Unraveling Belowground Community Assembly in Temperate Steppe Ecosystems
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Field Surveys and Index Determination
2.3. Statistical Analysis
3. Results
3.1. Community Cluster
3.2. Vegetation Association Classification
3.3. Life Form Spectrum Analysis
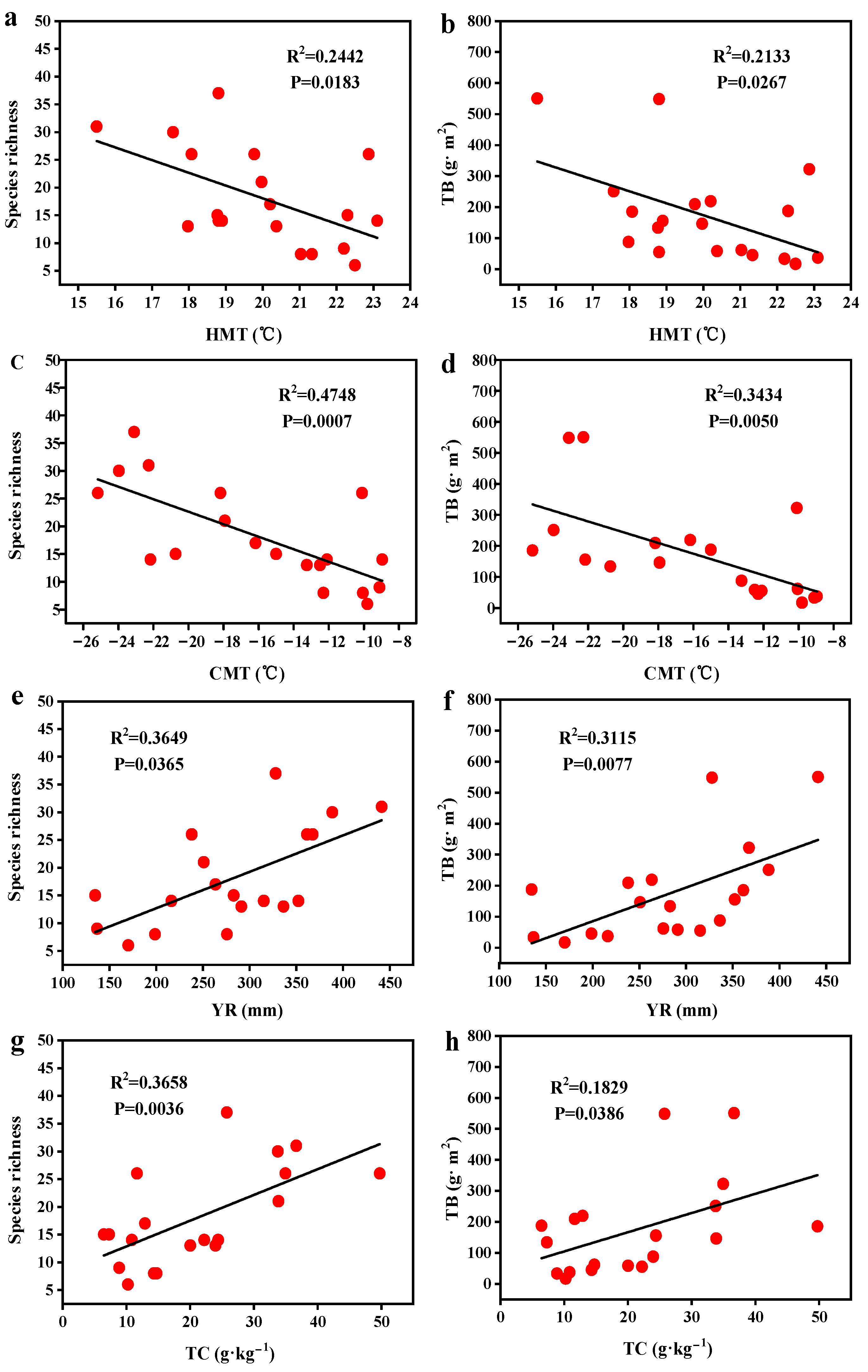
3.4. Single-Factor Analysis and Correspondence Analysis of Influencing Factors
4. Discussion
4.1. Root Traits and Spatial Distribution Patterns at the Community Scale
4.2. Driving Factors of Spatial Distribution Patterns
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, X.; Liu, Y.X.; Ma, M.; Zhen, H.C. Exploring the spatiotemporal variations and influential factors of net ecosystem productivity in the Inner Mongolian grassland ecosystem. Remote Sens. Nat. Resour. 2025, 37, 212–220. [Google Scholar] [CrossRef]
- Xin, X.P.; Lan, X.Q.; Li, L.H.; Tang, H.J.; Guo, H.N.; Jiang, C.X.; Liu, F.; Shao, C.L.; Qin, Y.F.; Liu, Z.L.; et al. Anthropogenic and climate impacts on carbon stocks of grassland ecosystems in Inner Mongolia and adjacent region. Sci. Total Environ. 2024, 946, 174054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Mu, W.P.; Zhao, R.; Ye, B.; Bai, Z. Understanding the shift in drivers of terrestrial water storage decline in the central Inner Mongolian steppe over the past two decades. J. Hydrol. 2024, 636, 131312. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Long, R.; Lin, H.; Ren, J.Z. Study on pastoral ecosystem security and its assessment. Acta Prataculturae Sin. 2008, 17, 143. [Google Scholar] [CrossRef]
- Pillar, V.D.; Sabatini, F.M.; Jandt, U.; Camiz, S.; Bruelheide, H.; Roberts, D. Revealing the functional traits that are linked to hidden environmental factors in community assembly. J. Veg. Sci. 2021, 32, e12976. [Google Scholar] [CrossRef]
- Pillar, V.D. Trait divergence in plant community assembly is generated by environmental factor interactions. J. Veg. Sci. 2024, 35, e13259. [Google Scholar] [CrossRef]
- Li, W.; Shen, Y.; Wang, G.H.; Ma, H.B.; Yang, Y.D.; Li, G.Q.; Huo, X.R.; Liu, Z. Plant species diversity and functional diversity relations in the degradation process of desert steppe in an arid area of northwest China. J. Environ. Manag. 2024, 365, 121534. [Google Scholar] [CrossRef]
- Orwin, K.H.; Buckland, S.M.; Johnson, D.; Turner, B.L.; Smart, S.; Oakley, S.; Bardgett, R.D. Linkages of plant traits to soil properties and the functioning of temperate grassland. J. Ecol. 2010, 98, 1074–1083. [Google Scholar] [CrossRef]
