Modelling Human Hair Follicles—Lessons from Animal Models and Beyond
Abstract
Simple Summary
Abstract
1. Background
2. Hair Follicle Development and Regeneration
2.1. Signaling Pathways
2.2. Complex Regulation of Hair Cycling beyond Epithelial-Mesenchymal Cross-Talk
2.3. Differences in Mouse and Human Hair Biology
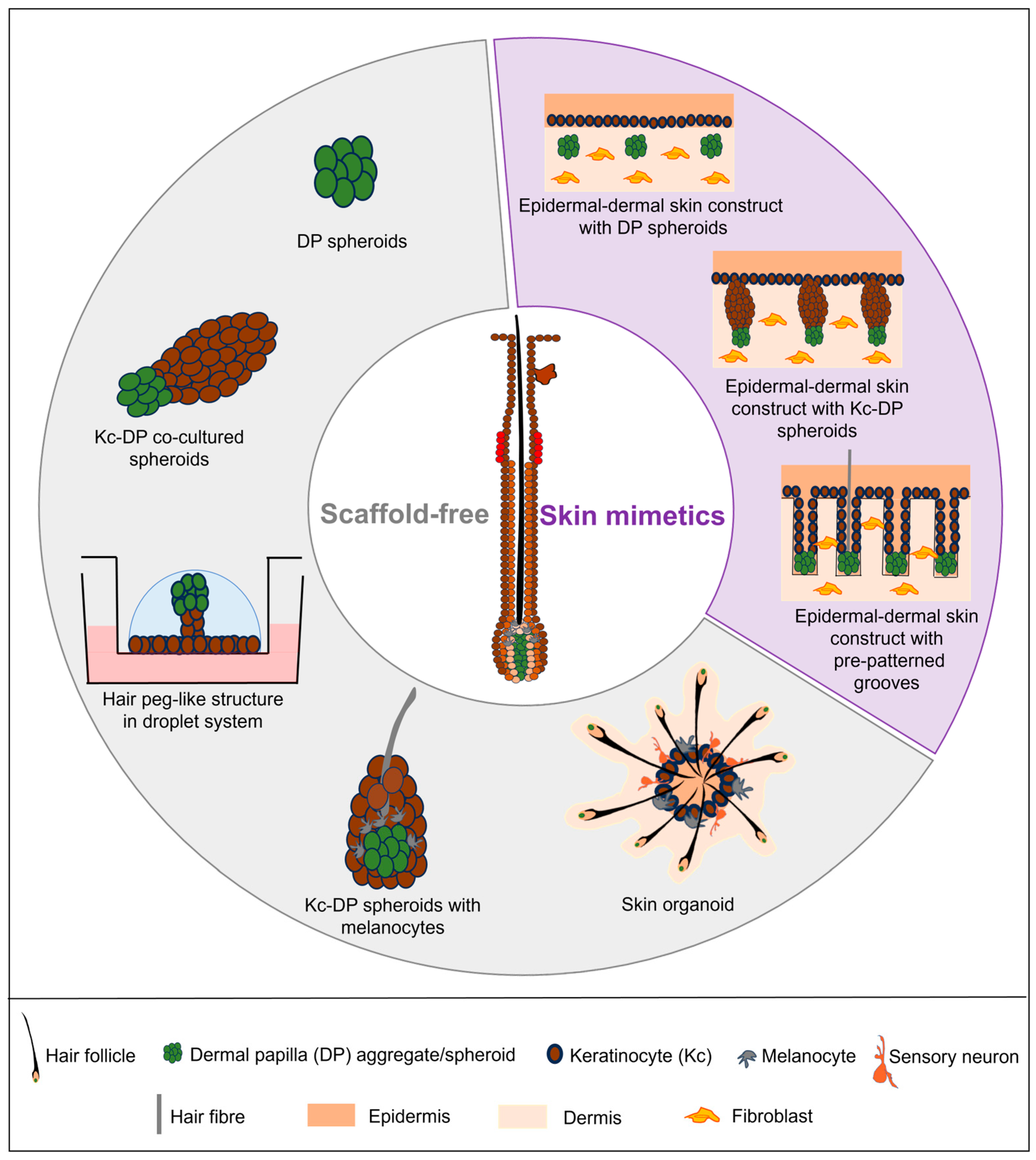
3. Current In Vitro Human Hair Models
3.1. Scaffold-Free Models
3.1.1. DP Spheroids
3.1.2. Co-Culture of DP Spheroids with Keratinocytes and Melanocytes
3.1.3. Skin Organoid
3.2. Skin-Mimetic Models
3.2.1. Epidermal-Dermal Skin Construct with DP or Keratinocyte-DP Spheroids
3.2.2. Epidermal-Dermal Skin Construct with Pre-Patterned Grooves
4. Improving Hair Follicle Models
4.1. Immune Cells
4.2. Vasculature
4.3. Biomechanical Forces
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martel, J.L.; Miao, J.H.; Badri, T. Anatomy, Hair Follicle—StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/pubmed/29261946 (accessed on 26 February 2024).
