Flexible Resource Allocation-Efficient Water Use Strategies Facilitate Invasion of Invasive Vine Sicyos angulatus L.
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Seeds
2.3. Measurements of Leaf Area, Photosynthetic Characteristics, and Water-Use Efficiency
2.4. Determination of Root Pressure and Root Activity
2.5. Statistical and Data Analyses
3. Results
3.1. Compared with the Other Three Plants, Sa Can Maintain Rapid Growth and Development
3.2. Photosynthetic Capacity of Sa Fruit Period Was Stronger Than That of In, Ip, and Td
3.3. Sa Had Higher Root Pressure and Root Activity Than In, Ip, and Td
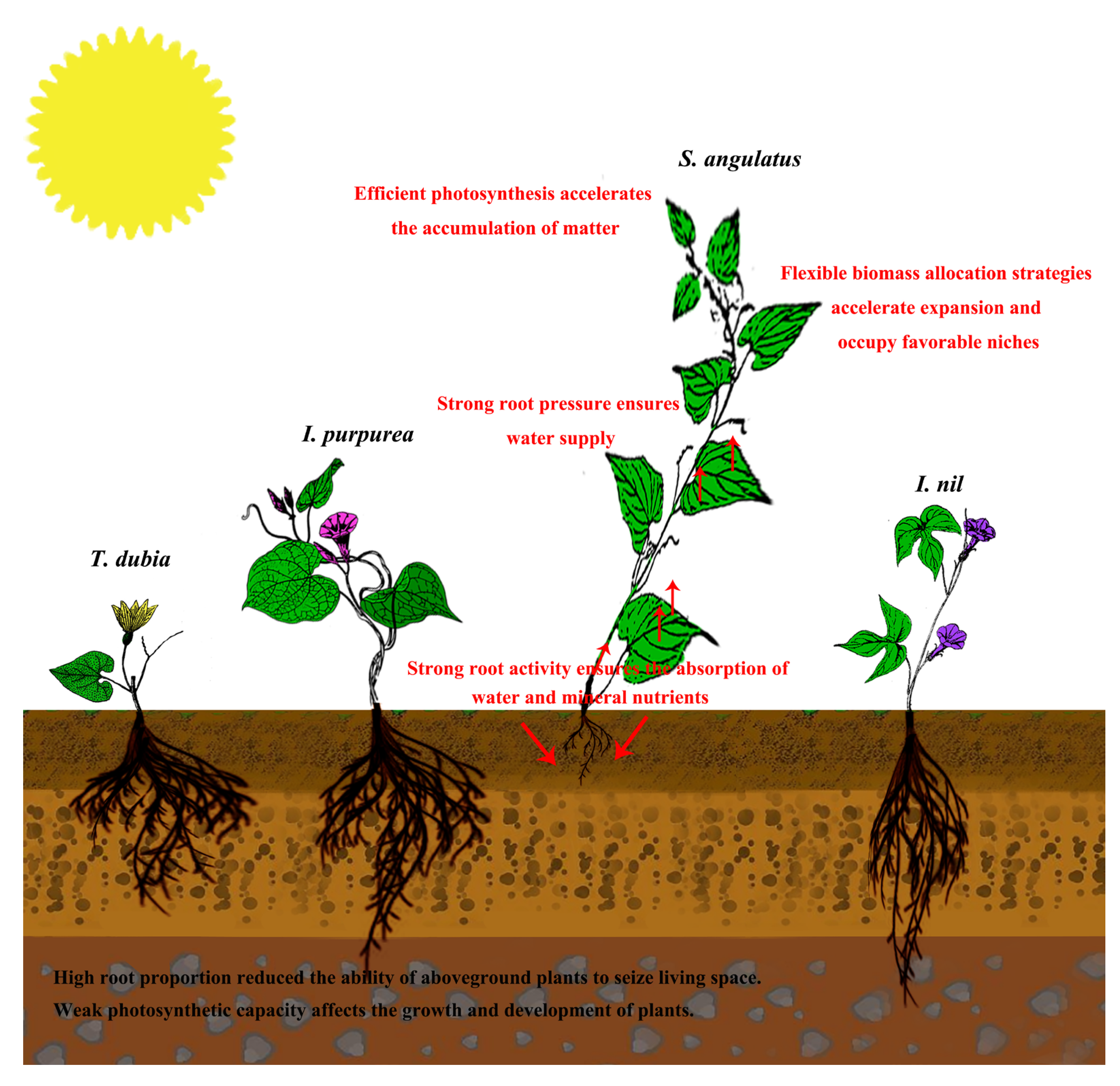
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Kleunen, M.; Dawson, W.; Essl, F.; Pergl, J.; Winter, M.; Weber, E.; Kreft, H.; Weigelt, P.; Kartesz, J.; Nishino, M.; et al. Global exchange and accumulation of non-native plants. Nature 2015, 525, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Miao, L.; Wu, P.; Yang, X.; Guo, W.; Li, S.; Feng, G. Effects of anthropogenic activities and climate factors on the distribution of invasive alien species in China. Sci. Sin. Vitae 2023, 53, 543–550. [Google Scholar] [CrossRef]
- Diagne, C.; Leroy, B.; Vaissière, A.C.; Gozlan, R.E.; Roiz, D.; Jarić, I.; Salles, J.M.; Bradshaw, C.J.A.; Courchamp, F. High and rising economic costs of biological invasions worldwide. Nature 2021, 592, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Welsh, M.J.; Turner, J.A.; Epanchin-Niell, R.S.; Monge, J.J.; Soliman, T.; Robinson, A.P.; Kean, J.M.; Phillips, C.; Stringer, L.D.; Vereijssen, J.; et al. Approaches for estimating benefits and costs of interventions in plant biosecurity across invasion phases. Ecol. Appl. 2021, 31, e02319. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.C.; Alpert, P.; Guo, W.; Yu, F.H. Effects of fragmentation on the survival and growth of the invasive, clonal plant Alternanthera philoxeroides. Biol. Invasions 2011, 14, 1101–1110. [Google Scholar] [CrossRef]
