Gamma-Aminobutyric Acid (GABA) Avoids Deterioration of Transport Water Quality, Regulates Plasma Biochemical Indices, Energy Metabolism, and Antioxidant Capacity of Tawny Puffer (Takifugui flavidus) under Transport Stress
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. GABA Pretreatment Experiment
2.3. Simulative Transportation Experiment
2.4. Sample Collection
2.5. Plasma Biochemical Indices
2.6. Energy Metabolism Indicators
2.7. Antioxidant Indexes
3. Statistical Analyses
4. Results
4.1. Water Quality Parameters
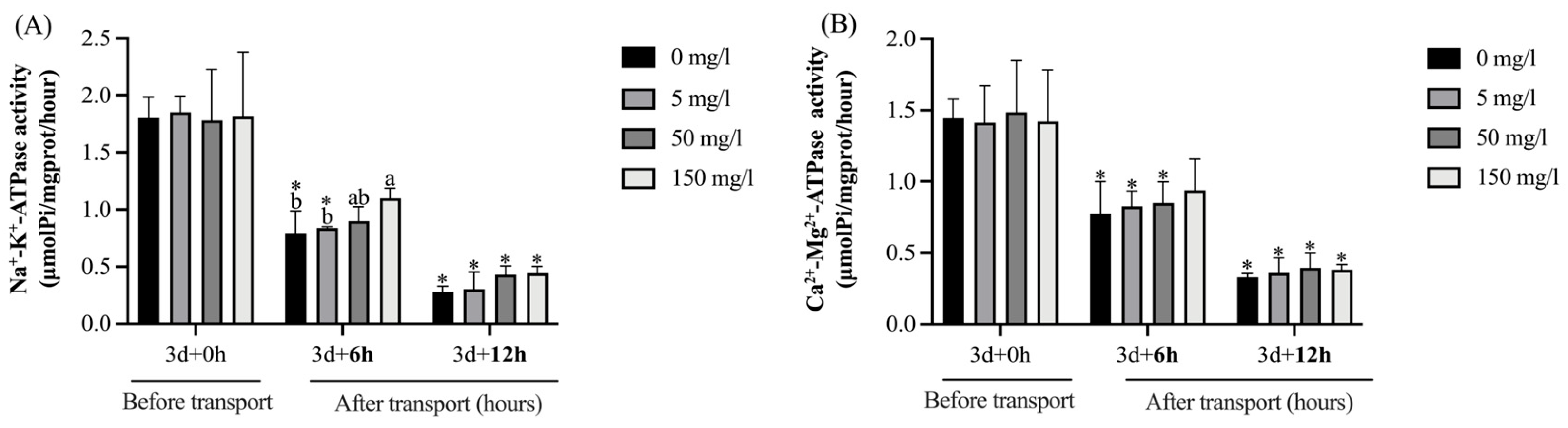
4.2. Plasma Biochemical Indices
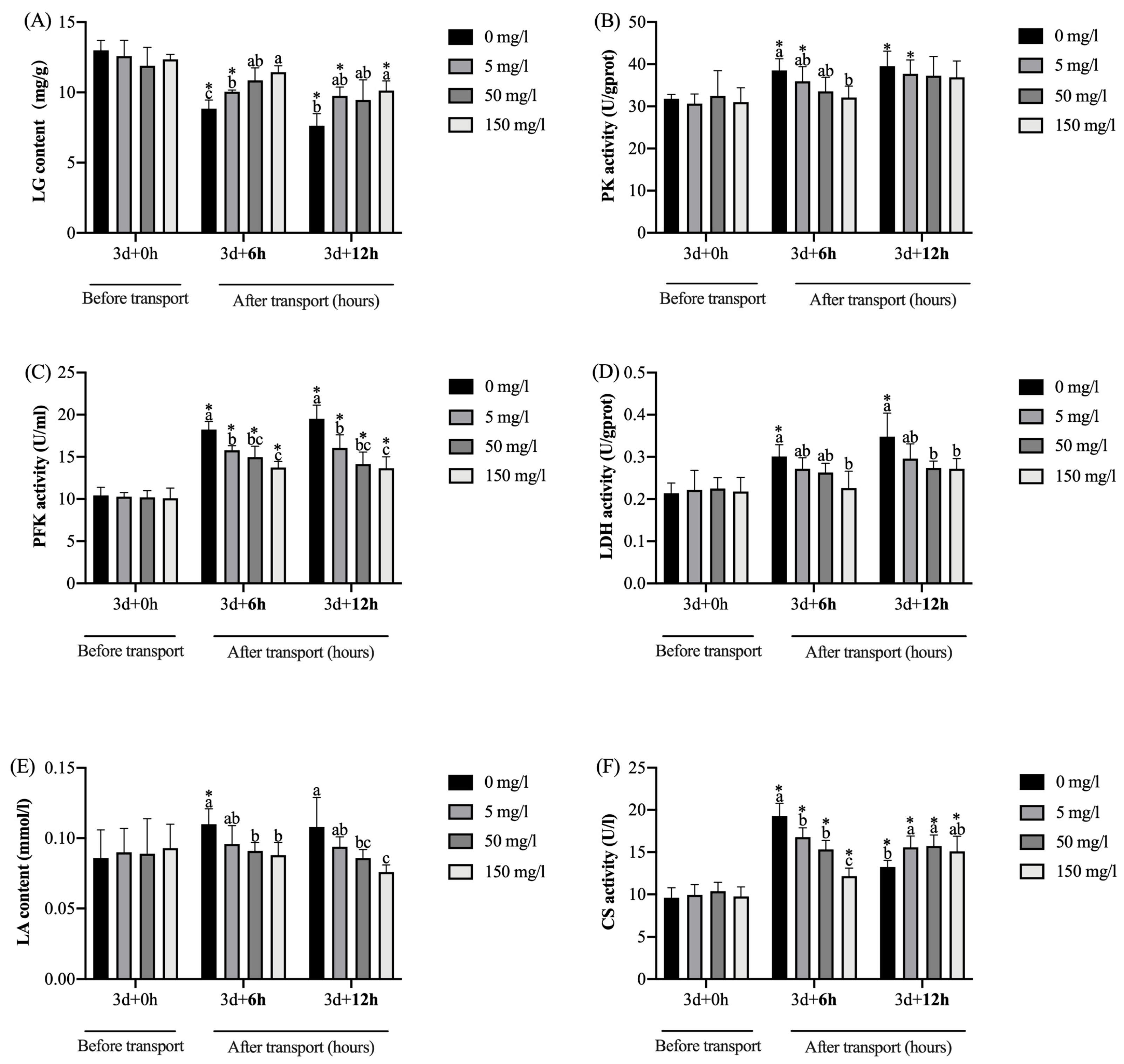
4.3. Energy Metabolism Indicators
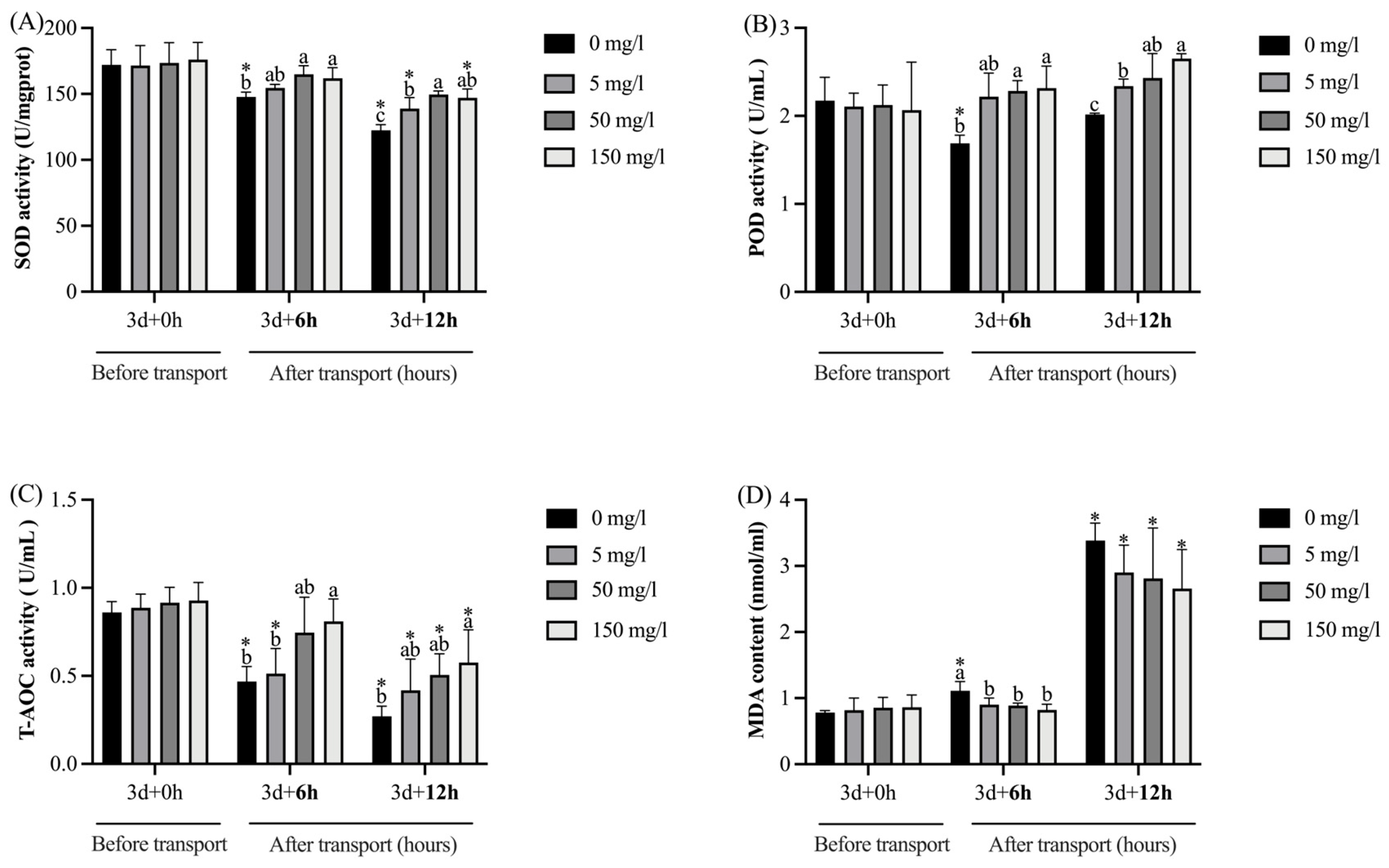
4.4. Antioxidant Indexes
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, Y.F.; Narayan, E.; Planellas, S.R.; Phillips, C.J.C.; Zheng, L.; Xu, B.Y.; Wang, L.; Liu, Y.C.; Sun, Y.X.; Sagada, G.; et al. Effects of stocking density during simulated transport on physiology and behavior of largemouth bass (Micropterus salmoides). J. World Aquacult. Soc. 2024, 55, e13054. [Google Scholar] [CrossRef]
- Zhang, H.Z.; Wang, Q.; Dong, Y.X.; Mei, J.; Xie, J. Effects of Tricaine Methanesulphonate (MS-222) on Physiological Stress and Fresh Quality of Sea Bass (Lateolabrax maculatus) under Simulated High-Density and Long-Distance Transport Stress. Biology 2023, 12, 223. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.Y.; Li, C.P.; Hou, T.T.; Wen, C.Q.; Kong, S.Z.; Ma, D.; Sun, C.B.; Li, S.D. Effects of Chitosan-Gentamicin Conjugate Supplement on Non-Specific Immunity, Aquaculture Water, Intestinal Histology and Microbiota of Pacific White Shrimp (Litopenaeus vannamei). Mar. Drugs 2020, 18, 419. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Tao, Y.F.; Lu, S.Q.; Qiang, J.; Xu, P. Integrated Transcriptome and 16S rDNA Analyses Reveal That Transport Stress Induces Oxidative Stress and Immune and Metabolic Disorders in the Intestine of Hybrid Yellow Catfish (Tachysurus fulvidraco♀ Pseudobagrus vachellii♂). Antioxidants 2022, 11, 1737. [Google Scholar] [CrossRef]
- Wang, Z.H.; Li, J.H.; Wang, Z.M.; Xue, L.Y.; Zhang, Y.; Chen, Y.J.; Su, J.; Li, Z.M. L-tyrosine improves neuroendocrine function in a mouse model of chronic stress. Neural. Regener. Res. 2012, 7, 1413–1419. [Google Scholar] [CrossRef]
