Integrated Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway in Relation to Color Mutation in Roses
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Materials
2.2. Extraction and Comparative Quantitative Analysis of Flavonoid Metabolites
2.3. RNA Extraction and cDNA Library Construction
2.4. RNA-Seq Analysis
2.5. Association Analysis of Metabolomic and Transcriptomic Data
3. Results
3.1. Quantitative Analysis of Rose Petal Metabolites Between AR and SR
3.2. Transcriptome Sequencing and Analysis
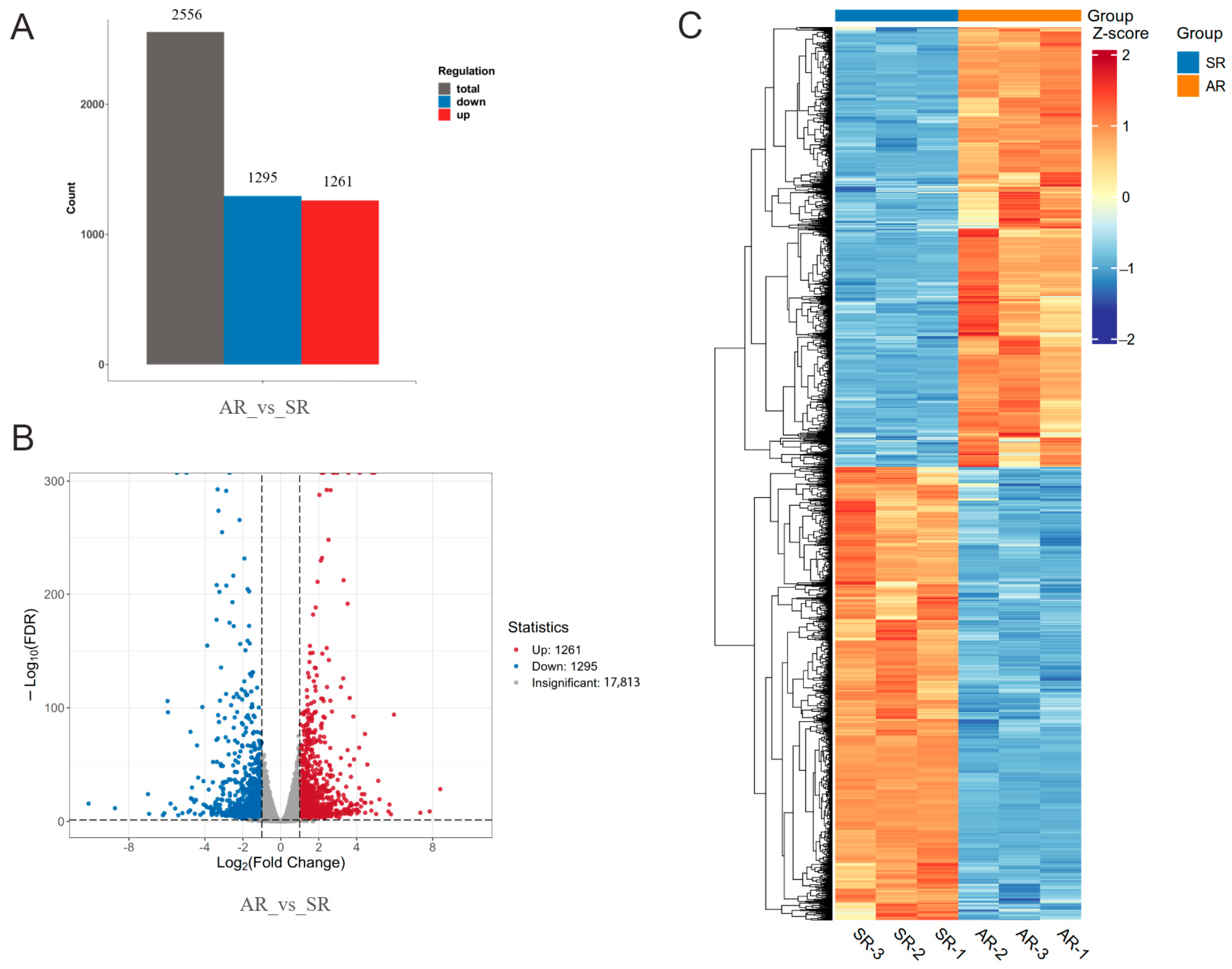
3.3. Identification of Differentially Expressed Genes (DEGs) in the Petals of AR and SR
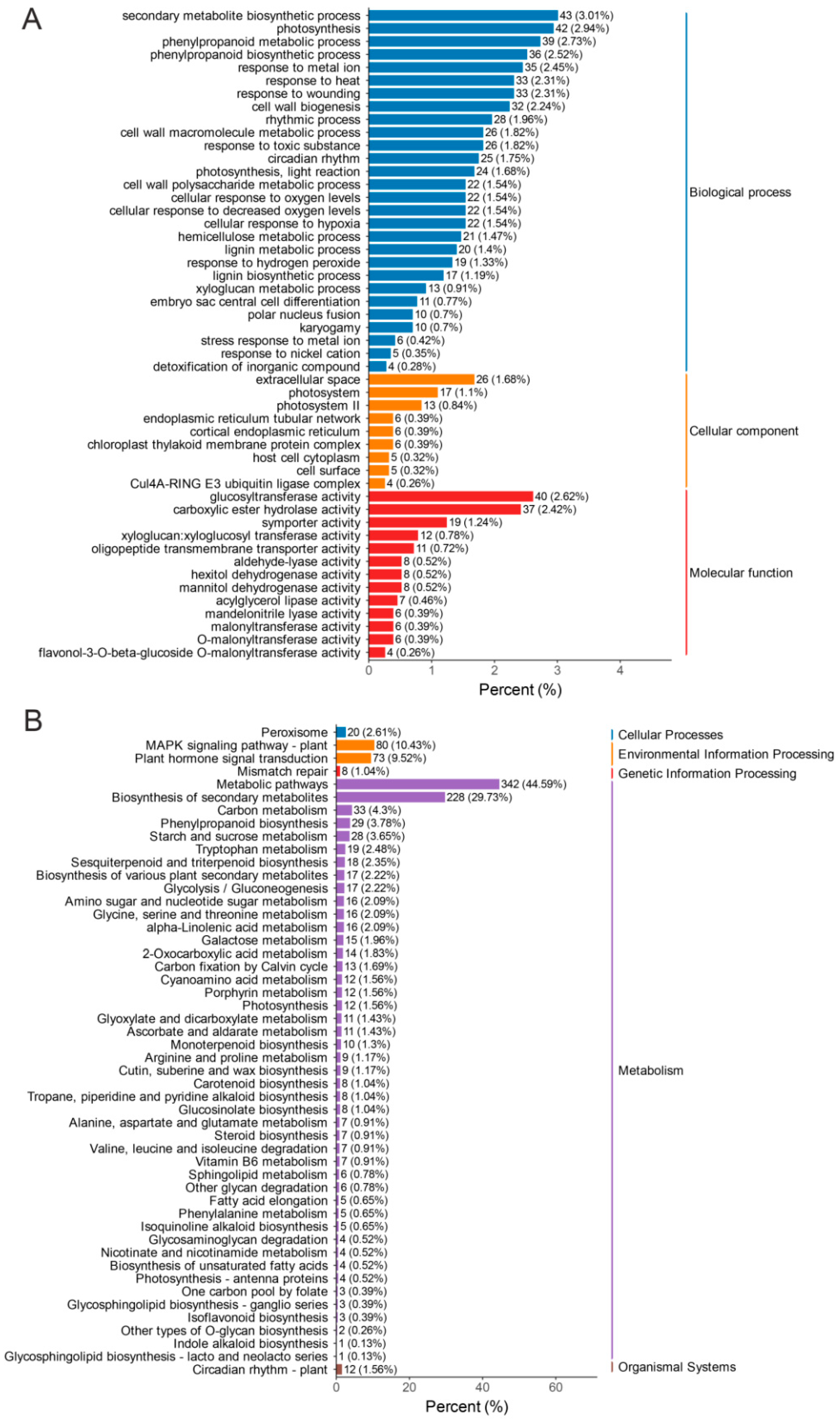
3.4. Expression of DEGs Related to Flavonoid Biosynthesis and Phenylpropanoid Biosynthesis
3.5. Expression Analysis of Transcription Factors (TFs)
3.6. Expression Analysis of GSTs, MATEs and ABCCs
3.7. Expression of Genes Related to the Biosynthetic Pathway of Flavonoids
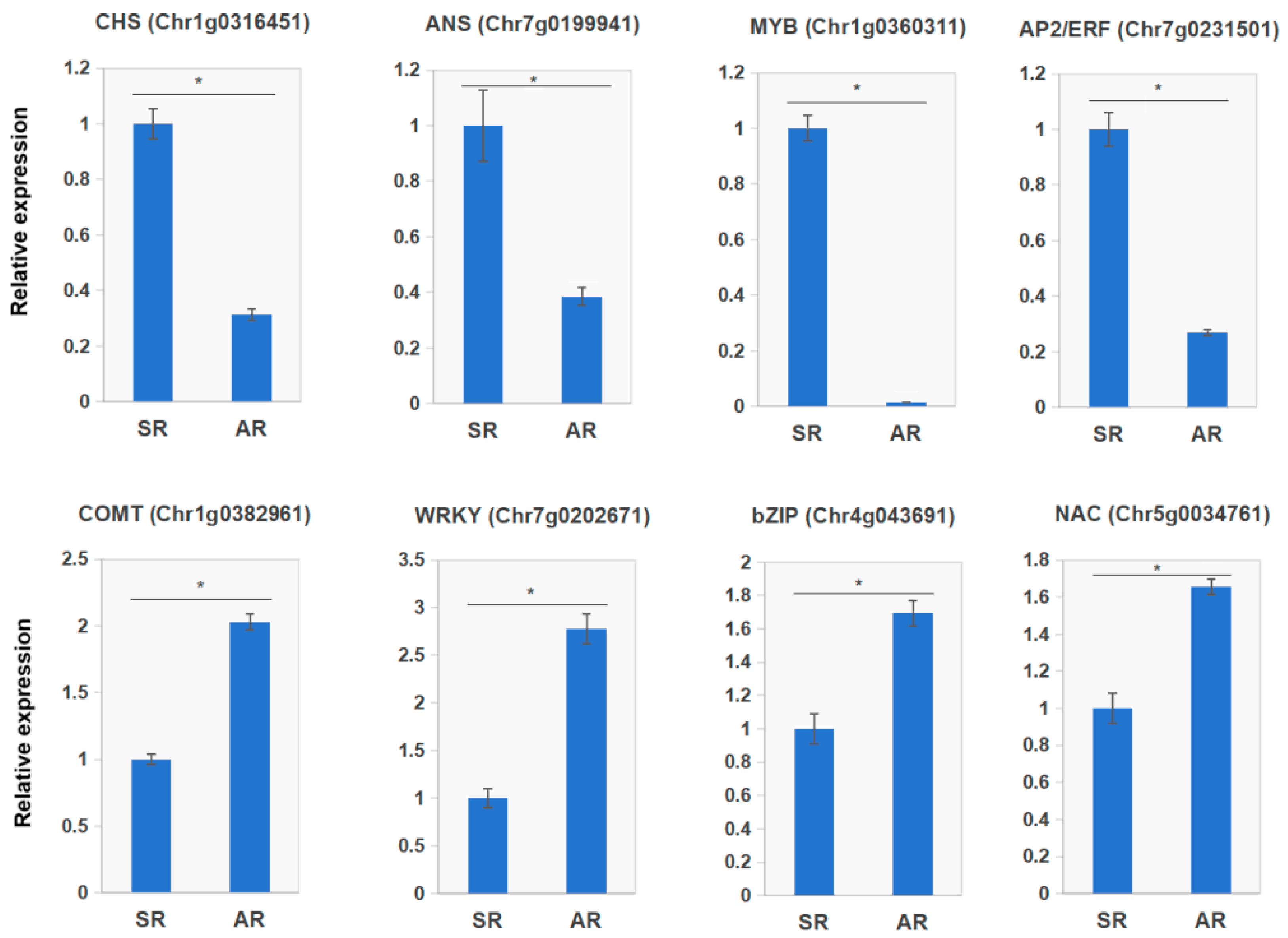
3.8. qRT-PCR Validation of Gene Expression Patterns
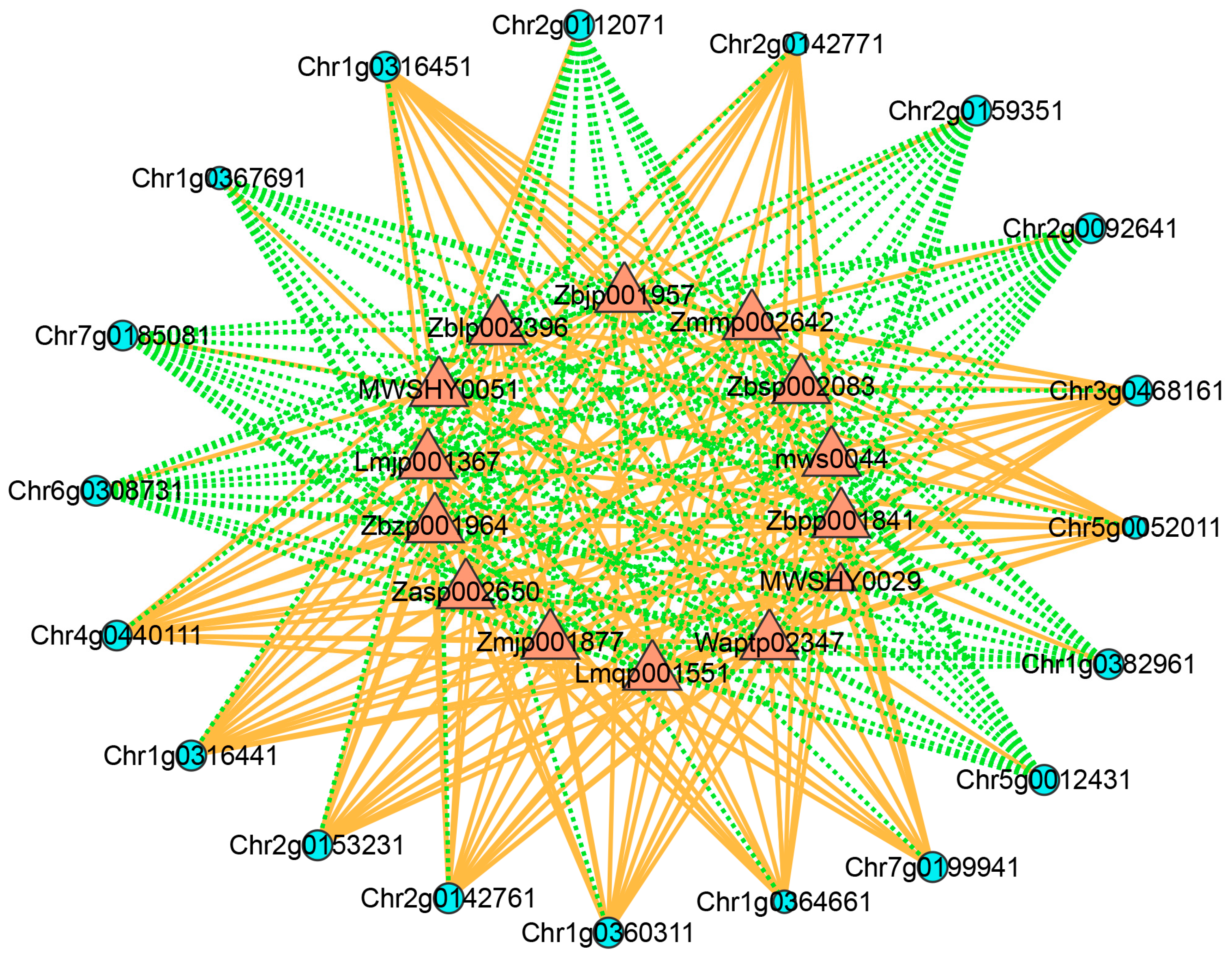
3.9. Network Analysis
4. Discussion
4.1. Effects of Flavonoid Content in Rose Petals of SR and AR on Flower Coloration
4.2. Anthocyanin-Related Genes Affect Flower Coloration in AR and SR
4.3. Downregulation of GST, ABCC and MATE Genes May Decrease Anthocyanin Storage in the Vacuoles of AR Flowers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kuppusamy, K.M.; Selvaraj, S.; Singaravelu, P.; John, C.M.; Racheal, K.; Varghese, K.; Kaliyamoorthy, D.; Perumal, E.; Gunasekaran, K. Anti-microbial and anti-cancer efficacy of acetone extract of Rosa chinensis against resistant strain and lung cancer cell line. BMC Complement. Med. Ther. 2023, 23, 406. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Barros, L.; Carvalho, A.M.; Ferreira, I.C.F.R. Studies on chemical constituents and bioactivity of Rosa micrantha, an alternative antioxidants source for food, pharmaceutical, or cosmetic applications. J. Agric. Food Chem. 2010, 58, 6277. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.; Choung, M. Anthocyanin compositions and biological activities from the red petals of Korean edible rose (Rosa hybrida cv. Noblered). Food Chem. 2011, 129, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sasaki, N.; Ohmiya, A. Biosynthesis of plant pigments, anthocyanins, betalains and carotenoids. Plant J. 2008, 54, 733–749. [Google Scholar] [CrossRef]
- Eugster, C.H.; Märki-Fischer, E. The Chemistry of Rose Pigments. Angew. Chem. Int. Ed. 1991, 30, 654–672. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Tian, Y.L.; Guo, P.Y.; Luo, J.J.; Xu, C.; Zhang, Y.; Chen, G.P.; Xie, Q.L.; Hu, Z.L. Novel insights into pigment composition and molecular mechanisms governing flower coloration in rose cultivars exhibiting diverse petal hues. Plants 2024, 13, 3353. [Google Scholar] [CrossRef]
- Miller, R.; Owens, S.J.; Rørslett, B. Plants and colour, flowers and pollination. Opt. Laser Technol. 2011, 43, 282–294. [Google Scholar] [CrossRef]
- Fan, X.P.; Fan, B.H.; Wang, Y.X.; Yang, W.C. Anthocyanin accumulation enhanced in Lc-transgenic cotton under light and increased resistance to bollworm. Plant Biotechnol. Rep. 2016, 10, 1–11. [Google Scholar] [CrossRef]
