A Preliminary Study on Quantitative Analysis of Collagen and Apoptosis Related Protein on 1064 nm Laser-Induced Skin Injury
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
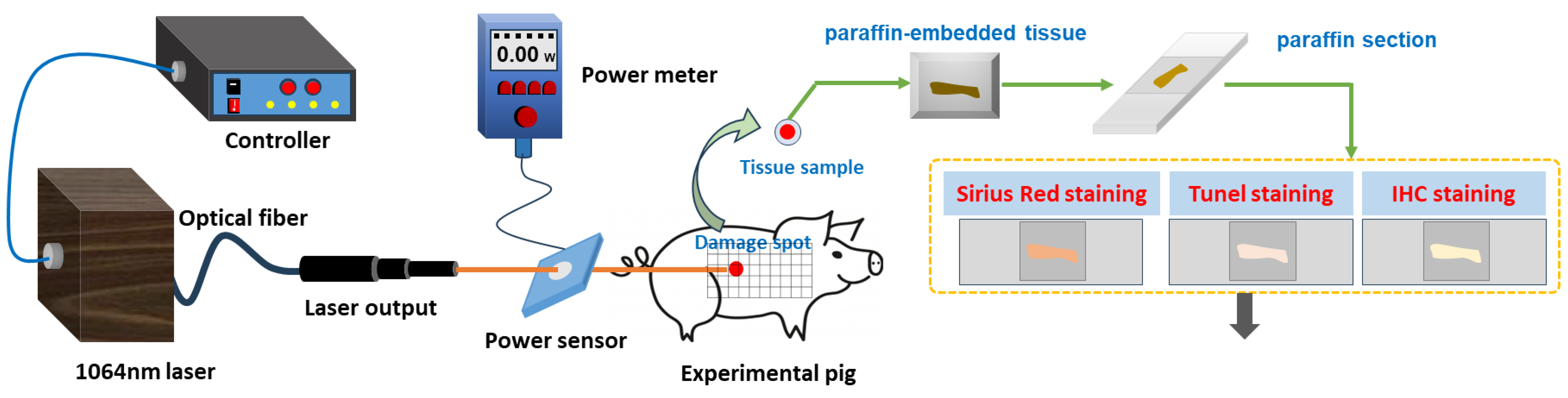
2.1. Experimental Animals
2.2. Laser Radiation Method
2.3. Method to Observe Damage Spots
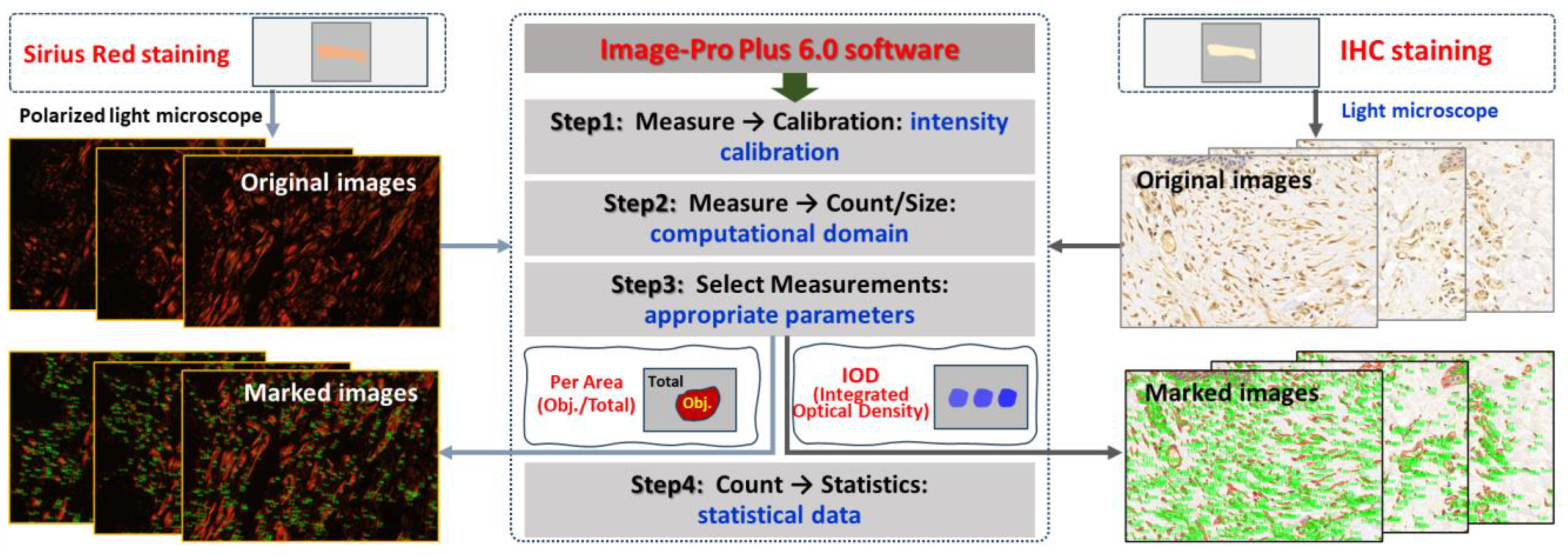
2.4. Quantitative Analysis of Collagen in Skin Wounds
2.5. Apoptosis Detection by TUNEL Staining
2.6. Immunohistochemical Detection
2.7. Statistical Analysis
3. Results
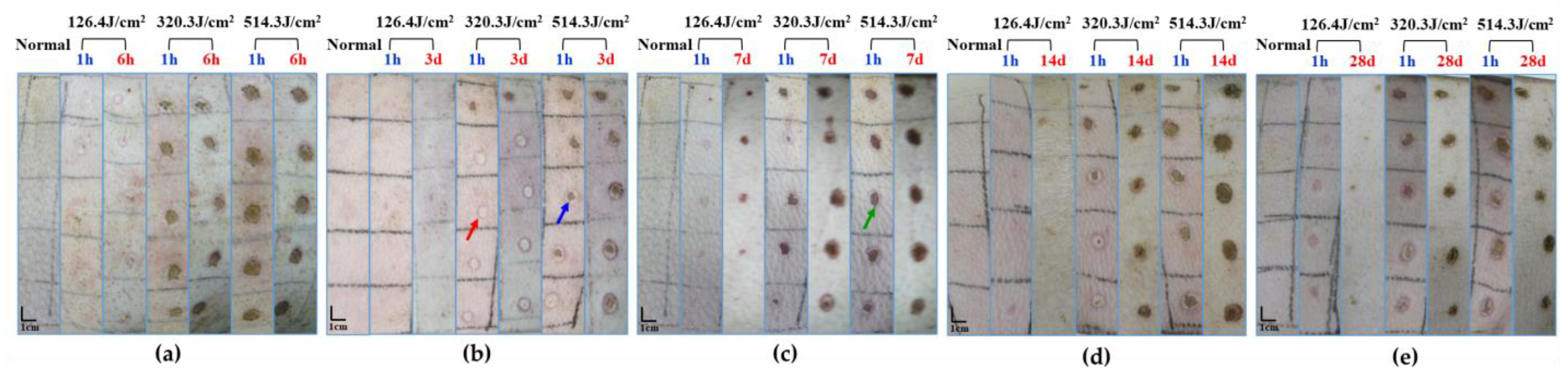
3.1. Macroscopic Observation of the Wound Healing
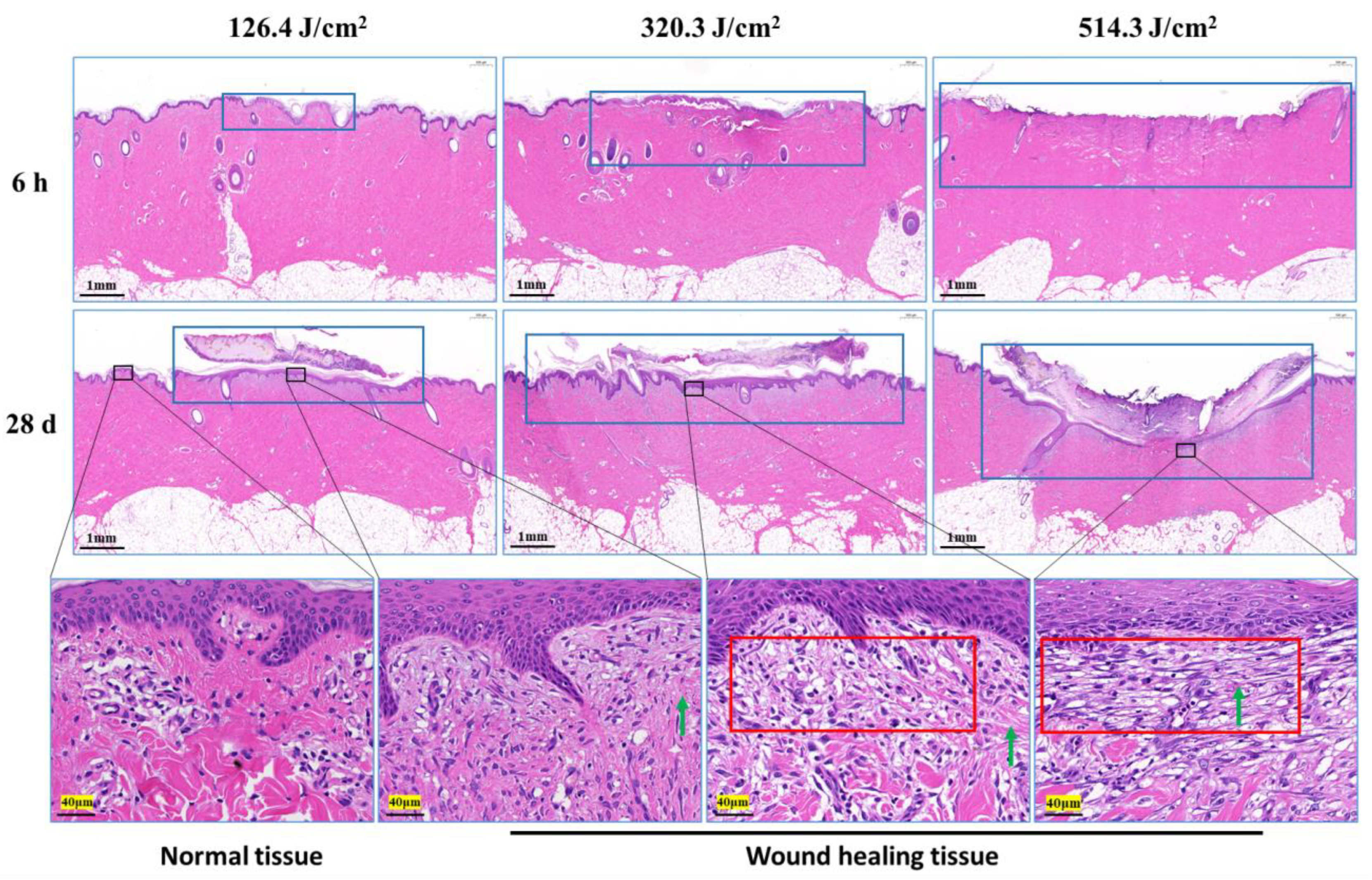
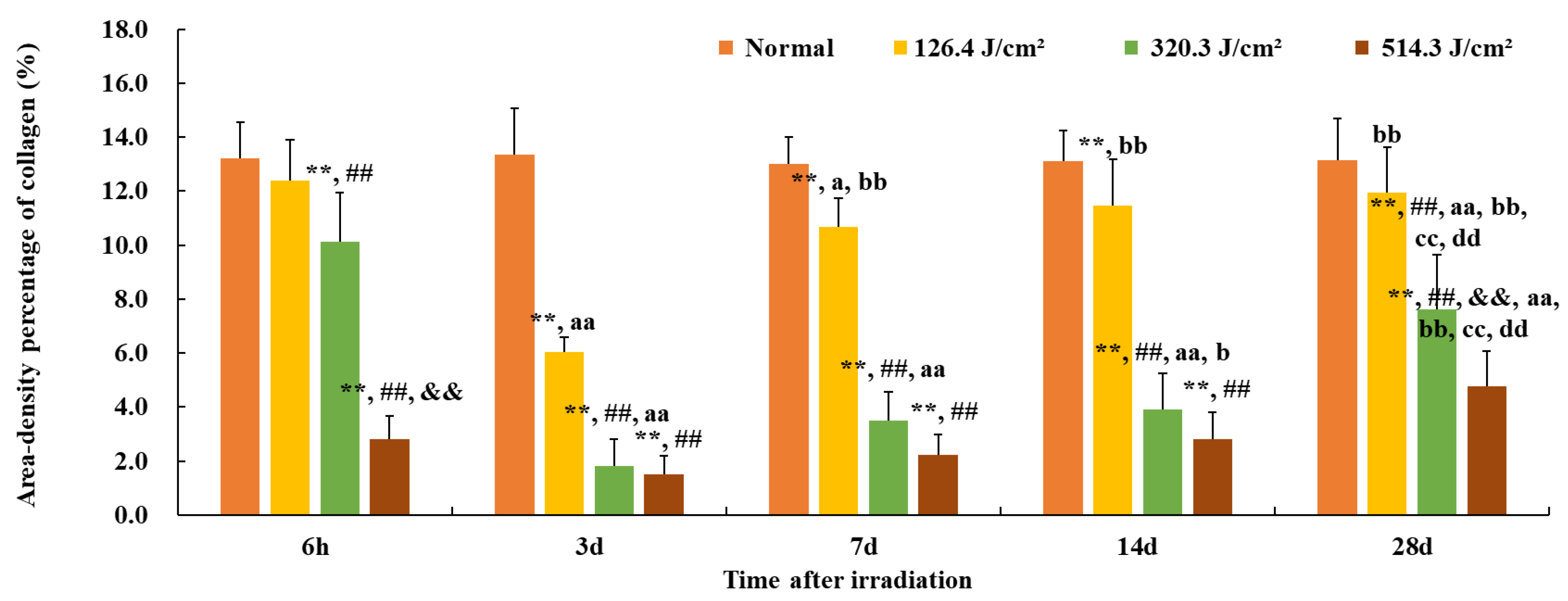
3.2. Structural and Quantitative Analysis of Collagen in the Skin Wounds after Irradiation
3.3. Apoptosis Analysis of Radiation Lesions
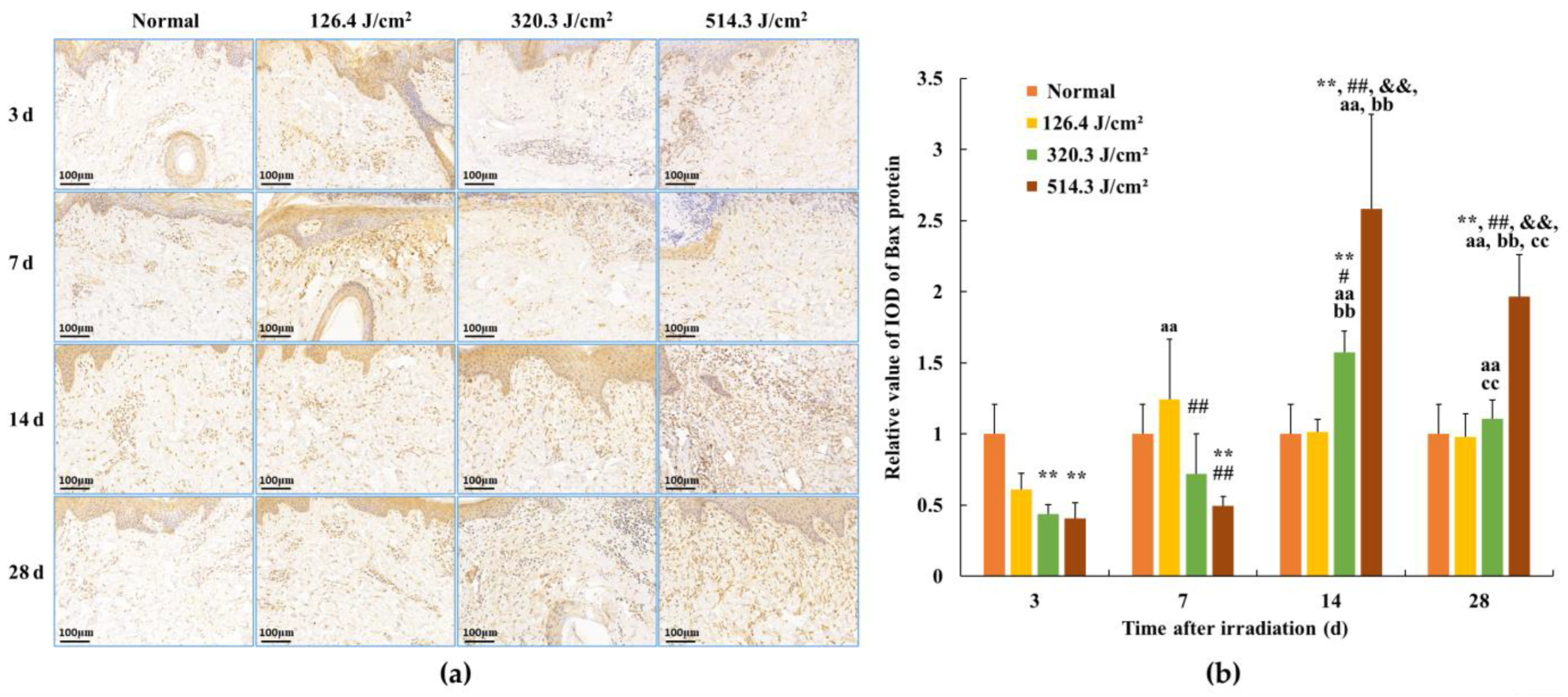
3.4. Expression of Bax Protein in Skin Wounds after Irradiation
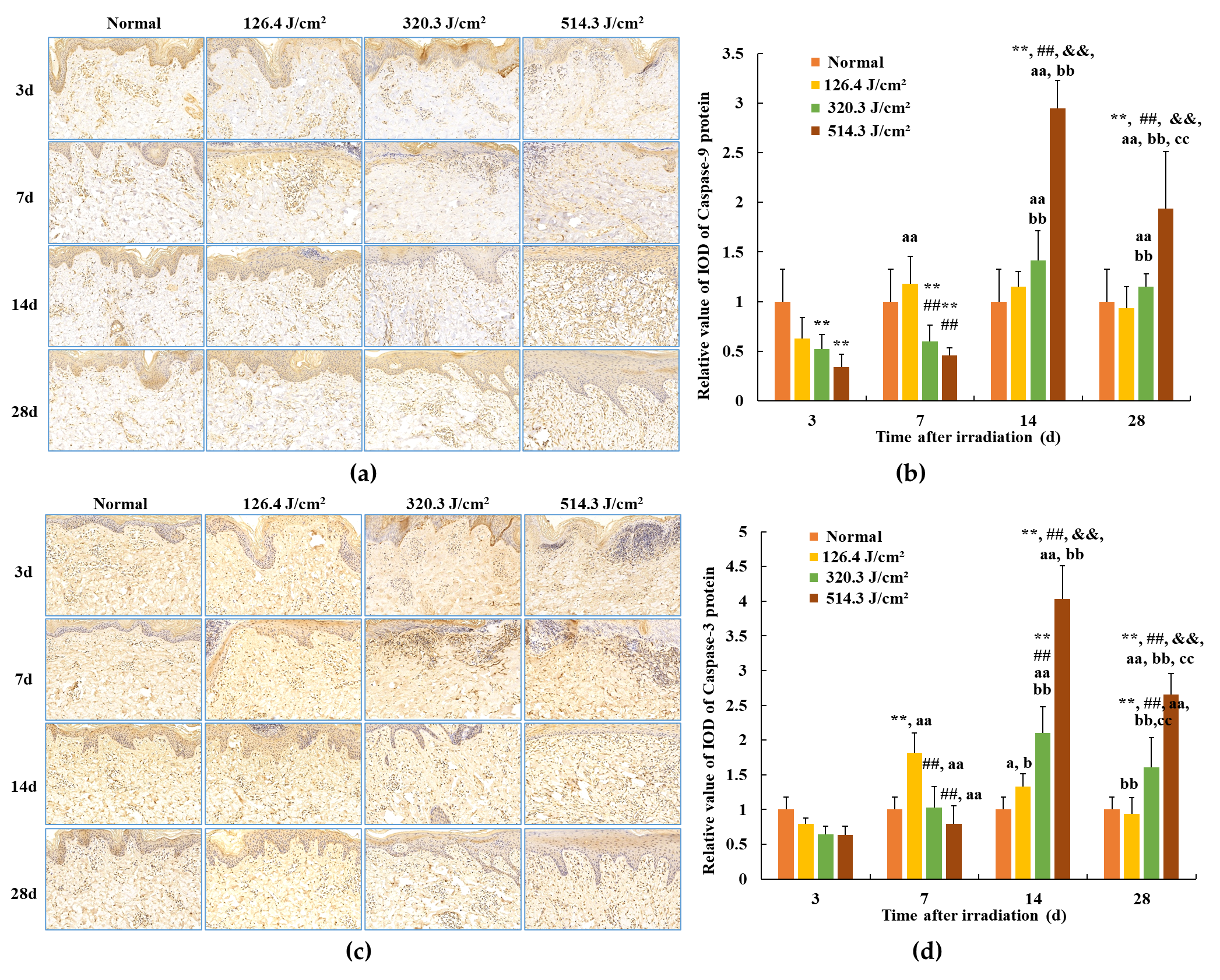
3.5. Expression of Caspase Proteins in Skin Wounds after Irradiation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaub, L.; Schmitz, C. Comparison of the penetration depth of 905 nm and 1064 nm laser light in surface layers of biological tissue ex vivo. Biomedicines 2023, 11, 1355. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Xu, J.; Wang, Z.; Yang, N.; Li, X.; Qian, Y.; Li, G.; Dai, R.; Xu, S. Penetrating effect of high-intensity infrared laser pulses through body tissue. RSC Adv. 2018, 8, 32344–32357. [Google Scholar] [CrossRef] [PubMed]
