Description of a Remarkable New Skate Species of Leucoraja Malm, 1877 (Rajiformes, Rajidae) from the Southwestern Indian Ocean: Introducing 3D Modeling as an Innovative Tool for the Visualization of Clasper Characters
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Genus Leucoraja Malm, 1877
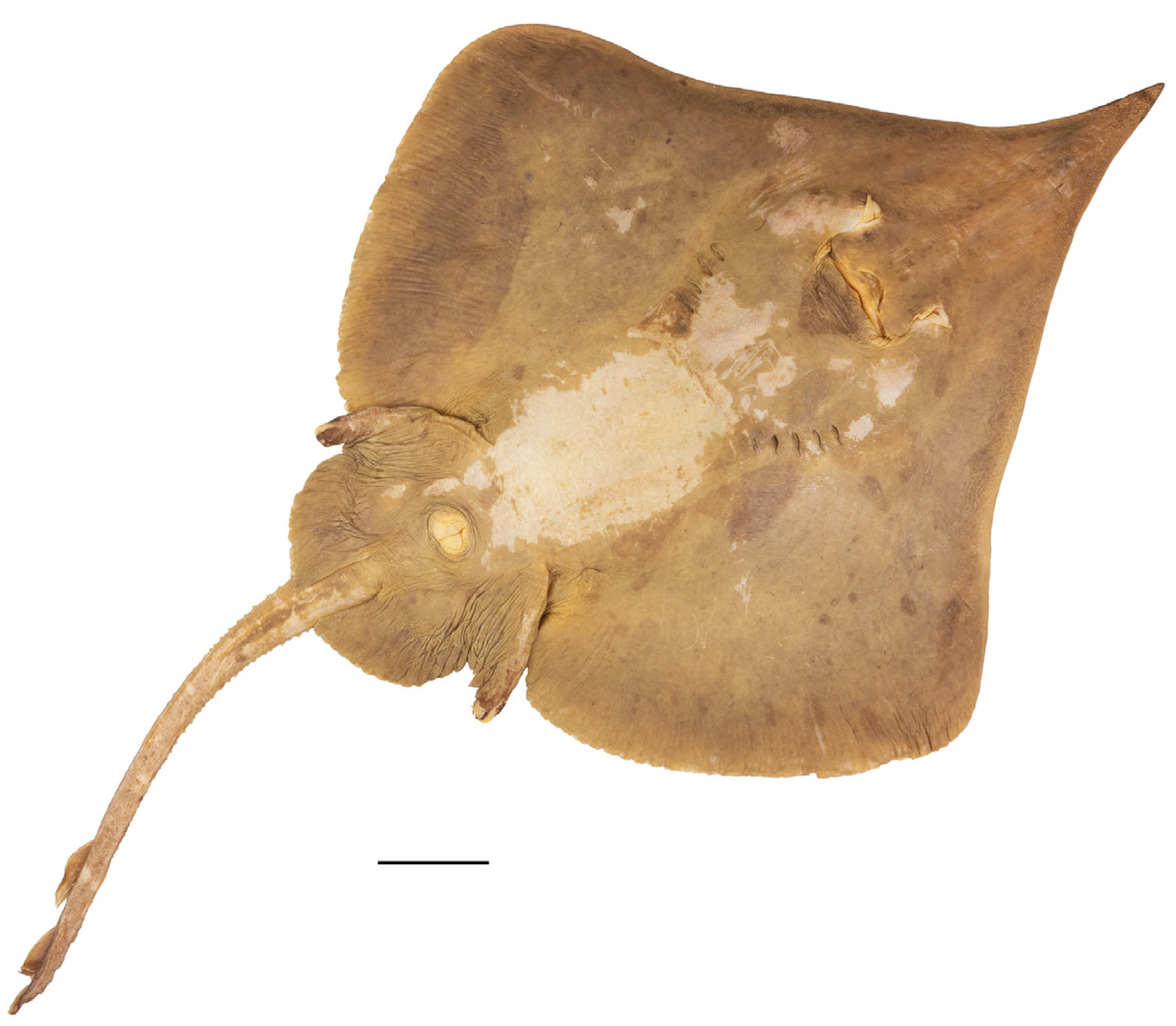
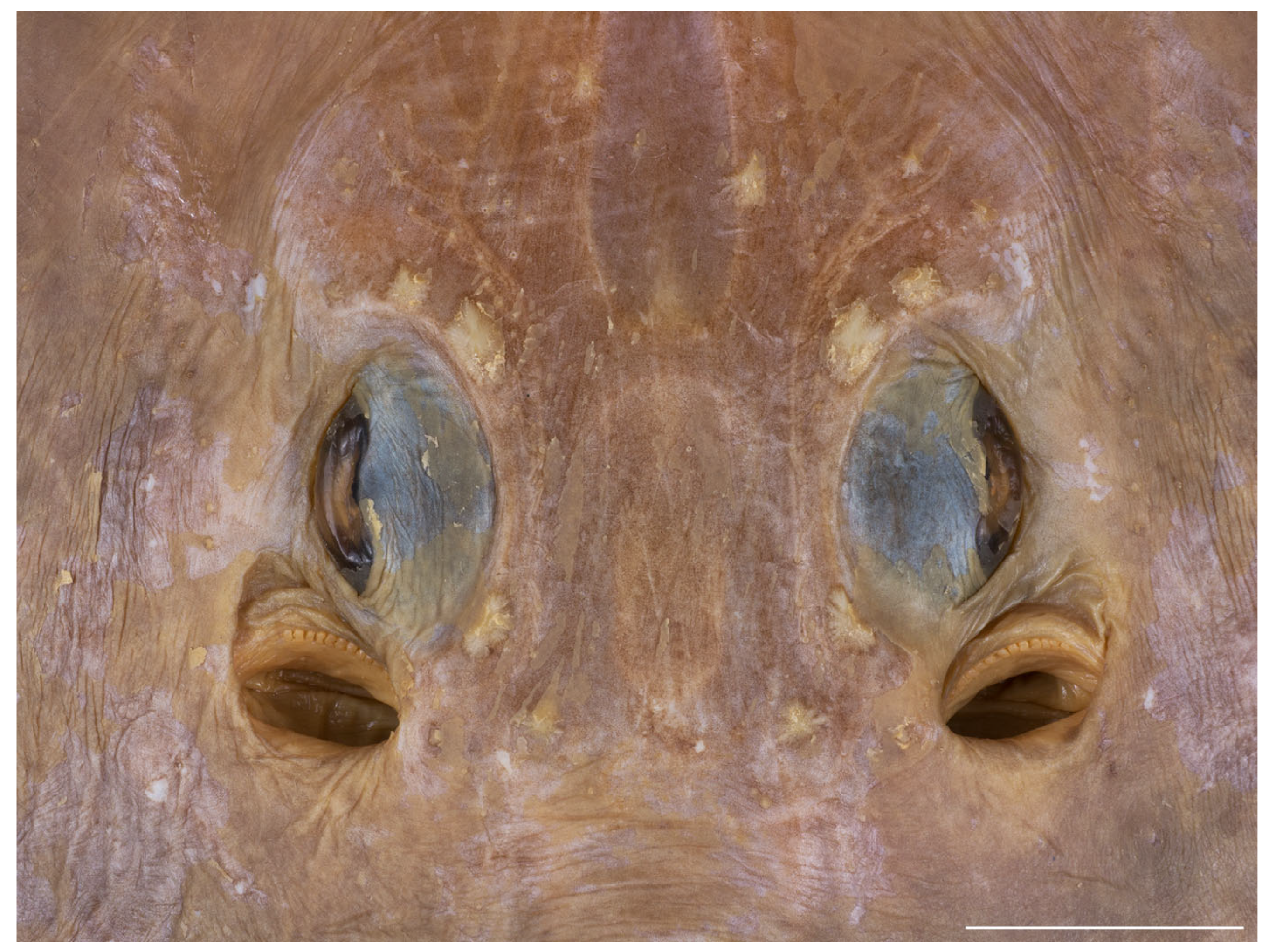
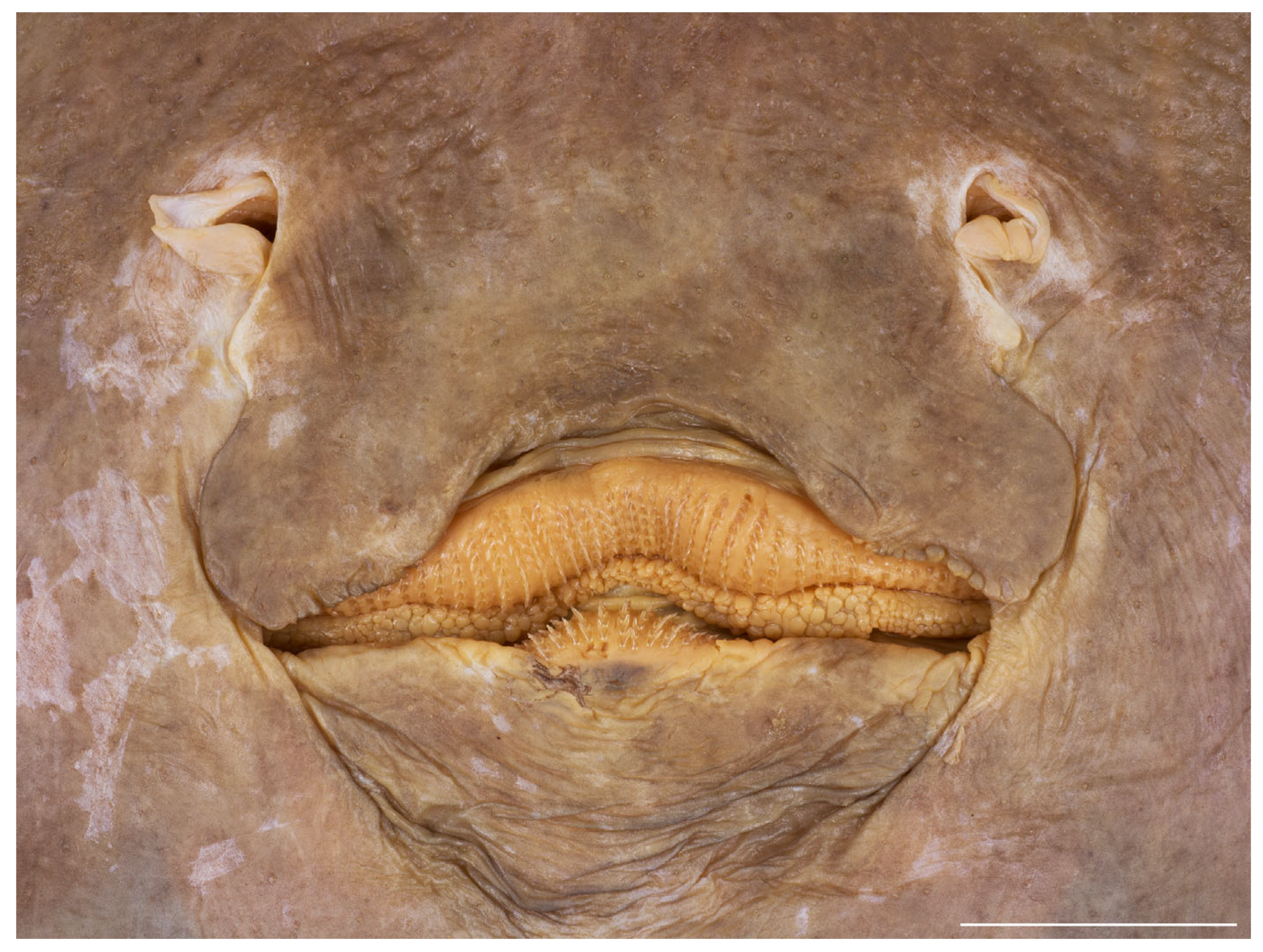
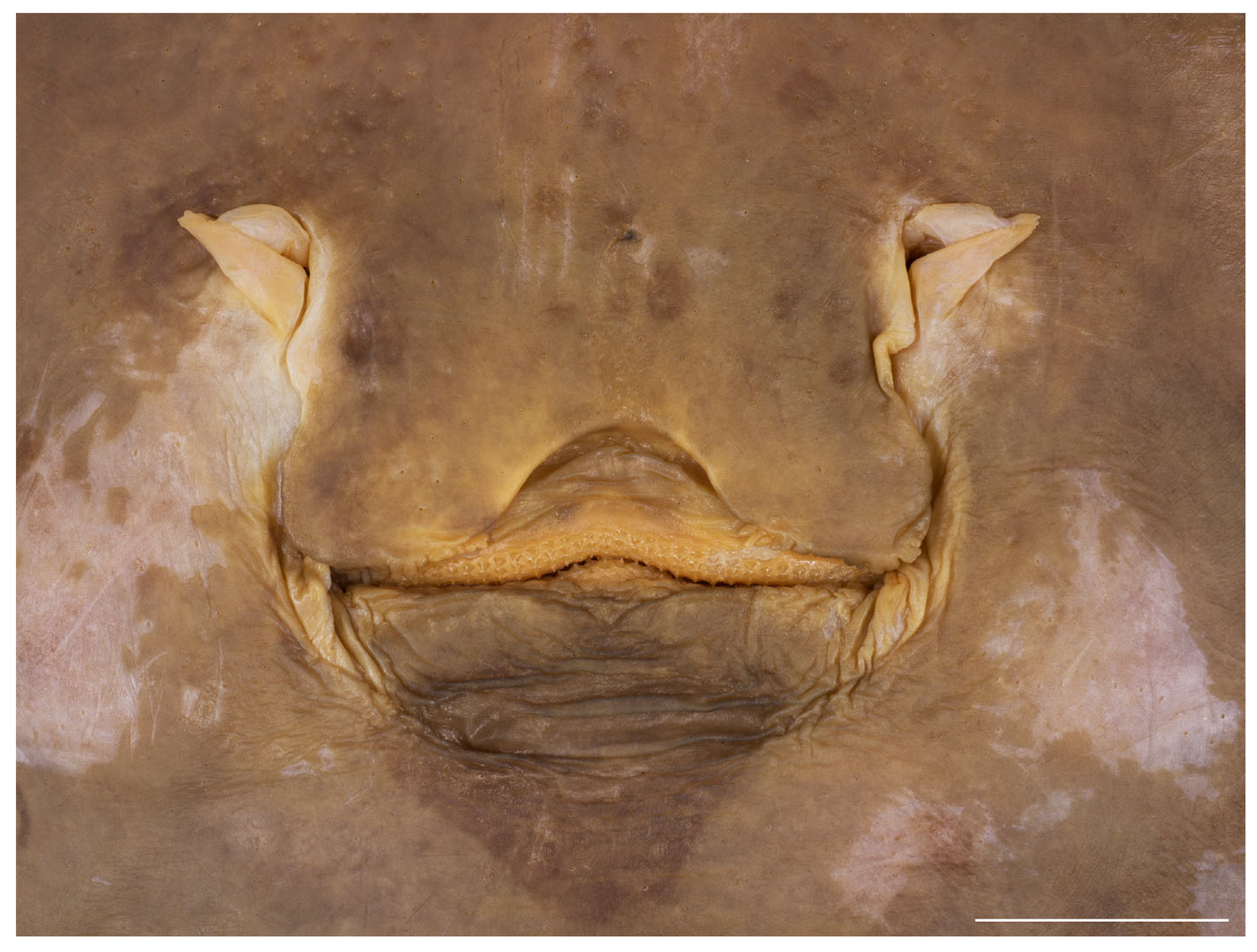
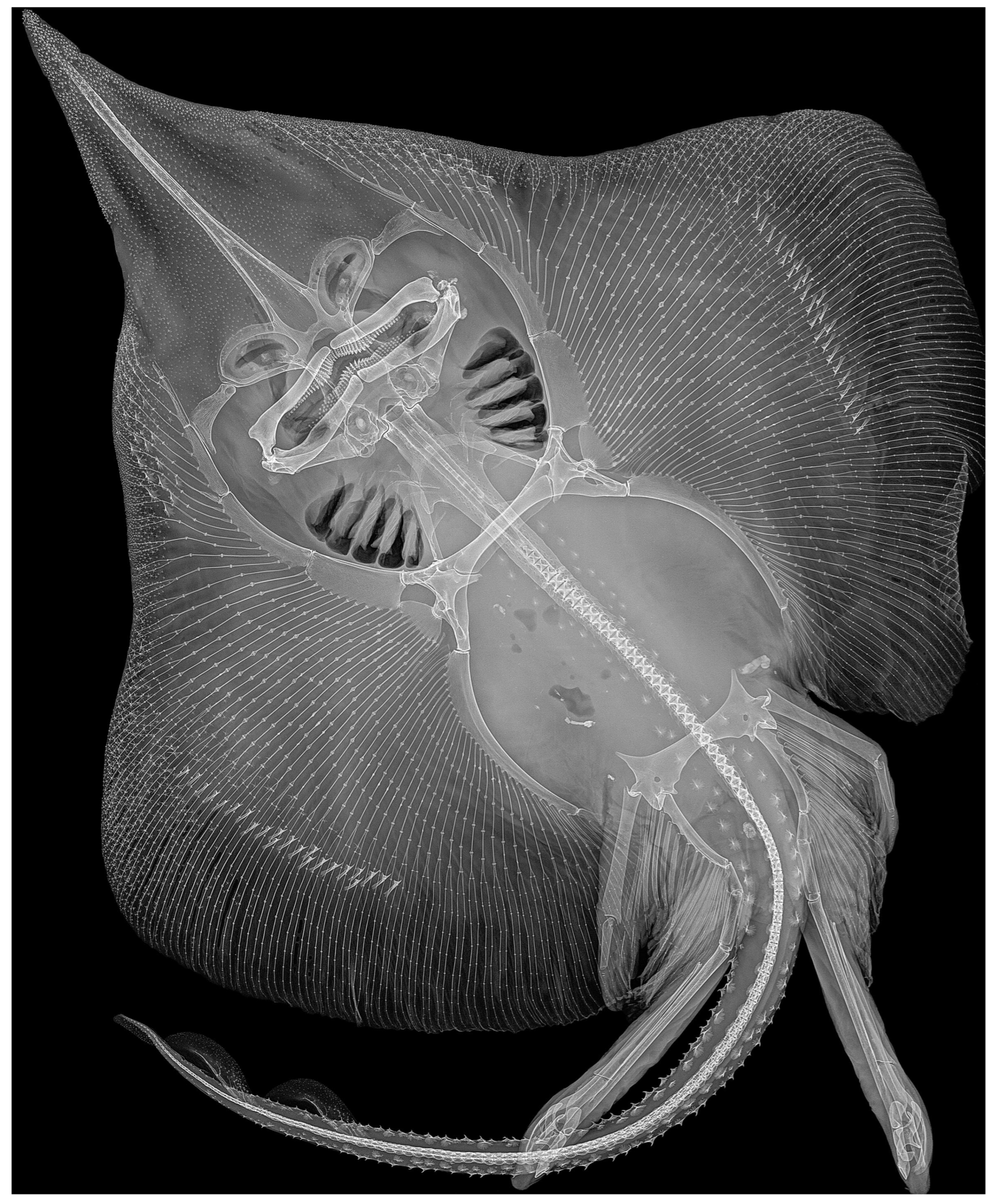
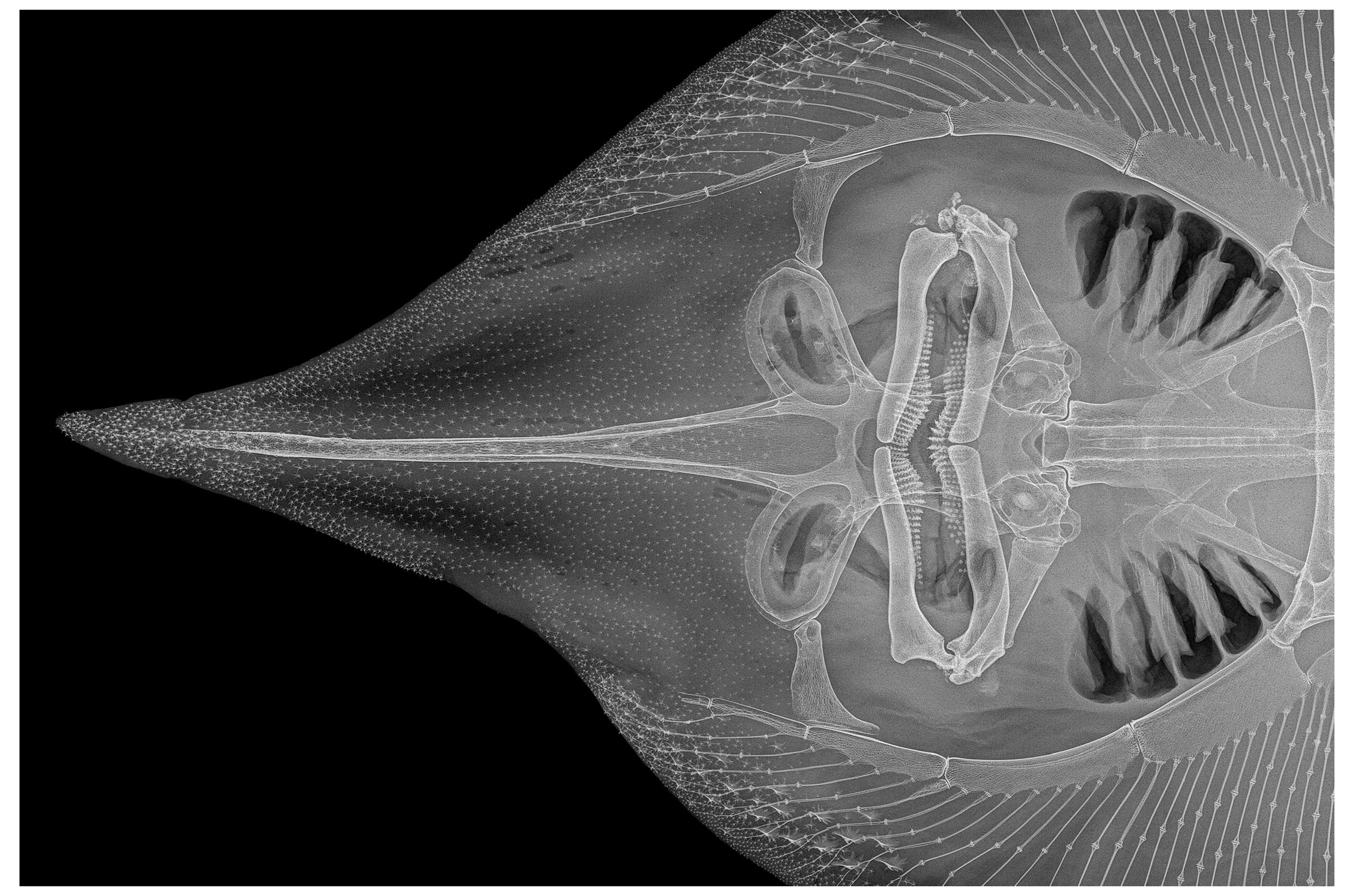
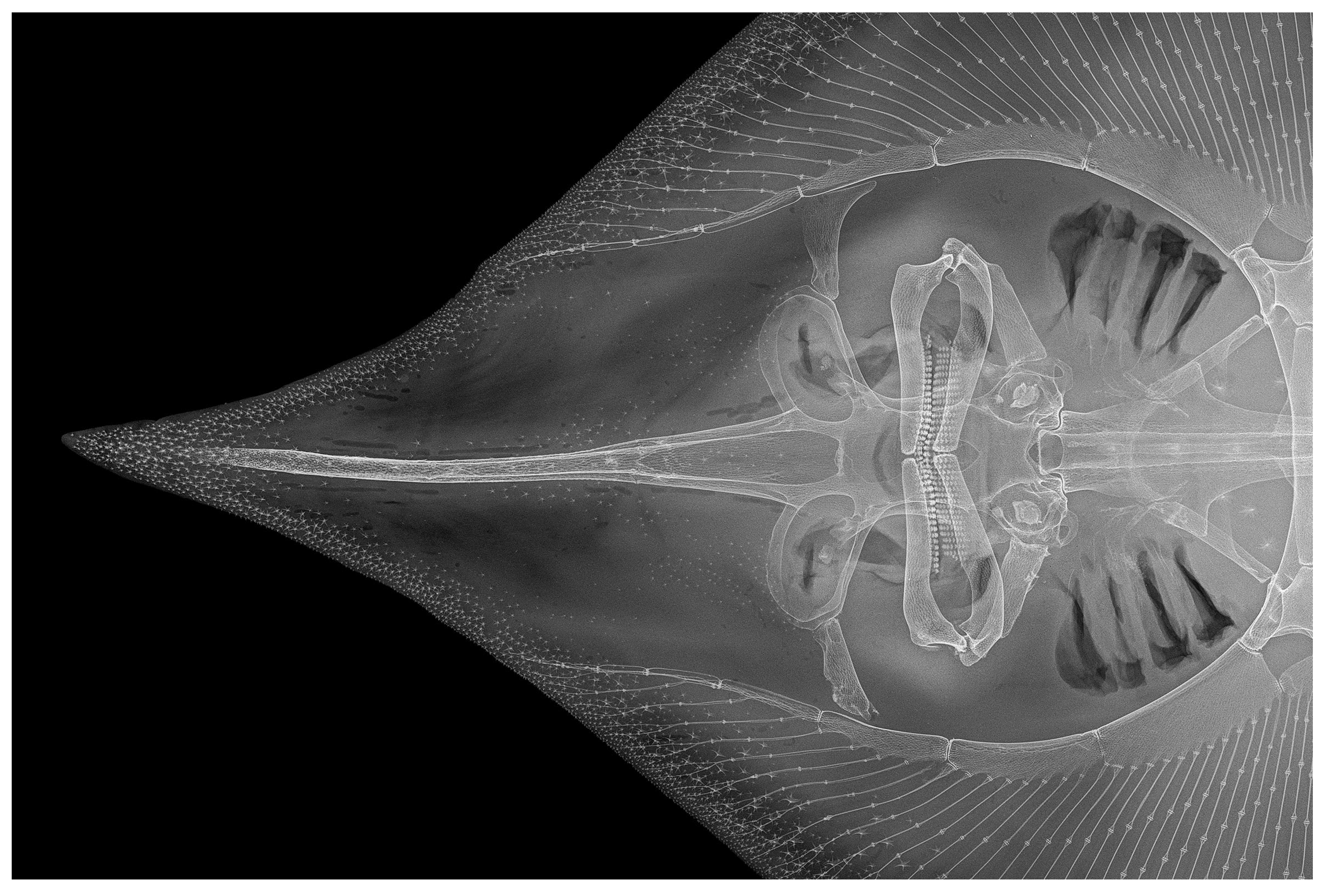
3.2. Leucoraja longirostris n. sp.
| Holotype, ZMH 26260, Adult Male | Paratype, ZMH 26263, Adult Female | Paratype, MTUF 30754, Early Subadult Male | Minimum (n = 5) | Maximum (n = 5) | Mean (n = 8) | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mm | % TL | mm | % TL | mm | % TL | % TL | % TL | % TL | ||
| total length (TL) | 711.0 | 100.0 | 700.0 | 100.0 | 592.0 | 100.0 | 276.0 | 477.0 | 482.8 | |
| disc, width | 382.0 | 53.7 | 388.0 | 55.4 | 315.0 | 53.2 | 53.0 | 54.5 | 54.0 | 0.8 |
| disc, length | 405.0 | 57.0 | 395.0 | 56.4 | 335.0 | 56.6 | 49.5 | 54.3 | 54.3 | 2.5 |
| snout length, preorbital | 153.5 | 21.6 | 161.0 | 23.0 | 132.0 | 22.3 | 17.2 | 20.7 | 20.6 | 1.8 |
| orbit, horizontal diameter | 25.6 | 3.6 | 26.5 | 3.8 | 23.0 | 3.9 | 3.5 | 4.1 | 3.7 | 0.2 |
| interorbital width | 24.0 | 3.4 | 23.8 | 3.4 | 20.0 | 3.4 | 3.2 | 3.8 | 3.5 | 0.2 |
| spiracle length, slit (opening proper) | 18.0 | 2.5 | 16.4 | 2.3 | 14.0 | 2.4 | 2.2 | 2.6 | 2.4 | 0.1 |
| interspiracular width | 38.6 | 5.4 | 40.6 | 5.8 | 32.0 | 5.4 | 5.9 | 6.4 | 5.9 | 0.4 |
| orbit + spiracle length | 34.4 | 4.8 | 30.5 | 4.4 | 30.0 | 5.1 | 4.2 | 4.5 | 4.5 | 0.3 |
| first dorsal fin (D1), height | 19.7 | 2.8 | 17.5 | 2.5 | 12.0 | 2.0 | 1.7 | 2.4 | 2.2 | 0.3 |
| first dorsal fin (D1), base length | 46.3 | 6.5 | 38.5 | 5.5 | 34.0 | 5.7 | 6.0 | 6.8 | 6.2 | 0.5 |
| second dorsal fin (D2), height | 16.5 | 2.3 | 16.6 | 2.4 | 12.0 | 2.0 | 1.6 | 2.3 | 2.1 | 0.3 |
| second dorsal fin (D2), base length | 42.7 | 6.0 | 39.5 | 5.6 | 32.0 | 5.4 | 5.2 | 6.0 | 5.7 | 0.3 |
| tail, postdorsal length | 22.5 | 3.2 | 19.0 | 2.7 | 15.0 | 2.5 | 2.6 | 3.8 | 3.1 | 0.5 |
| interdorsal space | 0.0 | 0.0 | 5.0 | 0.7 | 5.0 | 0.8 | 0.0 | 0.0 | 0.2 | 0.4 |
| caudal fin (C), base length | 22.5 | 3.2 | 19.0 | 2.7 | 15.0 | 2.5 | 2.6 | 3.8 | 3.1 | 0.5 |
| tail, height at pelvic tips | 10.4 | 1.5 | 11.7 | 1.7 | 10.0 | 1.7 | 1.5 | 1.8 | 1.7 | 0.1 |
| tail, width at pelvic tips | 19.2 | 2.7 | 22.3 | 3.2 | 18.0 | 3.0 | 3.0 | 3.4 | 3.1 | 0.2 |
| tail, height at D1 origin | 5.4 | 0.8 | 5.3 | 0.8 | 5.0 | 0.8 | 0.8 | 0.9 | 0.8 | 0.1 |
| tail, width at D1 origin | 11.0 | 1.5 | 11.5 | 1.6 | 8.0 | 1.4 | 1.3 | 1.6 | 1.4 | 0.2 |
| tail, lateral fold length | 212.0 | 29.8 | 182.5 | 26.1 | 170.0 | 28.7 | 30.8 | 35.8 | 31.7 | 3.4 |
| snout length, preoral | 157.0 | 22.1 | 166.0 | 23.7 | 139.0 | 23.5 | 18.4 | 21.4 | 21.3 | 1.8 |
| snout length, prenasal | 139.5 | 19.6 | 149.0 | 21.3 | 123.0 | 20.8 | 15.8 | 19.1 | 18.8 | 1.8 |
| head length, ventrally | 245.0 | 34.5 | 252.0 | 36.0 | 208.0 | 35.1 | 29.5 | 33.7 | 33.1 | 2.2 |
| mouth width | 53.4 | 7.5 | 46.7 | 6.7 | 47.0 | 7.9 | 6.6 | 7.0 | 7.0 | 0.5 |
| internarial width | 50.3 | 7.1 | 46.3 | 6.6 | 43.0 | 7.3 | 6.8 | 7.1 | 7.0 | 0.2 |
| nasal curtain, length | 33.8 | 4.8 | 33.3 | 4.8 | 24.0 | 4.1 | 3.4 | 4.4 | 4.2 | 0.5 |
