Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animals
2.3. Rationale for Dose Selection of Carbon Tetrachloride, Silymarin, and Dapagliflozin and Their Dissolution
2.4. Experimental Design
2.5. Collection of Blood and Serum Preparation
2.6. Collection of the Liver and Its Gross Examination
2.7. Liver Homogenate Preparation
2.8. Biochemical Estimations in Serum and Liver Homogenates
2.9. Qualitative Histopathological Examination of Liver
2.10. Statistical Analysis
3. Results
3.1. Impact on Liver Function Test
3.2. Effect on Fasting Blood Glucose Levels, Body Weight, and Mortality Rate
3.3. Influence on Inflammatory Cytokines
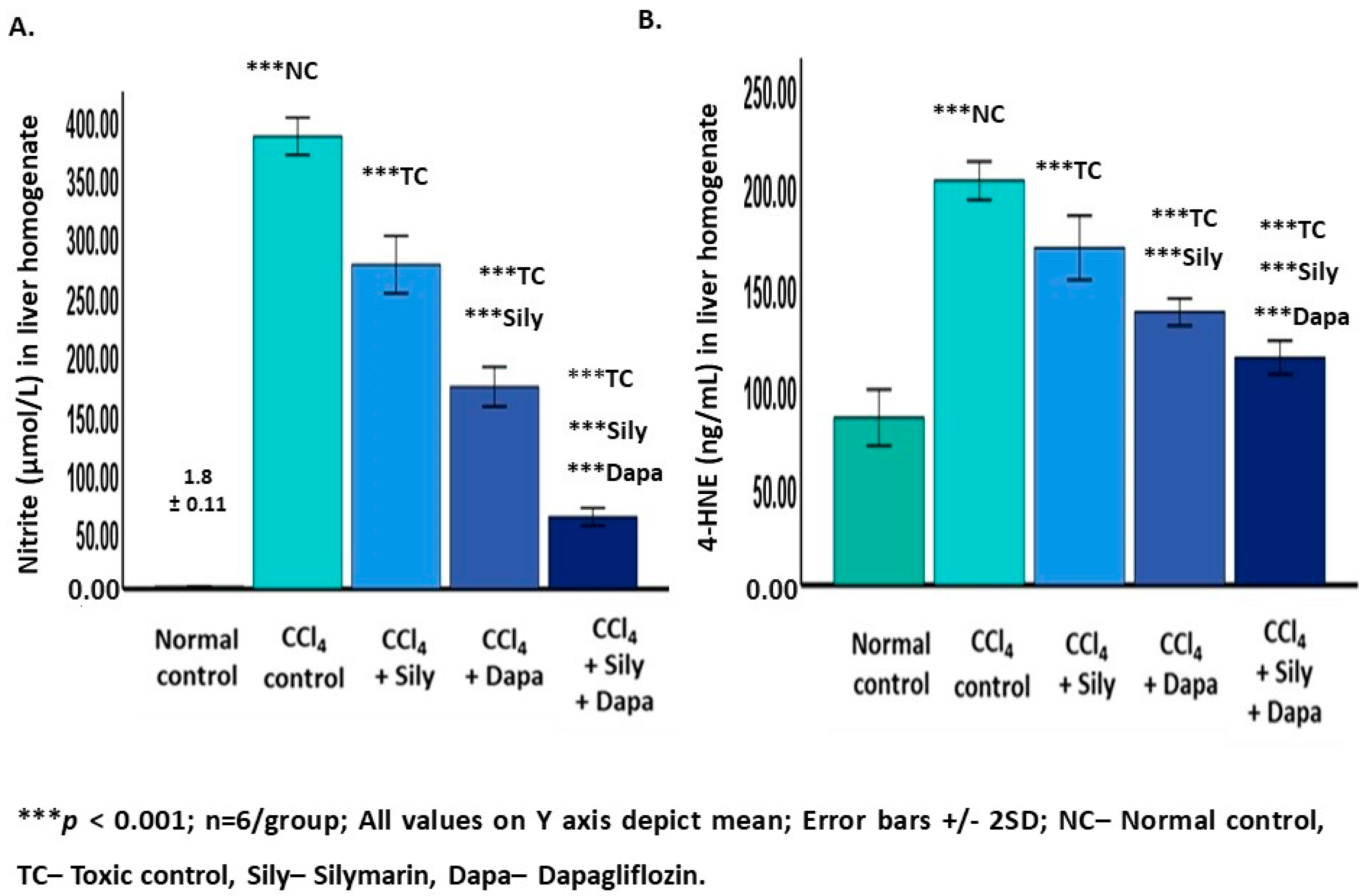
3.4. Effect on Oxidative Stress Biomarkers
3.5. Modulation of Nrf2/HO-1 Signaling Pathway
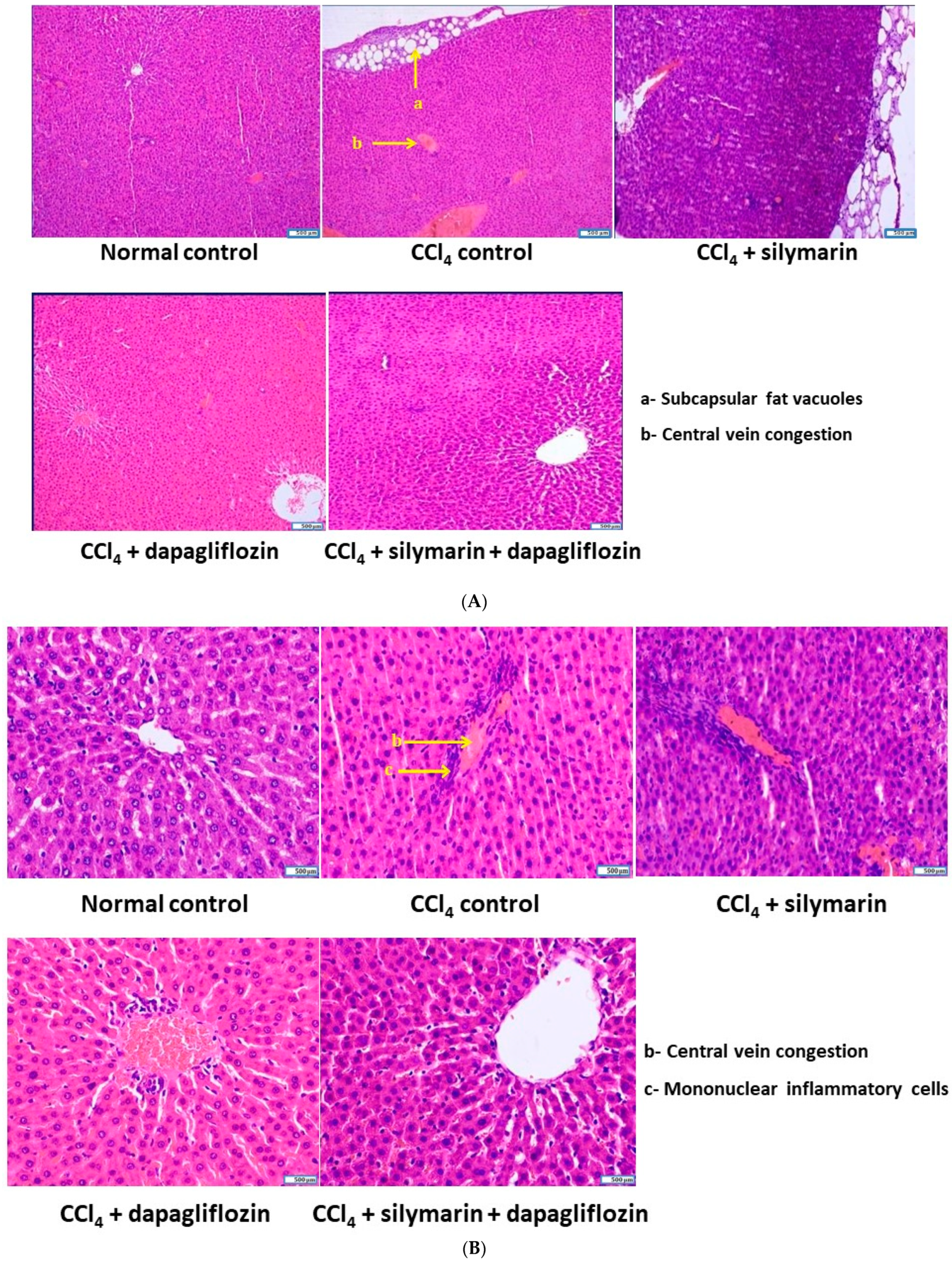
3.6. Impact on Gross Examination of the Liver
3.7. Effect on Cellular Architecture of Liver
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rusyn, I.; Arzuaga, X.; Cattley, R.C.; Corton, J.C.; Ferguson, S.S.; Godoy, P.; Guyton, K.Z.; Kaplowitz, N.; Khetani, S.R.; Roberts, R.A.; et al. Key characteristics of human hepatotoxicants as a basis for identification and characterization of the causes of liver toxicity. Hepatology 2021, 74, 3486–3496. [Google Scholar] [CrossRef]
- Devarbhavi, H.; Asrani, S.K.; Arab, J.P.; Nartey, Y.A.; Pose, E.; Kamath, P.S. Global burden of liver disease: 2023 update. J. Hepatol. 2023, 79, 516–537. [Google Scholar] [CrossRef]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Griffin, C.; Agbim, U.; Ramani, A.; Shankar, N.; Kanwal, F.; Asrani, S.K. Underestimation of Cirrhosis-Related Mortality in the Medicare Eligible Population, 1999–2018. Clin. Gastroenterol. Hepatol. 2023, 21, 223–225. [Google Scholar] [CrossRef]
- Dai, C.; Zhang, X.; Lin, J.; Shen, J. Nootkatone supplementation ameliorates carbon tetrachloride-induced acute liver injury via the inhibition of oxidative stress, NF-κB pathways, and the activation of Nrf2/HO-1 pathway. Antioxidants 2023, 12, 194. [Google Scholar] [CrossRef]