- Penberthy, W.T.; Vorwaller, C.E. Utilization of the 1064 nm wavelength in photobiomodulation: A systematic review and meta-analysis. J. Lasers Med. Sci 2021, 12, e86. [Google Scholar] [CrossRef] [PubMed]
- Majdabadi, A.; Abazari, M. Study of interaction of laser with tissue using monte carlo method for 1064 nm Neodymium-Doped Yttrium Aluminium Garnet (Nd:YAG) laser. J. Lasers Med. Sci. 2015, 6, 22–27. [Google Scholar] [PubMed]
- Husain, Z.; Alster, T.S. The role of lasers and intense pulsed light technology in dermatology. Clin. Cosmet. Investig. Dermatol. 2016, 9, 29–40. [Google Scholar] [PubMed]
- Kono, T.; Chan, H.H.L.; Groff, W.F.; Imagawa, K.; Hanai, U.; Akamatsu, T. Prospective comparison study of 532/1064 nm picosecond laser vs 532/1064 nm nanosecond laser in the treatment of professional tattoos in Asians. Laser Ther. 2020, 29, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Mengli Zhang, M.; Huang, Y.; Wu, Q.; Lin, T.; Gong, X.; Chen, H.; Wang, Y. Comparison of 1064-nm and dual-wavelength (532/1064-nm) picosecond-domain Nd:YAG lasers in the treatment of facial photoaging: A randomized controlled split-face study. Lasers Surg Med. 2021, 53, 1135–1295. [Google Scholar]
- Hammoda, T.M.; Ahmed, N.A.; Hamdino, M. Fractional CO2 laser versus 1064-nm long-pulsed Nd:YAG laser for inflammatory acne vulgaris treatment: A randomized clinical trial. Lasers Med. Sci. 2023, 38, 187. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Srishti, S.; Periyasamy, V.; Pramanik, M. Photoacoustic imaging depth cmparison at 532-, 800-, and 1064-nm wavelengths: Monte Carlo simulation and experimental validation. J. Biomed. Opt. 2019, 24, 121904. [Google Scholar] [CrossRef]