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 1999, 341, 491–497. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Jordan, S.A.; Oshima, H.; Yoshida, H.; Osawa, M.; Moriyama, M.; Jackson, I.J.; Barrandon, Y.; Miyachi, Y.; Nishikawa, S.-I. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 2002, 416, 854–860. [Google Scholar] [CrossRef]
- Tobin, D.J. The cell biology of human hair follicle pigmentation. Pigment. Cell Melanoma Res. 2011, 24, 75–88. [Google Scholar] [CrossRef]
- Chang, C.-Y.; Pasolli, H.A.; Giannopoulou, E.G.; Guasch, G.; Gronostajski, R.M.; Elemento, O.; Fuchs, E. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature 2013, 495, 98–102. [Google Scholar] [CrossRef]
- Taylor, G.; Lehrer, M.S.; Jensen, P.J.; Sun, T.T.; Lavker, R.M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 2000, 102, 451–461. [Google Scholar] [CrossRef]
- Ito, M.; Liu, Y.; Yang, Z.; Nguyen, J.; Liang, F.; Morris, R.J.; Cotsarelis, G. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat. Med. 2005, 11, 1351–1354. [Google Scholar] [CrossRef]
- Lisse, T.S.; Sharma, M.; Vishlaghi, N.; Pullagura, S.R.; Braun, R.E. GDNF promotes hair formation and cutaneous wound healing by targeting bulge stem cells. NPJ Regen. Med. 2020, 5, 13. [Google Scholar] [CrossRef]
- Gonzales, K.A.U.; Polak, L.; Matos, I.; Tierney, M.T.; Gola, A.; Wong, E.; Infarinato, N.R.; Nikolova, M.; Luo, S.; Liu, S.; et al. Stem cells expand potency and alter tissue fitness by accumulating diverse epigenetic memories. Science 2021, 374, eabh2444. [Google Scholar] [CrossRef]
- Leirós, G.J.; Kusinsky, A.G.; Drago, H.; Bossi, S.; Sturla, F.; Castellanos, M.L.; Stella, I.Y.; Balañá, M.E. Dermal papilla cells improve the wound healing process and generate hair bud-like structures in grafted skin substitutes using hair follicle stem cells. Stem Cells Transl. Med. 2014, 3, 1209–1219. [Google Scholar] [CrossRef]
- Lim, C.H.; Sun, Q.; Ratti, K.; Lee, S.-H.; Zheng, Y.; Takeo, M.; Lee, W.; Rabbani, P.; Plikus, M.V.; Cain, J.E.; et al. Hedgehog stimulates hair follicle neogenesis by creating inductive dermis during murine skin wound healing. Nat. Commun. 2018, 9, 4903. [Google Scholar] [CrossRef]
- Greco, V.; Chen, T.; Rendl, M.; Schober, M.; Pasolli, H.A.; Stokes, N.; dela Cruz-Racelis, J.; Fuchs, E. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 2009, 4, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.A.; Westgate, G.E.; Jahoda, C.A.B. From telogen to exogen: Mechanisms underlying formation and subsequent loss of the hair club fiber. J. Investig. Dermatol. 2009, 129, 2100–2108. [Google Scholar] [CrossRef] [PubMed]
- Sawaya, M.E.; Price, V.H. Different levels of 5alpha-reductase type I and II, aromatase, and androgen receptor in hair follicles of women and men with androgenetic alopecia. J. Investig. Dermatol. 1997, 109, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Ellis, J.A.; Stebbing, M.; Harrap, S.B. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J. Investig. Dermatol. 2001, 116, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Molecular mechanisms of androgenetic alopecia. Exp. Gerontol. 2002, 37, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Hillmer, A.M.; Hanneken, S.; Ritzmann, S.; Becker, T.; Freudenberg, J.; Brockschmidt, F.F.; Flaquer, A.; Freudenberg-Hua, Y.; Jamra, R.A.; Metzenet, C.; et al. Genetic variation in the human androgen receptor gene is the major determinant of common early-onset androgenetic alopecia. Am. J. Hum. Genet. 2005, 77, 140–148. [Google Scholar] [CrossRef]
