Observations on an Aggregation of Grey Reef Sharks (Carcharhinus amblyrhynchos) in the Mozambique Channel Off the Coast of Nosy Be (Madagascar) and Tools for Photo-Identification—A New Aggregation Nursery Site?
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
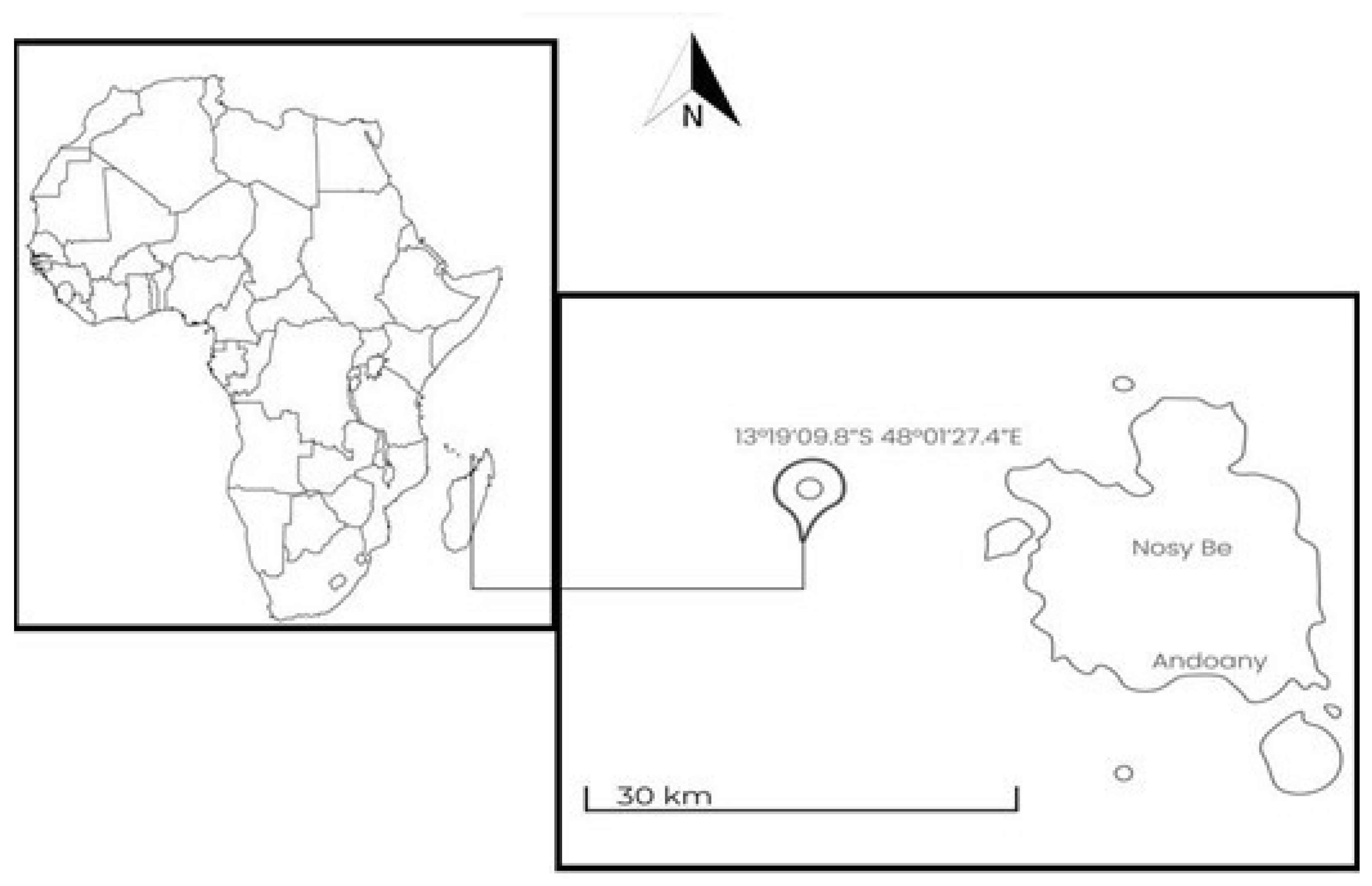
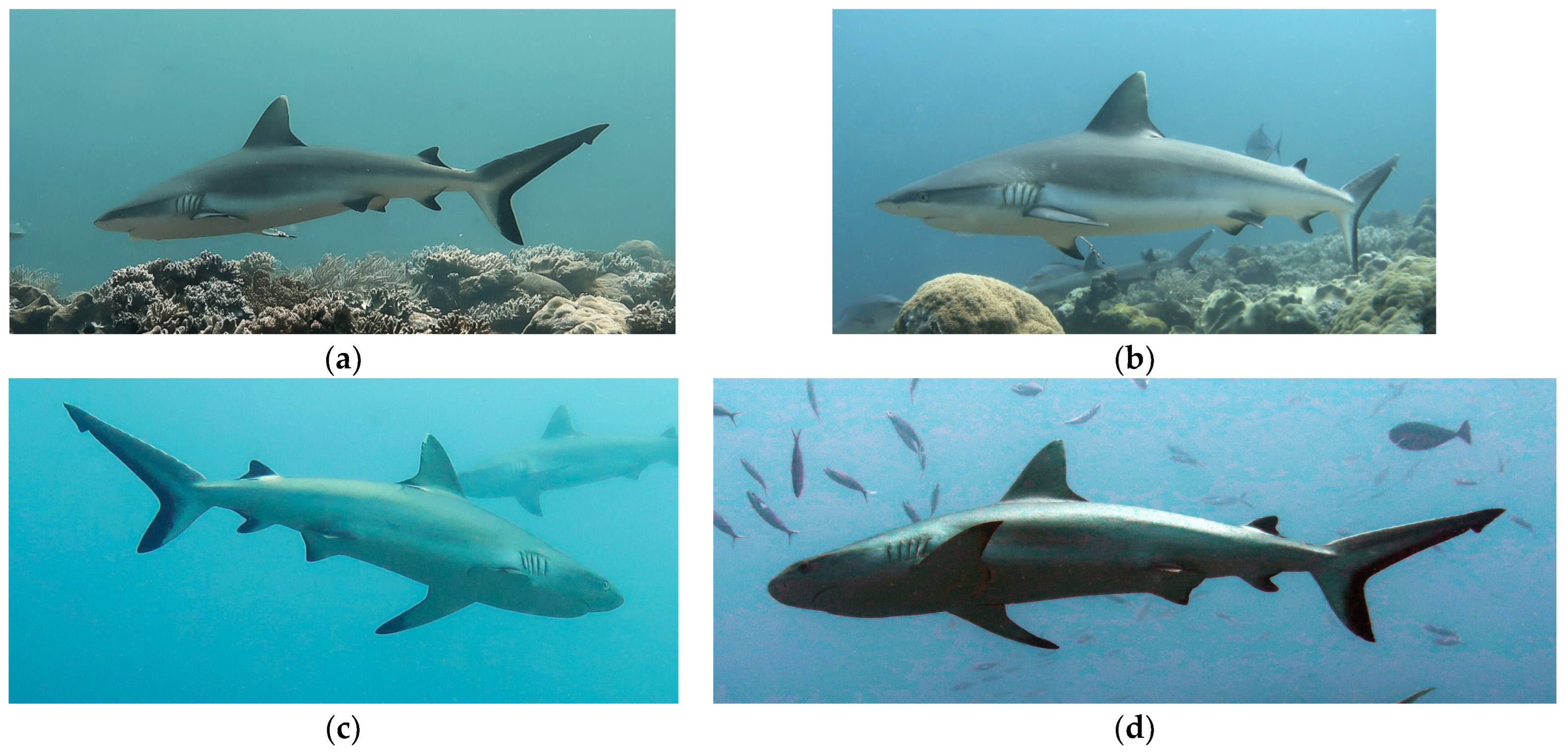
2.1. Sampling Area
2.2. Data Collection
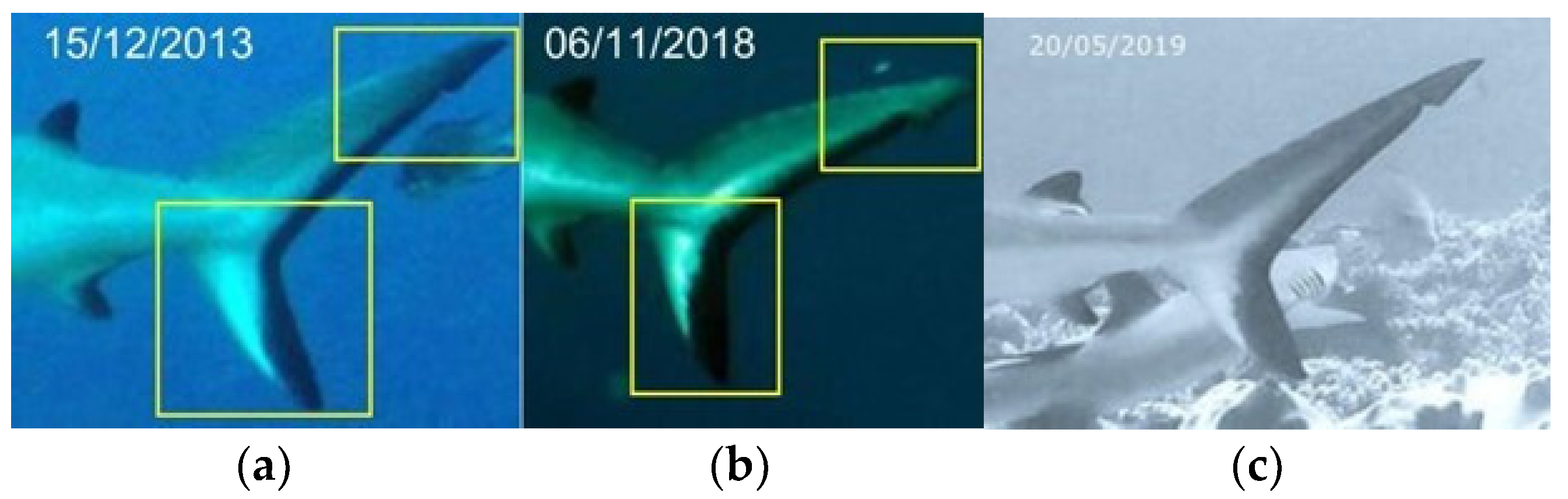
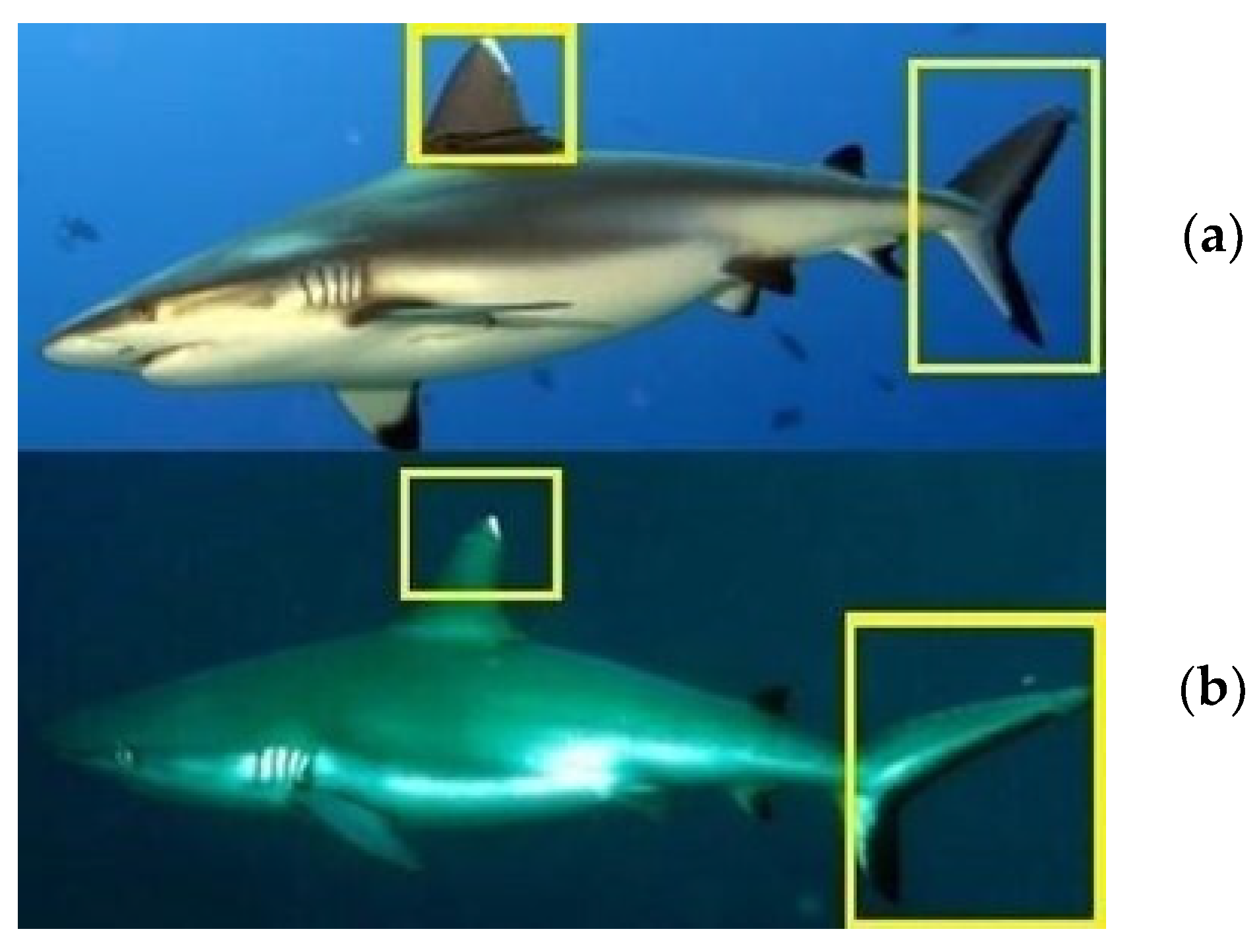
2.3. Photo Collection Procedure and Specimen Photo-Identification Protocol
- Videos were collected by each member of the team and divided by specimen and temporal sequence;
- Stills were taken from the videos in which the fundamental features for photo-ID were clearly visible;
- New specimens were compared with the ones already present in the database to verify that they were not a resighting, comparisons were performed using Excel;
- Photos of each shark were loaded into the Excel sheet relating to the year of sighting and any related data were loaded into the appropriate table. Image processing included removing the background and highlighting the first dorsal, especially the area with the white spot and caudal fin black spot. Additional excess parts were removed using the “mark areas to keep” or “mark areas to remove” operations.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simpfendorfer, C.; Fahmi, A.B.A.; Utzurrum, J.A.T.; Seyha, L.; Maung, A.; Bineesh, K.K.; Yuneni, R.R.; Sianipar, A.; Haque, A.B.; Tanay, D.; et al. Carcharhinus amblyrhynchos. The IUCN Red List of Threatened Species. 2020. e.T39365A173433550. Available online: http://www.iucnredlist.org/species/pdf/173433550 (accessed on 1 February 2024).
- Cripps, G.; Harris, A.; Humber, F.; Harding, S.; Thomas, T. A Preliminary Value Chain Analysis of Shark Fisheries in Madagascar. Programme for the Implementation of a Regional Fisheries Strategy for the Eastern and Southern Africa Indian Ocean Region; SF/2015/34; Indian Ocean Commission: Ebene, Mauritius, 2015; pp. 1–82. Available online: http://www.fao.org/3/ (accessed on 1 February 2024).
- Fourmanoir, P. Requins de la cote ouest de Madagascar. Mémoires de l’Institut Scientifique de Madagascar. Série F. Océanogr. 1961, 4, 1–81. [Google Scholar]
- Robinson, L.; Sauer, W. A first description of the artisanal shark fishery in northern Madagascar: Implications for management. Afr. J. Mar. Sci. 2013, 35, 9–15. [Google Scholar] [CrossRef]
- Nelson, D.R.; Johnson, R.H. Behavior of the reef sharks of Rangiroa, French Polynesia. Natl. Geogr. Soc. Res. Rep. 1980, 12, 479–499. [Google Scholar]
