Enhanced TfR1 Recognition of Myocardial Injury after Acute Myocardial Infarction with Cardiac Fibrosis via Pre-Degrading Excess Fibrotic Collagen
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Characterization of HSA-C
2.2. Synthesis of CI
2.3. Characterization of HSA-C and CI
2.4. Cell Counting Kit 8 (CCK8) Testing for Cytotoxicity
2.5. Ethics
2.6. MI Animal Model Establishment
2.7. Western Blot
2.8. Masson Staining
2.9. NIR Fluorescence Imaging
2.10. Statistical Analysis
3. Results
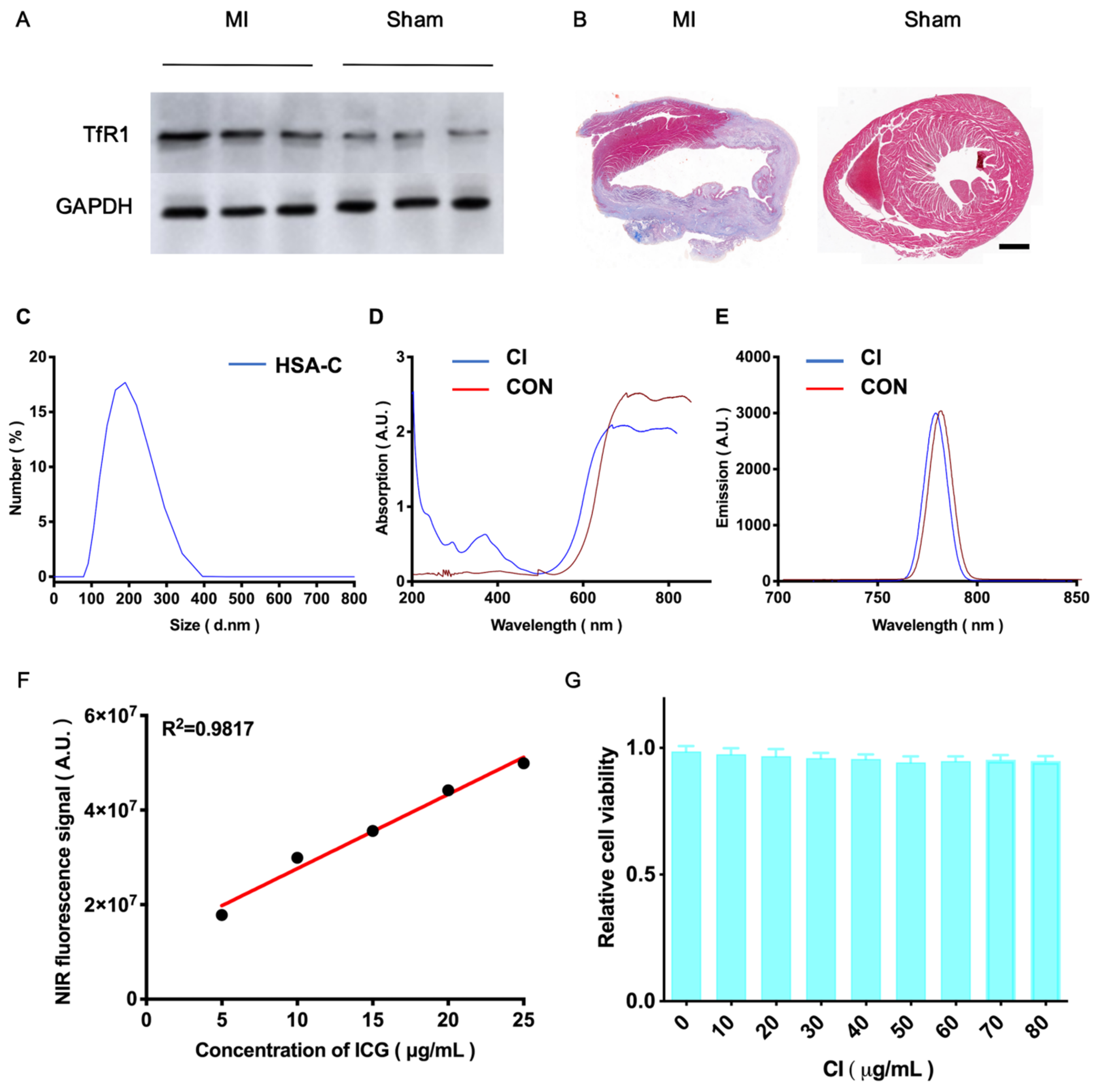
3.1. HSA-C and CI’s Synthesis and Characterization
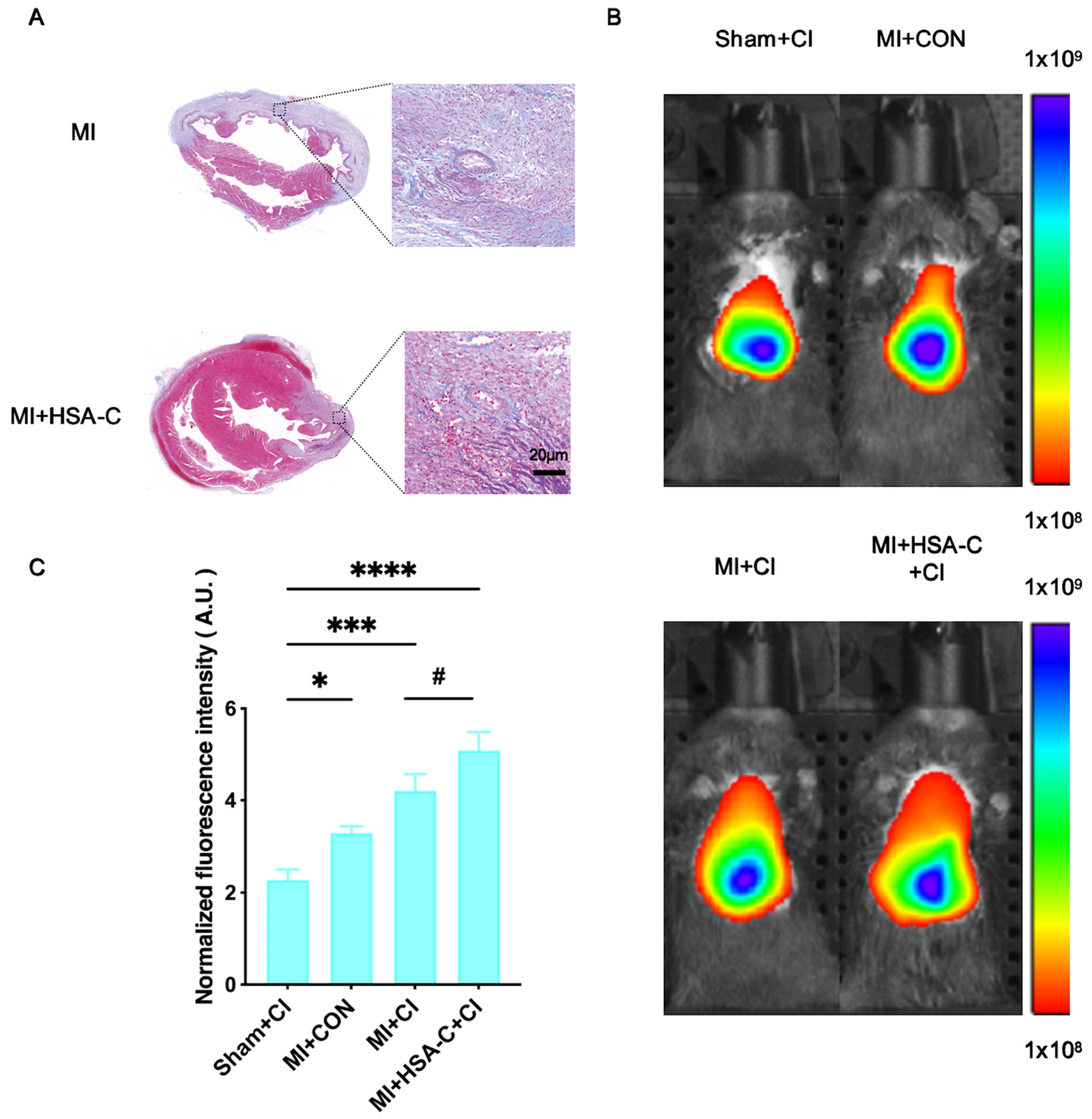
3.2. NIR Fluorescence Imaging in the MI Mouse Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghagolzadeh, P.; Plaisance, I.; Bernasconi, R.; Treibel, T.A.; Quetglas, C.P.; Wyss, T.; Wigger, L.; Nemir, M.; Sarre, A.; Chouvardas, P.; et al. Assessment of the Cardiac Noncoding Transcriptome by Single-Cell RNA Sequencing Identifies FIXER, a Conserved Profibrogenic Long Noncoding RNA. Circulation 2023, 148, 778–797. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. Pathophysiology of Myocardial Infarction. Compr. Physiol. 2015, 5, 1841–1875. [Google Scholar] [CrossRef]
