Evolutionary Fate of the Opine Synthesis Genes in the Arachis L. Genomes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA and RNA Isolation
2.3. PCR
2.4. DNA Sequencing
2.5. Reverse Transcription and Real-Time qPCR
2.6. Allele Reconstruction from SRA Data
2.7. Phylogenetic Analysis
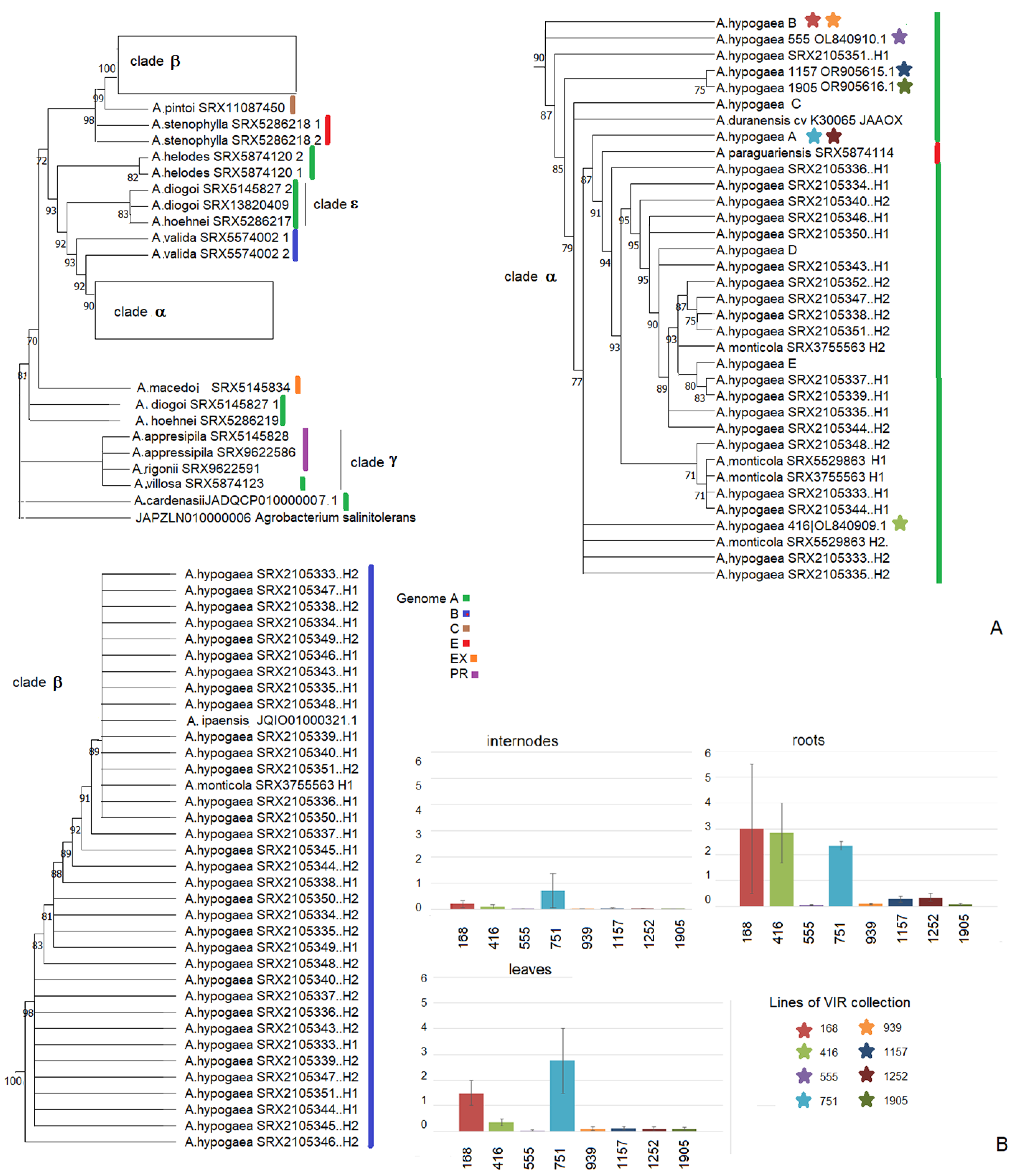
3. Results
3.1. Naturally Transgenic Species of the Arachis Genus
3.2. Intra- and Interspecific Variability of the Cus-like Gene
3.3. Expression of the Cus-like Gene
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nester, E.W. Agrobacterium: Nature’s genetic engineer. Front. Plant Sci. 2015, 5, 730. [Google Scholar] [CrossRef] [PubMed]
- White, F.F.; Garfinkel, D.J.; Huffman, G.A.; Gordon, M.P.; Nester, E.W. Sequence homologous to Agrobacterium rhizogenes T-DNA in the genomes of uninfected plants. Nature 1983, 301, 348–350. [Google Scholar] [CrossRef]
- Matveeva, T.V.; Bogomaz, D.I.; Pavlova, O.A.; Nester, E.W.; Lutova, L.A. Horizontal gene transfer from genus Agrobacterium to the plant Linaria in nature. Mol. Plant Microbe Interact. 2012, 25, 1542–1551. [Google Scholar] [CrossRef]
- Kyndt, T.; Quispe, D.; Zhai, H.; Jarret, R.; Ghislain, M.; Liu, Q.; Gheysen, G.; Kreuze, J.F. The genome of cultivated sweet potato contains Agrobacterium T-DNAs with expressed genes: An example of a naturally transgenic food crop. Proc. Natl. Acad. Sci. USA 2015, 112, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Matveeva, T.V. Agrobacterium-mediated transformation in the evolution of plants. Curr. Top. Microbiol. Immun. 2018, 418, 421–441. [Google Scholar] [CrossRef]
- Matveeva, T.V.; Otten, L. Widespread occurrence of natural transformation of plants by Agrobacterium. Plant Mol. Biol. 2019, 101, 415–437. [Google Scholar] [CrossRef]
- Matveeva, T.V. New naturally transgenic plants: 2020 up-date. Biol. Commun. 2021, 66, 36–46. [Google Scholar] [CrossRef]
- Lipatov, P.Y.; Bogomaz, F.D.; Gosudarev, K.D.; Kondrashova, S.A.; Kuchevsky, M.V.; Malyuga, N.L.; Myagkiy, E.V.; Sergeenkova, M.V.; Tverdokhlebova, V.R.; Shtina, A.D.; et al. New cellular T-DNAs in naturally transgenic plants. Ecol. Genet. 2022, 20, 40–41. [Google Scholar] [CrossRef]
- Matveeva, T.V.; Otten, L. Opine biosynthesis in naturally transgenic plants: Genes and products. Phytochemistry 2021, 189, 112813. [Google Scholar] [CrossRef]
- Kochert, G.; Stalker, H.T.; Gimenes, M.; Galgaro, L.; Lopes, C.R.; Moore, K. RFLP and cytogenetic evidence on the origin and evolution of allotetraploid domesticated peanut, Arachis hypogaea (Leguminosae). Am. J. Bot. 1996, 83, 1282–1291. [Google Scholar] [CrossRef]
- do Nascimento, E.F.M.B.; Dos Santos, B.V.; Marques, L.O.; Guimarães, P.M.; Brasileiro, A.C.; Leal-Bertioli, S.C.; Bertioli, D.J.; Araujo, A.C. The genome structure of Arachis hypogaea (Linnaeus, 1753) and an induced Arachis allotetraploid revealed by molecular cytogenetics. Comp. Cytogenet. 2018, 12, 111–140. [Google Scholar] [CrossRef]
