Effect of Nitrogen Application Rate on the Relationships between Multidimensional Plant Diversity and Ecosystem Production in a Temperate Steppe
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Community Survey and Plant Biomass Measurement
2.4. Plant Diversity Metrics
2.5. Statistical Analysis
3. Results
3.1. Biomass Production along the N Addition Gradient
3.2. Multiple Plant Diversity along the N Addition Gradient
3.3. Relationship between Plant Diversity and Biomass Production along the N Addition Gradient
4. Discussion
4.1. N Deposition Affects Biomass Production
4.2. Effect of N Addition Rate on Multidimensional Plant Diversity
4.3. Effect of N Addition Rate on the Relationship between Multidimensional Plant Diversity and Biomass Production
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grace, J.; Anderson, T.; Seabloom, E.; Borer, E.; Adler, P.; Harpole, W.; Hautier, Y.; Hillebrand, H.; Lind, E.; Pärtel, M.; et al. Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 2016, 529, 390–393. [Google Scholar] [CrossRef]
- Tilman, D.; Isbell, F.; Cowles, J. Biodiversity and ecosystem functioning. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 471–493. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Gross, K.; Fritschie, K.; Flombaum, P.; Fox, J.W.; Rixen, C.; van Ruijven, J.; Reich, P.B.; Scherer-Lorenzen, M.; Wilsey, B.J. Biodiversity simultaneously enhances the production and stability of community biomass, but the effects are independent. Ecol. Ecol. Soc. Am. 2013, 94, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Craven, D.; Isbell, F.; Manning, P.; Connolly, J.; Bruelheide, H.; Ebeling, A.; Roscher, C.; van Ruijven, J.; Weigelt, A.; Wilsey, B.; et al. Plant diversity effects on grassland productivity are robust to both nutrient enrichment and drought. Philos. T. R. Soc. B 2016, 371, 20150277. [Google Scholar] [CrossRef] [PubMed]
- Isbell, F.; Reich, P.B.; Tilman, D.; Hobbie, S.E.; Polasky, S.; Binder, S. Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc. Natl. Acad. Sci. USA 2013, 110, 11911–11916. [Google Scholar] [CrossRef]
