Basement Membranes, Brittlestar Tendons, and Their Mechanical Adaptability
Abstract
Simple Summary
Abstract
1. Introduction
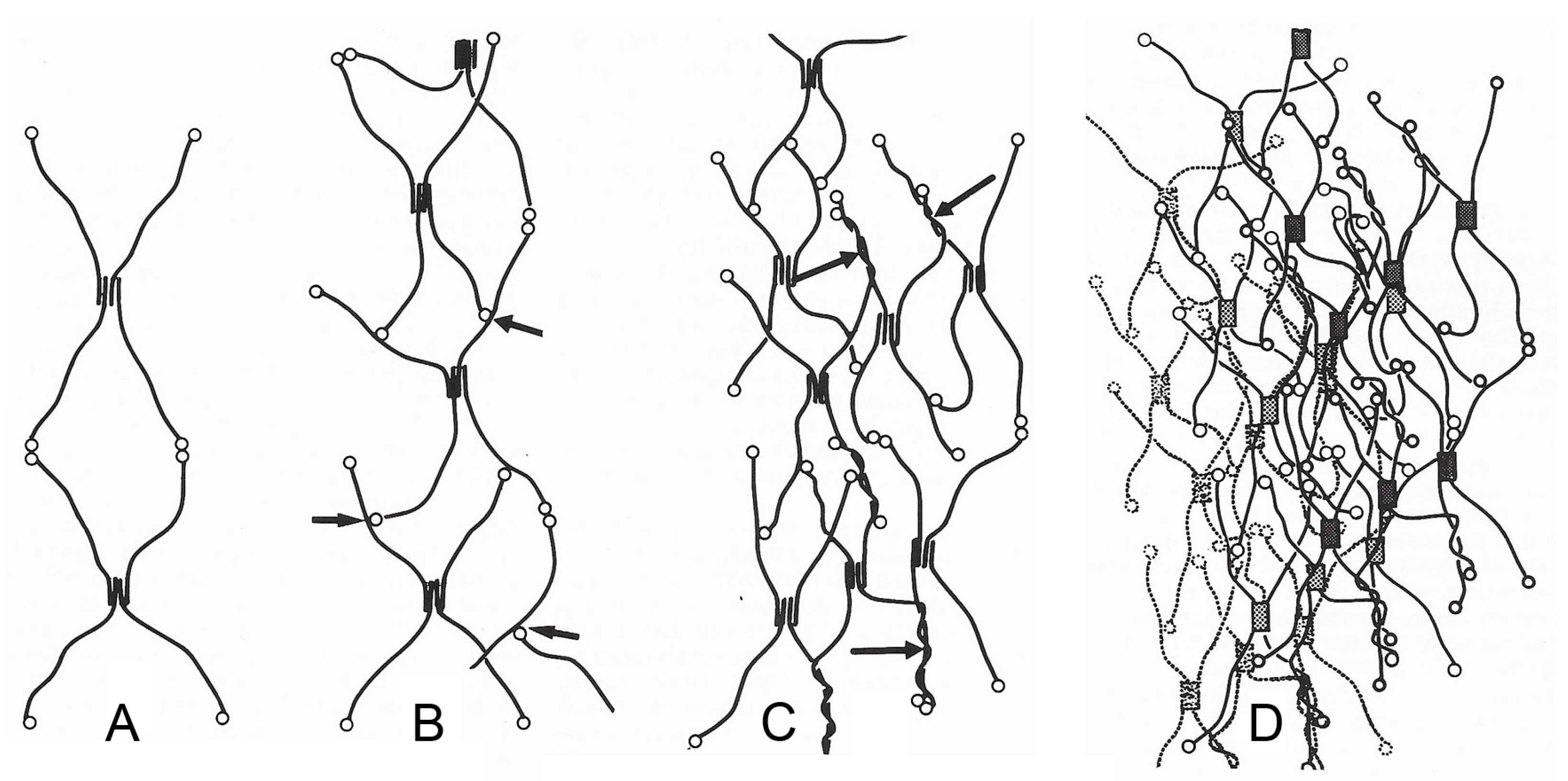
2. Supramolecular Organization
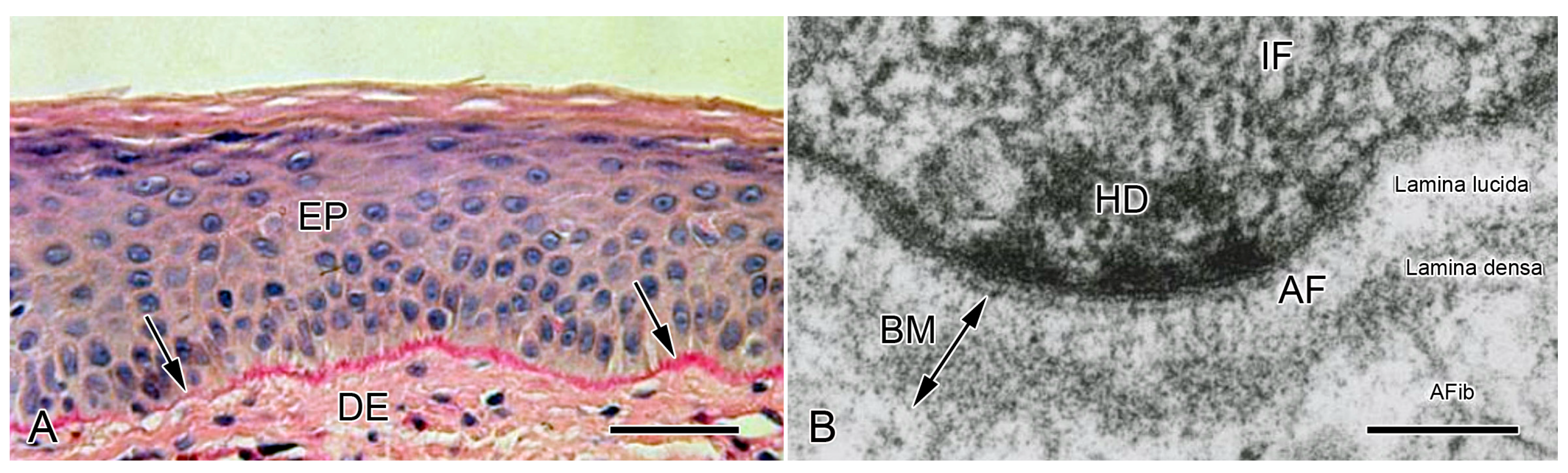
2.1. Non-Echinoderm BMs
2.1.1. Composition and Constitution
2.1.2. Configuration and Conformation
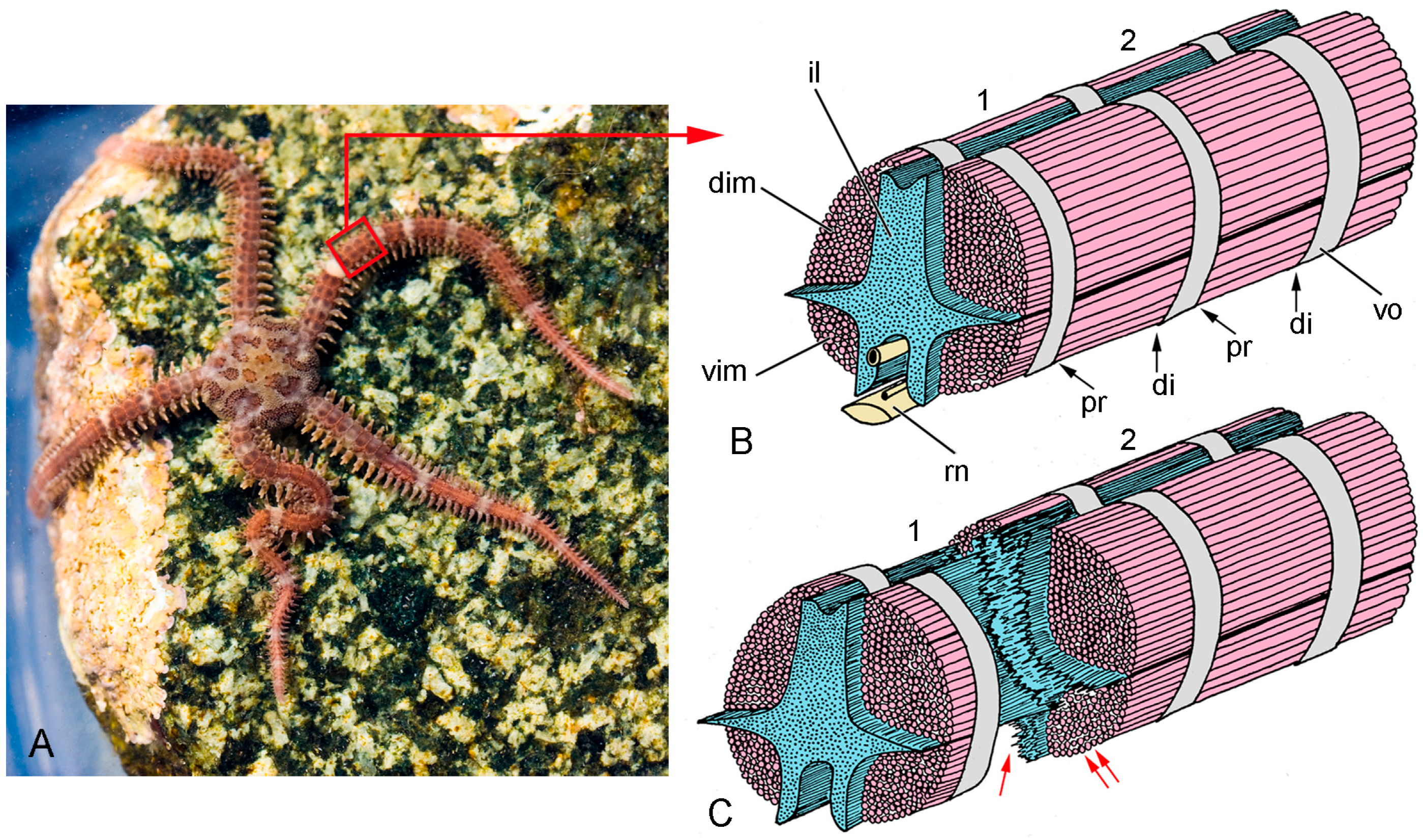
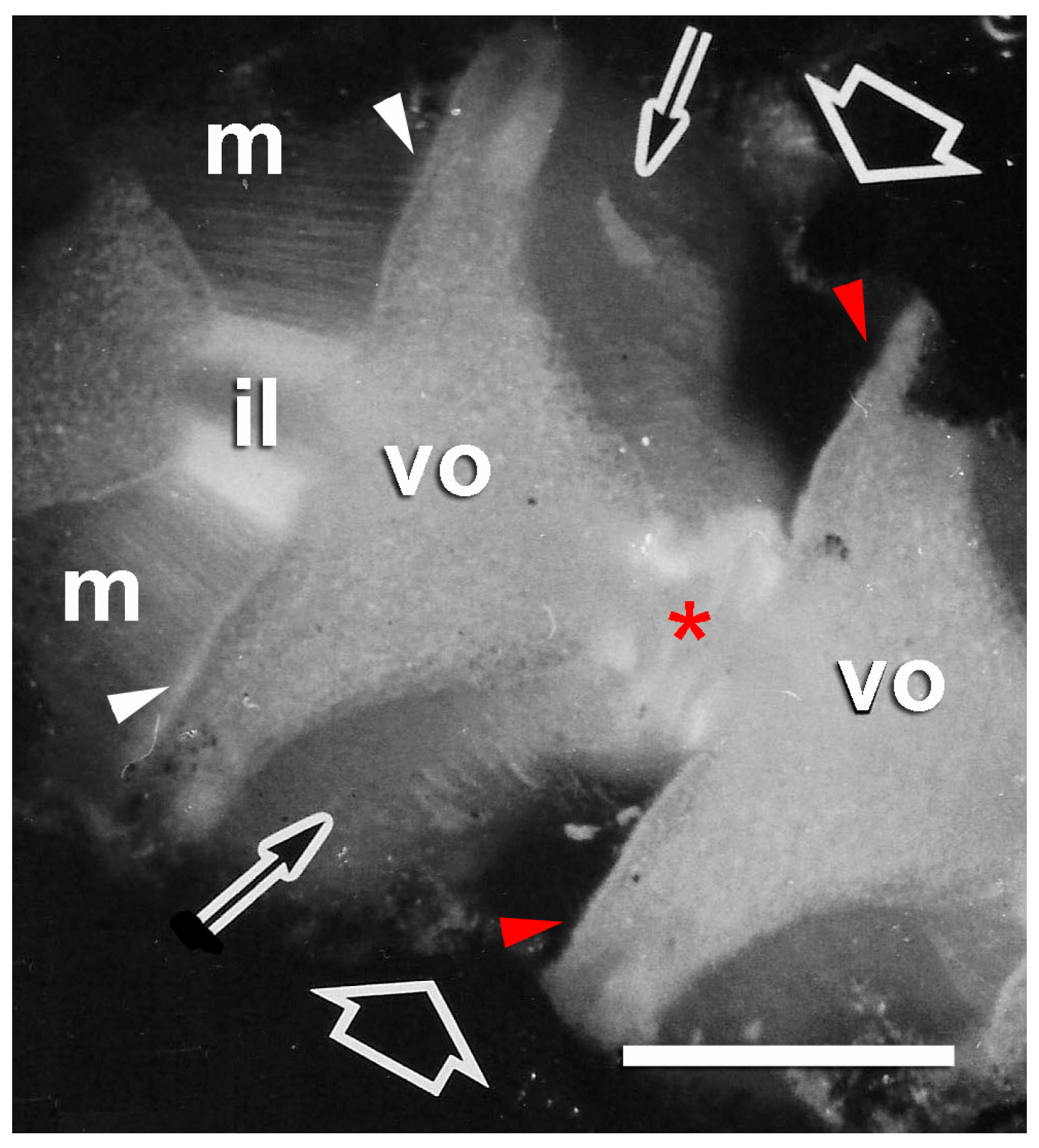
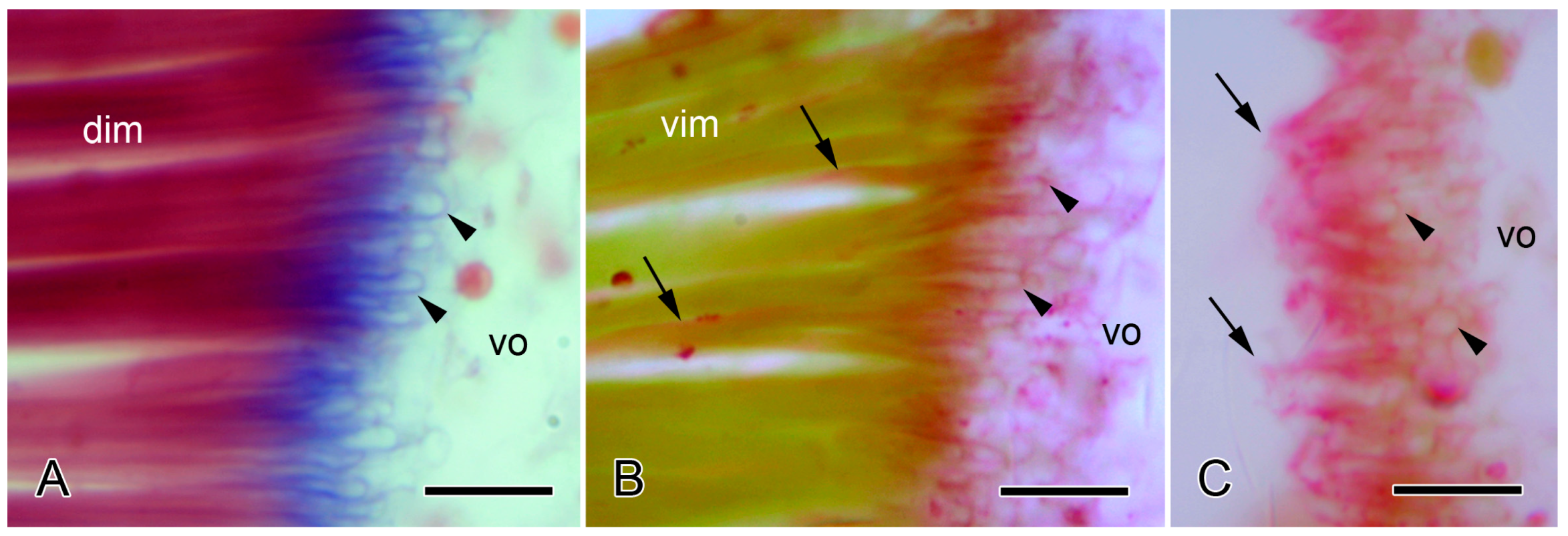
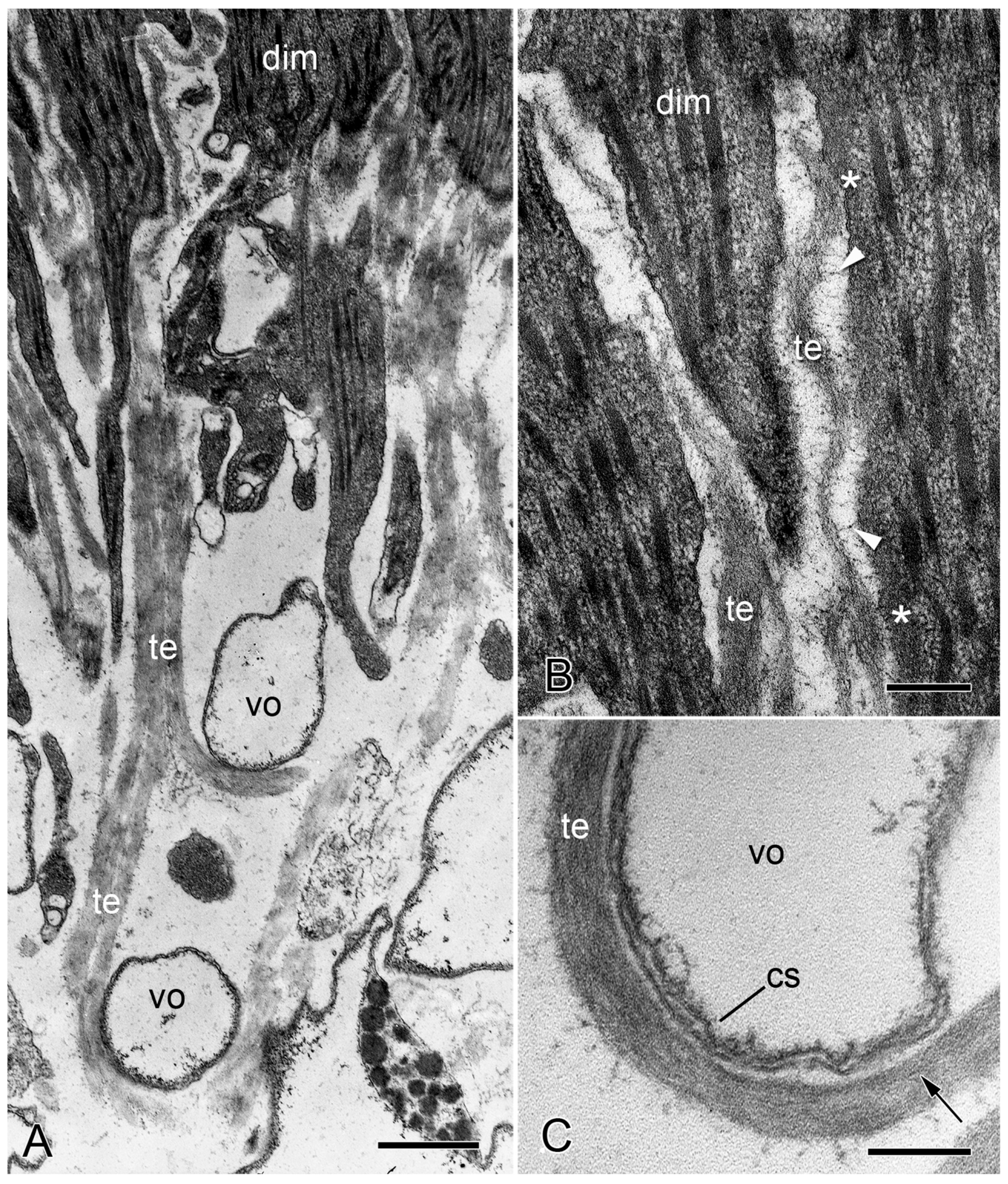
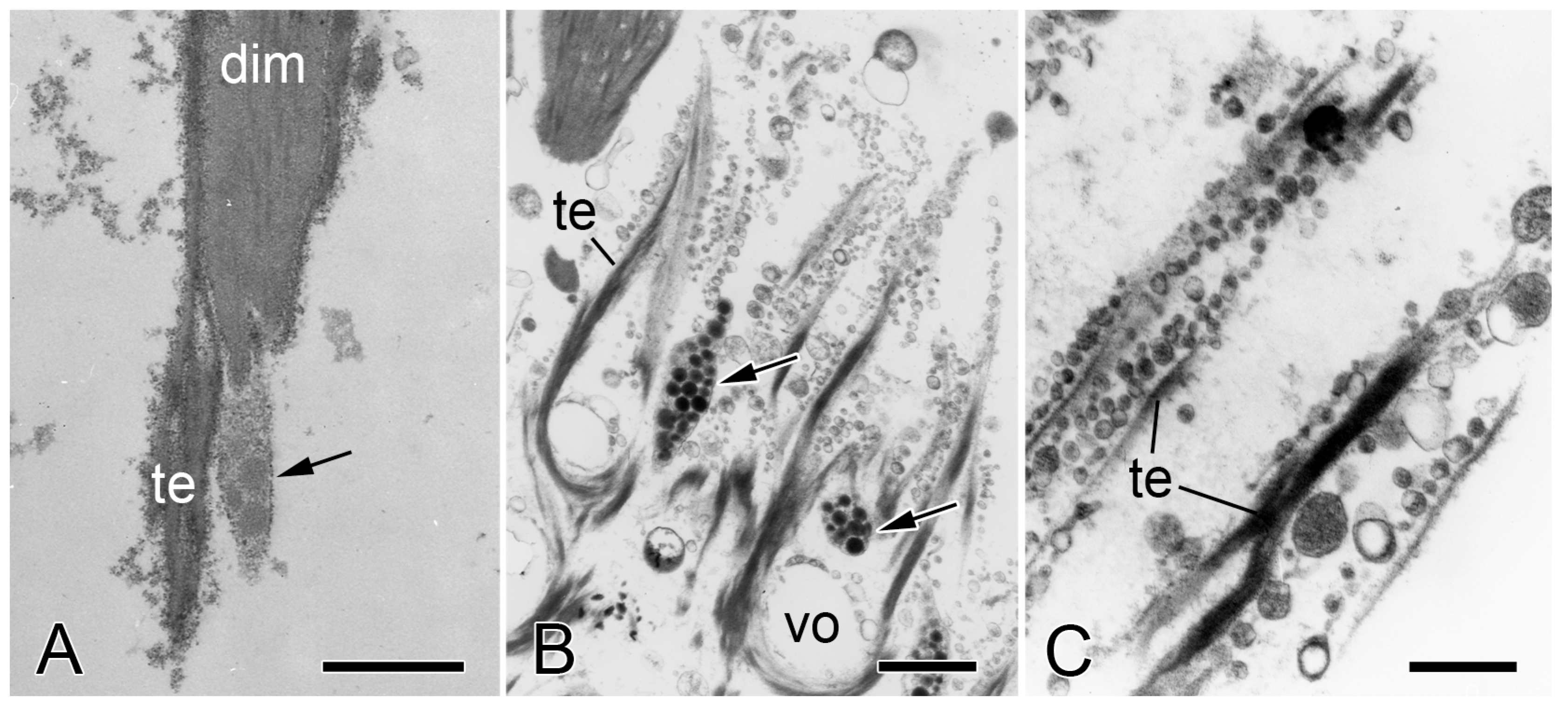
2.2. Brittlestar IMTs
2.2.1. Composition and Constitution
2.2.2. Configuration and Conformation
2.3. Comments on Supramolecular Organization
3. Biomechanical Aspects
3.1. Non-Echinoderm BMs
3.1.1. General Biomechanical Aspects
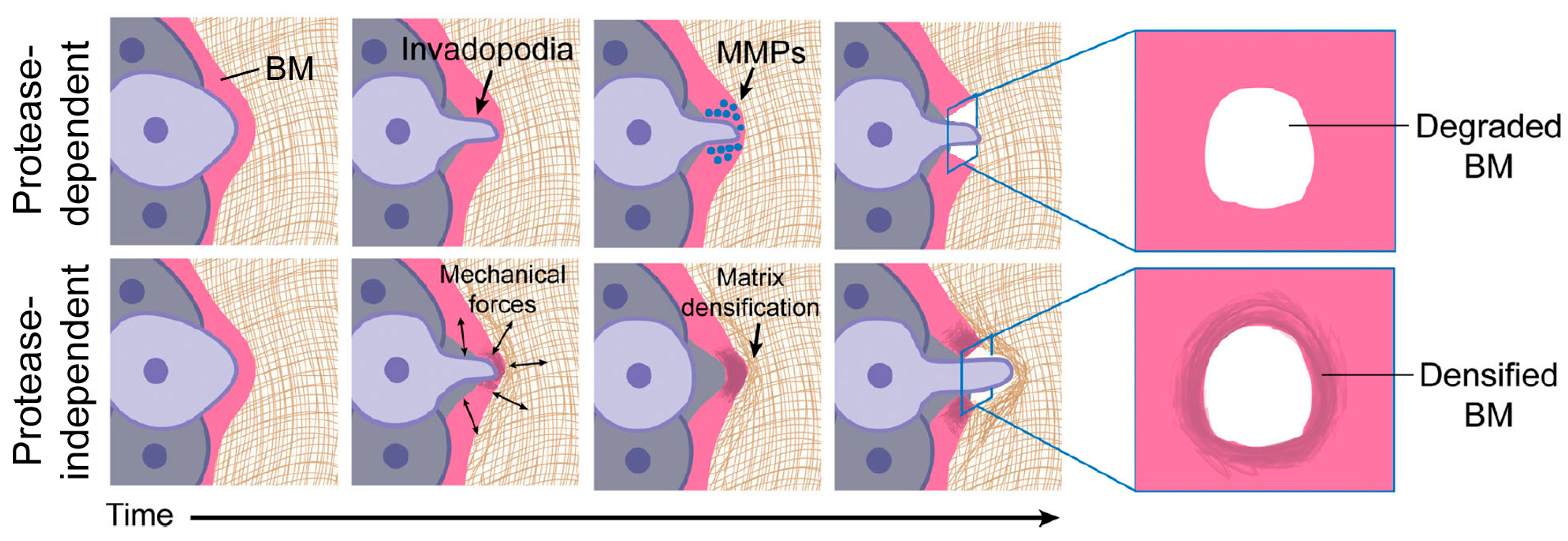
3.1.2. Mechanical Adaptability
3.2. Brittlestar IMTs
3.2.1. General Biomechanical Aspects
3.2.2. Mechanical Adaptability
3.3. Comments on Biomechanical Aspects
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkie, I.C.; Sugni, M.; Gupta, H.S.; Candia Carnevali, M.D.; Elphick, M.R. The mutable collagenous tissue of echinoderms: From biology to biomedical applications. In Soft Matter for Biomedical Applications; Azevedo, H.S., Mano, J.F., Borges, J., Eds.; Royal Society of Chemistry: Cambridge, UK, 2021; pp. 3–33. [Google Scholar]
- Motokawa, T.; Fuchigami, Y. Coordination between catch connective tissue and muscles through nerves in the spine joint of the sea urchin Diadema setosum. J. Exp. Biol. 2015, 218, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Holland, N.D.; Grimmer, J.C. Fine structure of syzygial articulations before and after arm autotomy in Florometra serratissima (Echinodermata: Crinoidea). Zoomorphology 1981, 98, 169–183. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Emson, R.H. The tendons of Ophiocomina nigra and their role in autotomy (Echinodermata, Ophiuroida). Zoomorphology 1987, 107, 33–44. [Google Scholar] [CrossRef]
- Pastor-Pareja, J.C. Atypical basement membranes and basement membrane diversity–What is normal anyway? J. Cell Sci. 2020, 133, jcs241794. [Google Scholar] [CrossRef] [PubMed]
- Fidler, A.L.; Darris, C.E.; Chetyrkin, S.V.; Pedchenk, V.K.; Boudko, S.P.; Brown, K.L.; Jerome, W.G.; Hudson, J.H.; Rokas, A.; Hudson, B.G. Collagen IV and basement membrane at the evolutionary dawn of metazoan tissues. eLife 2017, 6, e24176. [Google Scholar] [CrossRef] [PubMed]
- Khalilgharibi, N.; Mao, Y. To form and function: On the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol. 2021, 11, 200360. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, R.; Yamada, K.M. Basement membranes in development and disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef]
- Kyprianou, C.; Christodoulou, N.; Hamilton, R.S.; Nahaboo, W.; Boomgaard, D.S.; Amadei, G.; Migeotte, I.; Zernicka-Goetz, M. Basement membrane remodelling regulates mouse embryogenesis. Nature 2020, 582, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Roig-Rosello, E.; Rousselle, P. The human epidermal basement membrane: A shaped and cell instructive platform that aging slowly alters. Biomolecules 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Chaudhuri, O. Beyond proteases: Basement membrane mechanics and cancer invasion. J. Cell Biol. 2019, 218, 2456–2469. [Google Scholar] [CrossRef] [PubMed]
- Turro, N.J. Molecular structure as a blueprint for supramolecular structure chemistry in confined spaces. Proc. Natl. Acad. Sci. USA 2005, 102, 10766–10770. [Google Scholar] [CrossRef] [PubMed]
- Kramer, J.M. Basement membranes. In WormBook; The C. elegans Research Community, Ed.; WormBook: Pasadena, CA, USA, 2005; pp. 1–15. [Google Scholar] [CrossRef]
- Yurchenco, P.D. Basement membranes: Cell scaffoldings and signaling platforms. Cold Spring Harb. Perspect. Biol. 2011, 3, a004911. [Google Scholar] [CrossRef] [PubMed]
- Isabella, A.J.; Horne-Badovinac, S. Building from the ground up: Basement membranes in Drosophila development. Curr. Top. Membr. 2015, 76, 305–336. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.M.; Schneider, M.; Frock, R.; Castillejo-Lopez, C.; Gaman, E.A.; Baumgartner, S.; Ruohola-Baker, H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development 2003, 130, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.P.; Kang, S.H.; Kramer, J.M. C. elegans dystroglycan DGN-1 functions in epithelia and neurons, but not muscle, and independently of dystrophin. Development 2006, 133, 1911–1921. [Google Scholar] [CrossRef]
- Hrus, A.; Lau, G.; Hutter, H.; Schenk, S.; Ferralli, J.; Brown-Luedi, M.; Chiquet-Ehrismann, R.; Canevascini, S. C. elegans agrin is expressed in pharynx, IL1 neurons and distal tip cells and does not genetically interact with genes involved in synaptogenesis or muscle function. PLoS ONE 2007, 2, e731. [Google Scholar] [CrossRef] [PubMed]
