Antibacterial and Immunostimulatory Effects of Raziz Date Palm Pits in Streptococcus agalactiae-Infected Red Hybrid Tilapia
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Date Palm Pits
2.2. Preparation of Date Pit Extracts
2.3. GCMS Analysis
2.4. Bacterial Strain and Growth Condition
2.5. Antibiotics’ Efficacy Determination
2.6. Antibacterial Activity
2.7. In Vitro Immune Assays
2.7.1. Leucocyte Suspension Collection
2.7.2. Lysozyme Assay
2.7.3. Myeloperoxidase Assay
2.7.4. Respiratory Burst Assay
2.8. S. agalactiae Infection in Adult Red Hybrid Tilapia
2.8.1. Experimental Set-Up
S. agalactiae Culture
Infection of S. agalactiae
2.8.2. In Vivo Immune Assays
2.8.3. Bacterial Load Assay
2.9. Statistical Analysis
3. Results
3.1. Antibacterial Activity of Date Palm Pit Extracts
3.2. GC-MS Analysis
3.3. In Vitro Immune Assays
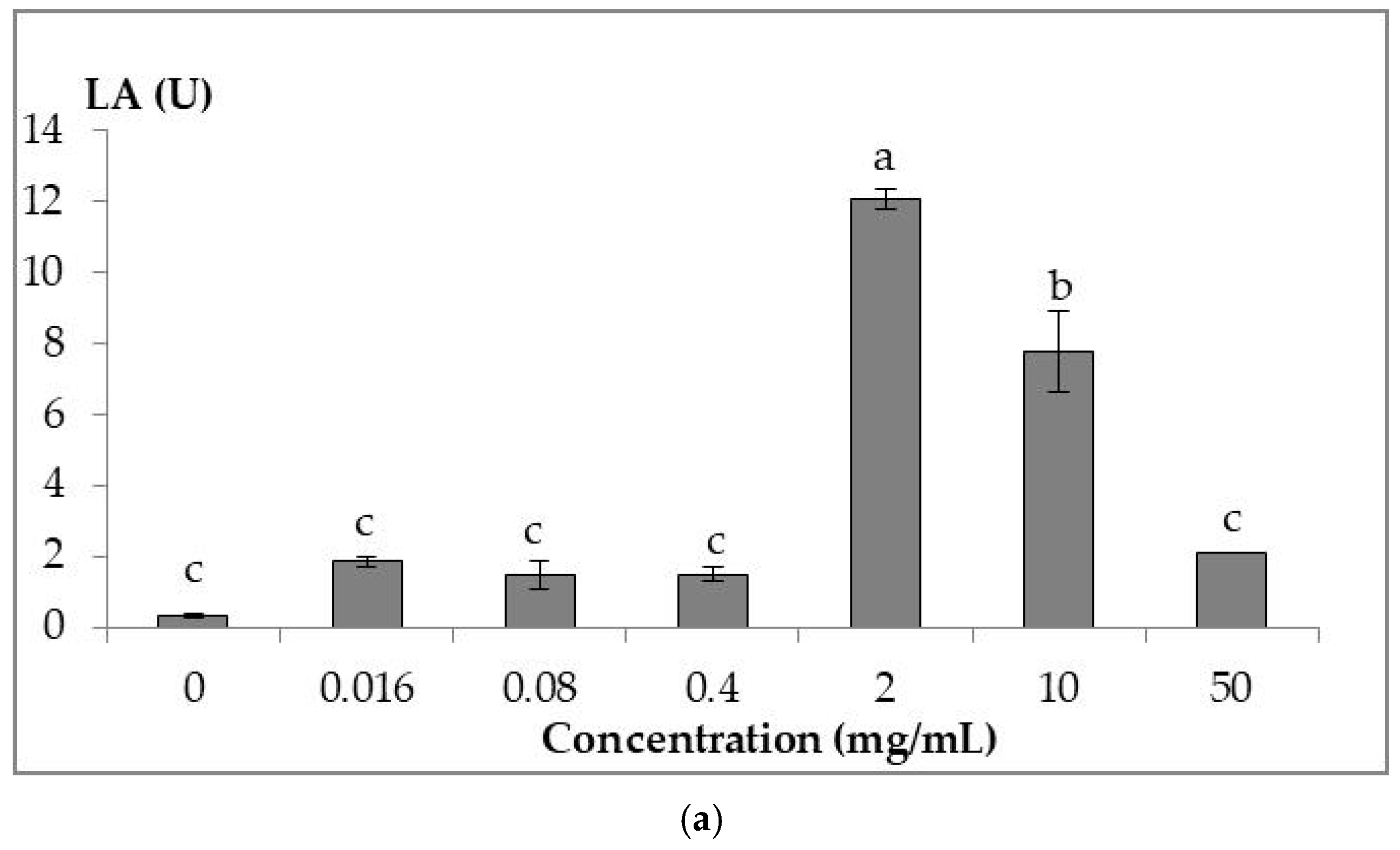
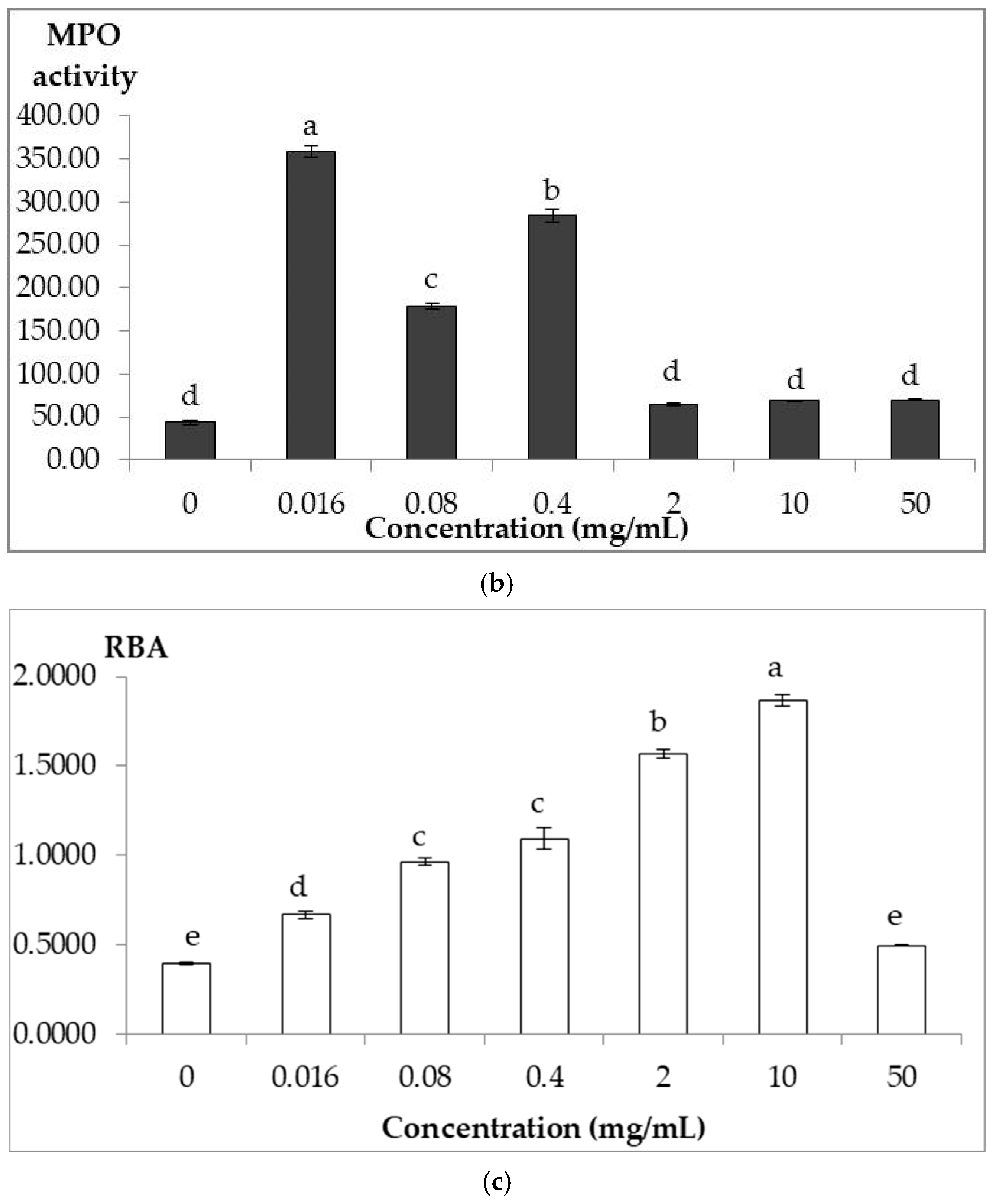
3.3.1. Lysozyme Assay
3.3.2. Myeloperoxidase Assay
3.3.3. Respiratory Burst Assay
