Neuroprotective Effect of Marrubium vulgare Extract in Scopolamine-Induced Cognitive Impairment in Rats: Behavioral and Biochemical Approaches
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extract Preparation
2.2. Determination of Marrubiin Content
| Time, min | A (%) | B (%) |
| 0–15 | 40–90 | 60–10 |
| 15–20 | 90–40 | 10–60 |
| 20–25 | 40 | 60 |
- A1 = the area of the marrubiin in the chromatogram of the test solution.
- A2 = the area of the marrubiin peak in the chromatogram of the reference solution.
- M1 = the mass of the extract being examined, in milligrams.
- M2 = the mass of marrubiin R, in milligrams.
- P = the percentage content of marrubiin in the marrubiin standard
2.3. Animals
2.4. Experimental Design
2.5. Assessment of Cognitive Function: Novel Object Recognition Test
2.6. Brain Dissection Technique
2.7. Determination of Acetylcholine and Monoamine Content
2.8. Determination of BDNF and pCREB Concentrations
2.9. RNA Extraction and Reverse Transcription
2.10. Quantitative Real-Time PCR
2.11. Statistical Analysis
3. Results
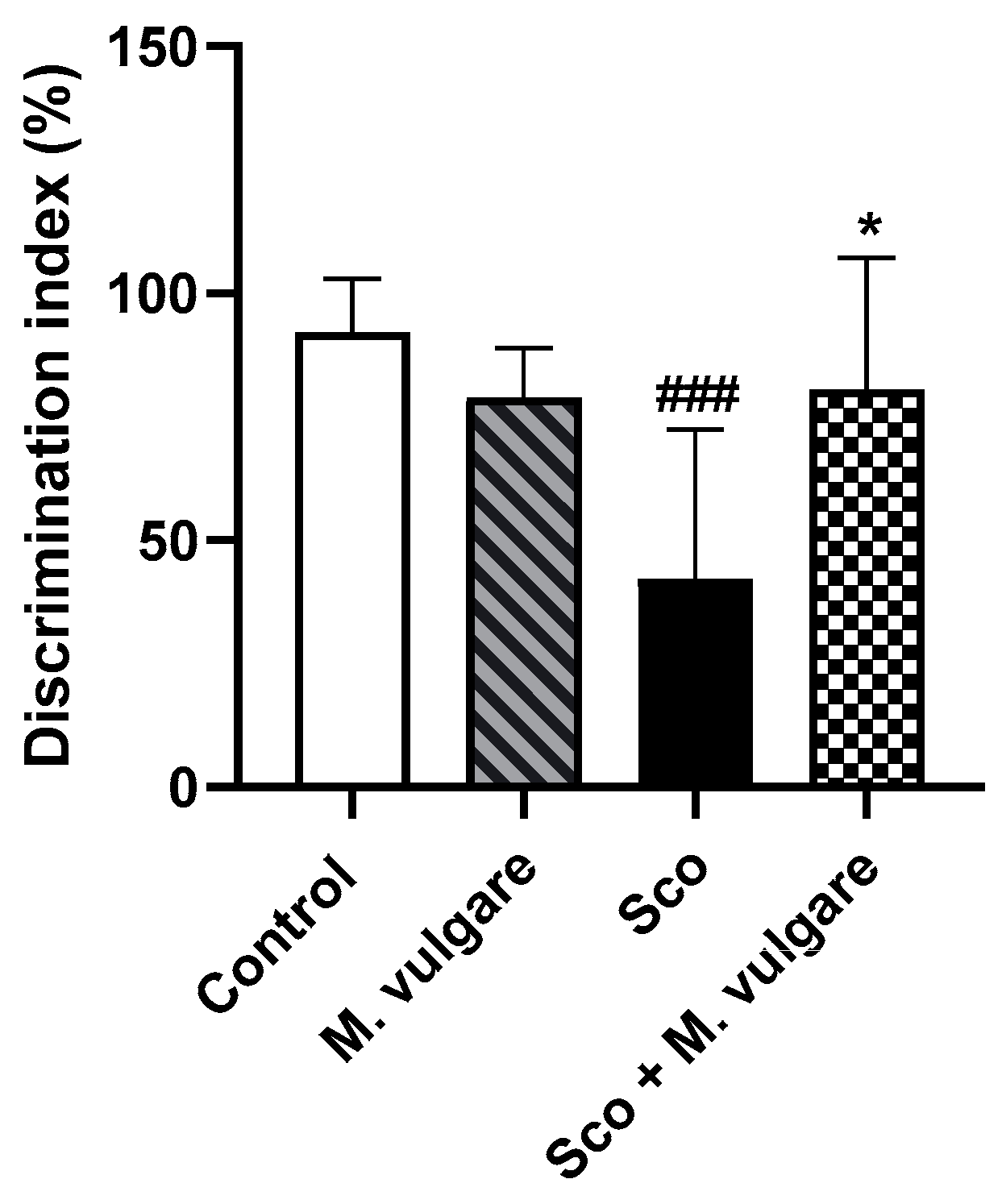
3.1. Effects of M. vulgare Treatment on Recognition Memory Performance in Healthy and Scopolamine-Treated Rats
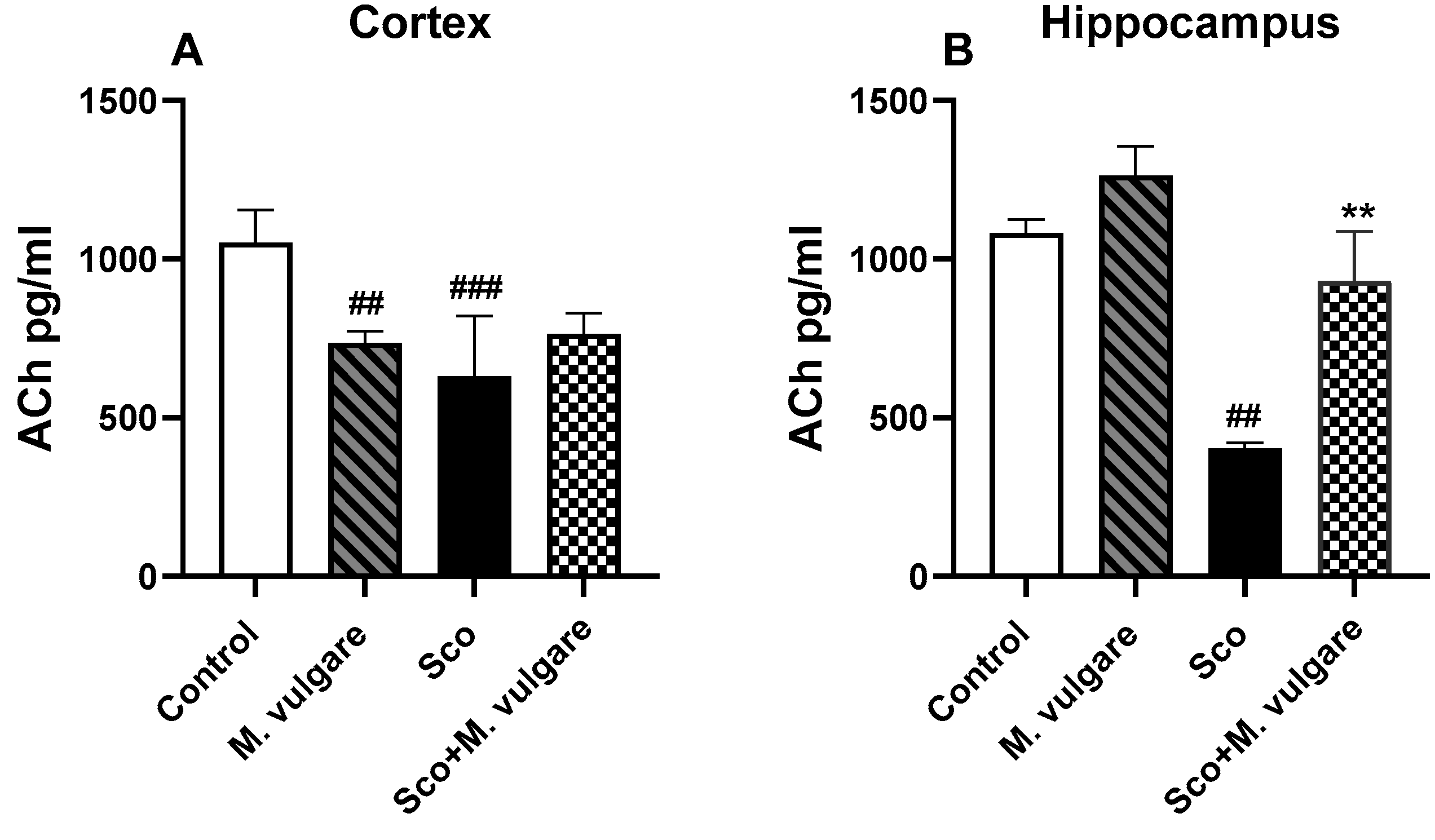
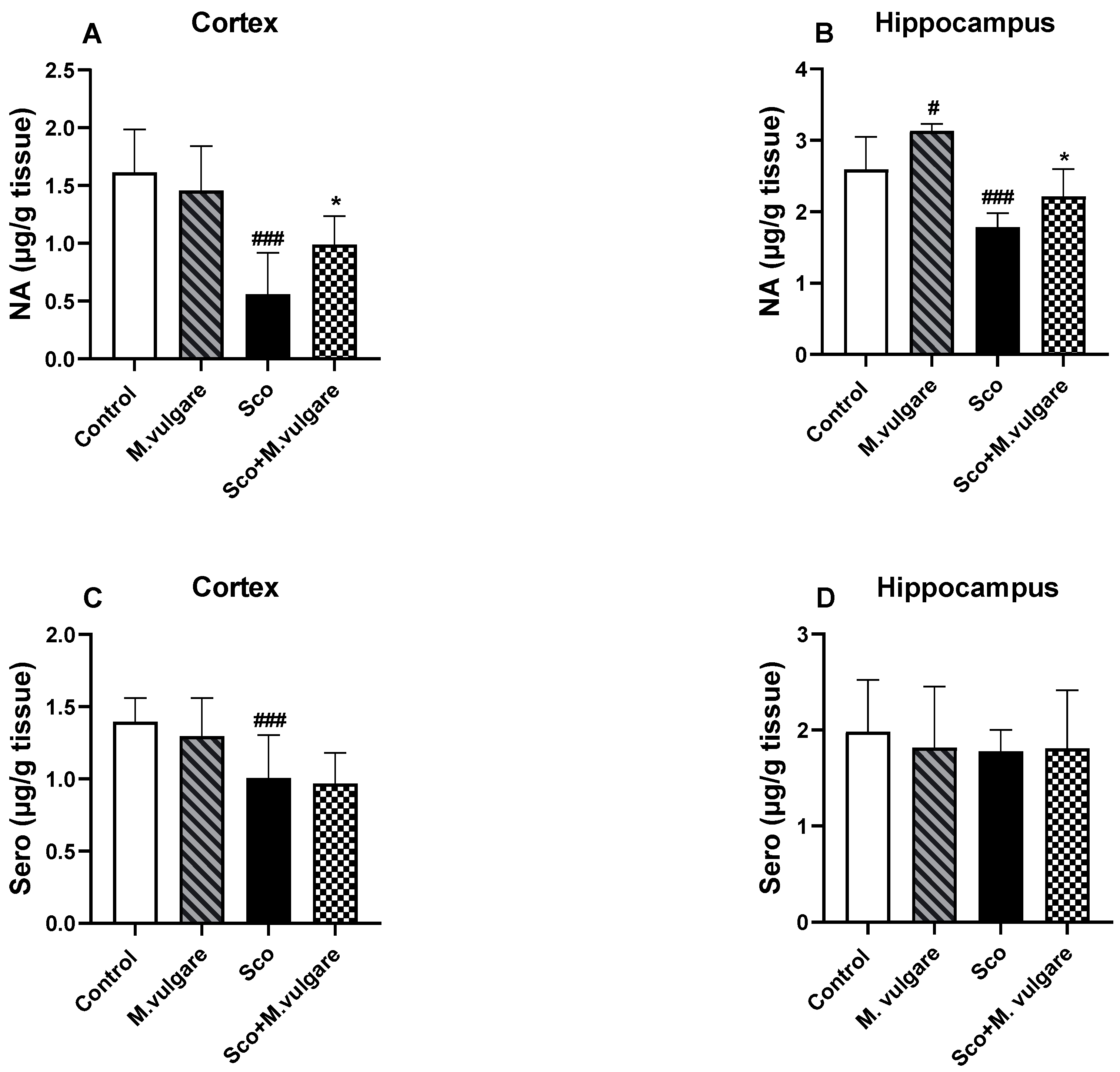
3.2. Neuromodulatory Activity of M. vulgare in the Frontal Cortex and Hippocampus of Healthy and Scopolamine-Treated Rats
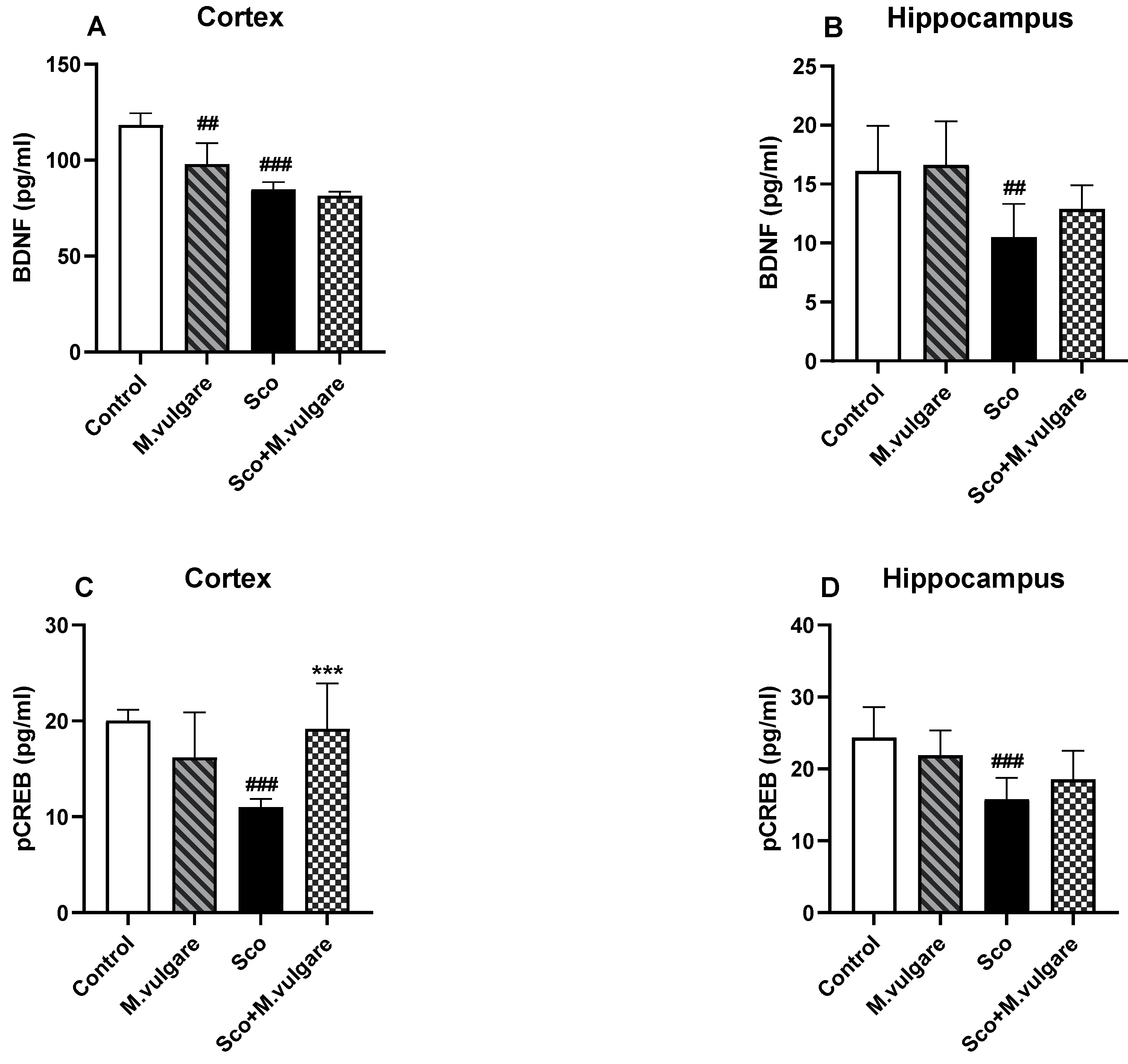
3.3. Effect of M. vulgare on the Expression Levels of BDNF and pCREB in the Frontal Cortex and Hippocampus of Healthy and Scopolamine-Treated Rats
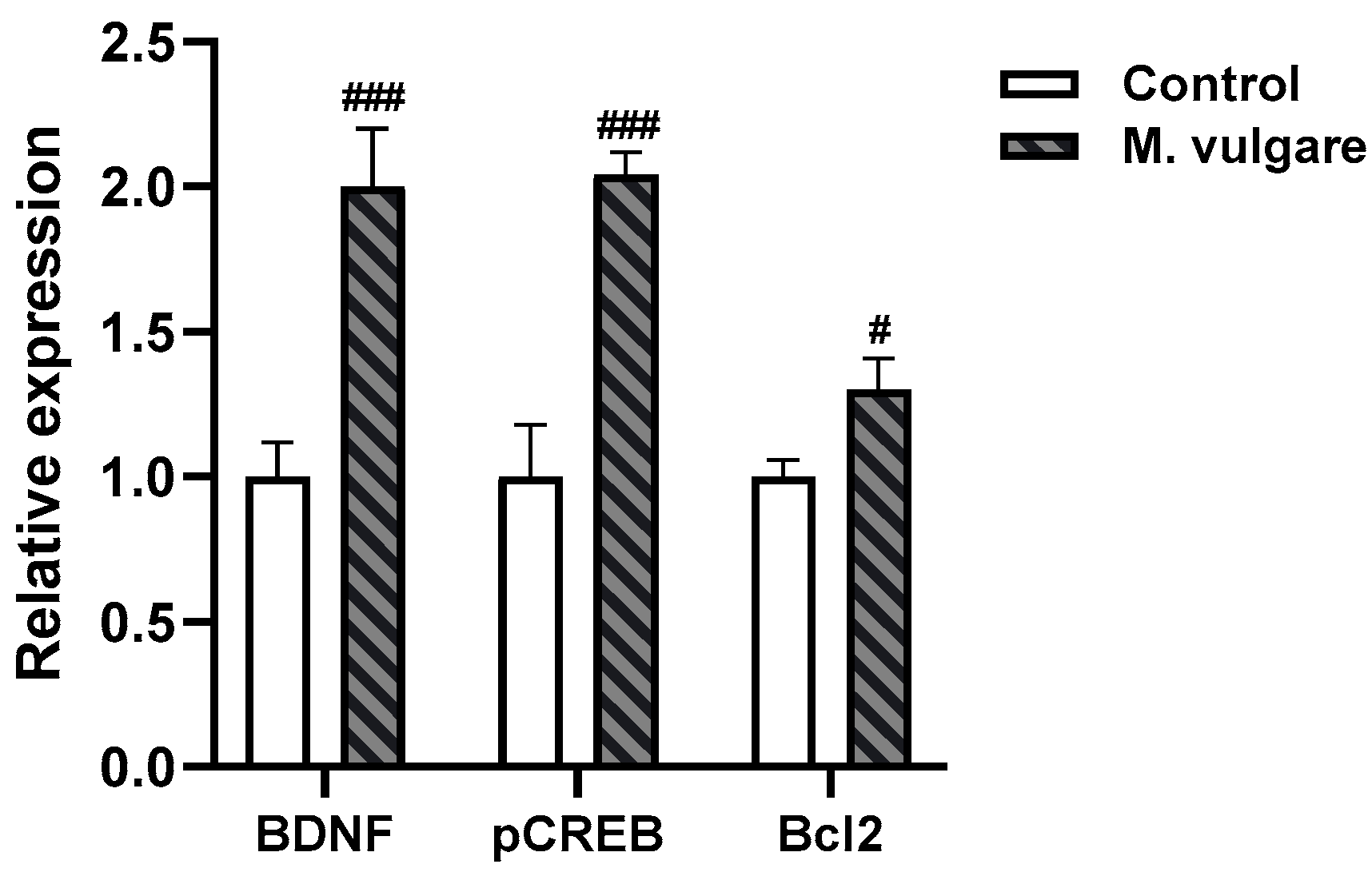
3.4. Effect of M. vulgare Treatment on Relative Expression Levels of BDNF, CREB, and Bcl2 in the Cortex of Healthy Rats
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand, A.; Khurana, P.; Chawla, J.; Sharma, N.; Khurana, N. Emerging treatments for the behavioral and psychological symptoms of dementia. CNS Spectr. 2018, 23, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Khurana, N.; Muthuraman, A. Lower vertebrate and invertebrate models of Alzheimer’s disease—A review. Eur. J. Pharmacol. 2017, 815, 312–323. [Google Scholar] [CrossRef]