- Momota, R.; Naito, I.; Ninomiya, Y.; Ohtsuka, A. Drosophila type XV/XVIII collagen, Mp, is involved in Wingless distribution. Matrix Biol. 2011, 30, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Kamimura, K.; Ueno, K.; Nakagawa, J.; Hamada, R.; Saitoe, M.; Maeda, N. Perlecan regulates bidirectional Wnt signaling at the Drosophila neuromuscular junction. J. Cell Biol. 2013, 200, 219–233. [Google Scholar] [CrossRef] [PubMed]
- Weber, M. Basement membrane proteins. Kidney Internat. 1992, 41, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.M.; Mei, R. Basement membrane type IV collagen and laminin: An overview of their biology and value as fibrosis biomarkers of liver disease. Anat. Rec. 2017, 300, 1371–1390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ge, G. Complexity of type IV collagens: From network assembly to function. Biol. Chem. 2019, 400, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Tsilibary, E.C.; Charonis, A.S. The role of the main noncollagenous domain (NC1) in type IV collagen self-assembly. J. Cell Biol. 1986, 103, 2467–2473. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Ruben, G.C. Basement membrane structure in situ: Evidence for lateral associations in the type IV collagen network. J. Cell Biol. 1987, 105, 2559–2568. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, E.; Yurchenco, P.D. Laminins in basement membrane assembly. Cell Adhes. Migr. 2013, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Merker, H.J. Morphology of the basement membrane. Microsc. Res. Tech. 1994, 28, 95–124. [Google Scholar] [CrossRef]
- Yurchenco, P.D.; Cheng, Y.S. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 1993, 268, 17286–17299. [Google Scholar] [CrossRef] [PubMed]
- Hohenester, E. Structural biology of laminins. Essays Biochem. 2019, 63, 285–295. [Google Scholar] [CrossRef]
- Lössl, P.; Kölbel, K.; Tänzler, D.; Nannemann, D.; Ihling, C.H.; Keller, M.V.; Schneider, M.; Zaucke, F.; Meiler, J.; Sinz, A. Analysis of nidogen-1/laminin γ1 interaction by cross-linking, mass spectrometry, and computational modeling reveals multiple binding modes. PLoS ONE 2014, 9, e112886. [Google Scholar] [CrossRef]
- Urbano, J.M.; Torgler, C.N.; Molnar, C.; Tepass, U.; López-Varea, A.; Brown, N.H.; de Celis, J.F.; Martín-Bermudo, M.D. Drosophila laminins act as key regulators of basement membrane assembly and morphogenesis. Development 2009, 136, 4165–4176. [Google Scholar] [CrossRef]
- Clay, M.R.; Sherwood, D.R. Basement membranes in the worm: A dynamic scaffolding that instructs cellular behaviors and shapes tissues. Curr. Top. Membr. 2015, 76, 337–371. [Google Scholar] [CrossRef]
- Aeschlimann, D.; Paulsson, M. Cross-linking of Laminin-nidogen complexes by tissue transglutaminase. A novel mechanism for basement membrane stabilization. J. Biol. Chem. 1991, 266, 15308–15317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, S.; Pei, Y.A.; Pei, M. Nidogen: A matrix protein with potential roles in musculoskeletal tissue regeneration. Genes Dis. 2022, 9, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Estrada, B.; Jacobs, S.; Sánchez-Sánchez, B.J.; Tang, J.; Ma, M.; Magadán-Corpas, P.; Pastor-Pareja, J.C.; Martín-Bermudo, M.D. Dissection of nidogen function in Drosophila reveals tissue-specific mechanisms of basement membrane assembly. PLoS Genet. 2018, 14, e1007483. [Google Scholar] [CrossRef] [PubMed]
- Olsen, B.R. Life without perlecan has its problems. J. Cell Biol. 1999, 147, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Bonche, R.; Smolen, P.; Chessel, A.; Boisivon, S.; Pisano, S.; Voigt, A.; Schaub, S.; Thérond, P.; Pizette, S. Regulation of the collagen IV network by the basement membrane protein perlecan is crucial for squamous epithelial cell morphogenesis and organ architecture. Matrix Biol. 2022, 114, 35–66. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Zoeller, J.J.; Nyström, A. Basement membrane proteoglycans: Modulators par excellence of cancer growth and angiogenesis. Mol. Cells 2009, 27, 503–513. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.J. The basement membrane proteoglycans perlecan and agrin: Something old, something new. Curr. Top. Membr. 2015, 76, 255–303. [Google Scholar] [CrossRef] [PubMed]
- Clementz, A.G.; Harris, A. Collagen XV: Exploring its structure and its role within the tumor microenvironment. Mol. Cancer Res. 2013, 11, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Iozzo, R.V.; Schaefer, L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. [Google Scholar] [CrossRef] [PubMed]
- Heljasvaara, R.; Aikio, M.; Ruotsalainen, H.; Pihlajaniemi, T. Collagen XVIII in tissue homeostasis and dysregulation—Lessons learned from model organisms and human patients. Matrix Biol. 2017, 57–58, 55–75. [Google Scholar] [CrossRef] [PubMed]
- Bretaud, S.; Guillon, E.; Karppinen, S.M.; Pihlajaniemi, T.; Ruggiero, F. Collagen XV, a multifaceted multiplexin present across tissues and species. Matrix Biol. Plus 2020, 6, 100023. [Google Scholar] [CrossRef] [PubMed]
