Simple Summary
This review mainly focuses on epigenetic changes in a few selective genes associated with insulin insensitivity in the periphery and how this peripheral insulin insensitivity is linked with impaired signaling of insulin in the brain, thus causing Alzheimer’s disease. DNA methylation and histone modifications are the focus of this review, with primary importance given to DNA methylation. Moreover, there has been a focus shift from the amyloid β hypothesis to epigenetic mechanisms. Furthermore, we discuss the advanced drug delivery systems that can be used for the delivery of drugs targeting the brain during Alzheimer’s disease. The advanced drug delivery systems discussed in this paper are nanoparticles, vesicular systems, network systems and dendrimers, hydrogel-based systems, and biologics.
Abstract
Alzheimer’s disease (AD) is a neurodegenerative condition that predominantly affects the hippocampus and the entorhinal complex, leading to memory lapse and cognitive impairment. This can have a negative impact on an individual’s behavior, speech, and ability to navigate their surroundings. AD is one of the principal causes of dementia. One of the most accepted theories in AD, the amyloid β (Aβ) hypothesis, assumes that the buildup of the peptide Aβ is the root cause of AD. Impaired insulin signaling in the periphery and central nervous system has been considered to have an effect on the pathophysiology of AD. Further, researchers have shifted their focus to epigenetic mechanisms that are responsible for dysregulating major biochemical pathways and intracellular signaling processes responsible for directly or indirectly causing AD. The prime epigenetic mechanisms encompass DNA methylation, histone modifications, and non-coding RNA, and are majorly responsible for impairing insulin signaling both centrally and peripherally, thus leading to AD. In this review, we provide insights into the major epigenetic mechanisms involved in causing AD, such as DNA methylation and histone deacetylation. We decipher how the mechanisms alter peripheral insulin signaling and brain insulin signaling, leading to AD pathophysiology. In addition, this review also discusses the need for newer drug delivery systems for the targeted delivery of epigenetic drugs and explores targeted drug delivery systems such as nanoparticles, vesicular systems, networks, and other nano formulations in AD. Further, this review also sheds light on the future approaches used for epigenetic drug delivery.
1. Introduction
AD is a progressively developing neurodegenerative condition mainly targeting the hippocampus, which involves neural circuits of memory processing and consolidation. AD pathology is characterized by intracellular neurofibrillary tangles (NT) and neuritic plaques due to excessive buildup of extraneuronal Aβ peptide in the neocortical regions and the medial temporal lobe. About one tenth of the individuals in the geriatric population beyond the age of sixty-five and four tenths of the individuals who are above eighty-five years of age are diagnosed with AD. By 2050, it is expected that the confirmed cases of AD will drastically increase [1]. AD is named after Alois Alzheimer, a German psychiatrist, who noticed the presence of an excess amount of amyloid plaques in the brain of a patient who previously suffered memory loss before death [2]. AD neuropathology involves positive and negative lesions. Positive lesions are due to the aggregation of deposits such as NT, dystrophic neurites, amyloid plaques, and other deposits in certain parts of the brains of AD patients. In contrast, negative lesions include the loss of neural, synaptic, and neuropil, leading to large atrophy [3,4,5]. A few tests for diagnosing AD for suspected patients include magnetic resonance imaging (MRI) for neurons, neurological examination, vitamin B12 deficiency, and patient examination [6,7].
AD can be categorized into early onset AD (EOAD), which is associated with cognitive impairment and develops before reaching the age of sixty-five years, and late onset AD (LOAD), associated with cognitive impairment and develops after sixty-five years of age. This stage accounts for more than 90% of confirmed AD patients [8]. The four clinical stages in AD are as follows: preclinical/presymptomatic, characterized by mild memory loss and absence of clinical signs [9,10,11]; early-stage AD, where concentration and memory loss become more frequent with slow depression [11,12]; moderate AD stage associated with severe memory loss and late-stage AD called the severe form of AD [11].
Several studies and research were conducted in view of the Aβ hypothesis to explore the etiology of AD and to find a treatment for AD. However, the conducted studies were unable to explain fully the pathogenesis of AD [13]. Waddington, in 1942, used the term “epigenetics” to illustrate the genetic control of embryonic development [14,15]. Some studies have put forward the argument that AD may not just be an advanced stage of aging but instead a disruption in the natural process of aging. Moreover, the natural process of aging may also protect against AD where epigenetics is involved [16]. Looking into the cellular level changes in an AD brain, various molecular pathways and intracellular signaling are dysregulated, which include Aβ and tau, inflammatory-immune responses, homeostasis and plasticity of synapses, and oxidative stress and their dysregulation results from genetic, environmental, and biologic interventions [17,18]. Furthermore, accumulating evidence has been pointing towards the possibility of an imbalance in epigenetic mechanisms responsible for abnormal synaptic plasticity and memory-associated genes in AD [19,20,21,22,23].
The role of insulin in maintaining glucose levels in liver, muscles, and the adipocytes, is well recognized. Recent research demonstrates that AD has been linked to aberrant brain insulin receptor signaling [24,25]. This implies the importance of comprehending how insulin/insulin receptor signaling affects neurodegeneration.
