Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
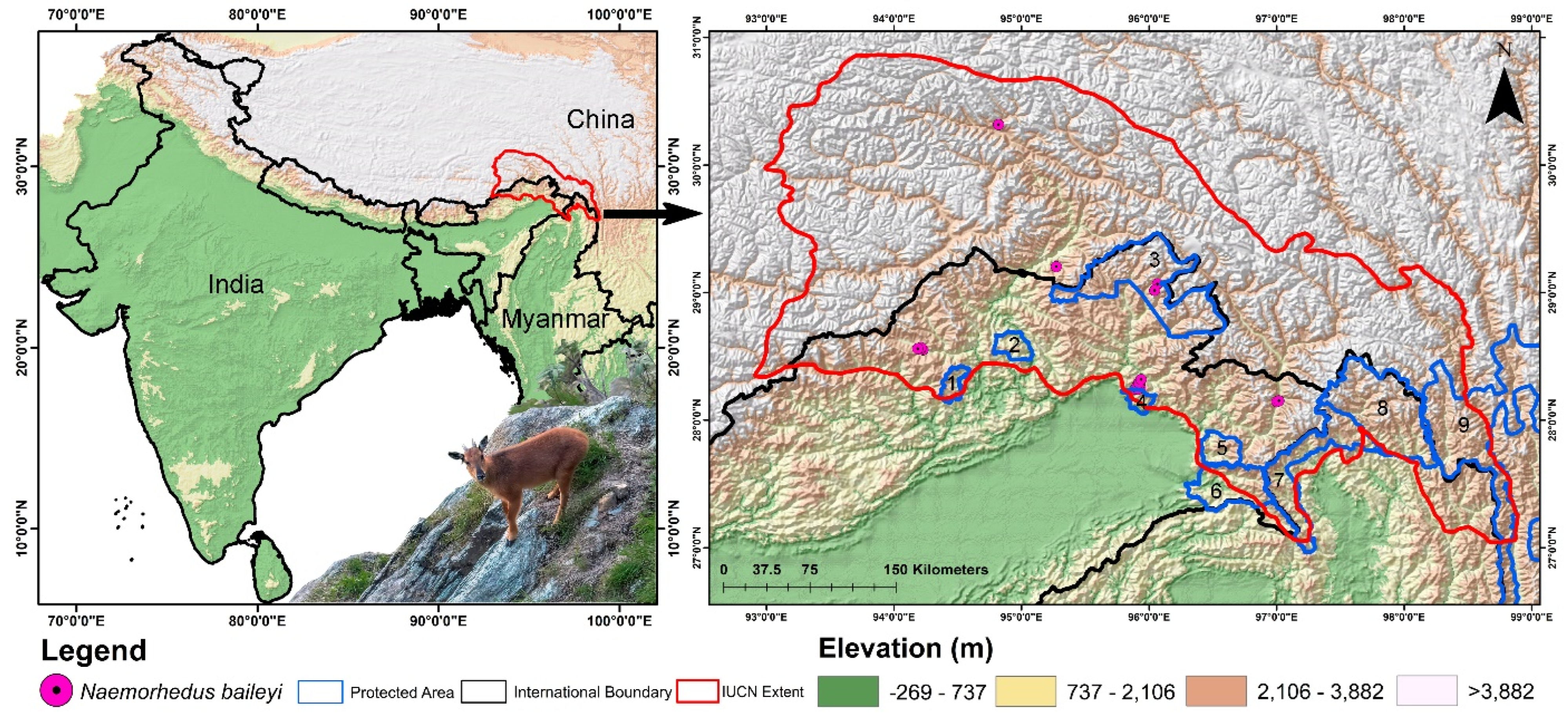
2.1. Study Area and Species Occurrence Data
2.2. Selection of Predictors for the Ensemble Model
2.3. SDM Utilizing Ensemble Approach
2.4. Assessment of Habitat Shape Geometry and Connectivity
3. Results
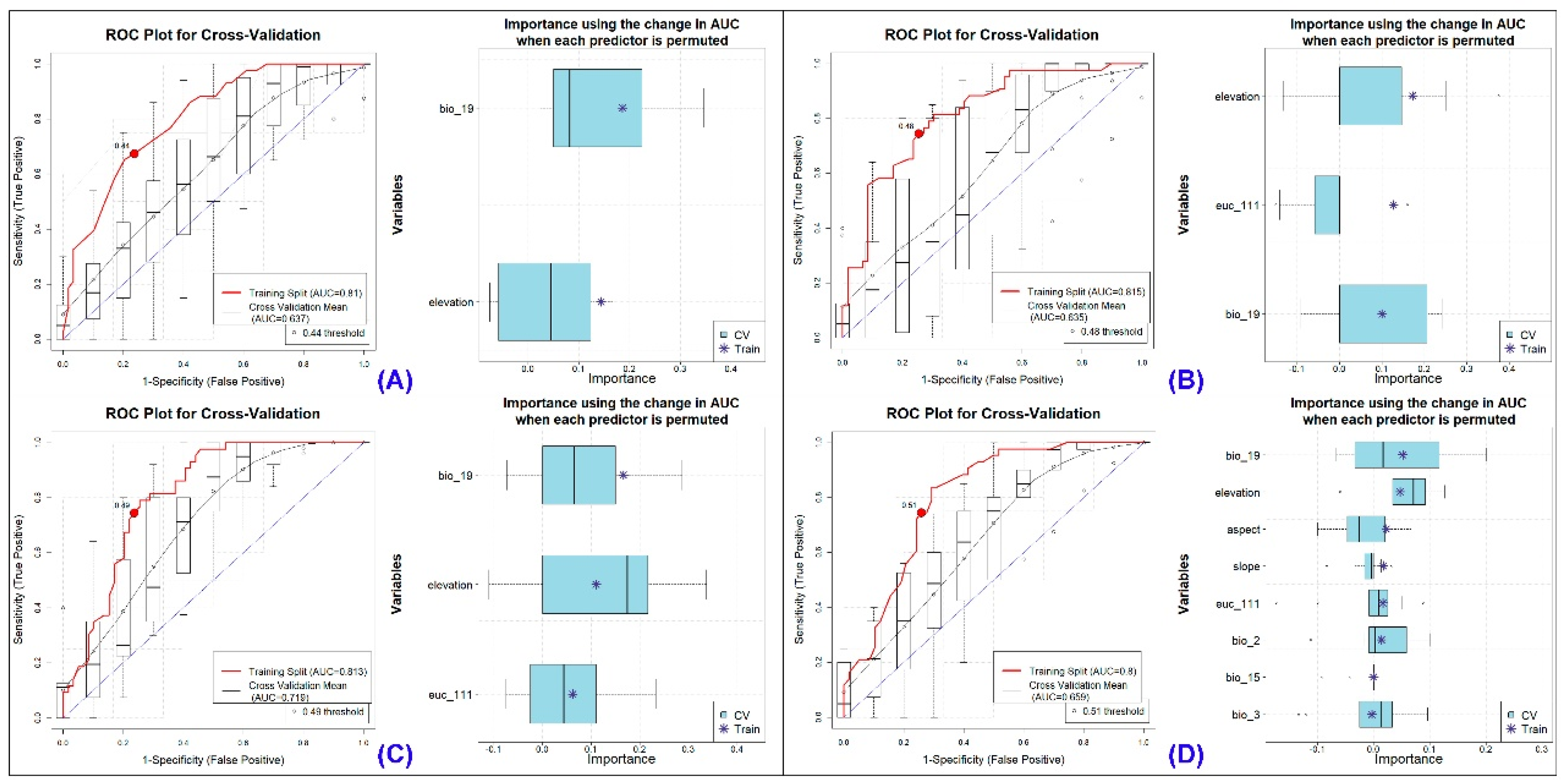
3.1. Ensemble Habitat Modeling
3.2. Effective Habitat Suitability Predictors
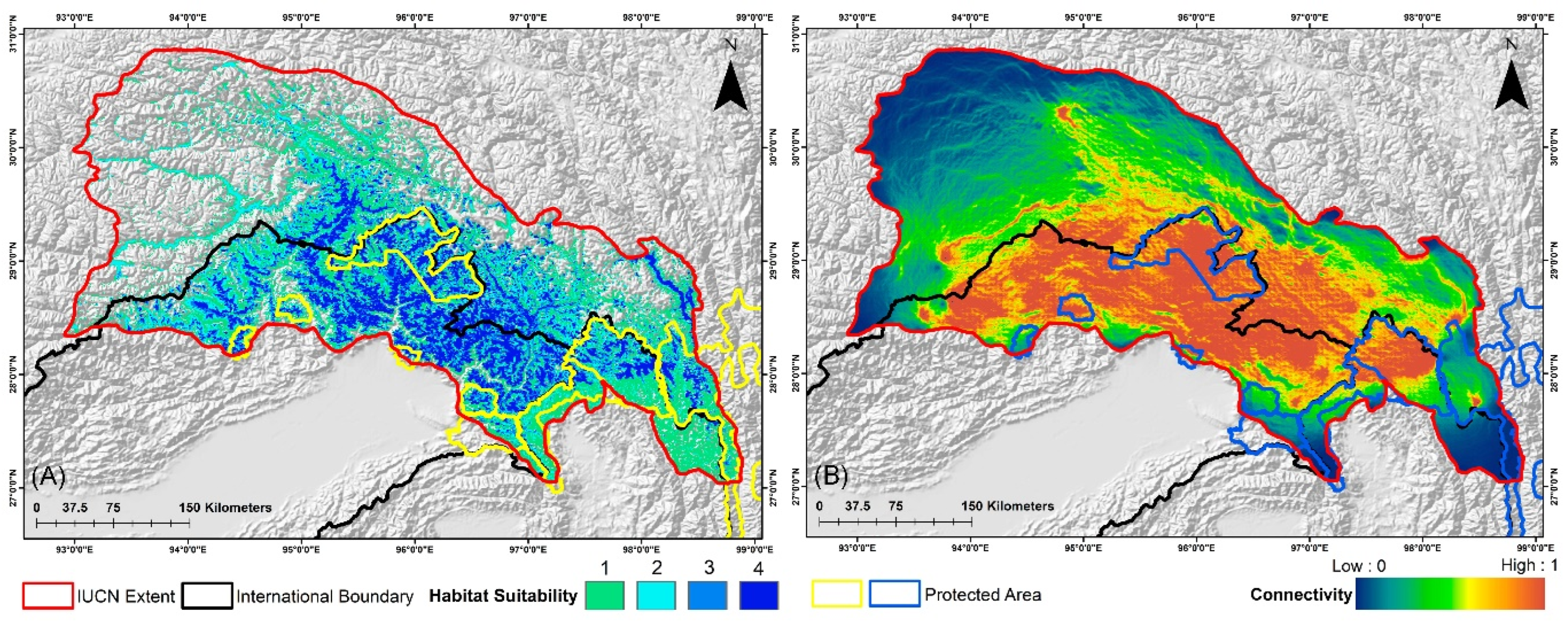
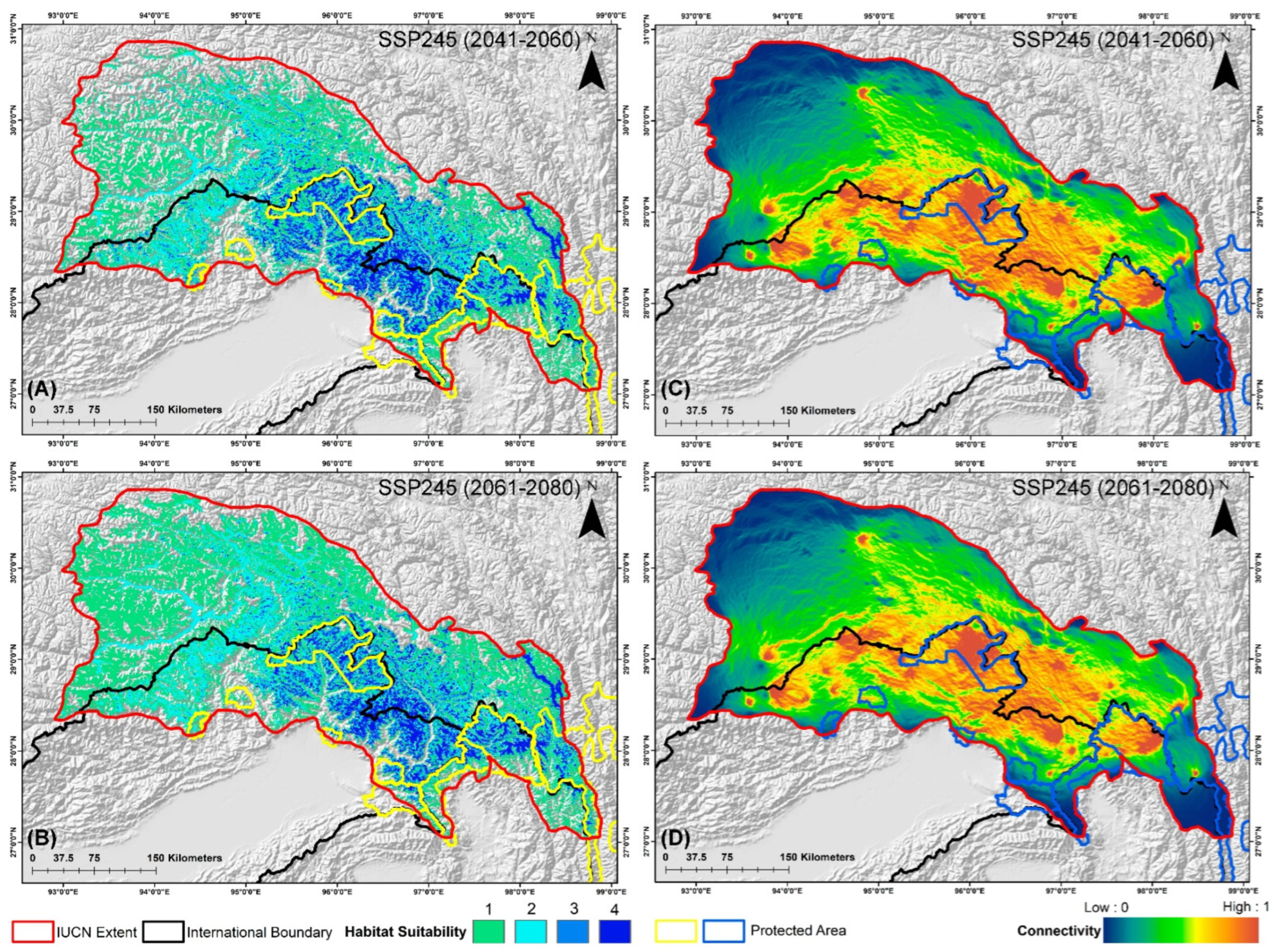
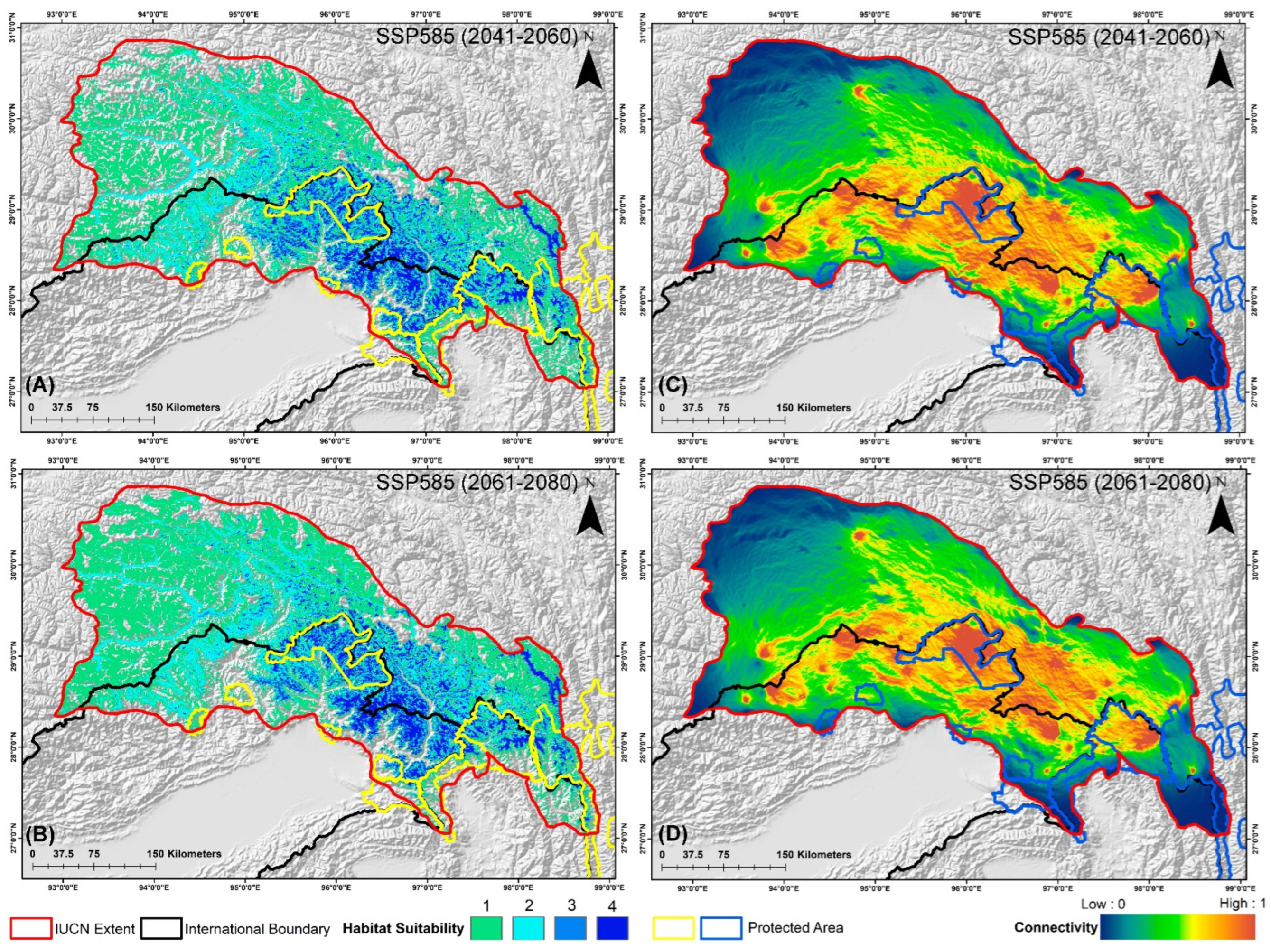
3.3. Suitable Habitat Extent in the IUCN Extent and Protected Areas
3.4. Habitat Fragmentation and Biological Corridors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, S.; Yang, L.; Khan, T.U.; Wanghe, K.; Li, M.; Luan, X. Using an ensemble modelling approach to predict the potential distribution of Himalayan gray goral (Naemorhedus goral bedfordi) in Pakistan. Glob. Ecol. Conserv. 2020, 21, e00845. [Google Scholar] [CrossRef]