- Hughes, N.M.; Neufeld, H.S.; Burkey, K.O. Functional role of anthocyanins in high-light winter leaves of the evergreen herb Galax urceolata. New Phytol. 2005, 168, 575–587. [Google Scholar] [CrossRef]
- Shao, L.; Shu, Z.; Sun, S.L.; Peng, C.L.; Wang, X.J.; Lin, Z.F. Antioxidation of anthocyanins in photosynthesis under high temperature stress. J. Integr. Plant Biol. 2007, 49, 1341–1351. [Google Scholar] [CrossRef]
- Cirillo, V.; D’Amelia, V.; Esposito, M.; Amitrano, C.; Carillo, P.; Carputo, D.; Maggio, A. Anthocyanins are key regulators of drought stress tolerance in Tobacco. Biology 2021, 10, 139. [Google Scholar] [CrossRef]
- Shih, P.H.; Yeh, C.T.; Yen, G.C. Anthocyanins induce the activation of phase II enzymes through the antioxidant response element pathway against oxidative stress-induced apoptosis. J. Agric. Food Chem. 2007, 55, 9427–9435. [Google Scholar] [CrossRef]
- Butelli, E.; Titta, L.; Giorgio, M.; Mock, H.P.; Matros, A.; Peterek, S.; Schijlen, E.G.W.M.; Hall, R.D.; Bovy, A.G.; Luo, J.; et al. Enrichment of tomato fruit with health-promoting anthocyanins by expression of select transcription factors. Nat. Biotechnol. 2008, 26, 1301–1308. [Google Scholar] [CrossRef]
- Li, W.F.; Mao, J.; Yang, S.J.; Guo, Z.G.; Ma, Z.H.; Dawuda, M.M.; Zuo, C.W.; Chu, M.Y.; Chen, B.H. Anthocyanin accumulation correlates with hormones in the fruit skin of ‘Red Delicious’ and its four generation bud sport mutants. BMC Plant Biol. 2018, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Chen, N.; Huang, Z.; Li, D.; Zhi, J.; Yu, B.; Liu, X.; Cao, B.; Qiu, Z. Anthocyanin fruit encodes an R2R3-MYB transcription factor, SlAN2-like, activating the transcription of SlMYBATV to fine-tune anthocyanin content in tomato fruit. New Phytol. 2019, 225, 2048–2063. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Deng, L.; Du, M.; Zhao, J.; Chen, Q.; Huang, T.; Jiang, H.; Li, C.B.; Li, C. A transcriptional network promotes anthocyanin biosynthesis in tomato flesh. Mol. Plant. 2020, 13, 42–58. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.H.; Kim, J.M.; Choi, C.Y.; Kim, C.Y.; Park, K.H. Ginkgo biloba extract and bilberry anthocyanins improve visual function in patients with normal tension glaucoma. J. Med. Food. 2012, 15, 818–823. [Google Scholar] [CrossRef]
- Tao, Y.; Chen, T.; Yang, G.Q.; Peng, G.H.; Yan, Z.J.; Huang, Y.F. Anthocyanin can arrest the cone photoreceptor degeneration and act as a novel treatment for retinitis pigmentosa. Int. J. Ophthalmol. 2016, 9, 153–158. [Google Scholar] [CrossRef]
- Jo, Y.N.; Jin, D.E.; Jeong, J.H.; Kim, H.J.; Kim, D.O.; Heo, H.J. Effect of anthocyanins from rabbit-eye blueberry (Vaccinium virgatum) on cognitive function in mice under trimethyltin-induced neurotoxicity. Food Sci. Biotechnol. 2015, 24, 1077–1085. [Google Scholar] [CrossRef]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef]
- Isaak, C.K.; Petkau, J.C.; Blewett, H.; Karmin, O.; Siow, Y.L. Lingonberry anthocyanins protect cardiac cells from oxidative- stress-induced apoptosis. Can. J. Physiol. Pharmacol. 2017, 95, 904–910. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.X.; Wang, C.L.; Feng, H.; Li, S.S.; Wang, L.S.; Wu, R.H.; Zhao, S.W. Research progress on flower color of rose. Acta Hortic. Sin. 2021, 48, 2044–2056. [Google Scholar]
