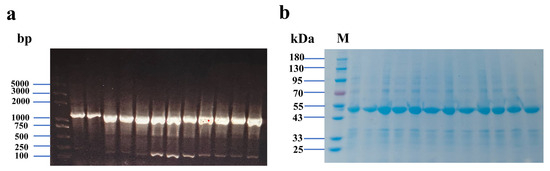
Figure 2.
Electrophoretic analysis of gene fragments. Lanes (left to right): marker, wild-type, truncation mutants Δ59 through Δ69 (a); SDS-PAGE analysis of protein Lanes (left to right): marker, wild-type, truncation mutants Δ59 through Δ69 (b).
3.2. Screening of Glycosyltransferase Mutants
By sequentially deleting each amino acid residue from positions 59 to 69 of UGTBL1, a total of 11 truncated mutants were generated. Their catalytic efficiencies in glycosylating p-hydroxybenzaldehyde were evaluated and compared with that of the wild-type. As illustrated in Figure 3, the mutants Δ59, Δ60, Δ63, and Δ64 exhibited the enhanced catalytic activities relative to the wild-type. Among them, mutant Δ60 displayed the highest relative activity, with an increase of approximately 30% compared to that of the wild-type. Residue 60 is located within the loop, and its deletion resulted in the observed change in enzymatic activity. Wang et al. reported that truncation mutations of γ-glutamyltranspeptidase II enhanced the hydrolytic activity in all mutants compared to the wild-type [23]. However, this research revealed that not all truncation mutants exhibited the improved activity, possibly due to the impaired substrate binding caused by loop region modifications. This suggested that not all mutation sites near the loop region are suitable for engineering. Based on above results, a whole-cell catalytic system was established using the engineered strain expressing the mutant Δ60 and subsequently optimized.
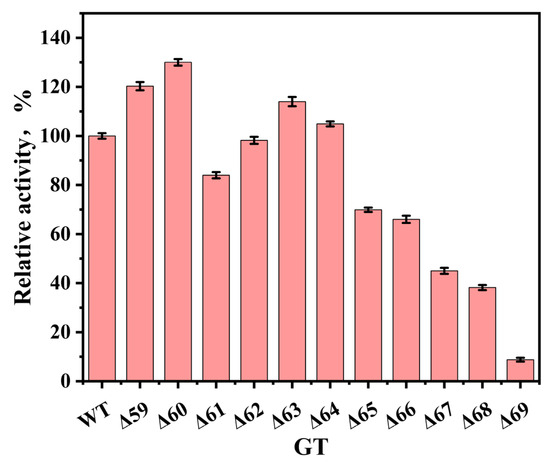
Figure 3.
Comparison of glycosylation activity toward p-hydroxybenzaldehyde between UGTBL1 wild-type and mutants.
3.3. Molecular Docking Results
Based on the molecular docking analysis of the wild-type glycosyltransferase UGTBL1 and p-hydroxybenzaldehyde (Figure 4a), the hydrogen-bonds were observed between substrates and residues (GLU322 and GLN323). Additionally, molecular docking reveals that p-hydroxybenzaldehyde forms Pi-Alkyl interactions with residues ALA235, and p-hydroxybenzaldehyde forms Pi-Cation interactions with residues HIS16, as well as Pi-Sigma interactions with residue PHE236. The docking results indicate that the binding energy between the p-hydroxybenzaldehyde molecule and the wild-type is −15.0 kcal/mol.
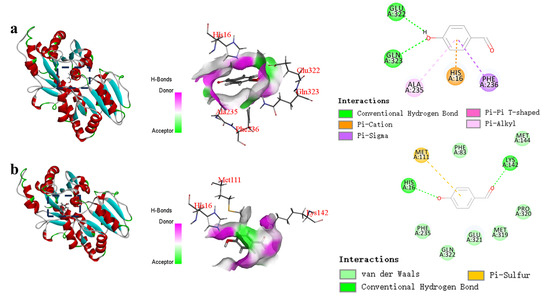
Figure 4.
Molecular docking results for wild-type (a) and mutant Δ60 (b).
In the case of the glycosyltransferase mutant Δ60, molecular docking reveals that p-hydroxybenzaldehyde forms hydrogen bonds with residues HIS16 and LYS142, as well as a Pi-Sulfur interaction with residue MET111. The calculated binding energy between p-hydroxybenzaldehyde and mutant Δ60 is −16.0 kcal/mol (Figure 4b). Notably, a key catalytic residue in the mutant, residue HIS16, can directly interact with p-hydroxybenzaldehyde.