| nasal curtain, width each lobe | 14.7 | 2.1 | 17.3 | 2.5 | 11.0 | 1.9 | 1.9 | 2.4 | 2.1 | 0.2 |
| nasal curtain, space between lobes | 33.7 | 4.7 | 20.4 | 2.9 | 27.0 | 4.6 | 3.9 | 4.4 | 4.1 | 0.6 |
| gill slit length, 1st | 8.0 | 1.1 | 10.4 | 1.5 | 7.0 | 1.2 | 1.2 | 1.4 | 1.3 | 0.1 |
| gill slit length, 3rd | 9.6 | 1.4 | 10.8 | 1.5 | 9.0 | 1.5 | 1.2 | 1.6 | 1.4 | 0.1 |
| gill slit length, 5th | 7.2 | 1.0 | 7.0 | 1.0 | 6.0 | 1.0 | 1.0 | 1.1 | 1.0 | 0.0 |
| interspace first gill slits | 87.8 | 12.3 | 86.8 | 12.4 | 70.0 | 11.8 | 11.6 | 12.9 | 12.3 | 0.4 |
| interspace fifth gill slits | 51.0 | 7.2 | 55.0 | 7.9 | 39.0 | 6.6 | 7.2 | 7.8 | 7.5 | 0.4 |
| pelvic lobe length from insertion at pelvis, left ant. lobe | 78.0 | 11.0 | 66.2 | 9.5 | 67.0 | 11.3 | 10.3 | 12.4 | 11.1 | 0.9 |
| pelvic lobe length from insertion at pelvis, left post. lobe | 111.0 | 15.6 | 102.4 | 14.6 | 93.0 | 15.7 | 13.6 | 15.2 | 14.8 | 0.8 |
| pelvic lobe length from insertion at pelvis, right ant. lobe | 79.5 | 11.2 | 70.0 | 10.0 | 67.0 | 11.3 | 10.1 | 11.8 | 11.1 | 0.7 |
| pelvic lobe length from insertion at pelvis, right post. lobe | 108.0 | 15.2 | 100.5 | 14.4 | 93.0 | 15.7 | 13.7 | 15.3 | 14.6 | 0.7 |
| clasper, postcloacal length | 152.0 | 21.4 | - | - | 52.0 | 8.8 | 8.1 | 8.4 | 11.7 | 6.5 |
| snout tip to mid-cloaca | 379.0 | 53.3 | 382.0 | 54.6 | 306.0 | 51.7 | 46.1 | 50.3 | 50.5 | 2.7 |
| mid-cloaca to D1 | 222.5 | 31.3 | 212.5 | 30.4 | 194.0 | 32.8 | 33.6 | 37.7 | 33.8 | 2.3 |
| mid-cloaca to D2 | 267.0 | 37.6 | 255.6 | 36.5 | 232.0 | 39.2 | 40.4 | 43.8 | 40.2 | 2.4 |
| mid-cloaca to tail tip | 333.0 | 46.8 | 314.0 | 44.9 | 281.0 | 47.5 | 49.7 | 53.6 | 49.3 | 2.8 |
| snout tip to axis max. disc width | 253.0 | 35.6 | 250.0 | 35.7 | 218.0 | 36.8 | 31.2 | 32.7 | 33.4 | 2.2 |
| snout angle, ° | 65.0 | 71.5 | 69.0 | 76.0 | 85.0 | 75.3 | 6.6 | |||
| pseudobranchial folds left/right | 13/13 | 13/13 | 13/13 | 12/12 | 14/14 | 12.9/13.0 | 0.8/0.8 | |||
| tooth rows, upper jaw | 46 | 41 | 41 | 44.0 | 49.0 | 44.9 | 2.9 | |||
| tooth rows, lower jaw | 42 | 37 | 39 | 41.0 | 46.0 | 42.4 | 3.4 | |||
| trunk vertebrae, Vtr | 29 | 27 | 30 | 27.0 | 30.0 | 28.6 | 1.2 | |||
| predorsal tail vert., Vprd | 62 | 61 | 61 | 60.0 | 64.0 | 61.8 | 1.2 | |||
| total predorsal vert. | 91 | 88 | 91 | 88.0 | 94.0 | 90.4 | 2.0 | |||
| terminal vert., Vterm (ca. in juveniles) | 45 | 46 | 36 | 42.0 | 47.0 | 43.8 | 3.5 | |||
| total vert., Vtotal (ca. in juveniles) | 136 | 134 | 127 | 134.0 | 136.0 | 134.1 | 3.0 | |||
| pectoral radials left/right | 70/70 | 70/70 | 69/70 | 69/70 | 72/73 | 70.3/70.8 | 1.2/1.2 | |||
| pelvic radials left/right | 3 + 16/3 + 16 | 3 + 17/3 + 17 | 3 + 16/3 + 16 | 3 + 15/3 + 15 | 3 + 18/3 + 18 | 16.5/16.4 | 1.1/0.9 | |||











| HT ZMH 26260 | PT ZMH 26261 | PT ZMH 26262 | PT ZMH 26263 | PT ZMH 26264 | PT ZMH 26265 | PT MTUF 30754 | PT ZMMU P-17803 | |
|---|---|---|---|---|---|---|---|---|
| rostral thorns | ~15 | ~20 | ~15 | ~8 | ~10 | 5 | ~10 | ~14 |
| preorbital thorns | 2/2 | 3/3 | 2/2 | 2/2 | 2/2 | 2/2 | 2/2 | 3/2 |
| supraorbital thorns | - | 1/1 | - | - | - | - | - | - |
| postorbital thorns | 1/1 | 1/1 | 2/1 | 1/1 | 1/2 | 1/1 | 2/2 | 1/1 |
| supraspiracular thorns | 1/1 | 1/1 | 1/0 (right one lost?) | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 |
| interspiracular thorns | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 1/1 | 0/1 (left one lost?) |
| median nuchal thorns | 4 (5th lost?) | 3 | 4 | 3 | 5 | 3 | 4 | 3 |
