Stock Assessment of the Commercial Small Pelagic Fishes in the Beibu Gulf, the South China Sea, 2006–2020
Abstract
Highlights
- The commercial small pelagic fishes (Decapterus maruadsi and Trachurus japonicus) in the Beibu Gulf were still miniaturizing.
- Fisheries management, characterized by reduced fishing efforts, cannot completely restore population structure in a short period.
- Continuing to maintain low fishing mortality and increasing the catchable length should be the key ways to achieve fishery resource conservation and recovery.
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
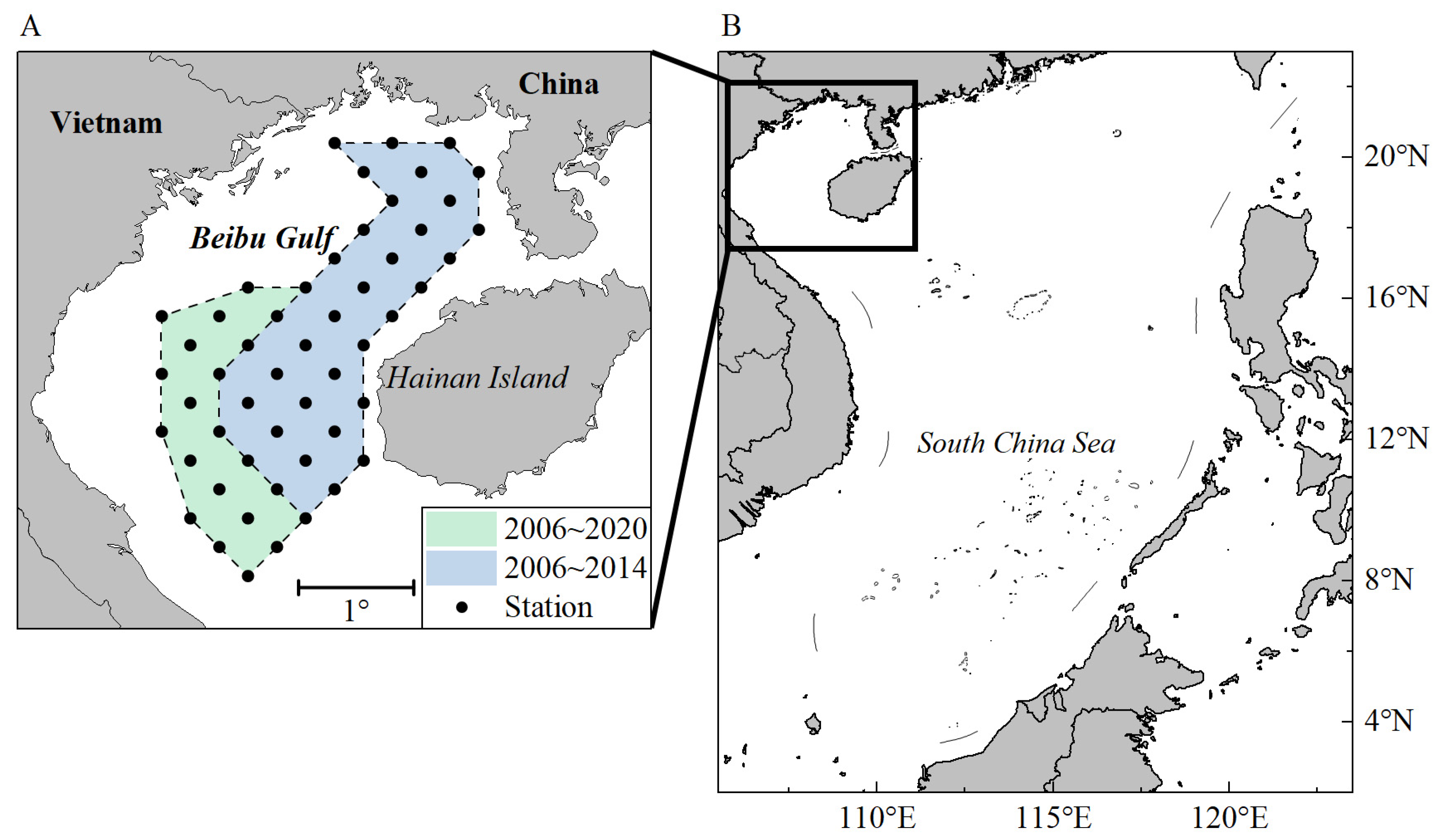
2.1. Study Area
2.2. Sampling
2.3. Data Analysis
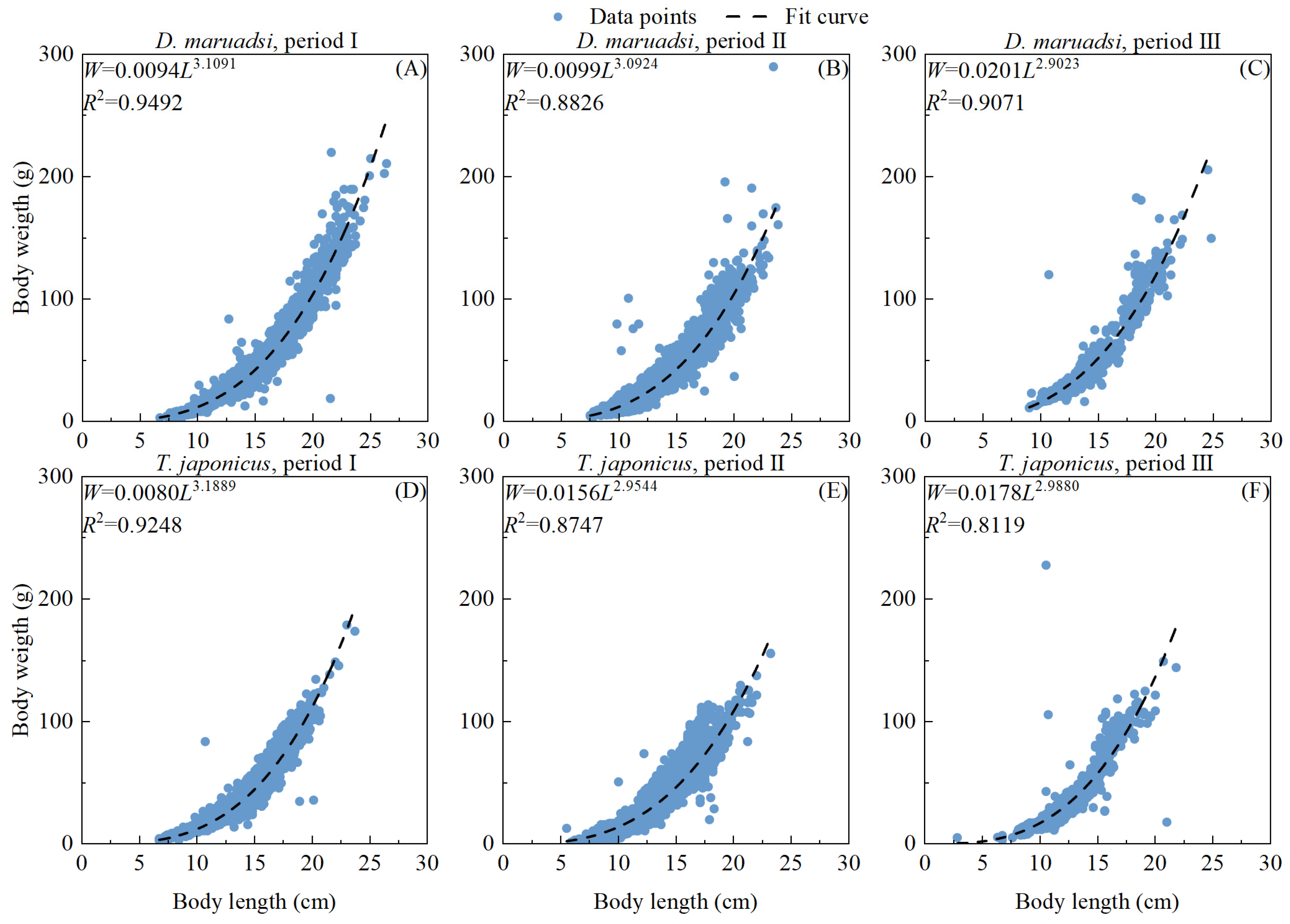
2.3.1. Length–Weight Relationship of Fish Samples
2.3.2. Stock Assessment by ELEFAN
3. Results
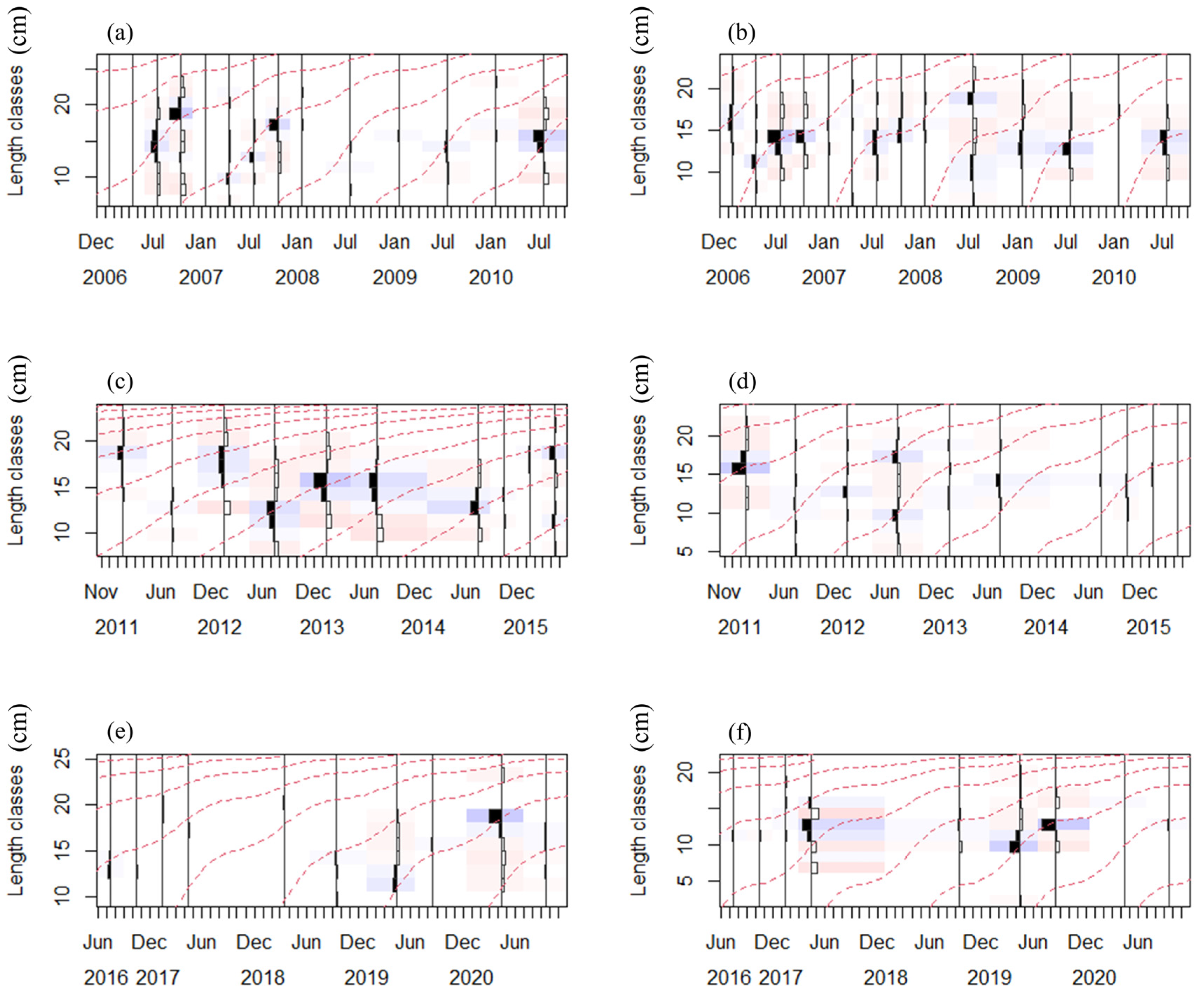
3.1. Size Distributions and Length–Weight Relationship of the Stocks
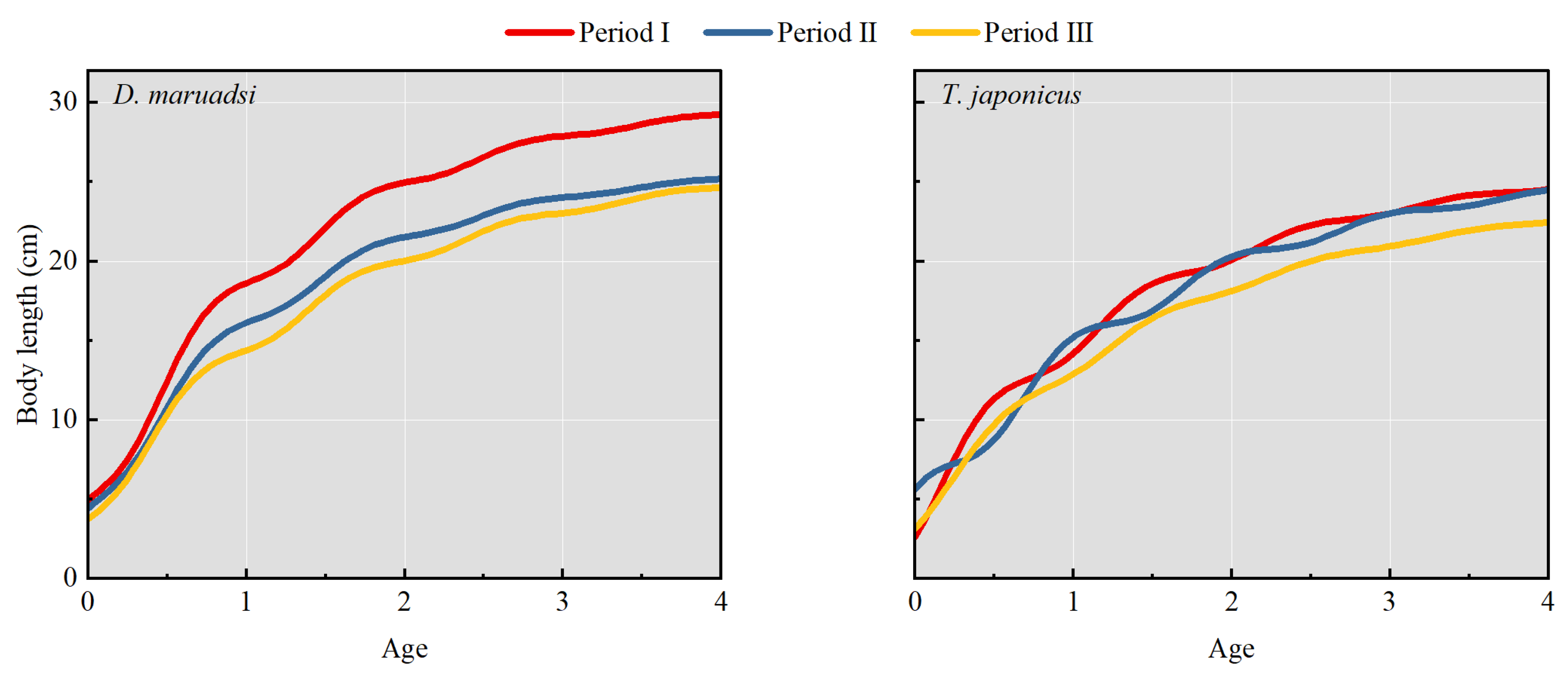
3.2. Growth Parameters and Growth Curves
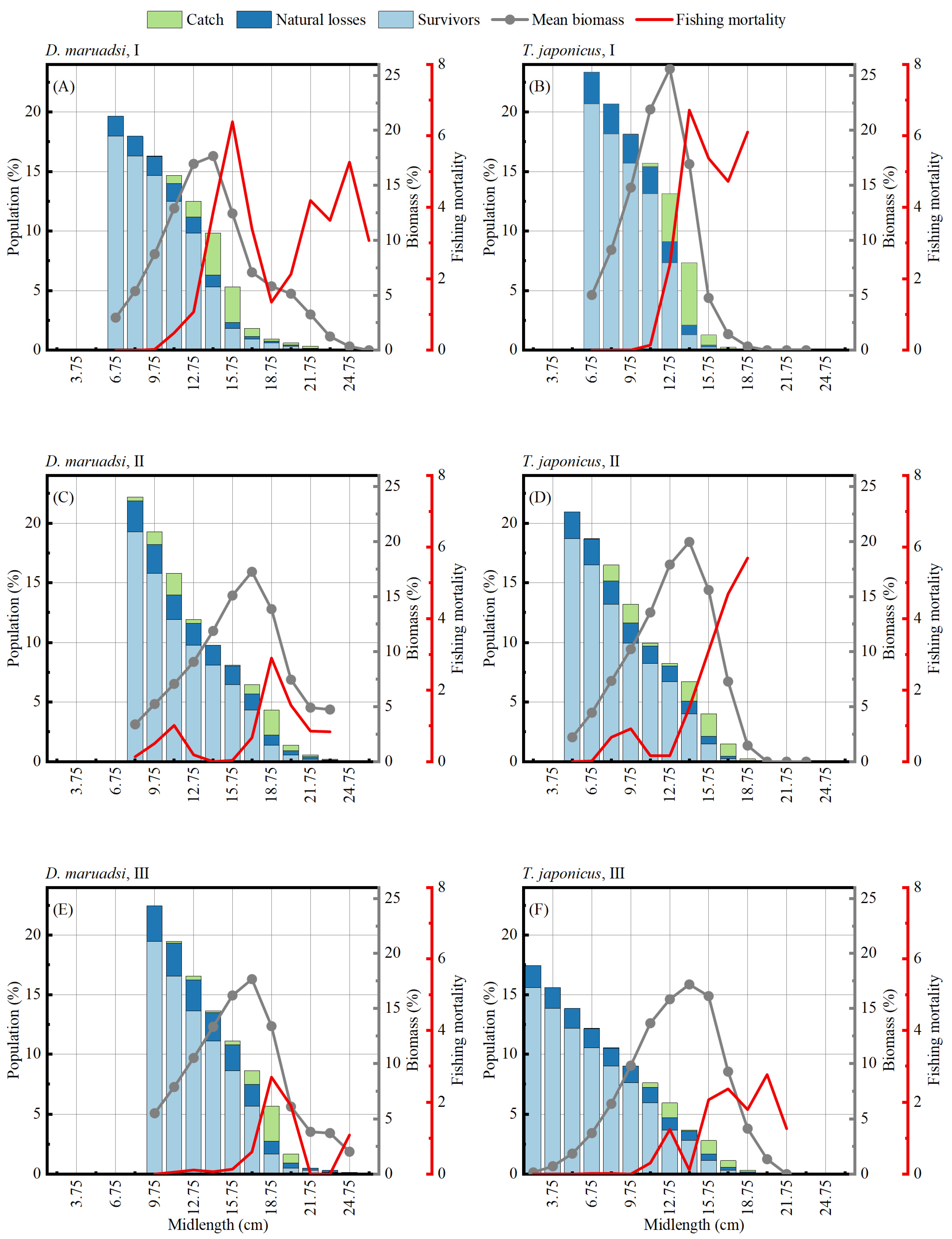
3.3. Mortality and Selectivity
3.4. Stock Status
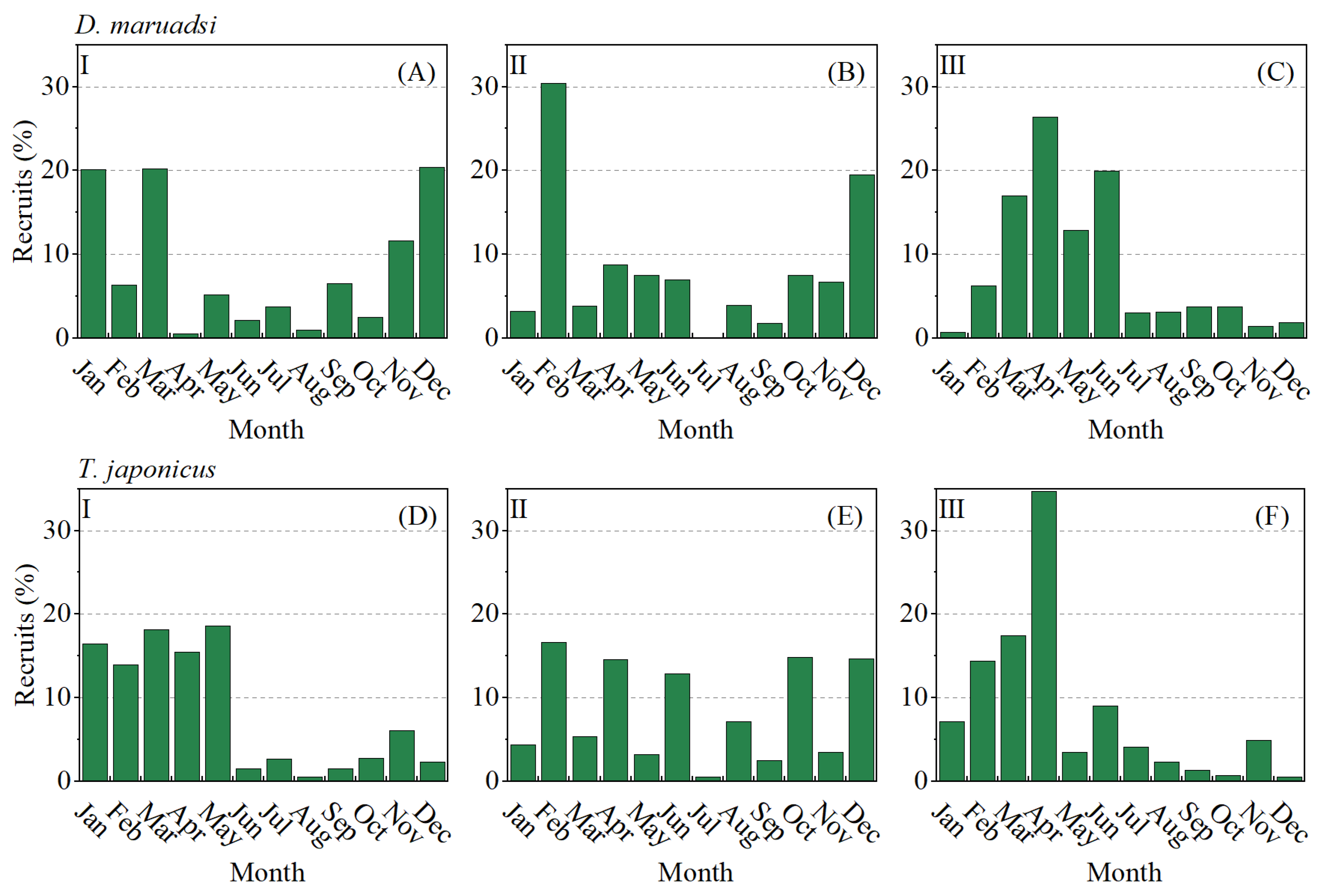
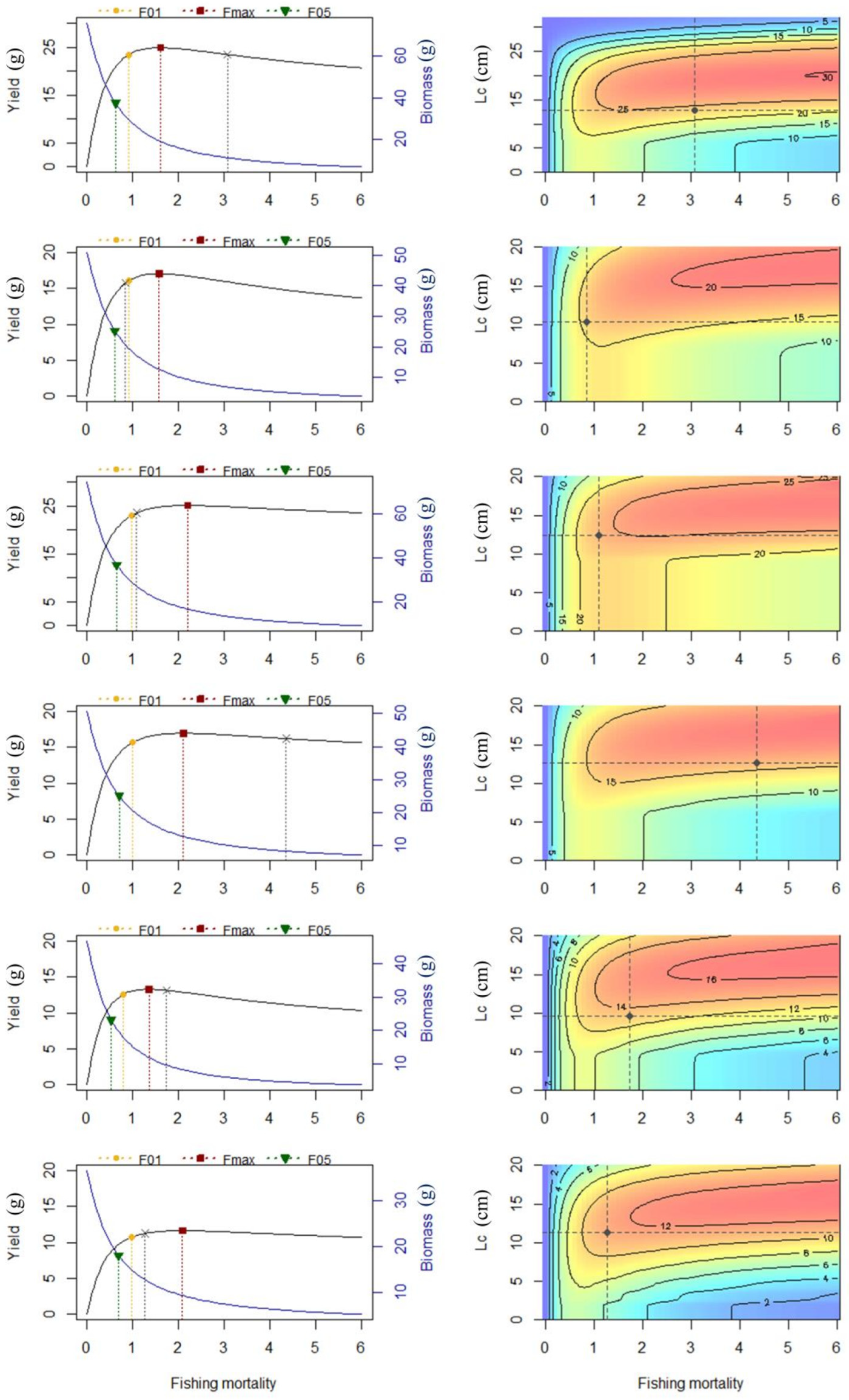
3.5. Recruitment and Yield per Recruit
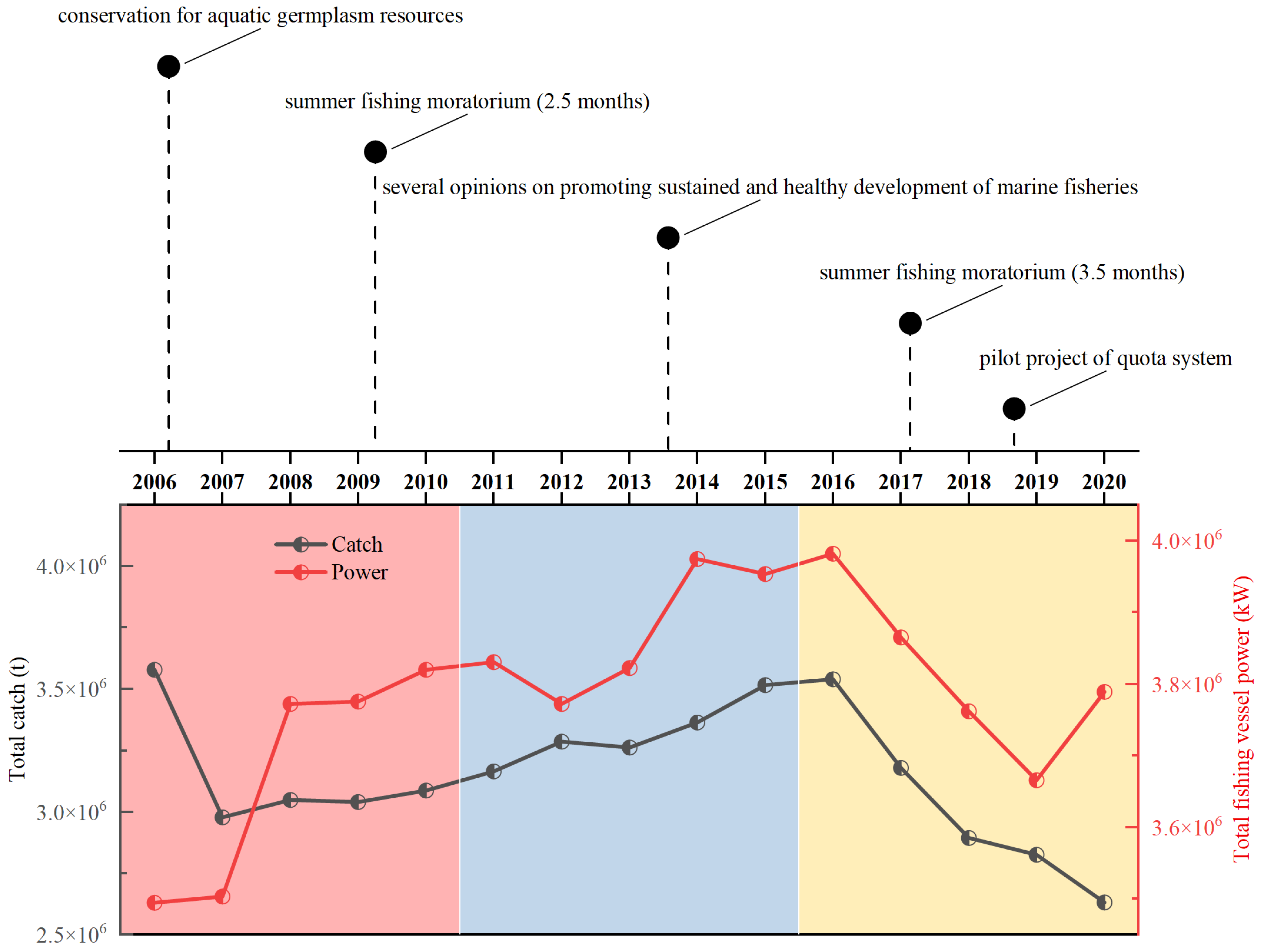
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fisher, B.; Naidoo, R.; Guernier, J.; Johnson, K.; Mullins, D.; Robinson, D.; Allison, E.H. Integrating fisheries and agricultural programs for food security. Agric. Food Secur. 2017, 6, 1. [Google Scholar] [CrossRef]
- Palomares, M.-L.D.; Pauly, D. Coastal Fisheries: The Past, Present, and Possible Futures. Coasts Estuaries 2019, 569–576. [Google Scholar]
- Pauly, D.; Zeller, D. Catch reconstructions reveal that global marine fisheries catches are higher than reported and declining. Nat. Commun. 2016, 7, 10244. [Google Scholar] [CrossRef]
- Watson, R.; Pang, L.; Pauly, D. The Marine Fisheries of China: Development and Reported Catches; Fisheries Centre, University of British Columbia: Vancouver, BC, Canada, 2001. [Google Scholar]
- Hu, F.; Zhong, H.; Wu, C.; Wang, S.; Guo, Z.; Tao, M.; Zhang, C.; Gong, D.; Gao, X.; Tang, C.; et al. Development of fisheries in China. Reprod. Breed. 2021, 1, 64–79. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2022; 266p. [Google Scholar]
- Liu, Z.; Sun, H.; Yue, D.; Geng, R.; Zhao, L.; Pan, P.; Cao, K. Research on China’s maintenance policy for marie capture fishery resources in the new era. J. Agric. Sci. Technol. 2018, 20, 1–8. [Google Scholar]
- Su, M.; Wang, L.; Xiang, J.; Ma, Y. Adjustment trend of China’s marine fishery policy since 2011. Mar. Policy 2021, 124, 104322. [Google Scholar] [CrossRef]
- Kang, B.; Wang, L.; Liu, M. Species traits determined different responses to “zero-growth” policy in China’s marine fisheries. Sci. Rep. 2022, 12, 20410. [Google Scholar] [CrossRef]