- English, R.S., Jr. A hypothetical pathogenesis model for androgenic alopecia: Clarifying the dihydrotestosterone paradox and rate-limiting recovery factors. Med. Hypotheses 2018, 111, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Harel, S.; Higgins, C.A.; Cerise, J.E.; Dai, Z.; Chen, J.C.; Clynes, R.; Christiano, A.M. Clinical Medicine: Pharmacologic inhibition of JAK-STAT signaling promotes hair growth. Sci. Adv. 2015, 1, e1500973. [Google Scholar] [CrossRef]
- Dai, Z.; Chen, J.; Chang, Y.; Christiano, A.M. Selective inhibition of JAK3 signaling is sufficient to reverse alopecia areata. JCI Insight 2021, 6, e142205. [Google Scholar] [CrossRef]
- Phillips, T.G.; Slomiany, W.P.; Allison, R. Hair Loss: Common Causes and Treatment. Am. Fam. Physician 2017, 96, 371–378. Available online: https://www.ncbi.nlm.nih.gov/pubmed/28925637 (accessed on 18 December 2023).
- Oh, J.W.; Kloepper, J.; Langan, E.A.; Kim, Y.; Yeo, J.; Kim, M.J.; Hsi, T.-C.; Rose, C.; Yoon, G.S.; Lee, S.J.; et al. A Guide to Studying Human Hair Follicle Cycling In Vivo. J. Investig. Dermatol. 2016, 136, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Van Genderen, C.; Okamura, R.M.; Fariñas, I.; Quo, R.G.; Parslow, T.G.; Bruhn, L.; Grosschedl, R. Development of several organs that require inductive epithelial-mesenchymal interactions is impaired in LEF-1-deficient mice. Genes Dev. 1994, 8, 2691–2703. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. beta-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT Signals Are Required for the Initiation of Hair Follicle Development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jarrell, A.; Guo, C.; Lang, R.; Atit, R. Dermal β-catenin activity in response to epidermal Wnt ligands is required for fibroblast proliferation and hair follicle initiation. Development 2012, 139, 1522–1533. [Google Scholar] [CrossRef]
- Schmidt-Ullrich, R.; Aebischer, T.; Hülsken, J.; Birchmeier, W.; Klemm, U.; Scheidereit, C. Requirement of NF-kappaB/Rel for the development of hair follicles and other epidermal appendices. Development 2001, 128, 3843–3853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tomann, P.; Andl, T.; Gallant, N.M.; Huelsken, J.; Jerchow, B.; Birchmeier, W.; Paus, R.; Piccolo, S.; Mikkola, M.L.; et al. Reciprocal requirements for EDA/EDAR/NF-kappaB and Wnt/beta-catenin signaling pathways in hair follicle induction. Dev. Cell 2009, 17, 49–61. [Google Scholar] [CrossRef]
- Biggs, L.C.; Mäkelä, O.J.; Myllymäki, S.-M.; Das Roy, R.; Närhi, K.; Pispa, J.; Mustonen, T.; Mikkola, M.L. Hair follicle dermal condensation forms via Fgf20 primed cell cycle exit, cell motility, and aggregation. eLife 2018, 7, e36468. [Google Scholar] [CrossRef]
- Woo, W.-M.; Zhen, H.H.; Oro, A.E. Shh maintains dermal papilla identity and hair morphogenesis via a Noggin-Shh regulatory loop. Genes Dev. 2012, 26, 1235–1246. [Google Scholar] [CrossRef] [PubMed]
- Glover, J.D.; Wells, K.L.; Matthäus, F.; Painter, K.J.; Ho, W.; Riddell, J.; Johansson, J.A.; Ford, M.J.; Jahoda, C.A.B.; Klika, V.; et al. Hierarchical patterning modes orchestrate hair follicle morphogenesis. PLoS Biol. 2017, 15, e2002117. [Google Scholar] [CrossRef] [PubMed]
