Effect of Different Larval Diets on Life History Traits and Nutritional Content in Anastrepha fraterculus (Diptera: Tephritidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Larval Diet Composition and Diet Elaboration
2.3. Experimental Procedure
2.3.1. Biological Traits
2.3.2. Female Fecundity
2.3.3. Wing Length
2.3.4. Dry Weight and Metabolite Content
2.4. Data Analysis
2.4.1. Biological Traits
2.4.2. Female Fecundity
2.4.3. Wing Length
2.4.4. Dry Weight
2.4.5. Metabolite Content
3. Results
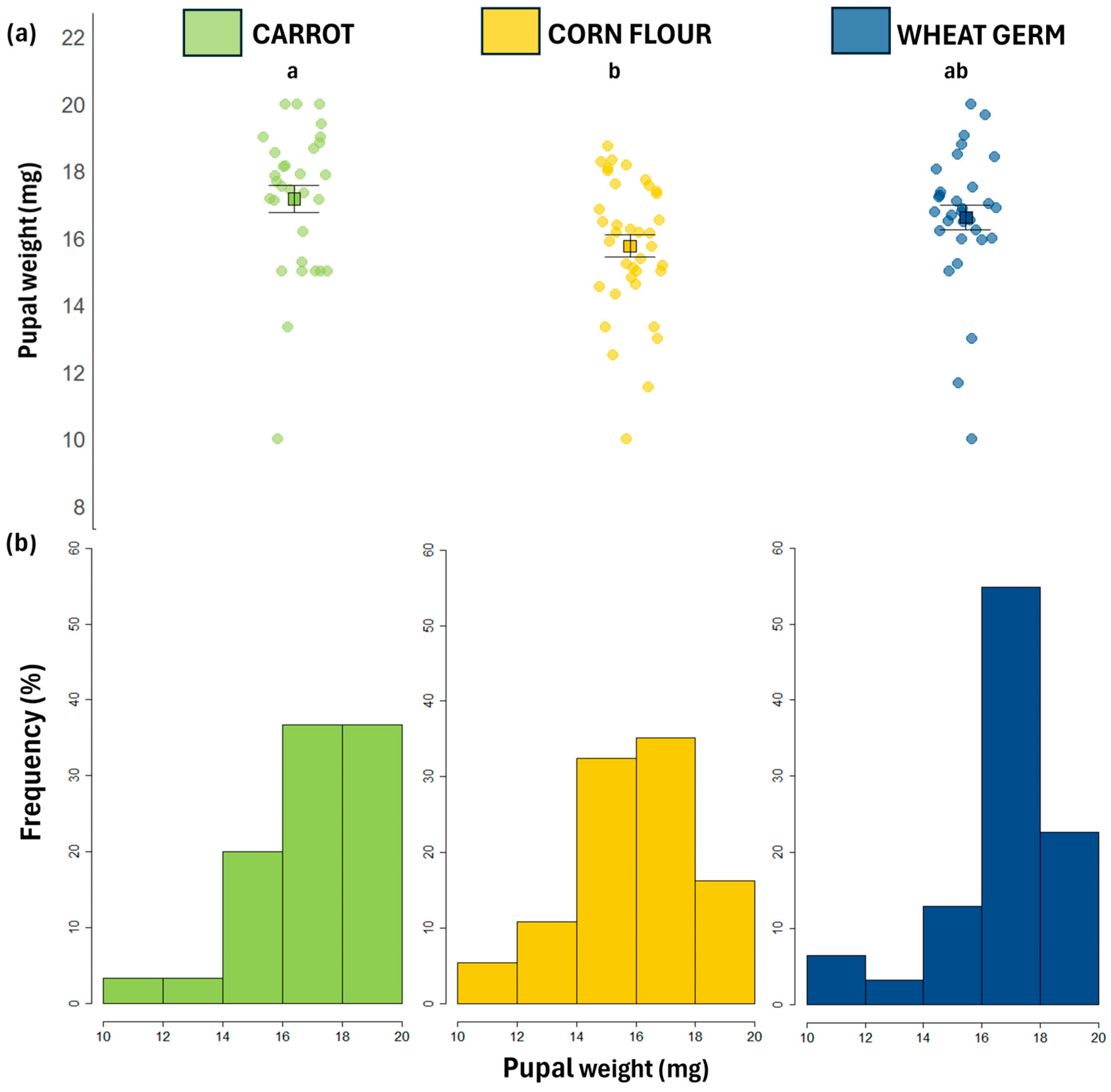
3.1. Biological Traits
3.2. Female Fecundity
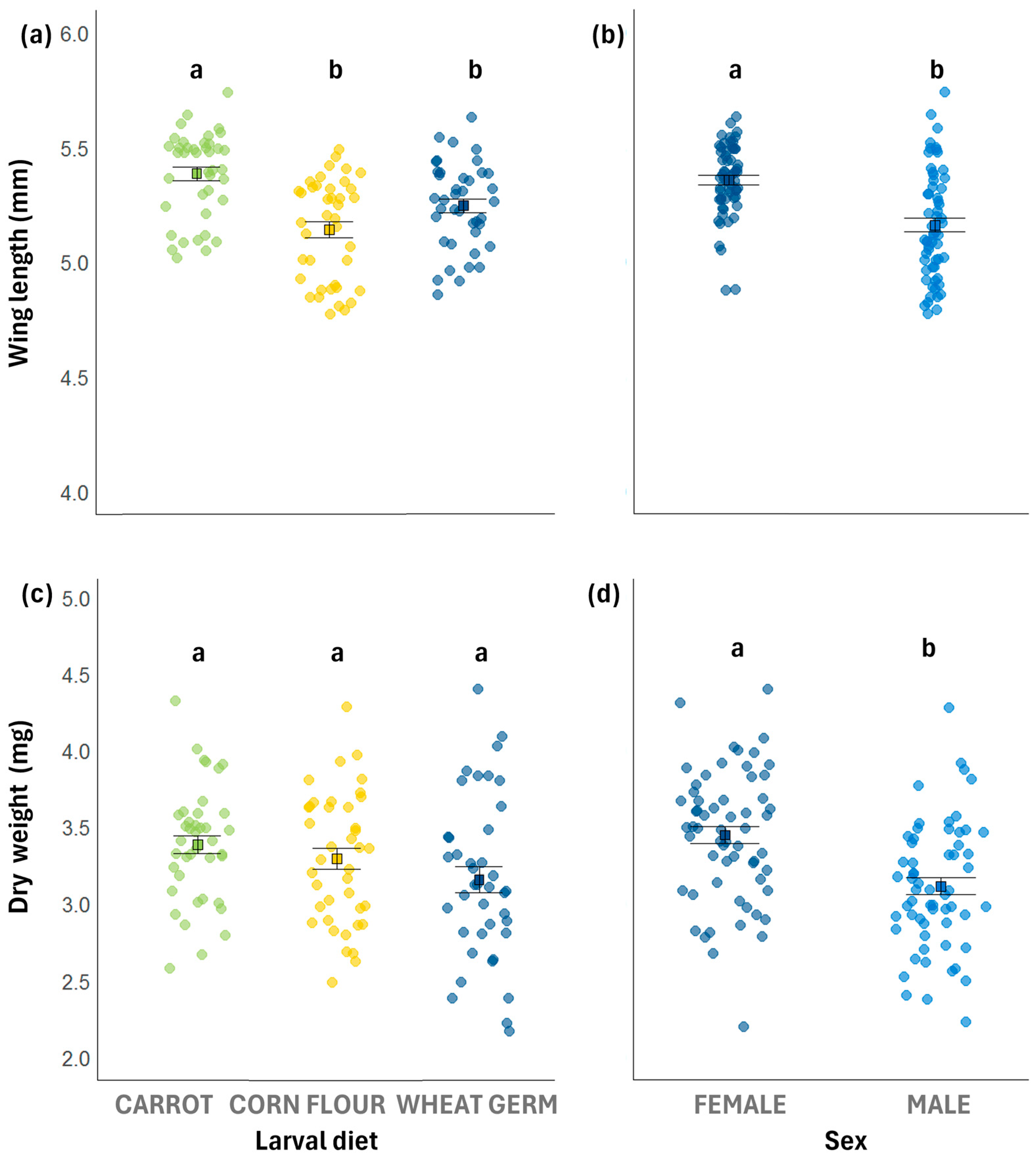
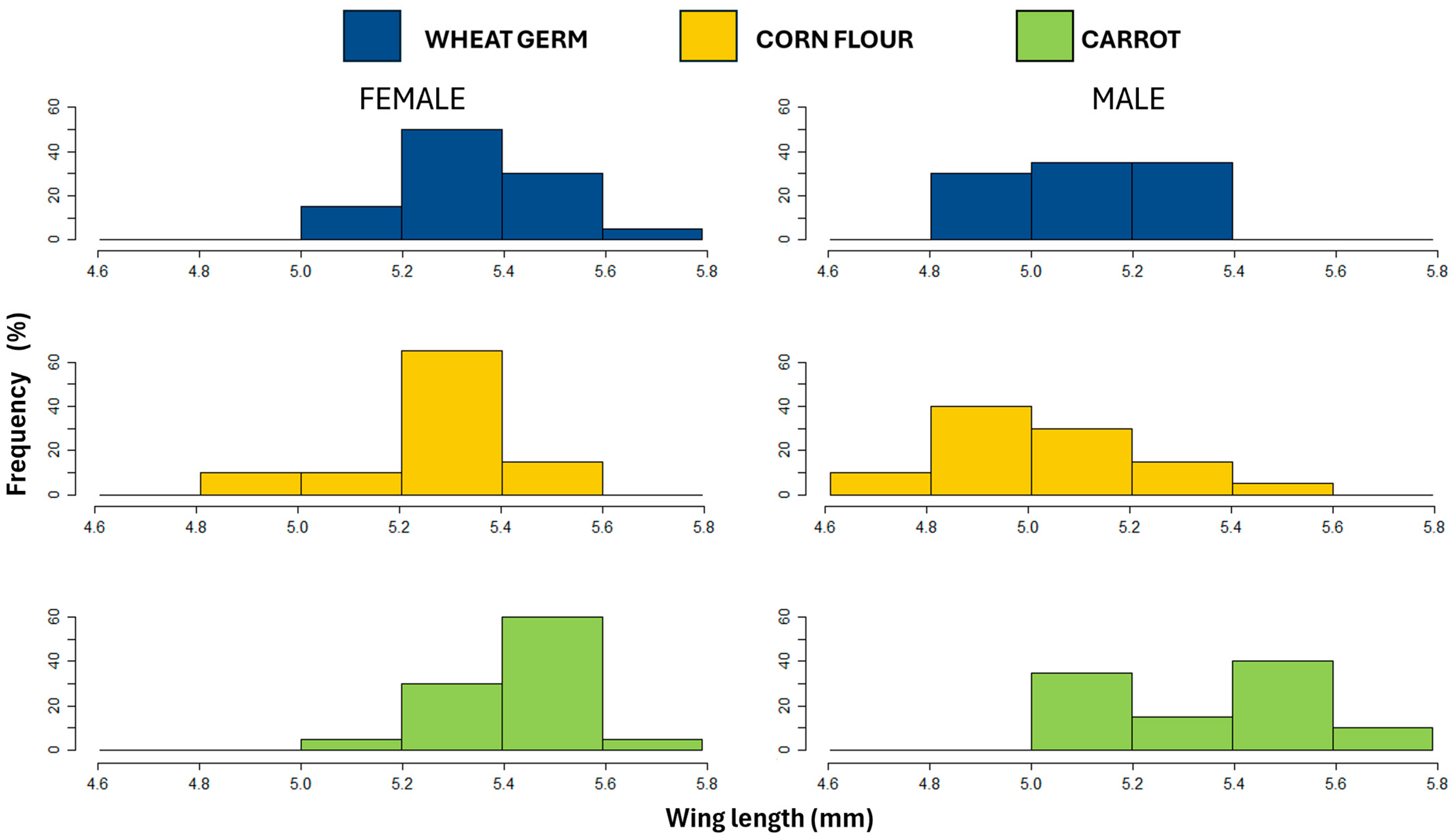
3.3. Wing Length
3.4. Dry Weight
3.5. Metabolite Content
3.5.1. Glycogen Content
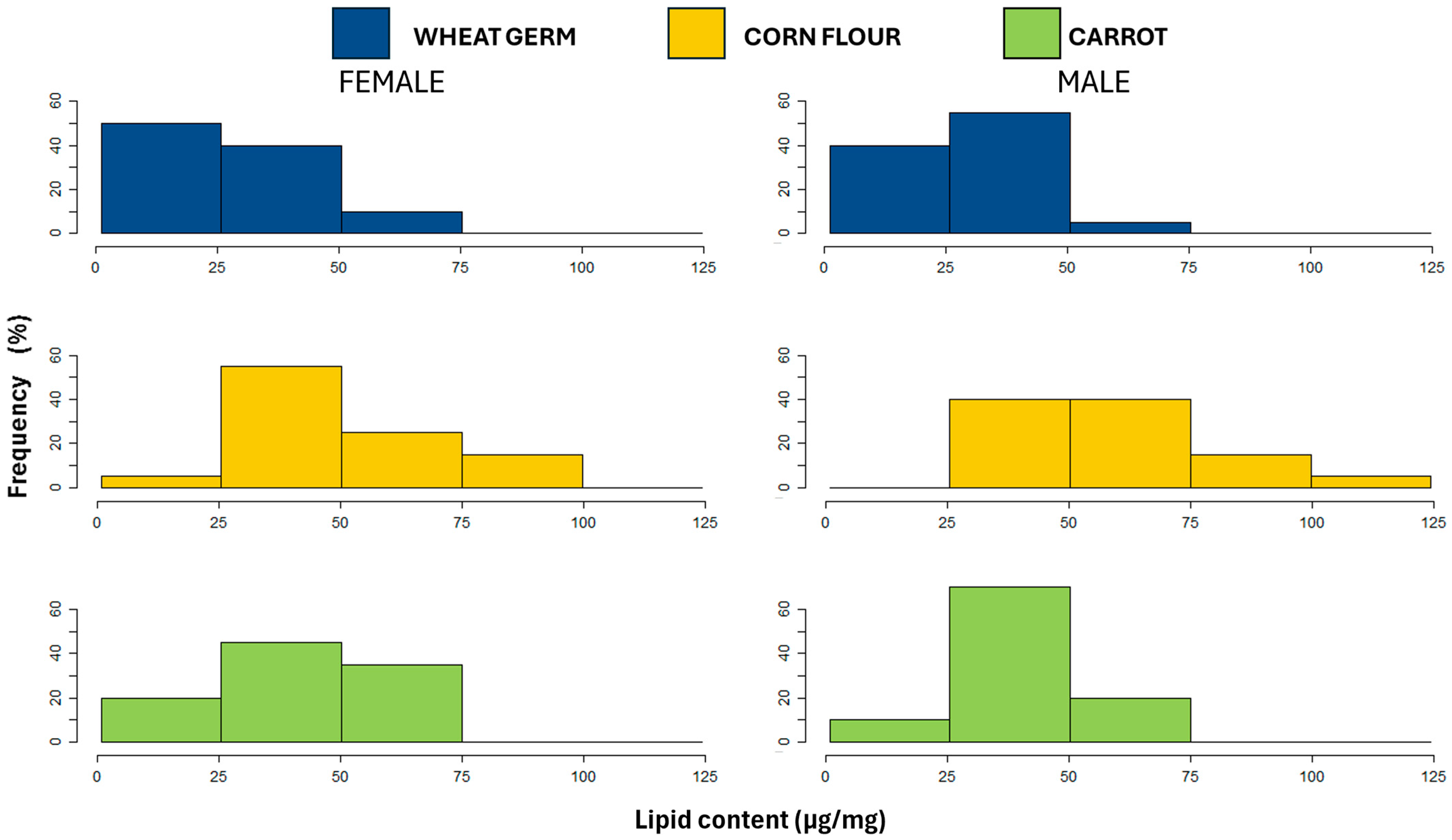
3.5.2. Lipid Content
3.5.3. Carbohydrate Content
3.5.4. Protein Content
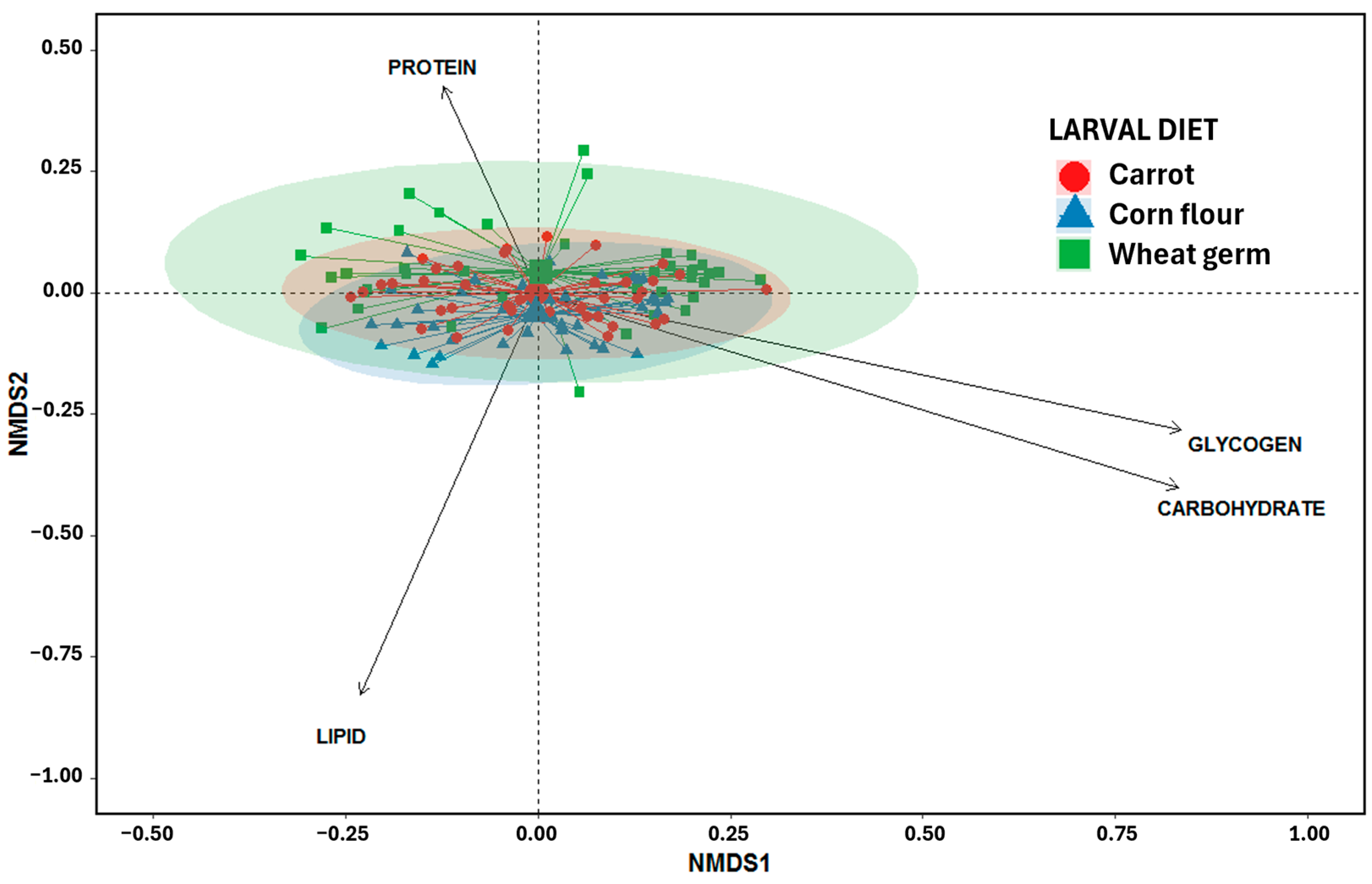
3.5.5. Non-Metric Multidimensional Scaling Analysis (NMDS)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SIT | Sterile insect technique |
Appendix A
| Larval Diet | Carbohydrates (g) | Proteins (g) | Lipids (g) |
|---|---|---|---|
| Wheat germ | 10.59 | 4.42 | 0.55 |
| Corn flour | 26.98 | 5.08 | 0.33 |
| Carrot | 16.93 | 5.21 | 0.71 |

References
- Kaspi, R.; Yuval, B. Post-teneral protein feeding improves sexual competitiveness but reduces longevity of mass-reared sterile male Mediterranean fruit flies (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2000, 93, 949–955. [Google Scholar] [CrossRef]
- Nash, W.J.; Chapman, T. Effect of Dietary Components on Larval Life History Characteristics in the Medfly (Ceratitis capitata: Diptera, Tephritidae). PLoS ONE 2014, 9, e86029. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Rincón-Betancurt, O.; Martínez-Salgado, R.T.; Hernández, E. A Novel, Low-Cost Coconut Fiber Larval Diet for Mass Rearing Anastrepha (Diptera: Tephritidae). J. Econ. Entomol. 2019, 112, 1112–1119. [Google Scholar] [CrossRef]
