Simple Summary
Cholangiocarcinomas, malignant tumours originating from the biliary epithelium, have had an increasing incidence during recent decades; thus, awareness represents the key operating factor nowadays. Our purpose was to overview the tumour field following the constellation of the risk factors and the paraneoplastic syndromes, emphasizing the endocrine features amid the entire multidisciplinary panel. The hormonal burden is reflected by complex interplays such as the galanin system, exposure to sex hormone therapy, interferences with vitamin D system, etc., while humoral hypercalcaemia of malignancy, although rare, has been reported due to parathyroid hormone-related protein over-production in addition to other elements such a fibroblast growth factor receptor-positive or even procalcitonin-positive tumour. The level of endocrine evidence across clinical trials remains far from generous. Further applications such as emergent hormonal biomarkers are expected for daily practice.
Abstract
Cholangiocarcinomas (CCAs), a heterogeneous group of challenging malignant tumours which originate from the biliary epithelium, are associated with an alarming increasing incidence during recent decades that varies between different regions of the globe. Thus, awareness represents the key operating factor. Our purpose was to overview the field of CCAs following a double perspective: the constellation of the risk factors, and the presence of the paraneoplastic syndromes, emphasizing the endocrine features amid the entire multidisciplinary panel. This is a narrative review. A PubMed-based search of English-language original articles offered the basis of this comprehensive approach. Multiple risk factors underlying different levels of statistical evidence have been listed such as chronic biliary diseases and liver conditions, inflammatory bowel disease, parasitic infections (e.g., Opisthorchis viverrini, Clonorchis sinensis), lifestyle influence (e.g., alcohol, smoking), environmental exposure (e.g., thorotrast, asbestos), and certain genetic and epigenetic interplays. With regard to the endocrine panel, a heterogeneous spectrum should be taken into consideration: non-alcoholic fatty liver disease, obesity, type 2 diabetes mellitus, and potential connections with vitamin D status, glucagon-like peptide 1 receptor, or the galanin system, respectively, with exposure to sex hormone therapy. Amid the numerous dermatologic, hematologic, renal, and neurologic paraneoplastic manifestations in CCAs, the endocrine panel is less described. Humoral hypercalcaemia of malignancy stands as the most frequent humoral paraneoplastic syndrome in CCAs, despite being exceptional when compared to other paraneoplastic (non-endocrine) manifestations and to its reported frequency in other (non-CCAs) cancers (it accompanies 20–30% of all cancers). It represents a poor prognosis marker in CCA; it may be episodic once the tumour relapses. In addition to the therapy that targets the originating malignancy, hypercalcaemia requires the administration of bisphosphonates (e.g., intravenous zoledronic acid) or denosumab. Early detection firstly helps the general wellbeing of a patient due to a prompt medical control of high serum calcium and it also provides a fine biomarker of disease status in selected cases that harbour the capacity of PTHrP secretion. The exact molecular biology and genetic configuration of CCAs that display such endocrine traits is still an open matter, but humoral hypercalcaemia adds to the overall disease burden.
Keywords:
cholangiocarcinoma; endocrine; paraneoplastic syndrome; risk factor; calcium; PTHrP; tumour biology 1. Introduction
Cholangiocarcinomas (CCAs), a heterogeneous group of challenging malignant tumours which originate from the biliary epithelium, are anatomically classified into intrahepatic (iCCAs), perihilar (pCCAs), and distal (dCCAs), the last two also being referred to as extrahepatic (eCCAs) [1,2]. iCCAs are located proximally to the second-order bile ducts and comprise 10–20% of all CCAs [1,3]. pCCA, also known as Klatskin tumour, is the most prevalent type of CCA (50–60%) and arises above the insertion of the cystic duct into the common bile duct [1,3,4]. dCCA can be located anywhere between the cystic duct and the ampulla of Vater and represents 20–30% of all CCAs [1,3,4]. Although iCCA is the least prevalent type of cholangiocarcinoma, it is the second most common type of hepatic malignancy after hepatocellular carcinoma [1,3]. Histologically, most CCAs are adenocarcinomas, with other subtypes, such as clear cell carcinomas or adenosquamous carcinomas, being very rare [1,2,3,4].
An alarming increasing incidence of this group of ailments has been reported during recent decades. It varies between different regions of the globe, with, for instance, between 0.3 and 6 cases per 100,000 inhabitants per year in Western countries and >6 cases per 100,000 inhabitants in Eastern Asia, especially Thailand, South Korea, and China [5,6]. Moreover, the disease burden, including a high mortality rate, requires a multidisciplinary awareness from the early stages for a better outcome. In Europe, data collected between 2008 and 2018 showed that the highest mortality rates were registered in Malta, Ireland, and Spain for male patients and in the UK and Switzerland for female patients, while Romania, Hungary, and Poland reported the lowest rates for both sexes, with a general increase in iCCA-related mortality for both sexes in all countries over the past decade [7,8]. This variation could be explained by geographical differences in associated risk factors as well as genetic determinants [1,2].
Our purpose was to overview the field of CCAs following a double perspective: the constellation of the risk factors, and the paraneoplastic syndromes, emphasizing the endocrine features amid the entire multidisciplinary panel. This is a narrative review. A PubMed-based search of English-language original articles offered the basis of this comprehensive multidisciplinary approach. The search was carried out from inception until June 2024, and most papers were published within the last decade.
2. CCA-Associated Risk Factors and Potential Contributors
Multiple risk factors have been reported in relationship with the group of CCAs. Some of them imply strong statistical evidence with the entire category of neoplasia while others have been related with a specific CCA subtype, but chronic inflammation of the biliary epithelium and bile stasis seem to be common characteristics amid the constellation of the multidisciplinary risk factors that have been identified so far [1,4,6]. Despite advancements in understanding the aetiology of this disease, around 50% of the cases diagnosed in Western countries are currently classified as sporadic in addition to lacking an identifiable risk factor, thus limiting the rate of early detection to a better outcome [1,6].
2.1. Chronic Biliary Diseases
Primary sclerosing cholangitis (PSC), an autoimmune disorder which affects the bile ducts causing inflammation and obstruction, cholestasis, and progressive liver failure, represents a well-established risk factor for the development of CCA, especially in Western countries, and a 400-fold increase in the risk for CCA in patients diagnosed with PSC compared to the general population was reported [7,8,9]. The association of other inflammatory conditions, such as inflammatory bowel disease, further increases this risk. It is recommended that the patients diagnosed with PSC should be included in surveillance programs and lifelong follow-up protocols. Hence, they should be evaluated at least annually by imaging techniques and CA 19-9 (Carbohydrate antigen 19-9) serum assays [1,2,9,10,11,12].
Bile ducts cysts or choledochal cysts, representing a rare congenital disorder characterized by the cystic dilation of the intrahepatic and/or extrahepatic bile ducts, are more frequently diagnosed in Asian female patients who typically develop CCA much earlier than the general population (at a median age of approximately 32 years) [1,6,9]. The tumour may arise from the cysts as well as from the normal parts of the biliary tree. Bile stasis with increased concentration of bile acids, pancreatic enzymes reflux, and bacterial infection are some of the pathogenic mechanisms that are currently considered to be involved in the malignant transformation of the bile duct epithelium [1,9,13,14]. A recent meta-analysis concluded that bile duct cysts were the most important associated risk factor for all types of CCA [15].
Moreover, Caroli’s disease (a phenotype of the choledochal cyst) involves a genetic autosomal recessive disorder in which segmental intrahepatic bile ducts develop gross non-obstructive dilation [16]. This ailment is associated with a 38-fold higher risk for iCCA and a 97-fold higher risk for eCCA [17]. Under these circumstances, the tumour development mostly occurs after the second decade of life, although a few cases have been reported among teenagers [1,9,13,14,15,16,17]. Additionally, hepatholithiasis, also known as intrahepatic biliary lithiasis, represents another disorder associated with a higher risk of developing iCCA. This relationship has been well documented, especially in East Asia, where the disorder is more common than in Western countries, with an overall incidence of CCA between 5% and 13% [1,9,15,18]. Finally, cholelithiasis and choledocholithiasis have also been linked to an increased risk of CCA, particularly with eCCA, although a strong association with iCCA has also been suggested. The risk is thought to increase with gallstone size, the degree of calcification of the epithelium, and the duration of the disease [1,9,13,15,19].
2.2. Chronic Liver Conditions
Cirrhosis, a well-established risk factor for hepatocellular carcinoma, has also been identified as a risk factor for CCA. This aspect may be explained by the increased cellular proliferation, fibrosis, and release of pro-inflammatory cytokines that occur in the cirrhotic liver [1,9,15,20,21,22]. A meta-analysis of fourteen case–control studies, as well as a meta-analysis of seven case–control studies from 2012 and a population-based case–control study in Asian patients from 2013, identified cirrhosis as a strong risk factor for iCCA. Another study conducted in the US population concluded that cirrhosis might increase the risk for eCCA as well [20,21,22].
Chronic hepatitis B (HBV) and C (HVC) virus infections stand for another well-established risk factor for hepatocellular carcinoma which has been proven to increase the risk for CCA development too, particularly for iCCA [23]. In Western populations, iCCA was found to be more often associated with HCV infection, while in Asian populations, there is a stronger association was detected with HBV (which is endemic in Asia) [24]. Some data have also identified a small increase in eCCA risk in patients with chronic viral hepatitis, but this is still an open matter [24,25]. The cause of this increased risk seems to be not only the development of cirrhosis, which is a frequent complication of these viral infections, but also a direct carcinogenic effect of HBV and HCV on target cells [1,9,15,23,24,25,26,27].
In addition, hemochromatosis, a genetic disorder characterized by pathological iron accumulation in multiple organs, particularly in the liver, has been listed by some authors as risk factor for CCA, but this is still a matter of debate [28]. Some studies suggested an association with iCCA development, but no increased risk for eCCA was confirmed [29,30,31]. This higher risk may be explained by the co-presence of cirrhosis, a common clinical manifestation of hemochromatosis, but some iCCA cases have also been reported in non-cirrhotic hemochromatosis patients, thus suggesting supplementary pathogenic elements that are less understood so far [1,28,29,30,31,32,33]. Similarly, Wilson’s disease, another genetic disorder which affects the liver by pathological accumulation of copper, was pinpointed to present sporadic cases of iCCA according, for instance, to a cohort study conducted on 1186 patients (0.5% of who were confirmed with iCCA) [34,35,36,37]. An excessive copper accumulation may induce DNA (deoxyribonucleic acid) damage through reactive oxygen species (ROS) generation, yet some evidence has also shown a protective role of copper (at certain levels) against other different types of malignancies [1,34,35,36,37].
2.3. Digestive Ailments
Inflammatory bowel disease, specifically ulcerative colitis, has been found to be associated with a higher risk for iCCA development compared to Crohn’s disease, while the risk for eCCA was identified as similar between ulcerative colitis and Crohn’s disease [38]. Both conditions may lead to CCA by inducing a state of chronic inflammation, ROS anomalies, and/or microbiome dysbiosis [39]. As mentioned before, these conditions are also associated with PSC, which otherwise stands as a well-known factor for CCA [1,15,38,39,40,41]. Chronic pancreatitis, as well as duodenal or gastric ulcer, is still incompletely understood in relationship with the field of CCA, but it has been linked to a higher risk for eCCA than iCCA according to some data (yet there are currently no unanimous results) [1,13,15]. The underlying mechanism may be the fact that the biliary strictures which appear in up to 23% of patients diagnosed with chronic pancreatitis might further lead to cholangitis and cholelithiasis, both being well-known risk factors for CCA [1,15]. Also, a modest association between CCA diagnosis and duodenal gastric ulcer was highlighted in Helicobacter pylori-positive subjects [1,13,15].
2.4. Parasitic Infections
Opisthorchis viverrini and Clonorchis sinensis, also known as liver flukes, are two species of trematodes which have been identified as major contributors for CCA in some regions of the world [42]. Infection with these parasites is endemic in Eastern Asian countries and occurs through consumption of raw or undercooked fish [1,6,9,15,42,43,44]. The majority of CCA cases in these regions are linked to liver fluke infections, which tend to become chronic, with multiple reinfections despite an adequate anti-helminthic treatment [42,43]. This has led to both species to be now classified as group 1 biological carcinogens [42,43,44]. The parasites colonize the bile ducts, thus causing chronic inflammation, cholangitis, and fibrosis of the periportal system. In these cases, CCA might develop anywhere along the biliary tree, and it presents as any of the three anatomical subsets, although a higher incidence of iCCA has been reported [1,6,9,15,43,44,45,46,47,48,49].
2.5. Lifestyle Influence
The association between chronic alcohol consumption and CCAs has not been clearly confirmed, but some studies reported an increased risk for the development of iCCA [1,6,9,15,44,45]. However, it is less understood if the association is related to alcohol-induced liver disease or to other carcinogenic mechanisms [44,45]. With regard to eCCA, a similar risk between drinkers and non-drinkers was found that may be explained by alcohol-induced inhibition of gallstone formation via its inhibition of cholesterol metabolism [9,15,50,51,52,53,54]. With concern to (traditional) cigarette smoking, older studies have suggested that smoking may display a carcinogenic effect on the biliary epithelium because of the carcinogenic compounds that are metabolized by the liver and excreted in the bile [55,56,57]. More recently, a positive association between smoking and iCCA, as well as eCCA, has been reported [55,56,57,58]. Currently, no specific data have been reported with regard to the long-term use of electronic cigarettes and electronic nicotine delivery systems (ENDSs) with/without (concurrent or prior) conventional tobacco smoking and CCAs-related risk, but recent evidence suggested the involvement of the heavy metals that are found in the refill liquids in certain types of carcinogenesis [58,59,60].