- Li, R.H.; Chu, S.H.; Wan, P.; Chang, X.Q.; Li, G.W. Survey on Alien Invasive Weeds in Farmlands in Hubei Province. Hubei Agric. Sci. 2011, 50, 3963–3966. [Google Scholar]
- Kazinczi, G.; Torma, M.; Beres, I.; Horvath, J. Competition between Xanthium italicum and crops under field conditions. Cereal Res. Commun. 2009, 37, 77–80. [Google Scholar]
- Ma, X.; Hou, M.C.; Ma, M. High interspecific competitiveness of the invasive plant Xanthium italicum Moretti severely reduces the yield and quality of Carthamus tinctorius L. Sci. Rep. 2023, 13, 4300. [Google Scholar] [CrossRef] [PubMed]
- Darwin, C. On the movements and habits of climbing plants. J. Linn. Soc. 1865, 9, 1–118. [Google Scholar] [CrossRef]
- Leicht Young, S.A.; Latimer, A.M.; Silander, J.A. Lianas escape self-thinning: Experimental evidence of positive density dependence in temperate lianas Celastrus orbiculatus and C. scandens. Perspect. Plant Ecol. Evol. Syst. 2011, 13, 163–172. [Google Scholar] [CrossRef]
- Harris, C.J.; Gallagher, R. Vines and lianas. In Encyclopedia of Biological Invasions; Simberlof, D., Rejmanek, M., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 608–684. [Google Scholar]
- Ichihashi, R.; Tateno, M. Biomass allocation and long-term growth patterns of temperate lianas in comparison with trees. New Phytol. 2015, 207, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lu, W.; Zheng, X.L. Research progress on biological characteristics, invasion mechanism and prevention and control technology of Ipomoea cairica. Guangxi Plant Prot. 2015, 27, 36–39. [Google Scholar]