- Bo, W.; Jing, X. The inducing factors and effects of stress response in the live transport of fish. Food Mach. 2018, 34, 169–172. [Google Scholar]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Barton, B.A.; Iwama, G.K. Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu. Rev. Fish Dis. 1991, 1, 3–26. [Google Scholar] [CrossRef]
- Aidos, L.; Cafiso, A.; Serra, V.; Vasconi, M.; Bertotto, D.; Bazzocchi, C.; Radaelli, G.; Di Giancamillo, A. How Different Stocking Densities Affect Growth and Stress Status of Acipenser baerii Early Stage Larvae. Animals 2020, 10, 1289. [Google Scholar] [CrossRef]
- Görlach, A.; Dimova, E.Y.; Petry, A.; Martínez-Ruiz, A.; Hernansanz-Agustín, P.; Rolo, A.P.; Palmeira, C.M.; Kietzmann, T. Reactive oxygen species, nutrition, hypoxia and diseases: Problems solved? Redox Biol. 2015, 6, 372–385. [Google Scholar] [CrossRef]
- Bai, C.; Qi, X.; Wang, Z.D.; Wang, J.G.; Qiu, L.; Li, H.H.; Zu, X.Y.; Li, H.L.; Xiong, G.Q.; Liao, T. Effect of density stress on the physiological, biochemical, and immunological parameters of juvenile Pelteobagrus fulvidraco during simulated transportation. Aquacult. Rep. 2024, 34, 101911. [Google Scholar] [CrossRef]
- Erikson, U.; Digre, H.; Misimi, E. Effects of Perimortem Stress on Farmed Atlantic Cod Product Quality: A Baseline Study. J. Food Sci. 2011, 76, S251–S261. [Google Scholar] [CrossRef] [PubMed]
- Bar, I.; Dutney, L.; Lee, P.; Yazawa, R.; Yoshizaki, G.; Takeuchi, Y.; Cummins, S.; Elizur, A. Small-scale capture, transport and tank adaptation of live, medium-sized Scombrids using “Tuna Tubes”. SpringerPlus 2015, 4, 604. [Google Scholar] [CrossRef][Green Version]
- de Oliveira, C.P.B.; Lemos, C.H.D.; Silva, A.F.E.; de Souza, S.A.; Albinati, A.C.L.; Lima, A.O.; Copatti, C.E. Use of eugenol for the anaesthesia and transportation of freshwater angelfish (Pterophyllum scalare). Aquaculture 2019, 513, 734409. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Diana, M.; Quílez, J.; Rafecas, M. Gamma-aminobutyric acid as a bioactive compound in foods: A review. J. Funct. Foods 2014, 10, 407–420. [Google Scholar] [CrossRef]
- Zhou, H.L.; Chen, H.Y.; Bao, D.P.; Shin, T.Y.; Zhong, Y.J.; Zhang, X.; Wu, Y.Y. Recent advances of γ-aminobutyric acid: Physiological and immunity function, enrichment, and metabolic pathway. Front. Nutr. 2022, 9, 1076223. [Google Scholar] [CrossRef]