2.6. Environmental Exposure
Thorotrast, a radiographic contrast agent that had been used during the first half of the 20th century, is currently banned because of its emission of alpha-radiation and subsequent carcinogenic effect [61,62]. The risk of CCA has been reported to be three hundred times higher in people exposed to this substance [61,62,63]. Regarding asbestos, several studies have found a link between asbestos exposure and CCA [64,65,66], results which have been confirmed by a case–control population-based study on the Nordic Occupational Cancer cohort, whereas a cumulative exposure to asbestos has been shown to increase the risk of iCCA but not eCCA [66].
2.7. Genetic and Epigenetic (Potential) Interplay
Certain genetic polymorphisms are associated with an increased risk for CCA. Some of the genes identified so far encode enzymes that are involved in xenobiotic detoxification, immune response, multidrug resistance, DNA repair, and folate metabolism [1,6,67,68,69,70]. Such genes vary from BRCA to TBX3 according to research ranging from small sample-sized clinical studies to human cell experimental models, with this issue currently representing an emergent topic to be further studied [69,70]. Recently, gut microbiota and associated metabolomics analysis and RNA (ribonucleic acid) profiling showed that glutamine-associated signal transduction pathways might play a pivotal role in the development of iCCA, while experimental evidence suggested that glutamine supplementation may inhibit ferroptosis and downregulate NOX1 (KNOTTED-like homebox) expression, which further inhibits NOX (NADPH oxidase) and ROS formation, as well as oncogene-related proteins such as p53 [67,71].
3. Metabolic and Endocrine Interferences in CCA Development
3.1. Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD, the accumulation of lipids in the liver in the absence of alcohol abuse, ranging from steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis, is a very frequent ailment nowadays, associated or not with other endocrine, metabolic, and even cardiovascular traits [72,73,74,75]. This metabolic disorder has been linked to hepatocellular carcinoma diagnosis, but its involvement in the pathogenic features of CCAs is less clear according to the current knowledge. A US population-based study reported a positive association between NAFLD and iCCA, while a case–control study conducted in Japan identified NASH as an independent risk factor for iCCA [76,77,78,79]. These results were confirmed by a meta-analysis of seven case–control studies which concluded that NAFLD has a stronger association with iCCA rather than eCCA [78]. However, a recent study which included 180 patients from Italy and France showed that NASH, but not NAFLD, increases the risk for iCCA and might affect its prognosis. In this study, NASH was found in >20% of patients without classical risk factors for CCA, and in 80% of the cases, the malignancy developed in the liver without severe fibrosis. Thus, to some extent, NASH might explain the increasing worldwide incidence of iCCA [79].
3.2. Obesity
A high body mass index, particularly in the obesity range, might increase the risk of certain cancers, including CCAs, through fat-related effects on leptin, adiponectin, and pro-inflammatory cytokines and various growth factors, but most studies consider that current evidence is too limited to draw any definitive conclusions [1,5,9,15,80,81,82,83]. However, a recent study based on data collected from eleven European countries reported that more than 50% of the patients were overweight or obese at the time of CCA diagnosis and almost 40% suffered from hypertension, both (obesity and high blood pressure) comorbidities being more frequent among patients diagnosed with iCCA [80]. Also, a meta-analysis of five cohort studies and five case–control studies reported a small, positive association between obesity and CCA [83].
3.3. Type 2 Diabetes Mellitus
An important part of the metabolic panel concerns the diagnosis of type 2 diabetes, which has been shown to increase the risk for all CCA subtypes, but especially for iCCA [84]. Diabetic patients treated with metformin had a lower risk for CCA compared to those not receiving this oral medication, suggesting a potential protective role of metformin [85]. Insulin, which has a compensatory hypersecretion in the early stages of type 2 diabetes (in addition to the insulin resistance at receptors levels), has been shown to potentially stimulate cancer cell growth (hence acting as a growth factor) [86]. Diabetes might also increase the risk of biliary stones that further represent a risk factor for eCCA [1,5,9,15,84,85,86].
Moreover, bile salts’ function as nutrient signalling hormones is to activate different G-protein coupled nuclear receptors such as FXR, PXR, or S1PR2 (sphingosine-1 phosphate receptor 2) and collaborate with insulin amid the regulation of nutrients metabolism at the hepatic level via the activation of AKT and ERK1/2 pathways [87,88,89]. Anomalies of these loops may cause NAFLD or even directly contribute to CCAs’ development (for instance, through activation of S1PR2) [88]. At the clinical level, we mention, for instance, a multicentre study from 2021 on 537 consecutive patients diagnosed with CCAs that pinpointed the potential anti-cancer benefits in individuals receiving metformin versus subjects who had never used it (hazard ratio of 0.7, p = 0.0162), the former of whom experienced an advanced stage correlated with a better overall survival as well as those who had a sequential therapy in terms of chemotherapy followed by metformin versus chemotherapy (metformin-free) (hazard ratio of 0.44, p = 0.0016) [89].
3.4. Vitamin D Status
Vitamin D metabolism has been related to different malignancies in terms of vitamin D deficiency and a higher proliferative risk on one hand, or, on the other hand, the modulation of vitamin D receptors (and variations of different polymorphisms) that are displayed all over the human body, some of which might be prone to cancer development [90,91]. However, interventional trials in terms of cancer prevention or improving a poor neoplasia-related outcome are less clear so far [90,91,92]. The above-mentioned analysis also showed a longer disease free survival in subjects who underwent vitamin D supplementation when compared to those who were never users (hazard ratio of 0.55, p = 0.02) [89]. Of note, vitamin D might act as an anti-tumour drug via its non-mineral effects, but the exact regime and time frame of exposure as well as the potential interferences in terms of efficacy and toxicity of standard chemotherapy still represent an open issue [93,94,95]. For instance, CCA cell lines and animal studies have shown that 1-alpha, 25-dihydroxyvitamin D3 (the active form of vitamin D) analogues such as MART-10 (which enhances the role of calcitriol by 10 times) mediate anti-growth functions in human CCAs cells via the generation of cell cycle arrest [96]. Moreover, a higher vitamin D receptor expression (as revealed by the immunohistochemistry analysis) in CCA specimens from humans was associated with a better prognosis [97]. Further clinical studies are necessary to provide the exact level of evidence with respect to the vitamin D applications amid daily practice in this devastating malignancy [96,97]. Most probably, this loop represents one of the multiple other pathogenic traits of the condition.
3.5. Glucagon-like Peptide 1 Receptor (GLP-1R)
Novel insights of emergent anti-diabetic drugs such as GLP-1R agonists have suggested that they might act as anti-CCA agents, but the results are not convincing yet. On one side, an immunohistochemistry study published in 2024 showed that iCCA tissue displayed a statistically significant correlation (p = 0.027) between GLP-1R expression and a poor histological grading [98]. On the other side, a candidate to GLP-1R agonists such as liraglutide or exendin-4 might show a clinical improvement since there are in vitro experiments confirming that liraglutide (not exendin-4) reduced the CCA cells migration by interacting with epithelial–mesenchymal transition [98]. In vivo (murine) data revealed that liraglutide-related GLP-1R downregulation reduced the tumour volume through suppression of Akt/STAT3 signal transduction pathways [98]. Noting these apparently paradoxical effects on CCA, other clinical studies raised the issue of incretins (such as dipeptidyl peptidase 4 inhibitors (DDP4)) and GLP-1R agonists in increasing the risk of CCA [99]. For instance, a nationwide, registry-based cohort in three European countries (Sweden, Denmark, and Norway) analysed data between 2007 and 2018 and concluded there was no increase in the CCA risk amid the use of DPP4 and GLP-1R agonist when compared to the use of sulfonylureas [99].
3.6. Galanin System
This system includes the galanin peptide and its receptors GAL1-3-R (which are G protein-coupled receptors) that have been studied in relationship with various tumours, and lately, some immunohistochemistry (antibodies-based) analyses identified GAL1-R in bile duct cells and GAL3-R in cholangiocytes and capillary vessels, while in pCCA, GAL3-R expression may work as a poor prognosis marker according to small-sample-sized studies [100,101]. Since GAL1-3-Rs are confirmed (but with different configurations) in both healthy humans and CCAs (particularly, pCCA), further experimental models are necessary to highlight their significance as prognostic biomarkers [100,101]. Similarly, the system of this neuropeptide of 30 amino acids has been studied in relationship with other endocrine and non-endocrine tumorigenesis such as glioma, meniningioma, and pituitary neuroendocrine neoplasia [102]. One of the most interesting mechanisms related to the development of tumours stands from the observation that GAL-Rs were identified at the level of tumour-related immune cells that might play an important role in the tumour microenvironment homeostasis, as seen with other peptides including amid the metastatic process [102,103,104,105].
3.7. Sex Hormone Therapy
Both oestrogen (alpha and beta) and androgen receptors are expressed in the liver and biliary duct cells; thus, the potential interferences with neoplasia originating at this level should be taken into account [106]. In patients confirmed with CCAs, prior or concurrent administration of oestrogens (associated or not with progestins) in terms of oral contraceptive or menopausal-related hormone replacement, respectively, or of testosterone (which was offered for different purposes such as hypogonadism-related substitution or gender-affirming hormone therapy), represents a very challenging, cross-disciplinary, and modern perspective that, to our understanding, includes at least three main turning points, as follows:
Firstly, there is the big picture of oestrogens use for women of reproductive age or early post-menopausal females, noting that oestrogens-associated signal transduction pathways are involved in the underlying pro-carcinogenetic mechanisms of different tumours, not only at the biliary tract level [106,107]. Combined oral contraceptives, since their introduction in the early 1960s, have been proven to associate a mixed panel of neoplasia interplay, while generally being regarded as safe and effective. Recent data have shown that the overall cancer risk might not be influenced in current and prior users [107]. A transitory risk in current/regular users has been reported for mammary cancer (with a 20–30% risk increase). A risk decrease was found with regard to endometrial, colorectal, and ovarian malignancies (apart from those subjects coming from high-risk families as seen in the carriers of different pathogenic variants such as BRCA, PTEN, etc.). Notably, the risk is related to the timeframe of administration; for instance, a higher risk of cervical cancer was reported in women who were exposed for more than 5 years, a risk that is no longer active when the medication is stopped. Similarly, conflicting data are described in relation to the confirmation of hepatic adenocarcinomas, adenomas, and CCAs, with some studies suggesting a higher risk of iCCA in certain subgroups [107,108,109]. The identification of oestrogen receptor 1 configuration as a key factor in iCCA recurrence might impact the prognosis by interfering with the JAK/STAT3 pathway [110].
Moreover, the expression of CYP19A1, the aromatase that converts androgens to oestrogens, was found to be high in association with elevated oestrogen-related proteins within CCA tissues that displayed a more aggressive outcome, specifically in males rather than in females, and further exploration of this topic is required [111,112]. With concern related to menopause hormone therapy, we mention a nested case–control study from the UK from 2022 showing that oestrogen-only formulas decreased the risk of CCA (odd ratio of 0.59, 95% CI between 0.34 and 0.93), while the dose, duration, and time since last prescription were not found to be associated with CCA risk [113]. Interestingly, data from the Liver Cancer Pooling Project and the UK Biobank pinpointed that a history of hysterectomy was associated with a two-time increase in iCCA risk (hazard ratio of 1.98, 95% CI between 1.27 and 3.09) compared to females with physiological menopause who were aged between 50 and 54 years. Of note, more than 9 years of oral contraceptive exposure induced a 62% increase in iCCA risk (hazard ratio of 1.62, 95% CI between 1.03 and 2.55) [114].
Secondarily, the use of chronic androgen therapy, including anabolic androgenic steroids, poses a low level of statistical evidence with respect to the potential risk elevation of developing CCA according to the data we have so far [115].
Thirdly, the recently developed gender-affirming hormone therapy for transgender males is under evaluation with concern to the risk of hepatobiliary neoplasms, but no clear conclusion has been established yet. The clinical data remain at isolated case reports [116]. An expansion of the published data in this particular multidisciplinary area is expected within the following years (Table 1).

Table 1.
Risk factors and potential contributors in CCA development (a qualitative analysis).