In this paper, we focus more on how epigenetic mechanisms alter the peripheral and central insulin signaling, leading to AD as well as a few important drug delivery systems which may be useful in combating the epigenetic changes responsible for AD.
2. Epigenetics
Epigenetics is the mitotically or meiotically heritable traits observed in gene functions due to alterations in the chromosome, which cannot be elucidated by modifications in DNA sequence [26,27]. Although there is no direct alteration in DNA patterns, the gene expression levels are altered due to a few epigenetic changes that include chemical modifications to the DNA bases and changes to the chromosomal structure where the packaging of DNA takes place [28]. One of the critical features of epigenetics is that they are inherited between mother and daughter cells, commonly referred to as mitotic inheritance, and between generations, commonly known as meiotic inheritance. Epigenetics explains how some organisms with identical DNA can have vast phenotype differences [29].
2.1. DNA Methylation
This is one of the most common mechanisms of epigenetic modification where a methyl group is added covalently from the S-adenyl methionine (SAM) to the cytosine-C5 position, the entire process being driven by DNA methyl transferases (Dnmts) to form 5-methylcytosine (5mC). Dnmt3b and Dnmt3a are also referred to as de novo Dnmts, which could establish new methylation patterns in unmodified DNA. At the same time, during DNA replication, Dnmt1 is involved in transferring the methylated sequence from the parent strand to the freshly produced daughter strand. As the cells approach terminal differentiation, Dnmt expression is reduced. Even though the highest levels of methylation in DNA occur in the brain, it makes up for hardly 1% of nucleic acids in the human genome [30,31]. DNA methylation was earlier thought to play the silencing role, where increased methylation levels were correlated with reduced expression [32]. Methylation of cytosines can occur at sites other than CpG and is involved in regulating the gene expression in embryonic stem cells [33]. Further, micronutrients like zinc have a significant impact on enzymes such as methionine synthase, and its deficiency may disrupt DNA methylation and histone modification [34].
2.2. Histone Modifications
Histones are those proteins to which the DNA strands wind up. These are further composed into structures called nucleosomes. The four types of histones that are important for the structural organization of DNA are H2A, H2B, H3, and H4 [35]. Histone modifications include a wide variety of modifications that happen after the translation process and regulate the chromatin structure. These include methylation, ubiquitination, phosphorylation, and acetylation [36]. The ‘histone code hypothesis’ states that DNA transcription is primarily controlled by a combination of post-translational modifications to the histone proteins [37].
Histones organize themselves as a unit of eight molecules called octamers. The DNA, which is negatively charged, winds around the positively charged histone octamer to form a nucleosome. The nucleosome contains one hundred and forty-seven base pairs of DNA associated with an octameric histone protein core. The two sets of chromatin are euchromatin and heterochromatin. Euchromatins are responsive to transcription, whereas heterochromatins are compact structures that are not transcribed [35,38].
Histones are protein structures consisting of an N-terminus and a C-terminus, comprising approximately thirty percent of the total mass of the histone protein. The histone tails protrude out from the chromatin surface, providing a higher surface area for chemical modifications to take place. These tails consist of the amino acid lysine, which supports the chemical modifications of methylation and acetylation. Histone methylation occurs when a -CH3 group is covalently added to the lysine residue and has a repressive action, leading to a heterochromatin state. In contrast, histone acetylation occurs when an acetyl group is added to the ε-amino lysine residue in H3 and H4 tails, which results in a euchromatin state [35,39]. Histone acetyltransferases (HAT) and its co-factors, such as CREB binding protein (CBP), GNAT, MYST, and p300, are recruited by DNA sequence-specific transcription factors that identify and target those regions of chromatin where acetylation takes place [40].
2.3. Non-Coding RNA
Non-coding RNA (ncRNA) is an epigenetic mechanism whereby a biologically active RNA molecule has undergone transcription but does not get translated or encode a protein [41]. ncRNAs include dicer-dependent microRNAs (miRNA) and short-interfering RNAs (siRNA), shaped by RNA interference pathways [42]. Literature suggests the presence of microRNAs in astrocytes that regulate glutamatergic and inflammatory signaling, which affect synaptic plasticity [43]. siRNAs include nucleotides less than thirty in number, while long non-coding RNAs (lncRNA) include more than two hundred nucleotides [44]. Apart from their role in gene silencing, reports suggest their role in DNA methylation and histone modifications.
3. Insulin and Epigenetic Mechanisms
Insulin is an anabolic hormone released by the pancreatic β-cells present which gets activated with the rise in glucose levels in the blood. The blood glucose level is reduced by insulin by boosting the uptake of glucose and favoring glycolysis in skeletal and adipose tissues. GLUT4 is a glucose transporter that does not require ATP and mediates glucose uptake from the blood. This kind of transfer is classified as facilitated diffusion [45,46]. Insulin also enhances the process of glycogen synthesis in skeletal muscle, adipose tissue, and liver [47]. Within the insulin promoter Cis-acting regulatory elements have demonstrated the ability to bind to a wide variety of transcription factors. Among these, cyclic adenosine monophosphate (cAMP) responsive element (CRE) is one of the regulatory elements that have the capability to bind to a wide array of transcription factors [48]. There are four CREs in humans, while in the case of rodents, only one site of CRE exists. Among these, CRE2 remains the only CRE conserved within humans and rodent species. cAMP responsive binding protein-1 (CREB-1) is a CRE-associated transcription factor having an inhibitory effect on transcription while activating transcription factor-2 (ATF-2), as the name suggests, activating transcription. Studies report that CRE2 mutation inhibits the ATF-2 effect and plays a significant part in gene regulation [48,49].