- Querejeta, R.; López, B.; González, A.; Sánchez, E.; Larman, M.; Ubago, J.L.M.; Díez, J. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: Relation to myocardial fibrosis. Circulation 2004, 110, 1263–1268. [Google Scholar] [CrossRef]
- Snider, J.C.; Riley, L.A.; Mallory, N.T.; Bersi, M.R.; Umbarkar, P.; Gautam, R.; Zhang, Q.; Mahadevan-Jansen, A.; Hatzopoulos, A.K.; Maroteaux, L.; et al. Targeting 5-HT 2B Receptor Signaling Prevents Border Zone Expansion and Improves Microstructural Remodeling After Myocardial Infarction. Circulation 2021, 143, 1317–1330. [Google Scholar] [CrossRef] [PubMed]
- Bengel, F.M.; Diekmann, J.; Hess, A.; Jerosch-Herold, M. Myocardial Fibrosis: Emerging Target for Cardiac Molecular Imaging and Opportunity for Image-Guided Therapy. J. Nucl. Med. 2023, 64 (Suppl. S2), 49S–58S. [Google Scholar] [CrossRef] [PubMed]
- Otto, G. Regenerative medicine: Stem cell-derived cardiomyocytes heal a broken heart. Nat. Rev. Drug Discov. 2018, 17, 622. [Google Scholar] [CrossRef]
- Barton, A.K.; Tzolos, E.; Bing, R.; Singh, T.; Weber, W.; Schwaiger, M.; Varasteh, Z.; Slart, R.H.J.A.; Newby, D.E.; Dweck, M.R. Emerging molecular imaging targets and tools for myocardial fibrosis detection. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 261–275. [Google Scholar] [CrossRef]