- Polanyi, T.G.; Bredemeier, H.C.; Davis, T.W., Jr. A CO2 laser for surgical research. Med. Biol. Eng. 1970, 8, 541–548. [Google Scholar] [CrossRef]
- Dang, Y.Y.; Ren, Q.S.; Liu, H.X.; Ma, J.B.; Zhang, J.S. Comparison of histologic, biochemical, and mechanical properties of murine skin treated with the 1064-nm and 1320-nm Nd: YAG lasers. Exp. Dermatol. 2005, 14, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, G.M.; Park, Y.L.; Lee, J.; Whang, K.U.; Lee, S. Comparison of wound repair after irradiation of rat skin with 1064 nm Nd: YAG, CO2, and Er: YAG lasers. Korean J. Dermatol. 2014, 52, 244–251. [Google Scholar]
- Karmisholt, K.E.; Taudorf, E.H.; Wulff, C.B.; Wenande, E.; Philipsen, P.A.; Haedersdal, M. Fractional CO2 laser treatment of caesarean section scars-A randomized controlled split-scar trial with long term follow-up assessment. Lasers Surg. Med. 2016, 49, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Champion, J. Laser safety management. Br. J. Perioper. Nurs. 2000, 10, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Lu, C.W.; Hu, X.; Zhou, D.D. A case of accidental retinal injury by cosmetic laser. Eye 2014, 28, 906–907. [Google Scholar] [CrossRef] [PubMed]
- Elgarhy, L.H.; EI-Tatawy, R.A.; Abdelaziz, D.; Dogheim, N.N. Pulsed dye laser versus ablative fractional CO2 laser in treatment of old hypertrophic scars: Clinicopathological study. Wound Repair Regen. 2021, 29, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Barkana, Y.; Belkin, M. Laser eye injurie. Surv. Ophthalmol. 2000, 44, 459–478. [Google Scholar] [CrossRef]