- Castro, A.L.; Rosa, R.S. Use of natural marks on population estimates of the nurse shark, Ginglymostoma cirratum, at Atol das Rocas Biological Reserve, Brazil. Environ. Biol. Fishes 2005, 72, 213–221. [Google Scholar] [CrossRef]
- Chapman, D.D.; Pikitch, E.K.; Babcock, E.; Shivji, M.S. Marine reserve design and evaluation using automated acoustic telemetry: A case-study involving coral reef-associated sharks in the Mesoamerican Caribbean. Mar. Technol. Soc. J. 2005, 39, 42–55. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Lowe, C.G.; Caselle, J.E.; Friedlander, A.M. Scale dependent effects of habitat on movements and path structure of reef sharks at a predator-dominated atoll. Ecology 2009, 90, 996–1008. [Google Scholar] [CrossRef]
- Fitzpatrick, R.; Abrantes, K.G.; Seymour, J.; Barnett, A. Variation in depth of whitetip reef sharks: Does provisioning ecotourism change their behaviour? Coral Reefs 2011, 30, 569–577. [Google Scholar] [CrossRef]
- Barnett, A.; Abrantes, K.G.; Seymour, J.; Fitzpatrick, R. Residency and Spatial Use by Reef Sharks of an Isolated Seamount and Its Implications for Conservation. PLoS ONE 2012, 7, e36574. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A. Science or slaughter: Need for lethal sampling of sharks. Conserv. Biol. 2010, 24, 1212–1218. [Google Scholar] [CrossRef]
- Speed, C.W.; Field, I.C.; Meekan, M.G.; Bradshaw, C.J.A. Complexities of coastal shark movements and their implications for management. Mar. Ecol. Prog. Ser. 2010, 408, 275–293. [Google Scholar] [CrossRef]
- Krause, J.; Hensor, E.M.A.; Ruxton, G.D. Fish as prey. In Handbook of Fish Biology and Fisheries. Fish Biology; Hart, P.J.B., Reynolds, J.D., Eds.; Blackwell Publishing: Malden, MA, USA, 2002; Volume 1, pp. 284–298. [Google Scholar] [CrossRef]
- McInturf, A.G.; Bowman, J.; Schulte, J.M.; Newton, K.C.; Vigil, B.; Honig, M.; Pelletier, S.; Cox, N.; Lester, O.; Cantor, M.; et al. A unified paradigm for defining elasmobranch aggregations. ICES J. Mar. Sci. 2023, 80, 1551–1566. [Google Scholar] [CrossRef]
- Vianna, G.M.S.; Meekan, M.G.; Meeuwig, J.J.; Speed, C.W. Environmental Influences on Patterns of Vertical Movement and Site Fidelity of Grey Reef Sharks (Carcharhinus amblyrhynchos) at Aggregation Sites. PLoS ONE 2013, 8, e60331. [Google Scholar] [CrossRef]
- Field, I.C.; Meekan, M.G.; Speed, C.W.; White, W.; Bradshaw, C. Quantifying movement patterns for shark conservation at remote coral atolls in the Indian Ocean. Coral Reefs 2011, 30, 61–71. [Google Scholar] [CrossRef]