- Biolley, J.P.; Jay, M. Anthocyanins in modern roses: Chemical and colorimetric features in relation to the colour range. J. Exp. Bot. 1993, 44, 1725–1734. [Google Scholar] [CrossRef]
- Schmitzer, V.; Robert, V.; Gregor, O.; Franci, S. Changes in the phenolic concentration during flower development of rose ‘KORcrisett’. J. Am. Soc. Hortic. Sci. 2009, 134, 491–496. [Google Scholar] [CrossRef]
- Yuki, M.; Yokoi, M.; Ueda, Y.; Saito, N. Anthocyanins in flowers of genus Rosa, sections Cinnamomeae (=Rosa), Chinenses, Gallicanae and some modern garden roses. Biochem. Syst. Ecol. 2000, 28, 887–902. [Google Scholar] [CrossRef]
- Cunja, V.; Mikulic-Petkovsek, M.; Stampar, F.; Schmitzer, V. Compound identification of selected rose species and cultivars: An insight to petal and leaf phenolic profiles. J. Am. Soc. Hortic. Sci. 2014, 139, 157–166. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Tao, J. Recent advances on the development and regulation of flower color in ornamental plants. Front. Plant Sci. 2015, 6, 261. [Google Scholar] [CrossRef]
- Paoli, E.D.; Dorantes-Acosta, A.; Zhai, J.; Accerbi, M.; Jeong, D.H.; Park, S.; Meyers, B.C.; Jorgensen, R.A.; Green, P.J. Distinct extremely abundant sirnas associated with cosuppression in petunia. RNA 2009, 15, 1965–1970. [Google Scholar] [CrossRef]
- Jaakola, L. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar] [CrossRef]
- Naing, A.H.; Park, D.Y.; Park, K.I.; Kim, C.K. Differential expression of anthocyanin structural genes and transcription factors determines coloration patterns in gerbera flowers. 3 Biotech. 2018, 8, 393. [Google Scholar] [CrossRef]
- Kaur, S.; Sharma, N.; Kapoor, P.; Chunduri, V.; Pandey, A.K.; Garg, M. Spotlight on the overlapping routes and partners for anthocyanin transport in plants. Physiol. Plant. 2021, 171, 868–881. [Google Scholar] [CrossRef]
- Xie, J.W.; Cao, X.Y.; Pan, W.Q.; Du, L.J. Advances in plant flavonoid transport and accumulation mechanism. Chin. Bull. Bot. 2024, 59, 463–480. [Google Scholar]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Xu, W.J.; Grain, D.; Bobet, S.; Le Gourrierec, J.; Thevenin, J.; Kelemen, Z.; Lepiniec, L.; Dubos, C. Complexity and robustness of the flavonoid transcriptional regulatory network revealed by comprehensive analyses of MYB-bHLH-WDR complexes and their targets in Arabidopsis seed. New Phytol. 2014, 202, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Li, X.; Fu, Y.; Li, C. Environmental stimuli and phytohormones in anthocyanin biosynthesis, a comprehensive review. Int. J. Mol. Sci. 2023, 24, 16415. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Zhang, H.; Yang, Y.; Wang, H.; Xue, Z.; Fan, Y.W.; Sun, P.; Zhang, H.; Zhang, X.Z. Rosa1, a transposable element-like insertion, produces red petal coloration in rose through altering RcMYB114 transcription. Front. Plant Sci. 2022, 13, 857684. [Google Scholar] [CrossRef] [PubMed]
- He, G.R.; Zhang, R.; Jiang, S.H.; Wang, H.H.; Ming, F. The MYB transcription factor RcMYB1 plays a central role in rose anthocyanin biosynthesis. Hortic. Res. 2023, 10, uhad080. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.; Kim, K.; Bang, G.; Cho, M.; Choi, S.B.; Choi, G.; Park, Y.I. HY5 regulates anthocyanin biosynthesis by inducing the transcriptional activation of the MYB75/PAP1 transcription factor in Arabidopsis. FEBS Lett. 2013, 587, 1543–1547. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Jeong, C.Y.; Kang, G.H.; Yoo, S.D.; Hong, S.W.; Lee, H. MYBD employed by HY5 increases anthocyanin accumulation via repression of MYBL2 in Arabidopsis. Plant J. 2015, 84, 1192–1205. [Google Scholar] [CrossRef]