- Ahluwalia, J.; Avram, M.M.; Ortiz, A.E. Lasers and energy-based devices marketed for vaginal rejuvenation: A cross-sectional analysis of the MAUDE database. Lasers Surg. Med. 2019, 51, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, J.N.; Kelly, K.M.; Holmes, J.D.; Zachary, C.B. Assessing the outcomes of focused heating of the skin by a long-pulsed 1064 nm laser with an integrated scanner, infrared thermal guidance, and optical coherence tomography. Lasers Surg. Med. 2021, 53, 806–814. [Google Scholar] [CrossRef]
- Leight-Dunn, H.; Chima, M.; Hoss, E. Wound healing treatments after ablative laser skin resurfacing: A review. J. Drugs Dermatol. 2020, 19, 1050–1055. [Google Scholar] [CrossRef]
- Glatter, R.D.; Goldberg, J.S.; Schomacker, K.T.; Compton, C.C.; Flotte, T.J.; Bua, D.P.; Greaves, K.W.; Nishioka, N.S.; Sheridan, R.L. Carbon dioxide laser ablation with immediate autografting in a full-thickness porcine burn model. Ann. Surg. 1998, 228, 257–265. [Google Scholar] [CrossRef]
- Baleg, S.M.; Bidin, N.; Suan, L.P.; Ahmad, M.F.; Krishnan, G.; Johari, A.R.; Hamid, A. The effect of CO2 laser treatment on skin tissue. J. Cosmet. Dermatol. 2015, 14, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Akhalaya, M.Y.; Maksimov, G.V.; Rubin, A.B.; Lademann, J.; Darvin, M.E. Molecular action mechanisms of solar infrared radiation and heat on human skin. Ageing Res. Rev. 2014, 16, 1–11. [Google Scholar] [CrossRef]
- Martin, P.; Nunan, R. Cellular and molecular mechanisms of repair in acute and chronic wound healing. Br. J. Dermatol. 2015, 173, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Martin, P.; Tomic-Canic, M. Wound repair and regeneration: Mechanisms, signaling, and translation. Sci. Transl. Med. 2014, 6, 265sr6. [Google Scholar] [CrossRef] [PubMed]