3.4. In Vivo Study of RDPME Effect on Tilapia
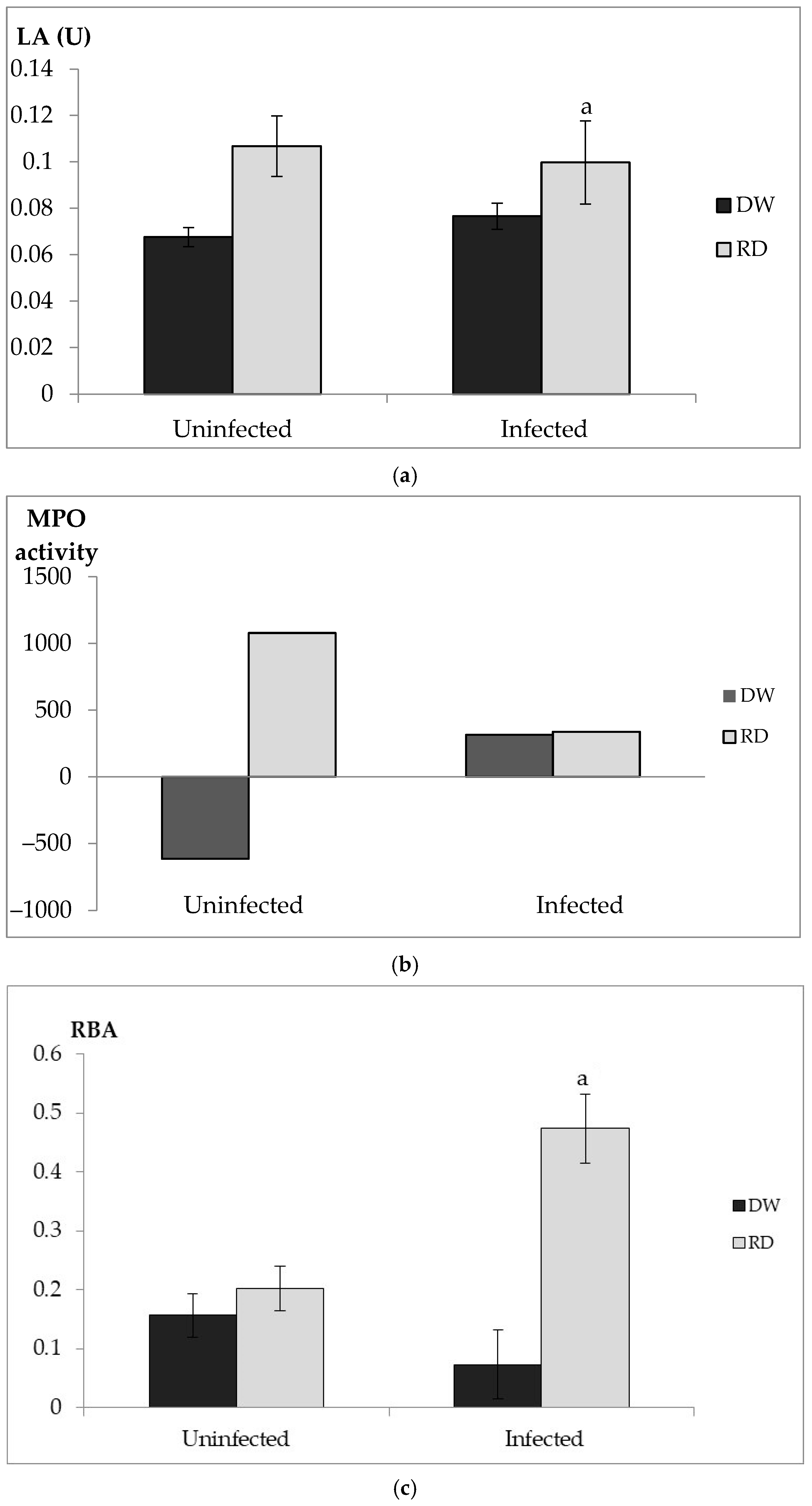
3.4.1. In Vivo Immune Assay
Lysozyme Assay
Myeloperoxidase Assay
Respiratory Burst Assay
3.4.2. Bacterial Load Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture. In FAO Yearbook of Fishery and Aquaculture Statistics; FAO: Rome, Italy, 2024. [Google Scholar]
- Amal, M.N.A.; Zamri-Saad, M. Streptococcosis in Tilapia (Oreochromis niloticus): A Review. Pertanika J. Trop. Agric. Sci. 2011, 34, 195–206. [Google Scholar]
- Zamri-Saad, M.; Amal, M.N.A.; Siti-Zahrah, A.; Zulkafli, A.R. Control and Prevention of Streptococcosis in Cultured Tilapia in Malaysia: A Review. Pertanika J. Trop. Agric. Sci. 2014, 37, 389–410. [Google Scholar]
- Debnath, S.C.; McMurtrie, J.; Temperton, B.; Delamare-Deboutteville, J.; Mohan, C.V.; Tyler, C.R. Tilapia Aquaculture, Emerging Diseases, and the Roles of the Skin Microbiomes in Health and Disease. Aquac. Int. 2023, 31, 2945–2976. [Google Scholar] [CrossRef]
- Slotved, H.C.; Møller, J.K.; Khalil, M.R.; Nielsen, S.Y. The Serotype Distribution of Streptococcus agalactiae (GBS) Carriage Isolates among Pregnant Women Having Risk Factors for Early-Onset GBS Disease: A Comparative Study with GBS Causing Invasive Infections during the Same Period in Denmark. BMC Infect. Dis. 2021, 21, 1129. [Google Scholar] [CrossRef]
- Raabe, V.N.; Shane, A.L. Group B Streptococcus (Streptococcus agalactiae). Microbiol. Spectr. 2019, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Delannoy, C.M.J.; Crumlish, M.; Fontaine, M.C.; Pollock, J.; Foster, G.; Dagleish, M.P.; Turnbull, J.F.; Zadoks, R.N. Human Streptococcus agalactiae Strains in Aquatic Mammals and Fish. BMC Microbiol. 2013, 13, 41. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Streptococcus agalactiae Activities. Available online: https://www.cdc.gov/strep-lab/php/group-b-strep/?CDC_AAref_Val=https://www.cdc.gov/streplab/groupb-strep/index.html (accessed on 7 July 2022).