- Crooks, K.R.; Burdett, C.L.; Theobald, D.M.; King, S.R.B.; Di Marco, M.; Rondinini, C.; Boitani, L. Quantification of habitat fragmentation reveals extinction risk in terrestrial mammals. Proc. Natl. Acad. Sci. USA 2017, 114, 7635–7640. [Google Scholar] [CrossRef]
- Santangeli, A.; Mammola, S.; Lehikoinen, A.; Rajasärkkä, A.; Lindén, A.; Saastamoinen, M. The effects of protected areas on the ecological niches of birds and mammals. Sci. Rep. 2022, 12, 11601. [Google Scholar] [CrossRef]
- Meehl, G.A.; Covey, C.; Delworth, T.; Latif, M.; McAvaney, B.; Mitchell, J.F.B.; Stouffer, R.J.; Taylor, K.E. The WCRP CMIP3 Multimodel Dataset: A New Era in Climate Change Research. Bull. Am. Meteorol. Soc. 2007, 88, 1383–1394. [Google Scholar] [CrossRef]
- Knight, J. Scientists’ warning of the impacts of climate change on mountains. PeerJ 2022, 10, e14253. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.P.; Lenoir, J.; Mai, G.S.; Kuo, H.C.; Chen, I.C.; Shen, S.F. Climate velocities and species tracking in global mountain regions. Nature 2024, 629, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Brivio, F.; Apollonio, M.; Anderwald, P.; Filli, F.; Bassano, B.; Bertolucci, C.; Grignolio, S. Seeking temporal refugia to heat stress: Increasing nocturnal activity despite predation risk. Proc. R. Soc. B 2024, 291, 20231587. [Google Scholar] [CrossRef]