- Cao, L.; Chen, Y.; Dong, S.; Hanson, A.; Huang, B.; Leadbitter, D.; Little, D.C.; Pikitch, E.K.; Qiu, Y.; Sadovy de Mitcheson, Y.; et al. Opportunity for marine fisheries reform in China. Proc. Natl. Acad. Sci. USA 2017, 114, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, H.; Weigel, B. Responses of coastal fishery resources to rapid environmental changes. J. Fish Biol. 2022, 101, 686–698. [Google Scholar] [CrossRef] [PubMed]
- Melnychuk, M.C.; Kurota, H.; Mace, P.M.; Pons, M.; Minto, C.; Osio, G.C.; Jensen, O.P.; de Moor, C.L.; Parma, A.M.; Richard Little, L.; et al. Identifying management actions that promote sustainable fisheries. Nat. Sustain. 2021, 4, 440–449. [Google Scholar] [CrossRef]
- Yoda, M.; Shiraishi, T.; Yukami, R.; Ohshimo, S. Age and maturation of jack mackerel Trachurus japonicus in the East China Sea. Fish. Sci. 2013, 80, 61–68. [Google Scholar] [CrossRef]
- Jamaludin, N.A.; Mohd-Arshaad, W.; Mohd Akib, N.A.; Zainal Abidin, D.H.; Nghia, N.V.; Nor, S.M. Phylogeography of the Japanese scad, Decapterus maruadsi (Teleostei; Carangidae) across the Central Indo-West Pacific: Evidence of strong regional structure and cryptic diversity. Mitochondrial DNA A DNA Mapp. Seq. Anal. 2020, 31, 298–310. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, M.; Li, J.; Sun, M.; Xu, Y.; Cai, Y.; Chen, Z.; Qiu, Y. Climate-induced small pelagic fish blooms in an overexploited marine ecosystem of the South China Sea. Ecol. Indic. 2022, 145, 109598. [Google Scholar] [CrossRef]
- Su, L.; Xu, Y.; Qiu, Y.; Sun, M.; Zhang, K.; Chen, Z. Long-Term Change of a Fish-Based Index of Biotic Integrity for a Semi-Enclosed Bay in the Beibu Gulf. Fishes 2022, 7, 124. [Google Scholar] [CrossRef]
- Hong, X.; Zhang, K.; Li, J.; Xu, Y.; Sun, M.; Wang, Y.; Xu, S.; Cai, Y.; Qiu, Y.; Chen, Z. Effects of Climate Events on Abundance and Distribution of Major Commercial Fishes in the Beibu Gulf, South China Sea. Diversity 2023, 15, 649. [Google Scholar] [CrossRef]
- Arnason, R. Fisheries management and operations research. Eur. J. Oper. Res. 2009, 193, 741–751. [Google Scholar] [CrossRef]
- Huang, M.; Ding, L.; Wang, J.; Ding, C.; Tao, J. The impacts of climate change on fish growth: A summary of conducted studies and current knowledge. Ecol. Indic. 2021, 121, 106976. [Google Scholar] [CrossRef]
- Brander, K. Climate and current anthropogenic impacts on fisheries. Clim. Change 2012, 119, 9–21. [Google Scholar] [CrossRef]
- Osathanunkul, M.; Suwannapoom, C. Sustainable fisheries management through reliable restocking and stock enhancement evaluation with environmental DNA. Sci. Rep. 2023, 13, 11297. [Google Scholar] [CrossRef]
- Lorenzen, K.; Cowx, I.G.; Entsua-Mensah, R.E.M.; Lester, N.P.; Koehn, J.D.; Randall, R.G.; So, N.; Bonar, S.A.; Bunnell, D.B.; Venturelli, P.; et al. Stock assessment in inland fisheries: A foundation for sustainable use and conservation. Rev. Fish Biol. Fish. 2016, 26, 405–440. [Google Scholar] [CrossRef]