| lateral nuchal thorns, left/right | 1/1 | 1/1 | 1/0 | 3/2 | 0 | 0 | 1/1 | 0 |
| suprascapular thorns | 1 | 2 | 2 | 2 | 1 | 2 | 1 | 2 |
| lateral scapular thorns, left/right | 1/1 | 0/1 | 2/2 | 2/2 | 1/1 | 1/1 | 1/1 | 0/1 |
| scapular thorns, left/right | 3/3 | 2/2 | 2/2 | 3/3 | 3/3 | 2/2 | 2/2 | 3/3 |
| median thorns on body | 9 | 7 | 7 | 7 | 7 | 9 | 8 | 9 |
| median thorns on tail | 28 | 27 | 27 | 30 | 30 | 32 | 30 | 29 |
| interdorsal thorns | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| parallel thorns on body, left/right | 15/15 | 6/6 | 1/1 | ~13/~13 | 0/1 | 0/1 | 2/2 | 0/0 |
| parallel thorns on tail, left/right | ~30/~30 | ~25/~30 | ~20/~20 | ~35/~35 | ~25/~25 | ~20/~20 | ~25/~20 | ~20/~20 |
| lateral thorns on tail, left/right | ~35/~35 | ~20/~20 | ~20/~25 | ~40/~40 | ~25/~25 | ~15/~15 | ~20/~20 | ~15/~15 |
| alar thorns | 2 rows of 20 each | - | - | - | - | - | - | - |
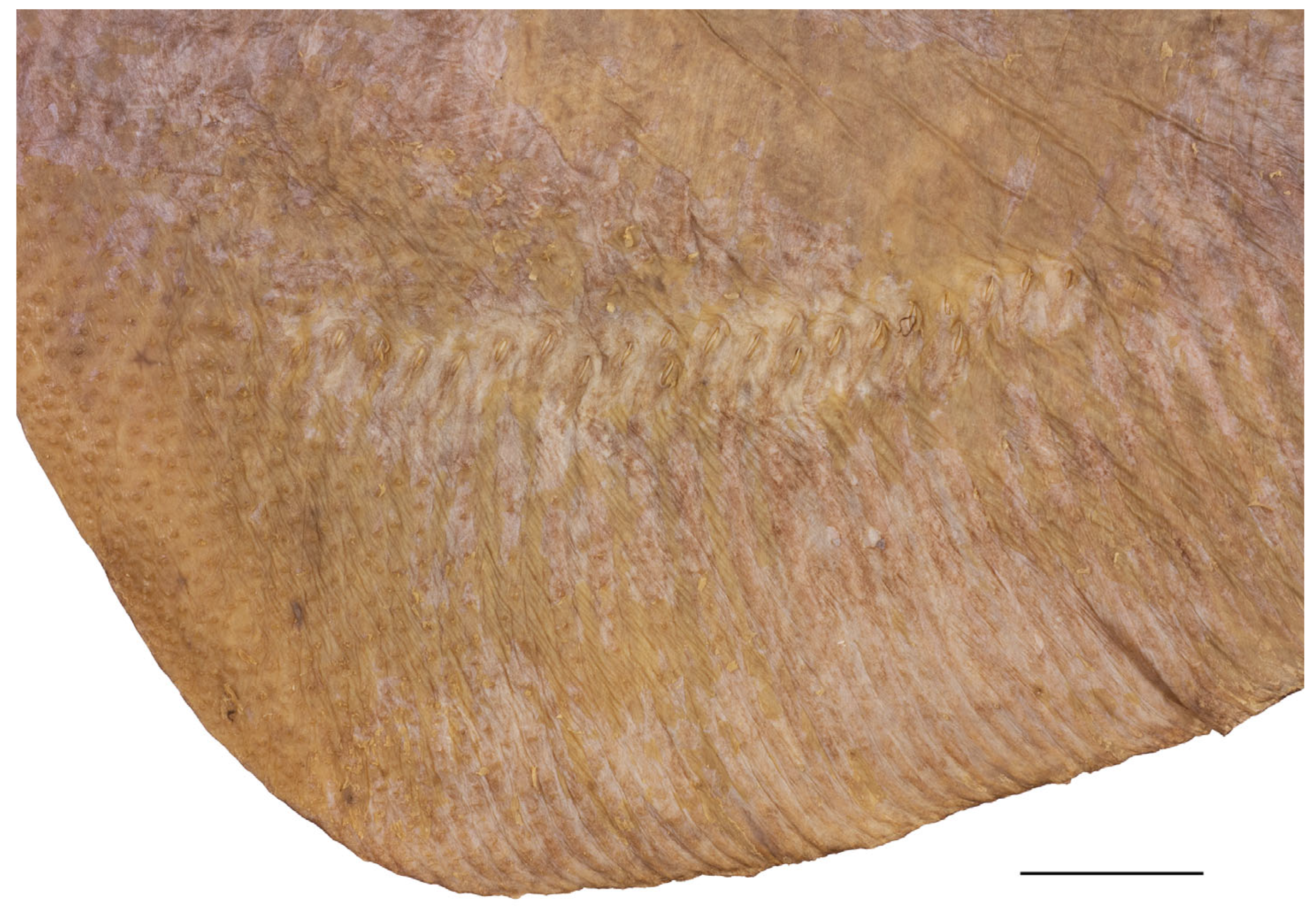
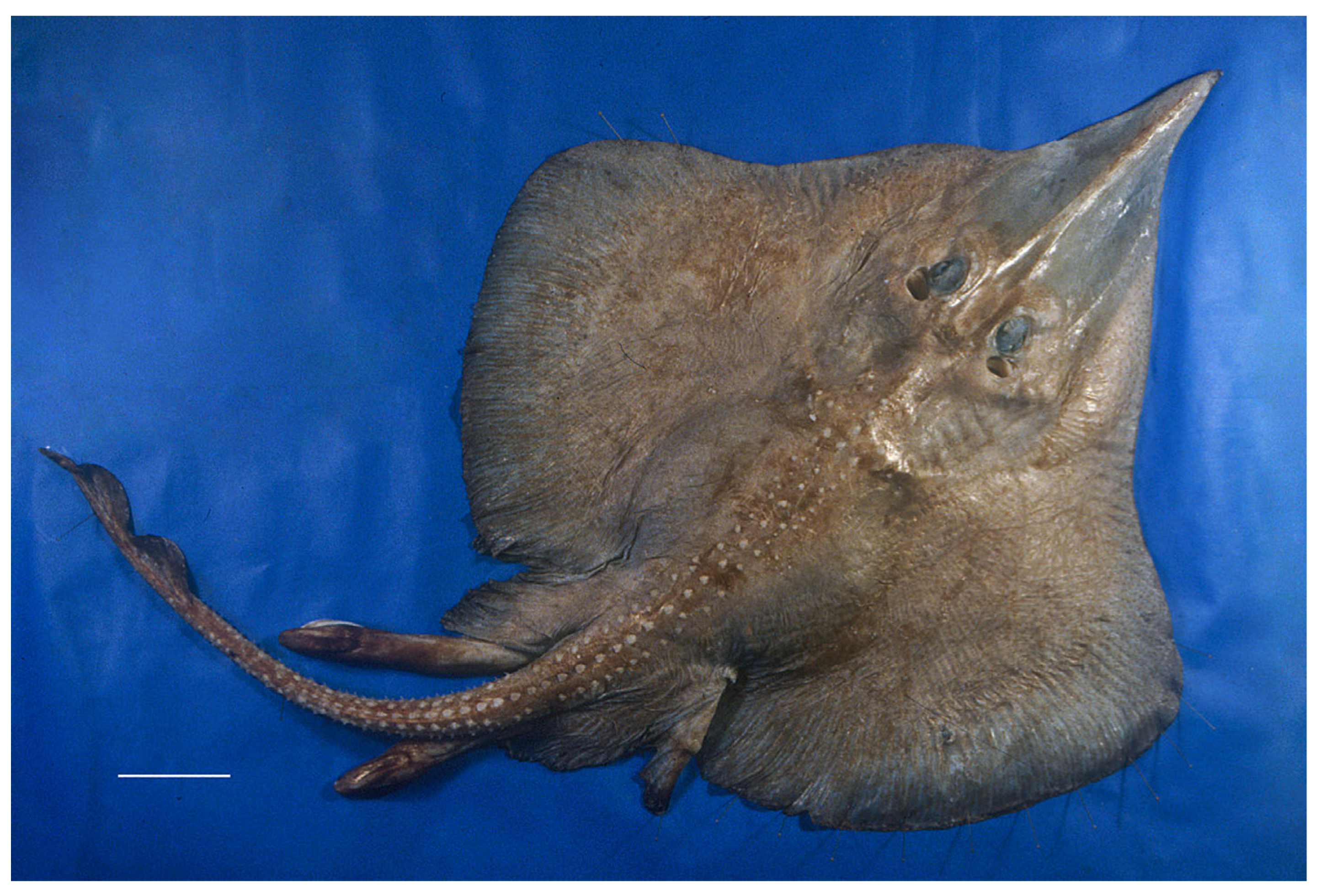

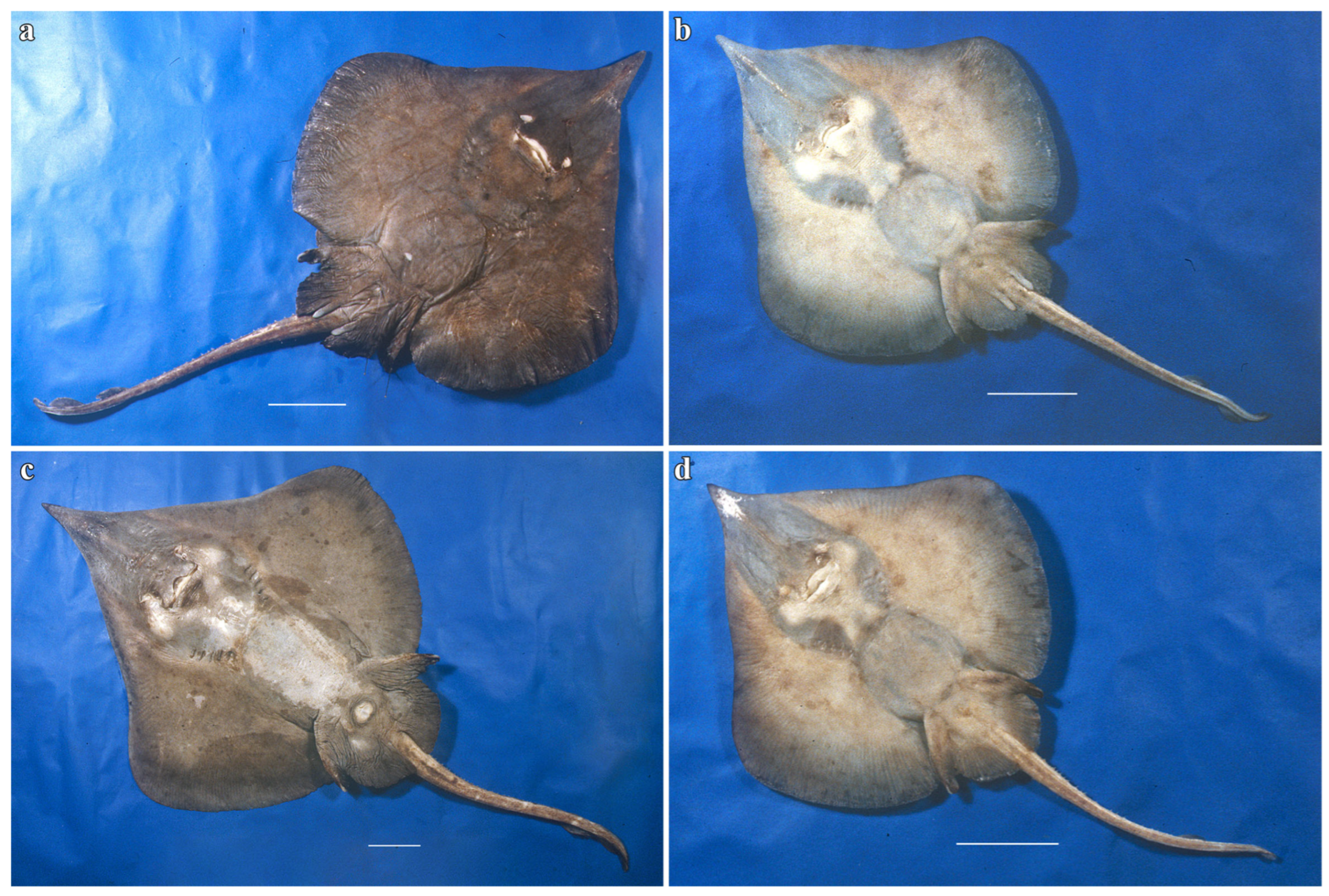
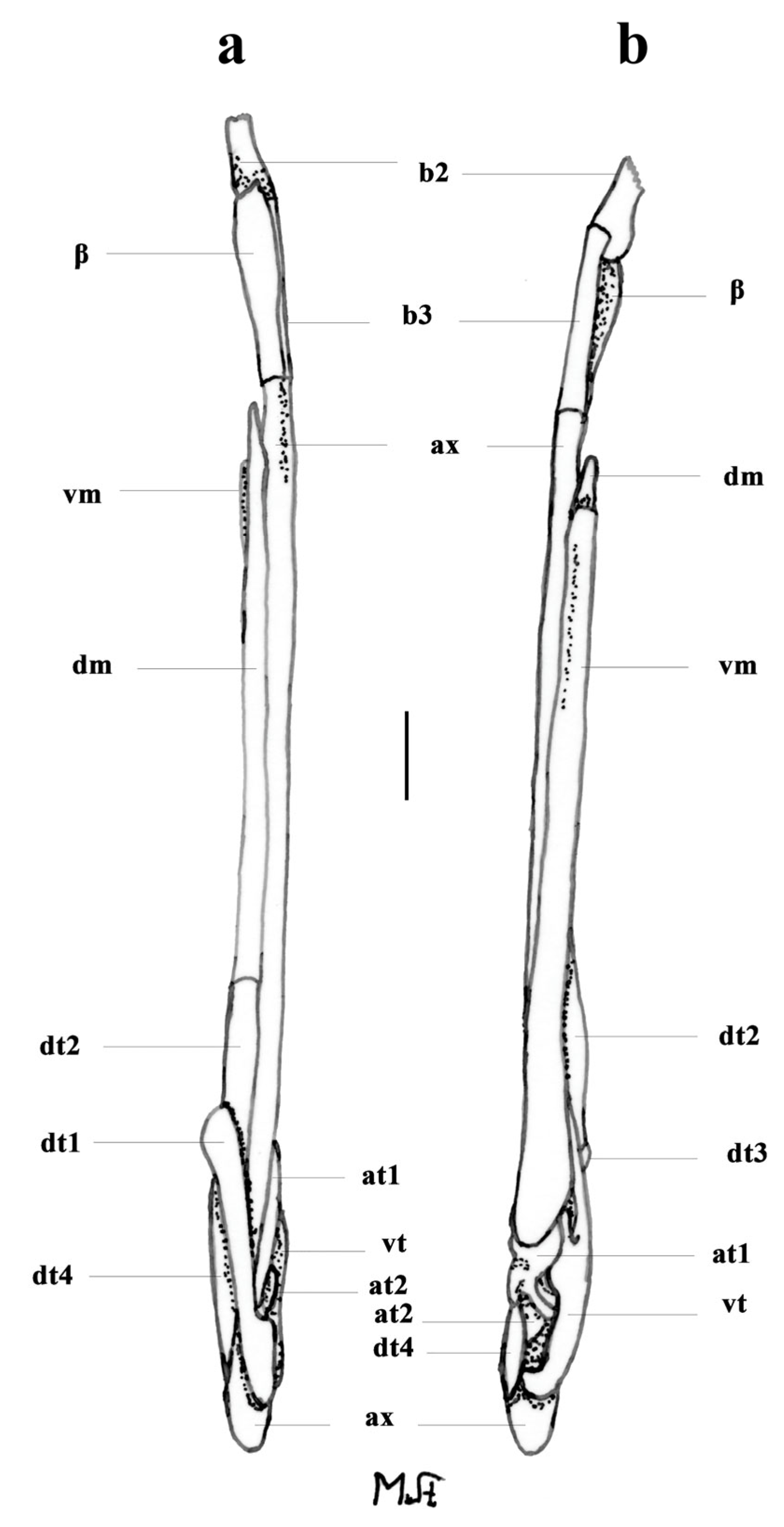
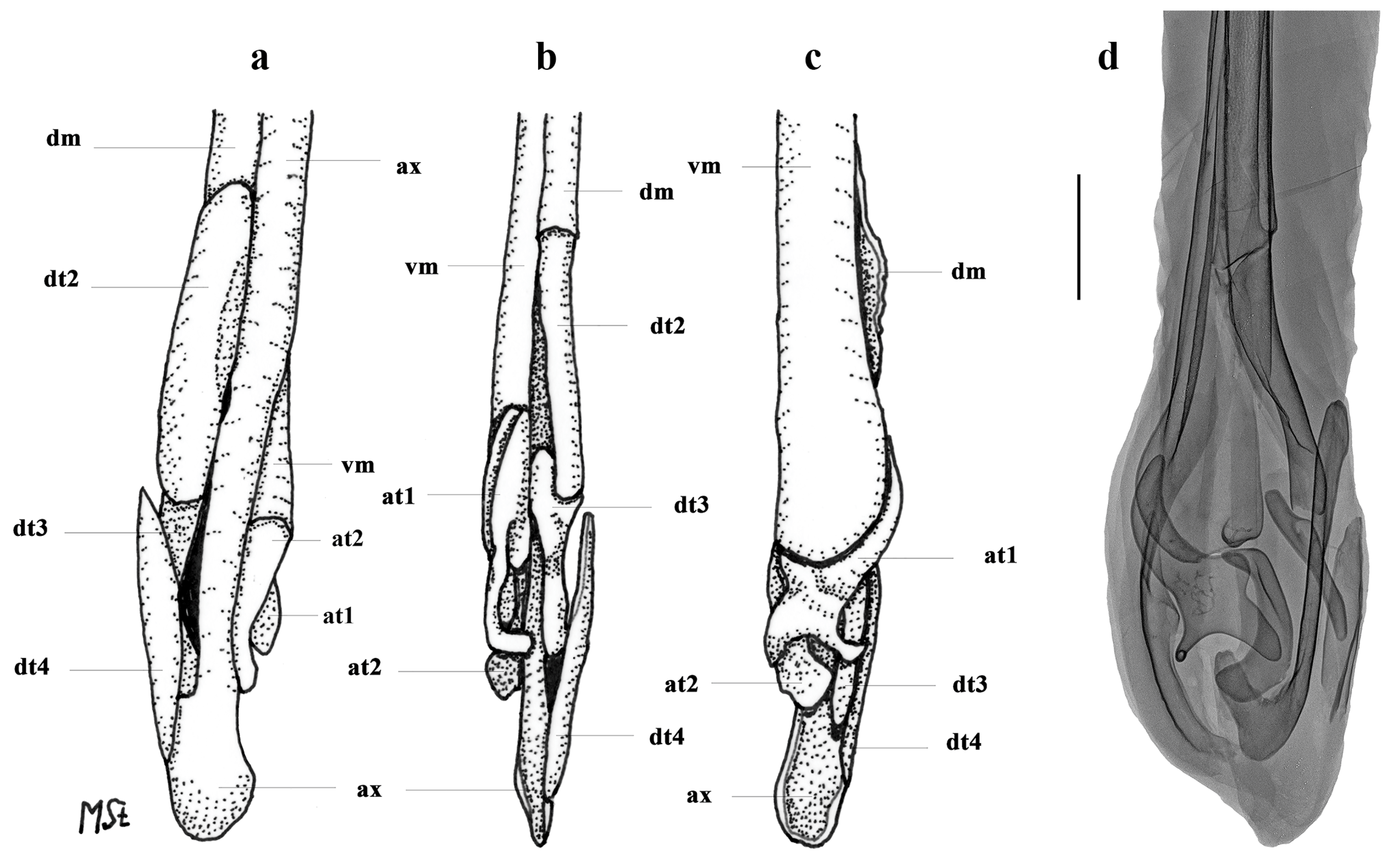
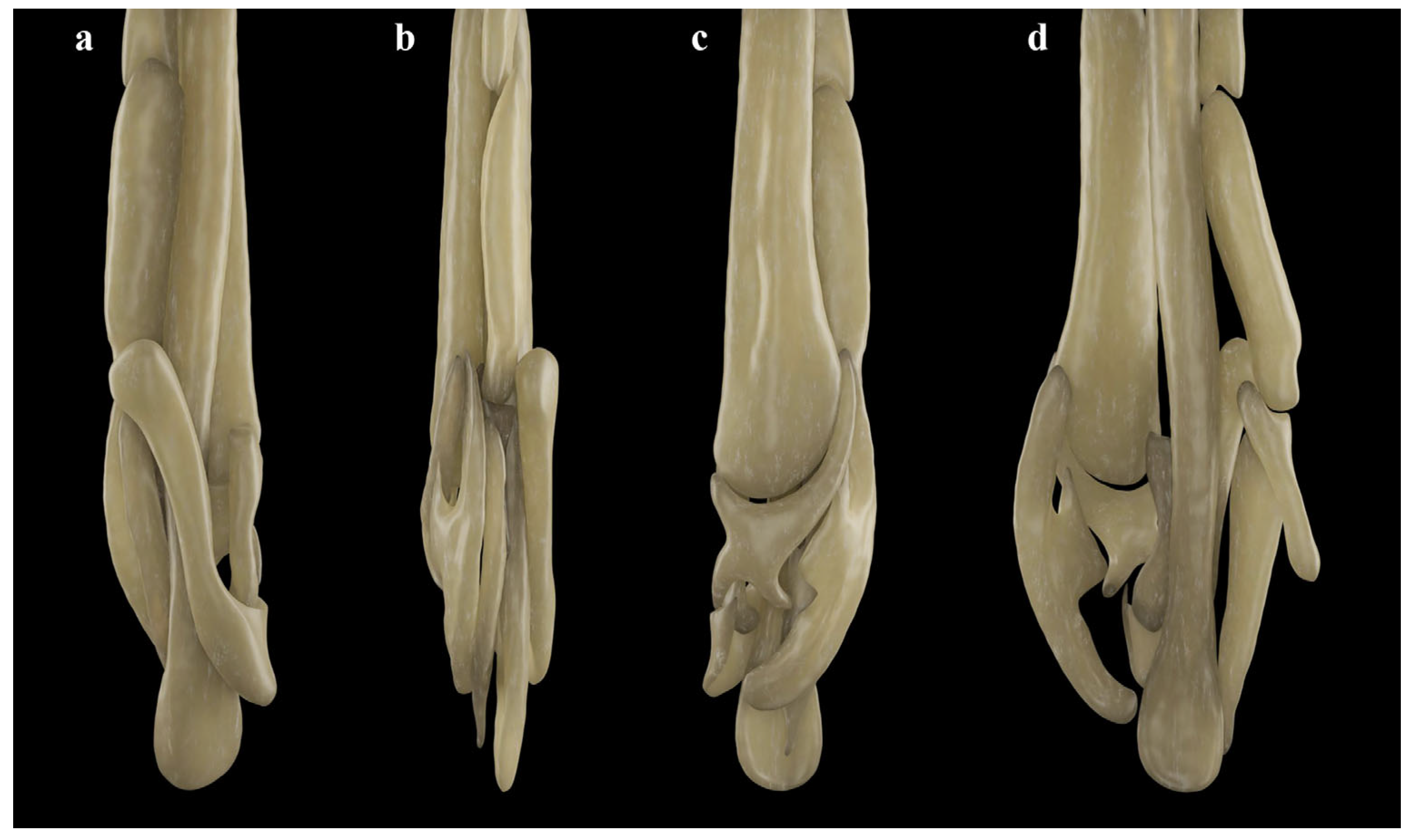
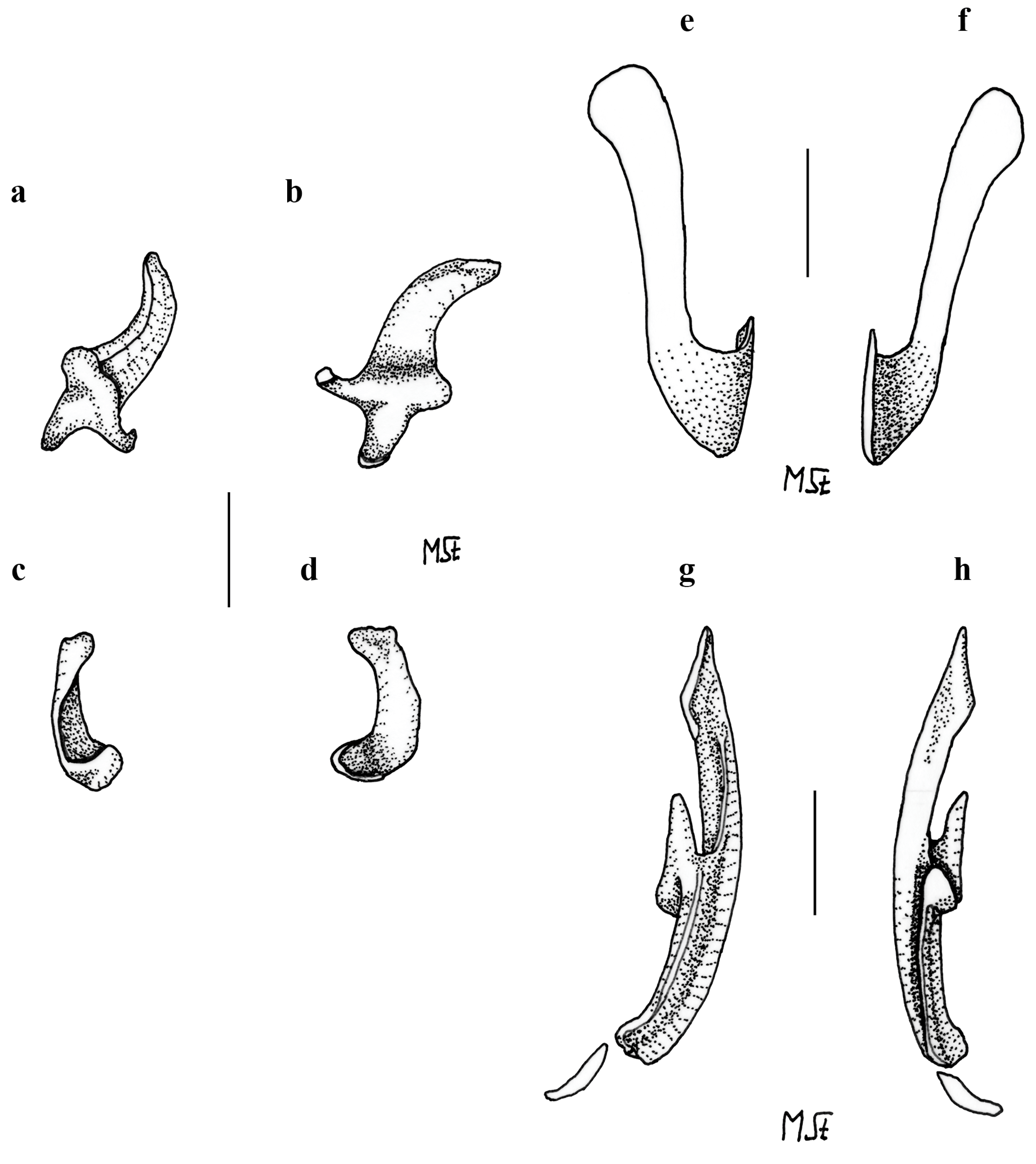
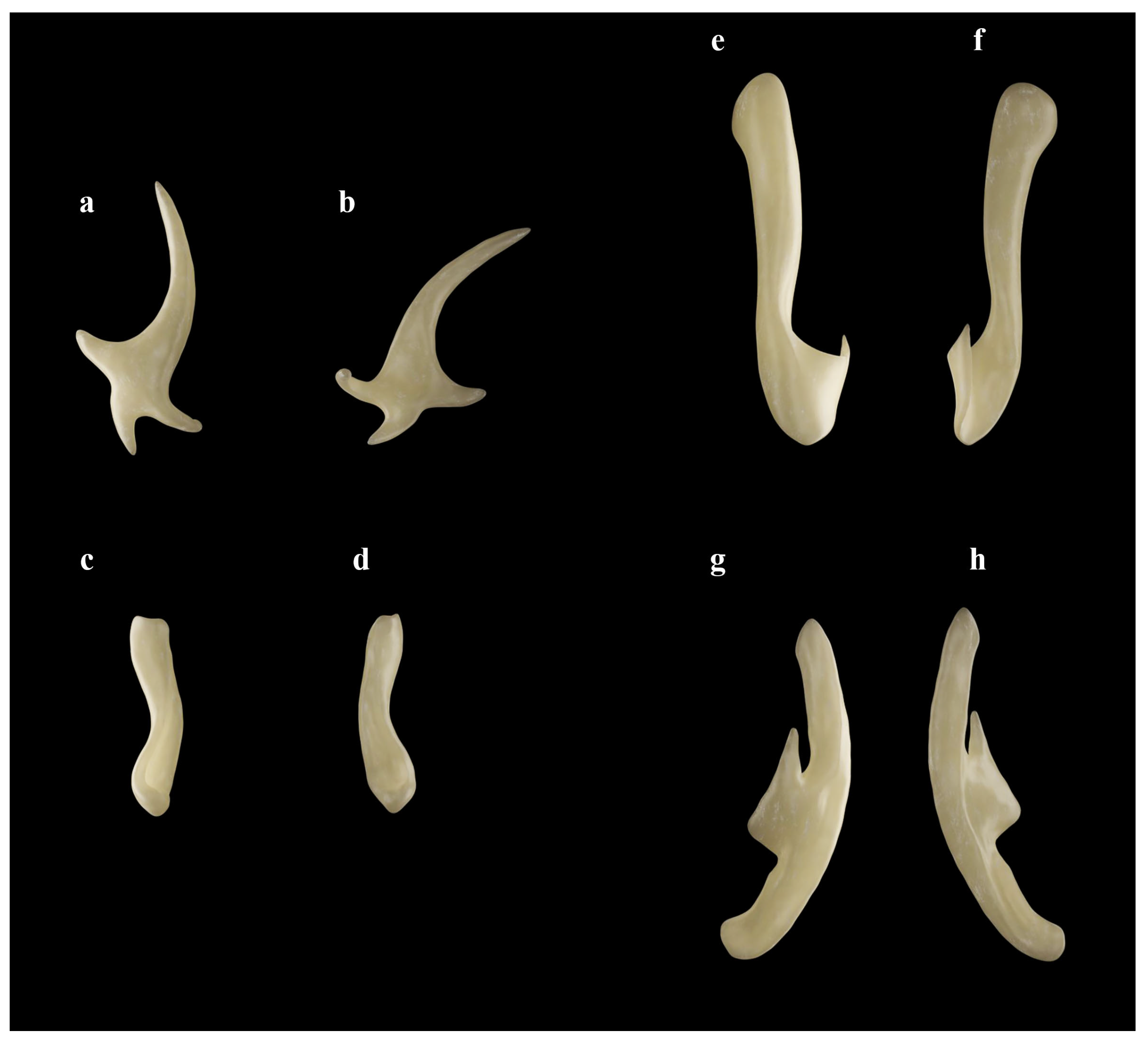
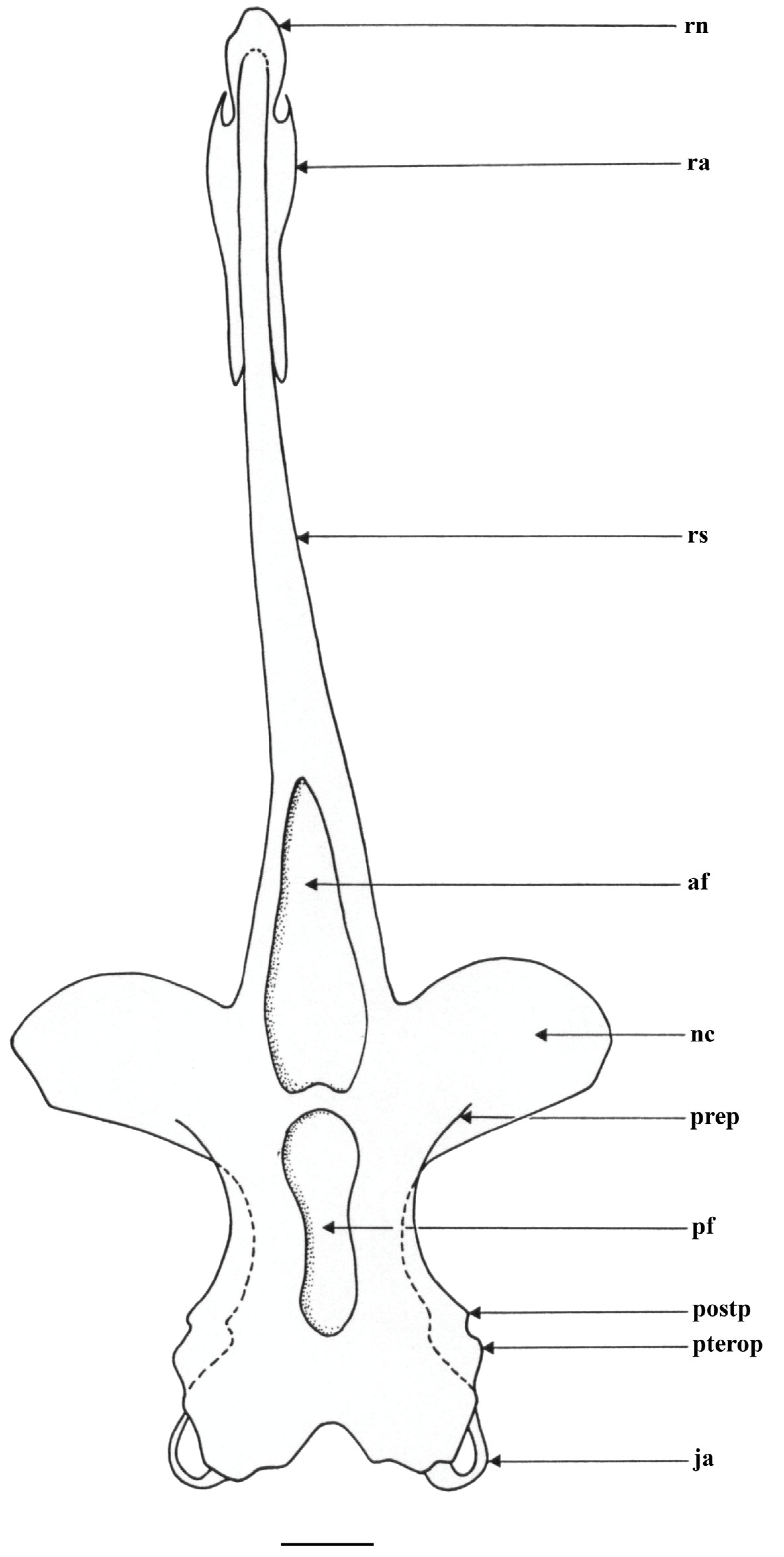
| Holotype, ZMH 26260, Adult Male, 711 mm TL | Paratype, ZMH 26263, Adult Female, 700 mm TL | Paratype, MTUF 30754, Early Subadult Male, 592 mm TL | Minimum (n = 5) | Maximum (n = 5) | Mean (n = 8) | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mm | % NBL | mm | % NBL | mm | % NBL | % NBL | % NBL | % NBL | ||
| cranium TL | 201.9 | 348.8 | 208.1 | 357.4 | 183.8 | 358.1 | 317.8 | 337.2 | 338.5 | 16.8 |
| nasobasal length (NBL) | 57.9 | 100.0 | 58.2 | 100.0 | 51.3 | 100.0 | 23.9 | 36.9 | 40.4 | |
| max. ethmoidal width | 73.4 | 126.8 | 71.4 | 122.6 | 66.7 | 130.0 | 127.8 | 130.4 | 128.1 | 2.5 |
| min. dorsal interorb. width | 24.0 | 41.5 | 23.8 | 40.9 | 20.0 | 39.0 | 40.1 | 43.9 | 41.1 | 1.4 |
| min. internasal width | 15.5 | 26.8 | 15.0 | 25.8 | 14.1 | 27.5 | 26.6 | 29.9 | 27.4 | 1.2 |
| max. width nasal apertures | 27.5 | 47.6 | 25.4 | 43.6 | 25.6 | 49.8 | 44.7 | 46.7 | 46.5 | 1.9 |
| min. ventral interorb./basal plate width | 19.2 | 33.2 | 18.6 | 31.9 | 17.2 | 33.5 | 32.1 | 35.5 | 33.3 | 1.4 |
| max. width otic region | 38.3 | 66.1 | 39.0 | 66.9 | 34.9 | 68.1 | 68.0 | 71.4 | 68.9 | 2.0 |
| max. width jugular | 40.2 | 69.5 | 39.7 | 68.1 | 36.2 | 70.6 | 71.9 | 74.6 | 71.5 | 2.0 |
| rostral shaft length | 143.7 | 248.3 | 149.6 | 256.9 | 132.3 | 257.8 | 217.8 | 237.2 | 238.2 | 16.7 |
| rostrum base width | 18.1 | 31.2 | 18.8 | 32.4 | 18.4 | 35.8 | 32.0 | 38.1 | 34.6 | 2.6 |
| postnasal length orbit region | 19.6 | 33.8 | 19.2 | 33.0 | 15.6 | 30.4 | 28.7 | 33.3 | 31.3 | 2.1 |
| length otic region | 17.9 | 31.0 | 18.6 | 31.9 | 17.0 | 33.2 | 32.2 | 36.8 | 33.7 | 2.1 |
| postoccipital length jugal arches | 2.1 | 3.7 | 2.0 | 3.4 | 1.8 | 3.5 | 1.3 | 3.0 | 2.6 | 0.9 |
| tip rostrum to tip ant. fontanelle | 114.6 | 198.0 | 119.7 | 205.6 | 105.9 | 206.4 | 171.1 | 185.9 | 188.3 | 15.1 |
| tip to end ant. fontanelle | 155.2 | 268.0 | 160.4 | 275.4 | 139.8 | 272.5 | 231.5 | 251.8 | 253.9 | 18.2 |
| tip to tip post. fontanelle | 159.0 | 274.6 | 163.6 | 281.0 | 142.0 | 276.7 | 236.5 | 259.3 | 259.5 | 18.5 |
| tip to end post. fontanelle | 183.5 | 317.1 | 189.8 | 326.0 | 167.9 | 327.2 | 283.8 | 304.5 | 305.7 | 18.3 |