- Anderwald, P.; Campell Andri, S.; Palme, R. Reflections of ecological differences? Stress responses of sympatric Alpine chamois and red deer to weather, forage quality, and human disturbance. Ecol. Evol. 2021, 11, 15740–15753. [Google Scholar] [CrossRef]
- Chatterjee, S.; Goswami, A.; Scotese, C.R. The longest voyage: Tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Res. 2013, 23, 238–267. [Google Scholar] [CrossRef]
- Haq, S.M.; Waheed, M.; Ahmad, R.; Bussmann, R.W.; Arshad, F.; Khan, A.M.; Casini, R.; Alataway, A.; Dewidar, A.Z.; Elansary, H.O. Climate Change and Human Activities, the Significant Dynamic Drivers of Himalayan Goral Distribution (Naemorhedus goral). Biology 2023, 12, 610. [Google Scholar] [CrossRef]
- Huntley, B.; Berry, P.M.; Cramer, W.; McDonald, A.P. Special Paper: Modelling Present and Potential Future Ranges of Some European Higher Plants Using Climate Response Surfaces. J. Biogeogr. 1995, 22, 967. [Google Scholar] [CrossRef]
- Pearson, R.G.; Dawson, T.P. Predicting the Impacts of Climate Change on the Distribution of Species: Are Bioclimate Envelope Models Useful? Glob. Ecol. Biogeogr. 2003, 12, 361–371. [Google Scholar] [CrossRef]
- Angert, A.L.; LaDeau, S.L.; Ostfeld, R.S. Climate change and species interactions: Ways forward. Ann. N. Y. Acad. Sci. 2013, 1297, 1–7. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, M.; Gao, K.; Gao, H.; Wei, F.; Nie, Y. Behavioural thermoregulation by montane ungulates under climate warming. Divers. Distrib. 2022, 28, 2229–2238. [Google Scholar] [CrossRef]
- Lehikoinen, P.; Tiusanen, M.; Santangeli, A.; Rajasärkkä, A.; Jaatinen, K.; Valkama, J.; Virkkala, R.; Lehikoinen, A. Increasing protected area coverage mitigates climate-driven community changes. Biol. Conserv. 2021, 253, 108892. [Google Scholar] [CrossRef]
- Nijhawan, S. Naemorhedus baileyi (Amended Version of 2020 Assessment). The IUCN Red List of Threatened Species 2020, e.T14294A179947455. Available online: https://www.iucnredlist.org/species/14294/179947455 (accessed on 30 June 2024).