- Li, S.R.; Ou, C.Q.; Wang, F.; Zhang, Y.J.; Ismail, O.; Abd Elaziz, Y.S.G.; Edris, S.; Li, H.; Jiang, S.L. Ppbbx24-del mutant positively regulates light-induced anthocyanin accumulation in the ‘Red Zaosu’ pear (Pyrus pyrifolia White Pear Group). J. Integr. Agric. 2025, 24, 2619–2639. [Google Scholar] [CrossRef]
- Hichri, I.; Barrieu, F.; Bogs, J.; Kappel, C.; Delrot, S.; Lauvergeat, V. Recent advances in the transcriptional regulation of the flavonoid biosynthetic pathway. J. Exp. Bot. 2011, 62, 2465–2483. [Google Scholar] [CrossRef]
- Wang, Y.T.; Li, S.P.; Zhu, Z.Q.; Xu, Z.D.; Qi, S.; Xing, S.T.; Yu, Y.Y.; Wu, Q.K. Transcriptome and chemical analyses revealed the mechanism of flower color formation in Rosa rugosa. Front. Plant Sci. 2022, 13, 1021521. [Google Scholar] [CrossRef]
- Jiang, S.H.; Wang, H.H.; Zhang, R.; Yang, Z.Y.; He, G.R.; Ming, F. Transcriptomic based analysis to identify candidate genes for blue color rose breeding. Plant Mol. Biol. 2023, 111, 439–454. [Google Scholar] [CrossRef]
- Cai, Y.; Xing, J.; Sun, M.; Zhan, Z.; Corke, H. Phenolic antioxidants (hydrolyzable tannins, flavonols, and anthocyanins) identified by LC-ESI-MS and MALDI-QIT-TOF MS from Rosa chinensis flowers. J. Agric. Food Chem. 2005, 53, 9940–9948. [Google Scholar] [CrossRef]
- Wan, H.H.; Yu, C.; Han, Y.; Guo, X.L.; Luo, L.; Pan, H.T.; Zheng, T.C.; Wang, J.; Cheng, T.R.; Zhang, Q.X. Determination of flavonoids and carotenoids and their contributions to various colors of rose cultivars (Rosa spp.). Front. Plant Sci. 2019, 10, 123. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Ren, J.; Chen, Z.; Shi, D. Integrated transcriptome and metabolome analyses provide molecular insights into the transition of flower color in the rose cultivar ‘Juicy Terrazza’. BMC Plant Biol. 2025, 25, 883. [Google Scholar] [CrossRef] [PubMed]
- Zan, W.X.; Wu, Q.K.; Dou, S.H.; Wang, Y.T.; Zhu, Z.Q.; Xing, S.T.; Yu, Y.Y. Analysis of flower color diversity revealed the co-regulation of cyanidin and peonidin in the red petals coloration of Rosa rugosa. Plant Physiol. Biochem. 2024, 216, 109126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Butelli, E.; De Stefano, R.; Schoonbeek, H.J.; Magusin, A.; Pagliarani, C.; Wellner, N.; Hill, L.; Orzaez, D.; Granell, A.; et al. Anthocyanins double the shelf life of tomatoes by delaying overripening and reducing susceptibility to gray mold. Curr. Biol. 2013, 23, 1094–1100. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Isayenkov, S.V. The role of anthocyanins in plant tolerance to drought and salt stresses. Plants 2023, 12, 2558. [Google Scholar] [CrossRef]
- Tanaka, Y.; Ohmiya, A. Seeing is believing, engineering anthocyanin and carotenoid biosynthetic pathways. Curr. Opin. Biotechnol. 2008, 19, 190–197. [Google Scholar] [CrossRef]