| tip to level ant. propterygia | 86.4 | 149.3 | 90.7 | 155.7 | 78.5 | 153.0 | 112.2 | 132.7 | 134.8 | 18.1 |
| tip to level max. ethmoidal width | 146.3 | 252.7 | 155.0 | 266.2 | 135.9 | 264.9 | 223.1 | 242.2 | 244.9 | 17.0 |
| tip to symphysis upper jaw | 163.1 | 281.7 | 174.0 | 298.8 | 148.7 | 289.8 | 250.8 | 263.3 | 270.7 | 19.2 |
| ant. fontanelle length | 40.5 | 70.0 | 40.8 | 70.1 | 33.8 | 65.8 | 59.9 | 66.8 | 65.7 | 3.7 |
| ant. fontanelle max. width | 12.1 | 21.0 | 11.3 | 19.5 | 12.3 | 24.0 | 18.9 | 25.9 | 22.5 | 2.6 |
| distance betw. ant. + post. fontanelles | 3.9 | 6.7 | 3.4 | 5.8 | 2.1 | 4.2 | 4.1 | 7.1 | 5.5 | 1.2 |
| post. fontanelle length | 24.6 | 42.4 | 26.4 | 45.3 | 26.1 | 50.8 | 45.7 | 47.2 | 46.4 | 2.3 |
| post. fontanelle min. width | 8.0 | 13.9 | 6.2 | 10.7 | 5.4 | 10.5 | 8.6 | 12.5 | 10.9 | 1.7 |
| post. fontanelle max. width anteriorly | 10.2 | 17.6 | 10.3 | 17.8 | 8.5 | 16.6 | 13.1 | 17.8 | 16.2 | 1.8 |
| post. fontanelle max. width posteriorly | 8.0 | 13.9 | 6.9 | 11.9 | 6.2 | 12.1 | 11.7 | 15.5 | 12.8 | 1.4 |
| angle post. edge nasal capsules, ° | 66.0 | 70.0 | 72.0 | 72.0 | 80.0 | 73.1 | 4.3 | |||
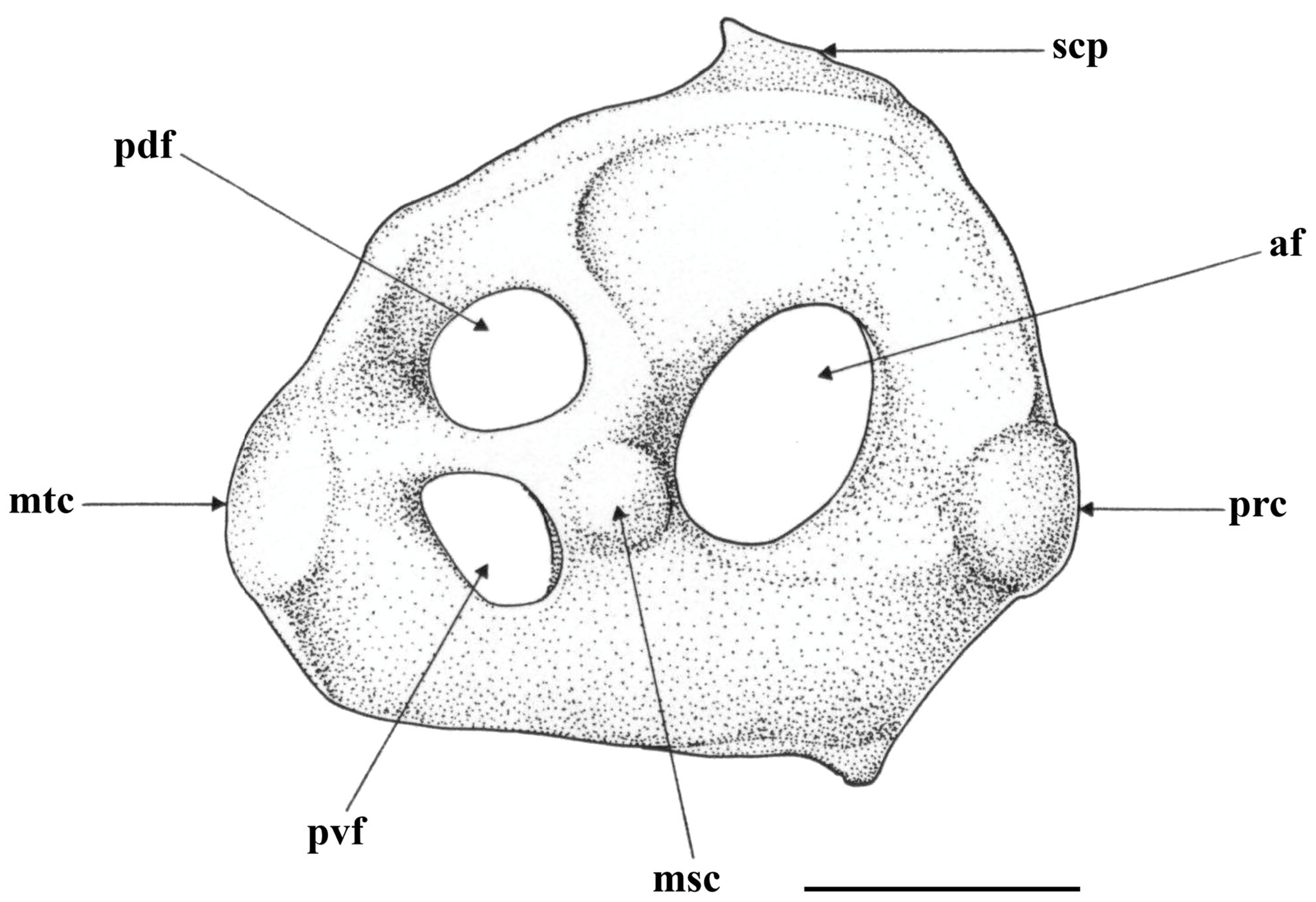
| mm | % max. Length | |
|---|---|---|
| maximum length | 30.6 | 100.0 |
| maximum height | 26.9 | 86.8 |
| height at rear corner | 18.7 | 60.3 |
| pre-mesocondyle length | 16.4 | 53.6 |
| post-metacondyle length | 14.2 | 46.4 |
| anterior fenestra height | 8.3 | 27.1 |
| anterior fenestra length | 7.0 | 22.9 |
| height postdorsal fenestra | 5.4 | 17.6 |
| length postdorsal fenestra | 6.5 | 21.2 |
| height postventral fenestra | 4.9 | 16.0 |
| length postventral fenestra | 5.1 | 16.7 |
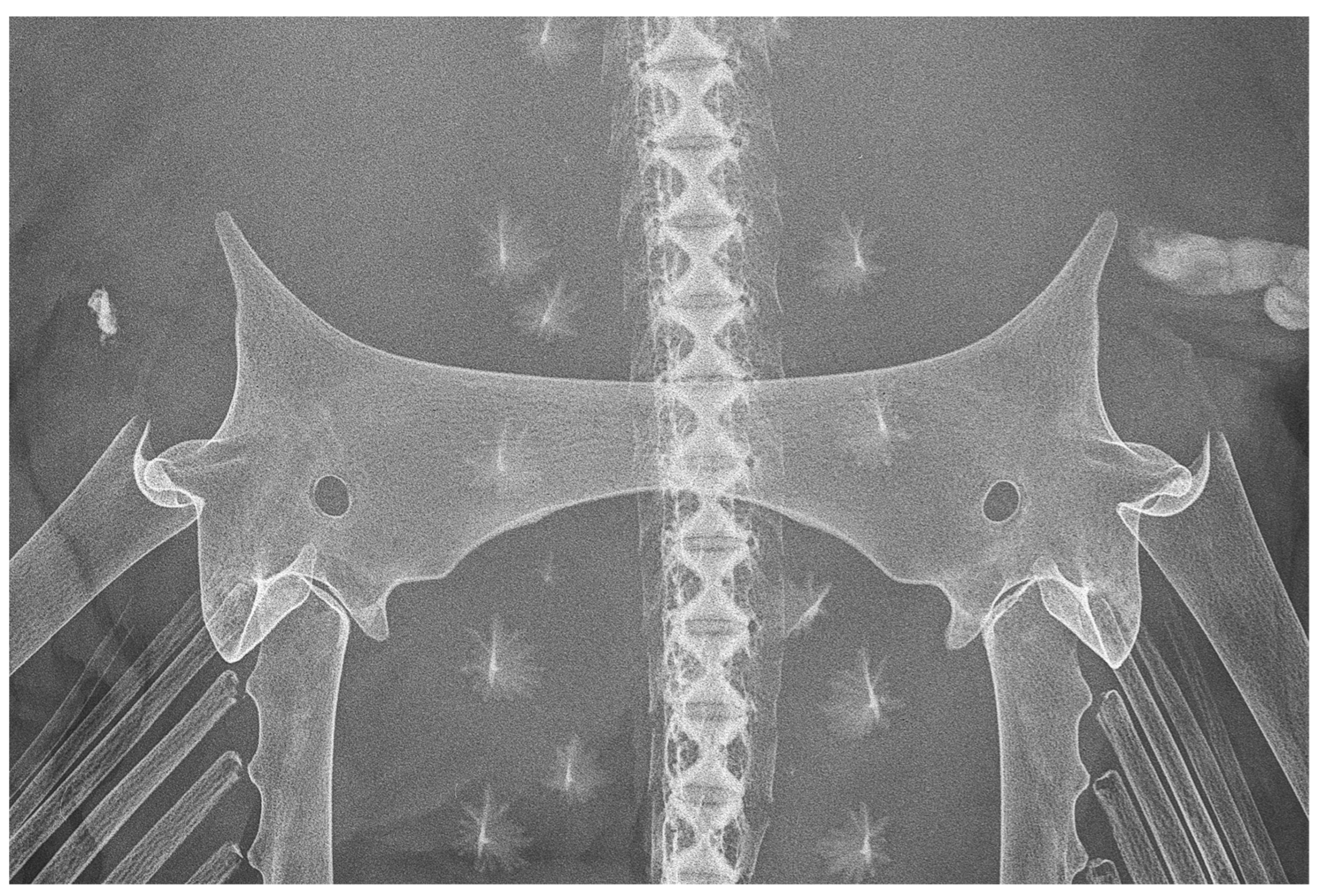
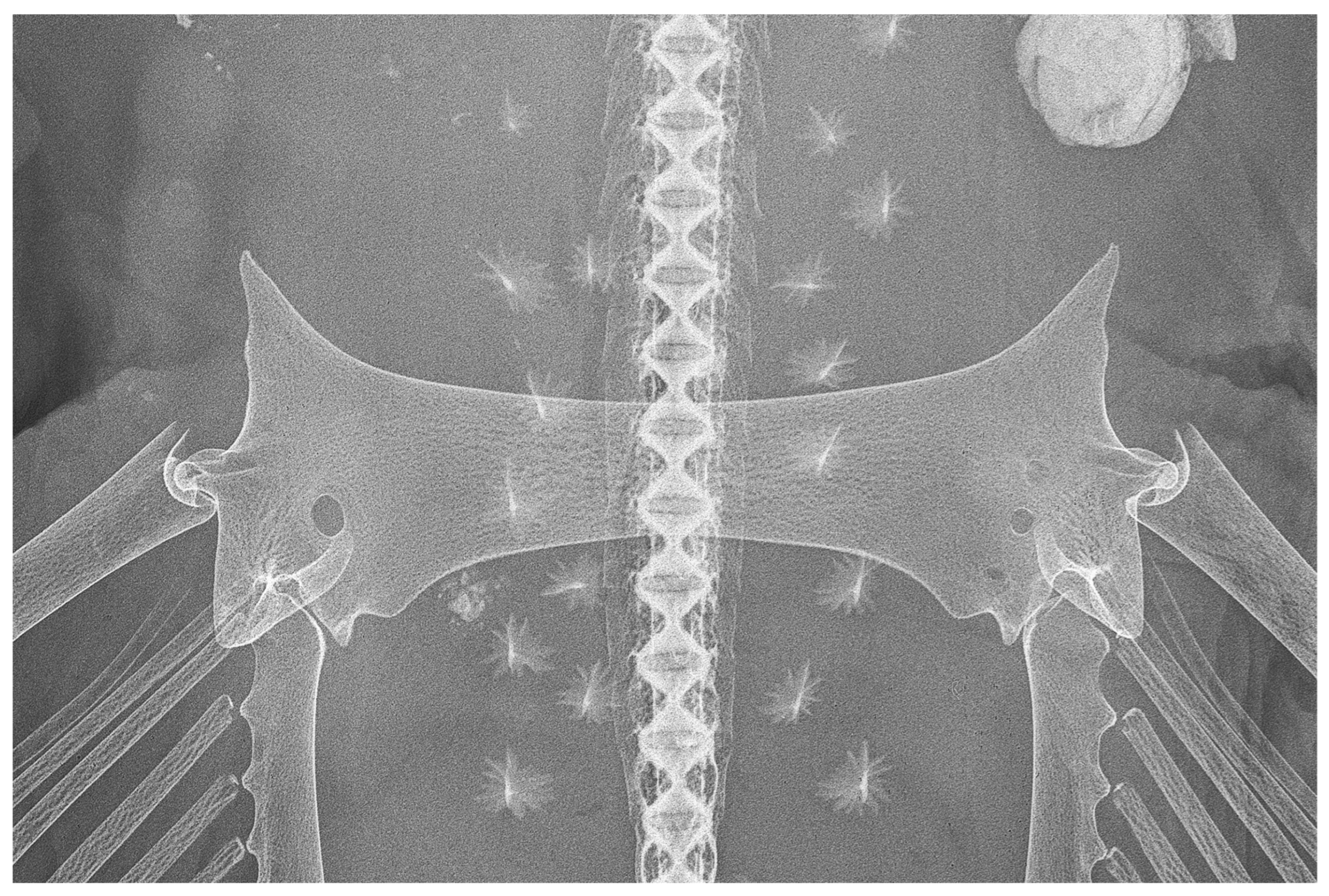
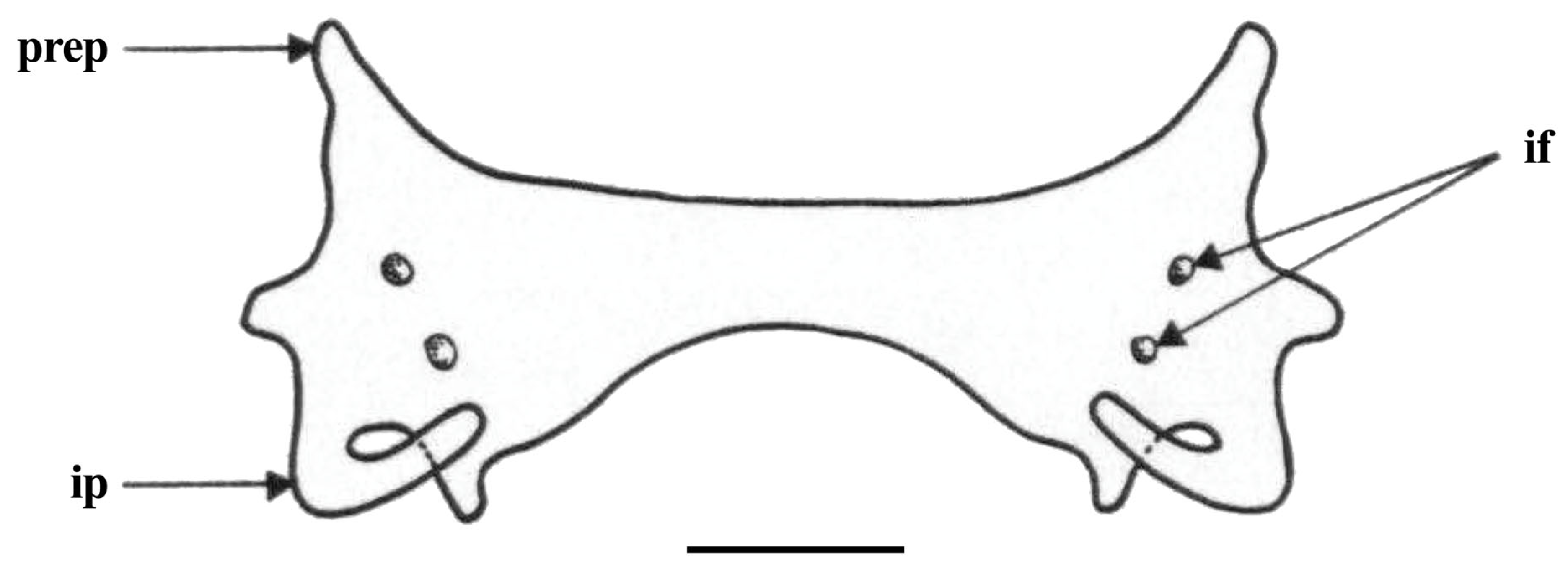
| Holotype, ZMH 26260, Adult Male, 711 mm TL | Paratype, ZMH 26263, Adult Female, 700 mm TL | Paratype, MTUF 30754, Early Subadult Male, 592 mm TL | Minimum (n = 5) | Maximum (n = 5) | Mean (n = 8) | SD | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| mm | % PGW | mm | % PGW | mm | % PGW | % PGW | % PGW | % PGW | ||
| interorbital width dorsally | 24.0 | 39.6 | 23.8 | 37.8 | 20.0 | 38.6 | 39.2 | 45.8 | 40.8 | 2.7 |
| shoulder girdle max. width | 85.1 | 140.6 | 98.5 | 156.3 | - | - | 154.1 | 165.0 | 155.3 | 7.4 |
| pelvic girdle max. width (PGW) | 60.6 | 100.0 | 63.0 | 100.0 | 51.8 | 100.0 | 22.9 | 38.0 | 41.2 | |
| median transverse thickness | 5.5 | 9.2 | 8.3 | 13.2 | 6.1 | 11.8 | 11.9 | 13.6 | 12.1 | 1.3 |
| length of prepelvic process (from level PGW) | 16.0 | 26.4 | 13.9 | 22.0 | 13.9 | 26.8 | 24.8 | 28.1 | 25.9 | 1.8 |
| length of prepelvic proc. (from level ant. edge pelvic girdle) | 10.0 | 16.5 | 9.9 | 15.7 | 7.9 | 15.2 | 13.3 | 17.8 | 15.8 | 1.4 |
| depth of posterior arc (from level PGW) | 9.6 | 15.8 | 10.9 | 17.3 | 8.3 | 16.0 | 15.3 | 17.9 | 16.4 | 1.0 |
| depth of post. arc (from level post. edge pelvic girdle) | 8.8 | 14.5 | 6.3 | 10.0 | 8.0 | 15.4 | 12.0 | 15.3 | 13.8 | 1.9 |
| iliac foramina number | 2 | 2 | 2 | 2 | 2 | 2 | ||||
| Species Authorship | Common Name | Maximum Size | Depth Distribution | Geographic Distribution | Remarks | |
|---|---|---|---|---|---|---|
| L. circularis | (Couch, 1838) | Sandy ray | 1200 mm | 10–800 m | NEA | |
| L. compagnoi | (Stehmann, 1995) | Tigertail skate | >517 mm | 480–625 m | WIO, SEA | |
| L. elaineae | Ebert and Leslie, 2019 | Elaine’s skate | >330 mm | 484 m | WIO | |