- Chen, M.; Wang, R.; Luo, F.G.; Huang, Y.; Liang, W.W.; Huang, T.; Lei, A.Y.; Gan, X.; Li, L.P. Streptococcus agalactiae Isolates of Serotypes Ia, III and V from Human and Cow Are Able to Infect Tilapia. Vet. Microbiol. 2015, 180, 129–135. [Google Scholar] [CrossRef]
- Woldu, Z.L.; Teklehaimanot, T.G.; Waji, S.T.; Gebremariam, M.Y. The Prevalence of Group B Streptococus Recto-Vaginal Colonization and Antimicrobial Susceptibility Pattern in Pregnant Mothers at Two Hospitals of Addis Ababa, Ethiopia. Reprod. Health 2014, 11, 2–5. [Google Scholar] [CrossRef]
- Gizachew, M.; Tiruneh, M.; Moges, F.; Tessema, B. Streptococcus agalactiae Maternal Colonization, Antibiotic Resistance and Serotype Profiles in Africa: A Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Bolukaoto, J.Y.; Monyama, C.M.; Chukwu, M.O.; Lekala, S.M.; Nchabeleng, M.; Maloba, M.R.B.; Mavenyengwa, R.T.; Lebelo, S.L.; Monokoane, S.T.; Tshepuwane, C.; et al. Antibiotic Resistance of Streptococcus agalactiae Isolated from Pregnant Women in Garankuwa, South Africa. BMC Res. Notes 2015, 8, 364. [Google Scholar] [CrossRef]
- Chu, C.; Huang, P.Y.; Chen, H.M.; Wang, Y.H.; Tsai, I.A.; Lu, C.C.; Chen, C.C. Genetic and Pathogenic Difference Between Streptococcus agalactiae Serotype Ia Fish and Human Isolates. BMC Microbiol. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef] [PubMed]
- Maniyappan, K.K.; Girijan, S.K.; Krishnan, R.; Gopan, A.; Pillai, D. Assessing Multi-Drug Resistance in Streptococcus agalactiae Infecting Farmed Nile Tilapia: Findings from Kerala, India. Microb. Pathog. 2025, 205, 107666. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Moradkasani, S.; Suliman, M.; Uthirapathy, S.; Zwamel, A.H.; Hjazi, A.; Vashishth, R.; Beig, M. Global Patterns of Antibiotic Resistance in Group B Streptococcus: A Systematic Review and Meta-Analysis. Front. Microbiol. 2025, 16, 1–25. [Google Scholar] [CrossRef]
- Meroni, G.; Sora, V.M.; Martino, P.A.; Sbernini, A.; Laterza, G.; Zaghen, F.; Soggiu, A.; Zecconi, A. Epidemiology of Antimicrobial Resistance Genes in Streptococcus agalactiae Sequences from a Public Database in a One Health Perspective. Antibiotics 2022, 11, 1236. [Google Scholar] [CrossRef]
- Varijakzhan, D.; Chong, C.M.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. Middle Eastern Plant Extracts: An Alternative to Modern Medicine Problems. Molecules 2020, 25, 1126. [Google Scholar] [CrossRef] [PubMed]
- Varijakzhan, D.; Yang, S.-K.; Chong, C.M.; Akseer, R.; Alhosani, M.S.; Thomas, W.; Lai, K.S.; Lim, S.H.E. Essential Oils as Potential Antimicrobial Agents. In Mitigation of Antimicrobial Resistance; Panwar, H., Sharma, C., Lichtfouse, E., Eds.; Springer: Cham, Switzerland, 2021; pp. 93–122. [Google Scholar] [CrossRef]
- Karasawa, K.; Otani, H. Anti-Allergic Properties of a Matured Fruit Extract of the Date Palm Tree (Phoenix dactylifera L.) in Mite-Sensitized Mice. J. Nutr. Sci. Vitaminol. 2012, 58, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, V.; Shanmugavadivu, M.; Ganesh, S. Preliminary Phytochemical Screening and Anti-Bacterial Activity of Date Seed Methanolic Extract. Int. J. Adv. Res. Biol. Sci 2018, 5, 209–215. [Google Scholar] [CrossRef]
- Saryono; Warsinah; Isworo, A.; Sarmoko. Anti-Inflammatory Activity of Date Palm Seed by Downregulating Interleukin-1β, TGF-β, Cyclooxygenase-1 and -2: A Study among Middle Age Women. Saudi Pharm. J. 2020, 28, 1014–1018. [Google Scholar] [CrossRef]
- Al-Maadhedy, A.T.A.; Nafea, H.H.; Alubaydi, T.S.M. Effect of Partial Replacement of Palm Kernel Powder, Oyster Mushrooms and Commercial Enzyme Instead of Corn in the Diets on the Blood Traits of Common Carp Fingerling Fish (Cyprinus carpio L.). IOP Conf. Ser. Earth Environ. Sci. 2021, 761, 012091. [Google Scholar] [CrossRef]
- Orabi, S.H.; Shawky, S.M. Effect Of Date Palm (Phoenix dactylifera) Seeds Extracts On Hematological, Biochemical Parameters And Some Fertility Indices In Male Rats. Int. J. Sci. Basic Appl. Res. 2014, 17, 137–147. [Google Scholar]