- Tong, X.; Li, Y.; Xiang, T.; Chen, Z.; Wang, L.; Zhang, C.; Feng, M.; Wu, L. The complete genome sequence of cucumopine-type Agrobacterium rhizogenes strain K599 (NCPPB2659), a nature’s genetic engineer inducing hairy roots. Int. J. Agric. Biol. 2018, 20, 1167–1174. [Google Scholar] [CrossRef]
- Valdes Franco, J.A.; Collier, R.; Wang, Y.; Huo, N.; Gu, Y.; Thilmony, R.; Thomson, J.G. Draft genome sequence of Agrobacterium rhizogenes strain NCPPB2659. Genome Announc. 2016, 4, 10–1128. [Google Scholar] [CrossRef]
- Bouchez, D.; Tourneur, J. Organization of the agropine synthesis region of the T-DNA of the Ri plasmid from Agrobacterium rhizogenes. Plasmid 1991, 25, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, A.J.; Davis, E.W.; Tabima, J.; Belcher, M.S.; Miller, M.; Kuo, C.H.; Loper, J.E.; Grünwald, N.J.; Putnam, M.L.; Chang, J.H. Unexpected conservation and global transmission of agrobacterial virulence plasmids. Science 2020, 368, eaba5256. [Google Scholar] [CrossRef]
- Stalker, H.T. Utilizing Wild Species for Peanut Improvement. Crop Sci. 2017, 57, 1102–1120. [Google Scholar] [CrossRef]
- Chen, K.; Zhurbenko, P.M.; Danilov, L.G.; Matveeva, T.V.; Otten, L. Conservation of an Agrobacterium cT-DNA insert in Camellia section Thea reveals the ancient origin of tea plants from a genetically modified ancestor. Front. Plant Sci. 2022, 13, 997762. [Google Scholar] [CrossRef] [PubMed]
- Zhidkin, R.R.; Zhurbenko, P.M.; Bogomaz, O.; Gorodilova, E.Y.; Katsapov, I.S.; Antropov, D.N.; Matveeva, T.V. Biodiversity of rolB/C-like Natural Transgene in the Genus Vaccinium L. and Its Application for Phylogenetic Studies. Int. J. Mol. Sci. 2023, 24, 6932. [Google Scholar] [CrossRef] [PubMed]
- Murray, M.G.; Thompson, W. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv 2013, arXiv:1303.3997. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef]
- Van der Auwera, G.A.; O’Connor, B.D. Genomics in the Cloud: Using Docker, GATK, and WDL in Terra, 1st ed.; O’Reilly Media: Sebastopol, CA, USA, 2020; pp. 191–222. [Google Scholar]
- Martin, M.; Patterson, M.; Garg, S.; OFischer, S.; Pisanti, N.; WKlau, G.; Schöenhuth, A.; Marschall, T. WhatsHap: Fast and accurate read-based phasing. bioRxiv 2016, 085050. [Google Scholar] [CrossRef]
- Edgar, R.C. MUSCLE v5 enables improved estimates of phylogenetic tree confidence by ensemble bootstrapping. bioRxiv 2021, 449169. [Google Scholar] [CrossRef]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Lefort, V.; Longueville, J.-E.; Gascuel, O. SMS: Smart Model Selection in PhyML. Mol. Biol. Evol. 2017, 34, 2422–2424. [Google Scholar] [CrossRef]