- Deyn, G.B.D.; Cornelissen, J.H.C.; Bardgett, R.D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008, 11, 516–531. [Google Scholar] [CrossRef]
- Bai, Y.F.; Wu, J.G.; Clark, C.M.; Pan, Q.M.; Zhang, L.X.; Chen, S.P.; Wang, Q.B.; Han, X.G. Grazing alters ecosystem functioning and C:N:P stoichiometry of grasslands along a regional precipitation gradient. J. Appl. Ecol. 2012, 49, 1204–1215. [Google Scholar] [CrossRef]
- Guo, X.Y.; Arshad, M.U.; Zhao, Y.F.; Gong, Y.F.; Li, H.Y. Effects of climate change and grazing intensity on grassland productivity—A case study of Inner Mongolia, China. Heliyon 2023, 9, e17814. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.L.; Li, J.F. Species richness and dominant functional groups enhance aboveground biomass, with no effect on belowground biomass in Qinghai-Tibet Plateau’s grasslands. Ecol. Inform. 2024, 82, 102688. [Google Scholar] [CrossRef]
- Chen, D.M.; Pan, Q.M.; Bai, Y.F.; Hu, S.J.; Huang, J.H.; Wang, Q.B.; Naeem, S.H.; Elser, J.J.; Wu, J.G.; Han, X.G. Effects of plant functional group loss on soil biota and net ecosystem exchange: A plant removal experiment in the Mongolian grassland. J. Ecol. 2016, 104, 734–743. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Liu, P.; Chi, Y.G.; Huang, Z.; Zhong, D.W.; Zhou, L. Multidimensional response of China’s grassland stability to drought. Glob. Ecol. Conserv. 2024, 52, e2961. [Google Scholar] [CrossRef]
- Li, W.H.; Xu, F.W.; Zheng, S.W.; Taube, F.H.; Bai, Y.F. Patterns and thresholds of grazing-induced changes in community structure and ecosystem functioning: Species-level responses and the critical role of species traits. J. Appl. Ecol. 2017, 54, 963–975. [Google Scholar] [CrossRef]
- Bai, Y.F.; Han, X.G.; Wu, J.G.; Chen, Z.Z.; Li, L.H. Ecosystem stability and compensatory effects in the Inner Mongolia grassland. Nature 2004, 431, 181–184. [Google Scholar] [CrossRef]
- Huang, X.Z.; Lu, Z.Y.; Li, F.B.; Deng, Y.; Wan, F.F.; Wang, Q.C.; Folega, F.; Wang, J.S.; Guo, Z.J. Evolution history dominantly regulates fine root lifespan in tree species across the world. For. Ecosyst. 2024, 11, 100211. [Google Scholar] [CrossRef]
- Gill, R.A.; Jackson, R.B. Global patterns of root turnover for terrestrial ecosystems. New Phytol. 2000, 147, 13–31. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ji, C.J.; Han, W.X. Above- and belowground biomass allocation in Tibetan grasslands. J. Veg. Sci. 2009, 20, 177–184. [Google Scholar] [CrossRef]