- Kari, Z.A.; Goh, K.W.; Edinur, H.A.; Mat, K.; Khalid, H.N.M.; Rusli, N.D.; Sukri, S.A.M.; Harun, H.C.; Wei, L.S.; Hanafiah, M.H.B.M.A.; et al. Palm Date Meal as a Non-Traditional Ingredient for Feeding Aquatic Animals: A Review. Aquac. Rep. 2022, 25, 101233. [Google Scholar] [CrossRef]
- Sejpal, M.A.; Shi, L.; Xie, R.; Ghafoor, K.; Ahmadi, F.; Suleria, H.A.R. Date Palm Fruit (Phoenix dactylifera L.): Structure, Ripening, Nutrition, and Applications. Discov. Chem. 2025, 2, 169. [Google Scholar] [CrossRef]
- Bitwell, C.; Indra, S.S.; Luke, C.; Kakoma, M.K. A Review of Modern and Conventional Extraction Techniques and Their Applications for Extracting Phytochemicals from Plants. Sci. Afr. 2023, 19, e01585. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Monir, M.S. Development of Feed-Based Whole-Cells Inactivated Bivalent Vaccine and Its Immunoprotective Ability Against Streptococcosis and Motile Aeromonad Septicemia in Red Hybrid Tilapia (Oreochromis spp.); University Putra Malaysia: Serdang, Malaysia, 2021. [Google Scholar]
- Quade, M.J.; Roth, J.A. A Rapid, Direct Assay to Measure Degranulation of Bovine Neutrophil Primary Granules. Vet. Immunol. Immunopathol. 1997, 58, 239–248. [Google Scholar] [CrossRef]
- Yakubu, Y.; Talba, A.M.; Chong, C.M.; Ismail, I.S.; Shaari, K. Effect of Terminalia Catappa Methanol Leaf Extract on Nonspecific Innate Immune Responses and Disease Resistance of Red Hybrid Tilapia against Streptococcus agalactiae. Aquac. Rep. 2020, 18, 100555. [Google Scholar] [CrossRef]
- Mohamad, A.; Yamkasem, J.; Paimeeka, S.; Khemthong, M.; Lertwanakarn, T.; Setthawong, P.; Nuez-Ortin, W.G.; Isern Subich, M.M.; Surachetpong, W. Efficacy of Feed Additives on Immune Modulation and Disease Resistance in Tilapia in Coinfection Model with Tilapia Lake Virus and Aeromonas Hydrophila. Biology 2024, 13, 938. [Google Scholar] [CrossRef]
- Wohlsen, T.; Bates, J.; Vesey, G.; Robinson, W.A.; Katouli, M. Evaluation of the Methods for Enumerating Coliform Bacteria from Water Samples Using Precise Reference Standards. Lett. Appl. Microbiol. 2006, 42, 350–356. [Google Scholar] [CrossRef]
- McFarland Srandard; 2014. Dalynn Biologicals. McFarland Standard. Available online: http://www.dalynn.com/dyn/ck_assets/files/tech/TM53.pdf (accessed on 26 April 2022).
- He, R.Z.; Li, Z.C.; Li, S.Y.; Li, A.X. Development of an Immersion Challenge Model for Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Aquaculture 2020, 531, 735877. [Google Scholar] [CrossRef]
- Li, C.; Sapugahawatte, D.N.; Yang, Y.; Wong, K.T.; Lo, N.W.S.; Ip, M. Multidrug-Resistant Streptococcus agalactiae Strains Found in Human and Fish with High Penicillin and Cefotaxime Non-Susceptibilities. Microorganisms 2020, 8, 1055. [Google Scholar] [CrossRef]
- Elma, S.N.; Badarusham, K.; Rosli, D.; Salvamani, S.; Hassan, M.S.; Hashim, R. Solvents Extraction Effects on Bioactive Compounds of Ajwa Date (Phoenix dactylifera L.) Flesh Using Mixture Design. Chem. Eng. Trans. 2018, 63, 817–822. [Google Scholar] [CrossRef]
- Nawaz, H.; Shad, M.A.; Rehman, N.; Andaleeb, H.; Ullah, N. Effect of Solvent Polarity on Extraction Yield and Antioxidant Properties of Phytochemicals from Bean (Phaseolus vulgaris) Seeds. Braz. J. Pharm. Sci. 2020, 56, e17129. [Google Scholar] [CrossRef]
- Wakeel, A.; Jan, S.A.; Ullah, I.; Shinwari, Z.K.; Xu, M. Solvent Polarity Mediates Phytochemical Yield and Antioxidant Capacity of Isatis tinctoria. PeerJ 2019, 7, e7857. [Google Scholar] [CrossRef]
- Selim, S.; Abdel-Mawgoud, M.; Al-Sharary, T.; Almuhayawi, M.S.; Alruhaili, M.H.; Al Jaouni, S.K.; Warrad, M.; Mohamed, H.S.; Akhtar, N.; Abdelgawad, H. Pits of Date Palm: Bioactive Composition, Antibacterial Activity and Antimutagenicity Potentials. Agronomy 2022, 12, 54. [Google Scholar] [CrossRef]