- Stevens, C.J.; David, T.I.; Storkey, J. Atmospheric nitrogen deposition in terrestrial ecosystems: Its impact on plant communities and consequences across trophic levels. Funct. Ecol. 2018, 32, 1757–1769. [Google Scholar] [CrossRef]
- Midolo, G.; Alkemade, R.; Schipper, A.M.; Benítez-López, A.; Perring, M.P.; De Vries, W. Impacts of nitrogen addition on plant species richness and abundance: A global meta-analysis. Glob. Ecol. Biogeogr. 2019, 28, 398–413. [Google Scholar] [CrossRef]
- Chen, Q.; Blowes, S.A.; Ladouceur, E.; Harpole, W.S.; Seabloom, E.W.; Tognetti, P.M.; MacDougall, A.; Daleo, P.; Hautier, Y.; Stevens, C.; et al. Nutrient addition in grasslands worldwide reveals proportional plant diversity decline across spatial scales but little change in beta diversity. BioRxiv 2024. [Google Scholar] [CrossRef]
- Ma, F.; Song, B.; Quan, Q.; Zhang, F.; Wang, J.; Zhou, Q.; Niu, S. Light competition and biodiversity loss cause saturation response of aboveground net primary productivity to nitrogen enrichment. JGR BiogeoSci. 2020, 125, e2019JG005556. [Google Scholar] [CrossRef]
- Namuhan; Wang, J.; Yang, G.; Song, Y.; Yu, Y.; Wang, J.; Wang, X.; Shi, Y.; Shen, Y.; Han, X.; et al. Mechanisms of biodiversity loss under nitrogen enrichment: Unveiling a shift from light competition to cation toxicity. N. Phytol. 2024. early view. [Google Scholar] [CrossRef]
- Lu, C.; Tian, H.; Liu, M.; Ren, W.; Xu, X.; Chen, G.; Zhang, C. Effect of nitrogen deposition on China’s terrestrial carbon uptake in the context of multifactor environmental changes. Ecol. Appl. 2012, 22, 53–75. [Google Scholar] [CrossRef] [PubMed]
- Hautier, Y.; Seabloom, E.W.; Borer, E.T.; Adler, P.B.; Harpole, W.S.; Hillebrand, H.; Lind, E.M.; MacDougall, A.S.; Stevens, C.J.; Bakker, J.D.; et al. Eutrophication weakens stabilizing effects of diversity in natural grasslands. Nature 2014, 508, 521–525. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Barfknecht, D.F.; Gibson, D.J. Disturbance effects on productivity-plant diversity relationships from a 22-year-old successional field. J. Veg. Sci. 2021, 32, e12970. [Google Scholar] [CrossRef]