Furthermore, studies have shown that there are nine CpG sites at regions upstream to the transcription start site (TSS) in human insulin (INS) promoters, whereas there are three CpG sites upstream to the transcription site of mouse Ins2 promoters. Detailed studies showed unmethylated CpG sites in β-cells of human INS and mouse Ins2, in contrast to the other tissues. These methylated CpG sites were shown to be responsible for suppressing ninety percent expression of insulin promoter-driven reporter genes [50].
More studies were conducted to evaluate how methylation events impact the overall expression of the insulin gene. The CpG sites that appeared in the mouse Ins2 promoters were methylated individually, as a result of which insulin promoter activity was suppressed by fifty percent. These results prove that along with methylation, there may be other mechanisms contributing to suppressing the insulin gene expression. Upon further analysis, it was observed that the methylation of CRE CpG hindered the in vivo interaction of CREB and ATF-2 with Ins2 in NIT-1 cells and increased the interaction of methyl CpG binding protein 2, thus decreasing the expression of insulin gene [50]. In vitro studies in Ins2 embryonic stem cells showed that initial methylation occurs in the insulin gene; nevertheless, upon maturation, it gets demethylated, resulting in differentiation into β-cells, producing insulin, thus suggesting the significant contribution of demethylation in the maturation of β-cells and insulin gene expression in specific tissues [50]. Suppressing the gene transcription at the INS gene promoter, specifically at CpG islands via DNA methylation, is an essential element that shapes the developmental process of type 1 diabetes [51]. These islands are frequently observed near the TSS. Dnmts drive the cytosine methylation. Dnmts include DNMT1, DNMT3A, and DNMT3B. These members are expressed in large numbers during the progression of diabetes due to DNA methylation [52]. Methylated DNA enters the circulation following the β-cell destruction by cytotoxic T-lymphocytes [53]. Moreover, another study, that examined the role of DNA methylation using pancreatic islet cells from type 2 diabetes mellitus (T2DM) and non-T2DM donors, reports a reduction in insulin secretion stimulated by glucose, expression of insulin mRNA, and assessing insulin concentrations in the pancreatic islets of both the donor groups. A rise in the levels of DNA methylation at four CpG sites, namely −234, −180, −102, and +63, was observed in T2DM pancreatic islets. At sites −234, −180, and +63, the degree of methylation and mRNA expression of insulin were negatively correlated. Additionally, the consequence of hyperglycemia on methylation levels at the promoter region was also investigated. Two CpG sites, namely −1057 and +58 in the INS gene promoter, were reported to be hypermethylated [54]. In a study involving Zucker diabetic fatty rats, the outcome of excessive nutrition on DNA methylation was analyzed. It was found that the expression of Dnmt1 mRNA had a direct correlation with higher glucose concentrations. As a result, five CpG positions present within the promoter region of Ins1 had higher methylation levels. These included CRE and the downregulation of Ins1 mRNA synthesis [55]. A rise in the methylation levels at the promoter region of Ins1 was observed in the islets in the pancreas isolated from Zucker diabetic fatty rats. Moreover, artificial methylation of the Ins1 promoter was reported to suppress the luciferase activity. Furthermore, it was observed that DNA methylation inhibitors reversed the methylation of the Ins1 promoter and showed a significant decline in the methylation levels [55].
4. DNA Methylation and Insulin Resistance
DNA methylation is the process of introducing a methyl (-CH3) group to the 5′-position of the cytosine within 5′-CpG-3′ covalently. This addition creates a 5′-methyl cytosine [56]. CpG sites arrange themselves in such a way to make up repetitive sequences known as CpG islands [57]. For transcription to take place in a gene, the accessibility of the promoter and the regulatory sites play a very crucial role. This introduction of the -CH3 groups decreases the accessibility of the DNA and prevents the DNA from becoming bound by the transcription factors, thus altering the gene expression [58]. Therefore, CpG island hypermethylation results in the silencing of transcription, whereas hypomethylation leads to the initiation of transcription [59].
4.1. DNA Methylation at IRS1/2
Insulin receptors become activated upon insulin binding to its receptor, followed by which, the insulin receptor substrates IRS1/IRS2 are brought to the plasma membrane [60]. In the liver, IRS2, which is present in the hepatocytes, regulates insulin signaling.
In a study conducted on mice, it was reported that the destruction of IRS2 does not cause IR in skeletal muscle, but the results were the opposite in the case of the liver [61]. A study done by Krause et al. on individuals with T2DM reported a reduction in the extent to which IRS2 is expressed in the liver of individuals with obesity and T2DM, and this downregulation of IRS2 correlated with DNA methylation of hepatic IRS2 at the CpG5 island present near the promoter region [62]. The same study also reported a hypomethylated CpG-containing binding interface for sterol regulatory element binding transcription factor 1 (SRBF1), which was previously reported to contribute to the repression of IRS2 promoter [63].