- Hussain, M.I.; Semreen, M.H.; Shanableh, A.; Khattak, M.N.K.; Saadoun, I.; Ahmady, I.M.; Mousa, M.; Darwish, N.; Radeef, W.; Soliman, S.S.M. Phenolic Composition and Antimicrobial Activity of Different Emirati Date (Phoenix dactylifera L.) Pits: A Comparative Study. Plants 2019, 8, 497. [Google Scholar] [CrossRef]
- Dilika, F.; Bremner, P.D.; Meyer, J.J.M. Antibacterial Activity of Linoleic and Oleic Acids Isolated from Helichrysum pedunculatum: A Plant Used during Circumcision Rites. Fitoterapia 2000, 71, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-H.; Cai, M.; Liu, Y.-S.; Sun, P.-L.; Luo, S.-L. Antibacterial Activity and Mechanisms of Essential Oil from Citrus medica L. var. sarcodactylis. Molecules 2019, 24, 1577. [Google Scholar] [CrossRef]
- Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M.J. Antibacterial and Antifungal Activities of Fatty Acid Methyl Esters of the Blind-Your-Eye Mangrove from India. Braz. J. Microbiol. 2007, 38, 739–742. [Google Scholar] [CrossRef]
- Zheng, C.J.; Yoo, J.-S.; Lee, T.-G.; Cho, H.-Y.; Kim, Y.-H.; Kim, W.-G. Fatty Acid Synthesis is a Target for Antibacterial Activity of Unsaturated Fatty Acids. FEBS Lett. 2005, 579, 5157–5162. [Google Scholar] [CrossRef]
- Casillas-Vargas, G.; Ocasio-Malavé, C.; Medina, S.; Morales-Guzmán, C.; Del Valle, R.G.; Carballeira, N.M.; Sanabria-Ríos, D.J. Antibacterial Fatty Acids: An Update of Possible Mechanisms of Action and Implications in the Development of the next-Generation of Antibacterial Agents. Prog. Lipid Res. 2021, 82, 101093. [Google Scholar] [CrossRef] [PubMed]
- Magnadóttir, B. Innate Immunity of Fish (Overview). Fish Shellfish Immunol. 2006, 20, 137–151. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.H.; Hong, J.M.; Guo, W.L.; Li, G.H.; Zhao, Z.C.; Zhou, Y.; Wang, S.F.; Sun, Y.; Li, J.L.; Zhang, D.D.; et al. The Screen Herbal Immunopotentiator and Research on Its Effect on the Innate Immune System and Disease Resistance of Nile Tilapia (Oreochromis niloticus) against Streptococcus agalactiae. Aquaculture 2021, 541, 736778. [Google Scholar] [CrossRef]
- Hampton, L.M.T.; Jeffries, M.K.S.; Venables, B.J. A Practical Guide for Assessing Respiratory Burst and Phagocytic Cell Activity in the Fathead Minnow, an Emerging Model for Immunotoxicity. MethodsX 2020, 7, 100992. [Google Scholar] [CrossRef] [PubMed]
- Ahmadifar, E.; Fallah, H.P.; Yousefi, M.; Dawood, M.A.O.; Hoseinifar, S.H.; Adineh, H.; Yilmaz, S.; Paolucci, M.; Van Doan, H. The Gene Regulatory Roles of Herbal Extracts on the Growth, Immune System, and Reproduction of Fish. Animals 2021, 11, 2167. [Google Scholar] [CrossRef]
- Mohammadi, M.; Soltani, M.; Siahpoosh, A.; Hosseini Shekarabi, S.P.; Shamsaie Mehrgan, M.; Lymbery, A. Effect of Date Palm (Phoenix dactylifera) Seed Extract as a Dietary Supplementation on Growth Performance Immunological Haematological Biochemical Parameters of Common Carp. Aquac. Res. 2018, 49, 2903–2912. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A.; El Asely, A.; Fadl, S.E.; Amin, A.A.; Paray, B.A.; Ahmed, H.A. Saccharomyces Cerevisiae Increases the Acceptability of Nile Tilapia (Oreochromis niloticus) to Date Palm Seed Meal. Aquac. Rep. 2020, 17, 100314. [Google Scholar] [CrossRef]
- Dawood, M.A.O.; Eweedah, N.M.; Khalafalla, M.M.; Khalid, A. Evaluation of Fermented Date Palm Seed Meal with Aspergillus Oryzae on the Growth, Digestion Capacity and Immune Response of Nile Tilapia (Oreochromis niloticus). Aquac. Nutr. 2020, 26, 828–841. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An Important Defence Molecule of Fish Innate Immune System. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Pulli, B.; Ali, M.; Forghani, R.; Schob, S.; Hsieh, K.L.C.; Wojtkiewicz, G.; Linnoila, J.J.; Chen, J.W. Measuring Myeloperoxidase Activity in Biological Samples. PLoS ONE 2013, 8, e67976. [Google Scholar] [CrossRef]
- García Beltrán, J.M.; Mahdhi, A.; Abdelkader, N.; Hatem, M.; Esteban, M.Á. Effect of the Administration of Date Palm Seeds (Phoenix dactylifera L.) in Gilthead Seabream (Sparus Aurata L.) Diets. J. Agric. Sci. Crop Res. 2020, 1, 1–20. [Google Scholar]