- Hayman, R.W. The Red Goral of the North-East Frontier Region. Proc. Zool. Soc. Lond. 1961, 136, 317–323. [Google Scholar] [CrossRef]
- Wiens, J.J. Climate-Related Local Extinctions Are Already Widespread among Plant and Animal Species. PLoS Biol. 2016, 14, e2001104. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, R.; Sarkar, M.S.; Hazra, P.; Rahman, M.; Banerjee, S.; John, R. Habitat Connectivity for the Conservation of Small Ungulates in a Human-Dominated Landscape. ISPRS Int. J. Geo-Inf. 2021, 10, 180. [Google Scholar] [CrossRef]
- Khadka, K.K.; James, D.A. Modeling and Mapping the Current and Future Climatic-Niche of Endangered Himalayan Musk Deer. Ecol. Inform. 2017, 40, 1–7. [Google Scholar] [CrossRef]
- Bao, S.; Yang, F. Identification of Potential Habitats and Adjustment of Protected Area Boundaries for Large Wild Herbivores in the Yellow-River-Source National Park, China. Land 2024, 13, 186. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Sayahnia, R.; Ranjbaran, Y.; Vaissi, S.; Ahmadzadeh, F. Dynamics of threatened mammalian distribution in Iran’s protected areas under climate change. Mamm. Biol. 2021, 101, 759–774. [Google Scholar] [CrossRef]
- Feng, B.; Xiao, Y.; Hu, L.; Yang, X.; Dong, X.; Zhang, J.; Yang, Z.; Qi, D.; Zhou, C.; Bai, W. Predicted Climate Change Impacts on Distribution and Habitat Structure of Forest Ungulates in Southwest China. Ecosyst. Health Sustain. 2024, 10, 0173. [Google Scholar] [CrossRef]
- Ye, X.; Yu, X.; Yu, C.; Tayibazhaer, A.; Xu, F.; Skidmore, A.K.; Wang, T. Impacts of Future Climate and Land Cover Changes on Threatened Mammals in the Semi-Arid Chinese Altai Mountains. Sci. Total Environ. 2018, 612, 775–787. [Google Scholar] [CrossRef]
- Loiseau, N.; Mouquet, N.; Casajus, N.; Grenié, M.; Guéguen, M.; Maitner, B.; Mouillot, D.; Ostling, A.; Renaud, J.; Tucker, C.; et al. Global Distribution and Conservation Status of Ecologically Rare Mammal and Bird Species. Nat. Commun. 2020, 11, 5071. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Almasieh, K.; Vaissi, S. Ungulates Conservation in the Face of Human Development: Mining and Roads’ Influences on Habitat and Connectivity in Iran’s Central Plateau. Ecol. Inform. 2024, 81, 102656. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R. Species Distribution Models: Ecological Explanation and Prediction Across Space and Time. Annu. Rev. Ecol. Evol. Syst. 2009, 40, 677–697. [Google Scholar] [CrossRef]
- Di Febbraro, M.; Bosso, L.; Fasola, M.; Santicchia, F.; Aloise, G.; Lioy, S.; Tricarico, E.; Ruggieri, L.; Bovero, S.; Mori, E.; et al. Different facets of the same niche: Integrating citizen science and scientific survey data to predict biological invasion risk under multiple global change drivers. Glob. Chang. Biol. 2023, 29, 5509–5523. [Google Scholar] [CrossRef]
- Hao, T.; Elith, J.; Lahoz-Monfort, J.J.; Guillera-Arroita, G. Testing Whether Ensemble Modelling Is Advantageous for Maximising Predictive Performance of Species Distribution Models. Ecography 2020, 43, 549–558. [Google Scholar] [CrossRef]
- Jamali, F.; Amininasab, S.M.; Taleshi, H.; Madadi, H. Using an ensemble modeling to predict the potential distribution and habitat suitability of caracal (Caracal caracal) in southwestern Iran. Glob. Ecol. Conserv. 2024, 52, e02968. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, F.; Li, G.; Qin, W.; Wu, T.; Xu, F.; Hou, Y.; Song, P.; Cai, Z.; Zhang, T. The Four Antelope Species on the Qinghai-Tibet Plateau Face Habitat Loss and Redistribution to Higher Latitudes under Climate Change. Ecol. Indic. 2021, 123, 107337. [Google Scholar] [CrossRef]
- Li, Z.; Khattak, R.H.; Han, X.; Zhang, N.; Wu, J.; Liu, Z.; Teng, L. Distribution Update of Water Deer (Hydropotes inermis) and Prediction of Their Potential Distribution in Northeast China. Sci. Rep. 2023, 13, 5610. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.; Moat, J.; Hill, A.W.; de la Torre, J.; Scott, B. Supporting red list threat assessments with GeoCAT: Geospatial conservation assessment tool. ZooKeys 2011, 150, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Aryal, A.; Hegab, I.M.; Shrestha, U.B.; Coogan, S.C.P.; Sathyakumar, S.; Dalannast, M.; Dou, Z.; Suo, Y.; Dabu, X.; et al. Decreasing Brown Bear (Ursus arctos) Habitat Due to Climate Change in Central Asia and the Asian Highlands. Ecol. Evol. 2018, 8, 11887–11899. [Google Scholar] [CrossRef] [PubMed]
- Buchhorn, M.; Bertels, L.; Smets, B.; De Roo, B.; Lesiv, M.; Tsendbazar, N.E.; Masiliunas, D.; Li, L. Copernicus Global Land Operations “Vegetation and Energy”: Algorithm Theoretical Basis Document—Moderate Dynamic Land Cover 100 m, version 3; Copernicus Global Land Service: Barcelona, Spain, 2020. [Google Scholar] [CrossRef]
- Mukherjee, T.; Sharma, L.K.; Kumar, V.; Sharief, A.; Dutta, R.; Kumar, M.; Joshi, B.D.; Thakur, M.; Venkatraman, C.; Chandra, K. Adaptive Spatial Planning of Protected Area Network for Conserving the Himalayan Brown Bear. Sci. Total Environ. 2021, 754, 142416. [Google Scholar] [CrossRef]
- Morisette, J.T.; Jarnevich, C.S.; Holcombe, T.R.; Talbert, C.B.; Ignizio, D.; Talbert, M.K.; Silva, C.; Koop, D.; Swanson, A.; Young, N.E. VisTrails SAHM: Visualization and Workflow Management for Species Habitat Modeling. Ecography 2013, 36, 129–135. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A Toolbox for Comparative Studies of Environmental Niche Models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- Guisan, A.; Zimmermann, N.E.; Elith, J.; Graham, C.H.; Phillips, S.; Peterson, A.T. What matters for predicting the occurrences of trees: Techniques, data, or species’ characteristics? Ecol. Monogr. 2007, 77, 615–630. [Google Scholar] [CrossRef]
- Miller, J. Species Distribution Modeling. Geogr. Compass 2010, 4, 490–509. [Google Scholar] [CrossRef]
- Talbert, C.B.; Talbert, M.K. User Manual for SAHM Package for VisTrails. 2012. Available online: https://pubs.usgs.gov/publication/70118102 (accessed on 25 June 2024).