| L. erinacea | (Mitchill, 1825) | Little skate/Hedgehog skate | 620 mm | 10–914 m | NWA | |
| L. fullonica | (Linnaeus, 1758) | Shagreen ray | 1200 mm | 30–600 m | NEA | Possibly occurs down to 800 m depth [43]. |
| L. garmani | (Whitley, 1939) | Freckled skate/Rosette skate | 570 mm | 33–530 m | NWA | L. garmani contains two valid subspecies: L. garmani caribbaea (McEachran, 1977) and L. garmani virginica (McEachran, 1977) [44,45,46]. |
| L. lentiginosa | (Bigelow and Schroeder, 1951) | Speckled skate | 440 mm | 53–588 m | NWA | |
| L. leucosticta | (Stehmann, 1971) | White-dappled skate | 800 mm | 70–704 m | NEA | Maximum depth of 600 m according to Stehmann [47] but one specimen from 704 m depth is present in the collection of the Fauna Marina del C. O. de Málaga. |
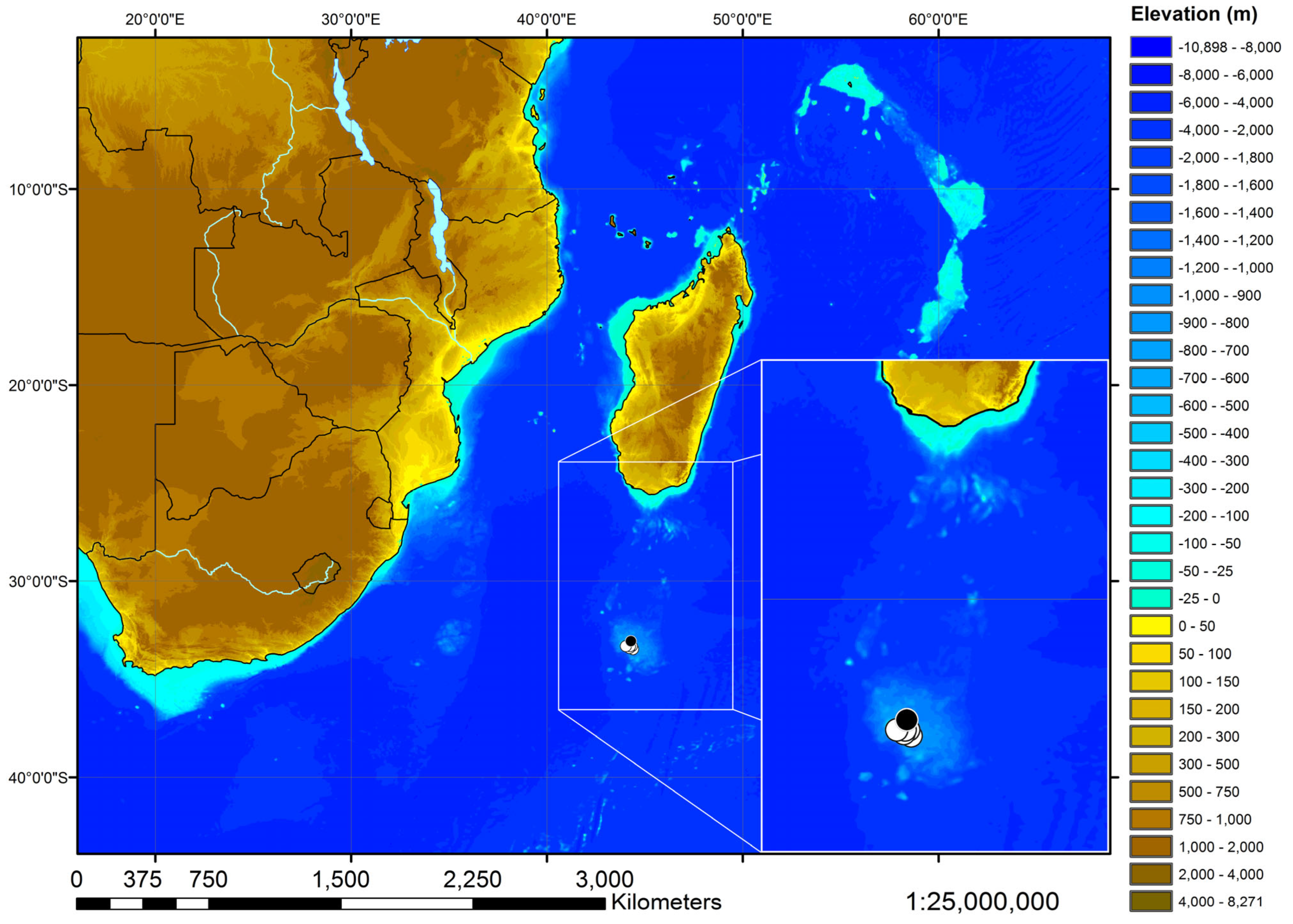
| L. longirostris n. sp. | Weigmann, Stehmann, Séret and Ishihara | Brown longnose skate | 711 mm | 750–1050 m | WIO | |
| L. melitensis | (Clark, 1926) | Maltese ray | 500 mm | 60–800 m | NEA (Mediterranean) | |
| L. naevus | (Müller and Henle, 1841) | Cuckoo ray | 810 mm | 12–900 m | NEA | Sizes of up to 1060 mm, occasionally reported for specimens from the North Sea [48], are probably erroneous. |
| L. ocellata | (Mitchill, 1815) | Winter skate | 1130 mm | 4–723 m | NWA | Much larger specimens of up to 1500 mm were captured by the Fisheries Research Board of Canada off central and northern Nova Scotia from 1960 to 1971 [49]. |
| L. pristispina | Last, Stehmann and Séret, 2008 | Sawback skate | 401 mm | 202–504 m | EIO | |
| L. wallacei | (Hulley, 1970) | Yellow-spotted skate | 963 mm | 73–517 m | WIO, SEA | |
| L. yucatanensis | (Bigelow and Schroeder, 1950) | Yucatan skate/Yucatan white skate | 300 mm | 192–457 m | NWA |
4. Discussion
- Dorsal coloration ornamented, or at least tail with dark cross-bands, snout short and obtusely angled (horizontal preorbital length 2.2–2.8 times orbit length, snout angle 110–120°)…………………………………………………………………………………… 2
- -
- Dorsal coloration uniformly brownish or grayish without any pattern, snout moderately long or long and slightly obtusely or acutely angled (horizontal preorbital length 2.8–6.1 times orbit length, snout angle 65–104°)…………………....................... 4
- 2.
- Dorsal disc plain colored, tail with broad cross-bands; anterior and posterior pelvic-fin lobes of nearly equal length; dorsal fins confluent; 38 tooth rows in the upper jaw …. L. compagnoi (southeastern Atlantic and southwestern Indian Ocean: South Africa)
- -
- Distinctive dorsal coloration of one large prominent ocellus about equal to orbit diameter on each pectoral fin mid-base or clusters of bright yellow spots; anterior pelvic-fin lobes much shorter than posterior lobes; dorsal fins separated by short but distinct interspace; 57–69 tooth rows in the upper jaw ………………………………... 3
- 3.
- Dorsal coloration variable, holotype uniformly brown with a few lighter spots and a prominent ocellus at each pectoral fin mid-base, but some specimens with more vivid pattern consisting of clusters of bright yellow spots and a dark blotch on the tip of the anterior lobe of the pelvic fin; tail with 3–4 colorful cross-bands formed by larger rosettes and whorls; ventral surface largely plain whitish; spinules ventrally on snout tip and along the anterior margins of the disc ………………............................ L. wallacei (southeastern Atlantic and southwestern Indian Ocean: Namibia to Mozambique)