- Guardiola, F.A.; Porcino, C.; Cerezuela, R.; Cuesta, A.; Faggio, C.; Esteban, M.A. Impact of Date Palm Fruits Extracts and Probiotic Enriched Diet on Antioxidant Status, Innate Immune Response and Immune-Related Gene Expression of European Seabass (Dicentrarchus labrax). Fish Shellfish Immunol. 2016, 52, 298–308. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the Use of Different Solvents for Phytochemical Constituents, Antioxidants, and In Vitro Anti-Inflammatory Activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Urszula Radzikowska, A.O.R.; Sözener, Z.Ç.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 79–95. [Google Scholar] [CrossRef]
- Jüttner, B.; Kröplin, J.; Coldewey, S.M.; Witt, L.; Osthaus, W.A.; Weilbach, C.; Scheinichen, D. Unsaturated Long-Chain Fatty Acids Induce the Respiratory Burst of Human Neutrophils and Monocytes in Whole Blood. Nutr. Metab. 2008, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Wanten, G.J.A.; Janssen, F.P.; Naber, A.H.J. Saturated Triglycerides and Fatty Acids Activate Neutrophils Depending on Carbon Chain-Length. Eur. J. Clin. Investig. 2002, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Lusiastuti, A.M.; Suhermanto, A.; Hastilestari, B.R.; Suryanto, S.; Mawardi, M.; Sugiani, D.; Syahidah, D.; Sudaryatma, P.E.; Caruso, D. Impact of Temperature on the Virulence of Streptococcus agalactiae in Indonesian Aquaculture: A Better Vaccine Design Is Required. Vet. World 2024, 17, 682–689. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, Q.; Han, S.; Shi, J.; Yan, H.; Hu, D.; Yi, Y. Omics Analysis Revealed the Possible Mechanism of Streptococcus Disease Outbreak in Tilapia Under High Temperature. Fish Shellfish Immunol. 2023, 134, 108639. [Google Scholar] [CrossRef] [PubMed]
- Kayansamruaj, P.; Pirarat, N.; Hirono, I.; Rodkhum, C. Increasing of Temperature Induces Pathogenicity of Streptococcus agalactiae and the Up-Regulation of Inflammatory Related Genes in Infected Nile Tilapia (Oreochromis niloticus). Vet. Microbiol. 2014, 172, 265–271. [Google Scholar] [CrossRef]
- Damascena, H.L.; Silveira, W.A.A.; Castro, M.S.; Fontes, W. Neutrophil Activated by the Famous and Potent PMA (Phorbol Myristate Acetate). Cells 2022, 11, 2889. [Google Scholar] [CrossRef]
- Hotea, I.; Dragomirescu, M.; Berbecea, A.; Radulov, I. Phytochemicals as Alternatives to Antibiotics in Animal Production. In Antibiotics and Probiotics in Animal Food-Impact and Regulation; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Lillehoj, H.; Liu, Y.; Calsamiglia, S.; Fernandez-Miyakawa, M.E.; Chi, F.; Cravens, R.L.; Oh, S.; Gay, C.G. Phytochemicals as Antibiotic Alternatives to Promote Growth and Enhance Host Health. Vet. Res. 2018, 49, 76. [Google Scholar] [CrossRef]
- Arowolo, M.A.; He, J. Use of Probiotics and Botanical Extracts to Improve Ruminant Production in the Tropics: A Review. Anim. Nutr. 2018, 4, 241–249. [Google Scholar] [CrossRef]
- Alyileili, S.R.; Belal, I.E.H.; Hussein, A.S.; El-Tarabily, K.A. Effect of Inclusion of Degraded and Non-Degraded Date Pits in Broilers’ Diet on Their Intestinal Microbiota and Growth Performance. Animals 2020, 10, 2041. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.H.; Tahir, M.; Naz, S.; Alhidary, I.A.; Khan, R.U.; Losacco, C.; Tufarelli, V. Dried Date (Phoenix dactylifera L.) Meal Inclusion in the Diets of Broilers Affects Growth Performance, Carcass Traits, Nutrients Digestibility, Fecal Microbiota and Economics. Agriculture 2023, 13, 1978. [Google Scholar] [CrossRef]
- Abo-Raya, M.H.; Alshehri, K.M.; Abdelhameed, R.F.A.; Elbialy, Z.I.; Elhady, S.S.; Mohamed, R.A. Assessment of Growth-Related Parameters and Immune-Biochemical Profile of Nile Tilapia (Oreochromis niloticus) Fed Dietary Ulva fasciata Extract. Aquac. Res. 2021, 52, 3233–3246. [Google Scholar] [CrossRef]