- Goo, M.J.; Choi, S.M.; Kim, S.H.; Ahn, B.O. Protective effects of acetyl-L-carnitine on neurodegenerative changes in chronic cerebral ischemia models and learning-memory impairment in aged rats. Arch. Pharm. Res. 2012, 35, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Puri, A.; Srivastava, P.; Pandey, P.; Yadav, R.S.; Bhatt, P.C. Scopolamine induced behavioral and biochemical modifications and protective effect of Celastrus paniculatous and Angelica glauca in rats. Int. J. Nutr. Pharmacol. Neurol. Dis. 2014, 4, 158–169. [Google Scholar]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T. The interplay of neurotransmitters in Alzheimer’s disease. CNS Spectr. 2005, 10, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Coyle, J.T.; Price, D.L.; DeLong, M.R. Alzheimer’s disease: A disorder of cortical cholinergic innervation. Science 1983, 219, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.R.; Bowen, D.M.; Allen, S.J.; Smith, C.C.T.; Neary, D.; Thomas, D.J.; Davison, A.N. Presynaptic cholinergic dysfunction in patients with dementia. J. Neurochem. 1983, 40, 503–509. [Google Scholar] [CrossRef]
- Davies, P.; Maloney, A.J. Selective loss of central cholinergic neurons in Alzheimer’s disease. Lancet 1976, 2, 1403. [Google Scholar] [CrossRef]
- Perry, E.K.; Gibson, P.H.; Blessed, G.; Perry, R.H.; Tomlinson, B.E. Neurotransmitter enzyme abnormalities in senile dementia. Choline acetyltransferase and glutamic acid decarboxylase activities in necropsy brain tissue. J. Neurol. Sci. 1977, 34, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Whitehouse, P.J.; Price, D.L.; Struble, R.G.; Clark, A.W.; Coyle, J.T.; Delon, M.R. Alzheimer’s disease and senile dementia: Loss of neurons in the basal forebrain. Science 1982, 215, 1237–1239. [Google Scholar] [CrossRef] [PubMed]
- Ikonomovic, M.D.; Wecker, L.; Abrahamson, E.E.; Wuu, J.; Counts, S.E.; Ginsberg, S.D.; Mufson, E.J.; Dekosky, S.T. Cortical alpha7 nicotinic acetylcholine receptor and beta-amyloid levels in early Alzheimer disease. Arch. Neurol. 2009, 6, 646–651. [Google Scholar]
