Comparative In Vitro Osteogenic Capacities of Bone Marrow- and Periosteal-Derived Progenitor Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bone Marrow Aspirate and Periosteum Collection
2.2. Sample Processing
2.3. Cellular Expansion and Proliferation Assays
2.4. In Vitro Osteogenesis
2.5. Reverse Transcription and Quantitative PCR
2.6. Alkaline Phosphatase (ALP) Enzymatic Activity Assay
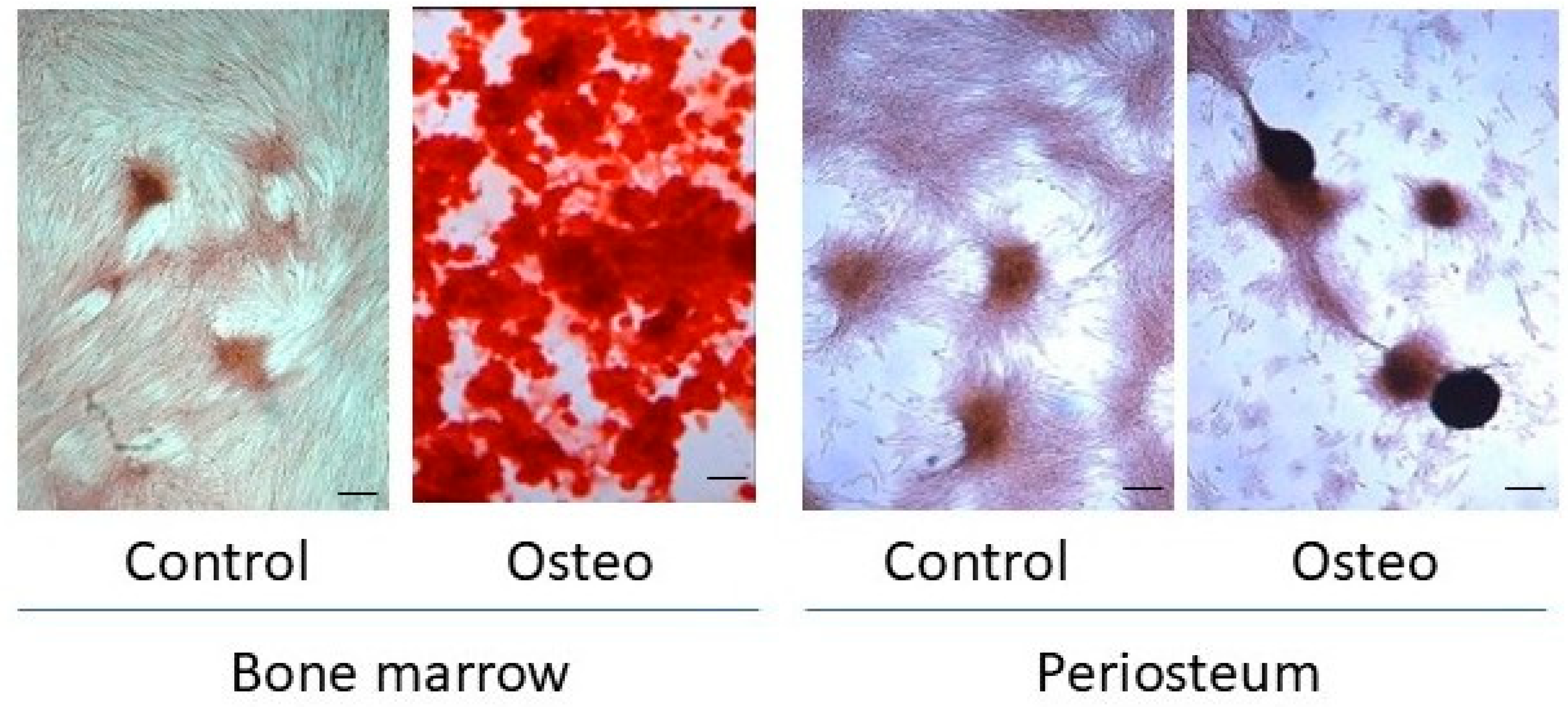
2.7. Alizarin Red Staining
2.8. Statistical Analyses
3. Results
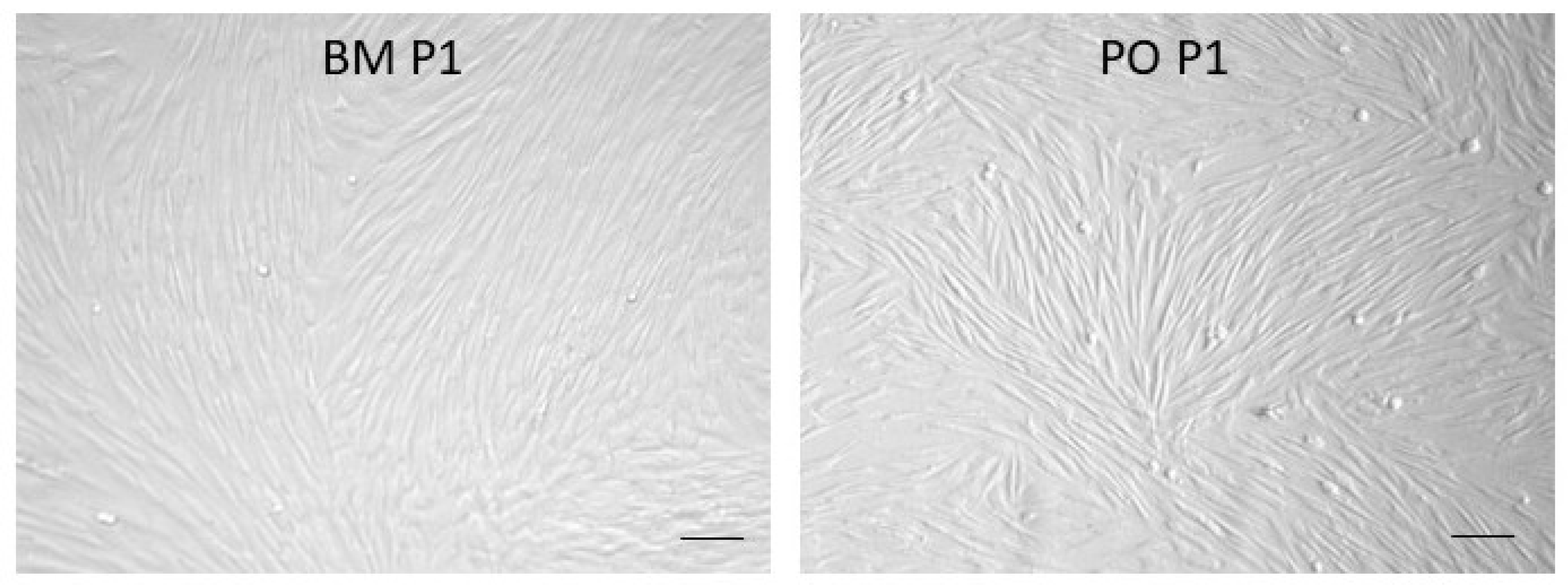
3.1. In Vitro Cell Expansion
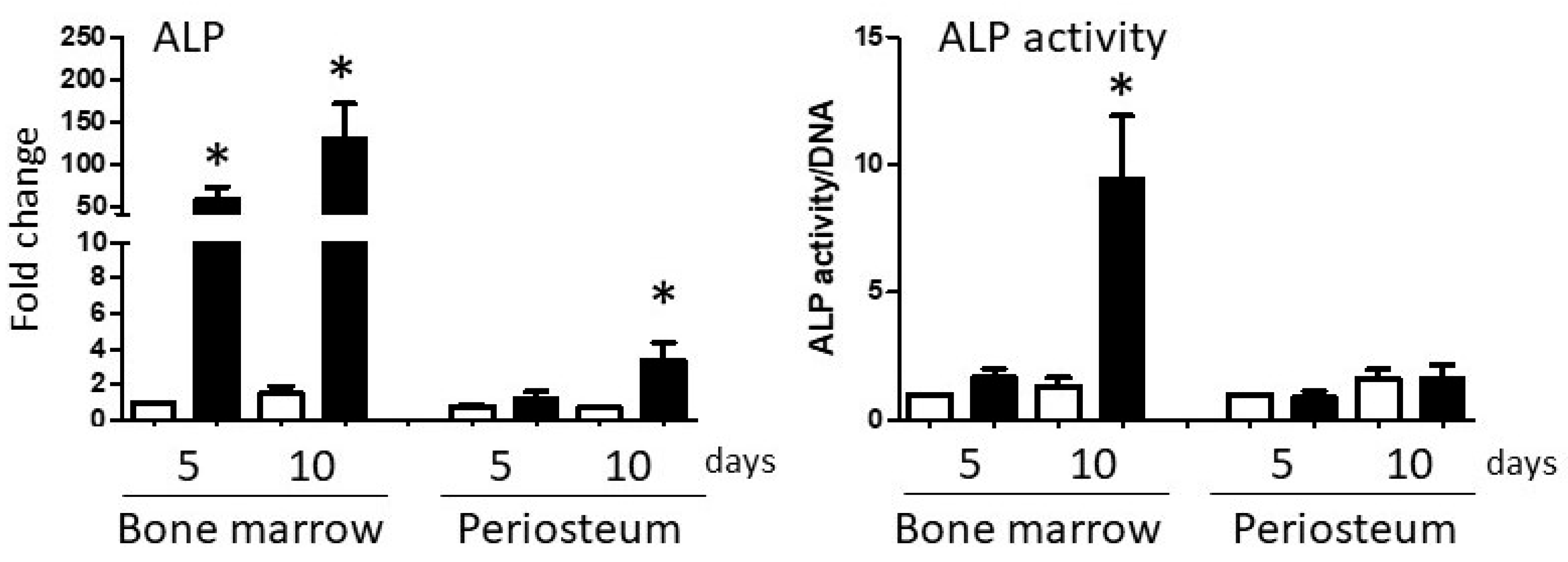
3.2. In Vitro Osteogenesis
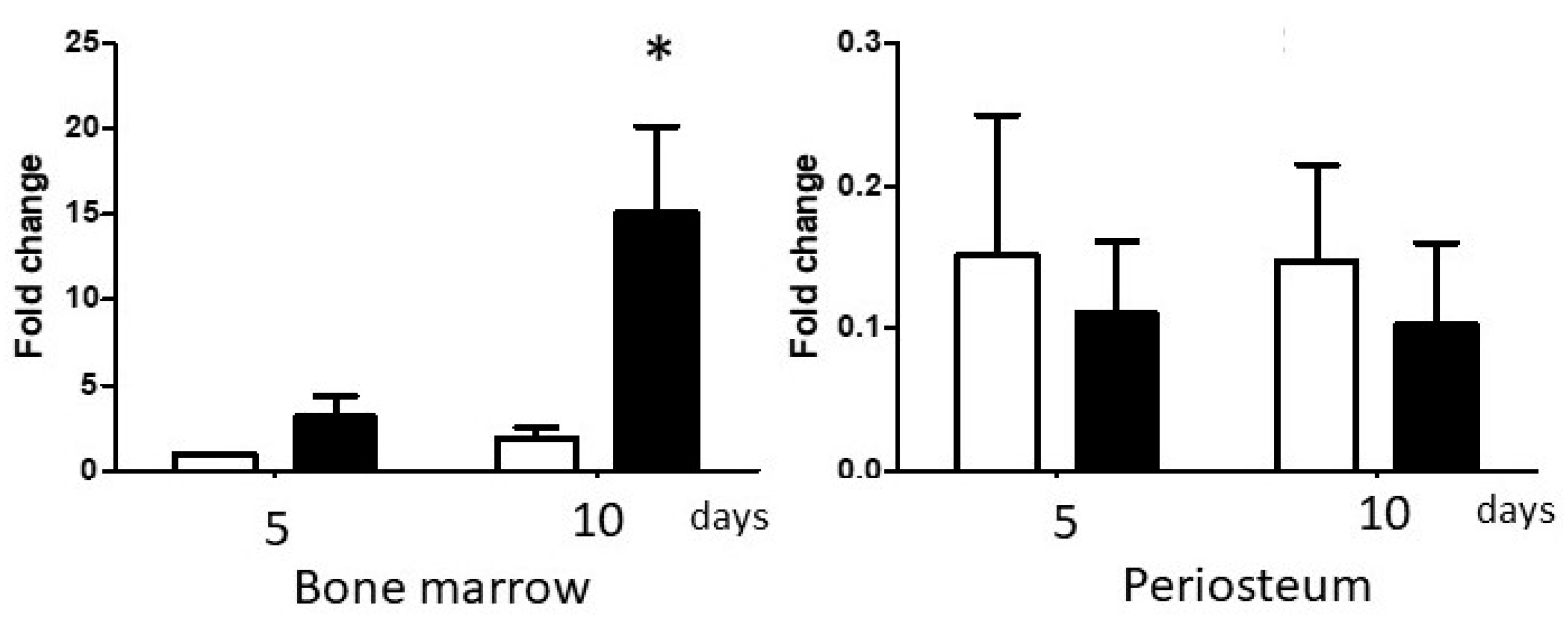
3.2.1. Differential Expression of BMP-2 in BM and PO Cell Cultures
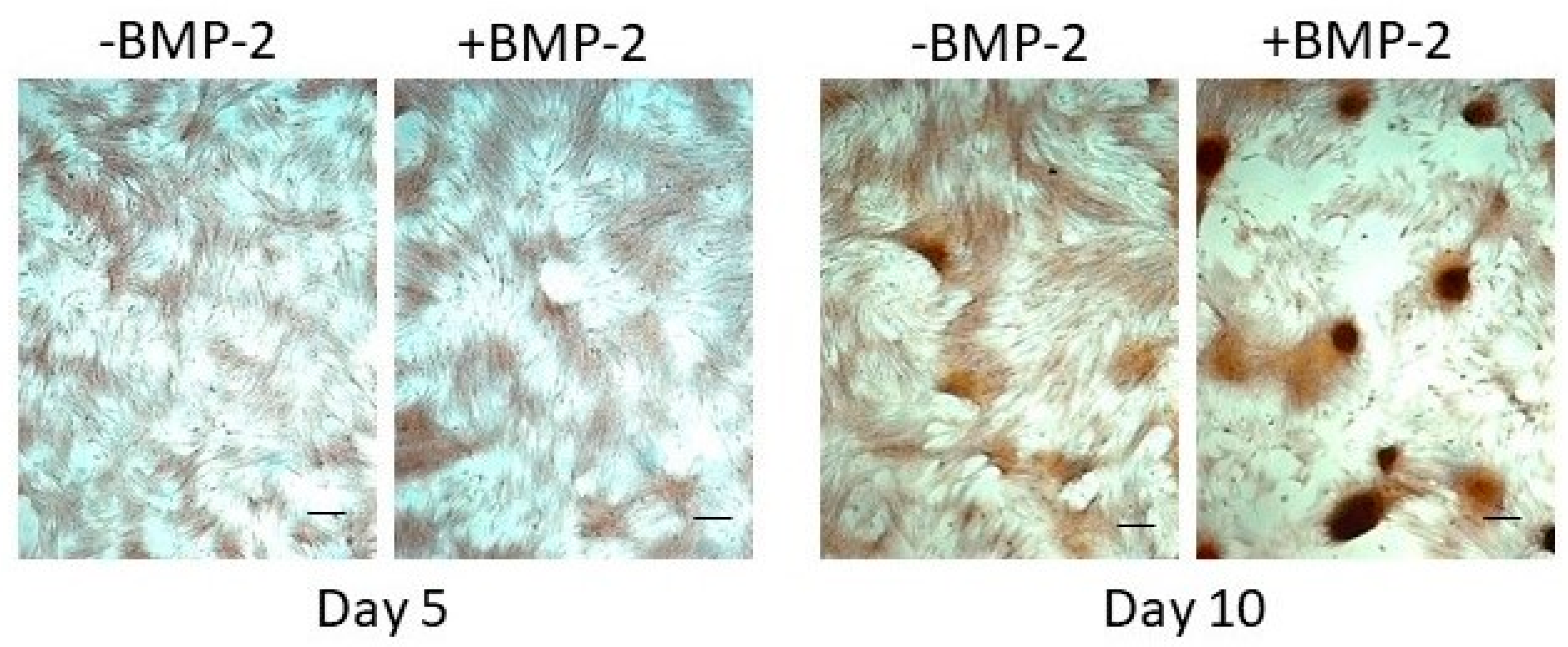
3.2.2. Exogenous BMP-2 Administration Does Not Restore Periosteal Cell Osteogenic Capacity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BM | Bone marrow-derived |
| IACUC | Institutional Animal Care and Use Committee |
| PO | Periosteal-derived |
| ALP | Alkaline phosphatase |
| ANOVA | Analysis of variance |
| CM | Centimeter |
| ML | Milliliter |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| FBS | Fetal bovine serum |
| Pen/Strep | Penicillin/Streptomycin |
| PBS | Phosphate-buffered saline |
| SEM | Standard error of the mean |
| RCF | Relative centrifugal field |
| IU | International unit |
| qPCR | Quantitative real-time polymerase chain reaction |
| TGF-β | Transforming growth factor beta |
References
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Debnath, S.; Yallowitz, A.R.; McCormick, J.; Lalani, S.; Zhang, T.; Xu, R.; Li, N.; Liu, Y.; Yang, Y.S.; Eiseman, M.; et al. Discovery of a periosteal stem cell mediating intramembranous bone formation. Nature 2018, 562, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Murao, H.; Yamamoto, K.; Matsuda, S.; Akiyama, H. Periosteal cells are a major source of soft callus in bone fracture. J. Bone Miner. Metab. 