- Izzi, V.; Heljasvaara, R.; Heikkinen, A.; Karppinen, S.M.; Koivunen, J.; Pihlajaniem, T. Exploring the roles of MACIT and multiplexin collagens in stem cells and cancer. Semin. Cancer Biol. 2020, 62, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Cole, G.J.; Halfter, W. Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol. 2010, 29, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.; Coulson-Thomas, Y.M.; Gesteira, T.F.; Coulson-Thomas, V.J. Distribution and function of glycosaminoglycans and proteoglycans in the development, homeostasis and pathology of the ocular surface. Front. Cell Dev. Biol. 2020, 8, 731. [Google Scholar] [CrossRef]
- Halfter, W.; Oertle, P.; Monnier, C.A.; Camenzind, L.; Reyes-Lua, M.; Hu, H.; Candiello, J.; Labilloy, A.; Balasubramini, M.; Henrich, P.B.; et al. New concepts in basement membrane biology. FEBS J. 2015, 282, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Monnier, C.; Müller, D.; Oertle, P.; Uechi, G.; Balasubramani, M.; Safi, F.; Lim, R.; Loparic, M.; Henrich, P.B. The bi-functional organization of human basement membranes. PLoS ONE 2013, 8, e67660. [Google Scholar] [CrossRef]
- Jayadev, R.; Sherwood, D.R. Basement membranes. Curr. Biol. 2017, 27, R207–R211. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.F.; Rutland, C.S.; Allegrucci, C.; Mongan, N.P.; Rakha, E. Defining invasion in breast cancer: The role of the basement membrane. J. Clin. Pathol. 2023, 76, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Uitto, J.; Pulkkinen, L. Molecular complexity of the cutaneous basement membrane zone. Mol. Biol. Rep. 1996, 23, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Inoue, S. Lamina lucida of basement membrane: An artefact. Microsc. Res. Tech. 1994, 28, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Miosge, N. The ultrastructural composition of basement membranes in vivo. Histol. Histopathol. 2001, 16, 1239–1248. [Google Scholar] [PubMed]
- Baumann, L. Basic science of the dermis. In Cosmetic Dermatology. Principles and Practice, 2nd ed.; Baumann, L., Ed.; McGraw Hill: New York, NY, USA, 2009; pp. 8–13. [Google Scholar]
- Vidal, E.M. The Basement Membrane Zone: Making the Connection, 1st ed.; version 1.3; American Academy of Dermatology: Rosemont, IL, USA, 2013; Available online: http://www.aad.org/education/the-basement-membrane-zone-video-lecture (accessed on 19 December 2023).
- Wilkie, I.C. A Study of the Process of Ophiuroid Arm Autotomy. Ph.D. Thesis, University of Glasgow, Glasgow, UK, 1976. [Google Scholar]
- Wilkie, I.C. Arm autotomy in brittlestars. J. Zool. Lond. 1978, 186, 311–330. [Google Scholar] [CrossRef]
- Wilkie, I.C. Autotomy as a prelude to regeneration in echinoderms. Microscop. Res. Tech. 2001, 55, 369–396. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, C.A.; Bergeron, K.F.; Whittle, J.; Brandhorst, B.P.; Burke, R.D.; Hynes, O. The echinoderm adhesome. Dev. Biol. 2006, 300, 252–266. [Google Scholar] [CrossRef]
- Dolmatov, I.Y.; Afanasyev, S.V.; Boyko, A.V. Molecular mechanisms of fission in echinoderms: Transcriptome analysis. PLoS ONE 2018, 13, e0195836. [Google Scholar] [CrossRef] [PubMed]
- Dolmatov, I.Y.; Nizhnichenko, V.A. Extracellular matrix of echinoderms. Mar. Drugs 2023, 21, 417. [Google Scholar] [CrossRef] [PubMed]
- Novinec, M.; Kordiš, D.; Turk, V.; Lenarčič, B. Diversity and evolution of the thyroglobulin type-1 domain superfamily. Mol. Biol. Evol. 2006, 23, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Bechtel, M.; Keller, M.V.; Bloch, W.; Sasaki, T.; Boukamp, P.; Zaucke, F.; Paulsson, M.; Nischt, R. Different domains in nidogen-1 and nidogen-2 drive basement membrane formation in skin organotypic cocultures. FASEB J. 2012, 26, 3637–3648. [Google Scholar] [CrossRef] [PubMed]
- Exposito, J.Y.; D’Alessio, M.; Di Liberto, M.; Ramirez, F. Complete primary structure of a sea urchin type IV collagen α chain and analysis of the 5′ end of its gene. J. Biol. Chem. 1993, 268, 5249–5254. [Google Scholar] [CrossRef] [PubMed]
- Fidler, A.L.; Vanacore, R.M.; Chetyrkin, S.V.; Pedchenko, V.K.; Bhave, G.; Yin, V.P.; Stothers, C.L.; Rose, K.L.; McDonald, W.H.; Clark, T.A.; et al. A unique covalent bond in basement membrane is a primordial innovation for tissue evolution. Proc. Natl. Acad. Sci. USA 2014, 111, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Stauber, M.; Märkel, K. Comparative morphology of muscle-skeleton attachments in the Echinodermata. Zoomorphology 1988, 108, 137–148. [Google Scholar] [CrossRef]
- Byrne, M. Ophiuroidea. In Microscopic Anatomy of Invertebrates Volume 14, Echinodermata; Harrison, F.W., Chia, F.S., Eds.; Wiley-Liss: New York, NY, USA, 1994; pp. 247–343. [Google Scholar]
- Wilkie, I.C. Functional morphology of the arm spine joint and adjacent structures of the brittlestar Ophiocomina nigra (Echinodermata: Ophiuroidea). PLoS ONE 2016, 11, e0167533. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cavey, M.J.; Wood, R.L. Organization of the adluminal and retractor cells in the coelomic lining from the tube foot of a phanerozonian starfish, Luidia foliolata. Can. J. Zool. 1991, 69, 911–923. [Google Scholar] [CrossRef]