- Zhang, K.; Li, J.; Hou, G.; Huang, Z.; Shi, D.; Chen, Z.; Qiu, Y. Length-Based Assessment of Fish Stocks in a Data-Poor, Jointly Exploited (China and Vietnam) Fishing Ground, Northern South China Sea. Front. Mar. Sci. 2021, 8, 718052. [Google Scholar] [CrossRef]
- Methot, R.D.; Wetzel, C.R. Stock synthesis: A biological and statistical framework for fish stock assessment and fishery management. Fish. Res. 2013, 142, 86–99. [Google Scholar] [CrossRef]
- Costello, C.; Ovando, D.; Hilborn, R.; Gaines, S.D.; Deschenes, O.; Lester, S.E. Status and Solutions for the World’s Unassessed Fisheries. Science 2012, 338, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef]
- Cotter, A.J.R.; Burt, L.; Paxton, C.G.M.; Fernandez, C.; Buckland, S.T.; Pan, J.X. Are stock assessment methods too complicated? Fish Fish. 2004, 5, 235–254. [Google Scholar] [CrossRef]
- Schwamborn, R.; Mildenberger, T.K.; Taylor, M.H. Assessing sources of uncertainty in length-based estimates of body growth in populations of fishes and macroinvertebrates with bootstrapped ELEFAN. Ecol. Model. 2019, 393, 37–51. [Google Scholar] [CrossRef]
- Pauly, D.; David, N. ELEFAN I, a BASIC program for the objective extraction of growth parameters from length-frequency data. Meeresforschung 1981, 28, 205–211. [Google Scholar]
- Chen, N.; Zhang, C.; Sun, M.; Xu, B.; Xue, Y.; Ren, Y.; Chen, Y. Evaluating the Performances of Size-Frequency-Based Methods for Estimating Fishing Mortality of Pholis fangi. Mar. Coast. Fish. 2019, 11, 305–314. [Google Scholar] [CrossRef]
- Schwamborn, R.; Moraes-Costa, D.F. Growth and mortality of endangered land crabs (Cardisoma guanhumi) assessed through tagging with PITs and novel bootstrapped methods. arXiv 2019, arXiv:1909.03311. [Google Scholar]
- Mildenberger, T.K.; Taylor, M.H.; Wolff, M.; Price, S. TropFishR: An R package for fisheries analysis with length-frequency data. Methods Ecol. Evol. 2017, 8, 1520–1527. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Xu, Y.; Jiang, Y.; Fan, J.; Xu, S.; Chen, Z. Long-term variations in fish community structure under multiple stressors in a semi-closed marine ecosystem in the South China Sea. Sci. Total Environ. 2020, 745, 140892. [Google Scholar] [CrossRef]
- Abobi, S.M.; Mildenberger, T.K.; Kolding, J.; Wolff, M. Assessing the exploitation status of main fisheries resources in Ghana’s reservoirs based on reconstructed catches and a length-based bootstrapping stock assessment method. Lake Reserv. Manag. 2019, 35, 415–434. [Google Scholar] [CrossRef]
- Scrucca, L. On Some Extensions to GA Package: Hybrid Optimisation, Parallelisation and Islands EvolutionOn some extensions to GA package: Hybrid optimisation, parallelisation and islands evolution. R J. 2017, 9, 187. [Google Scholar] [CrossRef]