- Moadeli, T.; Mainali, B.; Ponton, F.; Taylor, P.W. Effects of fatty acids and vitamin E in larval diets on development and performance of Queensland fruit fly. J. Insect Physiol. 2020, 125, 104058. [Google Scholar] [CrossRef]
- Weldon, C.W.; Manguni, S.; Démares, F.; Du Rand, E.E.; Malod, K.; Manrakhan, A.; Nicolson, S.W. Adult diet does not compensate for impact of a poor larval diet on stress resistance in a tephritid fruit fly. J. Exp. Biol. 2019, 222, jeb192534. [Google Scholar] [CrossRef]
- Simpson, S.J.; Raubenheimer, D. A multi-level analysis of feeding behavior: The geometry of nutritional decisions. Philos. Trans. R. Soc. B 1993, 342, 381–402. [Google Scholar]
- Pascacio-Villafán, C.; Williams, T.; Birke, A.; Aluja, M. Nutritional and non-nutritional food components modulate phenotypic variation but not physiological trade-offs in an insect. Sci. Rep. 2016, 6, 29413. [Google Scholar] [CrossRef]
- Kaspi, R.; Mossinson, S.; Drezner, T.; Kamensky, B.; Yuval, B. Effects of larval diet on development rates and reproductive maturation of male and female Mediterranean fruit flies. Physiol. Entomol. 2002, 27, 29–38. [Google Scholar] [CrossRef]
- Nestel, D.; Papadopoulos, N.T.; Liedo, P.; Gonzales-Ceron, L.; Carey, J.R. Trends in lipid and protein contents during medfly aging: An harmonic path to death. Arch. Insect Biochem. Physiol. 2005, 60, 130–139. [Google Scholar] [CrossRef]
- Nestel, D.; Nemny-Lavy, E. Nutrient balance in medfly, Ceratitis capitata, larval diets affect the ability of the developing insect to incorporate lipid and protein reserves. Entomol. Exp. Appl. 2008, 126, 53–60. [Google Scholar] [CrossRef]
- Morimoto, J.; Nguyen, B.; Lundbäck, I.; Than, A.T.; Tabrizi, S.T.; Ponton, F.; Taylor, P.W. Effects of carbohydrate types on larval development and adult traits in a polyphagous fruit fly. J. Insect Physiol. 2020, 120, 103969. [Google Scholar] [CrossRef]
- Moadeli, T.; Mainali, B.; Ponton, F.; Taylor, P.W. Canola Oil as an Economical Lipid Source in Gel Larval Diet for Queensland Fruit Fly. J. Econ. Entomol. 2018, 111, 2764–2771. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Hernández, E.; Rincón-Betancurt, O.; García-Fajardo, L.V.; Diego-García, E. Effects of a Bulking Agent on the Protein: Carbohydrate Ratio, Bioconversion, and Cost-effectiveness of a Larval Diet for Anastrepha ludens (Diptera: Tephritidae). J. Econ. Entomol. 2022, 115, 739–747. [Google Scholar] [CrossRef]