- Ishida, K.; Kaji, K.; Sato, S.; Ogawa, H.; Takagi, H.; Takaya, H.; Kawaratani, H.; Moriya, K.; Namisaki, T.; Akahane, T.; et al. Sulforaphane ameliorates ethanol plus carbon tetrachloride-induced liver fibrosis in mice through the Nrf2-mediated antioxidant response and acetaldehyde metabolization with inhibition of the LPS/TLR4 signaling pathway. J. Nutr. Biochem. 2021, 89, 108573. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Pirasanthan, R.; Vaishnav, R.L. Grape seed extract and zinc containing nutritional food supplement decreases the oxidative stress induced by carbon tetrachloride in rats. Int. J. Pharm. Pharm. Sci. 2013, 5, 626–631. [Google Scholar]
- Weber, L.W.; Boll, M.; Stampfl, A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003, 33, 105–136. [Google Scholar] [CrossRef] [PubMed]
- Satyam, S.M.; Bairy, K.L. Zincovit syrup ameliorates oxidative stress induced by carbon tetrachloride in rats. Int. J. Basic Clin. Pharmacol. 2015, 4, 449. [Google Scholar] [CrossRef]
- Elmowafy, M.; Viitala, T.; Ibrahim, H.M.; Abu-Elyazid, S.K.; Samy, A.; Kassem, A.; Yliperttula, M. Silymarin loaded liposomes for hepatic targeting: In vitro evaluation and HepG2 drug uptake. Eur. J. Pharm. Sci. 2013, 50, 161–171. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, L.K.; Ern, O.T.; Yen, Y.G.; Kanasin, A.; Muthaiah, T.; Ratnam, U.S.; Yadav, K. Influence of combination of docosahexaenoic acid supplement and a polyherbal formulation (Liv. 52) on carbon tetrachloride-induced hepatic injury: A preclinical study. J. Datta Meghe Inst. Med. Sci. Univ. 2020, 15, 114–117. [Google Scholar] [CrossRef]
- Satyam, S.M.; Bairy, K.L.; Vaishnav, R.L.; Rao, S.S. Hepatoprotective potential of Zincovit syrup against carbon tetrachloride induced hepatotoxicity in Wistar rats. Indian Med. Gaz. 2015, 149, 275–280. [Google Scholar]
- Satyam, S.M.; Bairy, K.L. Zincovit drop reduces oxidative stress induced by carbon tetrachloride in rats. AJPTT 2015, 3, 469–474. [Google Scholar]
- Satyam, S.M.; Bairy, K.L.; Pirasanthan, R.; Mohandas, R.K.; Nath, M. Grape seed extract and Zinc containing multivitamin-mineral nutritional food supplement ameliorates hepatic injury. Jokull J. 2014, 64, 184–195. [Google Scholar]
- Simeonova, R.; Vitcheva, V.; Kondeva-Burdina, M.; Krasteva, I.; Manov, V.; Mitcheva, M. Hepatoprotective and antioxidant effects of saponarin, isolated from Gypsophila trichotoma Wend. on paracetamol-induced liver damage in rats. BioMed Res. Int. 2013, 2013, 757126. [Google Scholar] [CrossRef] [PubMed]