- Bond, M.E.; Babcock, E.A.; Pikitch, E.K.; Abercrombie, D.L.; Lamb, N.F.; Chapman, D.D. Reef Sharks Exhibit Site-Fidelity and Higher Relative Abundance in Marine Reserves on the Mesoamerican Barrier Reef. PLoS ONE 2012, 7, e32983. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A. Importance of environmental and biological drivers in the presence and space use of a reef-associated shark. Mar. Ecol. Prog. Ser. 2014, 496, 47–57. [Google Scholar] [CrossRef]
- McKibben, J.N.; Nelson, D.R. Patterns of movement and grouping of grey reef sharks, Carcharhinus amblyrhynchos, at Enewetak, Marshall Islands. Bull. Mar. Sci. 1986, 38, 89–110. [Google Scholar]
- Awruch, C.A.; Frusher, S.D.; Pankhurst, N.W.; Stevens, J.D. Non-lethal assessment of reproductive characteristics for management and conservation of sharks. Mar. Ecol. Prog. Ser. 2008, 355, 277–285. [Google Scholar] [CrossRef]
- Barnett, A.; Redd, K.S.; Frusher, S.D.; Stevens, J.D.; Semmens, J.M. Non-lethal method to obtain stomach samples from a large marine predator and the use of DNA analysis to improve dietary information. J. Exp. Mar. Biol. Ecol. 2010, 393, 188–192. [Google Scholar] [CrossRef]
- Rowat, D.; Speed, C.W.; Meekan, M.G.; Gore, M. Population abundance and apparent survival of the Vulnerable whale shark, Rhincodon typus, in the Seychelles aggregation. Oryx 2009, 43, 591–598. [Google Scholar] [CrossRef]
- Sperone, E.; Micarelli, P.; Andreotti, S.; Brandmayr, P.; Bernabò, I.; Brunelli, E.; Tripepi, S. Surface behaviour of bait-attracted white sharks at Dyer Island (South Africa). Mar. Biol. Res. 2012, 8, 982–991. [Google Scholar] [CrossRef]
- Micarelli, P.; Sperone, E.; Pecchia JGiglio, G.; Mele, F.; Scuderi, A.; Romano, C.; Vespaziani, L. Dorsal fin photoidentification: Tool for long term studies of White shark (Carcharodon carcharias) behaviour. Biol. Mar. Mediterr. 2015, 22, 168–169. [Google Scholar] [CrossRef]
- Micarelli, P.; Bonsignori, D.; Compagno, L.J.V.; Pacifico, A.; Romano, C.; Reinero, F.R. Analysis of sightings of white sharks in Gansbaai (South Africa). Eur. Zool. J. 2021, 88, 363–374. [Google Scholar] [CrossRef]
- Arzoumanian, Z.; Holmberg, J.; Norman, B. An astronomical pattern-matching algorithm for computer-aided identification of whale sharks, Rhincodon typus. J. Appl. Ecol. 2005, 42, 999–1011. [Google Scholar] [CrossRef]
- Meekan, M.G.; Bradshaw, C.J.A.; Press, M.; McLean, C.; Richards, A.; Quasnichka, S.; Taylor, J.G. Population size and structure of whale sharks (Rhincodon typus) at Ningaloo Reef, Western Australia. Mar. Ecol. Prog. Ser. 2006, 319, 275–285. [Google Scholar] [CrossRef]
- Carraro, R.; Gladstone, W. Habitat preferences and site fidelity of the ornate wobbegong shark (Orectolobus ornatus) on rocky reefs of New South Wales. Pac. Sci. 2006, 60, 207–223. [Google Scholar] [CrossRef]