- Banerjee, J.; Al-Wadei, H.A.N.; Al-Wadei, M.H.; Dagnon, K.; Schuller, H.M. Differential modulation of nicotine-induced gemcitabine resistance by GABA receptor agonists in pancreatic cancer cell xenografts and in vitro. BMC Cancer 2014, 14, 725. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, J.; Wang, X.; Yang, Y.; Huang, Q.; Qiao, F.; Shi, Q.; Qin, J.; Chen, L. Gamma-aminobutyric acid enhances hypoxia tolerance of juvenile Chinese mitten crab (Eriocheir sinensis) by regulating respiratory metabolism and alleviating neural excitotoxicity. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 260, 109409. [Google Scholar] [CrossRef]
- Yan, F.B.; Dong, T.C.; Lu, Y.Y.; Liu, H.Z. Effects of-aminobutyric acid on blood biochemical parameters in Carassius auratus under transportation stress. G.D. Agric. Sci. 2014, 41, 127–130. [Google Scholar] [CrossRef]
- Wu, G.T. The Alleviation Effect of -Aminobutyric Acid and Guanidinoacetic Acid on the Transport Stress of Koi Carp (Cyprinus carpio). Master’s Thesis, Dalian Ocean University, Dalian, China, 2022. [Google Scholar]
- Shi, Y.H.; Zhang, G.Y.; Liu, J.Z.; Zhu, X.D. Effects of Photoperiod on Embryos and Larvae of Tawny Puffer, Takifugu flavidus. J. World Aquacult. Soc. 2012, 43, 278–285. [Google Scholar] [CrossRef]
- Molony, B.W.; Lenanton, R.; Jackson, G.; Norriss, J. Stock enhancement as a fisheries management tool. Rev. Fish Biol. Fisher. 2003, 13, 409–432. [Google Scholar] [CrossRef]
- Hong, J.W.; Zhou, S.J.; Yu, G.; Qin, C.X.; Zuo, T.; Ma, Z.H. Effects of transporting stress on the immune responses of Asian seabass Lates calcarifer fry. Aquacult. Res. 2021, 52, 2182–2193. [Google Scholar] [CrossRef]
- Zheng, T.; Song, Z.; Qiang, J.; Tao, Y.F.; Zhu, H.J.; Ma, J.L.; Xu, P. Transport Stress Induces Skin Innate Immunity Response in Hybrid Yellow Catfish (Tachysurus fulvidraco♀ × P. vachellii♂) Through TLR/NLR Signaling Pathways and Regulation of Mucus Secretion. Front. Immunol. 2021, 12, 740359. [Google Scholar] [CrossRef] [PubMed]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Yuen, V.G.; McNeill, J.H. Comparison of the glucose oxidase method for glucose determination by manual assay and automated analyzer. J. Pharmacol. Toxicol. Methods 2000, 44, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Roe, J.H.; Dailey, R.E. Determination of glycogen with the anthrone reagent. Anal. Biochem. 1966, 15, 245–250. [Google Scholar] [CrossRef]