- Wilkie, I.C.; Candia Carnevali, M.D.; Bonasoro, F. The compass depressors of Paracentrotus lividus (Echinodermata, Echinoida): Ultrastructural and mechanical aspects of their variable tensility and contractility. Zoomorphology 1992, 112, 143–153. [Google Scholar] [CrossRef]
- Trotter, J.A.; Corbett, K.; Avner, B.P. Structure and function of the murine muscle-tendon junction. Anat. Rec. 1981, 201, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Daniel, T.L. Myotendinous junctions of tonic muscle cells: Structure and loading. Cell Tissue Res. 1986, 25, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Charvet, B.; Ruggiero, F.; Le Guellec, D. The development of the myotendinous junction. A review. Muscle Ligaments Tendons J. 2012, 2, 53–63. [Google Scholar]
- Wilkie, I.C.; Candia Carnevali, M.D. The juxtaligamental cells of echinoderms and their role in the mechano-effector function of connective tissue. In Frontiers in Invertebrate Physiology: A Collection of Reviews, Volume 3, Annelida and Echinodermata; Saleuddin, A.S., Leys, S., Roer, R., Wilkie, I.C., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 345–430. [Google Scholar]
- Mashanov, V.S.; Charlina, N.A.; Dolmatov, I.Y.; Wilkie, I.C. Juxtaligamental cells in the arm of the brittlestar Amphipholis kochii Lütken, 1872 (Echinodermata: Ophiuroidea). Russ. J. Mar. Biol. 2007, 33, 110–117. [Google Scholar] [CrossRef]
- Wilkie, I.C. The juxtaligamental cells of Ophiocomina nigra and their possible role in mechano-effector function of collagenous tissue. Cell Tissue Res. 1979, 197, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, K. Über die Verbindung der Muskulatur mit dem Skelett bei dem Echinodermen Asterias rubens L. Z. Zellforsch. 1968, 87, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, I.C.; Candia Carnevali, M.D. Morphological and mechanical aspects of mutable collagenous tissue at the autotomy plane of the starfish Asterias rubens L. (Echinodermata, Asteroidea): An echinoderm paradigm. Mar. Drugs 2023, 21, 138. [Google Scholar] [CrossRef] [PubMed]
- Candiello, J.; Balasubramani, M.; Schreiber, E.M.; Cole, G.J.; Mayer, U.; Halfter, W.; Lin, H. Biomechanical properties of native basement membranes. FEBS J. 2007, 274, 2897–2908. [Google Scholar] [CrossRef] [PubMed]
- Aermes, C.; Hayn, A.; Fischer, T.; Mierke, C.T. Environmentally controlled magnetic nano-tweezer for living cells and extracellular matrices. Sci. Rep. 2020, 10, 13453. [Google Scholar] [CrossRef] [PubMed]
- Bevilacqua, C.; Gomez, J.M.; Fiuza, U.M.; Chan, C.J.; Wang, L.; Hambura, S.; Eguren, M.; Ellenberg, J.; Diz-Muñoz, A.; Leptin, M.; et al. High-resolution line-scan Brillouin microscopy for live imaging of mechanical properties during embryo development. Nat. Methods 2023, 20, 755–760. [Google Scholar] [CrossRef] [PubMed]
- Krag, S.; Andreassen, T.T. Mechanical properties of the human lens capsule. Prog. Ret. Eye Res. 2003, 22, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Halfter, W.; Candiello, J.; Hu, H.; Zhang, P.; Screiber, E.; Balasubramani, M. Protein composition and biomechanical properties on in vivo-derived basement membranes. Cell Adhes. Migr. 2013, 7, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Thomasy, S.M.; Raghunathan, V.K.; Winkler, M.; Reilly, C.M.; Sadeli, A.R.; Russell, P.; Jester, J.V.; Murphy, C.J. Elastic modulus and collagen organization of the rabbit cornea: Epithelium to endothelium. Acta Biomater. 2014, 10, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Smaje, L.H.; Fraser, P.A.; Clough, G. The distensibility of single capillaries and venules in the cat mesentery. Microvasc. Res. 1980, 20, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.F.; Wakely, J. The elastic constants and ultrastructural organization of a basement membrane (lens capsule). Proc. R. Soc. Lond. B 1976, 193, 335–358. [Google Scholar] [PubMed]
- To, M.; Goz, A.; Camenzind, L.; Oertle, P.; Candiello, J.; Sullivan, M.; Henrich, P.B.; Loparic, M.; Safi, F.; Eller, A.; et al. Diabetes-induced morphological, biomechanical, and compositional changes in ocular basement membranes. Exp. Eye Res. 2013, 116, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Last, J.A.; Liliensiek, S.J.; Nealey, P.F.; Murphy, C.J. Determining the mechanical properties of human corneal basement membranes with atomic force microscopy. J. Struct. Biol. 2009, 177, 19–24. [Google Scholar] [CrossRef]
- Bhave, G.; Colon, S.; Ferrell, N. The sulfilimine cross-link of collagen IV contributes to kidney tubular basement membrane stiffness. Am. J. Physiol. Ren. Physiol. 2017, 313, F596–F602. [Google Scholar] [CrossRef] [PubMed]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Comm. 2017, 8, 924. [Google Scholar] [CrossRef] [PubMed]
- Welling, L.W.; Grantham, J.J. Physical properties of isolated perfused renal tubules and tubular basement membranes. J. Clin. Investig. 1972, 51, 1063–1075. [Google Scholar] [CrossRef] [PubMed]