- Scrucca, L. GA: A Package for Genetic Algorithms in R. J. Stat. Softw. 2013, 53, 1–37. [Google Scholar] [CrossRef]
- Somers, I.F. On a Seasonally Oscillating Growth Function. Fishbyte 1988, 6, 8–11. [Google Scholar]
- Pauly, D. A Review of the ELEFAN System for Analysis of Length-Frequency Data in Fish and Aquatic Invertebrates. ICLARM Conf. Proc. 1987, 13, 7–34. [Google Scholar]
- Pauly, D. Gill Size and Temperature as Governing Factors in Fish Growth: A Generalization of von Bertalanffy’s Growth Formula; Berichte Institut Für Meereskunde: Kiel, Germany, 1979. [Google Scholar]
- Pauly, D.; Munro, J.L. Once More on the Comparison of Growth in Fish and Invertebrates. Fishbyte 1984, 2, 1–21. [Google Scholar]
- Pauly, D. Length-converted catch curves: A powerful tool for fisheries research in the tropics (part 1). Fishbyte 1983, 1, 9–13. [Google Scholar]
- Pauly, D. On the interrelationships between natural mortality, growth parameters, and mean environmental temperature in 175 fish stocks. ICES J. Mar. Sci. 1980, 39, 175–192. [Google Scholar] [CrossRef]
- Then, A.Y.; Hoenig, J.M.; Gedamke, T.; SAult, J. Comparison of two length-based estimators of total mortality: A simulation approach. Trans. Am. Fish. Soc. 2015, 144, 1206–1219. [Google Scholar] [CrossRef]
- Taylor, M.H.; Mildenberger, T.K. Extending electronic length frequency analysis in R. Fish. Manag. Ecol. 2017, 24, 330–338. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, C.; Xu, B.; Xue, Y.; Ren, Y. Selecting optimal bin size to account for growth variability in Electronic LEngth Frequency ANalysis (ELEFAN). Fish. Res. 2020, 225, 105474. [Google Scholar] [CrossRef]
- Beverton, R.J.; Holt, S.J. On the Dynamics of Exploited Fish Populations; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Beverton, R.J.H.; Holt, S.J. Manual of Methods for Fish Stock Assessment. Pt. 2: Tables of Yield Functions; FAO: Rome, Italy, 1979. [Google Scholar]
- Gulland, J.; Oerema, L.K.B. Scientific Advice on Catch Levels. Fish. Bull. 1973, 71, 325–336. [Google Scholar]
- Sparre, P. Introduction to Tropical Fish Stock Assessment, Part 1 Manual; FAO Fisheries Technical Paper 306/1; FAO: Rome, Italy, 1998; pp. 1–407. [Google Scholar]
- Morey, G.; Moranta, J.; Massutı, E.; Grau, A.; Linde, M.; Riera, F.; Morales-Nin, B. Weight–length relationships of littoral to lower slope fishes from the western Mediterranean. Fish. Res. 2003, 62, 89–96. [Google Scholar] [CrossRef]
- Froese, R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
- Munro, J.; Pauly, D. A simple method for comparing the growth of fishes and invertebrates. Fishbyte 1983, 1, 5–6. [Google Scholar]
- Bœuf, G.; Payan, P. How should salinity influence fish growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef]