- Engel, P.C.; Jones, J.B. Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: Improved conditions for the assay of L-glutamate, L-lactate, and other metabolites. Anal. Biochem. 1978, 88, 475–484. [Google Scholar] [CrossRef]
- Dalvi, R.S.; Das, T.; Debnath, D.; Yengkokpam, S.; Baruah, K.; Tiwari, L.R.; Pal, A.K. Metabolic and cellular stress responses of catfish, Horabagrus brachysoma (Gunther) acclimated to increasing temperatures. J. Therm. Biol. 2017, 65, 32–40. [Google Scholar] [CrossRef]
- Chifflet, S.; Torriglia, A.; Chiesa, R.; Tolosa, S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: Application to lens ATPases. Anal. Biochem. 1988, 168, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Duggleby, R.G.; Dennis, D.T. Pyruvate kinase, a possible regulatory enzyme in higher plants. Plant Physiol. 1973, 52, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Peskin, A.V.; Winterbourn, C.C. A microtiter plate assay for superoxide dismutase using a water-soluble tetrazolium salt (WST-1). Clin. Chim. Acta 2000, 293, 157–166. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yang, Y.; Hu, Z.; Xue, R.; Hu, Y. Resistance mechanisms and fitness of pyraclostrobin-resistant isolates of Lasiodiplodia theobromae from mango orchards. PLoS ONE 2021, 16, e0253659. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, A.; Santamaría, D.; Díaz-Muñoz, M.; Espinoza-González, V.; Rios, C. Effects of Nω-nitro-L-arginine and L-arginine on quinolinic acid-induced lipid peroxidation. Toxicol. Lett. 1997, 93, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Golombieski, J.I.; Silva, L.V.F.; Baldisserotto, B.; da Silva, J.H.S. Transport of silver catfish (Rhamdia quelen) fingerlings at different times, load densities, and temperatures. Aquaculture 2003, 216, 95–102. [Google Scholar] [CrossRef]
- Moran, D.; Gwells, R.M.; Pether, S.J. Low stress response exhibited by juvenile yellowtail kingfish (Seriola lalandi Valenciennes) exposed to hypercapnic conditions associated with transportation. Aquacult. Res. 2008, 39, 1399–1407. [Google Scholar] [CrossRef]
- Carneiro, P.C.F.; Urbinati, E.C. Salt as a stress response mitigator of matrinxa, Brycon cephalus (Gunther), during transport. Aquacult. Res. 2001, 32, 297–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, F.; Jia, W.; Li, N.; Zhang, J. Effect of Hydroxyl Groups and Rigid Structure in 1,4-Cyclohexanediol on Percutaneous Absorption of Metronidazole. AAPS PharmSciTech 2014, 15, 973–980. [Google Scholar] [CrossRef][Green Version]