- Cohen, J. Weighted kappa: Nominal scale agreement provision for scaled disagreement or partial credit. Psychol. Bull. 1968, 70, 213–220. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Phillips, S.J.; Elith, J. POC Plots: Calibrating Species Distribution Models with Presence-Only Data. Ecology 2010, 91, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Valverde, A.; Acevedo, P.; Barbosa, A.M.; Lobo, J.M.; Real, R. Discrimination Capacity in Species Distribution Models Depends on the Representativeness of the Environmental Domain. Glob. Ecol. Biogeogr. 2013, 22, 508–516. [Google Scholar] [CrossRef]
- O’Neill, B.C.; Kriegler, E.; Riahi, K.; Ebi, K.L.; Hallegatte, S.; Carter, T.R.; Mathur, R.; van Vuuren, D.P. A New Scenario Framework for Climate Change Research: The Concept of Shared Socioeconomic Pathways. Clim. Chang. 2014, 122, 387–400. [Google Scholar] [CrossRef]
- Riahi, K.; van Vuuren, D.P.; Kriegler, E.; Edmonds, J.; O’Neill, B.C.; Fujimori, S.; Bauer, N.; Calvin, K.; Dellink, R.; Fricko, O.; et al. The Shared Socioeconomic Pathways and Their Energy, Land Use, and Greenhouse Gas Emissions Implications: An Overview. Glob. Environ. Chang. 2017, 42, 153–168. [Google Scholar] [CrossRef]
- Andrews, M.B.; Ridley, J.K.; Wood, R.A.; Andrews, T.; Blockley, E.W.; Booth, B.; Burke, E.; Dittus, A.J.; Florek, P.; Gray, L.J.; et al. Historical Simulations with HadGEM3-GC3.1 for CMIP6. J. Adv. Model. Earth Syst. 2020, 12, e2019MS001995. [Google Scholar] [CrossRef]
- Desmet, Q.; Ngo-Duc, T. A novel method for ranking CMIP6 global climate models over the southeast Asian region. Int. J. Clim. 2022, 42, 97–117. [Google Scholar] [CrossRef]
- Norgate, M.; Tiwari, P.R.; Das, S.; Kumar, D. On the Heat Waves over India and Their Future Projections under Different SSP Scenarios from CMIP6 Models. Int. J. Clim. 2024, 44, 973–995. [Google Scholar] [CrossRef]
- Allen, B.J.; Hill, D.J.; Burke, A.M.; Clark, M.; Marchant, R.; Stringer, L.C.; Williams, D.R.; Lyon, C. Projected future climatic forcing on the global distribution of vegetation types. Philos. Trans. R. Soc. B Biol. Sci. 2024, 379, 20230011. [Google Scholar] [CrossRef]
- Atsawawaranunt, K.; Whibley, A.; Cain, K.E.; Major, R.E.; Santure, A.W. Projecting the current and potential future distribution of New Zealand’s invasive sturnids. Biol. Invasions 2024, 26, 1345–1366. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Kim, H.-W.; Kang, H.-E.; Kundu, S. Distribution Model Reveals Rapid Decline in Habitat Extent for Endangered Hispid Hare: Implications for Wildlife Management and Conservation Planning in Future Climate Change Scenarios. Biology 2024, 13, 198. [Google Scholar] [CrossRef]
- Liang, G.; Niu, H.; Li, Y. A multi-species approach for protected areas ecological network construction based on landscape connectivity. Glob. Ecol. Conserv. 2023, 46, e02569. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Kim, A.R.; Lee, S.R.; Kim, H.W.; Kundu, S. Fragile futures: Evaluating habitat and climate change response of hog badgers (Mustelidae: Arctonyx) in the conservation landscape of mainland Asia. Ecol. Evol. 2024, 14, e70160. [Google Scholar] [CrossRef] [PubMed]
- Zungu, M.M.; Maseko, M.S.T.; Kalle, R.; Ramesh, T.; Downs, C.T. Effects of landscape context on mammal richness in the urban forest mosaic of EThekwini Municipality, Durban, South Africa. Glob. Ecol. Conserv. 2020, 21, e00878. [Google Scholar] [CrossRef]
- McGarigal, K.; Marks, B.J. FRAGSTATS: Spatial Pattern Analysis Program for Quantifying Landscape Structure. In General Technical Report—PNW-GTR-351; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 1995; 122p. [Google Scholar] [CrossRef]
- Midha, N.; Mathur, P.K. Assessment of Forest Fragmentation in the Conservation Priority Dudhwa Landscape, India Using FRAGSTATS Computed Class Level Metrics. J. Indian Soc. Remote Sens. 2010, 38, 487–500. [Google Scholar] [CrossRef]
- Barwicka, S.; Milecka, M.; Chmielewski, S.; Olszewska-Guizzo, A.; Masoudi, M.; Szczepańska, M. The Use of Selected Landscape Metrics to Evaluate the Transformation of the Rural Landscape as a Result of the Development of the Mining Function—A Case Study of the Puchaczów Commune. Sustainability 2021, 13, 12279. [Google Scholar] [CrossRef]
- Abedin, I.; Mukherjee, T.; Kang, H.E.; Yoon, T.H.; Kim, H.W.; Kundu, S. Unraveling the unknown: Adaptive spatial planning to enhance climate resilience for the endangered Swamp Grass-babbler (Laticilla cinerascens) with habitat connectivity and complexity approach. Heliyon 2024, 10, e30273. [Google Scholar] [CrossRef]
- Kundu, S.; Mukherjee, T.; Kamalakannan, M.; Barhadiya, G.; Ghosh, C.; Kim, H.-W. Matrilineal Phylogeny and Habitat Suitability of the Endangered Spotted Pond Turtle (Geoclemys hamiltonii; Testudines: Geoemydidae): A Two-Dimensional Approach to Forecasting Future Conservation Consequences. PeerJ 2023, 11, e15975. [Google Scholar] [CrossRef]