- Aceituno-Medina, M.; Hernández, E.; Rincón-Betancurt, O.; García-Fajardo, L.V.; Diego-García, E. Larval nutritional effects on male and female survival and fecundity in Anastrepha ludens. Entomol. Exp. Appl. 2024, 172, 2–14. [Google Scholar] [CrossRef]
- Ongaratto, S.; Pinto, K.; Manica-Berto, R.; da Silva Gonçalves, R.; Nörnberg, S.D.; Nava, D.E.; Bernardi, D. Different protein sources of larval diet on the rearing of Anastrepha fraterculus (Diptera: Tephritidae): Biological and nutritional analyses. Neotrop. Entomol. 2024, 53, 1031–1044. [Google Scholar] [CrossRef]
- Maset, B.A. Eficiência de Dietas Larvais para a Produção de Ceratitis capitata e Anastrepha fraterculus (Diptera: Tephritidae). Master’s Thesis, University of São Paulo, Piracicaba, Brazil, 2019. [Google Scholar]
- Maset, B.A.; Demetrio, C.G.B.; Lopes, L.A.; Zamboni-Costa, M.L.; Botteon, V.W.; Mastrangelo, T. Which artificial larval diet is better for Ceratitis capitata (Diptera: Tephritidae) rearing? J. Basic Appl. Zool. 2022, 83, 48. [Google Scholar] [CrossRef]
- Orozco-Dávila, D.; Quintero, L.; Hernández, E.; Solís, E.; Artiaga, T.; Hernández, R.; Ortega, C.; Montoya, P. Mass rearing and sterile insect releases for the control of Anastrepha spp. pests in Mexico. Entomol. Exp. Appl. 2017, 164, 176–187. [Google Scholar] [CrossRef]
- Parra, J.R.P. A evolução das dietas artificiais e suas interações em ciência e tecnologia. In Bioecologia e Nutrição de Insetos: Base para o Manejo Integrado de Pragas, 2nd ed.; Panizzi, A.R., Ed.; Embrapa: Brasília, Brazil, 2009; pp. 91–174. [Google Scholar]
- Norrbom, A.L.; Zucchi, R.A.; Hernández-Ortiz, V. Phylogeny of the genera Anastrepha and Toxotrypana (Trypetinae: Toxotrypanini) based on morphology. In Fruit Flies (Tephritidae): Phylogeny and Evolution of Behavior; Aluja, M., Norrbom, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 299–342. [Google Scholar]