- Lemoine, F.; Domelevo Entfellner, J.B.; Wilkinson, E.; Correia, D.; Dávila Felipe, M.; De Oliveira, T.; Gascuel, O. Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 2018, 556, 452–456. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 58, 3022–3027. [Google Scholar] [CrossRef]
- Chen, K.; Dorlhac de Borne, F.; Julio, E.; Obszynski, J.; Pale, P.; Otten, L. Root-specific expression of opine genes and opine accumulation in some cultivars of the naturally occurring GMO Nicotiana tabacum. Plant J. 2016, 87, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Li, Y.; Yan, H.; Chen, W.F.; Zhang, X.; Wang, E.T.; Han, X.Z.; Xie, Z.H. Agrobacterium salinitolerans sp. nov., a saline-alkaline-tolerant bacterium isolated from root nodule of Sesbania cannabina. Int. J. Syst. Evol. Microbiol. 2017, 67, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.G.; Ryder, M.M.; Tate, M.E. Agrobacterium tumefaciens TR-DNA encodes a pathway for agropine biosynthesis. Mol. Gen. Genet. 1984, 195, 466–473. [Google Scholar] [CrossRef]
- Bertioli, D.J.; Seijo, G.; Freitas, F.O.; Valls, J.F.M.; Leal-Bertioli, S.C.M.; Moretzsohn, M.C. An overview of peanut and its wild relatives. Plant Genet. Resour. 2011, 9, 134–149. [Google Scholar] [CrossRef]
- Carvalho, P.V.; Brasileiro, A.C.M.; GuimarÃ, P.M.; da Silva, J.P.; Gimenes, M.A. Evidences that polyploidization and hybridization affected resveratrol content in Arachis interspecific hybrids. J. Plant Breed. Crop Sci. 2019, 11, 265–270. [Google Scholar] [CrossRef]
- Krapovickas, A.; Gregory, W.C.; Williams, D.E.; Simpson, C.E. Taxonomy of the genus Arachis (Leguminosae). Bonplandia 2007, 16, 1–205. [Google Scholar] [CrossRef]
- Tian, X.; Shi, L.; Guo, J.; Fu, L.; Du, P.; Huang, B.; Wu, Y.; Zhang, X.; Wang, Z. Chloroplast Phylogenomic Analyses Reveal a Maternal Hybridization Event Leading to the Formation of Cultivated Peanuts. Front. Plant Sci. 2021, 12, 804568. [Google Scholar] [CrossRef] [PubMed]
- Koppolu, R.; Upadhyaya, H.D.; Dwivedi, S.L.; Hoisington, D.A.; Varshney, R.K. Genetic relationships among seven sections of genus Arachis studied by using SSR markers. BMC Plant Biol. 2010, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.; Ji, C.; Song, Q.; Zhang, W.; Zhang, X.; Zhao, K.; Chen, C.Y.; Wang, C.; He, G.; Liang, Z.; et al. Comparison of Arachis monticola with Diploid and Cultivated Tetraploid Genomes Reveals Asymmetric Subgenome Evolution and Improvement of Peanut. Adv. Sci. 2019, 7, 1901672. [Google Scholar] [CrossRef]
- Stalker, H.T.; Dhesi, J.S.; Parry, D.C.; Hahn, J.H. Cytological and Interfertility Relationships of Arachis Section Arachis. Am. J. Bot. 1991, 78, 238–246. [Google Scholar] [CrossRef]
- Singh, A.K.; Gurtu, S.; Jambunathan, R. Phylogenetic relationships in the genus Arachis based on seed protein profiles. Euphytica 1994, 74, 219–225. [Google Scholar] [CrossRef]