- Wang, X.; Yan, X.; Huang, K.; Luo, X.; Zhang, Y.; Zhou, L.; Yang, F.; Xu, X.; Zhou, X.; Niu, K.; et al. Nitrogen enrichment and warming shift community functional composition via distinct mechanisms: The role of intraspecific trait variability and species turnover. Funct. Ecol. 2022, 36, 1230–1242. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Li, M.; Xiao, R.; Rao, K.; Wang, J.; Zhang, T.; Guo, J. Community composition, structure and productivity in response to nitrogen and phosphorus additions in a temperate meadow. Sci. Total Environ. 2019, 654, 863–871. [Google Scholar] [CrossRef]
- Barber, N.A.; Farrell, A.K.; Blackburn, R.C.; Bauer, J.T.; Groves, A.M.; Brudvig, L.A.; Jones, H.P. Grassland restoration characteristics influence phylogenetic and taxonomic structure of plant communities and suggest assembly mechanisms. J. Ecol. 2019, 107, 2105–2120. [Google Scholar] [CrossRef]
- Le Bagousse-Pinguet, Y.; Soliveres, S.; Gross, N.; Torices, R.; Berdugo, M.; Maestre, F.T. Phylogenetic, functional, and taxonomic richness have both positive and negative effects on ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2019, 116, 8419–8424. [Google Scholar] [CrossRef]
- Cadotte, M.W. Functional traits explain ecosystem function through opposing mechanisms. Ecol. Lett. 2017, 20, 989–996. [Google Scholar] [CrossRef]
- Liu, L.; Sun, K.; Sun, R.; Ma, Q.; Wang, Y.; Jia, B.; Zhou, G.; Xu, Z.; Zhang, F. Effects of nitrogen deposition with phosphorus addition on desert steppe plant communities. Agric. Ecosyst. Environ. 2024, 366, 108954. [Google Scholar] [CrossRef]
- Song, L.; Bao, X.; Liu, X.; Zhang, Y.; Christie, P.; Fangmeier, A.; Zhang, F.J.B. Nitrogen enrichment enhances the dominance of grasses over forbs in a temperate steppe ecosystem. Biogeosciences 2011, 8, 2341–2350. [Google Scholar] [CrossRef]
- Niu, S.; Wu, M.; Han, Y.; Xia, J.; Li, L.; Wan, S. Water-mediated responses of ecosystem carbon fluxes to climatic change in a temperate steppe. N. Phytol. 2008, 177, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Lu, P.; Ma, P.; Zhou, H.; Yang, M.; Zhai, X.; Chen, M.; Wang, H.; Li, W.; Bai, W.; et al. Processes at the soil–root interface determine the different responses of nutrient limitation and metal toxicity in forbs and grasses to nitrogen enrichment. J. Ecol. 2021, 109, 927–938. [Google Scholar] [CrossRef]