- Nan, Y.; Xiao, M.; Duan, Y.; Yang, Y. Toxicity of Ammonia Stress on the Physiological Homeostasis in the Gills of Litopenaeus vannamei under Seawater and Low-Salinity Conditions. Biology 2024, 13, 281. [Google Scholar] [CrossRef]
- Bolner, K.C.S.; Copatti, C.E.; Rosso, F.L.; Loro, V.L.; Baldisserotto, B. Water pH and metabolic parameters in silver catfish (Rhamdia quelen). Biochem. Syst. Ecol. 2014, 56, 202–208. [Google Scholar] [CrossRef]
- Gomes, L.C.; Brinn, R.P.; Marcon, J.L.; Dantas, L.A.; Brandao, F.R.; de Abreu, J.S.; Lemos, P.E.M.; McComb, D.M.; Baldisserotto, B. Benefits of using the probiotic Efinol®L during transportation of cardinal tetra, Paracheirodon axelrodi (Schultz), in the Amazon. Aquacult. Res. 2009, 40, 157–165. [Google Scholar] [CrossRef]
- Lim, L.C.; Dhert, P.; Sorgeloos, P. Recent developments and improvements in ornamental fish packaging systems for air transport. Aquacult. Res. 2003, 34, 923–935. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Loss of the glucocorticoid receptor in zebrafish improves muscle glucose availability and increases growth. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E1093–E1104. [Google Scholar] [CrossRef] [PubMed]
- Fatira, E.; Papandroulakis, N.; Pavlidis, M. Diel changes in plasma cortisol and effects of size and stress duration on the cortisol response in European sea bass (Dicentrarchus labrax). Fish Physiol. Biochem. 2014, 40, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Trenzado, C.E.; Carrick, T.R.; Pottinger, T.G. Divergence of endocrine and metabolic responses to stress in two rainbow trout lines selected for differing cortisol responsiveness to stress. Gen. Comp. Endocrinol. 2003, 133, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.W.; Sieghart, W. International union of pharmacology.: LXX.: Subtypes of γ-aminobutyric AcidA receptors:: Classification on the basis of subunit composition, pharmacology, and function.: Update. Pharmacol. Rev. 2008, 60, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.A.; Ahmed, I.; Jan, K.; Nabi, N.; Fazio, F. Haematological profile, blood cell characteristic and serum biochemical composition of cultured brown trout, Salmo trutta fario with respect to sex. Heliyon 2022, 8, e10247. [Google Scholar] [CrossRef]