The CDOCKER energy, a commonly used metric for evaluating ligand–receptor binding strength, suggests that a higher absolute value corresponds to a stronger binding affinity [24]. A comparative analysis of the CDOCKER energies reveals that the absolute value of binding energy of p-hydroxybenzaldehyde with mutant Δ60 is higher than that with the wild-type. The difference in binding energy between the wild type and mutant 60 is 1.0 kcal/mol, indicating a stronger affinity between the mutant and the substrate. The dominant forces governing molecular docking are salt bridges and hydrogen-bonds. The hydrogen-bonds stabilize ligand conformation within the binding pocket through polar interactions, with their strength exhibiting distance-dependent constraints [25]. Notably, the donor-acceptor distance profoundly impacts docking outcomes. In this research, the key residue HIS16 was identified as critical, with mutant Δ60 demonstrating the elevated binding affinity towards p-hydroxybenzaldehyde compared to the wild-type enzyme. This improvement might be due to the direct hydrogen-bond interaction between the residue HIS16 and the ligand. A higher binding energy between an enzyme and its substrate indicates a more stable interaction [26]. This enhanced binding affinity may contribute to the higher glycosylation efficiency of the mutant Δ60 toward p-hydroxybenzaldehyde compared to the wild-type.
3.4. Analysis of the Reaction Products
Based on the HPLC chromatogram of the reaction mixture, showing two distinct peaks (Figure S2, see Supplementary File). The peak observed at a retention time of 17.3 min corresponds to p-hydroxybenzaldehyde, which matches the retention time of the standard substrate. The peak, appearing at 11.9 min, was identified as p-hydroxybenzaldehyde β-glucoside and was further confirmed by high resolution mass spectrometry analysis.
Based on displays the mass spectrum of the substrate (Figure S3, see Supplementary File), p-hydroxybenzaldehyde, where the measured mass-to-charge ratio of 123.0449 corresponds to the protonated molecular ion [M+H]+, consistent with the theoretical value of 123.0446. Figure S4 (see Supplementary File) illustrates the mass spectrum of the product, p-hydroxybenzaldehyde β-glucoside, showing a mass-to-charge ratio of 307.0795, which closely matches the theoretical value of the sodium adduct ion [M+Na]+ at 307.0794. The molecular weight difference between the two compounds is 162, corresponding to the molecular weight of a single glucose unit (C6H10O5). These results confirm that p-hydroxybenzaldehyde was successfully glycosylated to form p-hydroxybenzaldehyde β-glucoside.
3.5. The Effect of Glucose Concentration on the Reaction System
During glycosyltransferase-catalyzed glycosylation reactions, UDPG serves as the glycosyl donor. However, UDPG is relatively costly. To address this issue, glucose was employed as the precursor of UDPG in the whole-cell catalytic system. Glucose can be metabolically converted to UDPG by E. coli. The relative activity of the system can be influenced by the concentration of glucose supplied [27]. The impact of glucose concentration on the reaction system is presented in Figure 5a. In the absence of glucose supplementation, the reaction proceeded to a minimal extent, with the trace amount of product likely originating from the basal level of UDPG naturally present in the cells. Upon adding glucose, the relative activity elevated with increasing glucose concentration. Maximum relative activity was observed in the occurrence of 200 mM glucose. However, further elevation in glucose concentration did not result in a continued increase in activity, and a decline was noted when glucose was supplemented in a higher concentration. Excessive glucose supplementary may have interfering effects on the whole-cell system. It seems that the concentration of glucose affects the relative activity of enzymes, with peak performance occurring at the optimal concentration and a progressive reduction in activity as the concentration deviates from this optimum [28]. This phenomenon may be attributed to the increased viscosity of the reaction medium accompanying the elevation in co-substrate concentration, which in turn may impair the activity of the glycosyltransferase [29].
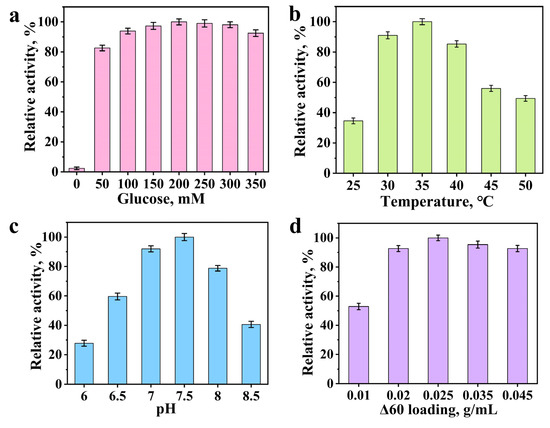
Figure 5.