- Rayamajhi, M.B.; Pratt, P.D.; Tipping, P.W.; Lake, E.; Smith, M.; Rohrig, E.; Dray, F.A.; Center, T.D. Seasonal Growth, Biomass Allocation, and Invasive Attributes Manifested by Dioscorea bulbifera L. (Air-Potato) Plants Generated from Bulbils in Florida. Invasive Plant Sci. Manag. 2016, 9, 195–204. [Google Scholar] [CrossRef]
- Kaneko, Y.; Homma, K. Differences in the allocation patterns between liana and shrub Hydrangea species. Plant Species Biol. 2006, 21, 147–153. [Google Scholar] [CrossRef]
- Feitosa, T.S.; de Carvalho, E.C.D.; Barreto, R.W.; Mantovani, W.; de Araújo, F.S.; da Costa, R.C. Use of support influences height and above-ground allometry but not biomass allocation to different aerial organs of an invasive vine. Trees 2022, 37, 373–383. [Google Scholar] [CrossRef]
- Zhu, S.D.; Cao, K.F. Hydraulic properties and photosynthetic rates in co-occurring lianas and trees in a seasonal tropical rainforest in southwestern China. Plant Ecol. 2009, 204, 295–304. [Google Scholar] [CrossRef]
- Putz, F.E. Liana stem diameter growth and mortality rates on Barro Colorado Island, Panama. Biotropica 1990, 22, 103–105. [Google Scholar] [CrossRef]
- Xu, Y.L.; Qin, Y.J.; Zhang, Y.; Zhang, Y.; Li, Z.H.; Fu, W.D.; Zhao, Z.H. Potential geographical distribution of alien invasive bur cucumber Sicyos angulatus in China based on MaxEnt model. J. Plant Prot. 2022, 49, 1440–1449. [Google Scholar]
- He, L.L.; Liu, J.C.; Chen, B. Potential Distribution and Agricultural Economic Loss Prediction of Alien Invasive Plant Sicyos angulatus in Liaoning Province. J. Shenyang Agric. Univ. 2022, 53, 119–127. [Google Scholar]
- Che, J.; Jia, F.; Liang, T. First Record of the Invasive Plant Sicyos angulatus in Beijing City. J. Weed Sci. 2013, 31, 66–68. [Google Scholar]