- Li, D. hillR: Taxonomic, functional, and phylogenetic diversity and similarity through Hill Numbers. J. Open Source Softw. 2018, 3, 1041. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Kembel, S.W.; Cowan, P.D.; Helmus, M.R.; Cornwell, W.K.; Morlon, H.; Ackerly, D.D.; Blomberg, S.P.; Webb, C.O. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 2010, 26, 1463–1464. [Google Scholar] [CrossRef] [PubMed]
- Paradis, E.; Schliep, K. ape: R package version 5.7.1 an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics 2019, 35. [Google Scholar] [CrossRef] [PubMed]
- Zanne, A.E.; Tank, D.C.; Cornwell, W.K.; Eastman, J.M.; Smith, S.A.; FitzJohn, R.G.; McGlinn, D.J.; O’Meara, B.C.; Moles, A.T.; Reich, P.B.; et al. Three keys to the radiation of angiosperms into freezing environments. Nature 2014, 506, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Qian, H.V. PhyloMaker: An R package that can generate very large phylogenies for vascular plants. Ecography 2019, 42, 1353–1359. [Google Scholar] [CrossRef]
- Webb, C.O. Exploring the phylogenetic structure of ecological communities: An example for rain forest trees. Am. Nat. 2000, 156, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Peng, Y.; Li, F.; Yang, G.; Wang, J.; Yu, J.; Zhou, G.; Yang, Y. Trait identity and functional diversity co-drive response of ecosystem productivity to nitrogen enrichment. J. Ecol. 2019, 107, 2402–2414. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P. A distance-based framework for measuring functional diversity from multiple traits. Ecology 2010, 91, 299–305. [Google Scholar] [CrossRef]
- Laliberté, E.; Legendre, P.; Shipley, B. FD: Measuring Functional Diversity from Multiple Traits, and Other Tools for Functional Ecology. R Package Version 1.0.12.3. 2014, Volume 1, 01–12. Available online: https://CRAN.R-project.org/package=FD (accessed on 13 February 2024).
- Pagel, M. Inferring the historical patterns of biological evolution. Nature 1999, 401, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar] [CrossRef]