- Crafts, T.D.; Jensen, A.R.; Blocher-Smith, E.C.; Markel, T.A. Vascular endothelial growth factor: Therapeutic possibilities and challenges for the treatment of ischemia. Cytokine 2015, 71, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Tsai, Y.C.; Korivi, M.; Chang, C.T.; Hseu, Y.C. Lucidone promotes the cutaneous wound healing process via activation of the PI3K/AKT, Wnt/β-catenin and NF-κB signaling pathways. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 151–168. [Google Scholar] [CrossRef]
- Chen, P.Y.; Qin, L.F.; Baeyens, N.; Li, G.; Afolabi, T.; Budatha, M.; Tellides, G.; Schwartz, M.A.; Simons, M. Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Investig. 2015, 125, 4514–4528. [Google Scholar] [CrossRef]
- Lagares, D.; Santos, A.; Grasberger, P.E.; Liu, F.; Probst, C.K.; Rahimi, R.A.; Sakai, N.; Kuehl, T.; Ryan, J.; Bhola, P.; et al. Targeted apoptosis of myofibroblasts with the BH3 mimetic ABT-263 reverses established fibrosis. Sci. Transl. Med. 2017, 9, eaal3765. [Google Scholar] [CrossRef]
- Zhao, J.C.; Zhang, B.R.; Hong, L.; Shi, K.; Wu, W.W.; Yu, J.A. Extracorporeal shock wave therapy with low-energy flux density inhibits hypertrophic scar formation in an animal model. Int. J. Mol. Med. 2018, 41, 1931–1938. [Google Scholar] [CrossRef]