Effect of glucose concentration (0–350 mM) on glycosylation of p-hydroxybenzaldehyde catalyzed by mutant Δ60 (35 °C, pH 7.5, 0.025 g/mL, 10 h reaction) (a); Effect of temperature (25–50 °C) on glycosylation of p-hydroxybenzaldehyde catalyzed by mutant Δ60 (200 mM glucose, pH 7.5, 0.025 g/mL, 10 h reaction) (b); Effect of pH (6–8.5) on glycosylation of p-hydroxybenzaldehyde catalyzed by mutant Δ60 (200 mM glucose, 35 °C, 0.025 g/mL, 10 h reaction) (c); Effect of mutant Δ60 cell loading (0.01–0.045 g/mL) on the glycosylation of p-hydroxybenzaldehyde (35 °C, pH 7.5, 200 mM glucose, 10 h reaction) (d).
3.6. Effects of Reaction Temperature and pH on the Glycosylation System
Enzyme activity is highly dependent on temperature, with distinct variations observed across different temperature ranges [30,31]. As illustrated in Figure 5b, the relative activity of glycosyltransferase in synthesizing p-hydroxybenzaldehyde β-glucoside was significantly influenced by reaction temperature. Between 25 °C and 35 °C, the relative activity increased with rising temperature, reaching its maximum at 35 °C. Upon raising temperature from 35 °C to 50 °C, a progressive decline in activity was observed. Over 35 °C, the elevated temperatures might lead to a marked reduction in catalytic performance. Beyond the optimal temperature, further temperature elevation may induce a progressive decline in bioreaction rate, as excessively high temperatures can trigger protein denaturation and subsequent structural alterations that lead to irreversible enzyme deactivation [32]. Consequently, 35 °C was the optimal temperature for the bioreaction.
The pH of bioreaction system significantly influences the glycosyltransferase activity. As illustrated in Figure 5c, the glycosylation activity gradually increased as the pH rose from 6.0 to 7.5, reaching maximum activity at pH 7.5. Beyond this optimal point, further increasing pH led to a progressive inhibition of enzymatic activity [33,34]. At pH 7.5, the glycosylation reaction activity began to be inhibited. Upon raising the pH from 7.5 to 8.5, the relative activity progressively declined. It can be concluded that the optimal pH for the bioreaction was 7.5.
3.7. The Impact of Bacterial Load on Reaction Outcomes
The cells loading could significantly influence the product yield of the relative activity (Figure 5d). Under the reaction temperature of 35 °C and pH 7.5, the relative activity was evaluated across varying bacterial concentrations in the presence of 2 mM p-hydroxybenzaldehyde (as substrate) and 200 mM glucose. The glycosylation activity elevated gradually as the bacterial cell loading was raised from 0.01 g/mL to 0.025 g/mL, reaching its maximum at 0.025 g/mL, followed by a gradual decline at higher loads. The enzyme exhibited the maximal relative activity when the bacterial concentration was 0.025 g/mL. This concentration represents the optimal bacterial concentration for the reaction. Any deviation from this optimal level might result in the reduced enzymatic activity [35,36,37]. It can be concluded that 0.025 g/mL represented the optimal bacterial concentration for the reaction.
3.8. Effects of Substrate Concentration on Biocatalytic Activity
The effect of substrate concentration on the reaction is evidenced in Figure 6a, depicting the reaction progress after 12 h for both the wild-type enzyme and the mutant Δ60. The impact of substrate concentration on the reaction is shown in Figure 6a, which presents the progress after 12 h of reaction. The activity of mutant Δ60 was higher than that of the wild type throughout. Notably, at a substrate concentration of 3 mM, the yield decreased significantly. As the substrate concentration further increased, the rate of the glycosylation reaction began to decline. Consequently, with increasing substrate loading, the yield progressively dropped at the same reaction time point (12 h). At high substrate concentrations, the reaction velocity decreased. This behavior indicated that the substrate inhibition occurred. The inhibition may arise because high substrate dose can disrupt the enzyme’s normal catalytic capacity, impairing its ability to catalyze the reaction efficiently [38]. Alternatively, it is possible that p-hydroxybenzaldehyde inhibited the activity of E. coli cells (or the enzyme within cell). As the substrate dose increased, this inhibitory effect would consequently weaken the relative activity [39]. A time-course experiment was conducted on mutant Δ60 (Figure 6b), and the results indicated that the response had not yet reached its maximum value before 24 h.