- Gupta, S.; Ge, Y.; Singh, A.; Gräni, C.; Kwong, R.Y. Multimodality Imaging Assessment of Myocardial Fibrosis. JACC Cardiovasc. Imaging 2021, 14, 2457–2469. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ardehali, H.; Min, J.; Wang, F. The molecular and metabolic landscape of iron and ferroptosis in cardiovascular disease. Nat. Rev Cardiol. 2023, 20, 7–23. [Google Scholar] [CrossRef]
- Li, N.; Jiang, W.; Wang, W.; Xiong, R.; Wu, X.; Geng, Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol. Res. 2021, 166, 105466. [Google Scholar] [CrossRef]
- Chen, L.; Ma, Y.; Ma, X.; Liu, L.; Jv, X.; Li, A.; Shen, Q.; Jia, W.; Qu, L.; Shi, L.; et al. TFEB regulates cellular labile iron and prevents ferroptosis in a TfR1-dependent manner. Free Radic. Biol. Med. 2023, 208, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Han, H.; Chen, F.; Cheng, L.; Ma, C.; Huang, H.; Chen, C.; Li, H.; Cai, H.; Huang, H.; et al. Amelioration of acute myocardial infarction injury through targeted ferritin nanocages loaded with an ALKBH5 inhibitor. Acta Biomater. 2022, 140, 481–491. [Google Scholar] [CrossRef]
- SNMMI Image of the Year 2022: PET/CT Biomarker Predicts Post-MI Cardiac Remodeling. J. Nucl. Med. 2022, 63, 16N.