- Boltana, S.; Sanhueza, N.; Aguilar, A.; Gallardo-Escarate, C.; Arriagada, G.; Valdes, J.A.; Soto, D.; Quinones, R.A. Influences of thermal environment on fish growth. Ecol. Evol. 2017, 7, 6814–6825. [Google Scholar] [CrossRef]
- Li, L.; Shen, Y.; Yang, W.; Xu, X.; Li, J. Effect of different stocking densities on fish growth performance: A meta-analysis. Aquaculture 2021, 544, 737152. [Google Scholar] [CrossRef]
- Steneck, R.S. Human influences on coastal ecosystems: Does overfishing create trophic cascades? Trends Ecol. Evol. 1998, 13, 429–430. [Google Scholar] [CrossRef]
- Jackson, J.B.; Kirby, M.X.; Berger, W.H.; Bjorndal, K.A.; Botsford, L.W.; Bourque, B.J.; Bradbury, R.H.; Cooke, R.; Erlandson, J.; Estes, J.A.; et al. Historical overfishing and the recent collapse of coastal ecosystems. Science 2001, 293, 629–637. [Google Scholar] [CrossRef]
- Zhang, K.; Cai, Y.; Liao, B.; Jiang, Y.; Sun, M.; Su, L.; Chen, Z. Population dynamics of threadfin porgy Evynnis cardinalis, an endangered species on the IUCN red list in the Beibu Gulf, South China Sea. J. Fish Biol. 2020, 97, 479–489. [Google Scholar] [CrossRef]
- Zhang, K.; Geng, P.; Li, J.; Xu, Y.; Kalhoro, M.A.; Sun, M.; Shi, D.; Chen, Z. Influences of fisheries management measures on biological characteristics of threadfin bream (Nemipterus virgatus) in the Beibu Gulf, South China Sea. Acta Oceanol. Sin. 2022, 41, 24–33. [Google Scholar] [CrossRef]
- Audzijonyte, A.; Richards, S.A.; Stuart-Smith, R.D.; Pecl, G.; Edgar, G.J.; Barrett, N.S.; Payne, N.; Blanchard, J.L. Fish body sizes change with temperature but not all species shrink with warming. Nat. Ecol. Evol. 2020, 4, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Enberg, K.; Jørgensen, C.; Dunlop, E.S.; Varpe, Ø.; Boukal, D.S.; Baulier, L.; Eliassen, S.; Heino, M. Fishing-induced evolution of growth: Concepts, mechanisms and the empirical evidence. Mar. Ecol. 2012, 33, 1–25. [Google Scholar] [CrossRef]
- Thambithurai, D.; Kuparinen, A. Environmental forcing alters fisheries selection. Trends Ecol. Evol. 2024, 39, 131–140. [Google Scholar] [CrossRef]
- Kraak, S.B.M.; Haase, S.; Minto, C.; Santos, J.; Anderson, E. The Rosa Lee phenomenon and its consequences for fisheries advice on changes in fishing mortality or gear selectivity. ICES J. Mar. Sci. 2019, 76, 2179–2192. [Google Scholar] [CrossRef]
- Yan, L.; Yang, B.; Zhang, P.; Li, J.; Wang, T. Size Selectivity of a Diamond-Mesh Codend of Demersal Trawl for Largehead Hairtail (Trichiurus lepturus Linnaeus, 1758) in the Beibu Gulf, in the South China Sea. J. Mar. Sci. Eng. 2023, 11, 1444. [Google Scholar] [CrossRef]
- Kang, B.; Liu, M.; Huang, X.-X.; Li, J.; Yan, Y.-R.; Han, C.-C.; Chen, S.-B. Fisheries in Chinese seas: What can we learn from controversial official fisheries statistics? Rev. Fish Biol. Fish. 2018, 28, 503–519. [Google Scholar] [CrossRef]