- St-Jacques, B.; Dassule, H.R.; Karavanova, I.; Botchkarev, V.A.; Li, J.; Danielian, P.S.; McMahon, J.A.; Lewis, P.M.; Paus, R.; McMahon, A.P. Sonic hedgehog signaling is essential for hair development. Curr. Biol. 1998, 8, 1058–1068. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.; Swan, R.Z.; Grachtchouk, M.; Bolinger, M.; Litingtung, Y.; Robertson, E.K.; Cooper, M.K.; Gaffield, W.; Westphal, H.; Beachy, P.A.; et al. Essential role for Sonic hedgehog during hair follicle morphogenesis. Dev. Biol. 1999, 205, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Foitzik, K.; Paus, R.; Doetschman, T.; Paolo Dotto, G. The TGF-β2 Isoform Is Both a Required and Sufficient Inducer of Murine Hair Follicle Morphogenesis. Dev. Biol. 1999, 212, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Li, X.; Tang, H.; Huang, A.S.; Panteleyev, A.A.; Owens, D.M.; Su, G.H. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J. Investig. Dermatol. 2011, 131, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Ouspenskaia, T.; Matos, I.; Mertz, A.F.; Fiore, V.F.; Fuchs, E. WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell 2016, 164, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Rendl, M.; Polak, L.; Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 2008, 22, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Plikus, M.V.; Mayer, J.A.; de la Cruz, D.; Baker, R.E.; Maini, P.K.; Maxson, R.; Chuong, C.-M. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 2008, 451, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Oshimori, N.; Fuchs, E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 2012, 10, 63–75. [Google Scholar] [CrossRef]
- Lien, W.-H.; Polak, L.; Lin, M.; Lay, K.; Zheng, D.; Fuchs, E. In vivo transcriptional governance of hair follicle stem cells by canonical Wnt regulators. Nat. Cell Biol. 2014, 16, 179–190. [Google Scholar] [CrossRef]
- Hsu, Y.-C.; Li, L.; Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014, 157, 935–949. [Google Scholar] [CrossRef] [PubMed]
- Kobielak, K.; Pasolli, H.A.; Alonso, L.; Polak, L.; Fuchs, E. Defining BMP functions in the hair follicle by conditional ablation of BMP receptor IA. J. Cell Biol. 2003, 163, 609–623. [Google Scholar] [CrossRef]
- Genander, M.; Cook, P.J.; Ramsköld, D.; Keyes, B.E.; Mertz, A.F.; Sandberg, R.; Fuchs, E. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell 2014, 15, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Adam, R.C.; Ge, Y.; Hua, Z.L.; Fuchs, E. Epithelial-Mesenchymal Micro-niches Govern Stem Cell Lineage Choices. Cell 2017, 169, 483–496.e13. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.E.; Dai, Z.; Ferrante, A.W.; Drake, C.G.; Christiano, A.M. A Subset of TREM2+ Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell 2019, 24, 654–669.e6. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Zirak, B.; Rodriguez, R.S.; Pauli, M.L.; Truong, H.-A.; Lai, K.; Ahn, R.; Corbin, K.; Lowe, M.M.; Scharschmidt, T.C.; et al. Regulatory T Cells in Skin Facilitate Epithelial Stem Cell Differentiation. Cell 2017, 169, 1119–1129.e11. [Google Scholar] [CrossRef]
- Festa, E.; Fretz, J.; Berry, R.; Schmidt, B.; Rodeheffer, M.; Horowitz, M.; Horsley, V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 2011, 146, 761–771. [Google Scholar] [CrossRef]
- Tanimura, S.; Tadokoro, Y.; Inomata, K.; Binh, N.T.; Nishie, W.; Yamazaki, S.; Nakauchi, H.; Tanaka, Y.; McMillan, J.R.; Sawamura, D.; et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 2011, 8, 177–187. [Google Scholar] [CrossRef]
- Gur-Cohen, S.; Yang, H.; Baksh, S.C.; Miao, Y.; Levorse, J.; Kataru, R.P.; Liu, X.; de la Cruz-Racelis, J.; Mehrara, B.J.; Fuchs, E. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 2019, 366, 1218–1225. [Google Scholar] [CrossRef]