2013, 31, 390–398. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, X.; Binkle, D.D. Osteogenic differentiation of periosteal cells during fracture healing. J. Cell. Physiol. 2017, 232, 913–921. [Google Scholar] [CrossRef]
- Jeyaraman, M.; Muthu, S.; Gangadaran, P.; Ranjan, R.; Jeyaraman, N.; Prajwal, G.S.; Mishra, P.C.; Rajendran, R.L.; Ahn, B.C. Osteogenic and chondrogenic potential of periosteum-derived mesenchymal stromal cells: Do they hold the key to the future? Pharmaceuticals 2021, 14, 1133. [Google Scholar] [CrossRef]
- GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990–2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet Healthy Longev. 2021, 2, e580–e592. [Google Scholar] [CrossRef]
- Einhorn, T.A.; Gerstenfeld, L.C. Fracture healing: Mechanisms and interventions. Nat. Rev. Rheumatol. 2015, 11, 45–54. [Google Scholar] [CrossRef]
- Tzioupis, C.; Giannoudis, P.V. Prevalence of long-bone non-unions. Injury 2007, 38 (Suppl. 2), S3–S9. [Google Scholar] [CrossRef]
- Westgeest, J.; Weber, D.; Dulai, S.K.; Bergman, J.W.; Buckley, R.; Beaupre, L.A. Factors associated with development of nonunion or delayed healing after an open long bone fracture: A prospective cohort study of 736 subjects. J. Orthop. Trauma. 2016, 30, 149–155. [Google Scholar] [CrossRef]
- Maeda, Y.; Hanada, M.; Oikawa, M.A. Epidemiology of racing injuries in Thoroughbred racehorses with special reference to bone fractures: Japanese experience from the 1980s to 2000s. J. Equine Sci. 2016, 27, 81–97. [Google Scholar] [CrossRef]