- Wang, F.; McShea, W.J.; Wang, D.; Li, S.; Zhao, Q.; Wang, H.; Lu, Z. Evaluating Landscape Options for Corridor Restoration between Giant Panda Reserves. PLoS ONE 2014, 9, e105086. [Google Scholar] [CrossRef]
- McRae, B.H.; Dickson, B.G.; Keitt, T.H.; Shah, V.B. Using Circuit Theory to Model Connectivity in Ecology, Evolution, and Conservation. Ecology 2008, 89, 2712–2724. [Google Scholar] [CrossRef]
- Ceballos, G.; Ehrlich, P.R.; Soberón, J.; Salazar, I.; Fay, J.P. Global mammal conservation: What must we manage? Science 2005, 309, 603–607. [Google Scholar] [CrossRef]
- Newbold, T.; Hudson, L.N.; Hill, S.L.L.; Contu, S.; Lysenko, I.; Senior, R.A.; Börger, L.; Bennett, D.J.; Choimes, A.; Collen, B.; et al. Global Effects of Land Use on Local Terrestrial Biodiversity. Nature 2015, 520, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Jiang, Z.; Tang, S. Impacts of Climate Change on Distributions and Diversity of Ungulates on the Tibetan Plateau. Ecol. Appl. 2015, 25, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, A.; Ghosal, S.; Raina, P.; Hore, U. Climate change impacts on high altitude wildlife distribution: Predicting range shifts for four ungulates in Changthang, eastern Ladakh. Ecol. Front. 2024, 44, 365–380. [Google Scholar] [CrossRef]
- Suggitt, A.J.; Wheatley, C.J.; Aucott, P.; Beale, C.M.; Fox, R.; Hill, J.K.; Isaac, N.J.B.; Martay, B.; Southall, H.; Thomas, C.D.; et al. Linking Climate Warming and Land Conversion to Species’ Range Changes across Great Britain. Nat. Commun. 2023, 14, 6759. [Google Scholar] [CrossRef]
- Cavallini, P. Survey of the goral Nemorhaedus goral (Hardwicke) in Himachal Pradesh. J. Bombay Nat. Hist. Soc. 1992, 89, 302–307. [Google Scholar]
- Hirzel, A.H.; Le Lay, G. Habitat Suitability Modelling and Niche Theory. J. Appl. Ecol. 2008, 45, 1372–1381. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Elith, J.; Phillips, S.J.; Hastie, T.; Dudík, M.; Chee, Y.E.; Yates, C.J. A statistical explanation of MaxEnt for ecologists. Divers. Distrib. 2011, 17, 43–57. [Google Scholar] [CrossRef]
- Taubert, F.; Fischer, R.; Groeneveld, J.; Lehmann, S.; Müller, M.S.; Rödig, E.; Wiegand, T.; Huth, A. Global Patterns of Tropical Forest Fragmentation. Nature 2018, 554, 519–522. [Google Scholar] [CrossRef]
- Jangtarwan, K.; Kamsongkram, P.; Subpayakom, N.; Sillapaprayoon, S.; Muangmai, N.; Kongphoemph, A.; Wongsodchuen, A.; Intapan, S.; Chamchumroon, W.; Safoowong, M.; et al. Predictive Genetic Plan for a Captive Population of the Chinese Goral (Naemorhedus griseus) and Prescriptive Action for Ex Situ and In Situ Conservation Management in Thailand. PLoS ONE 2020, 15, e0234064. [Google Scholar] [CrossRef]
- IUCN. Nature Conservation in Times of Conflict: Myanmar. 2013. Available online: https://www.iucn.nl/en/story/nature-conservation-in-times-of-conflict-myanmar/ (accessed on 11 July 2024).
- Yuan, Y.; Huang, K.; Liu, Q. Population Status and Genetic Analysis of Captive Red Goral (Naemorhedus baileyi) in Shanghai Zoo, China. Folia Zool. 2019, 68, 285–293. [Google Scholar] [CrossRef]
| Species | Model | Dataset | AUC | ΔAUC | PCC | TSS | Kappa | Specificity | Sensitivity |
|---|---|---|---|---|---|---|---|---|---|
| Naemorhedus baileyi | BRT | Train | 0.81 | 0.173 | 72.5 | 0.437 | 0.437 | 0.763 | 0.674 |
| CV | 0.637 | 64.7 | 0.275 | 0.273 | 0.68 | 0.595 | |||
| GLM | Train | 0.815 | 0.18 | 74.5 | 0.49 | 0.484 | 0.746 | 0.744 | |
| CV | 0.635 | 59.2 | 0.157 | 0.165 | 0.657 | 0.5 | |||
| MARS | Train | 0.813 | 0.094 | 75.5 | 0.507 | 0.502 | 0.763 | 0.744 | |
| CV | 0.719 | 65.3 | 0.288 | 0.284 | 0.683 | 0.605 | |||
| MaxEnt | Train | 0.8 | 0.141 | 74.3 | 0.486 | 0.48 | 0.741 | 0.744 | |
| CV | 0.939 | 88.1 | 0.746 | 0.697 | 0.887 | 0.859 |
| Variable | Abbreviation | BRT | GLM | MARS | MaxEnt | μ (Mean) | μ (Mean) % |
|---|---|---|---|---|---|---|---|
| Aspect | aspect | 0.000 | 0.000 | 0.000 | 0.023 | 0.006 | 1.62 |
| Precipitation Seasonality | bio_15 | 0.000 | 0.000 | 0.000 | 0.011 | 0.003 | 0.76 |
| Precipitation of Coldest Quarter | bio_19 | 0.186 | 0.100 | 0.167 | 0.052 | 0.126 | 35.87 |
| Mean Diurnal Range (Mean of Monthly (Max Temp Min Temp) | bio_2 | 0.000 | 0.000 | 0.000 | 0.014 | 0.003 | 0.98 |
| Isothermality | bio_3 | 0.000 | 0.000 | 0.000 | 0.003 | 0.001 | 0.23 |
| Elevation | elevation | 0.144 | 0.172 | 0.111 | 0.047 | 0.119 | 33.69 |
| Temperate Forest | euc_111 | 0.000 | 0.127 | 0.062 | 0.017 | 0.052 | 14.63 |
| Slope | slope | 0.000 | 0.000 | 0.000 | 0.172 | 0.043 | 12.21 |