- -
- Dorsal coloration similar to that of the holotype of L. wallacei, consisting of one large prominent ocellus about equal to the orbit diameter on each pectoral fin mid-base; tail without any banding; ventral surface whitish but with brownish disk margins and brownish-gray spots on the tail; ventral surface smooth, lacking any prickles ………...………………………………………. L. elaineae (western Indian Ocean: Kenya)
- 4.
- Snout moderately long and slightly obtusely angled, preorbital length 2.8–3.5 times orbit length, preoral length 1.4–1.7 times mouth width, snout angle 90–104°, dorsal disc very prickly, almost completely covered with fine denticles, ventrally naked, lateral tail folds confined to posterior half of tail, males mature at 33–34 cm TL, maximum size is 40 cm TL …. L. pristispina (southeastern Indian Ocean: western Australia)
- -
- Snout long and acutely angled, preorbital length 4.2–6.1 times orbit length, preoral length 2.8–3.6 times mouth width, snout angle 65–85°, dorsal disc largely devoid of denticles, ventrally spinules at least on the snout, lateral tail folds along posterior 58–72% of tail, males mature at 60 cm TL, maximum size is 71 cm TL ……… L. longirostris n. sp. (southwestern Indian Ocean: Madagascar Ridge)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Weigmann, S. Reply to Borsa (2017): Comment on ‘Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity by Weigmann (2016)’: Reply to borsa’s comment on weigmann (2016). J. Fish Biol. 2017, 90, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Last, P.R.; Weigmann, S.; Yang, L. Changes to the nomenclature of the skates (Chondrichthyes: Rajiformes). In Rays of the World: Supplementary Information; CSIRO Special Publication; CSIRO Australian National Fish Collection: Hobart, Australia, 2016; pp. 11–34. [Google Scholar]
- Last, P.R.; Séret, B.; Stehmann, M.F.W.; Weigmann, S. Skates, family Rajidae. In Rays of the World; Last, P.R., White, W.T., Carvalho, M.R., de Séret, B., Stehmann, M.F.W., Naylor, G.J.P., Eds.; CSIRO Publishing: Melbourne, Australia, 2016; pp. 204–363. [Google Scholar]
- Malm, A.W. Göteborgs Och Bohusläns Fauna; Ryggradsdjuren: Göteborg, Sweden, 1877. [Google Scholar]
- Jordan, D.S. The Genera of Fishes; Stanford University Publications: Stanford, CA, USA, 1919; Volume Part III. [Google Scholar]
- Stehmann, M. Vergleichend morphologische und anatomische Untersuchungen zur Neuordnung der Systematik der nordostatlantischen Rajidae (Chondrichthyes, Batoidei). Arch. Fisch. 1970, 21, 73–164. [Google Scholar]
- McEachran, J.D.; Dunn, K.A. Phylogenetic Analysis of Skates, a Morphologically Conservative Clade of Elasmobranchs (Chondrichthyes: Rajidae). Copeia 1998, 1998, 271–290. [Google Scholar] [CrossRef]
- Weigmann, S.; Stehmann, M.F.W.; Thiel, R. Rajella paucispinosa n. sp., a new deep-water skate (Elasmobranchii, Rajidae) from the western Indian Ocean off South Mozambique, and a revised generic diagnosis. Zootaxa 2014, 3847, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.K.; Jeong, D.; Chung, I.; Jung, J.W.; Oh, M.J.; Kim, S.; Lee, Y.-H.; Kim, C.-G.; Hwang, S.Y. Rapid Species Identification of Elasmobranch Fish (Skates and Rays) using Oligonucleotide Microarray. BioChip J. 2009, 3, 87–96. [Google Scholar]
- Pfaff, C.; Kriwet, J.; Martin, K.; Johanson, Z. Ontogenetic development of the otic region in the new model organism, Leucoraja erinacea (Chondrichthyes; Rajidae). Earth Environ. Sci. Trans. R. Soc. Edinb. 2018, 109, 105–114. [Google Scholar] [CrossRef]