- Hernández-Ortiz, V.; Aluja, M. Listado de especies del género neotropical Anastrepha (Diptera: Tephritidae) con notas sobre su distribución y plantas hospederas. Folia Entomol. Mex. 1993, 88, 89–105. [Google Scholar]
- Hernández-Ortiz, V.; Barradas-Juanz, N.; Díaz-Castelazo, C. A review of the natural host plants of the Anastrepha fraterculus complex in the Americas. In Area-Wide Management of Fruit Fly Pests; Perez-Staples, D., Diaz-Fleischer, F., Montoya, P., Vera, M.T., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 89–122. [Google Scholar]
- Cladera, J.L.; Vilardi, J.C.; Juri, M.; Paulin, L.E.; Giardini, M.C.; Gomez-Cendra, P.V.; Lanzavecchia, S.B. Genetics and biology of Anastrepha fraterculus: Research supporting the use of the sterile insect technique (SIT) to control this pest in Argentina. BMC Genet. 2014, 15, S12. [Google Scholar] [CrossRef]
- Hernández-Ortiz, V.; Bartolucci, A.F.; Morales-Valles, P.; Frías, D.; Selivon, D. Cryptic species of the Anastrepha fraterculus complex (Diptera: Tephritidae): A multivariate approach for the recognition of South American morphotypes. Ann. Entomol. Soc. Am. 2012, 105, 305–318. [Google Scholar] [CrossRef]
- Vaníčková, L.; Hernández-Ortiz, V.; Bravo, I.S.; Dias, V.; Roriz, A.K.; Laumann, R.A.; Mendonça-Ade, L.; Paranhos, B.A.; do Nascimento, R.R. Current knowledge of the species complex Anastrepha fraterculus (Diptera, Tephritidae) in Brazil. Zookeys 2015, 26, 211–237. [Google Scholar] [CrossRef]
- Vera, T.; Abraham, S.; Oviedo, A.; Willink, E. Demographic and quality control parameters of Anastrepha fraterculus (Diptera: Tephritidae) maintained under artificial rearing. Fla. Entomol. 2007, 90, 53–57. [Google Scholar] [CrossRef]
- Morelli, R.; Costa, K.Z.; Fagioni, K.M.; Costa, M.D.L.Z.; Nascimento, A.S.D.; Pimentel, R.M.D.A.; Walder, J.M.M. New protein sources in adults diet for mass-rearing of Anastrepha fraterculus (Diptera:Tephritidae). Braz. Arch. Biol. Technol. 2012, 55, 827–833. [Google Scholar] [CrossRef]