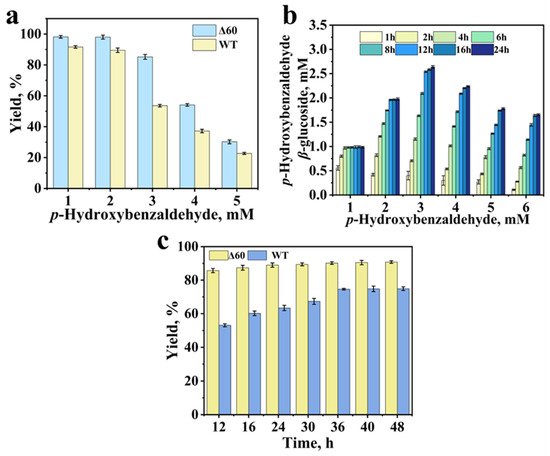
Figure 6.
Effect of substrate concentration on the catalytic synthesis of p-hydroxybenzaldehyde β-glucoside by wild-type and mutant Δ60 (12 h) (35 °C, pH 7.5, 200 mM glucose, 0.025 g/mL cell loading) (a); Time course of p-hydroxybenzaldehyde β-glucoside synthesis by mutant Δ60 at different substrate concentrations (0–24 h) (35 °C, pH 7.5, 200 mM glucose, 0.025 g/mL mutant Δ60 cell) (b); Time-dependent production of p-hydroxybenzaldehyde β-glucoside by wild-type and mutant Δ60 at 3 mM p-hydroxybenzaldehyde (0–48 h) (35 °C, pH 7.5, 200 mM glucose, 0.025 g/mL cell loading) (c).
A time-course experiment was implemented for converting 3 mM substrate (Figure 6c). The reaction essentially reached the maximum product concentration after 24 h. Figure 6a presents a comparison of the progress of the mutant and the wild type after 12 h of reaction, indicating that the substrate concentration has a significant impact on the reaction activity. Under the catalysis by Δ60, the accumulated maximum product concentration reached 2.7 mM.
Biomass represents an abundant and renewable biological resource primarily composed of cellulose, hemicellulose, and lignin [40,41,42]. While cellulose and hemicellulose have been extensively utilized [43,44,45], the valorization of lignin-derived compounds remains underdeveloped [46,47]. This study employed p-hydroxybenzaldehyde, a representative lignin derivative, as a model substrate to advance novel lignin valorization strategies. The biocatalytic transformation of p-hydroxybenzaldehyde into p-hydroxybenzaldehyde β-glucoside was implemented, simultaneously addressing limitations of traditional extraction methods and reducing environmental impacts inherent to chemical synthesis. Through the targeted mutagenesis of wild-type glycosyltransferase, the catalytic efficacy was enhanced, resulting in the accelerated glycosylation kinetics. Notably, substituting costly uridine diphosphate glucose (UDPG) with glucose as glycosyl donor can significantly reduce the production costs. Under optimized conditions, 2 mM p-hydroxybenzaldehyde was converted to the target product within 10 h with a yield of 97.8%. Furthermore, when the substrate concentration was increased to 3 mM, 2.7 mM (767.5 mg/L) of the product was obtained.
Further research efforts, guided by the results of this study, should leverage more systematic approaches to mutagenesis for advanced strain development. By strategically screening mutation sites to enhance substrate tolerance, a breakthrough increase in product yield and a simultaneous improvement in substrate loading capacity can ultimately be achieved [48,49,50]. Utilizing appropriate mutation strategy to significantly increase p-hydroxybenzaldehyde β-glucoside titers while maintaining relative activity, thereby advancing industrial feasibility of this bioprocess. The developed glycosylation methodology demonstrates both economic viability and operational robustness. Furthermore, expanding the substrate scope to include diverse aldehydes presents promising exploratory avenues. In a concise summary, this study establishes a green and efficient biocatalytic route for synthesizing p-hydroxybenzaldehyde β-glucoside while enabling high-value biomass utilization. To facilitate industrial-scale production, subsequent efforts should focus on reducing raw material costs and enhancing the relative activity of the enzyme through rational strain engineering.