- Watling, L.; Norse, E.A. Disturbance of the Seabed by Mobile Fishing Gear: A Comparison to Forest Clearcutting. Conserv. Biol. 2008, 12, 1180–1197. [Google Scholar] [CrossRef]
- Walsh, M.R.; Munch, S.B.; Chiba, S.; Conover, D.O. Maladaptive changes in multiple traits caused by fishing: Impediments to population recovery. Ecol. Lett. 2006, 9, 142–148. [Google Scholar] [CrossRef]
- Genner, M.J.; Sims, D.W.; Southward, A.J.; Budd, G.C.; Masterson, P.; McHugh, M.; Rendle, P.; Southall, E.J.; Wearmouth, V.J.; Hawkins, S.J. Body size-dependent responses of a marine fish assemblage to climate change and fishing over a century-long scale. Glob. Change Biol. 2010, 16, 517–527. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Sarmiento, J.L.; Dunne, J.; Frölicher, T.L.; Lam, V.W.Y.; Deng Palomares, M.L.; Watson, R.; Pauly, D. Shrinking of fishes exacerbates impacts of global ocean changes on marine ecosystems. Nat. Clim. Change 2012, 3, 254–258. [Google Scholar] [CrossRef]
- Tan, H.-J.; Cai, R.-S.; Wu, R.-G. Summer marine heatwaves in the South China Sea: Trend, variability and possible causes. Adv. Clim. Change Res. 2022, 13, 323–332. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Reiss, C.S.; Hunter, J.R.; Beddington, J.R.; May, R.M.; Sugihara, G. Fishing elevates variability in the abundance of exploited species. Nature 2006, 443, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Dou, M.; Xia, R.; Kuo, Y.-M.; Li, G.; Shen, L. Effects of hydrological alteration on fish population structure and habitat in river system: A case study in the mid-downstream of the Hanjiang River in China. Glob. Ecol. Conserv. 2020, 23, e01090. [Google Scholar] [CrossRef]
- Elliott, J.M.; Gulland, J. Fish Stock Assessment. A Manual of Basic Methods. J. Anim. Ecol. 1984, 53, 700. [Google Scholar] [CrossRef]
- Duarte, C.M.; Agusti, S.; Barbier, E.; Britten, G.L.; Castilla, J.C.; Gattuso, J.P.; Fulweiler, R.W.; Hughes, T.P.; Knowlton, N.; Lovelock, C.E.; et al. Rebuilding marine life. Nature 2020, 580, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Hutchings, J.A. Collapse and recovery of marine fishes. Nature 2000, 406, 882–885. [Google Scholar] [CrossRef] [PubMed]
- Muhling, B.; Lindegren, M.; Clausen, L.W.; Hobday, A.; Lehodey, P. Impacts of Climate Change on Pelagic Fish and Fisheries. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; Wiley: Hoboken, NJ, USA, 2017; Volume 2, pp. 771–814. [Google Scholar]
- Shannon, L.J.; Nelson, G.; Crawford, R.J.M.; Boyd, A.J. Possible impacts of environmental change on pelagic fish recruitment: Modelling anchovy transport by advective processes in the southern Benguela. Glob. Change Biol. 2006, 2, 407–420. [Google Scholar] [CrossRef]