- Rosanowski, S.M.; Chang, S.M.; Stirk, A.J.; Verheyen, K.L.P. Epidemiology of race-day distal limb fracture in flat racing Thoroughbreds in Great Britain (2000–2013). Equine Vet. J. 2019, 51, 83–89. [Google Scholar] [CrossRef]
- Claes, L. Improvement of clinical fracture healing—What can be learned from mechano-biological research? J. Biomech. 2021, 115, 110148. [Google Scholar] [CrossRef] [PubMed]
- Gerber, C.; Mast, J.W.; Ganz, R. Biological internal fixation of fractures. Arch. Orthop. Trauma. Surg. 1990, 109, 295–303, Erratum in Arch. Orthop. Trauma. Surg. 1991, 110, 226. [Google Scholar] [CrossRef] [PubMed]
- Maritato, K.C.; Barnhart, M.D. Minimally Invasive Fracture Repair. Vet. Clin. North. Am. Small Anim. Pract. 2020, 50, xiii–xiv. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.; Gronthos, S. Clinical Application of bone marrow mesenchymal stem/stromal cells to repair skeletal tissue. Int. J. Mol. Sci. 2020, 21, 9759. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef]
- Colnot, C.; Zhang, X.; Knothe Tate, M.L. Current insights on the regenerative potential of the periosteum: Molecular, cellular & endogenous engineering approaches. J. Orthop. Res. 2012, 30, 1869–1878. [Google Scholar] [CrossRef]
- Ferretti, C.; Mattioli-Belmonte, M. Periosteum derived stem cells for regenerative medicine proposals: Boosting current knowledge. World J. Stem Cells 2014, 6, 266–277. [Google Scholar] [CrossRef]
- Roberts, S.J.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Uncovering the periosteum for skeletal regeneration: The stem cell that lies beneath. Bone 2015, 70, 10–18. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, D.; Qi, S. Periosteum containing implicit stem cells: A progressive source of inspiration for bone tissue regeneration. Int. J. Mol. Sci. 2024, 25, 2162. [Google Scholar] [CrossRef]