- Binda, D.; Nicod, L.; Viollon-Abadie, C.; Rodriguez, S.; Berthalot, A.; Coassolo, P.; Richert, L. Strain difference (WKY, SPRD) in the hepatic antioxidant status in rat and effect of hypertension (SHR, DOCA). Ex vivo and in vitro data. Mol. Cell. Biochem. 2001, 218, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [PubMed]
- Tsai, K.F.; Chen, Y.L.; Chiou, T.T.; Chu, T.H.; Li, L.C.; Ng, H.Y.; Lee, W.C.; Lee, C.T. Emergence of SGLT2 inhibitors as powerful antioxidants in human diseases. Antioxidants 2021, 10, 1166. [Google Scholar] [CrossRef] [PubMed]
- Tanna, M.S.; Goldberg, L.R. The pleiotropic cardiovascular effects of sodium-glucose cotransporter-2 inhibitors. Curr. Opin. Cardiol. 2021, 36, 764–768. [Google Scholar] [CrossRef]
- Bae, J.H.; Park, E.G.; Kim, S.; Kim, S.G.; Hahn, S.; Kim, N.H. Effects of sodium-glucose cotransporter 2 inhibitors on renal outcomes in patients with type 2 diabetes: A systematic review and meta-analysis of randomized controlled trials. Sci. Rep. 2019, 9, 13009. [Google Scholar] [CrossRef]
- Zaibi, N.; Li, P.; Xu, S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef] [PubMed]
- Bilgic, Y.; Akbulut, S.; Aksungur, Z.; Erdemli, M.E.; Ozhan, O.; Parlakpinar, H.; Vardi, N.; Turkoz, Y. Protective effect of dexpanthenol against cisplatin-induced hepatotoxicity. Exp. Ther. Med. 2018, 16, 4049–4057. [Google Scholar] [CrossRef] [PubMed]
- Okada, J.; Yamada, E.; Saito, T.; Yokoo, H.; Osaki, A.; Shimoda, Y.; Ozawa, A.; Nakajima, Y.; Pessin, J.E.; Okada, S.; et al. Dapagliflozin inhibits cell adhesion to collagen I and IV and increases ectodomain proteolytic cleavage of DDR1 by increasing ADAM10 activity. Molecules 2020, 25, 495. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, P.; Wang, Y.; Chen, Y.; Niu, Y.; Ding, Y.; Xu, N.; Zhang, Y.; Xie, W. Dapagliflozin attenuates steatosis in livers of high-fat diet-induced mice and oleic acid-treated L02 cells via regulating AMPK/mTOR pathway. Eur. J. Pharmacol. 2021, 907, 174304. [Google Scholar] [CrossRef]