4. Conclusions
The glycosyltransferase UGTBL1 was engineered via truncation mutation, yielding the mutant designated as Δ60, which exhibited the highest activity towards p-hydroxybenzaldehyde among the screened variants. A whole-cell catalytic system was subsequently developed to efficiently convert p-hydroxybenzaldehyde into p-hydroxybenzaldehyde β-glucoside. To enhance the economic feasibility of the process, glucose was employed as an inexpensive glycosyl donor substitute for costly UDPG. Under optimized reaction conditions, a maximum product concentration of 2.7 mM (767.5 mg/L) could be achieved. There is limited information about the efficient transformation of p-hydroxybenzaldehyde into valuable p-hydroxybenzaldehyde β-glucoside. The truncated mutation method employed in this study could enhance the relative activity of glycosyltransferase, with a concentration of 2 mM of p-hydroxybenzaldehyde, the yield could reach 97.8% within 10 h, providing an eco-friendlier approach for the efficient synthesis of p-hydroxybenzaldehyde β-glucoside.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology14101358/s1. Figure S1: Electrophoresis profile of supernatant protein from cell lysate. Figure S2: HPLC chromatogram. Figure S3: HRMS spectrum of the substrate p-hydroxybenzaldehyde. Figure S4: HRMS spectrum of the product p-hydroxybenzaldehyde β-glucoside. Table S1: Primers employed in this study. Table S2: The amino acid sequence.
Author Contributions
Conceptualization, methodology and writing—original draft, B.F.; conceptualization, data curation, software, and writing—original draft, S.F.; data curation and software, Y.Z.; data curation and methodology, W.T.; supervision, review and revising manuscript, Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research is kindly funded by the National Natural Science Foundation of China (No. 22208031).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank the Analysis and Testing Center (Changzhou University) for analysis of biomass samples.
Conflicts of Interest
Author Yijun Zhu is a co-founder of Changzhou Pharmaceutical Factory Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Chen, Y.; Tang, Z.; Koffi, P.A.Y.; Tang, W.; Fan, B.; He, Y.-C. Promoted lignocellulose fractionation and improved enzymatic hydrolysis of corn stalks through cationic surfactant combined with deep eutectic solvent pretreatment. Int. J. Biol. Macromol. 2024, 282, 137150. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Ni, J.; He, Y.-C.; Ye, J. Chemoenzymatic catalytic synthesis of furfurylamine from hemicellulose in biomasses. Int. J. Biol. Macromol. 2022, 222, 1201–1210. [Google Scholar] [CrossRef] [PubMed]
- Batista-García, R.A.; del Rayo Sánchez-Carbente, M.; Talia, P.; Jackson, S.A.; O’Leary, N.D.; Dobson, A.D.W.; Folch-Mallol, J.L. From lignocellulosic metagenomes to lignocellulolytic genes: Trends, challenges and future prospects. Biofuels Bioprod. Biorefin. 2016, 10, 864–882. [Google Scholar] [CrossRef]
- Wu, M.; Di, J.; Gong, L.; He, Y.-C.; Ma, C.; Deng, Y. Enhanced adipic acid production from sugarcane bagasse by a rapid room temperature pretreatment. Chem. Eng. J. 2023, 452, 139320. [Google Scholar] [CrossRef]
- Yang, Q.; Fan, B.; He, Y.-C. Combination of solid acid and solvent pretreatment for co-production of furfural, xylooligosaccharide and reducing sugars from Phyllostachys edulis. Bioresour. Technol. 2024, 395, 130398. [Google Scholar] [CrossRef]
- D’Arrigo, P.; Rossato, L.A.M.; Strini, A.; Serra, S. From waste to value: Recent insights into producing vanillin from lignin. Molecules 2024, 29, 442. [Google Scholar] [CrossRef]