- Mastrangelo, T.; Kovaleski, A.; Maset, B.; Costa, M.D.L.Z.; Barros, C.; Lopes, L.A.; Caceres, C. Improvement of the mass-rearing protocols for the South American fruit fly for application of the sterile insect technique. Insects 2021, 12, 622. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho, R.B. Developing a mass-rearing system for Anastrepha fraterculus (Diptera:Tephritidae) in north-eastern Brazil. Int. J. Trop. Insect Sci. 2014, 34, S66–S72. [Google Scholar] [CrossRef]
- Turinetto, B.; Rodriguez, L.; Rivero, M.; Sosa, S.; Goane, L.; Milla, F.; Ruiz, M.J.; Segura, D.; Vera, M.T.; Asfennato, A. Puesta a punto de la cría semimasiva de Anastrepha fraterculus Wied. In Proceedings of the 11th Tephritid Workers of the Western Hemisphere (TWHH) Meeting, Montego Bay, Jamaica, 3–7 June 2024. [Google Scholar]
- Salles, L.A.B. Bioecologia e Controle da Moscadas-Frutas Sul-Americana; EMBRAPA-CPACT: Pelotas, Brazil, 1995; 58p. [Google Scholar]
- Cohen, A.C. Insect Diets: Science and Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar] [CrossRef]
- Gonzalez, J.; Vargas, C.; Lara, B. Estudios sobre la aplicación de la técnica de machos esteriles en el control de la mosca sudamericana de la fruta Anastrepha fraterculus (Wied). Anales ler congreso Latinoamericano de Entomología. Rev. Peru. Entom. 1971, 14, 66–71. [Google Scholar]
- Manso, F. Breeding technique of Anastrepha fraterculus (Wied.) for genetic studies. In Proceedings of the Workshop on the South American Fruit Fly, Anastrepha fraterculus (Wied.): Advances in Artificial Rearing, Taxonomic Status and Biological Studies, Viña del Mar, Chile, 1–2 November 1996; IAEA-TECDOC-1064. International Atomic Energy Agency: Vienna, Austria, 1998; pp. 25–32. [Google Scholar]
- Domínguez Gordillo, J.C. Mass rearing methods for fruit fly. In Proceedings of the Workshop on the South American Fruit Fly, Anastrepha fraterculus (Wied.): Advances in Artificial Rearing, Taxonomic Status and Biological Studies, Viña del Mar, Chile, 1–2 November 1996; IAEA-TECDOC-1064. International Atomic Energy Agency: Vienna, Austria, 1998; pp. 59–72. [Google Scholar]