- Wang, L.; Liu, M.; Yin, F.; Wang, Y.; Li, X.; Wu, Y.; Ye, C.; Liu, J. Trilobatin, a novel SGLT1/2 inhibitor, selectively induces the proliferation of human hepatoblastoma cells. Molecules 2019, 24, 3390. [Google Scholar] [CrossRef] [PubMed]
- Basu, S. Carbon tetrachloride-induced lipid peroxidation: Eicosanoid formation and their regulation by antioxidant nutrients. Toxicology 2003, 189, 113–127. [Google Scholar] [CrossRef]
- Sharma, S.K.; Vasudeva, N. Hepatoprotective activity of Vitis vinifera root extract against carbon tetrachloride-induced liver damage in rats. Acta Pol. Pharm. 2012, 69, 933–937. [Google Scholar]
- Ramaiah, S.K. A toxicologist guide to the diagnostic interpretation of hepatic biochemical parameters. Food Chem. Toxicol. 2007, 45, 1551–1557. [Google Scholar] [CrossRef]
- Ahmed, W.S.; Soliman, A.; Amer, A.A.; El Shahat, R.M.; Amin, M.M.; Taha, R.S.; Awad, M.M.; Hamid, A.A.; El-Sayed, M.S.; Eid, E.A.; et al. Effect of dapagliflozin against NAFLD and dyslipidemia in type 2 diabetic albino rats: Possible underlying mechanisms. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8101–8109. [Google Scholar] [CrossRef]
- Hazem, R.M.; Ibrahim, A.Z.; Ali, D.A.; Moustafa, Y.M. Dapagliflozin improves steatohepatitis in diabetic rats via inhibition of oxidative stress and inflammation. Int. Immunopharmacol. 2022, 104, 108503. [Google Scholar] [CrossRef]
- Li, L.; Li, Q.; Huang, W.; Han, Y.; Tan, H.; An, M.; Xiang, Q.; Zhou, R.; Yang, L.; Cheng, Y. Dapagliflozin alleviates hepatic steatosis by restoring autophagy via the AMPK-mTOR pathway. Front. Pharmacol. 2021, 12, 589273. [Google Scholar] [CrossRef] [PubMed]
- Ko, I.G.; Jin, J.J.; Hwang, L.; Kim, S.H.; Kim, C.J.; Han, J.H.; Lee, S.; Kim, H.I.; Shin, H.P.; Jeon, J.W. Polydeoxyribonucleotide exerts protective effect against CCl4-induced acute liver injury through inactivation of NF-κB/MAPK signaling pathway in mice. Int. J. Mol. Sci. 2020, 21, 7894. [Google Scholar] [CrossRef]
- Checa, J.; Aran, J.M. Reactive oxygen species: Drivers of physiological and pathological processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghani, M.; Del Prato, S.; Chilton, R.; DeFronzo, R.A. SGLT2 inhibitors and cardiovascular risk: Lessons learned from the EMPA-REG OUTCOME study. Diabetes Care 2016, 39, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, H.; Wang, W.; Zhang, X.; Liu, J.; Wang, Q.; Wang, Y.; Zhang, C.; Guo, X.; Qiao, Q.; et al. Effect of dapagliflozin on liver and pancreatic fat in patients with type 2 diabetes and non-alcoholic fatty liver disease. J. Diabetes Its Complicat. 2023, 37, 108610. [Google Scholar] [CrossRef] [PubMed]