- Jyoti; Dwivedi, P.; Negi, P.; Chauhan, R.; Gosavi, S.W.; Mishra, B.B. Alkaline hydrolysis of spent aromatic biomass for production of phenolic aldehydes, lignin, and cellulose. Bioresour. Technol. 2023, 387, 129659. [Google Scholar] [CrossRef]
- Sun, Q.; Fu, X.; Wang, P.; Li, K.; Wei, L.; Zhai, S.; An, Q. Selective production of p-hydroxybenzaldehyde in the oxidative depolymerization of alkali lignin catalyzed by copper-containing imidazolium-based ionic liquids. J. Mol. Liq. 2023, 385, 122378. [Google Scholar] [CrossRef]
- Li, Y.-X.; Zhu, J.-P.; Zhang, Z.-J.; Qu, Y.-S. Preparation of Syringaldehyde from Lignin by Catalytic Oxidation of Perovskite-Type Oxides. ACS Omega 2020, 5, 2107–2113. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yi, L.; Zhang, G.; Ye, B. Yeast-based whole-cell factory for the ecofriendly synthesis of yanillylamine as a critical step toward capsaicin production. ACS Sustain. Chem. Eng. 2023, 11, 7683–7691. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, S.; Yang, J.; Ye, N.; Gao, F.; Gallou, F.; Gao, L.; Lei, X. Chemoenzymatic synthesis of 2-aryl thiazolines from 4-hydroxybenzaldehydes using vanillyl alcohol oxidases. Angew. Chem. Int. Ed. 2024, 63, e202405833. [Google Scholar] [CrossRef]
- Harmandar, K.; Giray, G.; Önal, E.; Sengul, I.F.; Özdemir, S.; Atilla, D. New AB3-type porphyrins with piperidine and morpholine motifs; synthesis and photo-physicochemical and biological properties. Dalton Trans. 2023, 52, 2672–2683. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.; Zhou, Z.; Qi, W.; Jiang, S.; Yang, B.; Zhong, Z.; Jia, Y.; Li, X.; Xiong, L.; Nie, L. Antidepressant effect of helicid in chronic unpredictable mild stress model in rats. Int. Immunopharmacol. 2019, 67, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, H.; Gele, T.; Hu, C.; Liu, C.; Song, W.; Wei, L.; Song, D.; Jin, M.; Tang, Y.; et al. Helicid: A novel anti-staphylococcus aureus adjuvant. Biochimie 2025, 231, 46–60. [Google Scholar] [CrossRef]
- Wen, H.; Lin, C.; Que, L.; Ge, H.; Ma, L.; Cao, R.; Wan, Y.; Peng, W.; Wang, Z.; Song, H. Synthesis and biological evaluation of helicid analogues as novel acetylcholinesterase inhibitors. Eur. J. Med. Chem. 2008, 43, 166–173. [Google Scholar] [CrossRef]
- He, X.-Q.; Li, H.-B.; Li, T.; Chen, X.-Y.; Wang, Z.-Z.; Yao, X.-S.; Xiao, W.; Yu, Y. One undescribed glycoside benzofuran derivative and a new p-hydroxybenzoate glycoside from the leaves of Illicium dunnianum Tutcher. Nat. Prod. Res. 2024, 38, 3130–3139. [Google Scholar] [CrossRef]
- Yi, W.; Cao, R.; Wen, H.; Yan, Q.; Zhou, B.; Wan, Y.; Ma, L.; Song, H. Synthesis and biological evaluation of helicid analogues as mushroom tyrosinase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 6490–6493. [Google Scholar] [CrossRef]
- Ali, M.Y.; Liaqat, F.; Khazi, M.I.; Sethupathy, S.; Zhu, D. Utilization of glycosyltransferases as a seamless tool for synthesis and modification of the oligosaccharides—A review. Int. J. Biol. Macromol. 2023, 249, 125916. [Google Scholar] [CrossRef]
- Guidi, C.; Biarnés, X.; Planas, A.; De Mey, M. Controlled processivity in glycosyltransferases: A way to expand the enzymatic toolbox. Biotechnol. Adv. 2023, 63, 108081. [Google Scholar] [CrossRef]
- Madavi, T.B.; Chauhan, S.; Keshri, A.; Alavilli, H.; Choi, K.-Y.; Pamidimarri, S.D.V.N. Whole-cell biocatalysis: Advancements toward the biosynthesis of fuels. Biofuels Bioprod. Biorefin. 2022, 16, 859–876. [Google Scholar] [CrossRef]