4.2. DNA Methylation at PPARGC1A
Peroxisome proliferator-activated receptor gamma coactivator 1α (PGC1α) is a transcriptional coactivator that is induced by fasting and is encoded by the PPARGC1A gene. It is responsible for maintaining mitochondrial biogenesis [64]. The disruption of PGC1α has contributed to hepatic IR [65], and it was further observed that its affinity is higher at locations of MASLD-associated modifications in DNA methylation [66]. In a study carried out on fatty liver, the plasma insulin levels at fasting and the HOMA-IR correlated with the ratio of methylated to unmethylated DNA for liver PPARGC1A, whereas the ratio of methylated to unmethylated DNA for mitochondrial factor transcription factor A had an inverse correlation with IR [67].
4.3. FADS2
FADS2 gene encodes fatty acid desaturase 2(FADS2) [68] and metabolizes fatty acids through epigenetic modifications [69]. Accumulating genome-wide association studies have linked FADS2 and IR-related diseases [70,71,72]. SREBP1c is a major transcription factor regulating lipogenesis and lipid homeostasis [73]. FADS2-deficient animals showed the presence of disrupted SREBP1c and were also involved in the over-production of alternate enzymes accountable for the metabolism of fatty acids and, thus, IR [73]. Furthermore, there exists an inverse correlation between the expression of FADS2 and the activity of FADS2 in the serum, with the two CpG positions present in a neighboring enhancer becoming methylated along with the CpG-rich region present upstream to the FADS2 TSS. This indicates that the degree of methylation significantly contributes to modulating the FADS2 activity [74].
4.4. DNA Methylation and Insulin-Like Growth Factor Binding Proteins
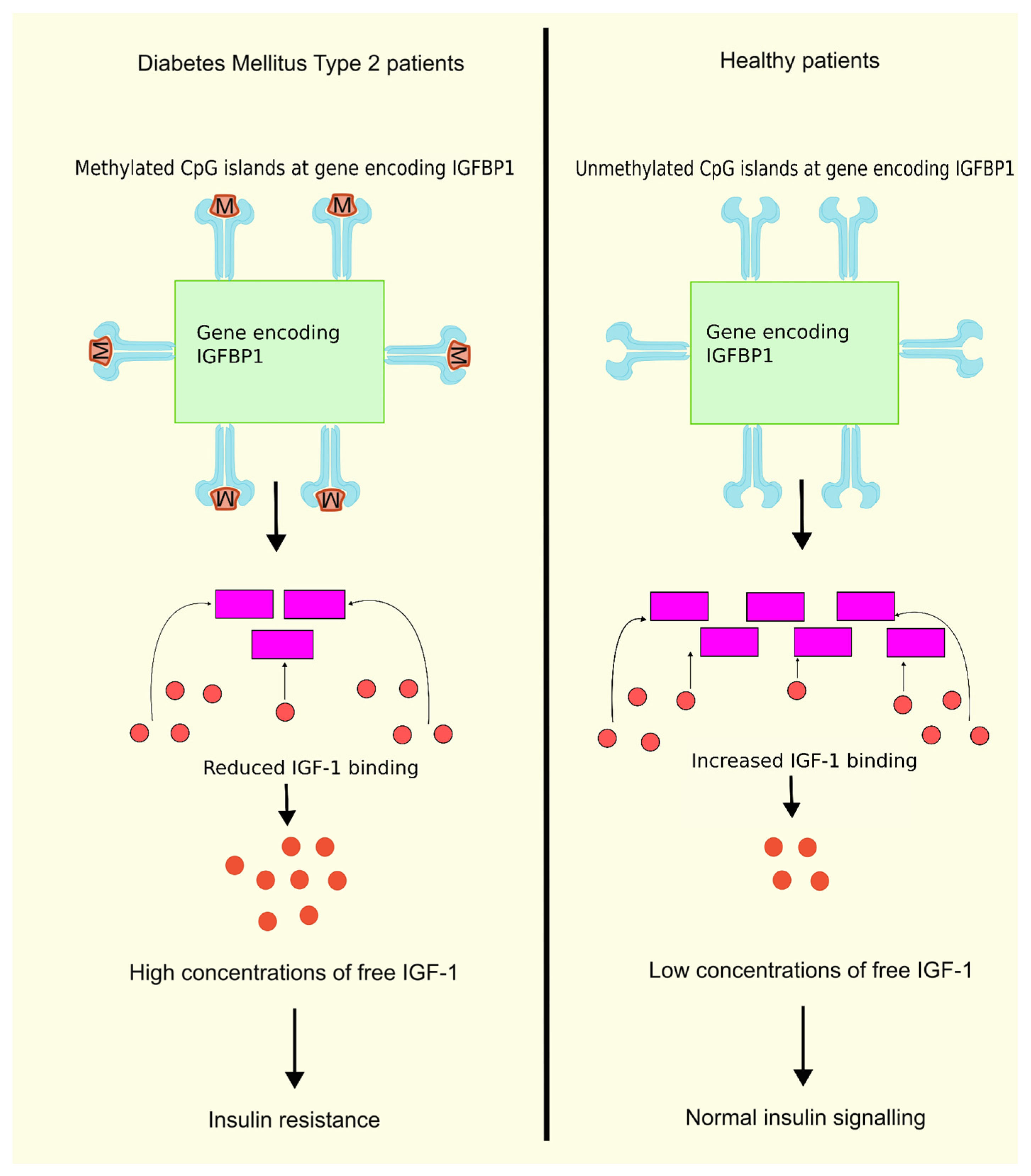
Insulin-like growth factor binding protein 1 (IGFBP1) is one among the family of structurally homologous proteins that attaches to IGF-1 with good affinity and serves as a transport protein for IGF-1. The family consists of six IGFBPs, namely, IGFBP1, IGFBP2, IGFBP3, IGFBP4, IGFBP5, and IGFBP6. There are, however, other IGFBPs that are misnomered, such as IGFBP7, IGFBP8, IGFBP9, etc. Due to their high binding affinity to IGFs and their conserved protein structure, the IGFBPs 1–6 are considered true IGFBPs [75]. An inverse correlation exists between the IGFBP1 levels and the free IGF1 levels. A reduction in the circulating glucose and free IGF1 levels is achieved by administering exogenous IGFBP1. Previous research showed a decline in the level of IGFBP1 in T2DM patients, whereas the free IGF1 levels were increased in T2DM patients. It was also observed that IR is linked with reduced production and