- Komori, T.; Yagi, H.; Nomura, S.; Yamaguchi, A.; Sasaki, K.; Deguchi, K.; Shimizu, Y.; Bronson, R.T.; Gao, Y.H.; Inada, M.; et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 1997, 89, 755–764. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Kasashima, Y.; Ueno, T.; Tomita, A.; Goodship, A.E.; Smith, R.K. Optimisation of bone marrow aspiration from the equine sternum for the safe recovery of mesenchymal stem cells. Equine Vet. J. 2011, 43, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Bianchessi, M.; Chen, Y.; Durgam, S.; Pondenis, H.; Stewart, M. Effect of Fibroblast Growth Factor 2 on equine synovial fluid chondroprogenitor expansion and chondrogenesis. Stem Cells Int. 2015, 2016, 9364974. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kloen, P.; Lauzier, D.; Hamdy, R.C. Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 2012, 51, 59–68. [Google Scholar] [CrossRef]
- Tsuji, K.; Bandyopadhyay, A.; Harfe, B.D.; Cox, K.; Kakar, S.; Gerstenfeld, L.; Einhorn, T.; Tabin, C.J.; Rosen, V. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat. Genet. 2006, 38, 1424–1429. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Lieu, S.; Lu, C.; Colnot, C. Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 2010, 47, 65–73. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, C.; Xue, M.; Zhang, X. Expression of endogenous BMP-2 in periosteal progenitor cells is essential for bone healing. Bone 2011, 48, 524–532. [Google Scholar] [CrossRef]
- van Gastel, N.; Torrekens, S.; Roberts, S.J.; Moermans, K.; Schrooten, J.; Carmeliet, P.; Luttun, A.; Luyten, F.P.; Carmeliet, G. Engineering vascularized bone: Osteogenic and proangiogenic potential of murine periosteal cells. Stem Cells 2012, 30, 2460–2471. [Google Scholar] [CrossRef] [PubMed]