- Dudgeon, C.L.; Noad, M.J.; Lanyon, J.M. Abundance and demography of a seasonal aggregation of zebra sharks Stegostoma fasciatum. Mar. Ecol. Prog. Ser. 2008, 368, 269–281. [Google Scholar] [CrossRef]
- Mourier, J.; Vercelloni, J.; Planes, S. Evidence of social communities in a spatially structured network of a free-ranging shark species. Anim. Behav. 2012, 83, 389–401. [Google Scholar] [CrossRef]
- Marshall, A.D.; Pierce, S.J. The use and abuse of photographic identification in sharks and rays. J. Fish Biol. 2012, 80, 1361–1379. [Google Scholar] [CrossRef]
- Martín, G.; Espinoza, M.; Heupel, M.; Simpfendorfer, C.A. Estimating marine protected area network benefits for reef sharks. J. Appl. Ecol. 2020, 57, 1969–1980. [Google Scholar] [CrossRef]
- Espinoza, M.; Heupel, M.R.; Tobin, A.J.; Simpfendorfer, C.A. Residency patterns and movements of grey reef sharks (Carcharhinus amblyrhynchos) in semi-isolated coral reef habitats. Mar. Biol. 2015, 162, 343–358. [Google Scholar] [CrossRef]
- Wetherbee, B.M.; Crow, G.L.; Lowe, C.G. Distribution, reproduction and diet of the Grey Reef Shark Carcharhinus amblyrhynchos in Hawaii. Mar. Ecol. Prog. Ser. 1997, 151, 181–189. [Google Scholar] [CrossRef]
- Ebert, D.A.; Fowler, S.L.; Compagno, L.J.V. Sharks of the World: A Fully Illustrated Guide; Wild Nature Press: Oxford, UK, 2013. [Google Scholar]
- Klimley, A.P.; Anderson, S.D. Residency patterns of white sharks at the South Farallon Islands, California. In Great White Sharks: The Biology of Carcharodon carcharias; Klimley, A.P., Ainley, D.G., Eds.; Academic Press: Cambridge, MA, USA, 1996; pp. 365–373. [Google Scholar]
- Sims, D.W.; Speedy, C.D.; Fox, A.M. Movements and growth of a female basking shark re-sighted after a three-year period. J. Mar. Biol. Assoc. 2000, 80, 1141–1142. [Google Scholar] [CrossRef]
- Anderson, S.D.; Chapple, T.K.; Jorgensen, S.J.; Klimley, A.P.; Block, B.A. Long-term individual identification and site fidelity of white sharks, Carcharodon carcharias, off California using dorsal fins. Mar. Biol. 2011, 158, 1233–1237. [Google Scholar] [CrossRef]
- Hussey, N.E.; Stroh, N.; Klaus, R.; Chekchak, T.; Kessel, S.T. SCUBA diver observations and placard tags to monitor grey reef sharks, Carcharhinus amblyrhynchos, at Sha’ab Rumi, The Sudan: Assessment and future directions. J. Mar. Biol. Assoc. 2013, 93, 299–308. [Google Scholar] [CrossRef]
- Mourier, J.; Maynard, J.; Parravicini, V.; Ballesta, L.; Clua, E.; Domeier, M.L.; Planes, S. Extreme Inverted Trophic Pyramid of Reef Sharks Supported by Spawning Groupers. Curr. Biol. 2016, 26, 2011–2016. [Google Scholar] [CrossRef]