- Amato, M.T.; Giménez, D. Quantifying root turnover in grasslands from biomass dynamics: Application of the growth-maintenance respiration paradigm and re-analysis of historical data. Eco. Model. 2022, 467, 109940. [Google Scholar] [CrossRef]
- West, J.B.; Espeleta, J.F.; Donovan, L.A. Fine root production and turnover across a complex edaphic gradient of a Pinus palustris-Aristida stricta savanna ecosystem. For. Ecol. Manag. 2004, 189, 397–406. [Google Scholar] [CrossRef]
- Paulus, E.L.; Vitousek, P.M. Manganese and soil organic carbon stability on a Hawaiian grassland rainfall gradient. Soil Biol. Biochem. 2024, 194, 109418. [Google Scholar] [CrossRef]
- Mora, J.L.; Armas-Herrera, C.M.; García, D.G.; Badía–Villas, D. Mosaic coexistence of two subalpine grassland types as a consequence of soil nutrient heterogeneity. Catena 2024, 243, 108192. [Google Scholar] [CrossRef]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef]
- Kramer-Walter, K.R.; Bellingham, P.J.; Millar, T.R.; Smissen, R.D.; Richardson, S.J.; Laughlin, D.C. Root traits are multidimensional: Specific root length is independent from root tissue density and the plant economic spectrum. J. Ecol. 2016, 104, 1299–1310. [Google Scholar] [CrossRef]
- Padilla, F.M.; Miranda, J.D.; Jorquera, M.J.; Pugnaire, F.I. Variability in amount and frequency of water supply affects roots but not growth of arid shrubs. Plant Ecol. 2009, 204, 261–270. [Google Scholar] [CrossRef]
- Yang, Y.H.; Fang, J.Y.; Ma, W.H.; Guo, D.L.; Mohammat, A. Large-scale pattern of biomass partitioning across China’s grasslands. Global Ecol. Biogeogr. 2010, 19, 268–277. [Google Scholar] [CrossRef]
- Wang, X.W.; Niu, M.M.; Zhang, L.W.; Bai, W.M.; Li, G.Y.; Chen, A.Q. Effects of multi-level nitrogen addition on plant above- and below-ground biomass allocation in a typical temperate steppe. J. Henan Norm. Univ. (Nat. Sci. Ed.) 2021, 49, 39–46. [Google Scholar] [CrossRef]
- Fu, Z.Y. Spatial Differentiation Characteristics of Vegetation and Ecological Stability in the Inner Mongolia Steppe. Ph.D. Thesis, China University of Mining and Technology-Beijing, Beijing, China, 2022. [Google Scholar]
- Zhao, F.J. Distribution Patterns and Determinants of Plants in the Temperate Steppe of China. Ph.D. Thesis, Peking University, Beijing, China, 2013. [Google Scholar]
- Weigelt, A.; Momme, L.; Andraczek, K.; Iversen, C.M.; Bergmann, J.; Bruelheide, H.; Fan, Y.; Freschet, G.T.; Guerrero-Ramírez, N.R.; Kattge, J.; et al. An integrated framework of plant form and function: The belowground perspective. New Phytol. 2021, 232, 42–59. [Google Scholar] [CrossRef]