- Sohrabipour, S.; Sharifi, M.R.; Talebi, A.; Sharifi, M.; Soltani, N. GABA dramatically improves glucose tolerance in streptozotocin-induced diabetic rats fed with high-fat diet. Eur. J. Pharmacol. 2018, 826, 75–84. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Foppen, E.; Schalij, I.; Van Heijningen, C.; van der Vliet, J.; Fliers, E.; Buijs, R.M. Circadian Control of the Daily Plasma Glucose Rhythm: An Interplay of GABA and Glutamate. PLoS ONE 2008, 3, e3194. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, T.; Abe, H. Physiological roles of free D- and L-alanine in the crayfish Procambarus clarkii with special reference to osmotic and anoxic stress responses. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, H.; He, K.; Yan, T.; Zhou, J.; Zhao, L.L.; Sun, J.L.; Lian, W.Q.; Zhang, D.M.; Du, Z.J.; et al. Response of AMP-activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Sci. Total Environ. 2019, 666, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate metabolism: Historical context, prior misinterpretations, and current understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef] [PubMed]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Hepatic glucokinase and glucose-6-phosphatase responses to dietary glucose and starch in gilthead sea bream (Sparus aurata) juveniles reared at two temperatures. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 149, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, T.D.; Hagemeyer, J.C.G.; Hoadley, K.D.; Marsh, A.G.; Warner, M.E. Partitioning of Respiration in an Animal-Algal Symbiosis: Implications for Different Aerobic Capacity between Symbiodinium spp. Front. Physiol. 2016, 7, 181784. [Google Scholar] [CrossRef] [PubMed]
- Schreck, C.B.; Jonsson, L.; Feist, G.; Reno, P. Conditioning improves performance of juvenile Chinook salmon, Oncorhynchus tshawytscha, to transportation stress. Aquaculture 1995, 135, 99–110. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Lutz, P.L. Role of GABA in hypoxia tolerance, metabolic depression and hibernation--possible links to neurotransmitter evolution. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 1993, 105, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Varghese, T.; Kumar, V.J.R.; Anand, G.; Dasgupta, S.; Pal, A.K. Dietary GABA enhances hypoxia tolerance of a bottom-dwelling carp, Cirrhinus mrigala by modulating HIF-1α, thyroid hormones and metabolic responses. Fish Physiol. Biochem. 2020, 46, 199–212. [Google Scholar] [CrossRef]