- Li, K.N.; Jain, P.; He, C.H.; Eun, F.C.; Kang, S.; Tumbar, T. Skin vasculature and hair follicle cross-talking associated with stem cell activation and tissue homeostasis. eLife 2019, 8, e45977. [Google Scholar] [CrossRef] [PubMed]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Shwartz, Y.; Gonzalez-Celeiro, M.; Chen, C.-L.; Pasolli, H.A.; Sheu, S.-H.; Fan, S.M.-Y.; Shamsi, F.; Assaad, S.; Lin, E.T.-Y.; Zhang, B.; et al. Cell Types Promoting Goosebumps Form a Niche to Regulate Hair Follicle Stem Cells. Cell 2020, 182, 578–593.e19. [Google Scholar] [CrossRef] [PubMed]
- Halloy, J.; Bernard, B.A.; Loussouarn, G.; Goldbeter, A. Modeling the dynamics of human hair cycles by a follicular automaton. Proc. Natl. Acad. Sci. USA 2000, 97, 8328–8333. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Foitzik, K.; Paus, R.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; McKay, I.A.; Stenn, K.S. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Blanpain, C.; Lowry, W.E.; Geoghegan, A.; Polak, L.; Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 2004, 118, 635–648. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Tumbar, T.; Guasch, G.; Greco, V.; Blanpain, C.; Lowry, W.E.; Rendl, M.; Fuchs, E. Defining the Epithelial Stem Cell Niche in Skin. Science 2004, 303, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, M.; Terunuma, A.; Tock, C.L.; Radonovich, M.F.; Pise-Masison, C.A.; Hopping, S.B.; Brady, J.N.; Udey, M.C.; Vogel, J.C. Characterization and isolation of stem cell-enriched human hair follicle bulge cells. J. Clin. Investig. 2006, 116, 249–260. [Google Scholar] [CrossRef]
- Kloepper, J.E.; Tiede, S.; Brinckmann, J.; Reinhardt, D.P.; Meyer, W.; Faessler, R.; Paus, R. Immunophenotyping of the human bulge region: The quest to define useful in situ markers for human epithelial hair follicle stem cells and their niche. Exp. Dermatol. 2008, 17, 592–609. [Google Scholar] [CrossRef]
- Inoue, K.; Aoi, N.; Sato, T.; Yamauchi, Y.; Suga, H.; Eto, H.; Kato, H.; Araki, J.; Yoshimura, K. Differential expression of stem-cell-associated markers in human hair follicle epithelial cells. Lab. Investig. 2009, 89, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Ohnemus, U.; Uenalan, M.; Inzunza, J.; Gustafsson, J.-A.; Paus, R. The hair follicle as an estrogen target and source. Endocr. Rev. 2006, 27, 677–706. [Google Scholar] [CrossRef] [PubMed]
- Langan, E.A.; Foitzik-Lau, K.; Goffin, V.; Ramot, Y.; Paus, R. Prolactin: An emerging force along the cutaneous-endocrine axis. Trends Endocrinol. Metab. 2010, 21, 569–577. [Google Scholar] [CrossRef]
- Sundberg, J.P.; Beamer, W.G.; Uno, H.; Van Neste, D.; King, L.E. Androgenetic alopecia: In vivo models. Exp. Mol. Pathol. 1999, 67, 118–130. [Google Scholar] [CrossRef] [PubMed]
- Crabtree, J.S.; Kilbourne, E.J.; Peano, B.J.; Chippari, S.; Kenney, T.; McNally, C.; Wang, W.; Harris, H.A.; Winneker, R.C.; Nagpal, S.; et al. A mouse model of androgenetic alopecia. Endocrinology 2010, 151, 2373–2380. [Google Scholar] [CrossRef] [PubMed]
- Higgins, C.A.; Richardson, G.D.; Ferdinando, D.; Westgate, G.E.; Jahoda, C.A.B. Modelling the hair follicle dermal papilla using spheroid cell cultures. Exp. Dermatol. 2010, 19, 546–548. [Google Scholar] [CrossRef]
- Higgins, C.A.; Chen, J.C.; Cerise, J.E.; Jahoda, C.A.B.; Christiano, A.M. Microenvironmental reprogramming by threedimensional culture enables dermal papilla cells to induce de novo human hair-follicle growth. Proc. Natl. Acad. Sci. USA 2013, 110, 19679–19688. [Google Scholar] [CrossRef]