circulating levels of IGFBP1 [76]. In another study on T2DM patients, higher levels of DNA methylation were observed in six CpG positions in the gene encoding IGFBP1 compared to the control group. Moreover, IGFBP1 gene methylation levels were greater in patients diagnosed with T2DM having a familial history of the disease compared to patients without a familial history of this disease [77], as shown in Figure 1. These findings are conclusive that higher levels of DNA methylation in the IGFBP1 gene can lead to lower serum IGFBP1 levels, leading to higher concentrations of free IGF-1, thus resulting in IR and T2DM.
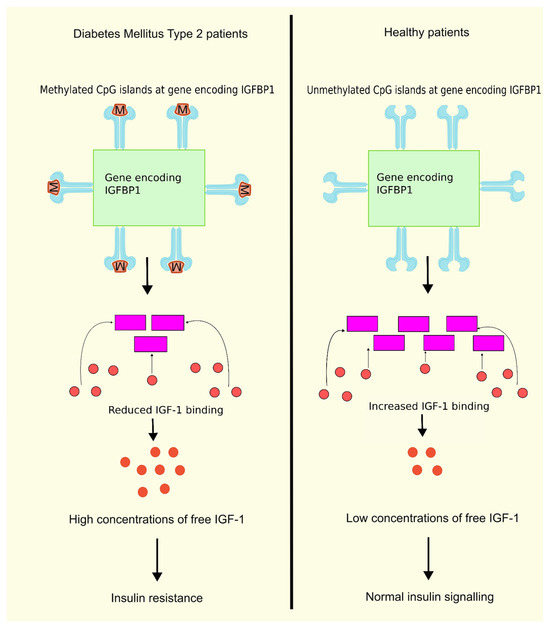
Figure 1.
DNA methylation of IGFBP1 and insulin resistance. DNA methylation of the gene encoding IGFBP1 leads to lower serum levels of IGFBP1, reducing the binding of IGF-1 to IGFBP1, further leading to higher concentrations of free IGF1, and finally resulting in IR compared to normal unmethylated DNA having normal insulin signaling due to binding of IFG-1 to IGFBP1. When IGF-1 binds to IGFBP1, the concentration of free IGF-1 decreases, resulting in normal insulin signaling. Created using inkscape.org (Accessed on 24 February 2024).
4.5. DNA Methylation and IGFBP 7
Insulin-like growth factor binding protein 7 (IGFBP7), encoded by the IGFBP7 gene, belongs to the IGFBP superfamily. It was one of the first IGFBP-related proteins discovered and is also designated as IGFBP-rp1 [78]. Studies on newly diagnosed men with T2DM exhibited increased levels of methylation at three CpG positions on IGFBP7 in comparison with the control group. However, the serum contents of IGFBP7 were similar across all the groups, i.e., the control group, the treated T2DM group, and the newly diagnosed T2DM group. They did not have any correlation with IGFBP7 DNA methylation levels. Moreover, the levels of IGFBP7 in the serum had a positive correlation with serum IGFBP1 levels, which is a marker to produce insulin found in men but not in women. In conclusion, low IGFBP7 levels may be linked with T2DM IR [79].
4.6. The Role of Dnmt3a in Insulin Resistance
DNA methyltransferases are a family of five members, namely Dnmt1, Dnmt2, Dnmt3a, Dnmt3b, and Dnmt3l [80]. Dnmt1 maintains DNA methyltransferase and mainly methylates hemimethylated DNA. Dnmt3a and 3b are called de novo DNA methyltransferases since they preferably act on unmethylated DNA. Dnmt3l lacks catalytic activity but is homologous to other Dnmt3s. Dnmt2 does not methylate DNA but methylates cytoplasmic tRNA [80]. In genetic knock-out studies, it was shown that administration of a Dnmt inhibitor demethylates the Adipoq promoter, improving insulin sensitivity [81].