- Crest, J.; Diz-Munõz, A.; Chen, D.Y.; Fletcher, D.A.; Bilder, D. Organ sculpting by patterned extracellular matrix stiffness. eLife 2017, 6, e24958. [Google Scholar] [CrossRef] [PubMed]
- Chlasta, J.; Milani, P.; Runel, G.; Duteyrat, J.L.; Arias, L.; Lamiré, L.A.; Boudaoud, A.; Grammont, M. Variations in basement membrane mechanics are linked to epithelial morphogenesis. Development 2017, 144, 4350–4362. [Google Scholar] [CrossRef] [PubMed]
- Howard, A.M.; LaFever, K.S.; Fenix, A.M.; Scurrah, C.R.; Lau, K.S.; Burnette, D.T.; Bhave, G.; Ferrell, N.; Page-McCaw, A. DSS-induced damage to basement membranes is repaired by matrix replacement and crosslinking. J. Cell Sci. 2019, 132, jcs226860. [Google Scholar] [CrossRef] [PubMed]
- Yoo, L.; Reed, J.; Shin, A.; Demer, J.L. Atomic force microscopy determination of Young’s modulus of bovine extra-ocular tendon fiber bundles. J. Biomech. 2014, 47, 1899–1903. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.B.; Ker, R.F.; Dimery, N.J.; Alexander, R.M. Mechanical properties of various mammalian tendons. J. Zool. Lond. A 1986, 209, 537–548. [Google Scholar] [CrossRef]
- Maganaris, C.N.; Paul, J.P. In vivo human tendon mechanical properties. J. Physiol. 1999, 521, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, G.A.; Sommer, G.; Gasser, C.T.; Regitnig, P. Determination of layer-specific mechanical properties of human coronary arteries with nonatherosclerotic intimal thickening and related constitutive modeling. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H2048–H2058. [Google Scholar] [CrossRef] [PubMed]
- Last, J.A.; Thomasy, S.M.; Croasdale, C.R.; Russell, P.; Murphy, C.J. Compliance profile of the human cornea as measured by atomic force microscopy. Micron 2012, 43, 1293–1298. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Li, G.Y.; Ramier, A.; Eltony, A.M.; Yun, S.H. In vivo stiffness measurement of epidermis, dermis, and hypodermis using broadband Rayleigh-wave optical coherence elastography. Acta Biomater. 2022, 146, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Silver, F.H.; Freeman, J.W.; DeVore, D. Viscoelastic properties of human skin and processed dermis. Ski. Res. Technol. 2001, 7, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, T.; Tsuchi, A. Dynamic mechanical properties of body-wall dermis in various mechanical states and their implications for the behavior of sea cucumbers. Biol. Bull. 2003, 205, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Shadwick, R.E.; Rapoport, H.S.; Fenger, J.M. Structure and function of tuna tail tendons. Comp. Biochem. Physiol. A 2002, 133, 1109–1125. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Andarawis-Puri, N.; Eppell, S.J. Method to extract minimally damaged collagen fibrils from tendon. J. Biol. Methods 2016, 3, e54. [Google Scholar] [CrossRef]
- Shen, Z.L.; Dodge, M.R.; Kahn, H.; Ballarini, R.; Eppell, S.J. Stress-strain experiments on individual collagen fibrils. Biophys. J. 2008, 95, 3956–3963. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, N.; Odajima, S. Stress-strain curve and Young’s modulus of a collagen molecule as determined by the X-ray diffraction technique. J. Biomech. 1996, 29, 655–658. [Google Scholar] [CrossRef]
- West, J.B.; Tsukimoto, K.; Mathieu-Costello, O.; Prediletto, R. Stress failure in pulmonary capillaries. J. Appl. Physiol. 1991, 70, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H. Mechanical properties of ligament, tendon, and fascia. In Strength of Biological Materials; Evans, F.G., Ed.; The Williams & Wilkins Co.: Baltimore, MD, USA, 1970; pp. 92–105. [Google Scholar]
- Wilkie, I.C. Design for disaster: The ophiuroid intervertebral ligament as a typical mutable collagenous structure. In Echinoderm Biology; Burke, R.D., Mladenov, P.V., Lambert, P., Parsley, R.M., Eds.; Balkema: Rotterdam, The Netherlands, 1988; pp. 25–38. [Google Scholar]
- Buehler, M.J. Nature designs tough collagen: Explaining the nanostructure of collagen fibrils. Proc. Natl. Acad. Sci. USA 2006, 103, 12285–12290. [Google Scholar] [CrossRef] [PubMed]
- Gould, D.B.; Phalan, F.C.; Breedveld, G.J.; van Mil, S.E.; Smith, R.S.; Schimenti, J.C.; Aguglia, U.; van der Knaap, M.S.; Heutink, P.; John, S.W.M. Mutations in Col4a1 cause perinatal cerebral hemorrhage and porencephaly. Science 2005, 308, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Bilguvar, K.; DiLuna, M.L.; Bizzarro, M.J.; Bayri, Y.; Schneider, K.C.; Lifton, R.P.; Gunel, M.; Ment, L.R. COL4A1 mutation in preterm intraventricular hemorrhage. J. Pediatr. 2009, 155, 743–745. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, U.; Santillán, K.Y.G.; Fischer-Friedrich, E.; Dahmann, C. Distinct contributions of ECM proteins to basement membrane mechanical properties in Drosophila. Development 2022, 149, dev200456. [Google Scholar] [CrossRef]