- Kalabusheva, E.; Terskikh, V.; Vorotelyak, E. Hair germ model in vitro via human postnatal keratinocyte-dermal papilla interactions: Impact of hyaluronic acid. Stem Cells Int. 2017, 2017, 9271869. [Google Scholar] [CrossRef]
- Weber, E.L.; Woolley, T.E.; Yeh, C.Y.; Ou, K.L.; Maini, P.K.; Chuong, C.M. Self-organizing hair peg-like structures from dissociated skin progenitor cells: New insights for human hair follicle organoid engineering and Turing patterning in an asymmetric morphogenetic field. Exp. Dermatol. 2019, 28, 355–366. [Google Scholar] [CrossRef]
- Lindner, G.; Horland, R.; Wagner, I.; Ataç, B.; Lauster, R. De novo formation and ultra-structural characterization of a fiber-producing human hair follicle equivalent in vitro. J. Biotechnol. 2011, 152, 108–112. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Vahav, I.; van den Broek, L.J.; Thon, M.; Monsuur, H.N.; Spiekstra, S.W.; Atac, B.; Scheper, R.J.; Lauster, R.; Lindner, G.; Marx, U.; et al. Reconstructed human skin shows epidermal invagination towards integrated neopapillae indicating early hair follicle formation in vitro. J. Tissue Eng. Regen. Med. 2020, 14, 761–773. [Google Scholar] [CrossRef]
- Tan, C.T.; Leo, Z.Y.; Lim, C.Y. Generation and integration of hair follicle-primed spheroids in bioengineered skin constructs. Biomed. Mater. 2022, 17, 061001. [Google Scholar] [CrossRef]
- Abaci, H.E.; Coffman, A.; Doucet, Y.; Chen, J.; Jacków, J.; Wang, E.; Guo, Z.; Shin, J.U.; Jahoda, C.A.; Christiano, A.M. Tissue engineering of human hair follicles using a biomimetic developmental approach. Nat. Commun. 2018, 9, 5301. [Google Scholar] [CrossRef] [PubMed]
- Abreu, C.M.; Cerqueira, M.T.; Pirraco, R.P.; Gasperini, L.; Reis, R.L.; Marques, A.P. Rescuing key native traits in cultured dermal papilla cells for human hair regeneration. J. Advert. Res. 2021, 30, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, J.; Kang, D.; Zhou, Y.; Du, L.; Qu, Q.; Wang, J.; Wen, L.; Fu, D.; Hu, Z. Microenvironmental reprogramming of human dermal papilla cells for hair follicle tissue engineering. Acta Biomater. 2023, 165, 31–49. [Google Scholar] [CrossRef]
- Gupta, A.C.; Chawla, S.; Hegde, A.; Singh, D.; Bandyopadhyay, B.; Lakshmanan, C.C.; Kalsi, G.; Ghosh, S. Establishment of an in vitro organoid model of dermal papilla of human hair follicle. J. Cell Physiol. 2018, 233, 9015–9030. [Google Scholar] [CrossRef] [PubMed]
- Betriu, N.; Jarrosson-Moral, C.; Semino, C.E. Culture and Differentiation of Human Hair Follicle Dermal Papilla Cells in a Soft 3D Self-Assembling Peptide Scaffold. Biomolecules 2020, 10, 684. [Google Scholar] [CrossRef]
- Quílez, C.; Valencia, L.; González-Rico, J.; Suárez-Cabrera, L.; Amigo-Morán, L.; Jorcano, J.L.; Velasco, D. In vitro induction of hair follicle signatures using human dermal papilla cells encapsulated in fibrin microgels. Cell Prolif. 2024, 57, e13528. [Google Scholar] [CrossRef]
- Jung, S.-Y.; You, H.J.; Kim, M.-J.; Ko, G.; Lee, S.; Kang, K.-S. Wnt-activating human skin organoid model of atopic dermatitis induced by Staphylococcus aureus and its protective effects by Cutibacterium acnes. iScience 2022, 25, 105150. [Google Scholar] [CrossRef]
- Ma, J.; Liu, J.; Gao, D.; Li, X.; Zhang, Q.; Lv, L.; Wang, Y.; Li, J.; Zhu, Y.; Wu, Z.; et al. Establishment of Human Pluripotent Stem Cell-Derived Skin Organoids Enabled Pathophysiological Model of SARS-CoV-2 Infection. Adv. Sci. 2022, 9, e2104192. [Google Scholar] [CrossRef] [PubMed]