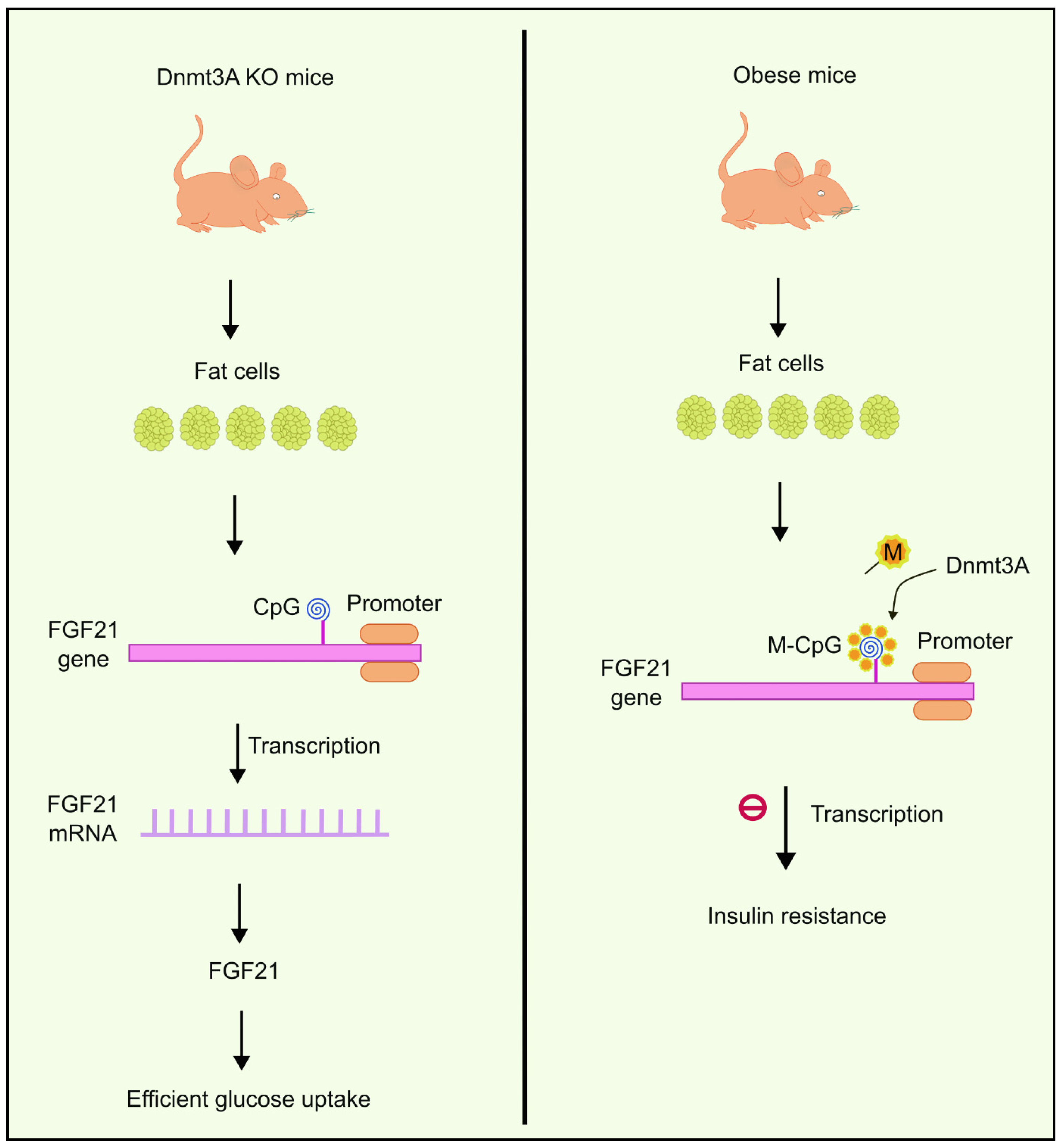
You et al. observed the existence of abnormally elevated levels of Dnmts in fat cells extracted from obese mice. One of the DNA methyltransferases, Dnmt3a, has been shown to contribute to developing IR. Dnmt3a knock-out mice had a better insulin-dependent glucose uptake profile compared to the wild type, and the results were like those of genetically engineered mice lacking Dnmt3a in their fat cells. It was also observed that overexpression of Dnmt3a had an inhibitory effect on the transcription of another gene called Fgf21 [82]. Fgf21 encodes secreted proteins that are essential in absorbing glucose to fat cells [83]. Based on this evidence, You et al. suggested the role of Dnmt3a in causing IR in the Fgf21 promoter via the methylation of CpG islands. Reduced expression of Fgf21 causes IR in fat cells. These results were also consistent with studies performed on human beings [82]. Higher methylation levels were observed near the Fgf21 gene (Figure 2) in diabetic individuals compared to people without diabetes. Moreover, the degree of methylation had an inverse correlation with expression levels of Fgf21 mRNA [82].