- Jiang, S.; Li, Y.; Zhang, C.; Zhao, Y.; Bu, G.; Xu, H.; Zhang, Y.W. M1 muscarinic acetylcholine receptor in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Stucky, A.; Liu, J.; Shen, C.; Trocme-Thibierge, C.; Morain, P. Dissociating beta-amyloid from alpha 7 nicotinic acetylcholine receptor by a novel therapeutic agent, S 24795, normalizes alpha 7 nicotinic acetylcholine and NMDA receptor function in Alzheimer’s disease brain. J. Neurosci. 2009, 29, 10961–10973. [Google Scholar] [CrossRef] [PubMed]
- Zuchner, T.; Schliebs, R.; Perez-Polo, J.R. Down-regulation of muscarinic acetylcholine receptor M2 adversely affects the expression of Alzheimer’s disease-relevant genes and proteins. J. Neurochem. 2005, 95, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, J.; Hahm, D.H.; Lee, H. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complement. Altern. Med. 2014, 14, 338. [Google Scholar] [CrossRef]
- Goverdhan, P.; Sravanthi, A.; Mamatha, T. Neuroprotective effects of meloxicam and selegiline in scopolamine-induced cognitive impairment and oxidative stress. Int. J. Alzheimer’s Dis. 2012, 2012, 974013. [Google Scholar] [CrossRef]
- Abd-El-Fattah, M.A.; Abdelakader, N.F.; Zaki, H.F. Pyrrolidine dithiocarbamate protects against scopolamine-induced cognitive impairment in rats. Eur. J. Pharmacol. 2014, 723, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Tsvetanova, E.; Georgieva, A.; Stefanova, M.; Uzunova, D.; Denev, P.; Vassileva, V.; Tasheva, K. Extracts of Sideritis scardica and Clinopodium vulgare alleviate cognitive impairments in scopolamine-induced rat dementia. Int. J. Mol. Sci. 2024, 25, 1840. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.I.; Tsvetanova, E.R.; Georgieva, A.P.; Stefanova, M.O.; Uzunova, D.N.; Denev, P.N.; Tasheva, K.N. Marrubium vulgare extract improves spatial working memory and oxidative stress damage in scopolamine-treated rats. J. Alzheimers Dis. 2024, 99, S157–S169. [Google Scholar] [CrossRef] [PubMed]
- Lazarova, M.; Tancheva, L.; Alexandrova, A.; Tsvetanova, E.; Georgieva, A.; Stefanova, M.; Tsekova, D.; Vezenkov, L.; Kalfin, R.; Uzunova, D.; et al. Effects of new galantamine derivatives in a scopolamine model of dementia in mice. J. Alzheimers Dis. 2021, 84, 671–690. [Google Scholar] [CrossRef] [PubMed]