- Wood, S.N. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Crawley, M.J. The R Book, 2nd ed.; John Wiley & Sons: San Jose, CA, USA, 2012; pp. 537–554. [Google Scholar] [CrossRef]
- Tatarko, A.R.; Knops, J.M.H. Nitrogen addition and ecosystem functioning: Both species abundances and traits alter community structure and function. Ecosphere 2018, 9, e02087. [Google Scholar] [CrossRef]
- Zhang, Y.; He, N.; Li, M.; Yan, P.; Yu, G. Community chlorophyll quantity determines the spatial variation of grassland productivity. Sci. Total Environ. 2021, 801, 149567. [Google Scholar] [CrossRef] [PubMed]
- Harpole, W.S.; Sullivan, L.L.; Lind, E.M.; Firn, J.; Adler, P.B.; Borer, E.T.; Chase, J.; Fay, P.A.; Hautier, Y.; Hillebrand, H.; et al. Addition of multiple limiting resources reduces grassland diversity. Nature 2016, 537, 93–96. [Google Scholar] [CrossRef]
- Xu, Z.; Li, M.-H.; Zimmermann, N.E.; Li, S.-P.; Li, H.; Ren, H.; Sun, H.; Han, X.; Jiang, Y.; Jiang, L. Plant functional diversity modulates global environmental change effects on grassland productivity. J. Ecol. 2018, 106, 1941–1951. [Google Scholar] [CrossRef]
- Tang, Z.; Deng, L.; An, H.; Yan, W.; Shangguan, Z. The effect of nitrogen addition on community structure and productivity in grasslands: A meta-analysis. Ecol. Eng. 2017, 99, 31–38. [Google Scholar] [CrossRef]
- Baert, J.M.; Eisenhauer, N.; Janssen, C.R.; De Laender, F. Biodiversity effects on ecosystem functioning respond unimodally to environmental stress. Ecol. Lett. 2018, 21, 1191–1199. [Google Scholar] [CrossRef]
- He, K.; Huang, Y.; Qi, Y.; Sheng, Z.; Chen, H. Effects of nitrogen addition on vegetation and soil and its linkages to plant diversity and productivity in a semi-arid steppe. Sci. Total Environ. 2021, 778, 146299. [Google Scholar] [CrossRef] [PubMed]
- DeMalach, N.; Zaady, E.; Kadmon, R. Light asymmetry explains the effect of nutrient enrichment on grassland diversity. Ecol. Lett. 2017, 20, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Chi, Y.; Yang, X.; Li, W.; Lan, Z.; Bai, Y. Direct and indirect effects of nitrogen enrichment and grazing on grassland productivity through intraspecific trait variability. J. Appl. Ecol. 2021, 59, 598–610. [Google Scholar] [CrossRef]