- Guo, D.M.; Wang, H.Y.; Zhang, S.M.; Lan, T. The type Ⅲ polyketide synthase supergene family in plants, complex evolutionary history and functional divergence. Plant J. 2022, 112, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Schijlen, E.G.; de Vos, C.H.; Martens, S.; Jonker, H.H.; Rosin, F.M.; Molthoff, J.W.; Tikunov, Y.M.; Angenent, G.C.; van Tunen, A.J.; Bovy, A.G. RNA interference silencing of chalcone synthase, the first step in the flavonoid biosynthesis pathway, leads to parthenocarpic tomato fruits. Plant Physiol. 2007, 144, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- He, L.Y.; Lai, G.T.; Lin, J.X.; Guo, A.L.; Yang, F.X.; Pan, R.; Che, J.M.; Lai, C.C. VdCHS2 overexpression enhances anthocyanin biosynthesis, modulates the composition ratio, and increases antioxidant activity in Vitis davidii cells. Antioxidants 2024, 13, 1472. [Google Scholar] [CrossRef] [PubMed]
- Aharoni, A.; De Vos, C.H.; Wein, M.; Sun, Z.; Greco, R.; Kroon, A.; Mol, J.N.; O’Connell, A.P. The strawberry FaMYB1 transcription factor suppresses anthocyanin and favonol accumulation in transgenic tobacco, strawberry Myb represses favonoid biosynthesis. Plant J. 2001, 28, 319–332. [Google Scholar] [CrossRef]
- Abrahams, S.; Lee, E.; Walker, A.R.; Tanner, G.J.; Larkin, P.J.; Ashton, A.R. The Arabidopsis TDS4 gene encodes leuco- anthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J. 2003, 35, 624–636. [Google Scholar] [CrossRef]
- Li, Q.; Wang, J.; Sun, H.Y.; Shang, X. Flower color patterning in pansy (Viola × wittrockiana Gams.) is caused by the diferential expression of three genes from the anthocyanin pathway in acyanic and cyanic flower areas. Plant Physiol. Biochem. 2014, 84, 134–141. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Yuan, Z.H.; Feng, L.J.; Fang, Y.M. Cloning and expression of anthocyanin biosynthetic genes in red and white pomegranate. J. Plant Res. 2015, 128, 687–696. [Google Scholar] [CrossRef]
- Yue, J.Y.; Zhu, C.X.; Zhou, Y.; Niu, X.L.; Miao, M.; Tang, X.F.; Chen, F.; Zhao, W.P.; Liu, Y.S. Transcriptome analysis of diferentially expressed unigenes involved in favonoid biosynthesis during fower development of Chrysanthemum morifolium ‘Chuju’. Sci. Rep. 2018, 8, 13414. [Google Scholar] [CrossRef]
- Zhang, H.L.; Zhao, X.J.; Zhang, J.P.; Yang, B.; Yu, Y.H.; Liu, T.F.; Nie, B.H.; Song, B.T. Functional analysis of an anthocyanin synthase gene StANS in potato. Sci. Hortic. 2020, 272, 109569. [Google Scholar] [CrossRef]
- Szankowski, I.; Flachowsky, H.; Li, H.; Halbwirth, H.; Treutter, D.; Regos, I.; Hanke, M.V.; Stich, K.; Fischer, T.C. Shift in polyphenol proffle and sublethal phenotype caused by silencing of anthocyanidin synthase in apple (Malus sp.). Planta 2009, 229, 681–692. [Google Scholar] [CrossRef]
- Zhang, J.; Han, Z.Y.; Tian, J.; Zhang, X.; Song, T.T.; Yao, Y.C. The expression level of anthocyanidin synthase determines the anthocyanin content of crabapple (Malus sp.) petals. Acta Physiol. Plant. 2015, 37, 109. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishimaru, M.; Ding, C.K.; Yakushiji, H.; Goto, N. Comparison of UDP-glucose: Flavonoid 3-O-glucosyltransferase (UFGT) gene sequences between white grapes (Vitis vinifera) and their sports with red skin. Plant Sci. 2001, 160, 543–550. [Google Scholar] [CrossRef]