- Ezugwu, J.A.; Okoro, U.C.; Ezeokonkwo, M.A.; Hariprasad, K.S.; Rudrapal, M.; Gogoi, N.; Chetia, D.; Ugwu, D.I.; Eze, F.U.; Onyeyilim, L.E.; et al. Design, Synthesis, Molecular Docking, Drug-Likeness/ADMET and Molecular Dynamics Studies of Thiazolyl Benzenesulfonamide Carboxylates as Antimalarial Agents. Chem. Afr. 2024, 7, 2353–2368. [Google Scholar] [CrossRef]
- Fan, B.; Dong, W.; Chen, T.; Chu, J.; He, B. Switching glycosyltransferase UGTBL1 regioselectivity toward polydatin synthesis using a semi-rational design. Org. Biomol. Chem. 2018, 16, 2464–2469. [Google Scholar] [CrossRef]
- Wang, X.; Hatta, S.; Matsui, D.; Imamura, H.; Wakayama, M. Expression and characterization of c-terminal truncated mutants of γ-glutamyltranspeptidase II (PaGGTII) from pseudomonas aeruginosa PAO1. Protein Expr. Purif. 2023, 210, 106321. [Google Scholar] [CrossRef]
- Tu, M.; Feng, L.; Wang, Z.; Qiao, M.; Shahidi, F.; Lu, W.; Du, M. Sequence analysis and molecular docking of antithrombotic peptides from casein hydrolysate by trypsin digestion. J. Funct. Foods 2017, 32, 313–323. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Chen, Z. Insights into the molecular mechanisms of protein-ligand interactions by molecular docking and molecular dynamics simulation: A case of oligopeptide binding protein. Comput. Math. Methods Med. 2018, 2018, 3502514. [Google Scholar] [CrossRef]
- Guo, J.; Kong, L.; Tian, L.; Han, Y.; Teng, C.; Ma, H.; Tao, B. Molecular docking and mutation sites of CYP57A1 enzyme with Fomesafen. Pestic. Biochem. Physiol. 2025, 209, 106328. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Z.; Xiong, J.; He, Y. Sustainable chemoenzymatic cascade transformation of corncob to furfuryl alcohol with rice husk-based heterogeneous catalyst UST-Sn-RH. Catalysts 2023, 13, 37. [Google Scholar] [CrossRef]
- Wu, Y.; Shen, J.; Yang, D.; Xu, D.; Huang, M.; He, Y. Production of furfuryl alcohol from corncob catalyzed by CCZU-KF cell via chemoenzymatic approach. Acad. J. Sci. Technol. 2023, 6, 132–138. [Google Scholar] [CrossRef]
- Fan, B.; Lin, R.; Gu, X.; Cui, R.; Yang, R.; He, Y.-C. Synthesis of biobased alcohols from biomass-derived aldehydes through the biocatalysis with E. coli KPADH in deep eutectic solvent ChCl:Glycerol-water. Mol. Catal. 2025, 584, 115305. [Google Scholar] [CrossRef]
- Yang, S.; Yan, T.; Zhao, L.; Wu, H.; Du, Z.; Yan, T.; Xiao, Q. Effects of temperature on activities of antioxidant enzymes and Na+/K+-ATPase, and hormone levels in Schizothorax prenanti. J. Therm. Biol. 2018, 72, 155–160. [Google Scholar] [CrossRef]
- McLeod, M.J.; Barwell, S.A.E.; Holyoak, T.; Thorne, R.E. A structural perspective on the temperature dependent activity of enzymes. Structure 2025, 33, 924–934.e922. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.F.; Ju, L.-K. On optimization of enzymatic processes: Temperature effects on activity and long-term deactivation kinetics. Process Biochem. 2023, 130, 734–746. [Google Scholar] [CrossRef]
- Tijskens, L.M.M.; Greiner, R.; Biekman, E.S.A.; Konietzny, U. Modeling the effect of temperature and pH on activity of enzymes: The case of phytases. Biotechnol. Bioeng. 2001, 72, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Ma, C.; He, Y.-C. Chemobiocatalytic transfromation of biomass into furfurylamine with mixed amine donor in an eco-friendly medium. Bioresour. Technol. 2023, 387, 129638. [Google Scholar] [CrossRef]
- Liang, J.; Ji, L.; He, J.; Tang, S.; He, Y. Chemoenzymatic conversion of biomass-derived D-Xylose to furfuryl alcohol with corn stalk-based solid acid catalyst and reductase biocatalyst in a deep eutectic solvent–water system. Processes 2022, 10, 113. [Google Scholar] [CrossRef]