- van den Bos, E.J.; Baks, T.; Moelker, A.D.; Kerver, W.; van Geuns, R.-J.; van der Giessen, W.J.; Duncker, D.J.; Wielopolski, P.A. Magnetic resonance imaging of haemorrhage within reperfused myocardial infarcts: Possible interference with iron oxide-labelled cell tracking? Eur. Heart J. 2006, 27, 1620–1626. [Google Scholar] [CrossRef]
- Desai, N.D.; Moussa, F.; Singh, S.K.; Chu, P.; Fremes, S.E. Intraoperative fluorescence angiography to determine the extent of injury after penetrating cardiac trauma. J. Thorac. Cardiovasc. Surg. 2008, 136, 218–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhuo, J.; Wang, Y.; Hui, H.; Li, C.; Yang, J.; Zhang, P.; Fang, C.; Tian, J. Enhanced glypican-3-targeted identification of hepatocellular carcinoma with liver fibrosis by pre-degrading excess fibrotic collagen. Acta Biomater. 2023, 158, 435–448. [Google Scholar] [CrossRef]
- Du, Y.; Gao, J.; Zhang, H.; Meng, X.; Qiu, D.; Gao, X.; Zheng, A. Brain-targeting delivery of MMB4 DMS using carrier-free nanomedicine CRT-MMB4@MDZ. Drug Deliv. 2021, 28, 1822–1835. [Google Scholar] [CrossRef]
- Sahebkar, A.; Foroutan, Z.; Katsiki, N.; Jamialahmadi, T.; Mantzoros, C.S. Ferroptosis, a new pathogenetic mechanism in cardiometabolic diseases and cancer: Is there a role for statin therapy? Metabolism 2023, 146, 155659. [Google Scholar] [CrossRef]
- Zhou, R.-P.; Chen, Y.; Wei, X.; Yu, B.; Xiong, Z.-G.; Lu, C.; Hu, W. Novel insights into ferroptosis: Implications for age-related diseases. Theranostics 2020, 10, 11976–11997. [Google Scholar] [CrossRef]
- Ryan, S.K.; Ugalde, C.L.; Rolland, A.-S.; Skidmore, J.; Devos, D.; Hammond, T.R. Therapeutic inhibition of ferroptosis in neurodegenerative disease. Trends Pharmacol. Sci. 2023, 44, 674–688. [Google Scholar] [CrossRef]
- Lillo-Moya, J.; Rojas-Solé, C.; Muñoz-Salamanca, D.; Panieri, E.; Saso, L.; Rodrigo, R. Targeting Ferroptosis against Ischemia/Reperfusion Cardiac Injury. Antioxidants 2021, 10, 667. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Neuhauser, H.; Ellert, U.; Berger, K. The role of the inflammatory markers ferritin, transferrin and fibrinogen in the relationship between major depression and cardiovascular disorders—The German Health Interview and Examination Survey. Acta Psychiatr. Scand. 2010, 121, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Stack, A.G.; Mutwali, A.I.; Nguyen, H.T.; Cronin, C.; Casserly, L.; Ferguson, J. Transferrin saturation ratio and risk of total and cardiovascular mortality in the general population. QJM 2014, 107, 623–633. [Google Scholar] [CrossRef]