- Stahl, W.; Sies, H. Antioxidant activity of carotenoids. Mol Aspects Med. 2003, 24, 345–351. [Google Scholar] [CrossRef]
- Rovai, D.; Ortgies, M.; Amin, S.; Kuwahara, S.; Schwartz, G.; Lesniauskas, R.; Garza, J.; Lammert, A. Utilization of Carrot Pomace to Grow Mealworm Larvae (Tenebrio molitor). Sustainability 2021, 13, 9341. [Google Scholar] [CrossRef]
- FAO; IAEA; USDA. Product Quality Control for Sterile Mass-Reared and Released Tephritid Fruit Flies, 7th ed.; International Atomic Energy Agency: Vienna, Austria, 2019; pp. 1–148.
- Sciurano, R.; Segura, D.; Rodriguero, M.; Gómez Cendra, P.; Allinghi, A.; Cladera, J.L.; Vilardi, J. Sexual selection on multivariate phenotypes in Anastrepha fraterculus (Diptera: Tephritidae) from Argentina. Fla. Entomol. 2007, 90, 163–170. [Google Scholar] [CrossRef]
- Foray, V.; Pelisson, P.F.; Bel-Venner, M.C.; Desouhant, E.; Venner, S.; Menu, F.; Giron, D.; Rey, B. A handbook for uncovering the complete energetic budget in insects: The van Handel’s method 1985 revisited. Physiol. Entomol. 2012, 37, 295–302. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Annu. Rev. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C. Determination of lipid, glycogen and sugars in mosquitoes. In Methods in Anopheles Research, 2nd ed.; MR4/BEI Resources, Ed.; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2015; pp. 1–7. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R.; Springer: New York, NY, USA, 2009. [Google Scholar]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Nielsen, A.; Skaug, H.J.; Mächler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Lenth, R.V. emmeans: Estimated Marginal Means, aka Least-Squares Means, R Package Version 1.8.3; 2022. Available online: https://CRAN.Rproject.org/package=emmeans (accessed on 30 July 2025).
- Graves, S.; Piepho, H.-P.; Selzer, L.; Dorai-Raj, S. multcompView: Visualizations of Paired Comparisons, R Package Version 0.1-8. 2019. Available online: https://cran.r-project.org/package=multcompView (accessed on 30 July 2025).