- ElMahdy, M.K.; Helal, M.G.; Ebrahim, T.M. Potential anti-inflammatory effect of dapagliflozin in HCHF diet-induced fatty liver degeneration through inhibition of TNF-α, IL-1β, and IL-18 in rat liver. Int. Immunopharmacol. 2020, 86, 106730. [Google Scholar] [CrossRef]
- Tang, L.; Wu, Y.; Tian, M.; Sjöström, C.D.; Johansson, U.; Peng, X.R.; Smith, D.M.; Huang, Y. Dapagliflozin slows the progression of the renal and liver fibrosis associated with type 2 diabetes. Am. J. Physiol.-Endocrinol. Metab. 2017, 313, E563–E576. [Google Scholar] [CrossRef] [PubMed]
- Khaznadar, F.; Petrovic, A.; Khaznadar, O.; Roguljic, H.; Bojanic, K.; Kuna Roguljic, L.; Siber, S.; Smolic, R.; Bilic-Curcic, I.; Wu, G.Y.; et al. Biomarkers for Assessing Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus on Sodium–Glucose Cotransporter 2 Inhibitor Therapy. J. Clin. Med. 2023, 12, 6561. [Google Scholar] [CrossRef] [PubMed]
- Saller, R.; Meier, R.; Brignoli, R. The use of silymarin in the treatment of liver diseases. Drugs 2001, 61, 2035–2063. [Google Scholar] [CrossRef]
- Yu, X.; Cui, L.; Zhang, Z.; Zhao, Q.; Li, S. α-Linolenic acid attenuates doxorubicin-induced cardiotoxicity in rats through suppression of oxidative stress and apoptosis. Acta Biochim. Biophys. Sin. 2013, 45, 817–826. [Google Scholar] [CrossRef]
- Wang, J.J.; Cui, P. Neohesperidin attenuates cerebral ischemia–reperfusion injury via inhibiting the apoptotic pathway and activating the Akt/Nrf2/HO-1 pathway. J. Asian Nat. Prod. Res. 2013, 15, 1023–1037. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Vieira, L.E.; Buttari, B.; Profumo, E.; Saso, L. The Nrf2 pathway in ischemic stroke: A review. Molecules 2021, 26, 5001. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Zhao, J.; Zhang, R.; Zhang, L.; Zhang, Q.; Yang, H.; An, J. Neuroprotective effects of natural compounds on neurotoxin-induced oxidative stress and cell apoptosis. Nutr. Neurosci. 