- Bao, Y.J.; Cao, M.; Li, Z.H.; Guo, P.; Zhang, J.; Qin, J. A comparative study of the response of Leymus chinensis and Stipa grandis root characteristics to moisture gradients. Acta Ecol. Sin. 2019, 39, 1063–1070. [Google Scholar] [CrossRef]
- Wang, X.F.; Wang, Z.Y.; Liang, J.H.; Wang, L.Q. Study on the root architecture of cespitose plants in the grassland of Inner Mongolia. J. Inner Mongolia Agric. Univ. 2013, 34, 77–82. [Google Scholar] [CrossRef]
- Götzenberger, L.; Bello, F.D.; Bråthen, K.A.; Davison, J.; Zobel, M. Ecological assembly rules in plant communities- approaches, patterns and prospects. Biol. Rev. 2012, 87, 111–127. [Google Scholar] [CrossRef]
- Mckinney, M.L. Effects of urbanization on species richness: A review of plants and animals. Urban Ecosys. 2008, 11, 161–176. [Google Scholar] [CrossRef]
- Helsen, K.; Hermy, M.; Honnay, O. Trait but not species convergence during community assembly in restored semi-natural grasslands. Oikos 2012, 121, 2121–2130. [Google Scholar] [CrossRef]
- Laliberté, E.; Norton, D.A.; Scott, D. Contrasting effects of productivity and disturbance on plant functional diversity at local and metacommunity scale. J. Veg. Sci. 2013, 24, 834–842. [Google Scholar] [CrossRef]
- Cao, C.Y.; Zhang, Y.; Qian, W.; Liang, C.P.; Wang, C.M.; Tao, S. Land-use changes influence soil bacterial communities in a meadow grassland in Northeast China. Solid Earth 2017, 8, 1119–1129. [Google Scholar] [CrossRef]
- Grace, J.B.; Michael, A.T.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hautier, Y.; Hillebrand, H.; Lind, E.M.; Pärtel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef]
- Pottier, J.; Dubuis, A.; Pellissier, L.; Maiorano, L.; Rossier, L.; Randin, C.F.; Vittoz, P.; Guisan, A.; Field, R. The accuracy of plant assemblage predictions from species distribution models varies along environmental gradients. Glob. Ecol. Biogeogr. 2013, 22, 52–63. [Google Scholar] [CrossRef]
- Bennett, J.A.; Lamb, E.G.; Hall, J.C.; Cardinal-Mcteague, W.M.; Cahill, J.F.; Rejmanek, M. Increased competition does not lead to increased phylogenetic verdispersion in a native grassland. Ecol. Lett. 2013, 16, 1168–1176. [Google Scholar] [CrossRef]
- Freschet, G.T.; Dias, A.T.C.; Ackerly, D.D.; Aerts, R.; van Bodegom, P.M.; Cornwell, W.K.; Dong, M.; Kurokawa, H.; Liu, G.F.; Onipchenko, V.G.; et al. Global to community scale differences in the prevalence of convergent over divergent leaf trait distributions in plant assemblages. Glob. Ecol. Biogeogr. 2011, 20, 755–765. [Google Scholar] [CrossRef]