- Midwood, K.S.; Williams, L.V.; Schwarzbauer, J.E. Tissue repair and the dynamics of the extracellular matrix. Int. J. Biochem. Cell Biol. 2004, 36, 1031–1037. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Korting, H.C.; Pavicic, T.; Ruzicka, T.; Jeschke, M.G. Hypertrophic scarring and keloids: Pathomechanisms and current and emerging treatment strategies. Mol. Med. 2011, 17, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Susanto, O.; Koh, Y.W.H.; Morrice, N.; Tumanov, S.; Thomason, P.A.; Nielson, M.; Tweedy, L.; Muinonen-Martin, A.J.; Kamphorst, J.J.; Mackay, G.M.; et al. LPP3 mediates self-generation of chemotactic LPA gradients by melanoma cells. J. Cell Sci. 2017, 130, 3455–3466. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.L.; Manhas, N.; Raghubir, R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res. Rev. 2007, 54, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; Santamaría, B.; Ruiz-Ortega, M.; Egido, J.; Ortiz, A. Mechanisms of renal apoptosis in health and disease. J. Am. Soc. Nephrol. 2008, 19, 1634–1642. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Ma, Q.; Liang, J.; Lu, Y.; Ni, B.; Luo, Z.; Cui, Y.; Kang, H. Quantitative and qualitative evaluation of recovery process of a 1064 nm laser on laser-induced skin injury: In vivo experimental research. Laser Phys. Lett. 2019, 16, 115604. [Google Scholar] [CrossRef]
- Ma, Q.; Fan, Y.; Luo, Z.; Cui, Y.; Kang, H. Quantitative analysis of collagen and capillaries of 3.8 μm laser-induced cutaneous thermal injury and wound healing. Lasers Med. Sci. 2021, 36, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Smatlikova, P.; Askeland, G.; Vaskovicova, M.; Klima, J.; Motlik, J.; Eide, L.; Ellederová, Z. Age-related oxidative changes in primary porcine fibroblasts expressing mutated