- Pei, Y.G.; Tang, W.J.; Huang, Y.D.; Li, H.F.; Liu, X.W.; Chen, H.X.; He, R.M.; Niu, W.Y.; Du, Q.Y.; Chu, Y.Z.; et al. The PavMYB.C2-UFGT module contributes to fruit coloration via modulating anthocyanin biosynthesis in sweet cherry. PLoS Genet. 2025, 21, e1011761. [Google Scholar] [CrossRef]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.M.; Robinson, S.P.; Barrieu, F. The transcription factor VvMYB5b contributes to the regulation of anthocyanin and proanthocyanidin biosynthesis in developing grape berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Li, M.F.; Yang, Y.; Wang, H.; Sun, P.; Zhou, S.T.; Kang, Y.H.; Sun, X.Y.; Jin, M.; Jin, W.M. The mutations in RcMYB114 affect anthocyanin glycoside accumulation in rose. Biology. 2025, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Vimolmangkang, S.; Han, Y.P.; Wei, G.C.; Korban, S.S. An apple MYB transcription factor; MdMYB3; is involved in regulation of anthocyanin biosynthesis and flower development. BMC Plant Biol. 2013, 13, 176. [Google Scholar] [CrossRef] [PubMed]
- Chagné, D.; Wang, K.L.; Espley, R.V.; Volz, R.K.; How, N.M.; Rouse, S.; Brendolise, C.; Carlisle, C.M.; Kumar, S.; De Silva, N.; et al. An ancient duplication of apple MYB transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol. 2013, 161, 225–239. [Google Scholar] [CrossRef]
- Kim, D.; Jeon, S.J.; Yanders, S.; Park, S.C.; Kim, H.S. MYB3 plays an important role in lignin and anthocyanin biosynthesis under salt stress condition in Arabidopsis. Plant Cell Rep. 2022, 41, 1549–1560. [Google Scholar] [CrossRef]
- Kitamura, S.; Shikazono, N.; Tanaka, A. TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J. 2004, 37, 104–114. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, H.; Sun, Y.J.; Zhang, P.H.; Li, L.X.; Luo, D.; Wu, Z. Identiffcation of the GST gene family and functional analysis of RcGSTF2 related to anthocyanin in Rosa chinensis ‘Old Blush’. Plants 2025, 14, 932. [Google Scholar] [CrossRef]
- Chai, Q.C.; Wang, X.L.; Gao, M.W.; Zhao, X.C.; Chen, Y.; Zhang, C.; Jiang, H.; Wang, J.B.; Wang, Y.C.; Zheng, M.N.; et al. A glutathione S-transferase GhTT19 determines flower petal pigmentation via regulating anthocyanin accumulation in cotton. Plant Biotechnol. J. 2023, 21, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.M.; Fan, J.W.; Lu, C.F.; Ren, J.S.; Qi, F.T.; Huang, H.; Dai, S.L. ScGST3 and multiple R2R3-MYB transcription factors function in anthocyanin accumulation in Senecio cruentus. Plant Sci. 2021, 313, 111094. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.; Xu, L.F.; Xu, H.; Yang, P.P.; He, G.R.; Tang, Y.C.; Qi, X.Y.; Song, M.; Ming, J. LhGST is an anthocyanin-related glutathione S-transferase gene in Asiatic hybrid lilies (Lilium spp.). Plant Cell Rep. 2021, 40, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.F.; Dai, C.; Li, Y.P.; Feng, J.; Liu, Z.C.; Kang, C.Y. Reduced anthocyanins in petioles codes for a GST anthocyanin transporter that is essential for the foliage and fruit coloration in strawberry. J. Exp. Bot. 2018, 69, 2595–2608. [Google Scholar] [CrossRef]
- Zhao, Y.; Dong, W.Q.; Zhu, Y.C.; Allan, A.C.; Kui, L.W.; Xu, C.J. PpGST1, an anthocyanin-related glutathione S-transferase gene, is essential for fruit coloration in peach. Plant Biotechnol. J. 2020, 18, 1284–1295. [Google Scholar] [CrossRef]
- Xue, L.; Huang, X.R.; Zhang, Z.H.; Lin, Q.H.; Zhong, Q.Z.; Zhao, Y.; Gao, Z.S.; Xu, C.J. An anthocyanin-related glutathione S-transferase, MrGST1, plays an essential role in fruit coloration in Chinese bayberry (Morella rubra). Front. Plant Sci. 2022, 13, 903333. [Google Scholar] [CrossRef]