- Goane, L.; Pereyra, P.; Castro, F.; Ruiz, M.J.; Juarez, M.L.; Segura, D.F.; Vera, M.T. Yeast derivatives and wheat germ in the adult diet modulates fecundity in a tephritid pest. Bull. Entomol. Res. 2019, 109, 178–190. [Google Scholar] [CrossRef] [PubMed]
- Goane, L.; Salgueiro, J.; Medina-Pereyra, P.; Arce-Osvaldo, E.A.; Ruiz, M.J.; Nussenbaum, A.L.; Segura, D.F.; Vera, M.T. Antibiotic treatment reduces fecundity and nutrient content in females of Anastrepha fraterculus (Diptera: Tephritidae) in a diet dependent way. J. Insect Physiol. 2022, 139, 104396. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Wickham, H.; François, R.; Henry, L.; Müller, K. dplyr: A Grammar of Data Manipulation, R Package Version 1.1.4; 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 30 July 2025).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package, R Package Version 2.6-4. 2022. Available online: https://CRAN.R-project.org/package=vegan (accessed on 30 July 2025).
- Anjos-Duarte, C.S.; Costa, A.M.; Joachim-Bravo, I.S. Sexual behaviour of the Mediterranean fruit fly (Diptera: Tephritidae): The influence of female size on mate choice. J. Appl. Entomol. 2011, 135, 367–373. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov (accessed on 15 June 2025).
- Wang, L.; Wei, D.D.; Wang, G.Q.; Huang, H.Q.; Wang, J.J. High-Sucrose Diet Exposure on Larvae Contributes to Adult Fecundity and Insecticide Tolerance in the Oriental Fruit Fly, Bactrocera dorsalis (Hendel). Insects 2023, 14, 407. [Google Scholar] [CrossRef]
- Chang, C.L. Effect of Amino Acids on Larvae and Adults of Ceratitis capitata (Diptera: Tephritidae). Ann. Entomol. Soc. Am. 2004, 97, 529–535. [Google Scholar] [CrossRef]
- Nestel, D.; Nemny-Lavy, E.; Chang, C.L. Lipid and protein loads in pupating larvae and emerging adults as affected by the composition of mediterranean fruit fly (Ceratitis capitata) meridic larval diets. Arch. Insect Biochem. Physiol. 2004, 56, 97–109. [Google Scholar] [CrossRef]
- Hernández, E.; Rivera, P.; Aceituno-Medina, M.; Aguilar-Laparra, R.; Quintero-Fong, L.; Orozco-Dávila, D. Yeast efficacy for mass rearing of Anastrepha ludens, A. obliqua, and Ceratitis capitata (Diptera: Tephritidae). Acta Zool. Mex. 2016, 32, 240–252. Available online: http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S0065-17372016000300240&lng=es&nrm=iso (accessed on 19 September 2025). [CrossRef][Green Version]
- Pašková, M. New larval agar-based diet for laboratory rearing of Mediterranean fruit fly Ceratitis capitata (Diptera, Tephritidae). Biologia 2007, 62, 477–481. [Google Scholar] [CrossRef]
- Fasce, B.; Ródenas, L.; López, M.C.; Moya, V.J.; Pascual, J.J.; Cambra-López, M. Nutritive Value of Wheat Bran Diets Supplemented with Fresh Carrots and Wet Brewers’ Grains in Yellow Mealworm. J. Insect Sci. 2022, 22, 7. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.D.; Maeda, S.; Holloway, J.R. Subsitution of dehydrated for fresh carrots in medium for rearing fruit flies. J. Econ. Entomol. 1956, 49, 135–136. [Google Scholar] [CrossRef]
- Felton, G.W.; Summers, C.B. Antioxidant Systems in insects. Arch. Insect Biochem. Physiol. 1995, 29, 995. [Google Scholar] [CrossRef] [PubMed]
- Shingleton, A.W.; Frankino, W.A.; Flatt, T.; Nijhout, H.F.; Emlen, D.J. Size and shape: The developmental regulation of static allometry in insects. BioEssays 2007, 29, 536–548. [Google Scholar] [CrossRef]
- Nestel, D.; Papadopoulos, N.T.; Pascacio-Villafán, C.; Righini, N.; Altuzar-Molina, A.R.; Aluja, M. Resource allocation and compensation during development in holometabolous insects. J. Insect Physiol. 2016, 95, 78–88. [Google Scholar] [CrossRef]
- Bangham, J.; Chapman, T.; Partridge, L. Effects of body size, accessory gland and testis size on pre- and postcopulatory success in Drosophila melanogaster. Anim. Behav. 2002, 64, 915–921. [Google Scholar] [CrossRef]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008, 7, 478–490. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Downer, R.G.H.; Matthews, J.R. Patterns of Lipid Distribution and Utilisation in Insects. Amer. Zool. 1976, 16, 733–745. [Google Scholar] [CrossRef]
| Constituent | Wheat Germ Diet | Corn Flour Diet | Carrot with Wheat Germ Diet |
|---|---|---|---|
| Non-hydrolyzed brewer’s yeast | 6.04 | 7 | 7 |