huntingtin. Neurodegener. Dis. 2019, 19, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Foubert, P.; Doyle-Eisele, M.; Gonzalez, A.; Berard, F.; Weber, W.; Zafra, D.; Alfonso, Z.; Zhao, S.; Tenenhaus, M.; Fraser, J.K. Development of a combined radiation and full thickness burn injury minipig model to study the effects of uncultured adipose-derived regenerative cell therapy in wound healing. Int. J. Radiat. Biol. 2017, 93, 340–350. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.K.; Adli, D.H.; Tumiran, M.A.; Abdulla, M.A.; Yusoff, K.M. The efficacy of gelam honey dressing towards excisional wound healing. Evid. Based Complement. Altern. Med. 2012, 2012, 805932. [Google Scholar] [CrossRef]
- Sorg, H.; Tilkorn, D.J.; Hager, S.; Hauser, J.; Mirastschijski, U. Skin wound healing: An update on the current knowledge and concepts. Eur. Surg. Res. 2017, 58, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Ruan, J.; Xiao, R.; Zhang, Q.; Huang, Y.S. Comparative study of 1,064-nm laser-induced skin burn and thermal skin burn. Cell Biochem. Biophys. 2013, 67, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dang, Y.; Wang, Z.; Chai, X.; Ren, Q. Laser induced collagen remodeling: A comparative study in vivo on mouse model. Lasers Surg. Med. 2008, 40, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.F.; Xia, G.Y.; Fu, X.B.; Yang, H.; Peng, R.Y.; Zhang, Y.; Gu, Q.Y.; Gao, Y.B.; Cui, X.M.; Hu, W.H. Relationship between expression of Bax and Bcl-2 proteins and apoptosis in radiation compound wound healing of rats. Chin. J. Traumatol. 2003, 6, 135–138. [Google Scholar] [PubMed]