- Liu, Y.F.; Qi, Y.W.; Zhang, A.L.; Wu, H.X.; Liu, Z.D.; Ren, X.L. Molecular cloning and functional characterization of AcGST1, an anthocyanin-related glutathione S-transferase gene in kiwifruit (Actinidia chinensis). Plant Mol. Biol. 2019, 100, 451–465. [Google Scholar] [CrossRef]
- Lai, B.; You, Y.; Zhang, L.L.; Wang, Q.X.; Chen, F.B.; Luo, G.J.; Du, L.N.; Wang, H.C. Identification and functional characterization of RsGST1, an anthocyanin-related glutathione S-transferase gene in radish. J. Plant Physiol. 2021, 263, 153468. [Google Scholar] [CrossRef]
- Pérez-Díaz, R.; Madrid-Espinoza, J.; Salinas-Cornejo, J.; González-Villanueva, E.; Ruiz-Lara, S. Differential roles for VviGST1, VviGST3, and VviGST4 in proanthocyanidin and anthocyanin transport in Vitis vinífera. Front. Plant Sci. 2016, 7, 1166. [Google Scholar] [CrossRef]
- Rea, P.A. MRP subfamily ABC transporters from plants and yeast. J. Exp. Bot. 1999, 50, 895–913. [Google Scholar] [CrossRef]
- Francisco, R.M.; Regalado, A.; Ageorges, A.; Burla, B.J.; Bassin, B.; Eisenach, C.; Zarrouk, O.; Vialet, S.; Marlin, T.; Chaves, M.M.; et al. ABCC1; an ATP binding cassette protein from grape berry; transports anthocyanidin 3-O-glucosides. Plant Cell. 2013, 25, 1840–1854. [Google Scholar] [CrossRef]
- Behrens, C.E.; Smith, K.E.; Iancu, C.V.; Choe, J.Y.; Dean, J.V. Transport of anthocyanins and other flavonoids by the Arabidopsis ATP-binding cassette transporter AtABCC2. Sci. Rep. 2019, 9, 437. [Google Scholar] [CrossRef]
- Sylvia, C.; Sun, J.L.; Zhang, Y.Q.; Ntini, C.; Ogutu, C.; Zhao, Y.; Han, Y.P. Genome-wide analysis of ATP Binding Cassette (ABC) transporters in peach (Prunus persica) and identification of a gene PpABCC1 involved in anthocyanin accumulation. Int. J. Mol. Sci. 2023, 24, 1931. [Google Scholar] [CrossRef]
- Takanashi, K.; Shitan, N.; Yazaki, K. The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol. 2014, 31, 417–430. [Google Scholar] [CrossRef]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell. 2007, 19, 2023–2038. [Google Scholar] [CrossRef]
- Zhao, J.; Dixon, R.A. MATE transporters facilitate vacuolar uptake of epicatechin 3′-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell. 2009, 21, 2323–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Huhman, D.; Shadle, G.; He, X.Z.; Sumner, L.W.; Tang, Y.H.; Dixon, R.A. MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell. 2011, 23, 1536–1555. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.W.; Qiu, Z.J.; Long, Y.; Liu, Y.Z.; Huang, J.J.; Liu, J.X.; Yu, Y.X. Functional identification of PhMATE1 in flower color formation in petunia. Physiol. Plant. 2023, 175, e13949. [Google Scholar] [CrossRef] [PubMed]
- Pal, L.; Dwivedi, V.; Gupta, S.K.; Saxena, S.; Pandey, A.; Chattopadhyay, D. Biochemical analysis of anthocyanin and proanthocyanidin and their regulation in determining chickpea flower and seed coat colour. J. Exp. Bot. 2023, 74, 130–148. [Google Scholar] [CrossRef]
- Ng, M.S.; Ku, Y.S.; Yung, W.S.; Cheng, S.S.; Man, C.K.; Yang, L.; Song, S.K.; Chung, G.; Lam, H.M. MATE-type proteins are responsible for isoflavone transportation and accumulation in soybean seeds. Int. J. Mol. Sci. 2021, 22, 12017. [Google Scholar] [CrossRef]
- Ku, Y.S.; Cheng, S.S.; Cheung, M.Y.; Niu, Y.C.; Liu, A.L.; Chung, G.; Lam, H.M. The poly-glutamate motif of GmMATE4 regulates its isoflavone transport activity. Membranes 2022, 12, 206. [Google Scholar] [CrossRef]