- Xue, C.; Sun, L.; Qu, B.; Gao, Y.; Liu, Z.; Guo, C.; Liu, W.; Chang, W.; Tai, P. Grafting with an invasive Xanthium strumarium improves tolerance and phytoremediation of native congener X. sibiricum to cadmium/copper/nickel tailings. Chemosphere 2022, 308, 136561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Guo, W.; Yang, S.J. Recent advances in research on root pressure of plants. Guihaia 2022, 42, 714–727. [Google Scholar]
- Fan, J.J.; Ruan, Y.Y. Plant Physiology Experiment Tutorial, 2nd ed.; China Agricultural University Press: Beijing, China, 2015. [Google Scholar]
- Gentry, A.G. The distribution and evolution of climbing plants. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 73–97. [Google Scholar]
- Di Tommaso, A.; Averill, K.M.; Qin, Z.; Ho, M.; Westbrook, A.S.; Mohler, C.L. Biomass allocation of Vincetoxicum rossicum and V. nigrum in contrasting competitive environments. Am. J. Bot. 2021, 108, 1646–1661. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Feng, Y.L. Effects of soil nitrogen leves on morphology, biomass allocation and photosynthesis in Ageratina adenophora and Chromoleana odorata. Acta Phytoecol. Sin. 2005, 29, 697–705. [Google Scholar]
- Zheng, Y.L.; Feng, Y.L.; Liu, W.X.; Liao, Z.Y. Growth, biomass allocation, morphology, and photosynthesis of invasive Eupatorium adenophorum and its native congeners grown at four irradiances. Plant Ecol. 2009, 203, 263–271. [Google Scholar] [CrossRef]
- Xue, C.; Xu, Y.; Qu, B. Comparison of morphology, photosynthesis, and growth among Xanthium strumarium, X. sibiricum and their hybrid under different nitrogen levels. Biodivers. Sci. 2018, 26, 554–563. [Google Scholar] [CrossRef]
- Qu, B.; Xue, C.; Xu, Y.; Gao, Y.; Chen, X.; Wang, W. Effects of Ambrosia trifida on the Early Spring Plant Community in Abandoned Farmland. J. Shenyang Agric. Univ. 2019, 50, 358–364. [Google Scholar]
- Wyka, T.P.; Oleksyn, J.; Karolewski, P.; Schnitzer, S.A. Phenotypic correlates of the lianescent growth form: A review. Ann. Bot. 2013, 112, 1667–1681. [Google Scholar] [CrossRef]