- Andrietti, A.L.P.; Durgam, S.S.; Naumann, B.; Stewart, M. Basal and inducible Osterix expression reflect equine mesenchymal progenitor cell osteogenic capacity. Front. Vet. Sci. 2023, 10, 1125893. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.; Ortinau, L.; Lei, K.; Park, D. Comparative analysis of gene expression identifies distinct molecular signatures of bone marrow- and periosteal-skeletal stem/progenitor cells. PLoS ONE 2018, 13, e0190909. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Docheve, D.; Knothe, U.R.; Knothe Tate, M.L. Arthritic periosteal tissue from joint replacement surgery: A novel, autologous source of stem cells. Stem Cells Transl. Med. 2014, 3, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Jaquiéry, C.; Schaeren, S.; Farhadi, J.; Mainil-Varlet, P.; Kunz, C.; Zeilhofer, H.F.; Heberer, M.; Martin, I. In vitro osteogenic differentiation and in vivo bone-forming capacity of human isogenic jaw periosteal cells and bone marrow stromal cells. Ann. Surg. 2005, 242, 859–868. [Google Scholar] [CrossRef]
- van Gastel, N.; Stegen, S.; Stockmans, I.; Moermans, K.; Schrooten, J.; Graf, D.; Luyten, F.P.; Carmeliet, G. Expansion of murine periosteal progenitor cells with fibroblast growth factor 2 reveals an intrinsic endochondral ossification program mediated by bone morphogenetic protein 2. Stem Cells 2014, 32, 2407–2418. [Google Scholar] [CrossRef]
- Ryu, Y.M.; Hah, Y.S.; Park, B.W.; Kim, D.R.; Roh, G.S.; Kim, J.R.; Kim, U.K.; Rho, G.J.; Maeng, G.H.; Byun, J.H. Osteogenic differentiation of human periosteal-derived cells in a three-dimensional collagen scaffold. Mol. Biol. Rep. 2011, 38, 2887–2894. [Google Scholar] [CrossRef]
- Roberts, S.J.; Chen, Y.; Moesen, M.; Schrooten, J.; Luyten, F.P. Enhancement of osteogenic gene expression for the differentiation of human periosteal derived cells. Stem Cell Res. 2011, 7, 137–144. [Google Scholar] [CrossRef]