- Fu, Z.Y.; Wang, F.; Lu, Z.H.; Zhang, M.; Zhang, L.; Hao, W.Y.; Zhao, L.; Jiang, Y.; Guo, B.; Chen, R. Community differentiation and ecological influencing factors along environmental gradients: Evidence from 1,200 km belt transect across Inner Mongolia grassland, China. Sustainability 2022, 14, 361. [Google Scholar] [CrossRef]
- Huang, J.; Zeng, H.; Xiong, Y.M.; Guo, D.L. Lower-order roots biomass of temperate steppe and the environmental controls in Inner Mongolia. Acta Sci. Nat. Univ. Pekin. 2012, 51, 931–938. [Google Scholar] [CrossRef]
- Yuan, Y.M.; Liu, J.Y.; Gao, X.L.; Xue, J.; Wang, R.Z. Root traits of seven stipa species and their relations with environmental factors in temperate grassland. Acta Ecol. Sin. 2022, 42, 8784–8794. [Google Scholar] [CrossRef]
- Bhaskar, R.; Dawson, T.E.; Balvanera, P. Community assembly and functional diversity along succession post-management. Funct. Ecol. 2014, 28, 1256–1265. [Google Scholar] [CrossRef]







| Index | Abbreviation | Average | Maximum | Minimum |
|---|---|---|---|---|
| Species richness | Sr | 10.9 | 21 | 3 |
| Margalef index | MI | 1.474 | 2.969 | 0.400 |
| Simpson index | D | 0.556 | 0.863 | 0.040 |
| Shannon-Wiener index | H | 1.212 | 2.207 | 1.112 |
| Pielou index | E | 0.519 | 0.844 | 0.099 |
| Total belowground biomass/(g·m−2) | TB | 173.83 | 717.20 | 15.80 |
| Total root length/(m·m−2) | TRL | 177.18 | 544.91 | 12.20 |
| Total root width/(m·m−2) | TRW | 129.33 | 441.29 | 7.59 |
| Total root volume/(m3·m−2) | TRV | 3.13 | 19.22 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Shang, S.; Rong, Z.; Sun, J.; Ma, J.; Lu, Z.; Wang, F.; Fu, Z. Unraveling Belowground Community Assembly in Temperate Steppe Ecosystems. Biology 2025, 14, 1350. https://doi.org/10.3390/biology14101350
Wang P, Shang S, Rong Z, Sun J, Ma J, Lu Z, Wang F, Fu Z. Unraveling Belowground Community Assembly in Temperate Steppe Ecosystems. Biology. 2025; 14(10):1350. https://doi.org/10.3390/biology14101350
Chicago/Turabian StyleWang, Ping, Shuai Shang, Zhengyang Rong, Jingkuan Sun, Jinzhao Ma, Zhaohua Lu, Fei Wang, and Zhanyong Fu. 2025. "Unraveling Belowground Community Assembly in Temperate Steppe Ecosystems" Biology 14, no. 10: 1350. https://doi.org/10.3390/biology14101350
APA StyleWang, P., Shang, S., Rong, Z., Sun, J., Ma, J., Lu, Z., Wang, F., & Fu, Z. (2025). Unraveling Belowground Community Assembly in Temperate Steppe Ecosystems. Biology, 14(10), 1350. https://doi.org/10.3390/biology14101350