- Leal-Bertioli, S.C.M.; Santos, S.P.; Dantas, K.M.; Inglis, P.W.; Nielen, S.; Araujo, A.C.G.; Silva, J.P.; Cavalcante, U.; Guimaraes, P.M.; Brasileiro, A.C.M.; et al. Arachis batizocoi: A study of its relationship to cultivated peanut (A. hypogaea) and its potential for introgression of wild genes into the peanut crop using induced allotetraploids. Ann. Bot. 2014, 115, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Li, C.; Yan, C.; Zhao, X.; Yuan, C.; Sun, Q.; Shi, C.; Shan, S. Twelve complete chloroplast genomes of wild peanuts: Great genetic resources and a better understanding of Arachis phylogeny. BMC Plant Biol. 2019, 19, 504. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, W.; Wang, X.; Paterson, A.H.; Chen, H.; Yang, M.; Zhang, C.; Sun, P.; Zheng, Y.; Wang, L.; Xie, W.; et al. Reply to: Evaluating two different models of peanut’s origin. Nat. Genet. 2020, 52, 560–563. [Google Scholar] [CrossRef] [PubMed]
- Moretzsohn, M.C.; Gouvea, E.G.; Inglis, P.W.; Leal-Bertioli, S.C.M.; Valls, J.F.M.; Bertioli, D.J. A study of the relationships of cultivated peanut (Arachis hypogaea) and its most closely related wild species using intron sequences and microsatellite markers. Ann. Bot. 2013, 111, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal-Bertioli, S.C.; Ren, L.; Farmer, A.D.; Pandey, M.K.; et al. The genome sequence of segmental allotetraploid peanut Arachis hypogaea. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhuang, Y.; Liu, T.; Chen, H.; Wang, L.; Varshney, R.K.; Zhuang, W.; Wang, X. Deciphering peanut complex genomes paves a way to understand its origin and domestication. Plant Biotechnol. J. 2023, 21, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Grabiele, M.; Chalup, L.; Robledo, G.; Seijo, G. Genetic and geographic origin of domesticated peanut, as evidenced by 5S rDNA and chloroplast DNA sequences. Plant Syst. Evol. 2012, 298, 1151–1165. [Google Scholar] [CrossRef]
- Chen, K.; Otten, L. Natural Agrobacterium Transformants: Recent Results and Some Theoretical Considerations. Front. Plant Sci. 2017, 8, 1600. [Google Scholar] [CrossRef]
- Aoki, S.; Syono, K. Function of Ngrol genes in the evolution of Nicotiana glauca: Conservation of the function of NgORF13 and NgORF14 after ancient infection by an Agrobacterium rhizogenes-like ancestor. Plant Cell Physiol. 1999, 40, 222–230. [Google Scholar] [CrossRef]
- Aoki, S.; Kawaoka, A.; Sekine, M.; Ichikawa, T.; Fujita, T.; Shinmyo, A.; Syono, K. Sequence of the cellular T-DNA in the untransformed genome of Nicotiana glauca that is homologous to ORFs 13 and 14 of the Ri plasmid and analysis of its expression in genetic tumors of N. glauca × N. langsdorffii. Mol. Gen. Genet. 1994, 243, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.D.; Ichikawa, T.; Meins, F. Horizontal gene transfer: Regulated expression of tobacco homologue of the Agrobacterium rhizogenes rolC gene. Mol. Gen. Genet. 