- Heupel, M.R.; Carlson, J.K.; Simpfendorfer, C.A. Shark nursery areas: Concepts, definition, characterization and assumptions. Mar. Ecol. Prog. Ser. 2007, 337, 287–297. [Google Scholar] [CrossRef]
- Orr, M. Potential Grey Reef Shark (Carcharhinus amblyrhynchos) Nursery on Seamounts Southwest of Guam. Micronesica 2019, 4, 1–8. Available online: http://micronesica.org/volumes/2019 (accessed on 1 February 2024).
- Lesturgie, P.; Braun, C.D.; Clua, E.; Mourier, J.; Thorrold, S.R.; Vignaud, T.; Planes, S.; Mona, S. Like a rolling stone: Colonization and migration dynamics of the grey reef shark (Carcharhinus amblyrhynchos). Ecol. Evol. 2023, 13, e9746. [Google Scholar] [CrossRef]
- Klimley, A.P. The determinants of sexual segregation in the scalloped hammerhead shark, Sphyrna lewini. Environ. Biol. Fishes 1987, 18, 27–40. [Google Scholar] [CrossRef]
- Mourier, J.; Mills, S.C.; Planes, S. Population structure, spatial distribution and life-history traits of blacktip reef sharks Carcharhinus melanopterus. J. Fish Biol. 2013, 82, 979–993. [Google Scholar] [CrossRef]
- Lester, E. Investigating the Influence of Reef Sharks on the Behavior of Mesopredatorsin Dynamic Coral Reef Seascapes. Ph.D. Thesis, The University of Western Australia, Perth, Australia, 2021; p. 169. [Google Scholar] [CrossRef]
- Heupel, M.R.; Simpfendorfer, C.A.; Olsen, E.M.; Moland, E. Consistent movement traits indicative of innate behavior in neonate sharks. J. Exp. Mar. Biol. Ecol. 2012, 432, 131–137. [Google Scholar] [CrossRef]
- Frisch, A.J.; Ireland, M.; Rizzari, J.R.; Lomstedt, O.M.; Magnenat, K.A.; Mirbach, C.E.; Hobbs, J.P.A. Reassessing the trophic role of reef sharks as apex predators on coral reefs. Coral Reefs 2016, 35, 459–472. [Google Scholar] [CrossRef]
- Casey, J.M.; Baird, A.H.; Brandl, S.J.; Hoogenboom, M.O.; Rizzari, J.R.; Frisch, A.J.; Mirbach, C.E.; Connolly, S.R. A test of trophic cascade theory: Fish and benthic assemblages across a predator density gradient on coral reefs. Oecologia 2017, 183, 161–175. [Google Scholar] [CrossRef]
- Bond, M.E.; Valentin-Albanese, J.; Babcock, E.A.; Hussey, N.E.; Heithaus, M.R.; Chapman, D.D. The trophic ecology of Caribbean reef sharks (Carcharhinus perezi) relative to other large teleost predators on an isolated coral atoll. Mar. Biol. 2018, 165, 1–13. [Google Scholar] [CrossRef]
- Roff, G.; Doropoulos, C.; Rogers, A.; Bozec, Y.M.; Krueck, N.C.; Aurellado, E.; Priest, M.; Birrel, C.; Mumby, P.J. The ecological role of sharks on coral reefs. Trends Ecol. Evol. 2016, 31, 395–407. [Google Scholar] [CrossRef] [PubMed]
- Mihari. MIAHRI−Madagascar’s Locally Managed Marine Area Network. 2016. Available online: http://mihari-network.org (accessed on 1 February 2024).