- Tancheva, L.; Lazarova, M.; Velkova, L.; Dolashki, A.; Uzunova, D.; Minchev, B.; Petkova-Kirova, P.; Hassanova, Y.; Gavrilova, P.; Tasheva, K.; et al. Beneficial effects of snail Helix aspersa extract in an experimental model of Alzheimer’s type dementia. J. Alzheimers Dis. 2022, 88, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.S. The cellular and molecular processes associated with scopolamine-induced memory deficit: A model of Alzheimer’s biomarkers. Life Sci. 2019, 233, 116695. [Google Scholar] [CrossRef] [PubMed]
- Campos, C.; Rocha, N.B.; Vieira, R.T.; Rocha, S.A.; Telles Correia, D.; Paes, F.; Yuan, T.; Nardi, A.E.; Arias-Carrion, O.; Machado, S.; et al. Treatment of cognitive deficits in Alzheimer’s disease: A psychopharmacological review. Psychiatr. Danub. 2016, 28, 2–12. [Google Scholar] [PubMed]
- McGleenon, B.M.; Dynan, K.B.; Passmore, A.P. Acetylcholinesterase inhibitors in Alzheimer’s disease. Br. J. Clin. Pharmacol. 1999, 8, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Craig, L.A.; Hong, N.S.; McDonald, R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011, 35, 1397–1409. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed]
- Corbett, A.; Smith, J.; Creese, B.; Ballard, C. Treatment of behavioral and psychological symptoms of Alzheimer’s disease. Curr. Treat. Options Neurol. 2012, 14, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, A.; Manera, V.; Koenig, A.; David, R. Pharmacologic approaches for the management of apathy in neurodegenerative disorders. Front. Pharmacol. 2020, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Fink, H.A.; Linskens, E.J.; MacDonald, R.; Silverman, P.C.; McCarten, J.R.; Talley, K.M.C.; Forte, M.L.; Desai, P.J.; Nelson, V.A.; Miller, M.A.; et al. Benefits and harms of prescription drugs and supplements for treatment of clinical Alzheimer-type dementia. Ann. Intern. Med. 2020, 172, 656–668. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, S. Cholinergic adverse effects of cholinesterase inhibitors in Alzheimer’s disease: Epidemiology and management. Drugs Aging 2001, 18, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer’s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement. 2020, 6, e12050. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.; Swanson, C.; Aisen, P.; Bateman, R.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Ayatollahi, S.A.; Varoni, E.M.; Salehi, B.; Kobarfard, F.; Sharifi-Rad, M.; Iriti, M.; Sharifi-Rad, M. Chemical composition and functional properties of essential oils from Nepeta schiraziana Boiss. Farmacia 2017, 65, 802–812. [Google Scholar]
- Sharifi-Rad, J.; Sharifi-Rad, M.; Salehi, B.; Iriti, M.; Roointan, A.; Mnayer, D.; Soltani-Nejad, A.; Afshari, A. In vitro and in vivo assessment of free radical scavenging and antioxidant activities of Veronica persica Poir. Cell. Mol. Biol. 2018, 64, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Taheri, Y.; Shaheen, S.; Kregiel, D.; Antolak, H.; Pawlikowska, E.; Brdar-Jokanovi’c, M.; Rajkovic, J.; et al. Malva species: Insights on its chemical composition towards pharmacological applications. Phytother. Res. 2020, 34, 546–567. [Google Scholar] [CrossRef]
- Patti, F.; Taheri, Y.; Sharifi-Rad, J.; Martorell, M.; C Cho, W.; Pezzani, R. Erythrina suberosa: Ethnopharmacology, phytochemistry and biological activities. Medicines 2019, 6, 105. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Stojanović-Radić, Z.; Matejić, J.; Sharopov, F.; Antolak, H.; Kręgiel, D.; Sen, S.; Sharifi-Rad, M.; Acharya, K.; Sharifi-Rad, R.; et al. Plants of genus Mentha: From farm to food factory. Plants 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Al-Megrin, W.A.; Alkhuriji, A.F.; Yousef, A.O.S.; Metwally, D.M.; Habotta, O.A.; Kassab, R.B.; Abdel Moneim, A.E.; El-Khadragy, M.F. Antagonistic efficacy of luteolin against lead acetate exposureassociated with hepatotoxicity is mediated via antioxidant, anti-inflammatory, and anti-apoptotic activities. Antioxidants 2019, 9, 10. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Pace, S.; Haskell, C.; Okello, E.J.; Milne, A.; Scholey, A.B. Effects of cholinesterase inhibiting sage (Salvia officinalis) on mood, anxiety and performance on a psychological stressor battery. Neuropsychopharmacology 2006, 31, 845–852. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Scholey, A.B.; Tildesley, N.T.J.; Perry, E.K.; Wesnes, K.A. Modulation of mood and cognitive performance following acute administration of Melissa officinalis (lemon balm). Pharmacol. Biochem. Behav. 2002, 72, 953–964. [Google Scholar] [CrossRef]