1995, 249, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Messens, E.; Lenaerts, A.; Hedges, R.W.; Van Montagu, M. Agrocinopine A, a phosphorylated opine is secreted from crown gall cells. EMBO J. 1985, 4, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Messens, E.; Lenaerts, A.; Van Montagu, M.; Hedges, R.W. Genetic basis for opine secretion from crown gall tumour cells. Mol. Gen. Genet. 1985, 199, 344–348. [Google Scholar] [CrossRef]
- Oger, P.M.; Mansouri, H.; Nesme, X.; Dessaux, Y. Engineering root exudation of Lotus toward the production of two novel carbon compounds leads to the selection of distinct microbial populations in the rhizosphere. Microb. Ecol. 2003, 47, 96–103. [Google Scholar] [CrossRef]
- Mondy, S.; Lenglet, A.; Beury-Cirou, A.; Libanga, C.; Ratet, P.; Faure, D.; Dessaux, Y. An increasing opine carbon bias in artificial exudation systems and genetically modified plant rhizospheres leads to an increasing reshaping of bacterial populations. Mol. Ecol. 2014, 23, 4846–4861. [Google Scholar] [CrossRef]
- Moore, L.W.; Chilton, W.S.; Canfield, M.L. Diversity of opines and opine-catabolizing bacteria isolated from naturally occurring crown gall tumors. Appl. Environ. Microbiol. 1997, 63, 201–207. [Google Scholar] [CrossRef]
| Section [16] | Genome [16] | Species | NGS Data | Transgenes Found |
SRR Accession Number(s) |
|---|---|---|---|---|---|
| Arachis | K | A. batizocoi Krapov. & W.C. Greg. | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX5286222, SRX5286221 |
| A | A. cardenasii Krapov. & W.C. Greg | cT-DNA was found in WGS data, and in new SRA data, coverage is sufficient to assemble full-length genes | cus | DRX231922, SRX5574006 | |
| A | A. correntina (Burkart) Krapov. & W.C. Greg. | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX5874118 | |
| A | A. diogoi Hoehne | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX1382040, SRX51458279 | |
| A | A. duranensis Krapov. & W.C. Greg. | cT-DNA was described earlier, and in new SRA data, coverage is sufficient to assemble full-length genes | cus | SRX5574003 | |
| D | A. glandulifera Stalker, | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX15154171 | |
| A | A. helodes Mart. ex Krapov. & Rigoni | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX5874120 | |
| A | A. hoehnei Krapov. & W.C. Greg. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus | SRX5286219 | |
| AB | A. hypogaea L. | cT-DNA was described earlier, and in new SRA data, coverage is sufficient to assemble full-length genes | cus, mas2′ | SRX2105338, SRX2105345, SRX2105341, SRX2105349, SRX2105334, SRX2105351, SRX2105347, SRX2105352, SRX2105333, SRX2105343, SRX2105344, SRX2105342, SRX2105348, SRX2105335, SRX2105337, SRX2105350, SRX2105340, SRX2105346, SRX2105336, SRX2105339, SRX4393155, SRX4393154, SRX4393152, SRX4393153 | |