- Nikkari, S.T.; Koivu, T.A.; Anttila, P.; Raunio, I.; Sillanaukee, P. Carbohydrate-deficient transferrin and gamma-glutamyltransferase are inversely associated with lipid markers of cardiovascular risk. Eur. J. Clin. Investig. 1998, 28, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, X.; Tian, Y.; Zou, J.; Wang, F.; Chen, X. Fluorescence Imaging-Incorporated Transcriptome Study of Glutathione Depletion-Enhanced Ferroptosis Therapy via Targeting Gold Nanoclusters. ACS Appl. Mater. Interfaces 2023, 15, 6385–6396. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.L.; Liu, F.; Li, N.; Fu, Q.; Wang, C.; Yang, S.; Xiao, H.; Tang, L.; Wang, F.; Zhou, W.; et al. Trisulfide Bond-Mediated Molecular Phototheranostic Platform for “Activatable” NIR-II Imaging-Guided Enhanced Gas/Chemo-Hypothermal Photothermal Therapy. Adv. Sci. 2023, 10, e2304104. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Wang, Y.; Fu, C.; Li, C.; Feng, F.; Li, H.; Tan, L.; Qu, H.; Hui, H.; Wang, J.; et al. Quantitative visualization of myocardial ischemia-reperfusion-induced cardiac lesions via ferroptosis magnetic particle imaging. Theranostics 2024, 14, 1081–1097. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, S.; Lai, M.; Ji, X.; Ye, Y.; Tang, J.; Liu, X.; Zhao, M. Fluorescent probes for lighting up ferroptotic cell death: A review. Talanta 2023, 260, 124628. [Google Scholar] [CrossRef]
- Zhou, R.; Liu, Y.; Wang, Z.; Lv, J.; Liao, W.; Shen, Z.; Rong, X. Nanoparticle-Based MRI-Guided Tumor Microenvironment Heating via the Synergistic Effect of Ferroptosis and Inhibition of TGF-β Signaling. Adv. Healthc. Mater. 2023, 12, e2300176. [Google Scholar] [CrossRef]
- Zeng, F.; Nijiati, S.; Liu, Y.; Yang, Q.; Liu, X.; Zhang, Q.; Chen, S.; Su, A.; Xiong, H.; Shi, C.; et al. Ferroptosis MRI for early detection of anticancer drug-induced acute cardiac/kidney injuries. Sci. Adv. 2023, 9, eadd8539. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Wang, Y.; Li, H.; Liao, F.; Peng, Y.; Lu, A.; Tan, L.; Qu, H.; Long, L.; Fu, C. Enhanced TfR1 Recognition of Myocardial Injury after Acute Myocardial Infarction with Cardiac Fibrosis via Pre-Degrading Excess Fibrotic Collagen. Biology 2024, 13, 213. https://doi.org/10.3390/biology13040213
Yang W, Wang Y, Li H, Liao F, Peng Y, Lu A, Tan L, Qu H, Long L, Fu C. Enhanced TfR1 Recognition of Myocardial Injury after Acute Myocardial Infarction with Cardiac Fibrosis via Pre-Degrading Excess Fibrotic Collagen. Biology. 2024; 13(4):213. https://doi.org/10.3390/biology13040213
Chicago/Turabian StyleYang, Wenwen, Yueqi Wang, Hongzheng Li, Feifei Liao, Yuxuan Peng, Aimei Lu, Ling Tan, Hua Qu, Linzi Long, and Changgeng Fu. 2024. "Enhanced TfR1 Recognition of Myocardial Injury after Acute Myocardial Infarction with Cardiac Fibrosis via Pre-Degrading Excess Fibrotic Collagen" Biology 13, no. 4: 213. https://doi.org/10.3390/biology13040213
APA StyleYang, W., Wang, Y., Li, H., Liao, F., Peng, Y., Lu, A., Tan, L., Qu, H., Long, L., & Fu, C. (2024). Enhanced TfR1 Recognition of Myocardial Injury after Acute Myocardial Infarction with Cardiac Fibrosis via Pre-Degrading Excess Fibrotic Collagen. Biology, 13(4), 213. https://doi.org/10.3390/biology13040213