- Schäfer, R.; DeBaun, M.R.; Fleck, E.; Centeno, C.J.; Kraft, D.; Leibacher, J.; Dieback, K.; Seifried, E.; Dragoo, J.L. Quantitation of progenitor cell populations and growth factors after bone marrow aspirate concentration. J. Transl. Med. 2019, 17, 115. [Google Scholar] [CrossRef]
- Hegde, V.; Shonuga, O.; Ellis, S.; Fragomen, A.; Kennedy, J.; Kudryashov, V.; Lane, J.M. A prospective comparison of 3 approved systems for autologous bone marrow concentration demonstrated nonequivalency in progenitor cell number and concentration. J. Orthop. Trauma 2014, 28, 591–598. [Google Scholar] [CrossRef]
- Wang, Z.; Li, X.; Yang, J.; Gong, Y.; Zhang, H.; Qiu, X.; Liu, Y.; Zhou, C.; Chen, Y.; Greenbaum, J.; et al. Single-cell RNA sequencing deconvolutes the in vivo heterogeneity of human bone marrow-derived mesenchymal stem cells. Int. J. Biol. Sci. 2021, 17, 4192–4206. [Google Scholar] [CrossRef] [PubMed]
- Bisseret, D.; Kaci, R.; Lafage-Proust, M.H.; Alison, M.; Parlier-Cuau, C.; Laredo, J.D.; Bousson, V. Periosteum: Characteristic imaging findings with emphasis on radiologic-pathologic comparisons. Skeletal Radiol. 2015, 44, 321–338. [Google Scholar] [CrossRef]
- Liu, Y.L.; Tang, X.T.; Shu, H.S.; Zou, W.; Zhou, B.O. Fibrous periosteum repairs bone fracture and maintains the healed bone throughout mouse adulthood. Dev. Cell 2024, 59, 1192–1209.e6. [Google Scholar] [CrossRef]
- Brownlow, H.C.; Reed, A.; Joyner, C.; Simpson, A.H.R.W. Anatomical effects of periosteal elevation. J. Orthop. Res. 2000, 18, 500–502. [Google Scholar] [CrossRef] [PubMed]