| B | A. ipaensis Krapov. & W.C. Greg. | cT-DNA was described earlier, and in new SRA data, coverage is sufficient to assemble full-length genes | cus, mas2′ | ||
| B | A. magna Krapov. et al. | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus, mas2′ | SRX5286220, SRX5573999 | |
| AB | A. monticola Krapov. & Rigoni | cT-DNA was described earlier, and in new SRA data, coverage is sufficient to assemble full-length genes | cus, mas2′ | SRX3755563, SRX3802089 | |
| A | A. stenosperma Krapov. & W.C. Greg. | cT-DNA was described earlier, and in new SRA data, coverage is sufficient to assemble full-length genes | cus | SRX5286218 | |
| F | A. trinitensis Krapov. & W.C. Greg. | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX15154170 | |
| B | A. valida Krapov. & W.C. Greg. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus, mas2′ | SRX5574002 | |
| A | A. villosa Benth. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus | SRX5874123 | |
| Erectoides | E | A.paraguariensis Chodat & Hassl | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus | SRX5874114 |
| E | A. stenophylla Krapov. & W.C. Greg. | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus | SRX5286218 | |
| Extranervosae | EX | A. macedoi Krapov. & W.C. Greg. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus, ags | SRX5145834 |
| Procumbentes | PR | A.appressipila Krapov. & W. C. Greg. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus, mas1′ | SRX5145828 |
| PR | A. rigonii Krapov. & W.C. Greg. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus, mas1′ | SRX9622591 | |
| Caulorrhizae | C | A. pintoi Krapov. & W.C. Greg. | cT-DNA is present in the genome, and coverage is sufficient to assemble a full-length gene | cus | SRX11087450 |
| Heteranthae | H | A. pusilla Benth. | cT-DNA is present in the genome, but coverage is not sufficient for assembly | cus, mas2′, ags | SRX5145826 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogomaz, O.D.; Bemova, V.D.; Mirgorodskii, N.A.; Matveeva, T.V. Evolutionary Fate of the Opine Synthesis Genes in the Arachis L. Genomes. Biology 2024, 13, 601. https://doi.org/10.3390/biology13080601
Bogomaz OD, Bemova VD, Mirgorodskii NA, Matveeva TV. Evolutionary Fate of the Opine Synthesis Genes in the Arachis L. Genomes. Biology. 2024; 13(8):601. https://doi.org/10.3390/biology13080601
Chicago/Turabian StyleBogomaz, Olesja D., Victoria D. Bemova, Nikita A. Mirgorodskii, and Tatiana V. Matveeva. 2024. "Evolutionary Fate of the Opine Synthesis Genes in the Arachis L. Genomes" Biology 13, no. 8: 601. https://doi.org/10.3390/biology13080601
APA StyleBogomaz, O. D., Bemova, V. D., Mirgorodskii, N. A., & Matveeva, T. V. (2024). Evolutionary Fate of the Opine Synthesis Genes in the Arachis L. Genomes. Biology, 13(8), 601. https://doi.org/10.3390/biology13080601