- Grime, J.P. Benefits of plant diversity to ecosystems: Immediate, filter and founder effects. J. Ecol. 1998, 86, 902–910. [Google Scholar] [CrossRef]
- Sonkoly, J.; Kelemen, A.; Valko, O.; Deak, B.; Kiss, R.; Toth, K.; Miglecz, T.; Tothmeresz, B.; Torok, P. Both mass ratio effects and community diversity drive biomass production in a grassland experiment. Sci. Rep. 2019, 9, 1848. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Lu, P.; Zhai, X.; Zhang, R.; Zheng, Y.; Wang, H.; Nie, B.; Bai, W.; Niu, S.; Shi, P.; et al. An integrated belowground trait-based understanding of nitrogen-driven plant diversity loss. Glob. Chang. Biol. 2022, 28, 3651–3664. [Google Scholar] [CrossRef] [PubMed]
- Band, N.; Kadmon, R.; Mandel, M.; DeMalach, N. Assessing the roles of nitrogen, biomass, and niche dimensionality as drivers of species loss in grassland communities. Proc. Natl. Acad. Sci. USA 2022, 119, e2112010119. [Google Scholar] [CrossRef] [PubMed]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef]
- Yue, K.; Jarvie, S.; Senior, A.M.; Van Meerbeek, K.; Peng, Y.; Ni, X.Y.; Wu, F.Z.; Svenning, J.C. Changes in plant diversity and its relationship with productivity in response to nitrogen addition, warming and increased rainfall. Oikos 2020, 129, 939–952. [Google Scholar] [CrossRef]
- Liu, X.; Shi, X.; Zhang, S. Soil abiotic properties and plant functional diversity co-regulate the impacts of nitrogen addition on ecosystem multifunctionality in an alpine meadow. Sci. Total Environ. 2021, 780, 146476. [Google Scholar] [CrossRef]
- Tian, Q.; Yang, L.; Ma, P.; Zhou, H.; Liu, N.; Bai, W.; Wang, H.; Ren, L.; Lu, P.; Han, W.; et al. Below-ground-mediated and phase-dependent processes drive nitrogen-evoked community changes in grasslands. J. Ecol. 2020, 108, 1874–1887. [Google Scholar] [CrossRef]