4. Paraneoplastic Syndrome in CCAs
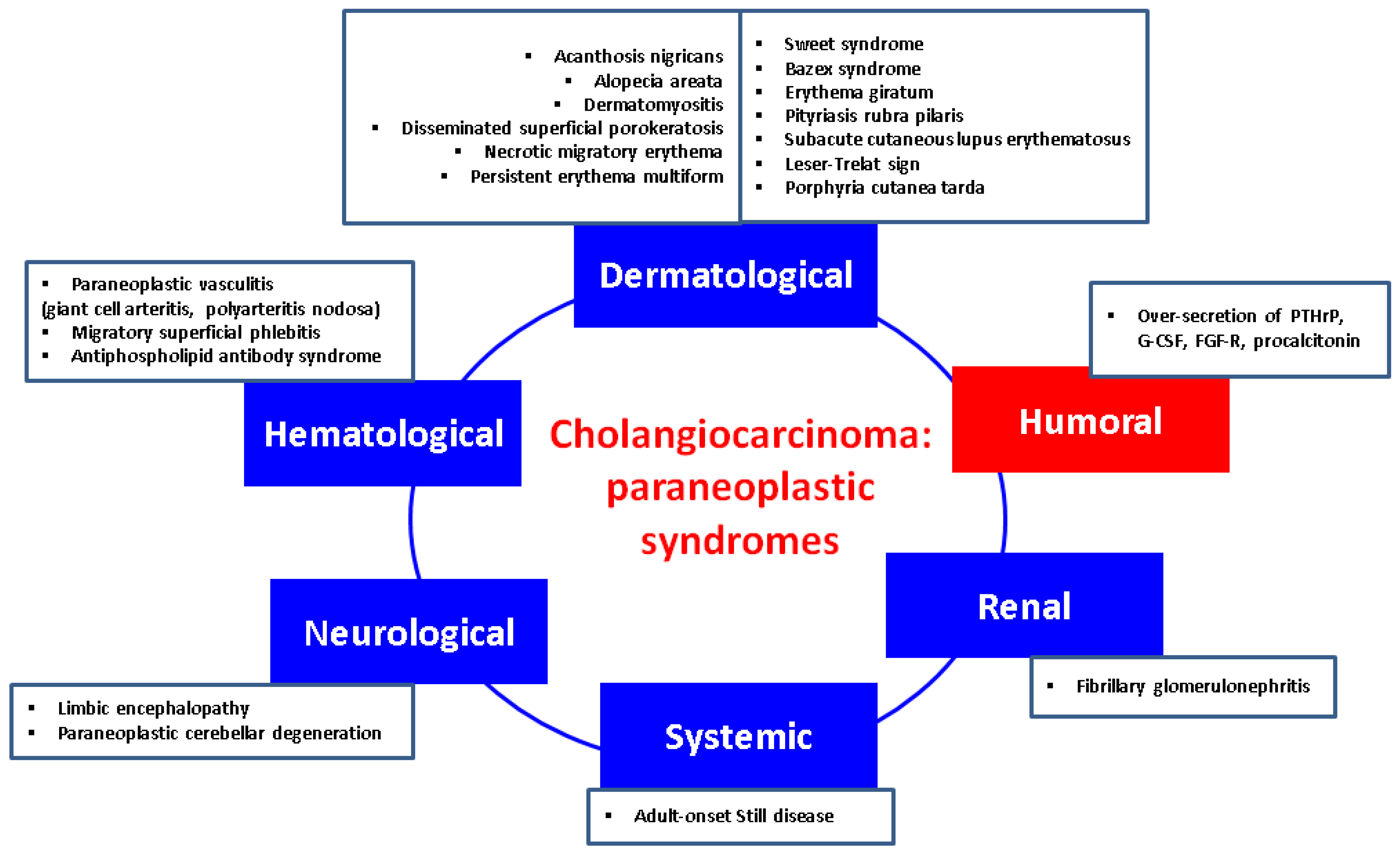
CCAs may cause a variety of paraneoplastic manifestations underlying various endocrine and non-endocrine mechanisms which are less or more understood at the present time. Recognition of these syndromes might lead to an early diagnosis of CCA, while their presence may predict the efficacy of treatment, including a relapse or recurrence of the disease. Although the available data on this specific subject are rather limited when compared to the malignancies of other origins, there are reports of dermatological, neurological, haematological, renal, and endocrine manifestations in CCAs. Of note, the current level of evidence remains mostly low, but their awareness is essential for daily basis [3,117].
4.1. Dermatological Features Have Been Found as Followings
4.1.1. Acanthosis
Acanthosis nigricans was associated with various solid malignancies including CCA. It presents as hyperkeratotic hyper-pigmented diffuse patches on the palms and soles, also affecting mucosa such as the oral cavity and oesophagus [3,118].
4.1.2. Alopecia
Alopecia areata is usually the type of hair loss associated with CCA (while not being specific), presenting as a round patch with well-shaped edges on top of the skull [119,120,121].
4.1.3. Dermatomyositis
Dermatomyositis represents a rare paraneoplastic manifestation of CCA, resulting from autoimmune response against the cancer cells which cross-reacts with healthy tissues. It is characterized by weakness of the proximal muscles, heliotrope rash (blue-purple skin lesions on the upper eyelids), and Gottron papules (erythematous papules on knuckles) [122,123].
4.1.4. Porokeratosis
Disseminated superficial porokeratosis is regarded as a benign hyperkeratotic skin tumour which has been linked to various malignancies. It displays itchy reddish-brown papules with elevated borders on the extensor surface of the limbs and trunk, sparing mucosal surfaces, palms, and soles. They become visible a few months before cancer-related symptoms appear; thus, prompt recognition and further investigations might help the overall outcome [124].
4.1.5. Necrotic Migratory Erythema
This lesion has been found in association with CCA too. It presents as annular erythematous erosive lesions of 1 to 2 cm that are surrounded by scaling, localized on the face, trunk, and extremities [125].
4.1.6. Persistent Erythema Multiform
This skin lesion is a disorder classified as paraneoplastic dermatosis because it appears in the setting of internal organ malignancies. In CCA, it usually occurs early in the course of the disease and presents as a painful erythematous rash with scaling, forming patches on the upper trunk and thighs; after a few days, it evolves into haemorrhagic bullae with violaceous edges which do not heal on their own; vital signs are normal and mucosal surfaces are not involved, as seen in other malignancies or as side effects to different drugs [126,127,128].
4.1.7. Sweet Syndrome
This ailment highlights an acute febrile neutrophilic dermatosis, occurring in approximately 15% of the people suffering from solid malignant tumours. It is characterized by rapidly growing painful erythematous plaques on the face, neck, and legs, associated with malaise, cough, and arthralgia. CCA and other malignancies involve an excessive production of granulocyte-colony stimulation factor (G-CSF) which stimulates neutrophils, causing this clinical presentation [129,130,131].
4.1.8. Bazex Syndrome
Also known as acrokeratosis paraneoplastica, this is another syndrome reported to be associated with CCA. It presents as hyperkeratotic pruritic scaly lesions of the face, ears, buttocks, palms, and soles, which can be associated with fatigue, abdominal pain, nausea, vomiting, constipation, and weight loss [132].
4.1.9. Erythema
Erythema giratum reflects a migratory erythema most commonly associated with oesophageal, lung, and breast carcinoma, but it has also been reported in association with CCA [133,134,135,136,137].
4.1.10. Pityriasis
Pityriasis rubra pilaris represents another dermatosis reported to be associated with CCA, presenting as a papulosquamous pruritic rash associated with mild fever [138].
4.1.11. Lupus
Subacute cutaneous lupus erythematosus, a rare paraneoplastic manifestation of CCA, involves a clinical presentation showing an explosive onset of a pruritic rash, with arthralgia associated with a lower limb oedema [139].
4.1.12. Leser–Trelat Sign
It is defined as the presence of multiple pigmented seborrheic keratosis associated with an underlying malignancy and was mostly reported in gastrointestinal adenocarcinoma and rarely with CCA. In association with acanthosis nigricans, it stands for an even stronger indicator of a malignant disease [140,141].
4.1.13. Porphyria
Porphyria cutanea tarda, the most common type of porphyria, resulting from a deficient activity of uroporphyrinogen decarboxylase, an enzyme involved in hem biosynthesis, poses clinical manifestations due to an increased mechanical fragility of the skin after sunlight exposure, leading to erosions and blistering, painful sores, milia, depigmentation, and scarring. Excretion of porphyrins in urine and stool is characteristically increased. There are many reports of porphyria in the setting of hepatocellular carcinoma, but in the case of CCA, porphyria may be considered an exceptional occurrence [3,142].
4.2. Neurological Paraneoplastic Elements
Neurological paraneoplastic elements are rare findings in cases diagnosed with CCAs, but limbic encephalopathy and paraneoplastic cerebellar degeneration have been reported. Limbic encephalopathy is a subacute cause of memory impairment and confusion characterized by autonomic seizures (pilomotor erection), delusion, and rapidly progressive dementia. Paraneoplastic cerebellar degeneration presents as sudden onset ataxia, dysarthria, diplopia, and dysphagia in a patient with underlying malignancy [143,144].
4.3. Renal Findings
Renal findings are extremely rare; a case of fibrillary glomerulonephritis has been reported in association with CCA; the clinical presentation was with oedema of the lower limbs and face, uncontrolled hypertension, nephrotic range proteinuria, and microscopic haematuria [145].
4.4. Haematological Manifestations
Haematological manifestations include a heterogeneous spectrum. For instance, paraneoplastic vasculitis represents less than 5% of all vasculitis cases and it is more commonly associated with haematological malignancies than with solid tumours. Most cases present as cutaneous forms, but internal organs may also be involved too. Anti-neutrophilic cytoplasmic antibodies (ANCA) titre is usually raised [146,147]. Giant cell arteritis has been reported to be more commonly associated with CCAs, but one study also reported polyarteritis nodosa (PAN) to be associated with this neoplasia. Early signs of PAN are bilateral numbness of lower limbs and gradually increasing fever. After two weeks, an arthritis-like disorder develops, with severe pain affecting the ankles, metatarsal, and phalangeal joints; skin involvement with necrosis and gangrene on distal phalanges is reported to develop after a few months; in late stages, severe abdominal pain, nausea, and vomiting appear as part of the gastrointestinal tract involvement. Paraneoplastic PAN is poorly responsive to steroids, with tumour removal or chemotherapy being needed for resolution of vasculitis [146,147].
Moreover, Trousseau’s syndrome, migratory superficial phlebitis, was identified as a sign of malignancy for the first time in 1865 by the French physician Armand Trousseau [147,148,149]. More than 100 years later, the definition of original syndrome was expanded to include any venous or arterial thromboembolism occurring in the presence of an underlying malignancy, including chronic disseminated intravascular coagulopathy, micro-angiopathic haemolytic anaemia, and Libman–Sacks endocarditis, while currently, the syndrome is defined as the occurrence of unexplained thrombotic events before or simultaneously with the diagnosis of a visceral malignancy such as those originating from the brain, lung, ovaries, and gastrointestinal tract, including hepatocellular carcinoma, with association with CCA being less common [148,149]. Some data showed that 10% of subjects with confirmed CCAs developed venous thromboembolism, which was associated with a reduced survival rate. It was hypothesized that the cause of Trousseau’s syndrome is hypoxia leading to activation of endothelial adhesion molecules and the coagulation pathways. Low-molecular-weight heparin and removal of the primary tumour have been found to be the most effective treatment strategies. On the other hand, using anticoagulants to treat venous thrombosis and prevent pulmonary thromboembolism while needing to perform a diagnostic liver biopsy can be challenging [148,150]. Additionally, antiphospholipid antibody syndrome, a cause of hypercoagulability due to formation of antibodies against platelet phospholipids, has been linked to various solid organ malignancies, including CCA [151,152]. We also mention the paraneoplastic leukemoid reaction, a manifestation defined by a number of white blood cells of more than 50,000/mm3, particularly neutrophils, in reaction to any infection or carcinoma. In the setting of CCA, it has been reported to mimic a pyogenic liver abscess, presenting as intermittent fever for at least a month, progressive generalized weakness, weight loss, and leucocytosis [153,154,155].
Of note, multi-systemic paraneoplastic syndrome might also include adult-onset Still disease, which was found in one study in relationship with CCA; clinical presentation included high-grade fever and chills, sore throat, cough, myalgia, pleuritic chest pain, and arthralgia involving various joints [156]. Newly diagnosed systemic lupus erythematosus with full blown clinical picture was reported as a paraneoplastic presentation in CCA too [157,158].
4.5. Humoral Manifestations
Humoral manifestations are mostly dominated by the diagnosis of hypercalcaemia of malignancy that overall had been reported in some CCA cases. Most of them are related to the over-production of parathyroid hormone-related protein (PTHrP), also named parathyroid hormone-like hormone (PTHLH), by the proliferating bile duct epithelial cells. PTHrP causes an elevation of the serum calcium via interacting with tumour growth factor alpha (TGF-α) and tumour necrosis factor alpha (TNF-α), with PTH levels being normal or suppressed by the blood calcium elevation [84,159]. The usual clinical signs are caused by the increased serum calcium, specifically abdominal discomfort, constipation, nausea, bone pain, polydipsia, polyuria, alteration of the mental status (even syncope [160]), and weakness [159,160,161,162,163].
As mentioned, the tumour-related secretion of G-CSF might serve as a paraneoplastic element as well as a biomarker of disease progress or recurrence. In 2017, a third-ever case of co-secretion with concern to CCA-related G-CSF and PTHrP was reported in a 78-year-old male who was initially suspected to have a liver abscess. After the histological diagnosis was confirmed via percutaneous needle biopsy, the subject started chemotherapy and died 134 days after its initiation, hence highlighting the importance of early ailment recognition and the fact that a humoral paraneoplastic syndrome usually indicates a severe outcome [161].
Additionally, we mention another interesting tumour biology that triggers hypercalcaemia: the presence of a fibroblast growth factor receptor-positive iCCA (the most recent report was provided in 2024) [162]. In 2020, a first-ever case of CCAs, specifically iCCA, was reported to associate humoral hypercalcaemia of malignancy with polycythaemia and leucocytosis, respectively, with elevated procalcitonin (in the absence of any infection) in an 80-year-old man [163] (Figure 1).
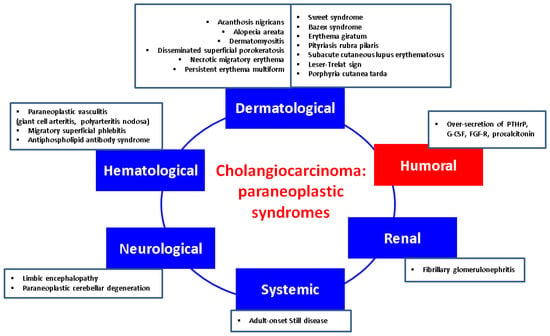
Figure 1.
Integrating humoral (endocrine) paraneoplastic syndrome amid the complex multidisciplinary panel of paraneoplastic elements in CCAs: a qualitative perspective [117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159,160,161,162,163]. (Abbreviations: FGF-R = fibroblast growth factor receptor; G-CSF = granulocyte-colony stimulation factor; PTHrP = parathyroid hormone-related protein).
5. Discussion
CCAs, very aggressive neoplasia, need to be integrated amid the modern medicine era, including the understanding of the particular features with respect to the tumour biology, the constellation of the risk factors, and the clinical picture, from the first presentation and the co-presence of paraneoplastic syndromes to the use of various imagery tools across an overall multidisciplinary management. This group of ailments still presents a large area of pitfalls and challenges despite recent progress in imagery assessment, biomarkers, surgical techniques, and chemotherapy/immunotherapy, and even liver transplantation in certain centres [164].