| Class | Number (AR vs. SR) | |
|---|---|---|
| Up | Down | |
| Anthocyanidins | 0 | 11 |
| Aurones | 3 | 3 |
| Chalcones | 5 | 4 |
| Flavonols | 88 | 38 |
| Flavones | 41 | 27 |
| Isoflavones | 1 | 3 |
| Flavanols | 5 | 11 |
| Flavanones | 3 | 6 |
| Flavanonols | 1 | 1 |
| Other Flavonoids | 2 | 5 |
| Proanthocyanidins | 0 | 11 |
| Tannins | 4 | 4 |
| Total | 153 | 124 |
| Class | Metabolite | Content | Log2(FC) | VIP | |
|---|---|---|---|---|---|
| AR_Mean | SR_Mean | ||||
| Anthocyanins | Cyanidin 3,5-O-diglucoside | 2.47 × 105 | 7.17 × 106 | −4.86 | 1.140 |
| Cyanidin 3-O-beta-D-sambubioside | 8.23 × 105 | 6.67 × 106 | −3.02 | 1.136 | |
| Cyanidin-3-O-galloyl-galactoside | 1.74 × 105 | 2.18 × 106 | −3.64 | 1.137 | |
| Cyanidin-3-diglucoside-5-glucoside | 3.60 × 103 | 3.67 × 104 | −3.35 | 1.139 | |
| Cyanidin 3,3′,5-tri-O-glucoside | 1.33 × 104 | 1.00 × 105 | −2.91 | 1.142 | |
| Cyanidin-3-O-(6″-O-feruloyl)glucoside | 6.18 × 104 | 5.79 × 105 | −3.23 | 1.142 | |
| Cyanidin 3-O-(beta-D-xylosyl-(1→2)-beta-D-galactoside) | 5.62 × 104 | 7.24 × 106 | −7.01 | 1.152 | |
| Pelargonidin 3,5-di-beta-D-glucoside | 8.00 × 104 | 6.32 × 105 | −2.98 | 1.137 | |
| Peonidin 3-O-sophoroside | 4.55 × 104 | 3.67 × 106 | −6.33 | 1.141 | |
| Peonidin 3-O-glucoside | 1.17 × 105 | 7.46 × 106 | −6.00 | 1.142 | |
| Peonidin-3,5-O-diglucoside | 4.49 × 104 | 3.38 × 106 | −6.23 | 1.141 | |
| Proanthocyanidins | Procyanidin B8 | 9.15 × 105 | 2.41 × 106 | −1.40 | 1.141 |
| Procyanidin A4 | 2.14 × 105 | 1.73 × 106 | −3.02 | 1.142 | |
| Procyanidin C2 | 3.71 × 104 | 1.42 × 105 | −1.94 | 1.122 | |
| procyanidin B4 3-O-gallate | 4.72 × 104 | 1.22 × 105 | −1.37 | 1.126 | |
| Cinnamtannin A1 | 1.06 × 104 | 4.41 × 104 | −2.05 | 1.112 | |
| Proanthocyanidin A2 | 1.53 × 104 | 2.02 × 105 | −3.72 | 1.085 | |
| 3-galloylProcyanidin B1 | 3.43 × 104 | 1.24 × 105 | −1.86 | 1.044 | |
| Procyanidin A1 | 5.42 × 103 | 4.31 × 104 | −2.99 | 1.125 | |
| 2α,3α-Epoxy-5,7,3′,4′-tetrahydroxyflavan-(4β→8)-catechin | 3.80 × 104 | 3.97 × 105 | −3.38 | 1.142 | |
| 2α,3α-Epoxy-5,7,3′,4′-tetrahydroxyflavan-(4β→8)-epicatechin | 2.74 × 104 | 2.45 × 105 | −3.16 | 1.111 | |
| 9,10-Dihydro-10-(4-hydroxyphenyl)-pyrano [2,3-h]epicatechin-8-one gallate | 1.84 × 105 | 2.70 × 106 | −3.88 | 1.139 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Y.; Ren, J.; Chen, Z.; Shi, D. Integrated Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway in Relation to Color Mutation in Roses. Biology 2025, 14, 1337. https://doi.org/10.3390/biology14101337
Xuan Y, Ren J, Chen Z, Shi D. Integrated Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway in Relation to Color Mutation in Roses. Biology. 2025; 14(10):1337. https://doi.org/10.3390/biology14101337
Chicago/Turabian StyleXuan, Yun, Jie Ren, Zhu Chen, and Dan Shi. 2025. "Integrated Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway in Relation to Color Mutation in Roses" Biology 14, no. 10: 1337. https://doi.org/10.3390/biology14101337
APA StyleXuan, Y., Ren, J., Chen, Z., & Shi, D. (2025). Integrated Metabolomic and Transcriptomic Analyses of the Flavonoid Biosynthetic Pathway in Relation to Color Mutation in Roses. Biology, 14(10), 1337. https://doi.org/10.3390/biology14101337