- Shang, N.; Bank, T.; Ding, X.; Breslin, P.; Li, J.; Shi, B.; Qiu, W. Caspase-3 suppresses diethylnitrosamine-induced hepatocyte death, compensatory proliferation and hepatocarcinogenesis through inhibiting p38 activation. Cell Death Dis. 2018, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Zhang, Y.; Yu, B.; Yang, Y.; Li, B.; Wu, J. Oocyte-G1 promotes male germ cell apoptosis through activation of Caspase-3. Gene 2018, 670, 22–30. [Google Scholar] [CrossRef]
- Kutscher, L.M.; Shaham, S. Non-apoptotic cell death in animal development. Cell Death Differ. 2017, 24, 1326–1336. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Fan, Y.; Cui, Y.; Luo, Z.; Kang, H. A Preliminary Study on Quantitative Analysis of Collagen and Apoptosis Related Protein on 1064 nm Laser-Induced Skin Injury. Biology 2024, 13, 217. https://doi.org/10.3390/biology13040217
Ma Q, Fan Y, Cui Y, Luo Z, Kang H. A Preliminary Study on Quantitative Analysis of Collagen and Apoptosis Related Protein on 1064 nm Laser-Induced Skin Injury. Biology. 2024; 13(4):217. https://doi.org/10.3390/biology13040217
Chicago/Turabian StyleMa, Qiong, Yingwei Fan, Yufang Cui, Zhenkun Luo, and Hongxiang Kang. 2024. "A Preliminary Study on Quantitative Analysis of Collagen and Apoptosis Related Protein on 1064 nm Laser-Induced Skin Injury" Biology 13, no. 4: 217. https://doi.org/10.3390/biology13040217
APA StyleMa, Q., Fan, Y., Cui, Y., Luo, Z., & Kang, H. (2024). A Preliminary Study on Quantitative Analysis of Collagen and Apoptosis Related Protein on 1064 nm Laser-Induced Skin Injury. Biology, 13(4), 217. https://doi.org/10.3390/biology13040217