- Ozarowski, M.; Mikolajczak, P.L.; Bogacz, A.; Gryszczynska, A.; Kujawska, M.; Jodynis-Liebert, J.; Piasecka, A.; Napieczynska, H.; Szulc, M.; Kujawski, R.; et al. Rosmarinus officinalis L. leaf extract improves memory impairment and affects acetylcholinesterase and butyrylcholinesterase activities in rat brain. Fitoterapia 2013, 91, 261–271. [Google Scholar] [CrossRef]
- Gürbüz, P.; Martinez, A.; Pérez, C.; Martínez-González, L.; Göger, F.; Ayran, İ. Potential anti-Alzheimer effects of selected Lamiaceae plants through polypharmacology on glycogen synthase kinase-3β, β-secretase, and casein kinase 1δ. Ind. Crops Prod. 2019, 138, 111431. [Google Scholar] [CrossRef]
- Topcu, G.; Kusman, T. Lamiaceae Family Plants as a Potential Anticholinesterase Source in the Treatment of Alzheimer’s Disease. Bezmialem Sci. 2014, 1, 1–25. [Google Scholar] [CrossRef]
- Perry, N.; Menzies, R.; Hodgson, F.; Wedgewood, P.; Howes, M.J.; Brooker, H.; Wesnes, K.; Perry, E. A randomised double-blind placebo-controlled pilot trial of a combined extract of sage, rosemary and melissa, traditional herbal medicines, on the enhancement of memory in normal healthy subjects, including influence of age. Phytomedicine 2018, 39, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Melissa officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomised, placebo controlled trial. J. Neurol. Neurosurg. Psychiatry 2003, 74, 863–866. [Google Scholar] [CrossRef] [PubMed]
- Akhondzadeh, S.; Noroozian, M.; Mohammadi, M.; Ohadinia, S.; Jamshidi, A.H.; Khani, M. Salvia officinalis extract in the treatment of patients with mild to moderate Alzheimer’s disease: A double blind, randomized and placebo-controlled trial. J. Clin. Pharm. Ther. 2003, 28, 53–59. [Google Scholar] [CrossRef] [PubMed]
- European Medicine Agency. Committee on Herbal Medicinal Products (HMPC) Community Herbal Monograph on Marrubium vulgare L., Herba, 604271/2012. 2013. Available online: https://www.ema.europa.eu/en/medicines/herbal/marrubii-herba (accessed on 20 May 2024).