- Checkley, D.; Alheit, J.; Oozeki, Y.; Roy, C. Climate Change and Small Pelagic Fish; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Zhao, H.; Feng, Y.; Dong, C.; Li, Z. Spatiotemporal distribution of Decapterus maruadsi in spring and autumn in response to environmental variation in the northern South China Sea. Reg. Stud. Mar. Sci. 2021, 45, 101811. [Google Scholar] [CrossRef]
- Ishikawa, K.; Watanabe, C.; Kameda, T.; Tokeshi, T.; Horie, H.; Hashida, D.; Ookawa, T.; Takeda, T.; Kuno, M.; Suzuki, Y.; et al. Spatiotemporal variability in the occurrence of juvenile Japanese jack mackerel Trachurus japonicus along coastal areas of the Kuroshio Current. Fish. Oceanogr. 2021, 30, 569–583. [Google Scholar] [CrossRef]
- Shoji, J.; Toshito, S.-I.; Mizuno, K.-i.; Kamimura, Y.; Hori, M.; Hirakawa, K. Possible effects of global warming on fish recruitment: Shifts in spawning season and latitudinal distribution can alter growth of fish early life stages through changes in daylength. ICES J. Mar. Sci. 2011, 68, 1165–1169. [Google Scholar] [CrossRef]
- Meehl, G.A.; Washington, W.M.; Collins, W.D.; Arblaster, J.M.; Hu, A.; Buja, L.E.; Strand, W.G.; Teng, H. How Much More Global Warming and Sea Level Rise? Science 2005, 307, 1769–1772. [Google Scholar] [CrossRef]
- Wang, G.; Cai, W.; Gan, B.; Wu, L.; Santoso, A.; Lin, X.; Chen, Z.; McPhaden, M.J. Continued increase of extreme El Niño frequency long after 1.5 °C warming stabilization. Nat. Clim. Change 2017, 7, 568–572. [Google Scholar] [CrossRef]
- Kritzer, J.P.; Liu, O.R. Fishery Management Strategies for Addressing Complex Spatial Structure in Marine Fish Stocks. In Stock Identification Methods; Academic Press: Cambridge, MA, USA, 2014; pp. 29–57. [Google Scholar]
- Millar, R.B. Estimating the Size-selectivity of Fishing Gear by Conditioning on the Total Catch. J. Am. Stat. Assoc. 1992, 87, 962–968. [Google Scholar] [CrossRef]
- Dunn, D.C.; Maxwell, S.M.; Boustany, A.M.; Halpin, P.N. Dynamic ocean management increases the efficiency and efficacy of fisheries management. Proc. Natl. Acad. Sci. USA 2016, 113, 668–673. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Hansen, L.J. Potential impacts of global climate change on freshwater fisheries. Rev. Fish Biol. Fish. 2007, 17, 581–613. [Google Scholar] [CrossRef]
- Lam, V.W.Y.; Allison, E.H.; Bell, J.D.; Blythe, J.; Cheung, W.W.L.; Frölicher, T.L.; Gasalla, M.A.; Sumaila, U.R. Climate change, tropical fisheries and prospects for sustainable development. Nat. Rev. Earth Environ. 2020, 1, 440–454. [Google Scholar] [CrossRef]
| Species | Years | Periods | Number | Lmin (cm) | Lmax (cm) | Lmedian (cm) | Lmean (±SD) (cm) |
|---|---|---|---|---|---|---|---|
| D. maruadsi | 2006–2010 | I | 4102 | 6.7 | 26.4 | 14.2 | 14.46 (±2.87) |
| 2011–2015 | II | 2475 | 7.5 | 23.8 | 14.2 | 14.41 (±2.78) | |
| 2016–2020 | III | 701 | 9.0 | 24.8 | 13.6 | 14.32 (±3.07) | |
| T. japonicus | 2006–2010 | I | 6178 | 6.7 | 23.7 | 13.6 | 13.54 (±2.24) |
| 2011–2015 | II | 3872 | 5.5 | 23.2 | 13.0 | 13.01 (±2.58) | |