5.1. Imagery Tools to Help the Clinical Assessment
An early diagnosis might improve the overall prognosis, and this hinges on the clinical evaluation as well as the imagery investigations in addition to the blood assays. Transabdominal ultrasound (TAUS) is often the first and most accessible imaging technique for the evaluation of patients with right upper abdominal pain with or without jaundice because it detects bile duct dilatation, the presence of gallstones, or other causes of biliary obstruction as well as other hepatic masses. In CCAs, depending on the anatomical type, TAUS might show bilateral intrahepatic duct non-union and segmental dilatation or polypoid masses inside the biliary tract representing papillary tumours or smooth masses with mural thickening in nodular pCCA. The intrahepatic form of CCA might present a large hepatic mass with irregular margins; depending on the amount of fibrosis, mucin, and calcification within the tumour, the echogenicity can be predominately hypo- or hyperechoic or mixed. The distal common bile duct may be often masked by the duodenal air; therefore, TAUS does not identify a tumour at this level. However, distention of the bile ducts is used as an indicator of distal biliary obstruction [165,166].
Contrast-enhanced ultrasound (CEUS) has been successfully used to characterize focal liver lesions based on specific enhancement and washout patterns, having a superior diagnostic ability compared to grey scale and Doppler ultrasound. CEUS might misdiagnose iCCA as hepatocarcinoma [167]. Computed tomography is applied in up to 90% of suspected CCAs due to its ability to assess tumour extension and potential resectability, as well as vascular involvement and lymph node metastases. In patients with eCCA, the tumour site is suggested by the level of biliary dilatation. Dilatation of both intrahepatic ducts and separation of the left and right hepatic ducts is suggestive for pCCA. Magnetic resonance imagery also provides a very good assessment of tumour extension, vascular involvement, and resectability. These imaging techniques also have an important role in the surveillance of patients with an increased CCA risk, such as those with PSC [167,168].
5.2. Is There a Place for Endocrine Considerations in CCAs?
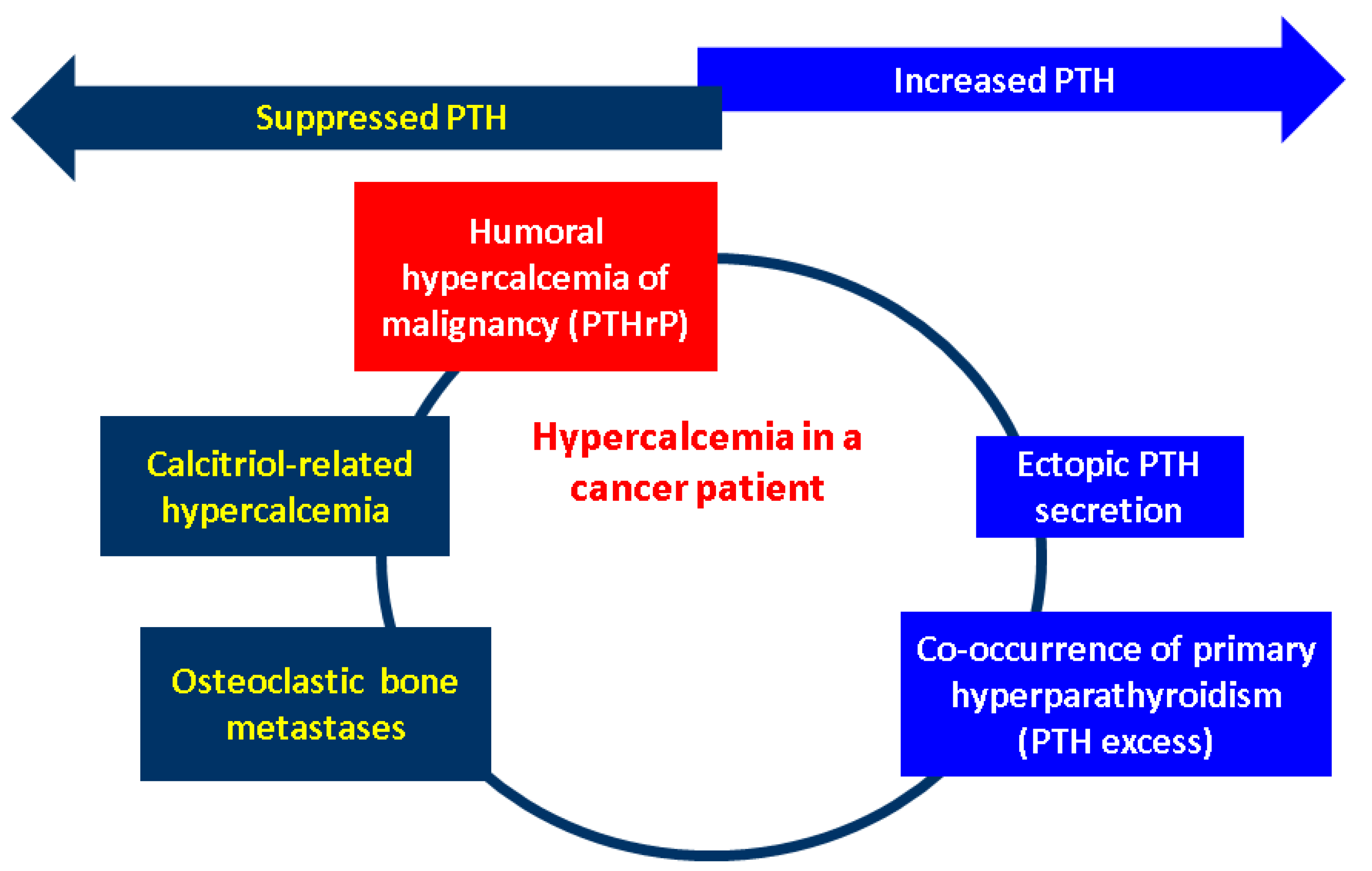
Nowadays, one of the most challenging sub-domains in the field of CCAs remains the potential link with the endocrine traits as tumour-related manifestations. An in-depth perspective of the hormonal paraneoplastic profile in CCAs showed that it remains at a very low level of statistical evidence; thus showing the importance of its awareness amid a multidisciplinary complex perspective. According to the data we have so far in CCAs, humoral hypercalcaemia of malignancy stands for the most frequent humoral paraneoplastic syndrome in CCAs, despite being exceptional amid the identification of other paraneoplastic (non-endocrine) manifestations and compared to its reported frequency in other (non-CCAs) cancers (it accompanies 20–30% of malignancies) [169,170]. It represents a poor prognosis marker in CCA as seen in other tumours (except for the majority of mammary cancers, where it might imply a long standing evolution). It may be episodic once the tumour relapses. In addition to the therapy that targets the originating malignancy, the control of elevated serum calcium requires the administration of a bisphosphonate such as intravenous zoledronic acid or subcutaneous denosumab, a human monoclonal antibody which acts as an inhibitor of RANKL (receptor activator of nuclear factor kappa B ligand), as similarly used in PTH-related hypercalcaemia (e.g., primary hyperparathyroidism) [171]. Early detection firstly helps the general wellbeing of a patient due to prompt medical control of hypercalcaemia and also provides a fine biomarker of disease status in selected cases that harbour the capacity of PTHrP secretion. The exact molecular biology and genetic configuration of CCAs that display such characteristics is still an open matter, but humoral hypercalcaemia adds to the overall disease burden [160,161,162,163,172].
Generally, the underlying mechanisms in humoral hypercalcaemia of malignancy are associated with PTHrP (PTHLH) over-secretion as described in CCAs which might act as growth promotor via activation of the ERK (extracellular signal-related kinase)–JNK (c-Jun N-terminal kinase)-ATF2 (activating transcription factor-2)-cyclinD1 pathway (this canonical path explains the paracrine effects of PTHrP) [173]. Other (non-CCAs) tumour biologies that also impair the blood calcium are represented by the ectopic PTH over-secretion (at the level of malignant cells, not by the parathyroid glands) or abnormal production of 1,25-dihydroxyvitamin D within malignancies (particularly hematologic neoplasia such as lymphomas, as similarly found in sarcoidosis and granulomatous diseases) that hold the enzymatic equipment for this final step of 25-hydroxyvitamin D activation (calcitriol-induced hypercalcaemia). In addition, the presence of bone (osteolytic) metastases might increase the serum calcium by displacing a large bone mass [174,175,176]. (Figure 2).
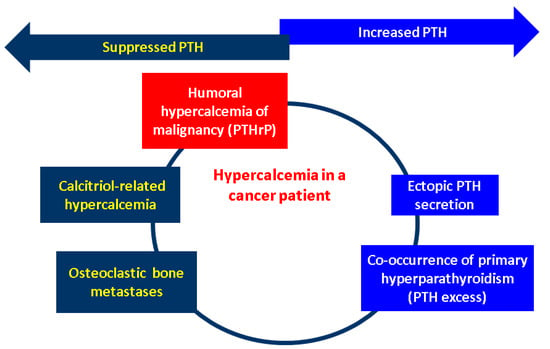
Figure 2.
Mechanisms of hypercalcaemia in patients diagnosed with a malignancy: humoral hypercalcaemia of malignancy due to PTHrP excess; ectopic PTH secretion (by a non-parathyroid tumour); osteolytic metastases; calcitriol-induced hypercalcaemia; and potential co-occurrence of the primary hyperparathyroidism-associated hypercalcaemia due to an orthotopic or ectopic parathyroid gland [170,171,172,173,174,175,176]. High calcium levels are induced by the PTHrP excess, which causes the suppression of the physiological PTH at the level of parathyroid glands via negative feedback. Calcitriol over-production also causes hypercalcaemia by interfering with the metabolism of vitamin D, and this also supresses the normal parathyroid-related PTH. Osteolytic metastases displace a large bone mass, and this comes with the serum release of the calcium, thus causing physiological PTH inhibition. One the other hand, a synchronous parathyroid tumour causes an abnormally high PTH that induces hypercalcaemia (primary hyperparathyroidism). The same phenomenon takes place in cases with ectopic PTH secretion (involving other cancers than parathyroid glands tumours). (Abbreviations: PTH = parathormone; PTHrP = PTH-related protein).
5.3. Current Limits and Further Expansion
The level of endocrine evidence across clinical trials remains far from generous in CCAs; thus, we performed a narrative analysis rather than a systematic review. We need more experimental models to understand the tumour biology with respect to the hormonal interplay, and further applications such as emergent biomarkers or new strategies are expected for daily practice. Other topics in this specific area that refer to the hormonal insights in CCAs (which remain out of the scope of the present work) might include thyroid hormones’ implications in the liver and biliary duct tract under physiological and pathological circumstances (such as goitre or thyroid cancer) or the analysis of the CCA subgroups that displays adrenal metastases complicated with adrenal insufficiency [177,178,179]. Moreover, the recent COVID-19 pandemic expanded the edges of the scientific knowledge as well as identifying new clinical/surgical entities; novel research has delved into system biology approaches to connect the viral infection with a higher risk of CCAs or to prove whether CCAs patients were prone to developing a more severe respiratory panel amid coronavirus complications [180,181,182,183,184,185].
This works brings novel information to bridge the gap toward the traditional data in CCAs that helps in understanding the tumour behaviour with respect to the clinical implications in terms of the paraneoplastic syndromes and with regard to a large panel of potential contributors that come from different medical areas. To our knowledge, a particular endocrine frame as a novel approach across this complex picture represents an emergent topic to further be studied and understood.
6. Conclusions
The hormonal burden in CCAs is reflected in a complex constellation of interplays such as the galanin system, exposure to sex hormone therapy, interferences with vitamin D system, etc., while humoral hypercalcaemia of malignancy, despite rare, has been reported due to PTHrP over-production in addition with other elements such a G-CSF, fibroblast growth factor receptor positivity, or even procalcitonin. The level of endocrine evidence across clinical trials remains far from generous in CCAs. Further applications such as emergent hormonal biomarkers or new strategies are expected for daily practice.