- Boudjelal, A.; Henchiri, C.; Siracusa, L.; Sari, M.; Ruberto, G. Compositional analysis and in vivo anti-diabetic activity of wild Algerian Marrubium vulgare L. infusion. Fitoterapia 2012, 83, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Ghedadba, N.; Bousselsela, H.; Hambaba, L.; Benbia, S.; Mouloud, Y. Évaluation de l’activité antioxydante et antimicrobienne des feuilles et des sommités fleuries de Marrubium vulgare L. Phytothérapie 2014, 12, 15–24. [Google Scholar] [CrossRef]
- Masoodi, M.; Ahmed, B.; Zargar, I.; Khan, S.; Khan, S.; Singh, P. Antibacterial activity of whole plant extract of Marrubium vulgare. AJB 2008, 7, 086–087. [Google Scholar]
- Paula de Oliveira, A.; Santin, J.R.; Lemos, M.; Klein Junior, L.C.; Couto, A.G.; Meyre da Silva Bittencourt, C.; Filho, V.C.; Faloni de Andrade, S. Gastroprotective activity of methanol extract and marrubiin obtained from leaves of Marrubium vulgare L. (Lamiaceae). J. Pharm. Pharmacol. 2011, 63, 1230–1237. [Google Scholar] [CrossRef]
- Kanyonga, P.; Faouzi, M.; Meddah, B.; Mpona, M.; Essassi, E.; Cherrah, Y. Assessment of methanolic extract of Marrubium vulgare for anti-inflammatory, analgesic and anti-microbiologic activities. J. Chem. Pharm. Res. 2011, 3, 199–204. [Google Scholar]
- Nidhi; Singh, G.; Valecha, R.; Shukla, G.; Kaushik, D.; Rahman, M.A.; Gautam, R.K.; Madan, K.; Mittal, V.; Singla, R.K. Neurobehavioral and biochemical evidences in support of protective effect of marrubiin (furan labdane diterpene) from Marrubium vulgare Linn. and its extracts after traumatic brain injury in experimental mice. Evid. Based Complement. Alternat Med. 2022, 2022, 4457973. [Google Scholar]
- White Horehound. Ph Eur Monograph 1835, European Pharmacopoeia, 7th ed.; Council Of Europe, European Directorate for the Quality of Medicines and Healthcare: Strasbourg, France, 2013. [Google Scholar]
- Tzvetanova, E.; Georgieva, A.; Alexandrova, A.; Tancheva, L.; Lazarova, M.; Dragomanova, S.; Alova, L.; Stefanova, M.; Kalfin, R. Antioxidant mechanisms in neuroprotective action of lipoic acid on learning and memory of rats with experimental dementia. Bul. Chem. Commun. 2018, 50, 52–57. [Google Scholar]
- Staykov, H.; Lazarova, M.; Hassanova, Y.; Stefanova, M.; Tancheva, L.; Nikolov, R. Neuromodulatory mechanisms of a memory loss-preventive effect of alpha-lipoic acid in an experimental rat model of dementia. J. Mol. Neurosci. 2022, 72, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J. Physiol. Pharmacol. 2012, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shukla, R.; Kavindra Tiwari, K.N.; Dubey, G.P.; Mishra, S.K. Neuroprotective effect of Reinwardtia indica against scopolamine induced memory-impairment in rat by attenuating oxidative stress. Metab. Brain Dis. 2020, 35, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, S.; Ilango, K.; Agrawal, A.; Dubey, G.P. Efect of hippophae rhamnoides on cognitive enhancement via neurochemical modulation in scopolamine induced Sprague Dawely rats. Int. J. Pharm. Sci. Res. 2014, 6, 4153–4158. [Google Scholar]
- Ennaceur, A.; Delacour, J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988, 31, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Jacobowitz, D.M.; Richardson, J.S. Method for the rapid determination of norepinephrine, dopamine and serotonin in the same brain region. Pharmacol. Biochem. Behav. 1978, 8, 515–519. [Google Scholar] [CrossRef] [PubMed]
- Ramezani, N.; Vanaky, B.; Shakeri, N.; Soltanian, Z.; Fakhari Rad, F.; Shams, Z. Evaluation of Bcl-2 and Bax expression in the heart of diabetic rats after four weeks of high intensity interval training. MLJ 2019, 13, 15–20. [Google Scholar] [CrossRef]
- El Kharroubi, A.; Piras, G.; Stewart, C.L. DNA demethylation reactivates a subset of imprinted genes in uniparental mouse embryonic fibroblasts. J. Biol. Chem. 2001, 276, 8674–8680. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in Real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Giacobini, E. Long-term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer’ disease. J. Neural Transm. Suppl. 2002, 62, 181–187. [Google Scholar]
- Yamada, K.; Nabeshima, T. Brain-derived neurotrophic factor/TrkB signaling in memory processes. J. Pharmacol. Sci. 2003, 91, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Bekinschtein, P.; Cammarota, M.; Izquierdo, I.; Medina, J.H. Reviews: BDNF and memory formation and storage. Neuroscientist 2008, 14, 147–156. [Google Scholar] [CrossRef] [PubMed]
- El-Sherbiny, D.A.; Khalifa, A.E.; Attia, A.S.; Eldenshary, D. Hypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolamine. Pharmacol. Biochem. Behav. 2003, 76, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Soellner, D.E.; Grandys, T.; Joseph, L.; Nunez, J.L. Chronic prenatal caffeine exposure impairs novel object recognition and radial arm maze behaviors in adult rats. Behav. Brain Res. 2009, 205, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Popoola, O.K.; Elbagory, A.M.; Ameer, F.; Hussein, A.A. Marrubiin. Molecules 2013, 18, 9049–9060. [Google Scholar] [CrossRef] [PubMed]