- McCall, A.S.; Cummings, C.F.; Bhave, G.; Vanacore, R.; Page-McCaw, A.; Hudson, B.G. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell 2014, 157, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Peebles, K.E.; LaFever, K.S.; Page-McCaw, P.S.; Colon, S.; Wang, D.; Stricker, A.M.; Ferrell, N.; Bhave, G.; Page-McCaw, A. Peroxidasin is required for full viability in development and for maintenance of tissue mechanics in adults. Matrix Biol. 2024, 125, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, Y.; Candiello, J.; Thorn, T.L.; Gray, N.; Halfter, W.M.; Hu, H. Biochemical and biophysical changes underlie the mechanisms of basement membrane disruptions in a mouse model of dystroglycanopathy. Matrix Biol. 2013, 32, 196–207. [Google Scholar] [CrossRef]
- Uspenskaia, O.; Liebetrau, M.; Herms, J.; Danek, A.; Hamann, G.F. Aging is associated with increased collagen type IV accumulation in the basal lamina of human cerebral microvessels. BMC Neurosci. 2004, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.K.; Cikic, S.; Rutkai, I.; Guidry, J.J.; Katakam, P.V.G. Effects of aging on protein expression in mice brain microvessels: ROS scavengers, mRNA/protein stability, glycolytic enzymes, mitochondrial complexes, and basement membrane components. GeroScience 2022, 44, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Töpfer, U. Basement membrane dynamics and mechanics in tissue morphogenesis. Biol. Open 2023, 12, bio059980. [Google Scholar] [CrossRef] [PubMed]
- Fessler, L.I.; Condic, M.L.; Nelson, R.E.; Fessler, J.H.; Fristrom, J.W. Site-specific cleavage of basement membrane collagen IV during Drosophila metamorphosis. Development 1993, 117, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Sui, L.; Alt, S.; Weigert, M.; Dye, N.; Eaton, S.; Jug, F.; Myers, E.W.; Jülicher, F.; Salbreux, G.; Dahmann, C. Differential lateral and basal tension drive folding of Drosophila wing discs through two distinct mechanisms. Nat. Comm. 2018, 9, 4620. [Google Scholar] [CrossRef] [PubMed]
- Kelley, L.C.; Lohmer, L.L.; Hagedorn, E.J.; Sherwood, D.R. Traversing the basement membrane in vivo: A diversity of strategies. J. Cell Biol. 2014, 204, 291–302. [Google Scholar] [CrossRef]
- Rosell, A.; Cuadrado, E.; Ortega-Aznar, A.; Hernández-Guillamon, M.; Lo, E.H.; Montaner, J. MMP-9–positive neutrophil infiltration is associated to blood–brain barrier breakdown and basal lamina type IV collagen degradation during hemorrhagic transformation after human ischemic stroke. Stroke 2008, 39, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for blood brain barrier disruption and hemorrhagic transformation following ischemic stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef]
- Kelley, L.C.; Chi, Q.; Cáceres, R.; Hastie, E.; Schidler, A.J.; Jiang, Y.; Matus, D.Q.; Plastino, J.; Sherwood, D.R. Adaptive F-Actin polymerization and localized ATP production drive basement membrane invasion in the absence of MMPs. Dev. Cell 2019, 48, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hartt, I.; Foltz, C.M.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Linklater, E.; Jewett, C.E.; Prekeris, R. Polarized membrane trafficking in development and disease: From epithelia polarization to cancer cell invasion. In Cell Polarity in Development and Disease; Houston, D.W., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 121–145. [Google Scholar]
- Augoff, K.; Hryniewicz-Jankowska, R.; Tabola, R. Invadopodia: Clearing the way for cancer cell invasion. Ann. Transl. Med. 2020, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Alexander, R.M. Tendon elasticity and muscle function. Comp. Biochem. Physiol. A 2002, 133, 1001–1011. [Google Scholar] [CrossRef]
- Ennos, R. Solid Biomechanics; Princeton University Press: Princeton, NJ, USA, 2012; pp. 64–66. [Google Scholar]
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582. [Google Scholar] [CrossRef] [PubMed]
- Paxton, J.Z.; Baar, K. Tendon mechanics: The argument heats up. J. Appl. Physiol. 2007, 103, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Warner, G. Food and feeding mechanisms: Ophiuroidea. In Echinoderm Nutrition; Jangoux, M., Lawrence, J.M., Eds.; Balkema: Rotterdam, The Netherlands, 1982; pp. 161–181. [Google Scholar]
- Yee, A.; Burkhardt, J.; Gilly, W.F. Mobilization of a coordinated escape response by giant axons in the ophiuroid, Ophiopteris papillosa. J. Exp. Biol. 1987, 128, 287–305. [Google Scholar] [CrossRef]
- Astley, H.C. Getting around when you’re round: Quantitative analysis of the locomotion of the blunt-spined brittle star, Ophiocoma echinata. J. Exp. Biol. 2012, 215, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Screen, H.R.C. Investigating load relaxation mechanics in tendon. J. Mech. Behav. Biomed. Mat. 2008, 1, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Cobb, J.L.S. Neurophysiological studies on photic responses in Ophiura ophiura. Comp. Biochem. Physiol. A 1985, 80, 11–16. [Google Scholar] [CrossRef]