- Clark, C.M.; Cleland, E.E.; Collins, S.L.; Fargione, J.E.; Gough, L.; Gross, K.L.; Pennings, S.C.; Suding, K.N.; Grace, J.B. Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol. Lett. 2007, 10, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liu, J.; Wang, J.; Ding, X. Effects of short-term nitrogen additions on biomass and soil phytochemical cycling in alpine grasslands of Tianshan, China. Plants 2024, 13, 1103. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, L.; Wisz, M.S.; Strandberg, B.; Damgaard, C. Herbicide and fertilizers promote analogous phylogenetic responses but opposite functional responses in plant communities. Environ. Res. Lett. 2014, 9, 024016. [Google Scholar] [CrossRef]
- Blomberg, S.P.; Garland, T., Jr.; Ives, A.R. Testing for phylogenetic signal in comparative data: Behavioral traits are more labile. Evolution 2003, 57, 717–745. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Dong, S.; Shen, H.; Xu, Y.; Gao, X.; Han, Y.; Zhang, J.; Yang, M.; Li, Y.; Zhao, Z.; et al. Nitrogen addition gradient can regulate the environmental filtering of soil potassium or phosphorus in shaping the community assembly of alpine meadow. Ecol. Indic. 2020, 109, 105774. [Google Scholar] [CrossRef]
- Li, S.; Dong, S.; Shen, H.; Han, Y.; Zhang, J.; Xu, Y.; Gao, X.; Yang, M.; Li, Y.; Zhao, Z.; et al. Different responses of multifaceted plant diversities of alpine meadow and alpine steppe to nitrogen addition gradients on Qinghai-Tibetan Plateau. Sci. Total Environ. 2019, 688, 1405–1412. [Google Scholar] [CrossRef]
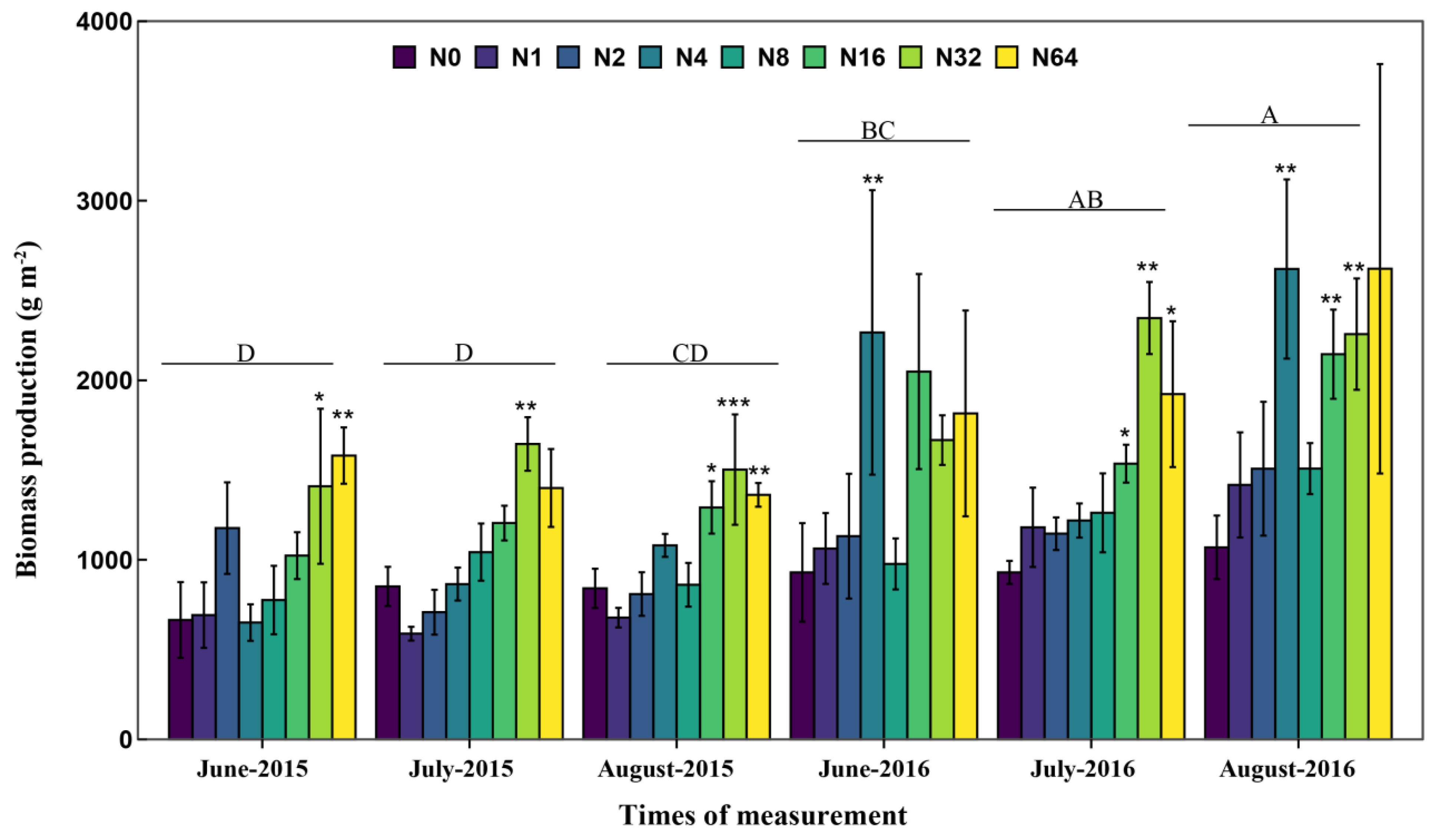

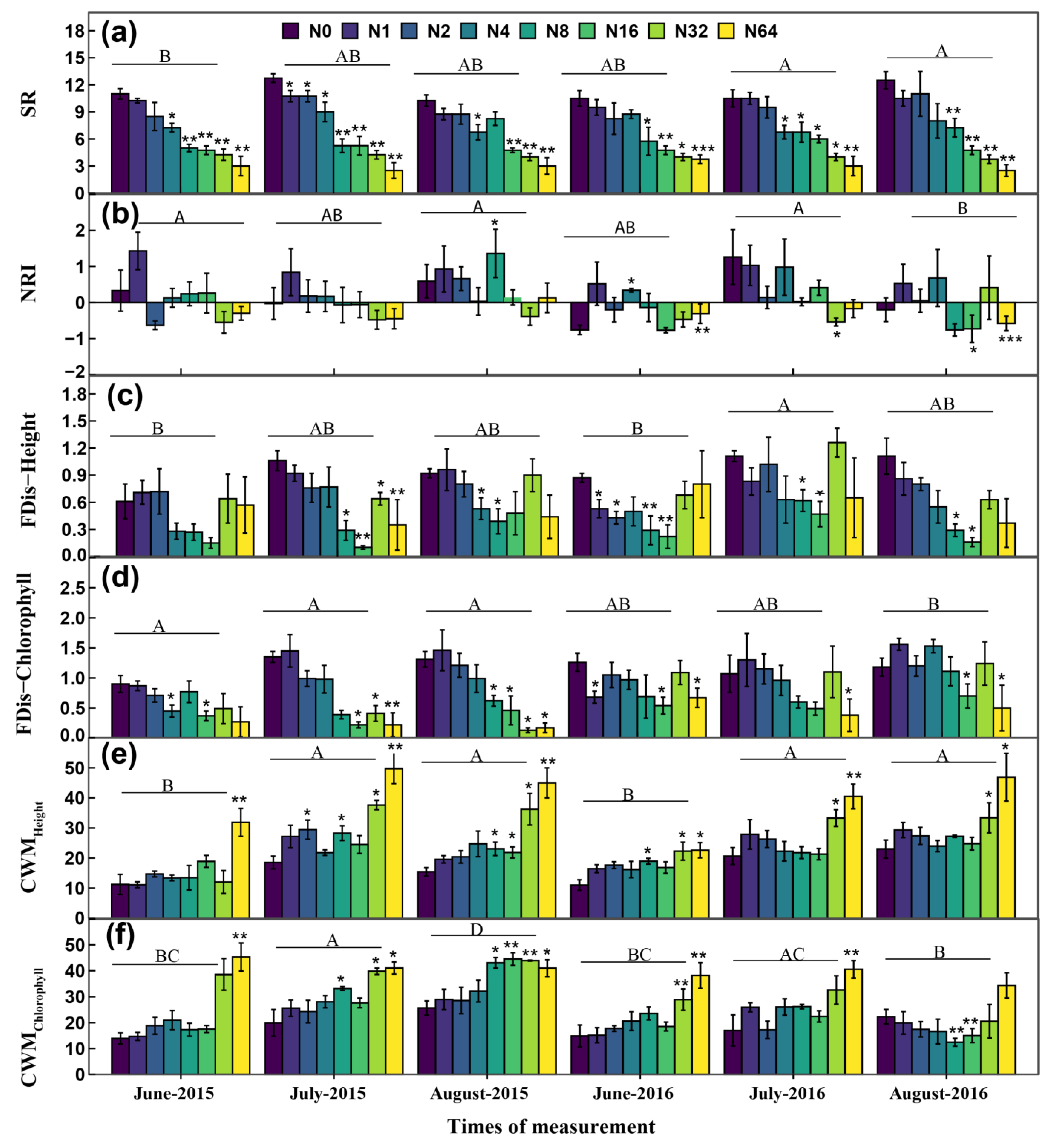
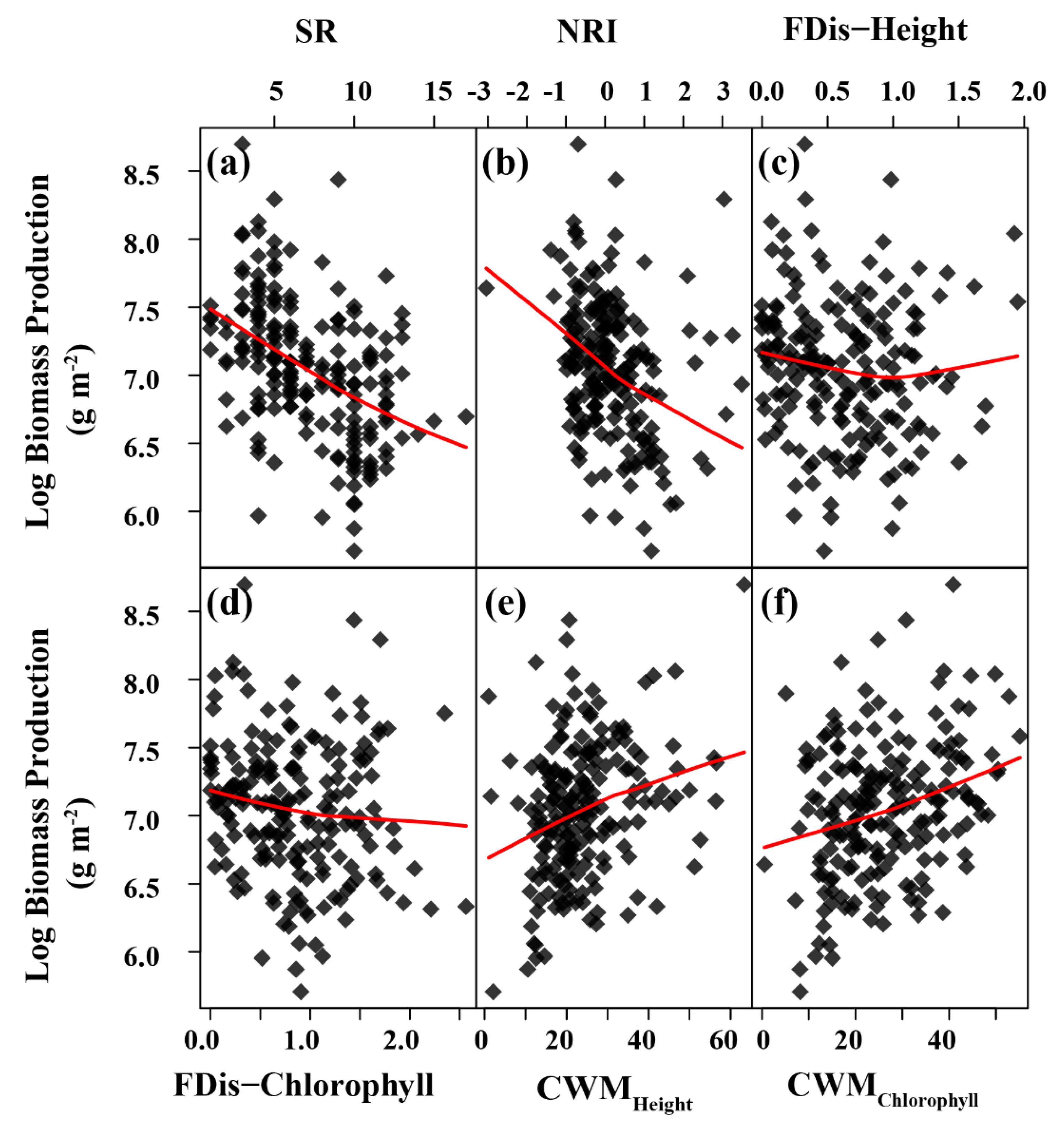
| Source | df | SR | NRI | CWMHeight | CWMChlorophyll | FDis-Height | FDis-Chlorophyll | Production |
|---|---|---|---|---|---|---|---|---|
| N | 7 | 32.79 *** | 4.08 ** | 11.66 *** | 15.38 *** | 4.49 ** | 5.36 *** | 8.66 *** |
| Time | 5 | 1.45 | 3.04 * | 44.23 *** | 24.17 *** | 4.18 ** | 7.70 *** | 15.56 *** |
| N × Time | 35 | 1.06 | 1.07 | 2.11 ** | 1.74 * | 0.87 | 1.78 * | 0.97 |
| Response | Term | Statistics | |||
|---|---|---|---|---|---|
| Biomass Production | Taxonomic diversity | df | F | p | AIC |
| s(SR) | 1 | 0.95 | 0.32 | 177.22 | |
| s(qSR) | 1 | 2.27 | 0.13 | ||
| N | 7 | 2.49 | <0.05 | ||
| Time | 5 | 16.27 | <0.001 | ||