- Eltahawy, N.; Ali, A.; Ibrahim, S.; Nafie, M.; Sindi, A.; Alkharobi, H.; Almalki, A.; Badr, J.; Elhady, S.; Abdelhameed, R. Analysis of marrubiin in Marrubium alysson L. extract using advanced HPTLC: Chemical profiling, acetylcholinesterase inhibitory activity, and molecular docking. Metabolites 2024, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Hasanein, P.; Mahtaj, A.K. Ameliorative effect of rosmarinic acid on scopolamineinduced memory impairment in rats. Neurosci. Lett. 2015, 585, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, J.L.; Liu, R.; Li, X.X.; Li, J.F.; Zhang, L. Neuroprotective, antiamyloidogenic and neurotrophic effects of apigenin in an Alzheimer’s disease mouse model. Molecules 2013, 18, 9949–9965. [Google Scholar] [CrossRef]
- Micheau, J.; Marighetto, A. Acetylcholine and memory: A long, complex and chaotic but still living relationship. Behav. Brain Res. 2011, 221, 424–429. [Google Scholar] [CrossRef]
- Eichenbaum, H. Memory: Organization and control. Annu. Rev. Psychol. 2016, 68, 19–45. [Google Scholar] [CrossRef]
- Loughlin, S.E.; Foote, S.L.; Grzanna, R. Efferent projections of nucleus locus coeruleus: Morphologic subpopulations have different efferent targets. Neuroscience 1986, 18, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Fallon, J.H.; Koziell, D.A.; Moore, R.Y. Catecholamine innervations of the basal forebrain. II. Amygdala, suprarhinal cortex and entorhinal cortex. J. Com. Neurol. 1978, 180, 509–532. [Google Scholar] [CrossRef]
- Loughlin, S.E.; Foote, S.L.; Fallon, J.H. Locus coeruleus projections to cortex: Topography, morphology and collateralization. Brain Res. Bull. 1982, 9, 287–294. [Google Scholar] [CrossRef]
- Vankov, A.; Hervé-Minvielle, A.; Sara, S.J. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur. J. Neurosci. 1995, 7, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Rajkowski, J.; Kubiak, P.; Aston-Jones, G. Locus coeruleus activity in monkey: Phasic and tonic changes are associated with altered vigilance. Brain Res. Bull. 1994, 35, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Theofilas, P.; Ehrenberg, A.J.; Dunlop, S.; Di Lorenzo Alho, A.T.; Nguy, A.; Leite, R.E.P.; Rodriguez, R.D.; Mejia, M.B.; Suemoto, C.K.; Ferretti-Rebustini, R.E.; et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017, 13, 236–246. [Google Scholar] [CrossRef]
- Tyler, W.J.; Alonso, M.; Bramham, C.R.; Pozzo-Miller, L.D. From acquisition to consolidation: On the role of brain-derived neurotrophic factor signaling in hippocampal-dependent learning. Learn. Mem. 2002, 9, 224–237. [Google Scholar] [CrossRef]
- Xu, J.; Rong, S.; Xie, B.; Sun, Z.; Deng, Q.; Wu, H.; Bao, W.; Wang, D.; Yao, P.; Huang, F.; et al. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: Reversal by procyanidins extracted from the lotus seedpod. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 933–940. [Google Scholar] [CrossRef]
- Vaynman, S.; Ying, Z.; Gomez-Pinilla, F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience 2003, 122, 647–657. [Google Scholar] [CrossRef]
- Kida, S.; Josselyn, S.A.; Peña de Ortiz, S.; Kogan, J.H.; Chevere, I.; Masushige, S.; Silva, A.J. CREB required for the stability of new and reactivated fear memories. Nat. Neurosci. 2002, 5, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Guzowski, J.F.; McGaugh, J.L. Antisense oligodeoxynucleotidemediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc. Natl. Acad. Sci. USA 1997, 94, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Thompson, M.A.; Greenberg, M.E. CREB: A Ca2+-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science 1991, 252, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.; Tavazoie, S.F.; Maloratsky, A.; Jacobs, K.M.; Harris, K.M.; Greenberg, M.E. CREB: A major mediator of neuronal neurotrophin responses. Neuron 1997, 19, 1031–1047. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, C.; Ding, W. Z-guggulsterone improves the scopolamine-induced memory impairments through enhancement of the BDNF signal in C57BL/6 J mice. Neurochem. Res. 2016, 41, 3322–3332. [Google Scholar] [CrossRef]

| Gene. | Primer sequences (5′-3′) | Annealing Temperature (°C) | Product Size (bp) |
|---|---|---|---|
| BDNF | TGGCTGACACTTTTGAGCAC | 58 | 188 |
| GTTTGCGGCATCCAGGTAAT | |||
| CREB | TGCACAGACCACTGATGGAC | 59 | 286 |
| TTCAAGCACTGCCACTCTGT | |||
| Bcl2 | GGATTGTGGCCTTCTTTGAGTTC | 59 | 88 |
| AGAGCGATGTTGTCCACCAG | |||
| GAPDH | ACCACAGTCCATGCCATCACTGCCAC | 58 | 447 |
| TCCACCACCCTGTTGCTGTA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazarova, M.; Stefanova, M.; Denev, P.; Taseva, T.; Vassileva, V.; Tasheva, K. Neuroprotective Effect of Marrubium vulgare Extract in Scopolamine-Induced Cognitive Impairment in Rats: Behavioral and Biochemical Approaches. Biology 2024, 13, 426. https://doi.org/10.3390/biology13060426
Lazarova M, Stefanova M, Denev P, Taseva T, Vassileva V, Tasheva K. Neuroprotective Effect of Marrubium vulgare Extract in Scopolamine-Induced Cognitive Impairment in Rats: Behavioral and Biochemical Approaches. Biology. 2024; 13(6):426. https://doi.org/10.3390/biology13060426
Chicago/Turabian StyleLazarova, Maria, Miroslava Stefanova, Petko Denev, Teodora Taseva, Valya Vassileva, and Krasimira Tasheva. 2024. "Neuroprotective Effect of Marrubium vulgare Extract in Scopolamine-Induced Cognitive Impairment in Rats: Behavioral and Biochemical Approaches" Biology 13, no. 6: 426. https://doi.org/10.3390/biology13060426
APA StyleLazarova, M., Stefanova, M., Denev, P., Taseva, T., Vassileva, V., & Tasheva, K. (2024). Neuroprotective Effect of Marrubium vulgare Extract in Scopolamine-Induced Cognitive Impairment in Rats: Behavioral and Biochemical Approaches. Biology, 13(6), 426. https://doi.org/10.3390/biology13060426