Author Contributions
Conceptualization, M.-L.C., B.-A.S., L.-M.C., M.I., M.-L.G., M.C., C.N. and A.-M.R.; methodology, M.-L.C., L.-M.C., M.I., M.-L.G., M.C., C.N. and A.-M.R.; software, M.-L.C., B.-A.S., M.I., M.-L.G., M.C., C.N. and A.-M.R.; validation, M.-L.C., M.-L.G., M.C., C.N. and A.-M.R.; formal analysis, M.-L.C., B.-A.S., L.-M.C., M.I., M.C., C.N. and A.-M.R.; investigation, M.-L.C., M.C., C.N. and A.-M.R.; resources, M.-L.C., B.-A.S., L.-M.C., M.I., M.-L.G., M.C., C.N. and A.-M.R.; data curation, M.-L.C., M.C., C.N. and A.-M.R.; writing—original draft preparation, A.-M.R. and M.C.; writing—review and editing, M.-L.C., M.C., C.N. and A.-M.R.; visualization, M.-L.C., B.-A.S., L.-M.C., M.I., M.-L.G., M.C., C.N. and A.-M.R.; supervision, M.-L.C., M.C., C.N. and A.-M.R.; project administration, M.-L.C., M.C., C.N. and A.-M.R.; funding acquisition, M.-L.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ANCA | anti-neutrophilic cytoplasmic antibodies |
| ATF2 | activating transcription factor-2 |
| CCA | cholangiocarcinoma |
| CA 19-9 | carbohydrate antigen 19-9 |
| CI | confidence interval |
| CEUS | contrast-enhanced ultrasound |
| dCCA | distal cholangiocarcinoma |
| DNA | deoxyribonucleic acid |
| DDP4 | dipeptidyl peptidase 4 inhibitors |
| ENDSs | electronic nicotine delivery systems |
| ERK | extracellular signal-related kinase |
| CCAs | extrahepatic cholangiocarcinoma |
| GLP-1R | glucagon-like peptide 1 receptor |
| GAL-R | galanin receptors |
| G-CSF | granulocyte-colony stimulation factor |
| HBV | hepatitis B virus chronic infection |
| HVC | hepatitis C virus chronic infection |
| iCCA | intrahepatic cholangiocarcinoma |
| JNK | c-Jun N-terminal kinase |
| NAFLD | non-alcoholic fatty liver disease |
| NASH | non-alcoholic steatohepatitis |
| NOX1 | KNOTTED-like homebox |
| NOX | NADPH oxidase |
| pCCA | perihilar cholangiocarcinoma |
| PSC | primary sclerosing cholangitis |
| PTHrP | parathyroid hormone-related protein |
| PTHLH | parathyroid hormone-like hormone |
| PAN | polyarteritis nodosa |
| RNA | ribonucleic acid |
| ROS | reactive oxygen species |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| S1PR2 | sphingosine-1 phosphate receptor 2 |
| TGF-α | tumour growth factor-alpha |
| TNF-α | tumour necrosis factor-alpha |
| TAUS | transabdominal ultrasound |
References
- Khan, S.A.; Tavolari, S.; Brandi, G. Cholangiocarcinoma: Epidemiology and risk factors. Liver Int. 2019, 39 (Suppl. S1), 19–31. [Google Scholar] [CrossRef] [PubMed]
- Dar, F.S.; Abbas, Z.; Ahmed, I.; Atique, M.; Aujla, U.I.; Azeemuddin, M.; Aziz, Z.; Bhatti, A.B.H.; Bangash, T.A.; Butt, A.S.; et al. National guidelines for the diagnosis and treatment of hilar cholangiocarcinoma. World J. Gastroenterol. 2024, 30, 1018–1042. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Moon, S.H.; Kim, J.H. Diagnosis of Cholangiocarcinoma. Diagnostics 2023, 13, 233. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Izquierdo-Sanchez, L.; Lamarca, A.; La Casta, A.; Buettner, S.; Utpatel, K.; Klümpen, H.J.; Adeva, J.; Vogel, A.; Lleo, A.; Fabris, L.; et al. Cholangiocarcinoma landscape in Europe: Diagnostic, prognostic and therapeutic insights from the ENSCCA Registry. J. Hepatol. 2022, 76, 1109–1121. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Vithayathil, M.; Khan, S.A. Current epidemiology of cholangiocarcinoma in Western countries. J. Hepatol. 2022, 77, 1690–1698. [Google Scholar] [CrossRef]
- WHO World Health Organization (WHO). Mortality Database Health Statistics and Information Systems. 2019. Available online: https://www.who.int/data/data-collection-tools/who-mortality-database (accessed on 10 June 2024).
- Elvevi, A.; Laffusa, A.; Scaravaglio, M.; Rossi, R.E.; Longarini, R.; Stagno, A.M.; Cristoferi, L.; Ciaccio, A.; Cortinovis, D.L.; Invernizzi, P.; et al. Clinical treatment of cholangiocarcinoma: An updated comprehensive review. Ann. Hepatol. 2022, 27, 100737. [Google Scholar] [CrossRef] [PubMed]
- Barner-Rasmussen, N.; Pukkala, E.; Jussila, A.; Färkkilä, M. Epidemiology, risk of malignancy and patient survival in primary sclerosing cholangitis: A population-based study in Finland. Scand. J. Gastroenterol. 2020, 55, 74–81. [Google Scholar] [CrossRef]
- Boonstra, K.; Weersma, R.K.; van Erpecum, K.J.; Rauws, E.A.; Spanier, B.W.; Poen, A.C.; van Nieuwkerk, K.M.; Drenth, J.P.; Witteman, B.J.; Tuynman, H.A.; et al. Population-based epidemiology, malignancy risk, and outcome of primary sclerosing cholangitis. Hepatology 2013, 58, 2045–2055. [Google Scholar] [CrossRef]
- Bergquist, A.; Weismüller, T.J.; Levy, C.; Rupp, C.; Joshi, D.; Nayagam, J.S.; Montano-Loza, A.J.; Lytvyak, E.; Wunsch, E.; Milkiewicz, P.; et al. Impact on follow-up strategies in patients with primary sclerosing cholangitis. Liver Int. 2023, 43, 127–138. [Google Scholar] [CrossRef]
- Petrick, J.L.; Yang, B.; Altekruse, S.F.; Van Dyke, A.L.; Koshiol, J.; Graubard, B.I.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based study in SEER-Medicare. PLoS ONE 2017, 12, e0186643. [Google Scholar] [CrossRef] [PubMed]
- Söreide, K.; Körner, H.; Havnen, J.; Söreide, J.A. Bile duct cysts in adults. Br. J. Surg. 2004, 91, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Clements, O.; Eliahoo, J.; Kim, J.U.; Taylor-Robinson, S.D.; Khan, S.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: A systematic review and meta-analysis. J. Hepatol. 2020, 72, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Lee, Y.J.; Kim, H. Intrahepatic cholangiocarcinoma arising in Caroli’s disease. Clin. Mol. Hepatol. 2014, 20, 402–405. [Google Scholar] [CrossRef]
- Mansour, J.C.; Aloia, T.A.; Crane, C.H.; Heimbach, J.K.; Nagino, M.; Vauthey, J.N. Hilar cholangiocarcinoma: Expert consensus statement. HPB 2015, 17, 691–699. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.S.; Joo, M.K.; Lee, B.J.; Kim, J.H.; Yeon, J.E.; Park, J.J.; Byun, K.S.; Bak, Y.T. Hepatolithiasis and intrahepatic cholangiocarcinoma: A review. World J. Gastroenterol. 2015, 21, 13418–13431. [Google Scholar] [CrossRef]
- Cai, H.; Kong, W.T.; Chen, C.B.; Shi, G.M.; Huang, C.; Shen, Y.H.; Sun, H.C. Cholelithiasis and the risk of intrahepatic cholangiocarcinoma: A meta-analysis of observational studies. BMC Cancer 2015, 15, 831. [Google Scholar] [CrossRef]
- Palmer, W.C.; Patel, T. Are common factors involved in the pathogenesis of primary liver cancers? A meta-analysis of risk factors for intrahepatic cholangiocarcinoma. J. Hepatol. 2012, 57, 69–76. [Google Scholar] [CrossRef]
- Chang, J.S.; Tsai, C.R.; Chen, L.T. Medical risk factors associated with cholangiocarcinoma in Taiwan: A population-based case-control study. PLoS ONE 2013, 8, e69981. [Google Scholar] [CrossRef]
- Welzel, T.M.; Graubard, B.I.; El–Serag, H.B.; Shaib, Y.H.; Hsing, A.W.; Davila, J.A.; McGlynn, K.A. Risk factors for intrahepatic and extrahepatic cholangiocarcinoma in the United States: A population-based case-control study. Clin. Gastroenterol. Hepatol. 2007, 5, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhao, Y.; Li, B.; Huang, J.; Wu, L.; Xu, D.; Yang, J.; He, J. Hepatitis viruses infection and risk of intrahepatic cholangiocarcinoma: Evidence from a meta-analysis. BMC Cancer 2012, 12, 289. [Google Scholar] [CrossRef]
- Li, M.; Li, J.; Li, P.; Li, H.; Su, T.; Zhu, R.; Gong, J. Hepatitis B virus infection increases the risk of cholangiocarcinoma: A meta-analysis and systematic review. J. Gastroenterol. Hepatol. 2012, 27, 1561–1568. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, B.; Zhang, H.; Liang, J.; Zeng, W. HBV Infection Status and the Risk of Cholangiocarcinoma in Asia: A Meta-Analysis. BioMed Res. Int. 2016, 2016, 3417976. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Hu, B.; Zhou, Z.Q.; Guan, J.; Zhang, Z.Y.; Zhou, G.W. Hepatitis C virus infection and the risk of intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma: Evidence from a systematic review and meta-analysis of 16 case-control studies. World J. Surg. Oncol. 2015, 13, 161. [Google Scholar] [CrossRef]
- Ralphs, S.; Khan, S.A. The role of the hepatitis viruses in cholangiocarcinoma. J. Viral Hepat. 2013, 20, 297–305. [Google Scholar] [CrossRef]
- Yaziji, N.; Martin, L.; Hillon, P.; Favre, J.P.; Henninger, J.F.; Piard, F. Cholangiocarcinoma arising from biliary micro-hamartomas in a man suffering from hemochromatosis. Ann. Pathol. 1997, 17, 346–349. [Google Scholar] [PubMed]
- Fernandez Pelaez, J.M.; Sanchez Martin, E.; Tirado Miranda, R.; Navarro Martinez, A.; Alamillo, S.A. Hemochromatosis and hilar cholangiocarcinoma: Report of a case. Rev. Esp. Enferm. Dig. 2000, 92, 474–475. [Google Scholar]
- Di Stefano, F.; Verna, N.; Balatsinou, L.; Schiavone, C.; Di Gioacchino, M. Genetic hemochromatosis with normal transferrin saturation in a man with cholangiocarcinoma and yellow nail syndrome. J. Gastroenterol. Hepatol. 2003, 18, 1221–1222. [Google Scholar] [CrossRef]
- Sulpice, L.; Rayar, M.; Boucher, E.; Pele, F.; Pracht, M.; Meunier, B.; Boudjema, K. Intrahepatic cholangiocarcinoma: Impact of genetic hemochromatosis on outcome and overall survival after surgical resection. J. Surg. Res. 2013, 180, 56–61. [Google Scholar] [CrossRef]
- Morcos, M.; Dubois, S.; Bralet, M.P.; Belghiti, J.; Degott, C.; Terris, B. Primary liver carcinoma in genetic hemochromatosis reveals a broad histologic spectrum. Am. J. Clin. Pathol. 2001, 116, 738–743. [Google Scholar] [CrossRef]
- Nkontchou, G.; Tran Van Nhieu, J.; Ziol, M.; Tengher, I.; Mahmoudi, A.; Roulot, D.; Bourcier, V.; Ganne Carrie, N.; Grando-Lemaire, V.; Trinchet, J.C.; et al. Peripheral intrahepatic cholangiocarcinoma occurring in patients without cirrhosis or chronic bile duct diseases: Epidemiology and histopathology of distant nontumoral liver in 57 White patients. Eur. J. Gastroenterol. Hepatol. 2013, 25, 94–98. [Google Scholar] [CrossRef]
- Pfeiffenberger, J.; Mogler, C.; Gotthardt, D.N.; Schulze-Bergkamen, H.; Litwin, T.; Reuner, U.; Hefter, H.; Huster, D.; Schemmer, P.; Członkowska, A.; et al. Hepatobiliary malignancies in Wilson disease. Liver Int. 2015, 35, 1615–1622. [Google Scholar] [CrossRef]
- Angele-Martinez, C.; Goodman, C.; Brumaghim, J. Metal-mediated DNA damage and cell death: Mechanisms, detection methods, and cellular consequences. Metallomics 2014, 6, 1358–1381. [Google Scholar] [CrossRef] [PubMed]
- Kamamoto, Y.; Makiura, S.; Sugihara, S.; Hiasa, Y.; Arai, M. The inhibitory effect of copper on DL-ethionine carcinogenesis in rats. Cancer Res. 1973, 33, 1129–1135. [Google Scholar]
- Wilkinson, M.L.; Portmann, B.; Williams, R. Wilson’s disease and hepatocellular carcinoma: Possible protective role of copper. Gut 1983, 24, 767–771. [Google Scholar] [CrossRef][Green Version]
- Huai, J.P.; Ding, J.; Ye, X.H.; Chen, Y.P. Inflammatory bowel disease and risk of cholangiocarcinoma: Evidence from a meta-analysis of population-based studies. Asian Pac. J. Cancer Prev. 2014, 15, 3477–3482. [Google Scholar] [CrossRef] [PubMed]
- Axelrad, J.E.; Lichtiger, S.; Yajnik, V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol. 2016, 22, 4794–4801. [Google Scholar] [CrossRef] [PubMed]
- Holmes, E.; Li, J.V.; Athanasiou, T.; Ashrafian, H.; Nicholson, J.K. Understanding the role of gut microbiome-host metabolic signal disruption in health and disease. Trends Microbiol. 2011, 19, 349–359. [Google Scholar] [CrossRef]