- Neupane, P.; Bhuju, S.; Thapa, N.; Bhattarai, H.K. ATP Synthase: Structure, Function and Inhibition. Biomol. Concepts 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Ulshoefer, R.; Bros, H.; Hauser, A.E.; Niesner, R.A.; Paul, F.; Malla, B.; Infante-Duarte, C. Preventing Axonal Sodium Overload or Mitochondrial Calcium Uptake Protects Axonal Mitochondria from Oxidative Stress-Induced Alterations. Oxid. Med. Cell. Longevity 2022, 2022, 6125711. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Luo, M.; Qin, L.; Guo, C.; Liu, J.; Zhang, T.; Feng, G.; Li, W. Effects of Hypoxia Stress on Survival, Antioxidant and Anaerobic Metabolic Enzymes, and Related Gene Expression of Red Swamp Crayfish Procambarus clarkii. Biology 2024, 13, 33. [Google Scholar] [CrossRef]
- Mandal, A.; Das, S.; Roy, S.; Ghosh, A.K.; Sardar, A.H.; Verma, S.; Saini, S.; Singh, R.; Abhishek, K.; Kumar, A.; et al. Deprivation of L-Arginine Induces Oxidative Stress Mediated Apoptosis in Leishmania donovani Promastigotes: Contribution of the Polyamine Pathway (Publication with Expression of Concern. See vol. 15, 2021). PLoS Neglected Trop. Dis. 2016, 10, e0004373. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.W.; Li, Y.T.; Zhou, W.W.; Tian, L.X.; Li, Y.M.; Zeng, S.L.; Liu, Y.J. Effect of γ-aminobutyric acid supplementation on growth performance, endocrine hormone and stress tolerance of juvenile Pacific white shrimp, Litopenaeus vannamei, fed low fishmeal diet. Aquacult. Nutr. 2017, 23, 54–62. [Google Scholar] [CrossRef]
- Wu, F.; Liu, M.M.; Chen, C.; Chen, J.J.; Tan, Q.S. Effects of Dietary Gamma Aminobutyric Acid on Growth Performance, Antioxidant Status, and Feeding-related Gene Expression of Juvenile Grass Carp, Ctenopharyngodon idellus. J. World Aquacult. Soc. 2016, 47, 820–829. [Google Scholar] [CrossRef]
- Bouché, N.; Fait, A.; Bouchez, D.; Moller, S.G.; Fromm, H. Mitochondrial succinic-semialdehyde dehydrogenase of the γ-aminobutyrate shunt is required to restrict levels of reactive oxygen intermediates in plants. Proc. Natl. Acad. Sci. USA 2003, 100, 6843–6848. [Google Scholar] [CrossRef]
- Fait, A.; Yellin, A.; Fromm, H. GABA shunt deficiencies and accumulation of reactive oxygen intermediates: Insight from Arabidopsis mutants. FEBS Lett. 2005, 579, 415–420. [Google Scholar] [CrossRef]