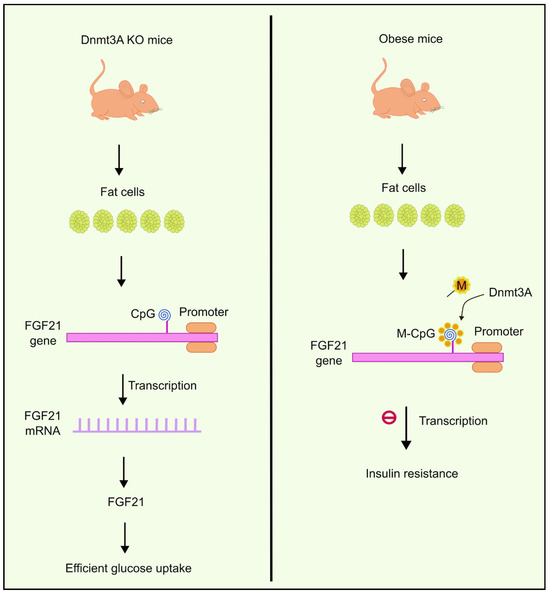
Figure 2.
Overexpression of Dnmt3a inhibits the transcription of the FGF21 gene, which is responsible for glucose uptake to fat cells. Dnmt3a KO mice, on the other hand, were observed to have efficient glucose uptake. Created using inkscape.org (Accessed on 17 November 2023).
4.7. DNA Methylation and Glucokinase
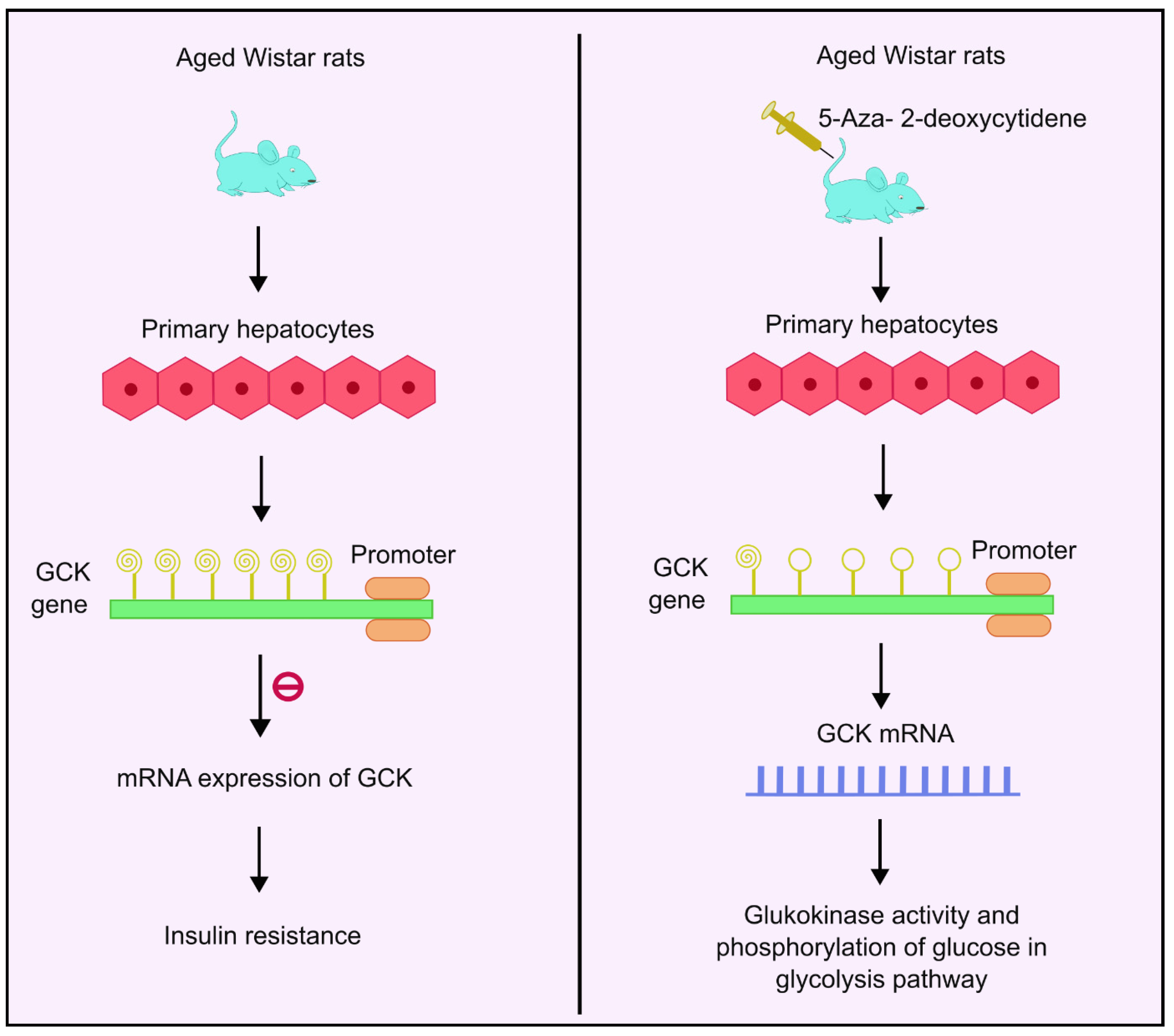
The glucokinase (GCK) gene regulates glucose homeostasis in humans by catalyzing phosphorylation of glucose. It acts as the rate-determining step in the glycolysis pathway in liver and pancreas [84]. It is a glucose sensor that helps maintain normoglycemia [85]. GCK is mainly concentrated in the liver and the pancreas [86]. Insulin stimulates GCK expression, which further activates glycolytic genes to increase glucose utilization. IR has been reported in T2DM patients with downregulated GCK activities [87]. Studies conducted in aged Wistar rats showed age-related methylation changes. As the aging progresses, the degree of methylation also increases, and a decline in GCK mRNA expression and glucokinase activity was observed. Analysis of 11 CpG sites showed increased methylation levels as the age progresses. Additionally, 5-aza-2-deoxycytidine, a DNA methyl transferase inhibitor, was used as the treatment. Administration of this drug enhanced the GCK expression four-fold in rat primary hepatocytes, as shown in Figure 3. Moreover, the analysis also showed that the age-related increased methylation of GCK in the liver had an inverse correlation with its expression, implying that there may be links between methylation and age-dependent hepatic IR [87].