- Gillis, J.A.; Bennett, S.; Criswell, K.E.; Rees, J.; Sleight, V.A.; Hirschberger, C.; Calzarette, D.; Kerr, S.; Dasen, J. Big insight from the little skate: Leucoraja erinacea as a developmental model system. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 147, pp. 595–630. ISBN 978-0-12-820154-1. [Google Scholar]
- Yoo, D.; Park, J.; Lee, C.; Song, I.; Lee, Y.H.; Yun, T.; Lee, H.; Heguy, A.; Han, J.Y.; Dasen, J.S.; et al. Little skate genome provides insights into genetic programs essential for limb-based locomotion. eLife 2022, 11, e78345. [Google Scholar] [CrossRef] [PubMed]
- Marlétaz, F.; De La Calle-Mustienes, E.; Acemel, R.D.; Paliou, C.; Naranjo, S.; Martínez-García, P.M.; Cases, I.; Sleight, V.A.; Hirschberger, C.; Marcet-Houben, M.; et al. The little skate genome and the evolutionary emergence of wing-like fins. Nature 2023, 616, 495–503. [Google Scholar] [CrossRef]
- Nykänen, M.; Dillane, E.; Reid, D.; Rogan, E. Genetic methods reveal high diversity and no evidence of stock structure among cuckoo rays (Leucoraja naevus) in the northern part of Northeast Atlantic. Fish. Res. 2020, 232, 105715. [Google Scholar] [CrossRef]
- Turan, C. Molecular Systematic Analyses of Mediterranean Skates (Rajiformes). Turk. J. Zool. 2008, 32, 437–442. [Google Scholar]
- Aschliman, N.C.; Nishida, M.; Miya, M.; Inoue, J.G.; Rosana, K.M.; Naylor, G.J.P. Body plan convergence in the evolution of skates and rays (Chondrichthyes: Batoidea). Mol. Phylogenet. Evol. 2012, 63, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Naylor, G.; Caira, J.; Jensen, K.; Rosana, K.; Straube, N.; Lakner, C. Elasmobranch Phylogeny: A Mitochondrial Estimate Based on 595 Species. In Biology of Sharks and Their Relatives; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 31–56. ISBN 978-1-4398-3924-9. [Google Scholar]
- Naylor, G.J.P.; Caira, J.N.; Jensen, K.; Rosana, K.A.M.; White, W.T.; Last, P.R. A DNA sequence–based approach to the identification of shark and ray species and its implications for global elasmobranch diversity and parasitology. Bull. Am. Mus. Nat. Hist. 2012, 367, 1–262. [Google Scholar] [CrossRef] [PubMed]
- Chiquillo, K.L.; Ebert, D.A.; Slager, C.J.; Crow, K.D. The secret of the mermaid’s purse: Phylogenetic affinities within the Rajidae and the evolution of a novel reproductive strategy in skates. Mol. Phylogenet. Evol. 2014, 75, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Lynghammar, A.; Christiansen, J.S.; Griffiths, A.M.; Fevolden, S.; Hop, H.; Bakken, T. DNA barcoding of the northern Northeast Atlantic skates (Chondrichthyes, Rajiformes), with remarks on the widely distributed starry ray. Zool. Scr. 2014, 43, 485–495. [Google Scholar] [CrossRef]
- Compagno, L.J.V.; Ebert, D.A. Southern African skate biodiversity and distribution. Environ. Biol. Fishes 2007, 80, 125–145. [Google Scholar] [CrossRef]
- Compagno, L.J.V.; Stehmann, M.; Ebert, D.A. Rhinochimaera africana, a new longnose chimaera from southern Africa, with comments on the systematics and distribution of the genus Rhinochimaera Garman, 1901 (Chondrichthyes, Chimaeriformes, Rhinochimaeridae). S. Afr. J. Mar. Sci. 1990, 9, 201–222. [Google Scholar] [CrossRef]
- Fricke, R.; Eschmeyer, W.N. Eschmeyer’s Catalog of Fishes: Guide to Fish Collections. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/collections.asp (accessed on 16 April 2024).