- Heidarieh, M.; Gholamhosseini, A.; Sheikhzadeh, N.; Esteban, M.A. Effects of γ-Irradiated Date (Phoenix dactylifera) Fruit on Growth, Immunological and Antioxidant Parameters of Goldfish (Carassius auratus). Fishes 2023, 8, 251. [Google Scholar] [CrossRef]
- Natnan, M.E.; Low, C.F.; Chong, C.M.; Rungrassamee, W.; Baharum, S.N. The Effect of Oleic Acid-Enriched Diet in Hybrid Groupers (Epinephelus fuscoguttatus × Epinephelus lanceolatus) upon Infection with Vibrio vulnificus Using an LC-QTOF-MS Approach. J. Mar. Sci. Eng. 2023, 11, 1563. [Google Scholar] [CrossRef]
- Natnan, M.E.; Low, C.F.; Chong, C.M.; Daud, N.I.N.A.A.; Om, A.D.; Baharum, S.N. Comparison of Different Dietary Fatty Acids Supplement on the Immune Response of Hybrid Grouper (Epinephelus fuscoguttatus × Epinephelus lanceolatus) Challenged with Vibrio vulnificus. Biology 2022, 11, 1288. [Google Scholar] [CrossRef]
- Pereira, L.M.; Hatanaka, E.; Martins, E.F.; Oliveira, F.; Liberti, E.A.; Farsky, S.H.; Curi, R.; Pithon-Curi, T.C. Effect of Oleic and Linoleic Acids on the Inflammatory Phase of Wound Healing in Rats. Cell Biochem. Funct. 2008, 26, 197–204. [Google Scholar] [CrossRef]
- Reddy, K.V.K.; Naidu, K.A. Oleic Acid, Hydroxytyrosol and n-3 Fatty Acids Collectively Modulate Colitis through Reduction of Oxidative Stress and IL-8 Synthesis; In Vitro and In Vivo Studies. Int. Immunopharmacol. 2016, 35, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Zhan, W.; Peng, H.; Xie, S.; Deng, Y.; Zhu, T.; Cui, Y.; Cao, H.; Tang, Z.; Jin, M.; Zhou, Q. Dietary Lauric Acid Promoted Antioxidant and Immune Capacity by Improving Intestinal Structure and Microbial Population of Swimming Crab (Portunus trituberculatus). Fish Shellfish Immunol. 2024, 151, 109739. [Google Scholar] [CrossRef]

| Concentration (g/mL) | Zone of Inhibition # (mm) |
|---|---|
| Control | 20.222 ± 0.401 |
| 0.5 | 1.000 ± 1.00 b |
| 1 | 9.167 ± 0.441 a |
| 2 | 10.333 ± 0.333 a |
| No. | ID | Compounds Classification | Average Composition (%) |
|---|---|---|---|
| 1 | Furfural | Aldehyde, Furan | 0.54 |
| 2 | 4H-Pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- | Saponin (Triterpene glycoside) | 4.42 |
| 3 | 5-Hydroxymethylfurfural | Furan | 6.07 |
| 4 | Dodecanoic acid, methyl ester | Fatty acid | 1.27 |
| 5 | Dodecanoic acid | Saturated medium chain fatty acid | 14.73 |
| 6 | Tetradecanoic acid | Saturated long chain fatty acid | 7.78 |
| 7 | Hexadecanoic acid, methyl ester | Fatty acid | 0.23 |
| 8 | n-Hexadecanoic acid | Saturated long chain fatty acid | 7.91 |
| 9 | Oleic acid | Unsaturated fatty acid | 23.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varijakzhan, D.; Chong, C.-M.; Christianus, A.; Abushelaibi, A.; Lim, S.-H.E.; Cheng, W.-H.; Wangkahart, E.; Lai, K.-S. Antibacterial and Immunostimulatory Effects of Raziz Date Palm Pits in Streptococcus agalactiae-Infected Red Hybrid Tilapia. Biology 2025, 14, 1356. https://doi.org/10.3390/biology14101356
Varijakzhan D, Chong C-M, Christianus A, Abushelaibi A, Lim S-HE, Cheng W-H, Wangkahart E, Lai K-S. Antibacterial and Immunostimulatory Effects of Raziz Date Palm Pits in Streptococcus agalactiae-Infected Red Hybrid Tilapia. Biology. 2025; 14(10):1356. https://doi.org/10.3390/biology14101356
Chicago/Turabian StyleVarijakzhan, Disha, Chou-Min Chong, Annie Christianus, Aisha Abushelaibi, Swee-Hua Erin Lim, Wan-Hee Cheng, Eakapol Wangkahart, and Kok-Song Lai. 2025. "Antibacterial and Immunostimulatory Effects of Raziz Date Palm Pits in Streptococcus agalactiae-Infected Red Hybrid Tilapia" Biology 14, no. 10: 1356. https://doi.org/10.3390/biology14101356
APA StyleVarijakzhan, D., Chong, C.-M., Christianus, A., Abushelaibi, A., Lim, S.-H. E., Cheng, W.-H., Wangkahart, E., & Lai, K.-S. (2025). Antibacterial and Immunostimulatory Effects of Raziz Date Palm Pits in Streptococcus agalactiae-Infected Red Hybrid Tilapia. Biology, 14(10), 1356. https://doi.org/10.3390/biology14101356