- Deng, Y.; Xu, L.J.; Zeng, X.; Li, Z.Y.; Qin, B.B.; He, N.Y. New Perspective of GABA as an Inhibitor of Formation of Advanced Lipoxidation End-Products: It’s Interaction with Malondiadehyde. J. Biomed. Nanotechnol. 2010, 6, 318–324. [Google Scholar] [CrossRef]


| Indexes | 0 mg/L GABA | 5 mg/L GABA | 50 mg/L GABA | 150 mg/L GABA |
|---|---|---|---|---|
| 3 d + 0 h | ||||
| pH | 7.48 ± 0.02 | 7.55 ± 0.18 | 7.46 ± 0.13 | 7.49 ± 0.28 |
| DO (mg/L) | 8.77 ± 0.15 | 8.57 ± 0.25 | 8.63 ± 0.25 | 8.60 ± 0.44 |
| TAN (mg/L) | 0.27 ± 0.06 | 0.24 ± 0.09 | 0.27 ± 0.03 | 0.26 ± 0.04 |
| 3 d + 6 h | ||||
| pH | 7.08 ± 0.03 b | 7.16 ± 0.06 ab | 7.20 ± 0.06 ab | 7.26 ± 0.11 a |
| DO (mg/L) | 6.47 ± 0.15 | 6.73 ± 0.15 | 6.80 ± 0.26 | 6.77 ± 0.25 |
| TAN (mg/L) | 7.31 ± 0.22 a | 6.79 ± 0.96 ab | 6.44 ± 1.09 ab | 5.97 ± 0.32 b |
| 3 d + 12 h | ||||
| pH | 6.62 ± 0.19 b | 6.75 ± 0.23 ab | 7.08 ± 0.03 a | 7.05 ± 0.02 a |
| DO (mg/L) | 3.37 ± 0.15 c | 3.60 ± 0.20 bc | 3.87 ± 0.21 ab | 4.23 ± 0.31 a |
| TAN (mg/L) | 9.79 ± 1.24 a | 9.00 ± 0.75 ab | 8.49 ± 0.97 ab | 7.95 ± 0.44 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Hou, W.; Xiao, L. Gamma-Aminobutyric Acid (GABA) Avoids Deterioration of Transport Water Quality, Regulates Plasma Biochemical Indices, Energy Metabolism, and Antioxidant Capacity of Tawny Puffer (Takifugui flavidus) under Transport Stress. Biology 2024, 13, 474. https://doi.org/10.3390/biology13070474
Yu X, Hou W, Xiao L. Gamma-Aminobutyric Acid (GABA) Avoids Deterioration of Transport Water Quality, Regulates Plasma Biochemical Indices, Energy Metabolism, and Antioxidant Capacity of Tawny Puffer (Takifugui flavidus) under Transport Stress. Biology. 2024; 13(7):474. https://doi.org/10.3390/biology13070474
Chicago/Turabian StyleYu, Xiaowen, Wenjie Hou, and Lixia Xiao. 2024. "Gamma-Aminobutyric Acid (GABA) Avoids Deterioration of Transport Water Quality, Regulates Plasma Biochemical Indices, Energy Metabolism, and Antioxidant Capacity of Tawny Puffer (Takifugui flavidus) under Transport Stress" Biology 13, no. 7: 474. https://doi.org/10.3390/biology13070474
APA StyleYu, X., Hou, W., & Xiao, L. (2024). Gamma-Aminobutyric Acid (GABA) Avoids Deterioration of Transport Water Quality, Regulates Plasma Biochemical Indices, Energy Metabolism, and Antioxidant Capacity of Tawny Puffer (Takifugui flavidus) under Transport Stress. Biology, 13(7), 474. https://doi.org/10.3390/biology13070474