- Bigelow, H.B.; Schroeder, W.C. Fishes of the Western North Atlantic Part Two: Sawfishes, Guitarfishes, Skates and Rays, Chimaeroids; Sears Foundation for Marine Research, Yale University: New Haven, CT, USA, 1953. [Google Scholar]
- Clark, R.S. Rays and skates. A revision of the European species. Fish. Board Scotl. Sci. Investig. 1926, 1, 1–66. [Google Scholar]
- Ishiyama, R. Studies on the rajid fishes (Rajidae) found in the waters around Japan. J. Shimonoseki Coll. Fish. 1958, 7, 193–394. [Google Scholar]
- Hubbs, C.L.; Ishiyama, R. Methods for the Taxonomic Study and Description of Skates (Rajidae). Copeia 1968, 1968, 483–491. [Google Scholar] [CrossRef]
- Stehmann, M. Ergebnisse der Forschungsreisen des FFS “Walther Herwig” nach Südamerika. LXIV. Bathyraja papilionifera sp. n. (Pisces, Batoidea, Rajidae), eine weitere neue Rochenart aus dem Südwestatlantik vom nordargentinischen Kontinentalabhang. Arch. Fisch. 1985, 36, 195–211. [Google Scholar]
- Hulley, P.A. An investigation of the Rajidae of the west and south coasts of Southern Africa. Ann. S. Afr. Mus. 1970, 55, 151–220. [Google Scholar]
- Hulley, P.A. The origin, interrelationship and distribution of Southern African Rajidae (Chondrichthyes, Batoidei). Ann. S. Afr. Mus. 1972, 60, 1–103. [Google Scholar]
- McEachran, J.D.; Compagno, L.J.V. A Further Description of Gurgesiella furvescens with Comments on the Interrelationships of Gurgesiellidae and Pseudorajidae (Pisces, Rajoidei). Bull. Mar. Sci. 1979, 29, 530–553. [Google Scholar]
- Stehmann, M.F.W.; Séret, B.; Costa, E.M.; Baro, J. Neoraja iberica n. sp., a new species of pygmy skate (Elasmobranchii, Rajidae) from the southern upper slope of the Iberian Peninsula (Eastern North Atlantic). Cybium 2008, 32, 51–71. [Google Scholar] [CrossRef]
- Springer, V.G.; Garrick, J.A.F. A Survey of Vertebral Numbers in Sharks. Proc. United States Natl. Mus. 1964, 116, 73–96. [Google Scholar] [CrossRef]
- Krefft, G. Knorpelfische (Chondrichthyes) aus dem tropischen Ostatlantik. Atlantide Rep. 1968, 10, 33–76. [Google Scholar]
- Amante, C.; Eakins, B.W. ETOPO1 1 Arc-Minute Global Relief Model: Procedures, Data Sources and Analysis. In NOAA Technical Memorandum NESDIS NGDC-24; NOAA: Washington, DC, USA, 2009; Volume 19. [Google Scholar] [CrossRef]
- Weigmann, S.; Stehmann, M.F.W.; Thiel, R. Planonasus parini n. g. and n. sp., a new genus and species of false cat sharks (Carchariniformes, Pseudotriakidae) from the deep northwestern Indian Ocean off Socotra Islands. Zootaxa 2013, 3609, 163–181. [Google Scholar] [CrossRef] [PubMed]
- McEachran, J.D. Variation in Raja garmani and the Status of Raja lentiginosa (Pisces: Rajidae). Bull. Mar. Sci. 1977, 27, 423–439. [Google Scholar]
- McEachran, J.D.; Miyake, T. Interrelationships within a putative monophyletic groups of skates (Chondrichthyes, Rajoidei, Rajini). In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes; Uyeno, T., Arai, R., Taniuchi, T., Matsuura, K., Eds.; Ichthyological Society of Japan: Tokyo, Japan, 1986; pp. 281–290. [Google Scholar]
- Leible, M.D.; Stehmann, M. First records of Raja (Dipturus) trachyderma Krefft & Stehmann, 1975 from the Southeastern pacific off Chile, with first descriptions of its clasper characters and additional skeletal and morphological details (Pisces, Rajiformes, Rajidae). Stud. Neotrop. Fauna Environ. 1987, 22, 169–188. [Google Scholar] [CrossRef]
- Backman, G.V. Die Bauchflosse der Selachier. Erste Abtheilung. Die Bauchflosse der Batoidei. K. Sven. Vetenskabsakad. Handl. 1913, 50, 1–141. [Google Scholar]
- Ebert, D.A.; Leslie, R.W. Leucoraja elaineae sp. nov., a new rough skate (Rajiformes: Rajidae) from the Western Indian Ocean. Zootaxa 2019, 4691, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Relini, G.; Mannini, A.; de Ranieri, S.; Bitetto, S.; Follesa, M.C.; Gancitano, V.; Manfredi, C.; Casciaro, L.; Sion, L. Chondrichthyes caught during the MEDITS surveys in Italian waters. Biol. Mar. Mediterr. 2010, 17, 186–204. [Google Scholar]
- Schmitter-Soto, J.J.; Vásquez-Yeomans, L.; Aguilar-Perera, A.; Curiel-Mondragón, C.; Caballero-Vázquez, J.A. Lista de peces marinos del Caribe mexicano. An. Inst. Biología. Ser. Zool. 2000, 71, 143–177. [Google Scholar]
- McEachran, J.D. Skates. Family Rajidae. In Bigelow and Schroeder’s Fishes of the Gulf of Maine; Collette, B.B., Klein-MacPhee, G., Eds.; Smithsonian Institution Press: Washington, DC, USA, 2002; pp. 60–75. [Google Scholar]
- Moore, J.A.; Hartel, K.E.; Craddock, J.E.; Galbraith, J.K. An Annotated List of Deepwater Fishes from off the New England Region, with New Area Records. Northeast. Natl. 2003, 10, 159–248. [Google Scholar] [CrossRef]
- Stehmann, M. First and new records of skates (Chondrichthyes, Rajiformes, Rajidae) from the West African continental slope (Morocco to South Africa), with descriptions of two new species. Arch. Fish. Mar. Res. 1995, 43, 1–119. [Google Scholar]
- ICES ICES Database on Trawl Surveys (DATRAS). Available online: https://datras.ices.dk (accessed on 8 May 2024).
- McEachran, J.D.; Martin, C.O. Possible occurrence of character displacement in the sympatric skates Raja erinacea and R. ocellata (Pisces: Rajidae). Environ. Biol. Fish. 1977, 2, 121–130. [Google Scholar] [CrossRef]
- McEachran, J.D.; Martin, C.O. Interrelationships and Subgeneric Classification of Raja erinacea and R. ocellata Based on Claspers, Neurocrania and Pelvic Girdles (Pisces: Rajidae). Copeia 1978, 1978, 593–601. [Google Scholar] [CrossRef]
- Stehmann, M. Raja (Leucoraja) leucosticta spec. nov. (Pisces, Batoidei, Rajidae), eine neue Rochenart aus dem Seegebiet des tropischen Westafrika; gleichzeitig zur Frage des Vorkommens von Raja ackleyi Garman, 1881, im mittleren Ostatlantik. Arch. Fisch. 1971, 22, 1–16. [Google Scholar]
- Last, P.R.; Stehmann, M.; Séret, B. Leucoraja pristispina sp. nov., a new deepwater skate from Western Australia. CSIRO Mar. Atmos. Res. Pap. 2008, 21, 145–154. [Google Scholar]
- Weigmann, S.; Stehmann, M.F.W.; Thiel, R. Okamejei ornata n. sp., a new deep-water skate (Elasmobranchii, Rajidae) from the northwestern Indian Ocean off Socotra Islands. Deep Sea Res. II Top. Stud. Oceanogr. 2015, 115, 18–29. [Google Scholar] [CrossRef]
- Last, P.R.; Weigmann, S.; Dumale, D. A new skate genus Orbiraja (Rajiformes: Rajidae) from the Indo-West Pacific. Zootaxa 2016, 4184, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, R. Fauna Japonica/Rajidae (Pisces); Biogeographical Society of Japan: Tokyo, Japan, 1967. [Google Scholar]
- Stehmann, M. Revision der Rajoiden-Arten des nördlichen Indischen Ozean und Indopazifik (Elasmobranchii, Batoidea, Rajiformes). Beaufortia 1976, 24, 133–175. [Google Scholar]
- Ishihara, H.; Ishiyama, R. Systematics and Distribution of the Skates of the North Pacific (Chondrichthyes, Rajoidei). In Indo-Pacific Fish Biology: Proceedings of the Second International Conference on Indo-Pacific Fishes; Uyeno, T., Arai, R., Taniuchi, T., Matsuura, K., Eds.; Ichthyological Society of Japan: Tokyo, Japan, 1986; pp. 269–280. [Google Scholar]
- Weigmann, S.; Stehmann, M.F.W.; Thiel, R. Complementary redescription of Anacanthobatis ori (Wallace, 1967) and its assignment to Indobatis n. g. (Elasmobranchii, Anacanthobatidae), with comments on other legskates. Zootaxa 2014, 3779, 101–132. [Google Scholar] [CrossRef] [PubMed]
- Leigh-Sharpe, W.H. The comparative morphology of the secondary sexual characters of elasmobranch fishes. The claspers, clasper siphons, and clasper glands. Memoir VII. J. Morphol. 1924, 39, 567–577. [Google Scholar] [CrossRef]
- Hulley, P.A. Interrelationships within the Anacanthobatidae (Chondrichthyes, Rajoidea), with a description of the lectotype of Anacanthobatis marmoratus von Bonde & Swart, 1923. Ann. S. Afr. Mus. 1973, 62, 131–158. [Google Scholar]
- Stehmann, M. Raja „bathyphila“, eine Doppelart des Subgenus Rajella: Wiederbeschreibung von R. bathyphila Holt & Byrne, 1908 und Raja bigelowi spec. nov. (Pisces, Rajiformes, Rajidae). Arch. Fisch. 1978, 29, 23–58. [Google Scholar]
- Stehmann, M.F.W. Complementary redescription of Raja lintea Fries, 1839 (Elasmobranchii, Rajidae) and its revised generic assignment. Zootaxa 2012, 3331, 44–68. [Google Scholar] [CrossRef]
- Stehmann, M.F.W.; Weigmann, S. A new deepwater legskate, Sinobatis kotlyari n. sp. (Rajiformes, Anacanthobatidae) from the southeastern Indian Ocean on Broken Ridge. Zootaxa 2016, 4189, 327–347. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species; Version 2023-1; IUCN: Gland, Switzerland, 2023. [Google Scholar]
- Pollom, R.; Bennett, R.; Da Silva, C.; Ebert, D.A.; Gledhill, K.; Leslie, R.; McCord, M.E.; Weigmann, S.; Winker, H. Bythaelurus bachi. In The IUCN Red List of Threatened Species; e. T124396026A124552532; IUCN: Gland, Switzerland, 2019. [Google Scholar]
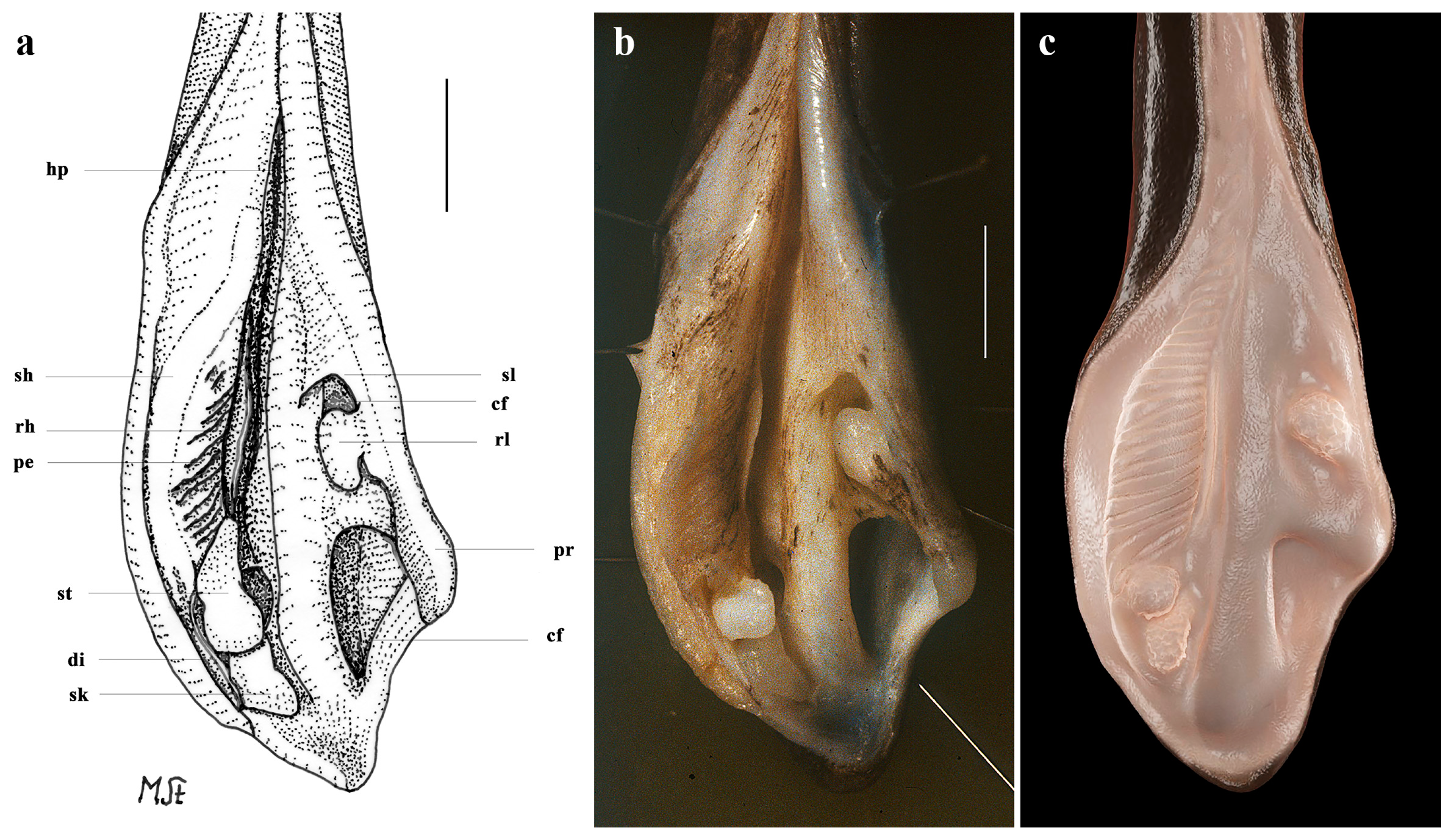
| ps | sh | di | rh | ep | cf | se | sl | st | sk | pt | rl | sp | pe | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. circularis | + | + | - | + | - | + | - | + | + | + | + | + | + | - |
| L. erinacea | +? | + | - | + | + | ++ | + | + | + | + | + | + | - | + |
| L. fullonica | + | + | - | + | - | ++ | - | + | + | + | + | + | + | - |
| L. garmani | - | + | - | + | - | + | + | + | + | + | ? | ? | - | + |
| L. lentiginosa | - | + | - | + | - | + | + | + | + | + | ? | ? | - | + |
| L. leucosticta | + | + | - | + | - | + | + | + | + | + | + | ? | + | - |
| L. longirostris n. sp. | - | + | + | + | - | ++ | - | + | + | + | + | + | - | + |
| L. naevus | + | + | - | + | - | ++ | - | + | + | + | +* | + | + | - |
| L. ocellata | + | + | - | + | + | ++ | + | + | + | + | + | + | - | + |
| L. pristispina | - | + | - | + | + | + | - | + | + | + | + | + | - | - |
| L. wallacei | - | + | - | + | - | + | -? | + | + | + | + | + | + | +? |
| dt1 | dt2 | dt3 | dt4 | vt | at1 | at2 | fc | |
|---|---|---|---|---|---|---|---|---|
| L. circularis | + | + | ++ | - | + | ++ | + | ? |
| L. erinacea | + | + | + | - | +* | +++ | + | ? |
| L. fullonica | + | + | ++ | - | + | ++ | + | + |
| L. garmani | + | + | + | - | +* | ++** | + | ? |
| L. lentiginosa | + | + | ? | - | + | ++** | + | ? |
| L. leucosticta | + | + | ++ | - | + | ++ | + | ? |
| L. longirostris n. sp. | + | + | + | + | + | ++++ | + | + |
| L. naevus | + | ++ | ++ | - | + | ++ | + | ? |
| L. ocellata | + | + | + | - | + | ++ | + | ? |
| L. wallacei | + | + | ++ | + | + | ++ | + | ? |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weigmann, S.; Stehmann, M.F.W.; Séret, B.; Ishihara, H. Description of a Remarkable New Skate Species of Leucoraja Malm, 1877 (Rajiformes, Rajidae) from the Southwestern Indian Ocean: Introducing 3D Modeling as an Innovative Tool for the Visualization of Clasper Characters. Biology 2024, 13, 405. https://doi.org/10.3390/biology13060405
Weigmann S, Stehmann MFW, Séret B, Ishihara H. Description of a Remarkable New Skate Species of Leucoraja Malm, 1877 (Rajiformes, Rajidae) from the Southwestern Indian Ocean: Introducing 3D Modeling as an Innovative Tool for the Visualization of Clasper Characters. Biology. 2024; 13(6):405. https://doi.org/10.3390/biology13060405
Chicago/Turabian StyleWeigmann, Simon, Matthias F. W. Stehmann, Bernard Séret, and Hajime Ishihara. 2024. "Description of a Remarkable New Skate Species of Leucoraja Malm, 1877 (Rajiformes, Rajidae) from the Southwestern Indian Ocean: Introducing 3D Modeling as an Innovative Tool for the Visualization of Clasper Characters" Biology 13, no. 6: 405. https://doi.org/10.3390/biology13060405
APA StyleWeigmann, S., Stehmann, M. F. W., Séret, B., & Ishihara, H. (2024). Description of a Remarkable New Skate Species of Leucoraja Malm, 1877 (Rajiformes, Rajidae) from the Southwestern Indian Ocean: Introducing 3D Modeling as an Innovative Tool for the Visualization of Clasper Characters. Biology, 13(6), 405. https://doi.org/10.3390/biology13060405