- Ramovs, V.; Janssen, H.; Fuentes, I.; Pitaval, A.; Rachidi, W.; Chuva de Sousa Lopes, S.M.; Freund, C.; Gidrol, X.; Mummery, C.L.; Raymond, K. Characterization of the epidermal-dermal junction in hiPSC-derived skin organoids. Stem Cell Rep. 2022, 17, 1279–1288. [Google Scholar] [CrossRef]
- Li, P.; Pachis, S.T.; Xu, G.; Schraauwen, R.; Incitti, R.; de Vries, A.C.; Bruno, M.J.; Peppelenbosch, M.P.; Alam, I.; Raymond, K.; et al. Mpox virus infection and drug treatment modelled in human skin organoids. Nat. Microbiol. 2023, 8, 2067–2079. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Sun, J.; Ahmed, I.A.; Phua, F.; Rossi, G.R.; Lin, C.-Y.; Souza-Fonseca-Guimaraes, F.; Wolvetang, E.J.; Brown, J.; Khosrotehrani, K. Development of Physiologically Relevant Skin Organoids from Human Induced Pluripotent Stem Cells. Small 2023, 20, e2304879. [Google Scholar] [CrossRef] [PubMed]
- Bell, E.; Ehrlich, H.P.; Buttle, D.J.; Nakatsuji, T. Living tissue formed in vitro and accepted as skin-equivalent tissue of full thickness. Science 1981, 211, 1052–1054. [Google Scholar] [CrossRef] [PubMed]
- Chau, D.Y.S.; Johnson, C.; MacNeil, S.; Haycock, J.W.; Ghaemmaghami, A.M. The development of a 3D immunocompetent model of human skin. Biofabrication 2013, 5, 035011. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, E.H.; Tjabringa, G.S.; Joosten, I.; Vonk-Bergers, M.; van Rijssen, E.; Tijssen, H.J.; Erkens, M.; Schalkwijk, J.; Koenen, H.J.P.M. Crosstalk between keratinocytes and T cells in a 3D microenvironment: A model to study inflammatory skin diseases. J. Investig. Dermatol. 2014, 134, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Jin, L.; Zheng, Y.; Zhu, J. Modeling human HSV infection via a vascularized immune-competent skin-on-chip platform. Nat. Commun. 2022, 13, 5481. [Google Scholar] [CrossRef] [PubMed]
- Headington, J.T. Hair follicle biology and topical minoxidil: Possible mechanisms of action. Dermatologica 1987, 175 (Suppl. S2), 19–22. [Google Scholar] [CrossRef]
- Messenger, A.G.; Rundegren, J. Minoxidil: Mechanisms of action on hair growth. Br. J. Dermatol. 2004, 150, 186–194. [Google Scholar] [CrossRef]
- Redmond, L.C.; Limbu, S.; Farjo, B.; Messenger, A.G.; Higgins, C.A. Male pattern hair loss: Can developmental origins explain the pattern? Exp. Dermatol. 2023, 32, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Ataç, B.; Wagner, I.; Horland, R.; Lauster, R.; Marx, U.; Tonevitsky, A.G.; Azar, R.P.; Lindner, G. Skin and hair on-a-chip: In vitro skin models versus ex vivo tissue maintenance with dynamic perfusion. Lab Chip 2013, 13, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Kim, J.; Kim, H.; Sung, G.Y. Effect of α-Lipoic Acid on the Development of Human Skin Equivalents Using a Pumpless Skin-on-a-Chip Model. Int. J. Mol. Sci. 2021, 22, 2160. [Google Scholar] [CrossRef] [PubMed]
- Risueño, I.; Valencia, L.; Jorcano, J.L.; Velasco, D. Skin-on-a-chip models: General overview and future perspectives. APL Bioeng. 2021, 5, 030901. [Google Scholar] [CrossRef]