- Saich, R.; Chapman, R. Primary sclerosing cholangitis, autoimmune hepatitis and overlap syndromes in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 331–337. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver fluke induces cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, D.A. Helminths in the induction of cancer: Opisthorchis viverrini, Clonorchis sinensis and cholangiocarcinoma. Trop. Geogr. Med. 1980, 32, 95–100. [Google Scholar] [PubMed]
- Shin, H.R.; Oh, J.K.; Masuyer, E.; Curado, M.P.; Bouvard, V.; Fang, Y.Y.; Wiangnon, S.; Sripa, B.; Hong, S.T. Epidemiology of cholangiocarcinoma: An update focusing on risk factors. Cancer Sci. 2010, 101, 579–585. [Google Scholar] [CrossRef] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Biological agents. IARC Monogr. Eval. Carcinog. Risks Hum. 2012, 100B, 341–370. [Google Scholar]
- Shin, H.R.; Oh, J.K.; Lim, M.K.; Shin, A.; Kong, H.J.; Jung, K.W.; Won, Y.J.; Park, S.; Park, S.J.; Hong, S.T. Descriptive epidemiology of cholangiocarcinoma and clonorchiasis in Korea. J. Korean Med. Sci. 2010, 25, 1011–1016. [Google Scholar] [CrossRef]
- Sithithaworn, P.; Yongvanit, P.; Duenngai, K.; Kiatsopit, N.; Pairojkul, C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 2014, 21, 301–308. [Google Scholar] [CrossRef]
- Marahatta, S.B.; Punyarit, P.; Bhudisawasdi, V.; Paupairoj, A.; Wongkham, S.; Petmitr, S. Polymorphism of glutathione S-transferase omega gene and risk of cancer. Cancer Lett. 2006, 236, 276–281. [Google Scholar] [CrossRef]
- Songserm, N.; Promthet, S.; Sithithaworn, P.; Pientong, C.; Ekalaksananan, T.; Chopjitt, P.; Parkin, D.M. MTHFR polymorphisms and Opisthorchis viverrini infection: A relationship with increased susceptibility to cholangiocarcinoma in Thailand. Asian Pac. J. Cancer Prev. 2011, 12, 1341–1345. [Google Scholar]
- Petrick, J.L.; Campbell, P.T.; Koshiol, J.; Thistle, J.E.; Andreotti, G.; Beane-Freeman, L.E.; Buring, J.E.; Chan, A.T.; Chong, D.Q.; Doody, M.M.; et al. Tobacco, alcohol use and risk of hepatocellular carcinoma and intrahepatic cholangiocarcinoma: The liver cancer pooling project. Br. J. Cancer 2018, 118, 1005–1012. [Google Scholar] [CrossRef]
- Makiuchi, T.; Sobue, T.; Kitamura, T.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Shimazu, T.; Inoue, M.; Tsugane, S. Smoking, Alcohol Consumption, and Risks for Biliary Tract Cancer and Intrahepatic Bile Duct Cancer. J. Epidemiol. 2019, 29, 180–186. [Google Scholar] [CrossRef]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol. Res. Health 2006, 29, 245–254. [Google Scholar]
- Ye, X.H.; Huai, J.P.; Ding, J.; Chen, Y.P.; Sun, X.C. Smoking, alcohol consumption, and the risk of extrahepatic cholangiocarcinoma: A meta-analysis. World J. Gastroenterol. 2013, 19, 8780–8788. [Google Scholar] [CrossRef] [PubMed]
- Jinga, M.; Jurcuţ, C.; Vasilescu, F.; Becheanu, G.; Stancu, S.H.; Ciobaca, L.; Mircescu, G.; Jinga, V. A rare case of digestive hemorrhage in an elderly patient: Diagnosis and treatment difficulties. Rom. J. Morphol. Embryol. 2012, 53 (Suppl. S3), 831–834. [Google Scholar] [PubMed]
- Huang, Y.; You, L.; Xie, W.; Ning, L.; Lang, J. Smoking and risk of cholangiocarcinoma: A systematic review and meta-analysis. Oncotarget 2017, 8, 100570–100581. [Google Scholar] [CrossRef]
- Staretz, M.E.; Murphy, S.E.; Patten, C.J.; Nunes, M.G.; Koehl, W.; Amin, S.; Koenig, L.A.; Guengerich, F.P.; Hecht, S.S. Comparative metabolism of the tobacco-related carcinogens benzo[a]pyrene, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone, 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol, and N′-nitrosonornicotine in human hepatic microsomes. Drug Metab. Dispos. 1997, 25, 154–162. [Google Scholar]
- Park, J.H.; Hong, J.Y.; Han, K. Association between Smoking Cessation and the Risk of Cholangiocarcinoma and Ampulla of Vater Cancer: A Nationwide Cohort Study. Liver Cancer 2023, 12, 457–466. [Google Scholar] [CrossRef]
- Granata, S.; Vivarelli, F.; Morosini, C.; Canistro, D.; Paolini, M.; Fairclough, L.C. Toxicological Aspects Associated with Consumption from Electronic Nicotine Delivery System (ENDS): Focus on Heavy Metals Exposure and Cancer Risk. Int. J. Mol. Sci. 2024, 25, 2737. [Google Scholar] [CrossRef]
- Gallagher, K.P.; Vargas, P.A.; Santos-Silva, A.R. The use of E-cigarettes as a risk factor for oral potentially malignant disorders and oral cancer: A rapid review of clinical evidence. Med. Oral. Patol. Oral. Cir. Bucal 2024, 29, e18–e26. [Google Scholar] [CrossRef] [PubMed]
- Muthumalage, T.; Noel, A.; Thanavala, Y.; Alcheva, A.; Rahman, I. Challenges in current inhalable tobacco toxicity assessment models: A narrative review. Tob. Induc. Dis. 2024, 22, 102. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Franceschi, S. Cholangiocarcinoma: Descriptive epidemiology and risk factors. Gastroenterol. Clin. Biol. 2010, 34, 173–180. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Chikawa, J.; Uegaki, Y.; Usuda, N.; Kuwahara, Y.; Fukumoto, M. Histological type of Thorotrast-induced liver tumors associated with the translocation of deposited radionuclides. Cancer Sci. 2010, 101, 336–340. [Google Scholar] [CrossRef]
- Khan, S.A.; Toledano, M.B.; Taylor-Robinson, S.D. Epidemiology, risk factors, and pathogenesis of cholangiocarcinoma. HPB 2008, 10, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.T.; Lin, Y.J.; Li, C.Y.; Tsai, P.J.; Yang, C.Y.; Liou, S.H.; Wu, T.N. Cancer attributable to asbestos exposure in shipbreaking workers: A matched-cohort study. PLoS ONE 2015, 10, e0133128. [Google Scholar] [CrossRef]
- Boulanger, M.; Morlais, F.; Bouvier, V.; Galateau-Salle, F.; Guittet, L.; Marquignon, M.F.; Paris, C.; Raffaelli, C.; Launoy, G.; Clin, B. Digestive cancers and occupational asbestos exposure: Incidence study in a cohort of asbestos plant workers. Occup. Environ. Med. 2015, 72, 792–797. [Google Scholar] [CrossRef]
- Brandi, G.; Di Girolamo, S.; Farioli, A.; de Rosa, F.; Curti, S.; Pinna, A.D.; Ercolani, G.; Violante, F.S.; Biasco, G.; Mattioli, S. Asbestos: A hidden player behind the cholangiocarcinoma increase? Findings from a case-control analysis. Cancer Causes Control 2013, 24, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, H.; Park, J.S. Beyond the Bile: Exploring the Microbiome and Metabolites in Cholangiocarcinoma. Life 2024, 14, 698. [Google Scholar] [CrossRef] [PubMed]
- Kerdkumthong, K.; Nanarong, S.; Roytrakul, S.; Pitakpornpreecha, T.; Tantimetta, P.; Runsaeng, P.; Obchoei, S. Quantitative proteomics analysis reveals possible anticancer mechanisms of 5′-deoxy-5′-methylthioadenosine in cholangiocarcinoma cells. PLoS ONE 2024, 19, e0306060. [Google Scholar] [CrossRef]
- Deng, S.; Lu, X.; Wang, X.; Liang, B.; Xu, H.; Yang, D.; Cui, G.; Yonemura, A.; Paine, H.; Zhou, Y.; et al. Overexpression of TBX3 suppresses tumorigenesis in experimental and human cholangiocarcinoma. Cell Death Dis. 2024, 15, 441. [Google Scholar] [CrossRef]
- Prabhakar, N.; Chiang, H.; Nabrinsky, E.; Eklund, J. Report of Cholangiocarcinoma with Transheterozygous BRCA1 and BRCA2 Co-mutation. Cureus 2024, 16, e60767. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, J.; Zhai, D.; Jiang, Q.; Yang, M.; Zhou, M. Gut microbiota regulates the ALK5/NOX1 axis by altering glutamine metabolism to inhibit ferroptosis of intrahepatic cholangiocarcinoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167152. [Google Scholar] [CrossRef]
- Zenoaga-Barbăroșie, C.; Berca, L.; Vassu-Dimov, T.; Toma, M.; Nica, M.I.; Alexiu-Toma, O.A.; Ciornei, C.; Albu, A.; Nica, S.; Nistor, C.; et al. The Predisposition for Type 2 Diabetes Mellitus and Metabolic Syndrome. Balkan J. Med. Genet. 2023, 26, 21–26. [Google Scholar] [CrossRef]
- Anghel, D.; Ciobîcă, L.M.; Stanciu, S.M.; Jurcuț, C.V.; Stoicescu, G.D.; Răduță, I.A.; Coca, A. Ankylosing spondylitis and cardiovascular risk—Case report. Rom. J. Mil. Med. 2016, 119, 39–42. [Google Scholar]
- Ionescu, O.P.; Stanciu, S.M.; Ciobîcă, M.L. Atherosclerosis in rheumatoid arthritis—The importance of imaging testing. Rom. J. Mil. Med. 2020, 123, 26–31. [Google Scholar]
- Zeng, L.; You, G.; Tanaka, H.; Srivatanakul, P.; Ohta, E.; Viwatthanasittiphong, C.; Matharit, M.; Chenvidhya, D.; Jedpiyawongse, A.; Tanaka, M.; et al. Combined effects of polymorphisms of DNA-repair protein genes and metabolic enzyme genes on the risk of cholangiocarcinoma. Jpn. J. Clin. Oncol. 2013, 43, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.H.; Kim, N.K.; Yim, D.J.; Hong, S.P.; Park, P.W.; Rim, K.S.; Kim, S.; Hwang, S.G. Polymorphisms of 5,10-methylenetetrahydrofolate reductase (MTHFR C677T) and thymidylate synthase enhancer region (TSER) as a risk factor of cholangiocarcinoma in a Korean population. Anticancer Res. 2006, 26, 4229–4233. [Google Scholar]
- Kinoshita, M.; Kubo, S.; Tanaka, S.; Takemura, S.; Nishioka, T.; Hamano, G.; Ito, T.; Tanaka, S.; Ohsawa, M.; Shibata, T. The association between non-alcoholic steatohepatitis and intrahepatic cholangiocarcinoma: A hospital based case-control study. J. Surg. Oncol. 2016, 113, 779–783. [Google Scholar] [CrossRef]
- Wongjarupong, N.; Assavapongpaiboon, B.; Susantitaphong, P.; Cheungpasitporn, W.; Treeprasertsuk, S.; Rerknimitr, R.; Chaiteerakij, R. Non-alcoholic fatty liver disease as a risk factor for cholangiocarcinoma: A systematic review and meta-analysis. BMC Gastroenterol. 2017, 17, 149. [Google Scholar] [CrossRef]
- De Lorenzo, S.; Tovoli, F.; Mazzotta, A.; Vasuri, F.; Edeline, J.; Malvi, D.; Boudjema, K.; Renzulli, M.; Jeddou, H.; D'Errico, A.; et al. Non-Alcoholic Steatohepatitis as a Risk Factor for Intrahepatic Cholangiocarcinoma and Its Prognostic Role. Cancers 2020, 12, 3182. [Google Scholar] [CrossRef]
- Parsi, M.A. Obesity and cholangiocarcinoma. World J. Gastroenterol. 2013, 19, 457–462. [Google Scholar] [CrossRef]
- Ciobîcă, L.M.; Sârbu, I.; Stanciu, S.M.; Coca, A. Behçet disease—Case presentation. Rom. J. Mil. Med. 2016, 119, 43–46. [Google Scholar] [CrossRef]
- Li, J.S.; Han, T.J.; Jing, N.; Li, L.; Zhang, X.H.; Ma, F.Z.; Liu, J.Y. Obesity and the risk of cholangiocarcinoma: A meta-analysis. Tumour Biol. 2014, 35, 6831–6838. [Google Scholar] [CrossRef]
- Pati, S.; Irfan, W.; Jameel, A.; Ahmed, S.; Shahid, R.K. Obesity and Cancer: A Current Overview of Epidemiology, Pathogenesis, Outcomes, and Management. Cancers 2023, 15, 485. [Google Scholar] [CrossRef]
- Jing, W.; Jin, G.; Zhou, X.; Zhou, Y.; Zhang, Y.; Shao, C.; Liu, R.; Hu, X. Diabetes mellitus and increased risk of cholangiocarcinoma: A meta-analysis. Eur. J. Cancer Prev. 2012, 21, 24–31. [Google Scholar] [CrossRef]
- Chaiteerakij, R.; Yang, J.D.; Harmsen, W.S.; Slettedahl, S.W.; Mettler, T.A.; Fredericksen, Z.S.; Kim, W.R.; Gores, G.J.; Roberts, R.O.; Olson, J.E.; et al. Risk factors for intrahepatic cholangiocarcinoma: Association between metformin use and reduced cancer risk. Hepatology 2013, 57, 648–655. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Kasimis, J.C.; Lopez, D.S.; Ntzani, E.E.; Ioannidis, J.P.A. Type 2 diabetes and cancer: Umbrella review of meta-analyses of observational studies. BMJ 2015, 350, g7607. [Google Scholar] [CrossRef]
- Rahman, S.U.; Sana, M.K.; Tahir, Z.; Ali, A.; Shah, P.A. Paraneoplastic syndromes in cholangiocarcinoma. World J. Hepatol. 2020, 12, 897–907. [Google Scholar] [CrossRef]
- Zhou, H.; Hylemon, P.B. Bile acids are nutrient signaling hormones. Steroids 2014, 86, 62–68. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Filippi, R.; Rimini, M.; Rapposelli, I.G.; Fornaro, L.; Silvestris, N.; Aldrighetti, L.; Aimar, G.; Rovesti, G.; Bartolini, G.; et al. Effects of Metformin and Vitamin D on Clinical Outcome in Cholangiocarcinoma Patients. Oncology 2021, 99, 292–299. [Google Scholar] [CrossRef]