- Toledo-Aceves, T.; Swaine, M.D. Above-and below-ground competition between the liana Acacia kamerunensis and tree seedlings in contrasting light environments. Plant Ecol. 2008, 196, 233–244. [Google Scholar] [CrossRef]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- den Dubbelden, K.C.; Verburg, R.W. Inherent allocation patterns and potential growth rates of herbaceous climbing plants. Plant Soil 1996, 184, 341–347. [Google Scholar] [CrossRef]
- Poorter, H.; Sack, L. Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Front. Plant Sci. 2012, 3, 259. [Google Scholar] [CrossRef] [PubMed]
- Isnard, S.; Silk, W.K. Moving with climbing plants from Charles Darwin’s time into the 21st century. Am. J. Bot. 2009, 96, 1205–1221. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, K.G.; Jesson, L.K.; Kubien, D.S. Photosynthesis and water-use efficiency: A comparison between invasive (exotic) and non-invasive (native) species. Austral Ecol. 2008, 33, 10–19. [Google Scholar] [CrossRef]
- Poorter, H.; Evans, J.R. Photosynthetic nitrogen-use efficiency of species that differ inherently in specific leaf area. Oecologia 1998, 116, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.D.; Cao, K.F. Contrasting cost-benefit strategy between lianas and trees in a tropical seasonal rain forest in southwestern China. Oecologia 2010, 163, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Santiago, L.S.; Wright, S.J. Leaf functional traits of tropical forest plants in relation to growth forms. Funct. Ecol. 2007, 21, 19–27. [Google Scholar] [CrossRef]
- Stratton, L.C.; Goldstein, G. Carbon uptake, growth and resource-use efficiency in one invasive and six native Hawaiian dry forest tree species. Tree Physiol. 2001, 21, 1327–1334. [Google Scholar] [CrossRef]