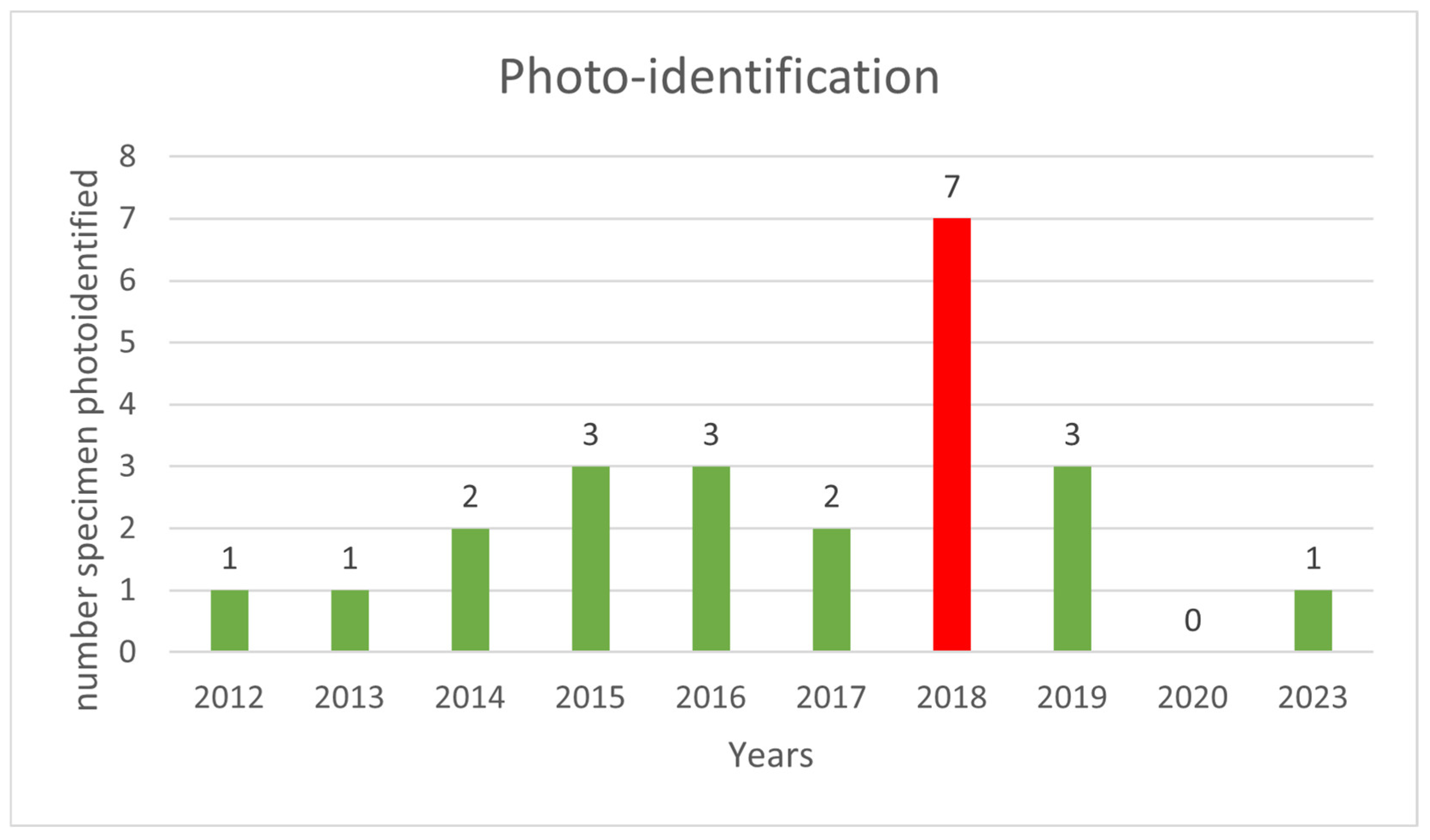
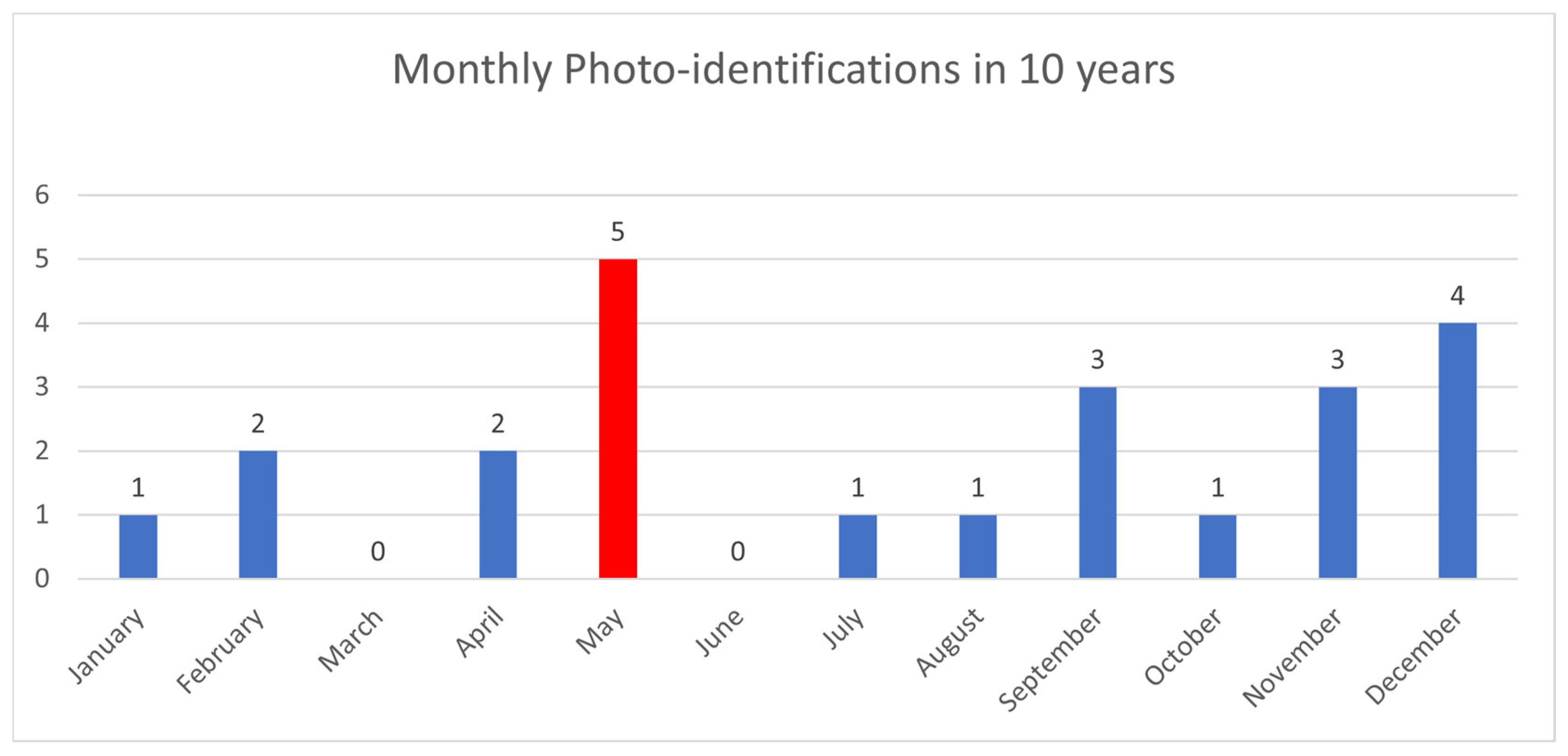
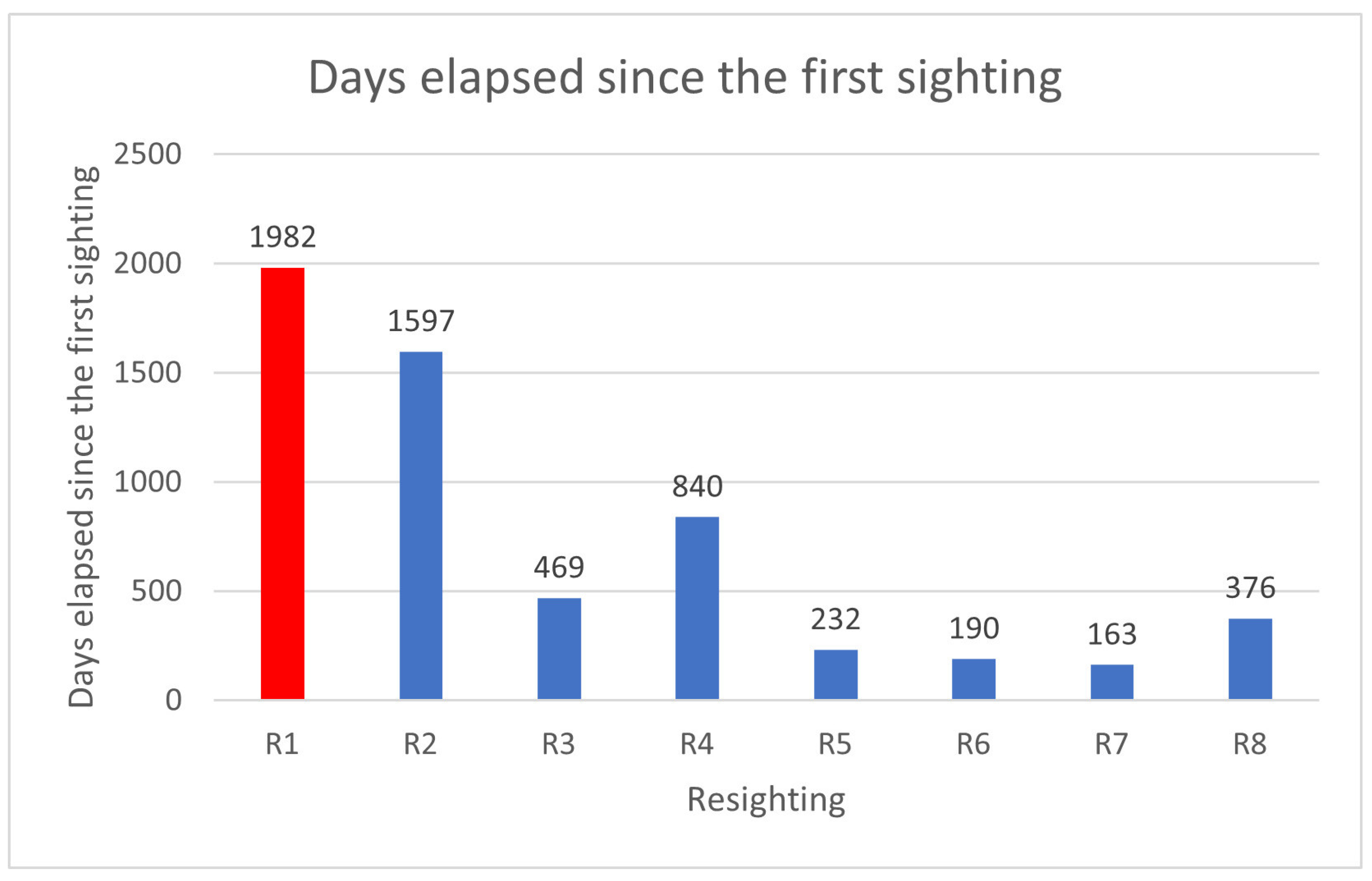
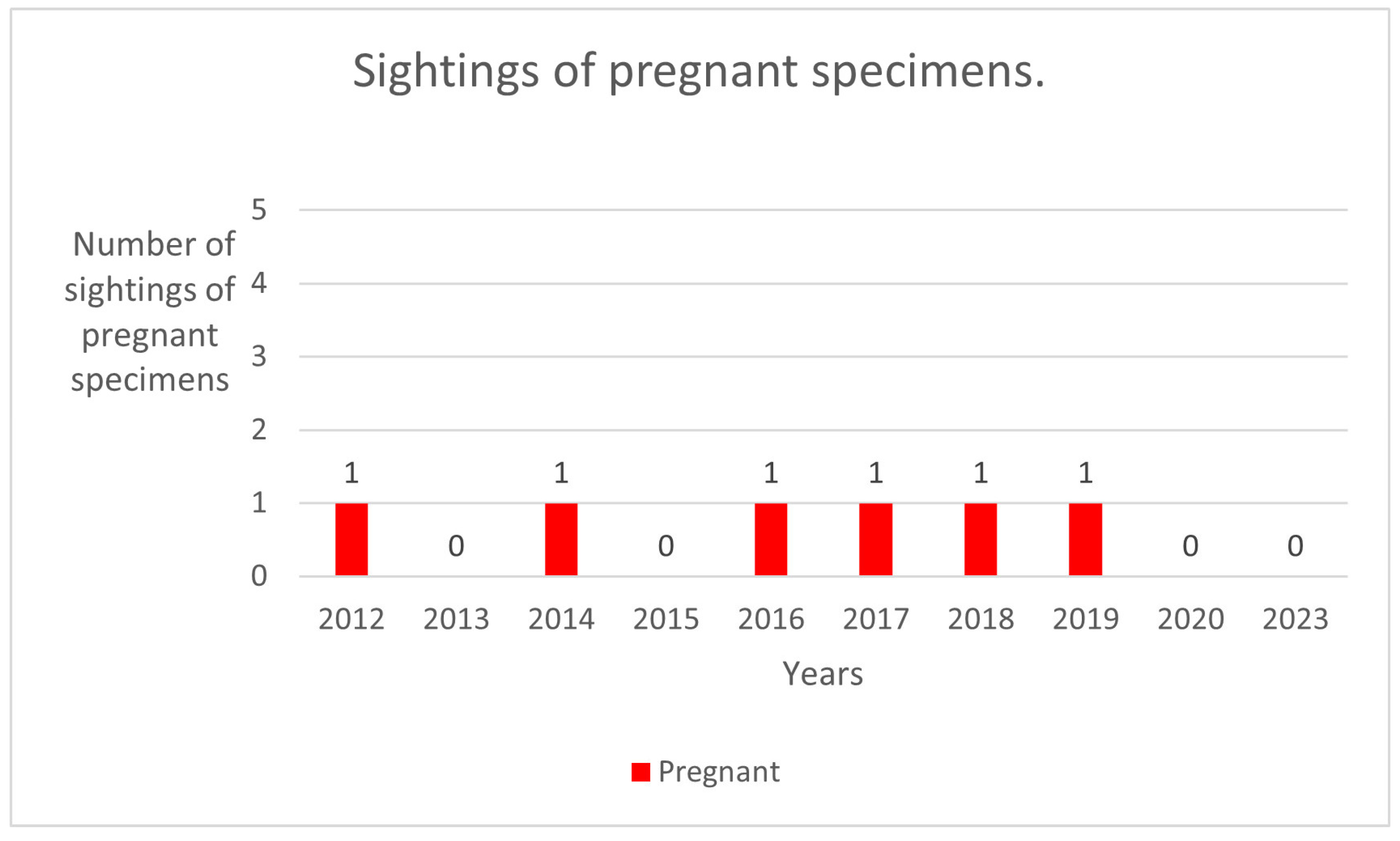
| Year | Sightings | Identifications |
|---|---|---|
| 2012 | 1 | 1 |
| 2013 | 1 | 1 |
| 2014 | 2 | 2 |
| 2015 | 5 | 3 |
| 2016 | 6 | 3 |
| 2017 | 5 | 2 |
| 2018 | 19 | 7 |
| 2019 | 13 | 3 |
| 2020 | 1 | 0 |
| 2023 | 1 | 1 |
| Total | 54 | 23 |
| N. Photo-ID | Date First Photo-ID | Date Second Photo-ID | Date Third Photo-ID |
|---|---|---|---|
| N.2-R1 | 15 December 2013 | 6 November 2018 Pregnant | 20 May 2019 |
| N.5-R2 | 2 January 2015 | 4 November 2018 | 18 May 2019 Pregnant |
| N.8-R3 | 26 May 2016 Pregnant | 7 September 2017 | |
| N.9-R4 | 17 July 2016 | 21 August 2017 Pregnant | 4 November 2018 |
| N.13-R5 | 28 September 2018 | 18 May 2019 | |
| N.16-R6 | 11 November 2018 | 20 May 2019 | |
| N.17-R7 | 6 December 2018 | 18 May 2019 | |