| Country | Protected Areas | Present | SSP 245 (2041–2060) | GR from Present (%) | SSP 245 (2061–2080) | GR from Present (%) | SSP 585 (2041–2060) | GR from Present (%) | SSP 585 (2061–2080) | GR from Present (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| India | Dibang WLS | 1852 | 1443 | −22.084 | 1195 | −35.475 | 1252 | −32.397 | 1304 | −29.590 |
| Myanmar | Hkakaborazi NP | 1191 | 1081 | −9.236 | 1050 | −11.839 | 1129 | −5.206 | 1004 | −15.701 |
| China | Three Parallel Rivers of Yunnan PA | 825 | 607 | −26.424 | 514 | −37.697 | 700 | −15.152 | 592 | −28.242 |
| Myanmar | Hponkanrazi WLS | 257 | 269 | +4.669 | 410 | +59.533 | 331 | +28.794 | 281 | +9.339 |
| India | Kamlang WLS | 210 | 120 | −42.857 | 116 | −44.762 | 109 | −48.095 | 79 | −62.381 |
| India | Yardi-Rabe Supse WLS | 134 | 14 | −89.552 | 2 | −98.507 | 4 | −97.015 | 2 | −98.507 |
| India | Namdapha NP | 126 | 120 | −4.762 | 115 | −8.730 | 112 | −11.111 | 93 | −26.190 |
| India | Mouling NP | 96 | 1 | −98.958 | 0 | −100.000 | 0 | −100.000 | 0 | −100.000 |
| India | Mehao WLS | 71 | 31 | −56.338 | 27 | −61.972 | 27 | −61.972 | 10 | −85.915 |
| Country | Protected Areas | Present | SSP 245 (2041–2060) | GR from Present (%) | SSP 245 (2061–2080) | GR from Present (%) | SSP 585 (2041–2060) | GR from Present (%) | SSP 585 (2061–2080) | GR from Present (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| India | Yardi-Rabe Supse WLS | 2.497 | 1.081 | −56.705 | 0.976 | −60.911 | 1.147 | −54.075 | 0.755 | −69.763 |
| India | Dibang WLS | 2.361 | 2.225 | −5.759 | 2.212 | −6.315 | 2.231 | −5.498 | 2.161 | −8.461 |
| India | Kamlang WLS | 2.059 | 1.400 | −31.996 | 1.322 | −35.800 | 1.315 | −36.113 | 1.011 | −50.912 |
| India | Mehao WLS | 2.028 | 1.284 | −36.682 | 0.905 | −55.374 | 1.000 | −50.701 | 0.616 | −69.626 |
| Myanmar | Hkakaborazi NP | 2.028 | 1.735 | −14.425 | 1.886 | −6.996 | 1.796 | −11.430 | 1.628 | −19.716 |
| India | Namdapha NP | 1.905 | 1.356 | −28.781 | 1.253 | −34.230 | 1.431 | −24.858 | 0.956 | −49.811 |
| China | Three Parallel Rivers of Yunnan PA | 1.784 | 1.484 | −16.823 | 1.659 | −7.032 | 1.556 | −12.787 | 1.339 | −24.975 |
| Myanmar | Hponkanrazi WLS | 1.710 | 1.201 | −29.752 | 1.282 | −25.051 | 1.226 | −28.320 | 0.841 | −50.836 |
| India | Mouling NP | 1.445 | 0.246 | −82.977 | 0.244 | −83.079 | 0.396 | −72.579 | 0.180 | −87.564 |
| Scenario | NP | PD | LPI | TE | ED | LSI | AI |
|---|---|---|---|---|---|---|---|
| Present | 1239 | 96,210,504.14 | 16.135 | 236.352 | 1,728,689.791 | 50.416 | 65.878 |
| SSP 245 (2041–2060) | 1566 | 1,757,433,743 | 14.473 | 194.032 | 2,177,512.031 | 51.386 | 56.932 |
| SSP 245 (2061–2080) | 1459 | 1,825,941,129 | 11.782 | 177.648 | 2,223,267.922 | 49.567 | 56.037 |
| SSP 585 (2041–2060) | 1504 | 1,823,684,619 | 13.651 | 180.944 | 2,194,047.804 | 49.601 | 56.620 |
| SSP 585 (2061–2080) | 1407 | 1,692,277,346 | 10.854 | 176.992 | 2,128,781.464 | 48.518 | 57.933 |
| Corridors | Present | SSP 245 (2041–2060) | GR from Present (%) | SSP 245 (2061–2080) | GR from Present (%) | SSP 585 (2041–2060) | GR from Present (%) | SSP 585 (2061–2080) | GR from Present (%) |
|---|---|---|---|---|---|---|---|---|---|
| YRSWLS_MNP | 0.0426 | 0.0317 | −25.60 | 0.0292 | −31.35 | 0.0310 | −27.15 | 0.0255 | −40.11 |
| MNP_DWLS | 0.0538 | 0.0481 | −10.57 | 0.0460 | −14.54 | 0.0477 | −11.39 | 0.0430 | −20.02 |
| DWLS_MWLS | 0.0583 | 0.0532 | −8.81 | 0.0505 | −13.40 | 0.0516 | −11.51 | 0.0468 | −19.86 |
| DWLS_KWLS | 0.0446 | 0.0401 | −10.12 | 0.0383 | −14.10 | 0.0390 | −12.60 | 0.0348 | −22.09 |
| KWLS_NNP | 0.0172 | 0.0151 | −12.05 | 0.0147 | −14.74 | 0.0147 | −14.29 | 0.0128 | −25.84 |
| NNP_HpWLS | 0.0332 | 0.0316 | −4.88 | 0.0319 | −3.97 | 0.0316 | −4.84 | 0.0290 | −12.67 |
| HpWLS_HkNP | 0.0482 | 0.0461 | −4.45 | 0.0466 | −3.32 | 0.0461 | −4.35 | 0.0434 | −10.05 |
| HkNP_TPRYPA | 0.0493 | 0.0490 | −0.49 | 0.0487 | −1.22 | 0.0490 | −0.55 | 0.0494 | 0.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedin, I.; Mukherjee, T.; Abedin, J.; Kim, H.-W.; Kundu, S. Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia. Biology 2024, 13, 667. https://doi.org/10.3390/biology13090667
Abedin I, Mukherjee T, Abedin J, Kim H-W, Kundu S. Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia. Biology. 2024; 13(9):667. https://doi.org/10.3390/biology13090667
Chicago/Turabian StyleAbedin, Imon, Tanoy Mukherjee, Joynal Abedin, Hyun-Woo Kim, and Shantanu Kundu. 2024. "Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia" Biology 13, no. 9: 667. https://doi.org/10.3390/biology13090667
APA StyleAbedin, I., Mukherjee, T., Abedin, J., Kim, H.-W., & Kundu, S. (2024). Habitat Loss in the IUCN Extent: Climate Change-Induced Threat on the Red Goral (Naemorhedus baileyi) in the Temperate Mountains of South Asia. Biology, 13(9), 667. https://doi.org/10.3390/biology13090667