2022, 25, 1078–1099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, J.; Deng, H.; Zheng, L.; Yang, H.; Lv, X. The role of Nrf2 in pulmonary fibrosis: Molecular mechanisms and treatment approaches. Antioxidants 2022, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; He, X.; Ren, J.; Chi, M.; Deng, G.; Li, G.; Nasser, M.I. Activation of the Nrf-2/HO-1 signalling axis can alleviate metabolic syndrome in cardiovascular disease. Ann. Med. 2023, 55, 2284890. [Google Scholar] [CrossRef] [PubMed]
- Ayala, A.; Munoz, M.F.; Arguelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Botchway, B.O.; Zhang, Y.; Wang, X.; Huang, M.; Liu, X. Resveratrol can improve spinal cord injury by activating Nrf2/HO-1 signaling pathway. Ann. Anat.-Anat. Anz. 2024, 251, 152180. [Google Scholar] [CrossRef]
- Yang, L.; Liu, D.; Yan, H.; Chen, K. Dapagliflozin attenuates cholesterol overloading-induced injury in mice hepatocytes with type 2 diabetes mellitus (T2DM) via eliminating oxidative damages. Cell Cycle 2022, 21, 641–654. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satyam, S.M.; Bairy, L.K.; Rehman, A.; Attia, M.; Ahmed, L.; Emad, K.; Jaafer, Y.; Bahaaeldin, A. Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats. Biology 2024, 13, 473. https://doi.org/10.3390/biology13070473
Satyam SM, Bairy LK, Rehman A, Attia M, Ahmed L, Emad K, Jaafer Y, Bahaaeldin A. Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats. Biology. 2024; 13(7):473. https://doi.org/10.3390/biology13070473
Chicago/Turabian StyleSatyam, Shakta Mani, Laxminarayana Kurady Bairy, Abdul Rehman, Mohamed Attia, Layth Ahmed, Karam Emad, Yusuf Jaafer, and Abdelrehman Bahaaeldin. 2024. "Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats" Biology 13, no. 7: 473. https://doi.org/10.3390/biology13070473
APA StyleSatyam, S. M., Bairy, L. K., Rehman, A., Attia, M., Ahmed, L., Emad, K., Jaafer, Y., & Bahaaeldin, A. (2024). Unlocking Synergistic Hepatoprotection: Dapagliflozin and Silymarin Combination Therapy Modulates Nuclear Erythroid 2-Related Factor 2/Heme Oxygenase-1 Pathway in Carbon Tetrachloride-Induced Hepatotoxicity in Wistar Rats. Biology, 13(7), 473. https://doi.org/10.3390/biology13070473