| 2016–2020 | III | 1477 | 2.8 | 21.8 | 12.3 | 12.30 (±2.07) |
| Species | Periods | L∞ (cm) (95% CI) | K (a−1) (95% CI) | C (95% CI) | ts (95% CI) | φ′ (95% CI) |
|---|---|---|---|---|---|---|
| D. maruadsi | I | 30.43 (23.94–32.69) | 0.77 (0.24–0.95) | 0.57 (0.32–0.88) | 0.54 (0.28–0.72) | 2.87 (2.33–2.94) |
| II | 26.19 (19.52–28.04) | 0.77 (0.32–0.86) | 0.51 (0.22–0.74) | 0.55 (0.12–0.77) | 2.43 (2.39–2.74) | |
| III | 26.48 (22.70–28.89) | 0.63 (0.50–0.85) | 0.54 (0.22–0.90) | 0.46 (0.05–0.90) | 2.65 (2.59–2.72) | |
| T. japonicus | I | 26.08 (24.18–27.82) | 0.68 (0.52–0.86) | 0.58 (0.32–0.85) | 0.23 (0.14–0.33) | 2.65 (2.59–2.74) |
| II | 26.13 (19.64–28.86) | 0.63 (0.25–0.86) | 0.75 (0.29–0.91) | 0.75 (0.14–0.92) | 2.66 (2.31–2.82) | |
| III | 24.22 (22.06–26.66) | 0.62 (0.20–0.81) | 0.36 (0.17–0.89) | 0.32 (0.03–0.97) | 2.58 (2.08–2.69) |
| Species | Periods | Z | F | M | E | L50 (cm) | L75 (cm) |
|---|---|---|---|---|---|---|---|
| D. maruadsi | I | 4.17 | 3.07 | 1.10 | 0.74 | 12.82 | 13.48 |
| II | 1.99 | 0.83 | 1.16 | 0.42 | 10.36 | 11.20 | |
| III | 2.08 | 1.08 | 1.00 | 0.52 | 12.35 | 12.64 | |
| T. japonicus | I | 5.41 | 4.35 | 1.06 | 0.80 | 12.66 | 13.23 |
| II | 2.74 | 1.74 | 1.00 | 0.63 | 9.63 | 10.19 | |
| III | 2.28 | 1.26 | 1.02 | 0.55 | 11.30 | 12.00 |
| Species | Periods | F0.1 | Fmax | Emax | YPR0.1 | YPRmax | YPRc |
|---|---|---|---|---|---|---|---|
| D. maruadsi | I | 0.91 | 1.60 | 0.38 | 23.44 | 24.87 | 24.89 |
| II | 0.91 | 1.57 | 0.79 | 16.10 | 17.06 | 15.92 | |
| III | 0.98 | 2.19 | 1.05 | 23.05 | 25.08 | 23.50 | |
| T. japonicus | I | 0.99 | 2.10 | 0.39 | 15.66 | 16.96 | 15.70 |
| II | 0.79 | 1.35 | 0.49 | 12.60 | 13.31 | 12.73 | |
| III | 0.97 | 2.08 | 0.92 | 10.74 | 11.64 | 11.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, X.; Zhang, K.; Li, J.; Xu, Y.; Sun, M.; Xu, S.; Cai, Y.; Qiu, Y.; Chen, Z. Stock Assessment of the Commercial Small Pelagic Fishes in the Beibu Gulf, the South China Sea, 2006–2020. Biology 2024, 13, 226. https://doi.org/10.3390/biology13040226
Hong X, Zhang K, Li J, Xu Y, Sun M, Xu S, Cai Y, Qiu Y, Chen Z. Stock Assessment of the Commercial Small Pelagic Fishes in the Beibu Gulf, the South China Sea, 2006–2020. Biology. 2024; 13(4):226. https://doi.org/10.3390/biology13040226
Chicago/Turabian StyleHong, Xiaofan, Kui Zhang, Jiajun Li, Youwei Xu, Mingshuai Sun, Shannan Xu, Yancong Cai, Yongsong Qiu, and Zuozhi Chen. 2024. "Stock Assessment of the Commercial Small Pelagic Fishes in the Beibu Gulf, the South China Sea, 2006–2020" Biology 13, no. 4: 226. https://doi.org/10.3390/biology13040226
APA StyleHong, X., Zhang, K., Li, J., Xu, Y., Sun, M., Xu, S., Cai, Y., Qiu, Y., & Chen, Z. (2024). Stock Assessment of the Commercial Small Pelagic Fishes in the Beibu Gulf, the South China Sea, 2006–2020. Biology, 13(4), 226. https://doi.org/10.3390/biology13040226