- Gleason, S.M.; Wiggans, D.R.; Bliss, C.A.; Young, J.S.; Cooper, M.; Willi, K.R.; Comas, L.H. Embolized Stems Recover Overnight in Zea mays: The Role of Soil Water, Root Pressure, and Nighttime Transpiration. Front. Plant Sci. 2017, 8, 00662. [Google Scholar] [CrossRef]
- Holmlund, H.I.; Davis, S.D.; Ewers, F.W.; Aguirre, N.M.; Sapes, G.; Sala, A.; Pittermann, J.; Griffiths, H. Positive root pressure is critical for whole-plant desiccation recovery in two species of terrestrial resurrection ferns. J. Exp. Bot. 2020, 71, 1139–1150. [Google Scholar] [CrossRef]
- Fisher, J.B.; Angeles, A.G.; Ewers, F.W.; Lopez, P.J. Surveyof rootpressure in tropical vines and woody species. Int. J. Plant Sci. 1997, 158, 44–50. [Google Scholar] [CrossRef]
- Jimenez-Castillo, M.; Lusk, C.H. Vascular performance of woody plants in a temperate rain-forest: Lianas suffer higher levels of freeze-thaw embolism than associated trees. Funct. Ecol. 2013, 27, 403–412. [Google Scholar] [CrossRef]
- Teramura, A.H.; Gold, W.G.; Forseth, I.N. Physiological ecology of mesic, temperate woody vines. In The Biology of Vines; Putz, F.E., Mooney, H.A., Eds.; Cambridge University Press: Cambridge, UK, 1991; pp. 245–285. [Google Scholar]

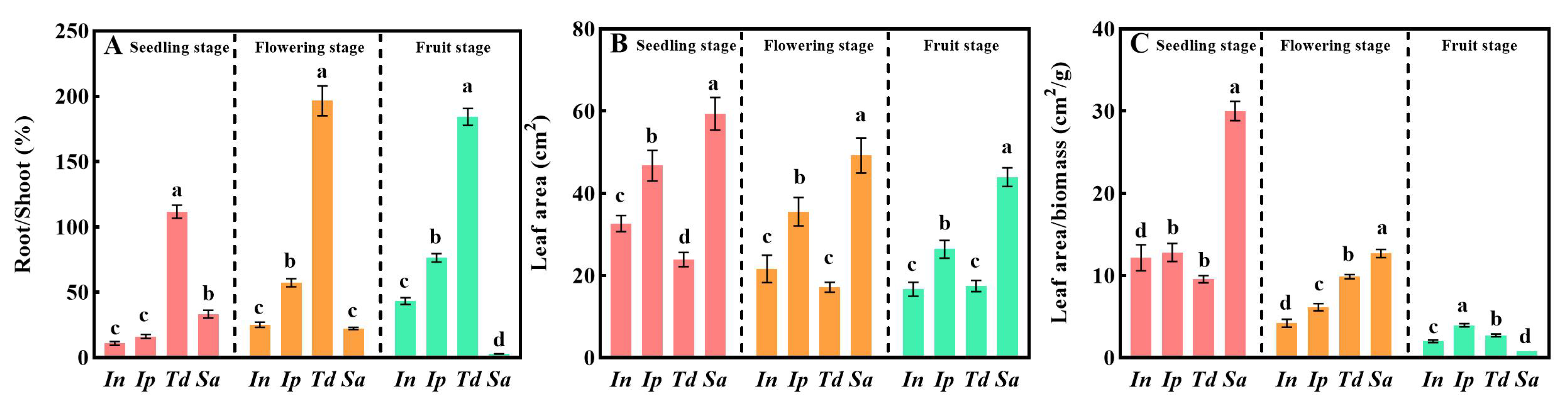
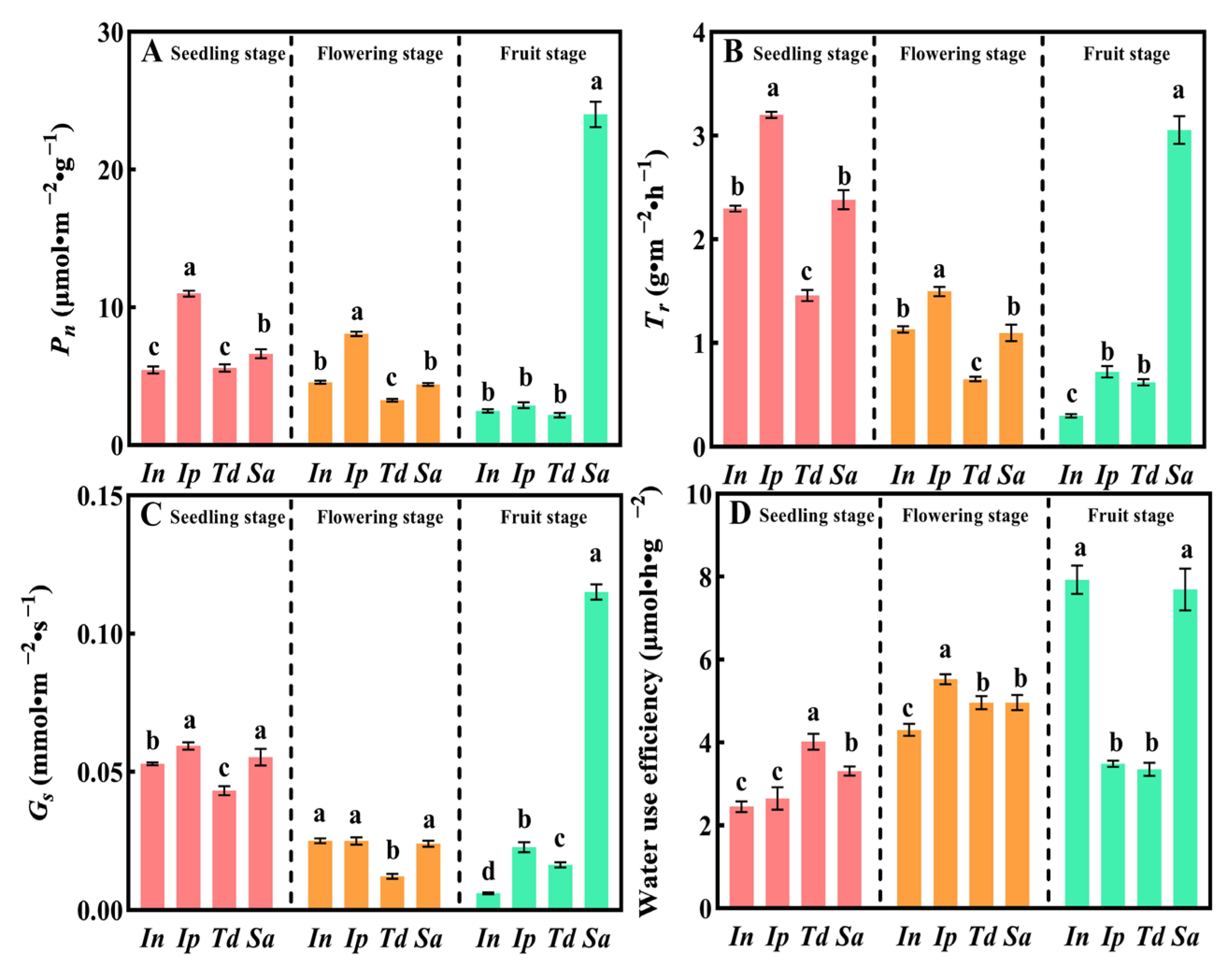
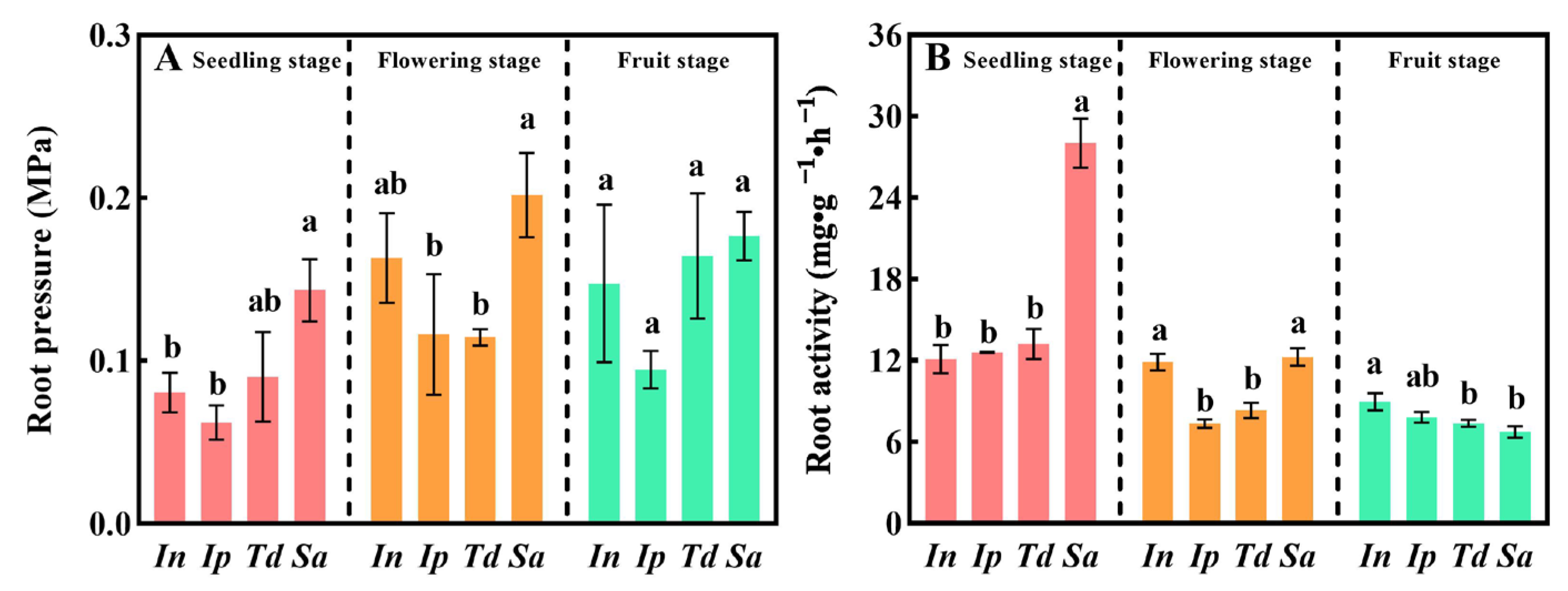
| Plant | Index | Seedling Stage | Flowering Stage | Fruit Stage |
|---|---|---|---|---|
| In | Total biomass (g) | 5.03 ± 1.94 b | 22.85 ± 2.86 b | 31.85 ± 6.32 c |
| Root ratio (%) | 23.35 ± 3.98 d | 17.52 ± 2.01 c | 29.16 ± 3.37 c | |
| Steam ratio (%) | 2.55 ± 0.41 c | 53.61 ± 2.26 b | 46.94 ± 4.00 b | |
| Leaf ratio (%) | 74.10 ± 1.40 a | 28.87 ± 0.69 a | 23.90 ± 0.62 b | |
| Ip | Total biomass (g) | 4.97 ± 0.64 b | 51.54 ± 7.03 a | 63.62 ± 12.24 b |
| Root ratio (%) | 27.74 ± 5.19 c | 36.22 ± 2.75 b | 42.61 ± 2.33 b | |