| Wheat germ | 6.04 | - | 5 |
| Corn flour | - | 30 | - |
| Sucrose | 6.04 | 3 | 5 |
| Sodium benzoate | 0.30 | 0.20 | 0.20 |
| Nipagin | 0.30 | 0.35 | 0.20 |
| Citric acid | 0.40 | 0.60 | 0.90 |
| Agar | 0.40 | - | - |
| Mashed carrot | - | - | 72.67 |
| Water | 80.48 | 58.85 | 9.08 |
| pH | 4.53 ± 0.06 | 3.83 ± 0.05 | 3.76 ± 0.06 |
| Biological Traits | Wheat Germ Diet | Corn Flour Diet | Carrot Diet | Statistical Parameters | |
|---|---|---|---|---|---|
| Egg hatch (%) | 87.8 ± 1.7 a | 87.1 ± 1.8 a | 86.2 ± 1.9 a | χ2 = 1.3846 | p = 0.5004 |
| Number of pupae | 108 ± 13.5 a | 109 ± 13.4 a | 122 ± 14.6 a | χ2 = 1.0905 | p = 05797 |
| Pupal weight (mg) | 16.50 ± 0.46 ab | 15.60 ± 0.44 b | 17.00 ± 0.47 a | χ2 = 7.2127 | p = 0.0271 |
| Larva-to-pupa survival (proportion) | 0.41 ± 0.04 a | 0.43 ± 0.04 a | 0.46 ± 0.04 a | χ2 = 1.0595 | p = 0.5888 |
| Larval development time (days) | 11.3 ± 0.65 b | 12.7 ± 0.73 a | 10.3 ± 0.60 c | χ2 = 761.99 | p < 0.0001 |
| Pupal development time (days) | 15.9 ± 0.67 b | 16.8 ± 0.71 a | 15.0 ± 0.63 c | χ2 = 839.84 | p < 0.0001 |
| Larva-to-adult survival (proportion) | 0.31 ± 0.05 a | 0.30 ± 0.05 a | 0.30 ± 0.05 a | χ2 = 0.0579 | p = 0.9714 |
| Adult emergence (proportion) | 0.72 ± 0.05 a | 0.68 ± 0.05 a | 0.68 ± 0.05 a | χ2 = 0.4433 | p = 0.8012 |
| Non-deformed adult emergence (proportion) | 0.966 ± 0.014 ab | 0.939 ± 0.024 b | 0.985 ± 0.007 a | χ2 = 17.976 | p = 0.0001 |
| Deformed adult emergence (proportion) | 0.034± 0.014 ab | 0.061 ± 0.024 b | 0.014 ± 0.007 a | χ2 = 17.976 | p = 0.0001 |
| Partially emerged adults (proportion) | 0.003 ± 0.001 a | 0.003 ± 0.001 a | 0.004 ± 0.001 a | χ2 = 0.2532 | p = 0.8811 |
| Sex ratio (proportion) | 0.49 ± 0.01 a | 0.48 ± 0.01 a | 0.49 ± 0.01 a | χ2 = 0.3275 | p = 0.8490 |
| Statistical Parameters | ||||||
|---|---|---|---|---|---|---|
| Wheat Germ Diet | Corn Flour Diet | Carrot Diet | Larval Diet | Sex | Larval Diet * Sex | |
| Wing length | 5.25 ± 0.03 b | 5.14 ± 0.03 b | 5.39 ± 0.03 a | χ2 = 7.9619 | χ2 = 16.0296 | χ2 = 5.6060 |
| p = 0.0186 | p < 0.0001 | p = 0.0606 | ||||
| Dry weight | 3.16 ± 0.06 a | 3.30 ± 0.06 a | 3.38 ± 0.06 a | F = 3.0010 | F = 19.2490 | F = 0.6770 |
| p = 0.5370 | p < 0.0001 | p = 0.5101 | ||||
| Glycogen | 2.35 ± 0.91 a | 2.79 ± 1.04 a | 2.54 ± 0.98 a | χ2 = 5.4336 | χ2 = 1.7351 | χ2 = 2.3335 |
| p = 0.0660 | p = 0.1877 | p = 0.3113 | ||||
| Lipid | 28.14 ± 3.22 c | 51.71 ± 5.92 a | 39.79 ± 4.55 b | χ2 = 45.040 | χ2 = 0.9660 | χ2 = 0.4359 |
| p < 0.0001 | p = 0.3257 | p = 0.8042 | ||||
| Carbohydrate | 18.82 ± 7.08 b | 25.76 ± 9.69 a | 22.82 ± 8.58 ab | χ2 = 12.2979 | χ2 = 1.0005 | χ2 = 0.3213 |
| p = 0.0021 | p = 0.3171 | p = 0.8516 | ||||
| Protein | 102.41 ± 3.98 a | 100.37 ± 4.06 a | 107.46 ± 4.26 a | χ2 = 2.5259 | χ2 = 5.2669 | χ2 = 4.2419 |
| p = 0.2828 | p = 0.0217 | p = 0.1199 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández, F.L.; Ruiz, M.J.; Medina Pereyra, P.; Milla, F.H.; Scannapieco, A.C.; Segura, D.F.; Vera, M.T.; Nestel, D.; Goane, L. Effect of Different Larval Diets on Life History Traits and Nutritional Content in Anastrepha fraterculus (Diptera: Tephritidae). Biology 2025, 14, 1332. https://doi.org/10.3390/biology14101332
Fernández FL, Ruiz MJ, Medina Pereyra P, Milla FH, Scannapieco AC, Segura DF, Vera MT, Nestel D, Goane L. Effect of Different Larval Diets on Life History Traits and Nutritional Content in Anastrepha fraterculus (Diptera: Tephritidae). Biology. 2025; 14(10):1332. https://doi.org/10.3390/biology14101332
Chicago/Turabian StyleFernández, Fátima L., María Josefina Ruiz, Pilar Medina Pereyra, Fabián H. Milla, Alejandra C. Scannapieco, Diego F. Segura, María Teresa Vera, David Nestel, and Lucía Goane. 2025. "Effect of Different Larval Diets on Life History Traits and Nutritional Content in Anastrepha fraterculus (Diptera: Tephritidae)" Biology 14, no. 10: 1332. https://doi.org/10.3390/biology14101332
APA StyleFernández, F. L., Ruiz, M. J., Medina Pereyra, P., Milla, F. H., Scannapieco, A. C., Segura, D. F., Vera, M. T., Nestel, D., & Goane, L. (2025). Effect of Different Larval Diets on Life History Traits and Nutritional Content in Anastrepha fraterculus (Diptera: Tephritidae). Biology, 14(10), 1332. https://doi.org/10.3390/biology14101332