- Wilkie, I.C. Nervously mediated change in the mechanical properties of a brittlestar ligament. Mar. Behav. Physiol. 1978, 5, 289–306. [Google Scholar] [CrossRef]
- Wilkie, I.C. Variable tensility of the oral arm plate ligaments of the brittlestar Ophiura ophiura L. (Echinodermata: Ophiuroidea). J. Zool. Lond. 1992, 228, 5–26. [Google Scholar] [CrossRef]
- Ker, R.F.; Alexander, R.M.; Bennet, M.B. Why are mammalian tendons so thick? J. Zool. Lond. 1988, 216, 309–324. [Google Scholar] [CrossRef]
- Takemae, N.; Nakaya, F.; Motokawa, T. Low oxygen consumption and high body content of catch connective tissue contribute to low metabolic rate of sea cucumbers. Biol. Bull. 2009, 216, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, E.; Haesaerts, D.; Dubois, P.; Flammang, P. Evaluation of the different forces brought into play during tube foot activities in sea stars. J. Exp. Biol. 2010, 213, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Candia Carnevali, M.D.; Sugni, M.; Bonasoro, F.; Wilkie, I.C. Mutable collagenous tissue: A concept generator for biomimetic materials and devices. Mar. Drugs 2024, 22, 37. [Google Scholar] [CrossRef] [PubMed]
- Tamboline, C.R.; Burke, R.D. Secondary mesenchyme of the sea urchin embryo: Ontogeny of blastocoelar cells. J. Exp. Zool. 1992, 262, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Katow, H. Mechanisms of the epithelial-to-mesenchymal transition in sea urchin embryos. Tissue Barriers 2015, 3, 4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tamori, M.; Ishida, K.; Matsuura, E.; Ogasawara, K.; Hanasaka, T.; Takehana, Y.; Motokawa, T.; Osawa, T. Ultrastructural changes associated with reversible stiffening in catch connective tissue of sea cucumbers. PLoS ONE 2016, 11, e0155673. [Google Scholar] [CrossRef] [PubMed]
- Motokawa, T. Skin of sea cucumbers: The smart connective tissue that alters mechanical properties in response to external stimuli. J. Aero Aqua Bio-Mech. 2019, 8, 1–5. [Google Scholar] [CrossRef][Green Version]
- Bonneel, M.; Hennebert, E.; Byrne, M.; Flammang, P. Mutable collagenous tissues in sea cucumbers. In The World of Sea Cucumbers; Mercier, A., Hamel, J.F., Suhrbier, A., Pearce, C., Eds.; Academic Press: Cambridge, MA, USA, 2024; pp. 573–584. [Google Scholar] [CrossRef]
- Ingersoll, E.P.; Pendharkar, N.C. Characterization and expression of two matrix metalloproteinase genes during sea urchin development. Gene Expr. Patterns 2005, 5, 727–732. [Google Scholar] [CrossRef] [PubMed]
- McClay, D.R.; Warner, J.; Martik, M.; Miranda, E.; Slota, L. Gastrulation in the sea urchin. Curr. Top. Dev. Biol. 2020, 136, 195–218. [Google Scholar] [PubMed]
- Formery, L.; Wakefield, A.; Gesson, M.; Toisoul, L.; Lhomond, G.; Gilletta, L.; Lasbleiz, R.; Schubert, M.; Croce, J.C. Developmental atlas of the indirect-developing sea urchin Paracentrotus lividus: From fertilization to juvenile stages. Front. Cell Dev. Biol. 2022, 10, 966408. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.M.; Ramsey, M.A.; Miranpuri, G.S.; Resnick, D.K. Regulation and function of matrix metalloproteinases in nervous system injury and neuropathic pain. Ann. Neurosci. 2008, 15, 94–105. [Google Scholar] [CrossRef]
- Miyata, S.; Nakatani, Y.; Hayashi, N.; Nakashima, T. Matrix-degrading enzymes tissue plasminogen activator and matrix metalloprotease-3 in the hypothalamo-neurohypophysial system. Brain Res. 2005, 1058, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sbai, O.; Ferhat, L.; Bernard, A.; Gueye, Y.; Ould-Yahoui, A.; Thiolloy, S.; Charrat, E.; Charton, G.; Tremblay, E.; Risso, J.J.; et al. Vesicular trafficking and secretion of metalloproteinases-2, -9 and tissue inhibitor of metalloproteinase-1 in neuronal cells. Mol. Cell. Neurosci. 2008, 39, 549–568. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, J.L.; Bark, S.J.; Funkelstein, L.; Mosier, C.; Yap, A.; Kazemi-Esfarjani, P.; La Spada, A.R.; Sigurdson, C.; O’Connor, D.T.; Hook, V. Proteomics of dense core secretory vesicles reveal distinct protein categories for secretion of neuroeffectors for cell-cell communication. J. Proteome Res. 2021, 9, 5002–5024. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, E.J.; Sherwood, D.R. Cell invasion through basement membrane: The anchor cell breaches the barrier. Curr. Opin. Cell Biol. 2011, 23, 589–596. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Adhikari, A.S.; Chai, J.; Dunn, A.R. Mechanical load induces a 100-fold increase in the rate of collagen proteolysis by MMP-1. J. Amer. Chem. Soc. 2011, 133, 1686–1689. [Google Scholar] [CrossRef]
- Zucker, S.; Hymowitz, M.; Conner, C.E.; DiYanni, E.A.; Cao, J. Rapid trafficking of membrane type 1-matrix metalloproteinase to the cell surface regulates progelatinase A activation. Lab. Investig. 2002, 82, 1673–1684. [Google Scholar] [CrossRef][Green Version]
- Gu, B.J.; Wiley, J.S. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 2006, 107, 4946–4953. [Google Scholar] [CrossRef] [PubMed]