- Powała, A.; Żołek, T.; Brown, G.; Kutner, A. Structure and the Anticancer Activity of Vitamin D Receptor Agonists. Int. J. Mol. Sci. 2024, 25, 6624. [Google Scholar] [CrossRef]
- Zhao, S.; Qian, F.; Wan, Z.; Chen, X.; Pan, A.; Liu, G. Vitamin D and major chronic diseases. Trends Endocrinol. Metab. 2024; online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bird, R.P. Vitamin D and cancer. Adv. Food Nutr. Res. 2024, 109, 92–159. [Google Scholar] [CrossRef]
- Chiang, K.C.; Yeh, C.N.; Lin, K.J.; Su, L.J.; Yen, T.C.; Pang, J.H.; Kittaka, A.; Sun, C.C.; Chen, M.F.; Jan, Y.Y.; et al. Chemopreventive and chemotherapeutic effect of dietary supplementation of vitamin D on cholangiocarcinoma in a Chemical-Induced animal model. Oncotarget 2014, 5, 3849–3861. [Google Scholar] [CrossRef]
- Kennedy, L.; Baker, K.; Hodges, K.; Graf, A.; Venter, J.; Hargrove, L.; Harris, R.; Harnish, E.; Meng, F.; Francis, H. Dysregulation of vitamin D3 synthesis leads to enhanced cholangiocarcinoma growth. Dig. Liver Dis. 2013, 45, 316–322. [Google Scholar] [CrossRef]
- Sookprasert, A.; Pugkhem, A.; Khuntikeo, N.; Chur-in, S.; Chamadol, N.; Prawan, A.; Janeklang, S.; Vaeteewoottacharn, K.; Kukongviriyapan, V.; Pairojkul, C.; et al. Evaluation of efficacy, safety and tolerability of high dose-intermittent calcitriol supplementation to advanced intrahepatic cholangiocarcinoma patients-a pilot study. Asian Pac. J. Cancer Prev. 2012, 13, 161–167. [Google Scholar]
- Chiang, K.C.; Yeh, T.S.; Huang, C.C.; Chang, Y.C.; Juang, H.H.; Cheng, C.T.; Pang, J.S.; Hsu, J.T.; Takano, M.; Chen, T.C.; et al. MART-10 represses cholangiocarcinoma cell growth and high vitamin D receptor expression indicates better prognosis for cholangiocarcinoma. Sci. Rep. 2017, 7, 43773. [Google Scholar] [CrossRef] [PubMed]
- Chiang, K.C.; Yeh, C.N.; Huang, C.C.; Yeh, T.S.; SPang, J.H.; Hsu, J.T.; Chen, L.W.; Kuo, S.F.; Kittaka, A.; Chen, T.C.; et al. 25(OH)D Is Effective to Repress Human Cholangiocarcinoma Cell Growth through the Conversion of 25(OH)D to 1alpha,25(OH)2D3. Int. J. Mol. Sci. 2016, 17, 1326. [Google Scholar] [CrossRef]
- Trakoonsenathong, R.; Kunprom, W.; Aphivatanasiri, C.; Yueangchantuek, P.; Pimkeeree, P.; Sorin, S.; Khawkhiaw, K.; Chiu, C.F.; Okada, S.; Wongkham, S.; et al. Liraglutide exhibits potential anti-tumor effects on the progression of intrahepatic cholangiocarcinoma, in vitro and in vivo. Sci. Rep. 2024, 14, 13726. [Google Scholar] [CrossRef]
- Ueda, P.; Wintzell, V.; Melbye, M.; Eliasson, B.; Svensson, A.M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Svanström, H.; et al. Use of incretin-based drugs and risk of cholangiocarcinoma: Scandinavian cohort study. Diabetologia 2021, 64, 2204–2214. [Google Scholar] [CrossRef]
- Huber, S.; Fitzner, T.; Feichtinger, R.G.; Hochmann, S.; Kraus, T.; Sotlar, K.; Kofler, B.; Varga, M. Galanin System in the Human Bile Duct and Perihilar Cholangiocarcinoma. Cells 2023, 12, 1678. [Google Scholar] [CrossRef]
- Petrescu, A.D.; Grant, S.; Williams, E.; Frampton, G.; Parks, N.; Blaney, H.; Davies, M.; John, R.; Reinhart, E.H.; McMillin, M.; et al. Coordinated Targeting of Galanin Receptors on Cholangiocytes and Hepatic Stellate Cells Ameliorates Liver Fibrosis in Multidrug Resistance Protein 2 Knockout Mice. Am. J. Pathol. 2020, 190, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Falkenstetter, S.; Leitner, J.; Brunner, S.M.; Rieder, T.N.; Kofler, B.; Weis, S. Galanin System in Human Glioma and Pituitary Adenoma. Front. Endocrinol. 2020, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Sandru, F.; Carsote, M.; Dumitrascu, M.C.; Albu, S.E.; Valea, A. Glucocorticoids and Trabecular Bone Score. J. Med. Life 2020, 13, 449–453. [Google Scholar] [CrossRef]
- Nistor, C.E.; Ciuche, A.; Cucu, A.P.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Clavicular Malignancies: A Borderline Surgical Management. Medicina 2022, 58, 910. [Google Scholar] [CrossRef]
- Carsote, M.; Valea, A.; Dumitru, N.; Terzea, D.; Petrova, E.; Albu, S.; Buruiana, A.; Ghemigian, A. Metastases in daily endocrine practice. Arch. Balk. Med. Union. 2016, 51, 476–480. [Google Scholar]
- Rubino, J.G.; Flemming, J.A. Menopausal hormone therapy and risk of biliary tract cancers: Addressing the ant, not the elephant in the room. Hepatology 2022, 75, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Kamani, M.; Akgor, U.; Gültekin, M. Review of the literature on combined oral contraceptives and cancer. Ecancermedicalscience 2022, 16, 1416. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Jiang, L.; Li, F.; Li, Q.; Yuan, S.; Huang, S.; Fu, Y.; Yan, X.; Chen, J.; Li, H.; et al. The epidemiological trends of biliary tract cancers in the United States of America. BMC Gastroenterol. 2022, 22, 546. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Chen, J.L.; Luo, Y.; Mathur, M.B.; Anagnostis, P.; Nurmatov, U.; Talibov, M.; Zhang, J.; Hawrylowicz, C.M.; Lumsden, M.A.; et al. Menopausal hormone therapy and women’s health: An umbrella review. PLoS Med. 2021, 18, e1003731. [Google Scholar] [CrossRef]
- Li, F.; Chen, Q.; Yang, Y.; Li, M.; Zhang, L.; Yan, Z.; Zhang, J.; Wang, K. ESR1 as a recurrence-related gene in intrahepatic cholangiocarcinoma: A weighted gene coexpression network analysis. Cancer Cell Int. 2021, 21, 225. [Google Scholar] [CrossRef]
- Kaewlert, W.; Sakonsinsiri, C.; Namwat, N.; Sawanyawisuth, K.; Ungarreevittaya, P.; Khuntikeo, N.; Armartmuntree, N.; Thanan, R. The Importance of CYP19A1 in Estrogen Receptor-Positive Cholangiocarcinoma. Horm. Cancer 2018, 9, 408–419. [Google Scholar] [CrossRef]
- Lu, C.; Miao, J.; Li, M.; Zheng, Q.; Xu, F.; Pan, Y.; Wang, Y.; Yang, Z.; Xia, X.; Zhu, H.; et al. Characterization of the Estrogen Response Helps to Predict Prognosis and Identify Potential Therapeutic Targets in Cholangiocarcinoma. Front. Oncol. 2022, 12, 870840. [Google Scholar] [CrossRef]
- Jackson, S.S.; Pfeiffer, R.M.; Gabbi, C.; Anderson, L.; Gadalla, S.M.; Koshiol, J. Menopausal hormone therapy and risk of biliary tract cancers. Hepatology 2022, 75, 309–321. [Google Scholar] [CrossRef]
- Petrick, J.L.; McMenamin, Ú.C.; Zhang, X.; Zeleniuch-Jacquotte, A.; Wactawski-Wende, J.; Simon, T.G.; Sinha, R.; Sesso, H.D.; Schairer, C.; Rosenberg, L.; et al. Exogenous hormone use, reproductive factors and risk of intrahepatic cholangiocarcinoma among women: Results from cohort studies in the Liver Cancer Pooling Project and the UK Biobank. Br. J. Cancer 2020, 123, 316–324. [Google Scholar] [CrossRef]
- Khalid, S.; Laput, G.; Khorfan, K.; Roytman, M. Development of Liver Cancers as an Unexpected Consequence of Anabolic Androgenic Steroid Use. Cureus 2023, 15, e34357. [Google Scholar] [CrossRef] [PubMed]
- Pothuri, V.S.; Anzelmo, M.; Gallaher, E.; Ogunlana, Y.; Aliabadi-Wahle, S.; Tan, B.; Crippin, J.S.; Hammill, C.W. Transgender Males on Gender-Affirming Hormone Therapy and Hepatobiliary Neoplasms: A Systematic Review. Endocr. Pract. 2023, 29, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Fadahunsi, O.O.; Ibitoye, B.O.; Adisa, A.O.; Alatise, O.I.; Adetiloye, V.A.; Idowu, B.M. Diagnostic accuracy of ultrasonography in adults with obstructive jaundice. J. Ultrason. 2020, 20, e100–e105. [Google Scholar] [CrossRef]
- Costa, M.; Valente, A.; Freire Coelho, A.; Meireles, S.; Barbosa, M. Acanthosis Nigricans Manifesting as a Paraneoplastic Syndrome Associated With Cholangiocarcinoma. Cureus 2023, 15, e35853. [Google Scholar] [CrossRef]
- Suchonwanit, P.; McMichael, A.J. Alopecia in Association with Malignancy: A Review. Am. J. Clin. Dermatol. 2018, 19, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, E.; Paraskeva, P.; Smyrnis, A.; Konstantopoulos, K. Alopecia: A common paraneoplastic manifestation of cholangiocarcinoma in humans and animals. BMJ Case Rep. 2012, 2012, bcr2012006217. [Google Scholar] [CrossRef]
- Ciobîcă, M.L.; Ionescu, O.P.; Săndulescu, B.A. Osteoporosis and the fracture risk in systemic lupus erythematosus. Rom. J. Mil. Med. 2020, 123, 341–347. [Google Scholar]
- Suh, K.J.; Park, J.K.; Cho, S.; Park, H.; Baek, H.W.; Lee, K.; Lee, D.S.; Lee, K.H. Dermatomyositis in a Patient with Cholangiocarcinoma Detected by an [(18)F]-Fluorodeoxyglucose Positron Emission Tomography-Computed Tomography Scan. Cancer Res. Treat. 2016, 48, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Staden, R.S.; Dumitru, A.V.; Stanciu Găvan, C. A Screening Test for Early Diagnosis of Microcellular Bronchopulmonary Cancer-Pilot Study. J. Clin. Med. 2019, 9, 76. [Google Scholar] [CrossRef]
- Sotoodian, B.; Mahmood, M.N.; Salopek, T.G. Clinical and Dermoscopic Features of Pigmented Disseminated Superficial Actinic Porokeratosis: Case Report and Literature Review. J. Cutan. Med. Surg. 2018, 22, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Chiyomaru, K.; Takai, T.; Ohashi, A.; Nishigori, C. Necrolytic migratory erythema with cholangiocarcinoma: Pseudoglucagonoma syndrome. Eur. J. Dermatol. 2010, 20, 238–239. [Google Scholar] [CrossRef]
- Tzovaras, V.; Liberopoulos, E.N.; Zioga, A.; Pavlidis, N.; Elisaf, M. Persistent erythema multiforme in a patient with extrahepatic cholangiocarcinoma. Oncology 2007, 73, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Ciuche, A.; Cucu, A.P.; Nitipir, C.; Slavu, C.; Serban, B.; Cursaru, A.; Cretu, B.; Cirstoiu, C. Management of Lung Cancer Presenting with Solitary Bone Metastasis. Medicina 2022, 58, 1463. [Google Scholar] [CrossRef]
- Chacko, A.M.; Carrero, G.; Akhouri, S. A Case Report of Erythema Multiforme Secondary to Atorvastatin Use. Cureus 2024, 16, e52175. [Google Scholar] [CrossRef]
- Cohen, P.R.; Holder, W.R.; Tucker, S.B.; Kono, S.; Kurzrock, R. Sweet syndrome in patients with solid tumors. Cancer 1993, 72, 2723–2731. [Google Scholar] [CrossRef]
- Shinojima, Y.; Toma, Y.; Terui, T. Sweet syndrome associated with intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor. Br. J. Dermatol. 2006, 155, 1103–1104. [Google Scholar] [CrossRef]
- Lemaire, C.C.; Portilho, A.L.C.; Pinheiro, L.V.; Vivas, R.A.; Britto, M.; Montenegro, M.; Rodrigues, L.F.F.; Arruda, S.; Lyra, A.C.; Cavalcante, L.N. Sweet syndrome as a paraneoplastic manifestation of cholangiocarcinoma: A case report. World J. Clin. Cases 2020, 8, 4122–4127. [Google Scholar] [CrossRef]
- Holzgruber, J.; Oberneder-Popper, J.; Guenova, E.; Hötzenecker, W. Acrokeratosis Paraneoplastica (Bazex Syndrome): A Case Report. Case Rep. Dermatol. 2022, 14, 307–312. [Google Scholar] [CrossRef]
- Ciuche, A.; Nistor, C.; Motaş, C.; Horvat, T. Minimally invasive surgery in the treatment of malignant pleuro-pericardial effusions. Chirurgia 2012, 107, 206–212. [Google Scholar] [PubMed]
- Stone, S.P.; Buescher, L.S. Life-threatening paraneoplastic cutaneous syndromes. Clin. Dermatol. 2005, 23, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.S.; Neldner, K.H.; Menter, A. Erythema gyratum repens: A paraneoplastic eruption. J. Am. Acad. Dermatol. 1992, 26 Pt 1, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.E.; Găvan, C.S.; Ciritel, A.A.; Nemes, A.F.; Ciuche, A. The Association of Minimally Invasive Surgical Approaches and Mortality in Patients with Malignant Pleuropericarditis-A 10 Year Retrospective Observational Study. Medicina 2022, 58, 718. [Google Scholar] [CrossRef]