- Ball, M.D.; Bonzani, I.C.; Bovis, M.J.; Williams, A.; Stevens, M.M. Human periosteum is a source of cells for orthopaedic tissue engineering: A pilot study. Clin. Orthop. Relat. Res. 2011, 469, 3085–3093. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, M.; Rieger, M.; Reinert, S.; Alexander, D. Isolation of osteoprogenitors from human jaw periosteal cells: A comparison of two magnetic separation methods. PLoS ONE 2012, 7, e47176. [Google Scholar] [CrossRef] [PubMed]
- Vozzi, G.; Lucarini, G.; Dicarlo, M.; Andreoni, C.; Salvolini, E.; Ferretti, C.; Mattioli-Belmonte, M. In vitro lifespan and senescent behaviour of human periosteal derived stem cells. Bone 2016, 88, 1–12. [Google Scholar] [CrossRef]
- Choi, Y.S.; Lim, S.M.; Shin, H.C.; Lee, C.W.; Kim, S.L.; Kim, D.I. Chondrogenesis of human periosteum-derived progenitor cells in atelocollagen. Biotechnol. Lett. 2007, 29, 323–329. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Luyten, F.P. Human periosteum-derived cells maintain phenotypic stability and chondrogenic potential throughout expansion regardless of donor age. Arthritis Rheum. 2001, 44, 85–95. [Google Scholar] [CrossRef]
- De Bari, C.; Dell’Accio, F.; Vanlauwe, J.; Eyckmans, J.; Khan, I.M.; Archer, C.W.; Jones, E.A.; McGonagle, D.; Mitsiadis, T.A.; Pitzalis, C.; et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006, 54, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- McDuffee, L.A.; Pack, L.; Lores, M.; Wright, G.M.; Esparza-Gonzalez, B.; Masaoud, E. Osteoprogenitor cell therapy in an equine fracture model. Vet. Surg. 2012, 41, 773–783. [Google Scholar] [CrossRef]
- Moore, S.R.; Heu, C.; Yu, N.Y.; Whan, R.M.; Knothe, U.R.; Milz, S.; Knothe Tate, M.L. Translating periosteum’s regenerative power: Insights from quantitative analysis of tissue genesis with a periosteum substitute implant. Stem Cells Transl. Med. 2016, 5, 1739–1749. [Google Scholar] [CrossRef]