- Tang, Y.; Luo, B.; Deng, Z.; Wang, B.; Liu, F.; Li, J.; Shi, W.; Xie, H.; Hu, X.; Li, J. Mitochondrial aerobic respiration is activated during hair follicle stem cell differentiation, and its dysfunction retards hair regeneration. PeerJ 2016, 4, e1821. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.E.; Bossano, C.M.; Johnston, C.M.; Reis, R.L.; Mikos, A.G. In vitro localization of bone growth factors in constructs of biodegradable scaffolds seeded with marrow stromal cells and cultured in a flow perfusion bioreactor. Tissue Eng. 2006, 12, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Moran, C.A.; Wick, T.M. Transient Growth Factor Stimulation Improves Chondrogenesis in Static Culture and Under Dynamic Conditions in a Novel Shear and Perfusion Bioreactor. Cell. Mol. Bioeng. 2015, 8, 267–277. [Google Scholar] [CrossRef]
- Hoffman, B.D.; Grashoff, C.; Schwartz, M.A. Dynamic molecular processes mediate cellular mechanotransduction. Nature 2011, 475, 316–323. [Google Scholar] [CrossRef] [PubMed]
- LeGoff, L.; Lecuit, T. Mechanical Forces and Growth in Animal Tissues. Cold Spring Harb. Perspect. Biol. 2015, 8, a019232. [Google Scholar] [CrossRef]
- Vining, K.H.; Mooney, D.J. Mechanical forces direct stem cell behaviour in development and regeneration. Nat. Rev. Mol. Cell Biol. 2017, 18, 728–742. [Google Scholar] [CrossRef]
- Ito, M.; Yang, Z.; Andl, T.; Cui, C.; Kim, N.; Millar, S.E.; Cotsarelis, G. Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 2007, 447, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Gay, D.; Kwon, O.; Zhang, Z.; Spata, M.; Plikus, M.V.; Holler, P.D.; Ito, M.; Yang, Z.; Treffeisen, E.; Kim, C.D.; et al. Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat. Med. 2013, 19, 916–923. [Google Scholar] [CrossRef]
- Harn, H.I.-C.; Wang, S.-P.; Lai, Y.-C.; Van Handel, B.; Liang, Y.-C.; Tsai, S.; Schiessl, I.M.; Sarkar, A.; Xi, H.; Hughes, M.; et al. Symmetry breaking of tissue mechanics in wound induced hair follicle regeneration of laboratory and spiny mice. Nat. Commun. 2021, 12, 2595. [Google Scholar] [CrossRef] [PubMed]
- Harn, H.I.-C.; Chiu, P.-Y.; Lin, C.-H.; Chen, H.-Y.; Lai, Y.-C.; Yang, F.-S.; Wu, C.-C.; Tang, M.-J.; Chuong, C.-M.; Hughes, M.W. Topological Distribution of Wound Stiffness Modulates Wound-Induced Hair Follicle Neogenesis. Pharmaceutics 2022, 14, 1926. [Google Scholar] [CrossRef] [PubMed]
- Thangapazham, R.L.; Klover, P.; Wang, J.A.; Zheng, Y.; Devine, A.; Li, S.; Sperling, L.; Cotsarelis, G.; Darling, T.N. Dissociated human dermal papilla cells induce hair follicle neogenesis in grafted dermal-epidermal composites. J. Investig. Dermatol. 2014, 134, 538. [Google Scholar] [CrossRef]
- Correia Carreira, S.; Taghavi, M.; Pavez Loriè, E.; Rossiter, J. FleXert: A Soft, Actuatable Multiwell Plate Insert for Cell Culture under Stretch. ACS Biomater. Sci. Eng. 2021, 7, 2225–2245. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Perfusable and stretchable 3D culture system for skin-equivalent. Biofabrication 2018, 11, 011001. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, C.T.; Lim, C.Y.; Lay, K. Modelling Human Hair Follicles—Lessons from Animal Models and Beyond. Biology 2024, 13, 312. https://doi.org/10.3390/biology13050312
Tan CT, Lim CY, Lay K. Modelling Human Hair Follicles—Lessons from Animal Models and Beyond. Biology. 2024; 13(5):312. https://doi.org/10.3390/biology13050312
Chicago/Turabian StyleTan, Chew Teng, Chin Yan Lim, and Kenneth Lay. 2024. "Modelling Human Hair Follicles—Lessons from Animal Models and Beyond" Biology 13, no. 5: 312. https://doi.org/10.3390/biology13050312
APA StyleTan, C. T., Lim, C. Y., & Lay, K. (2024). Modelling Human Hair Follicles—Lessons from Animal Models and Beyond. Biology, 13(5), 312. https://doi.org/10.3390/biology13050312