| Phylogenetic pattern | df | F | p | AIC | |
| s(NRI) | 1 | 0.27 | 0.61 | 179.17 | |
| N | 7 | 7.74 | <0.001 | ||
| Time | 5 | 15.15 | <0.001 | ||
| Functional Diversity | df | F | p | ||
| s(FDis-Height) | 1 | 1.68 | 0.90 | 178.80 | |
| s(qFDis-Height) | 1 | 1.75 | 0.18 | ||
| N | 7 | 7.10 | <0.001 | ||
| Time | 5 | 15.72 | <0.001 | ||
| s(FDis-Chlorophyll) | 1 | 5.26 | <0.05 | 170.20 | |
| FDis-Chlorophyll × N | 7 | 3.28 | <0.01 | ||
| N | 7 | 4.72 | <0.001 | ||
| Time | 5 | 15.00 | <0.001 | ||
| Functional composition | df | F | p | AIC | |
| s(CWMHeight) | 1 | 5.17 | 0.05 | 183.57 | |
| s(qCWMHeight) | 1 | 4.84 | <0.05 | ||
| CWMHeight × N | 7 | 2.50 | <0.05 | ||
| qCWMHeight × N | 7 | 2.27 | <0.05 | ||
| N | 7 | 3.21 | <0.01 | ||
| Time | 5 | 14.63 | <0.001 | ||
| s(CWMChlorophyll) | 1 | 32.65 | <0.001 | 141.64 | |
| CWMChlorophyll × N | 5 | 8.35 | <0.001 | ||
| N | 7 | 2.22 | <0.05 | ||
| Time | 5 | 9.32 | <0.001 | ||
| N × Time | 35 | 2.33 | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debaba, G.H.; Li, K.; Wang, X.; Wang, Y.; Bai, W.; Li, G. Effect of Nitrogen Application Rate on the Relationships between Multidimensional Plant Diversity and Ecosystem Production in a Temperate Steppe. Biology 2024, 13, 554. https://doi.org/10.3390/biology13080554
Debaba GH, Li K, Wang X, Wang Y, Bai W, Li G. Effect of Nitrogen Application Rate on the Relationships between Multidimensional Plant Diversity and Ecosystem Production in a Temperate Steppe. Biology. 2024; 13(8):554. https://doi.org/10.3390/biology13080554
Chicago/Turabian StyleDebaba, Gossaye Hailu, Kunyu Li, Xiaowei Wang, Yanan Wang, Wenming Bai, and Guoyong Li. 2024. "Effect of Nitrogen Application Rate on the Relationships between Multidimensional Plant Diversity and Ecosystem Production in a Temperate Steppe" Biology 13, no. 8: 554. https://doi.org/10.3390/biology13080554
APA StyleDebaba, G. H., Li, K., Wang, X., Wang, Y., Bai, W., & Li, G. (2024). Effect of Nitrogen Application Rate on the Relationships between Multidimensional Plant Diversity and Ecosystem Production in a Temperate Steppe. Biology, 13(8), 554. https://doi.org/10.3390/biology13080554