- Mashanov, V.; Machado, D.J.; Reid, R.; Brouwer, C.; Kofsky, J.; Janies, D.A. Twinkle twinkle brittle star: The draft genome of Ophioderma brevispinum (Echinodermata: Ophiuroidea) as a resource for regeneration research. BMC Genom. 2022, 23, 574. [Google Scholar] [CrossRef] [PubMed]
- Mah, C.L.; McKnight, D.G.; Eagle, M.K.; Pawson, D.L.; Améziane, N.; Vance, D.J.; Baker, A.N.; Clark, H.E.S.; Davey, N. Phylum Echinodermata. In New Zealand Inventory of Biodiversity Volume 1, Kingdom Animalia. Radiata, Lophotrochozoa, Deuterostomia; Gordon, D.P., Ed.; Canterbury University Press: Christchurch, New Zealand, 2009; pp. 371–400. [Google Scholar]
- Tyson, E. Phocaena, or the Anatomy of a Porpess, Dissected at Gresham Colledge: With a Praeliminary Discourse Concerning Anatomy, and a Natural History of Animals; B. Tooke: London, UK, 1680. [Google Scholar]


| Feature | Supramolecular Structure | Basement Membranes |
|---|---|---|
| Composition | Numbers and kinds of molecules | Collagens IV, XV and XVIII, laminin, nidogen, perlecan, agrin etc. |
| Constitution | Connections between the molecules | Intermolecular stabilization by covalent (collagen IV network only) and non-covalent bonds |
| Configuration | Orientation of neighboring molecules about each other in 3D space | Independent collagen IV and laminin networks linked to each other and cell surface by nidogen, perlecan, agrin etc. |
| Conformation | Overall shape of supramolecular structure in 3D space | Observed in TEM as lamina densa (comprising finely granular and filamentous material) and lamina lucida |
| Test | Detects | Reaction | |
|---|---|---|---|
| Tendon | Ligament | ||
| PAS after amylase | neutral 1,2 glycols | +++ | ++ |
| Toluidine blue (alcoholic) | acidic groups | β | β |
| Toluidine blue (aqueous) | acidic groups | γ | γ |
| Toluidine blue after hyaluronidase | CSA, CSC, HA | − | − |
| Alcian blue, pH 1.0 | sulfate groups | + | ++ |
| Alcian blue, pH 2.5 | carboxyl groups | + 1 | + |
| (A) Basement Membranes | |||
| Animal | BM Location | Stiffness MPa | Reference |
| Cat 1 | MCV | 1.8–5.4 | [85] |
| Cat 2 | Lens capsule | 0.82–7.74 | [86] |
| Chick | ILM | 0.95–3.30 | [79] |
| Human | ILM | 1.5–5 | [45] |
| Human | ILM | 0.024 | [87] |
| Human | Lens capsule | 3.92–4.37 | [45] |
| Human | Anterior cornea | 0.002–0.015 | [88] |
| Human | Descemet’s membrane | 0.02–0.08 | [88] |
| Mouse (neonatal) | ILM | 3.81 | [79] |
| Mouse (adult) | ILM | 4.07 | [79] |
| Mouse 3 | Renal tubule | 0.438–3.230 | [89] |
| Mouse | Mesentery | 0.055 | [90] |
| Rabbit | Anterior cornea | 0.0045 | [84] |
| Rabbit | Descemet’s membrane | 0.0117 | [84] |
| Rabbit 1 | Renal tubule | 7–10 | [91] |
| Drosophila | Egg chamber | 0.03–0.07 | [92] |
| Drosophila | Egg chamber | 0.02–0.8 | [93] |
| Drosophila3 | Malpighian tubule | 1.4 | [94] |
| (B) Other structures | |||
| Animal | Structure | Stiffness MPa | Reference |
| Cow | Tendon (extra-ocular) | 59 | [95] |
| Dolphin | Tendon (sacrocaudalis) | 1430 | [96] |
| Human | Tendon (tibialis anterior) | 450–1200 | [97] |
| Human | Adventitia (arterial) | 1.30–1.43 | [98] |
| Human | Corneal stroma | 0.033 | [99] |
| Human | Dermis | 0.03–0.15 | [100] |
| Human | Dermis | 0.1–18.4 | [101] |
| Rabbit | Corneal stroma | 0.0004–0.0095 | [84] |
| Sea cucumber | Dermis | 0.3–3 | [102] |
| Sea urchin | CDL | 1.43 | [70] |
| Tuna | Tendon (caudal) | 1310 | [103] |
| Rat | Collagen fibril | 39–130 | [104] |
| Sea cucumber | Collagen fibril | 360–1600 | [105] |
| Cow | Collagen I molecule | 2900 | [106] |
| Rat | Collagen I molecule | 5100–9000 | [106] |
| (A) Basement Membranes | |||
| Animal | BM Location | UTS MPa | Reference |
| Cat | Lens capsule | 0.17 | [86] |
| Human | Anterior lens capsule | 1.5–17.5 | [82] |
| Rabbit | Renal tubule | 1.8–2.0 | [91] |
| Rabbit | Alveolar capillary | 0.8 | [107] |
| (B) Other structures | |||
| Animal | Structure | UTS MPa | Reference |
| Dolphin | Tendon (sacrocaudalis) | 62–95 | [96] |
| Human | Tendon (calcaneal) | 60 | [108] |
| Human | Adventitia (arterial) | 1.30–1.43 | [98] |
| Brittlestar | IAL | 6.17 | [109] |
| Sea urchin | CDL | 0.14 | [70] |
| Tuna | Tendon (caudal) | 22–33 | [103] |
| Rat | Collagen fibril | 39–130 | [104] |
| Sea cucumber | Collagen fibril | 70–470 | [105] |
| Atomistic model | Collagen molecule | 11,200 | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkie, I.C. Basement Membranes, Brittlestar Tendons, and Their Mechanical Adaptability. Biology 2024, 13, 375. https://doi.org/10.3390/biology13060375
Wilkie IC. Basement Membranes, Brittlestar Tendons, and Their Mechanical Adaptability. Biology. 2024; 13(6):375. https://doi.org/10.3390/biology13060375
Chicago/Turabian StyleWilkie, Iain C. 2024. "Basement Membranes, Brittlestar Tendons, and Their Mechanical Adaptability" Biology 13, no. 6: 375. https://doi.org/10.3390/biology13060375
APA StyleWilkie, I. C. (2024). Basement Membranes, Brittlestar Tendons, and Their Mechanical Adaptability. Biology, 13(6), 375. https://doi.org/10.3390/biology13060375