| N.20-R8 | 16 May 2019 | 26 June 2020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Micarelli, P.; Pireddu, M.; Persia, D.; Sanna, M.; Vicariotto, C.; Pacifico, A.; Storelli, P.; Mahrer, M.; Venanzi, E.; Reinero, F.R. Observations on an Aggregation of Grey Reef Sharks (Carcharhinus amblyrhynchos) in the Mozambique Channel Off the Coast of Nosy Be (Madagascar) and Tools for Photo-Identification—A New Aggregation Nursery Site? Biology 2024, 13, 661. https://doi.org/10.3390/biology13090661
Micarelli P, Pireddu M, Persia D, Sanna M, Vicariotto C, Pacifico A, Storelli P, Mahrer M, Venanzi E, Reinero FR. Observations on an Aggregation of Grey Reef Sharks (Carcharhinus amblyrhynchos) in the Mozambique Channel Off the Coast of Nosy Be (Madagascar) and Tools for Photo-Identification—A New Aggregation Nursery Site? Biology. 2024; 13(9):661. https://doi.org/10.3390/biology13090661
Chicago/Turabian StyleMicarelli, Primo, Marco Pireddu, Damiano Persia, Marco Sanna, Consuelo Vicariotto, Antonio Pacifico, Pietro Storelli, Makenna Mahrer, Emanuele Venanzi, and Francesca Romana Reinero. 2024. "Observations on an Aggregation of Grey Reef Sharks (Carcharhinus amblyrhynchos) in the Mozambique Channel Off the Coast of Nosy Be (Madagascar) and Tools for Photo-Identification—A New Aggregation Nursery Site?" Biology 13, no. 9: 661. https://doi.org/10.3390/biology13090661
APA StyleMicarelli, P., Pireddu, M., Persia, D., Sanna, M., Vicariotto, C., Pacifico, A., Storelli, P., Mahrer, M., Venanzi, E., & Reinero, F. R. (2024). Observations on an Aggregation of Grey Reef Sharks (Carcharhinus amblyrhynchos) in the Mozambique Channel Off the Coast of Nosy Be (Madagascar) and Tools for Photo-Identification—A New Aggregation Nursery Site? Biology, 13(9), 661. https://doi.org/10.3390/biology13090661