- Gomes e Silva, N.C.; de Albuquerque, T.L.; Neto, C.A.G.; Gonçalves, L.R.B.; Rocha, M.V.P.; Fernandez-Lafuente, R. Effect of biocatalysts β-galactosidase loading in their performance in the kinetically controlled synthesis of lactulose. Process Biochem. 2024, 146, 169–175. [Google Scholar] [CrossRef]
- Tang, Z.; Li, Q.; Di, J.; Ma, C.; He, Y.-C. An efficient chemoenzymatic cascade strategy for transforming biomass into furfurylamine with lobster shell-based chemocatalyst and mutated ω-transaminase biocatalyst in methyl isobutyl ketone-water. Bioresour. Technol. 2023, 369, 128424. [Google Scholar] [CrossRef]
- Li, Q.; Jing, N.; Leng, X.; Liu, W.; Li, Q.; Yang, K.; Wang, X.; Yao, J. Substrate inhibition of the highly efficient PET hydrolase. Appl. Biochem. Microbiol. 2024, 60, 280–286. [Google Scholar] [CrossRef]
- Le, H.M.; Do, Q.T.; Doan, M.H.; Vu, Q.T.; Nguyen, M.A.; Vu, T.H.; Nguyen, H.D.; Duong, N.T.; Tran, M.H.; Chau, V.M.; et al. Chemical composition and biological activities of metabolites from the marine fungi penicillium sp. isolated from sediments of co to island, vietnam. Molecules 2019, 24, 3830. [Google Scholar] [CrossRef]
- Wang, N.; Xu, A.; Liu, K.; Zhao, Z.; Li, H.; Gao, X. Performance of green solvents in microwave-assisted pretreatment of lignocellulose. Chem. Eng. J. 2024, 482, 148786. [Google Scholar] [CrossRef]
- Ji, Q.; Yu, X.; Yagoub, A.E.-G.A.; Chen, L.; Zhou, C. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Capetti, C.C.D.M.; Pellegrini, V.O.A.; Espirito Santo, M.C.; Cortez, A.A.; Falvo, M.; Curvelo, A.A.D.S.; Campos, E.; Filgueiras, J.G.; Guimaraes, F.E.G.; de Azevedo, E.R.; et al. Enzymatic production of xylooligosaccharides from corn cobs: Assessment of two different pretreatment strategies. Carbohydr. Polym. 2023, 299, 120174. [Google Scholar] [CrossRef] [PubMed]
- Bian, H.; Yang, Y.; Tu, P.; Chen, J.Y. Value-added utilization of wheat straw: From cellulose and cellulose nanofiber to all-cellulose nanocomposite film. Membranes 2022, 12, 475. [Google Scholar] [CrossRef]
- Avanthi, A.; Kumar, S.; Sherpa, K.C.; Banerjee, R. Bioconversion of hemicelluloses of lignocellulosic biomass to ethanol: An attempt to utilize pentose sugars. Biofuels 2017, 8, 431–444. [Google Scholar] [CrossRef]
- Chibrikov, V.; Pieczywek, P.M.; Cybulska, J.; Zdunek, A. The effect of hemicelluloses on biosynthesis, structure and mechanical performance of bacterial cellulose-hemicellulose hydrogels. Sci. Rep. 2024, 14, 21671. [Google Scholar] [CrossRef]
- Ekielski, A.; Mishra, P.K. Lignin for Bioeconomy: The Present and Future Role of Technical Lignin. Int. J. Mol. Sci. 2021, 22, 63. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Li, L.; Ye, L.; Lin, Y.; Zhang, W.; Liao, X.; Liang, S. Enhancing the substrate tolerance of DszC by a combination of alanine scanning and site-directed saturation mutagenesis. J. Ind. Microbiol. Biotechnol. 2020, 47, 395–402. [Google Scholar] [CrossRef]
- Dong, T.-T.; Gong, J.-S.; Gu, B.-C.; Zhang, Q.; Li, H.; Lu, Z.-M.; Lu, M.-L.; Shi, J.-S.; Xu, Z.-H. Significantly enhanced substrate tolerance of Pseudomonas putida nitrilase via atmospheric and room temperature plasma and cell immobilization. Bioresour. Technol. 2017, 244, 1104–1110. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, Q.; Zhou, J.-H.; Xu, G. Enhanced activity and substrate tolerance of 7α-hydroxysteroid dehydrogenase by directed evolution for 7-ketolithocholic acid production. Appl. Microbiol. Biotechnol. 2019, 103, 2665–2674. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).