- Liau, M.M.; Long, V.; Yang, S.S. Erythema gyratum repens: A paraneoplastic eruption. BMJ Case Rep. 2016, 2016, bcr2016214665. [Google Scholar] [CrossRef]
- Bar-Ilan, E.; Gat, A.; Sprecher, E.; Zeeli, T. Paraneoplastic pityriasis rubra pilaris: Case report and literature review. Clin. Exp. Dermatol. 2017, 42, 54–57. [Google Scholar] [CrossRef]
- Opneja, A.; Mahajan, S.; Kapoor, S.; Marur, S.; Yang, S.H.; Manno, R. Unusual Paraneoplastic Presentation of Cholangiocarcinoma. Case Rep. Med. 2015, 2015, 806835. [Google Scholar] [CrossRef]
- Morgenthau, A.; Almudaires, A. Klatskin's cholangiocarcinoma presenting with the sign of Leser-Trelat. BMJ Case Rep. 2019, 12, e232507. [Google Scholar] [CrossRef]
- Scully, C.; Barrett, W.A.; Gilkes, J.; Rees, M.; Sarner, M.; Southcott, R.J. Oral acanthosis nigricans, the sign of Leser-Trélat and cholangiocarcinoma. Br. J. Dermatol. 2001, 145, 506–507. [Google Scholar] [CrossRef]
- Sökmen, M.; Demirsoy, H.; Ersoy, O.; Gökdemir, G.; Akbayir, N.; Karaca, C.; Ozdil, K.; Kesici, B.; Calişkan, C.; Yilmaz, B. Paraneoplastic porphyria cutanea tarda associated with cholangiocarcinoma: Case report. Turk. J. Gastroenterol. 2007, 18, 200–205. [Google Scholar] [PubMed]
- Schmidt, S.L.; Schmidt, J.J.; Tolentino, J.C.; Ferreira, C.G.; de Almeida, S.A.; Alvarenga, R.P.; Simoes, E.N.; Schmidt, G.J.; Canedo, N.H.; Chimelli, L. Cholangiocarcinoma associated with limbic encephalitis and early cerebral abnormalities detected by 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with computed tomography-positron emission tomography: A case report. J. Med. Case Rep. 2016, 10, 200. [Google Scholar] [CrossRef]
- Bruhnding, A.; Notch, D.; Beard, A. Anti-Yo positive paraneoplastic cerebellar degeneration in the setting of cholangiocarcinoma. J. Clin. Neurosci. 2017, 36, 71–72. [Google Scholar] [CrossRef]
- Normand, G.; Jolivot, A.; Rabeyrin, M.; Hervieu, V.; Valette, P.J.; Scoazec, J.Y.; Gougon, J.M.; Juillard, L.; Dumortier, J. Paraneoplastic fibrillary glomerulonephritis associated with intrahepatic cholangiocarcinoma: When diagnosis of a rare kidney disease leads to successful hepatic cancer treatment. Clin. Res. Hepatol. Gastroenterol. 2017, 41, e8–e11. [Google Scholar] [CrossRef] [PubMed]
- Solans-Laqué, R.; Bosch-Gil, J.A.; Pérez-Bocanegra, C.; Selva-O'Callaghan, A.; Simeón-Aznar, C.P.; Vilardell-Tarres, M. Paraneoplastic vasculitis in patients with solid tumors: Report of 15 cases. J. Rheumatol. 2008, 35, 294–304. [Google Scholar]
- Hatzis, G.S.; Papachristodoulou, A.; Delladetsima, I.K.; Moutsopoulos, H.M. Polyarteritis nodosa associated with cholangiocarcinoma. Lupus 1998, 7, 301–306. [Google Scholar] [CrossRef]
- Horsted, F.; West, J.; Grainge, M.J. Risk of venous thromboembolism in patients with cancer: A systematic review and meta-analysis. PLoS Med. 2012, 9, e1001275. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.K.; Kim, D.U.; Baek, D.H.; Ha, D.W.; Lee, B.E.; Ryu, D.Y.; Cheong, J.H.; Kim, G.H.; Song, G.A.; Jang, A.L. Venous thromboembolism in patients with cholangiocarcinoma: Focus on risk factors and impact on survival. Eur. J. Gastroenterol. Hepatol. 2012, 24, 444–449. [Google Scholar] [CrossRef]
- Blum, M.F.; Ma, V.Y.; Betbadal, A.M.; Bonomo, R.A.; Raju, R.R.; Packer, C.D. Trousseau's Syndrome in Cholangiocarcinoma: The Risk of Making the Diagnosis. Clin. Med. Res. 2016, 14, 53–59. [Google Scholar] [CrossRef]
- Zahidin, M.A.; Iberahim, S.; Hassan, M.N.; Zulkafli, Z.; Mohd Noor, N.H. Clinical and Laboratory Diagnosis of Antiphospholipid Syndrome: A Review. Cureus 2024, 16, e61713. [Google Scholar] [CrossRef]
- Samadian, S.; Estcourt, L. Recurrent thrombo-embolic episodes: The association of cholangiocarcinoma with antiphospholipid syndrome. Postgrad. Med. J. 1999, 75, 45–46. [Google Scholar] [CrossRef]
- Ham, H.; Kim, H.Y.; Seo, K.J.; Lee, S.L.; Kim, C.W. Cholangiocarcinoma with a paraneoplastic leukemoid reaction mimicking a pyogenic liver abscess. Korean J. Intern. Med. 2015, 30, 110–113. [Google Scholar] [CrossRef]
- Sohda, T.; Shiga, H.; Nakane, H.; Watanabe, H.; Takeshita, M.; Sakisaka, S. Cholangiocellular carcinoma that produced both granulocyte-colony-stimulating factor and parathyroid hormone-related protein. Int. J. Clin. Oncol. 2006, 11, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.F.; Jin, Y.; Li, J.T. Intrahepatic sarcomatoid cholangiocarcinoma: A case report of the youngest patient on record and a review of the condition's characteristics. Front. Surg. 2022, 9, 963952. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Houk, L.; Yousaf, W.; Smiley, D.; Coberly, L. Unusual para-neoplastic manifestation of cholangiocarcinoma. J. Gastrointest. Cancer 2013, 44, 228–230. [Google Scholar] [CrossRef] [PubMed]
- González Amores, Y.; Hernando Rebollar, S.; Casado Bernabeu, A. Lupus as a paraneoplastic manifestation of cholangiocarcinoma. Rev. Esp. Enferm. Dig. 2016, 108, 292. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.; McPherson, T. Paraneoplastic subacute cutaneous lupus erythematosus associated with cholangiocarcinoma. Australas. J. Dermatol. 2016, 57, e5–e7. [Google Scholar] [CrossRef]
- Erdinc, B.; Ramachandran, P.; Yadav, R.; Sahni, S.; Joseph, G. Cholangiocarcinoma Presenting as Humoral Hypercalcemia of Malignancy: A Case Report and Literature Review. Cureus 2019, 11, e6481. [Google Scholar] [CrossRef]
- Harsch, I.A.; Konturek, P.C. Humoral Hypercalcemia in a Patient with Cholangiocellular Carcinoma—Effective Therapy with Denosumab. Am. J. Case Rep. 2019, 20, 1325–1330. [Google Scholar] [CrossRef]
- Ozawa, N.; Doi, S.; Tsujikawa, T.; Mabuchi, M.; Kajiyama, Y.; Sato, K.; Kikuchi, K.; Takahashi, M.; Kawamoto, M.; Yasuda, I. Intrahepatic cholangiocarcinoma producing granulocyte colony-stimulating factor and parathyroid hormone-related protein. Nihon Shokakibyo Gakkai Zasshi 2017, 114, 1285–1292. [Google Scholar]
- Chauhan, A.; Likasitwatanakul, P.; Ahmed, A.; Sibley, S.D. A Case of Fibroblast Growth Factor Receptor Fusion-Positive Intrahepatic Cholangiocarcinoma With Humoral Hypercalcemia of Malignancy. Cureus 2024, 16, e58741. [Google Scholar] [CrossRef]
- Meegada, S.; Eisen, R.; Coons, G.; Verma, R. Intrahepatic Cholangiocarcinoma Associated with High Procalcitonin, Hypercalcemia, Polycythemia and Leukocytosis. Cureus 2020, 12, e6587. [Google Scholar] [CrossRef] [PubMed]
- Baiocchi, L.; Sato, K.; Ekser, B.; Kennedy, L.; Francis, H.; Ceci, L.; Lenci, I.; Alvaro, D.; Franchitto, A.; Onori, P.; et al. Cholangiocarcinoma: Bridging the translational gap from preclinical to clinical development and implications for future therapy. Expert. Opin. Investig. Drugs 2021, 30, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Joo, I.; Lee, J.M.; Yoon, J.H. Imaging Diagnosis of Intrahepatic and Perihilar Cholangiocarcinoma: Recent Advances and Challenges. Radiology 2018, 288, 7–13. [Google Scholar] [CrossRef]
- Razumilava, N.; Gores, G.J.; Lindor, K.D. Cancer surveillance in patients with primary sclerosing cholangitis. Hepatology 2011, 54, 1842–1852. [Google Scholar] [CrossRef]
- Liu, G.J.; Wang, W.; Lu, M.D.; Xie, X.Y.; Xu, H.X.; Xu, Z.F.; Chen, L.D.; Wang, Z.; Liang, J.Y.; Huang, Y.; et al. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer 2015, 4, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Olthof, S.C.; Othman, A.; Clasen, S.; Schraml, C.; Nikolaou, K.; Bongers, M. Imaging of Cholangiocarcinoma. Visc. Med. 2016, 32, 402–410. [Google Scholar] [CrossRef]
- Pfrepper, C. Paraneoplastic Thromboembolism and Thrombophilia: Significance in Visceral Medicine. Visc. Med. 2020, 36, 280–287. [Google Scholar] [CrossRef]
- Tai, N.; Inoue, D. Bone and calcium metabolism associated with malignancy. Malignancy-associated hypercalcemia. Clin. Calcium 2018, 28, 1503–1508. [Google Scholar]
- Nistor, C.E.; Stanciu-Găvan, C.; Vasilescu, F.; Dumitru, A.V.; Ciuche, A. Attitude of the surgical approach in hyperparathyroidism: A retrospective study. Exp. Ther. Med. 2021, 22, 959. [Google Scholar] [CrossRef]
- Ashihara, N.; Nakajima, K.; Nakamura, Y.; Kobayashi, M.; Shirahata, K.; Maeda, C.; Uehara, T.; Gomi, D.; Ito, N. Denosumab is Effective for Controlling Serum Calcium Levels in Patients with Humoral Hypercalcemia of Malignancy Syndrome: A Case Report on Parathyroid Hormone-related Protein-producing Cholangiocarcinoma. Intern. Med. 2016, 55, 3453–3457. [Google Scholar] [CrossRef]
- Tang, J.; Liao, Y.; He, S.; Shi, J.; Peng, L.; Xu, X.; Xie, F.; Diao, N.; Huang, J.; Xie, Q.; et al. Autocrine parathyroid hormone-like hormone promotes intrahepatic cholangiocarcinoma cell proliferation via increased ERK/JNK-ATF2-cyclinD1 signaling. J. Transl. Med. 2017, 15, 238. [Google Scholar] [CrossRef] [PubMed]
- Kane, J.F.; Johnson, R.W. e-Evaluating the Role of PTHrP in Breast Cancer. Cancers 2023, 15, 2670. [Google Scholar] [CrossRef] [PubMed]
- Mc Donald, D.; Drake, M.T.; Crowley, R.K. Treatment of hypercalcaemia of malignancy in adults. Clin. Med. 2023, 23, 503–507. [Google Scholar] [CrossRef]
- El-Hajj Fuleihan, G.; Clines, G.A.; Hu, M.I.; Marcocci, C.; Murad, M.H.; Piggott, T.; Van Poznak, C.; Wu, J.Y.; Drake, M.T. Treatment of Hypercalcemia of Malignancy in Adults: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2023, 108, 507–528. [Google Scholar] [CrossRef] [PubMed]
- Nistor, C.; Ciuche, A.; Constantinescu, I. Emergency surgical tracheal decompression in a huge retrosternal goiter. Acta Endocrinol. 2017, 13, 370–374. [Google Scholar] [CrossRef]
- Piantanida, E.; Ippolito, S.; Gallo, D.; Masiello, E.; Premoli, P.; Cusini, C.; Rosetti, S.; Sabatino, J.; Segato, S.; Trimarchi, F.; et al. The interplay between thyroid and liver: Implications for clinical practice. J. Endocrinol. Investig. 2020, 43, 885–899. [Google Scholar] [CrossRef]
- Benner, B.J.M.; Alsma, J.; Feelders, R.A. Hyponatraemia and hyperpigmentation in primary adrenal insufficiency. BMJ Case Rep. 2019, 12, e227200. [Google Scholar] [CrossRef]
- Nistor, C.E.; Pantile, D.; Gavan, C.S.; Ciuche, A. Pneumothorax on COVID-19 patients-retrospective clinical observations. Rom. J. Leg. Med. 2022, 30, 112–116. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, T.; Pan, H.; Du, A.; Wu, T.; Lan, J.; Song, Y.; Lv, Y.; He, F.; Yuan, K. Bioinformatics and system biology approaches to determine the connection of SARS-CoV-2 infection and intrahepatic cholangiocarcinoma. PLoS ONE 2024, 19, e0300441. [Google Scholar] [CrossRef]
- Wiwanitkit, V. Thioredoxin reductase-1 and incidence of COVID-19 among cholangiocarcinoma patients in tropical endemic area. J. Cancer Res. Ther. 2023, 19, 845. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, J.; Xiao, W.; Zhang, Y.; Lv, Y.; Yu, X.; Zheng, J. The Therapeutic Potential of Galectin-3 in the Treatment of Intrahepatic Cholangiocarcinoma Patients and Those Compromised with COVID-19. Front. Mol. Biosci. 2021, 8, 666054. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Pavuluri, S.; Srinivasan, K.; Ghouse, M. Thrombotic Microangiopathy in a Patient with COVID-19 Infection and Metastatic Cholangiocarcinoma. J. Hematol. 2021, 10, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.; Gokhale, P.; Katte, T.; Acharya, S.; Rasalkar, A.A.; Vidapanakal, S.; Manas, R.; Chinnam, S.; Narayanan, P.; Shettihalli, A.K.; et al. Assessing the Vulnerability of Cancer Patients for COVID-19. ACS Omega 2022, 7, 35735–35742. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).