- Reynders, P.; Becker, J.H.; Broos, P. Osteogenic ability of free periosteal autografts in tibial fractures with severe soft tissue damage: An experimental study. J. Orthop. Trauma. 1999, 13, 121–128. [Google Scholar] [CrossRef]
- Thabet, A.M.; Paley, D.; Kocaoglu, M.; Eralp, L.; Herzenberg, J.E.; Ergin, O.M. Periosteal grafting for congenital pseudarthrosis of the tibia: A preliminary report. Clin. Orthop. Relat. Res. 2008, 466, 2981–2994. [Google Scholar] [CrossRef]




| Gene Target | Sense (S) and Anti-Sense (A) Primer Sequences | Amplicon Size |
|---|---|---|
| EF1α | S: 5′-CCCGGACACAGAGACTTCAT-3′ A: 5′-AGCATGTTGTCACCATTCCA-3′ | 328 |
| Runx2 | S: 5′-CAGACCAGCAGCACTCCATA-3′ A: 5′-CAGCGTCAACACCATCATTC-3′ | 177 |
| Osterix | S: 5′-GGCTATGCCAATGACTACCC-3′ A: 5′-GGTGAGATGCCTGCATGGA-3′ | 207 |
| ALP | S: 5′-TGGGGTGAAGGCTAATGAGG-3′ A: 5′-GGCATCTCGTTGTCCGAGTA-3′ | 221 |
| BMP-2 | S: 5′-TAACCACGCCATTGTTCAGA-3′ A: 5′-ACAACCCTCCACAACCATGT-3′ | 160 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herzog, K.; Nguyen-Edquilang, J.; Stewart, M. Comparative In Vitro Osteogenic Capacities of Bone Marrow- and Periosteal-Derived Progenitor Cells. Biology 2025, 14, 1354. https://doi.org/10.3390/biology14101354
Herzog K, Nguyen-Edquilang J, Stewart M. Comparative In Vitro Osteogenic Capacities of Bone Marrow- and Periosteal-Derived Progenitor Cells. Biology. 2025; 14(10):1354. https://doi.org/10.3390/biology14101354
Chicago/Turabian StyleHerzog, Kalyn, Julie Nguyen-Edquilang, and Matthew Stewart. 2025. "Comparative In Vitro Osteogenic Capacities of Bone Marrow- and Periosteal-Derived Progenitor Cells" Biology 14, no. 10: 1354. https://doi.org/10.3390/biology14101354
APA StyleHerzog, K., Nguyen-Edquilang, J., & Stewart, M. (2025). Comparative In Vitro Osteogenic Capacities of Bone Marrow- and Periosteal-Derived Progenitor Cells. Biology, 14(10), 1354. https://doi.org/10.3390/biology14101354