| Steam ratio (%) | 3.21 ± 0.52 c | 52.7 ± 2.30 b | 46.95 ± 2.27 b | |
| Leaf ratio (%) | 69.05 ± 1.79 b | 11.08 ± 0.20 c | 10.44 ± 0.14 c | |
| Td | Total biomass (g) | 6.90 ± 1.54 a | 6.99 ± 0.90 d | 59.98 ± 10.46 b |
| Root ratio (%) | 49.77 ± 3.10 a | 66.06 ± 4.25 a | 65.29 ± 1.30 a | |
| Steam ratio (%) | 15.27 ± 1.13 a | 10.57 ± 1.13 c | 23.84 ± 1.94 c | |
| Leaf ratio (%) | 34.96 ± 0.51 d | 23.37 ± 1.10 b | 10.87 ± 0.32 c | |
| Sa | Total biomass (g) | 4.27 ± 0.82 b | 16.80 ± 1.92 c | 193.41 ± 29.88 a |
| Root ratio (%) | 41.99 ± 2.39 b | 18.23 ± 0.59 c | 2.90 ± 0.30 d | |
| Steam ratio (%) | 7.74 ± 0.83 b | 58.96 ± 1.95 a | 67.33 ± 1.02 a | |
| Leaf ratio (%) | 50.27 ± 0.73 c | 22.81 ± 0.71 b | 29.77 ± 0.28 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Q.; Xue, C.; Meng, L.; Gao, Y.; Yu, M.; Geng, L.; Guan, P.; Qu, B. Flexible Resource Allocation-Efficient Water Use Strategies Facilitate Invasion of Invasive Vine Sicyos angulatus L. Biology 2024, 13, 392. https://doi.org/10.3390/biology13060392
Pan Q, Xue C, Meng L, Gao Y, Yu M, Geng L, Guan P, Qu B. Flexible Resource Allocation-Efficient Water Use Strategies Facilitate Invasion of Invasive Vine Sicyos angulatus L. Biology. 2024; 13(6):392. https://doi.org/10.3390/biology13060392
Chicago/Turabian StylePan, Qingmin, Chenyang Xue, Lin Meng, Ying Gao, Mengyang Yu, Lin Geng, Ping Guan, and Bo Qu. 2024. "Flexible Resource Allocation-Efficient Water Use Strategies Facilitate Invasion of Invasive Vine Sicyos angulatus L." Biology 13, no. 6: 392. https://doi.org/10.3390/biology13060392
APA StylePan, Q., Xue, C., Meng, L., Gao, Y., Yu, M., Geng, L., Guan, P., & Qu, B. (2024). Flexible Resource Allocation-Efficient Water Use Strategies Facilitate Invasion of Invasive Vine Sicyos angulatus L. Biology, 13(6), 392. https://doi.org/10.3390/biology13060392