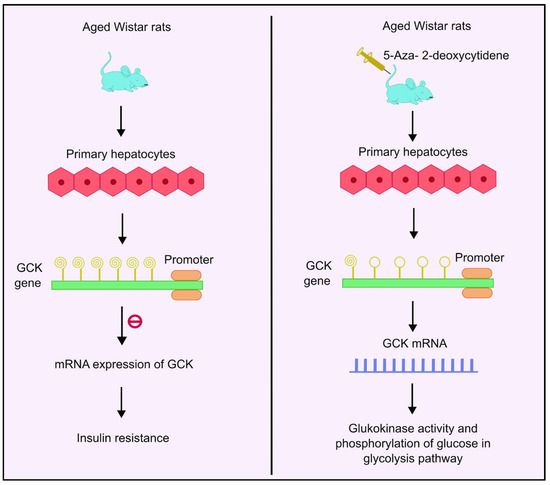
Figure 3.
DNA methylation of GCK gene and insulin resistance. Higher methylation levels of the GCK gene inhibit mRNA expression of GCK, which is responsible for glucokinase activity and phosphorylation of glucose, thus leading to insulin resistance. Administration with 5-aza-2-deoxycytidene enhances the GCK expression by four times, thus leading to enhanced mRNA expression followed by higher glucokinase activity and phosphorylation of glucose in the glycolysis pathway. Created using inkscape.org (Accessed on 17 November 2023).
4.8. DNA Methylation and Glucagon like Peptide 1
Glucagon-like peptide-1(GLP-1) is a thirty to thirty-one amino acid-long peptide hormone produced by the α-cells located in the pancreas. Along with the pancreas, enteroendocrine L cells, which are present in the intestines as well as the central nervous system, also secrete GLP-1 [88]. GLP-1 is mainly responsible for maintaining glucose homeostasis [89]. Studies conducted on rats and humans with T2DM have reported a decline in the presence of GLP-1 receptors. Studies have been conducted to link DNA methylation and GLP-1 mRNA expression. Pancreatic islets were isolated from adults who were grouped into T2DM and non-T2DM, and the expression levels for mRNA of Dnmts-1, 3a, and 3b, along with MECP2, were analyzed. In addition, a few CpG methylation levels were associated with the GLP-1 TSS. Further analysis of CpG positions at the +199 and +205 base pairs showed a reduction in glucose homeostasis and the presence of GLP-1 receptors in the islets isolated from T2DM donors. A slight increment in the methylation levels in T2DM islets was also reported but was not significant. Additionally, the methylation levels analyzed at the GLP-1 receptor promoter receptor were analyzed using α- and β-cells isolated from pancreatic cells. The DNA methylation levels at position 376 of the CpG site at the GLP-1 receptor were significantly elevated in α-cells compared to β-cells, and this had an inverse correlation with GLP-1 receptor expression [90].
4.9. Histone Modifications: Acetylation and Deacetylation
Histone proteins undergo acetylation and deacetylation via the action of HAT and histone deacetylases (HDACs), respectively. These modifications contribute a vital role in the epigenetic modulation of transcription [91]. When acetylation occurs in histones, it mainly relaxes the DNA and results in the initiation of transcription, while deacetylation, on the contrary, condenses the DNA protein and represses transcription [92]. In humans, there exist four main classes of histone deacetylases. These include class Ⅰ HDACs, which comprises HDACs 1–3 and 8; class Ⅱ HDACs can be further categorized into two subclasses—class Ⅱa and class Ⅱb HDACs. Class Ⅱa comprises HDACs 4, 5, 7, and 9, while class Ⅱb comprises of HDACs 6 and 10. Class Ⅲ HDACs are alternatively recognized by the term sirtuins. They include seven classes of sirtuins from SIRT1 to SIRT7. On the other hand, HDAC11 represents class Ⅳ HDAC [93].
HDACs, regardless of their class, take part in a vital role in regulating insulin signaling. Previous research showed that there have been interactions between HDAC2 and IRS-1 in yeast and ob/ob mice [94]. This interaction further leads to diminished acetylation and tyrosine phosphorylation (insulin-mediated) of the IRS-1 protein [94]. Trichostatin A is an antifungal antibiotic that selectively and reversibly inhibits mammalian class Ⅰ and Ⅱ HDACs [95]. This causes acetylation of IRS-1 and attenuation of IR [94]. Similarly, the role of HDAC3 in IR was also studied. A clinical study on 568 subjects in China demonstrated a significant association between HDAC3 and T2DM [96]. The study results were consistent with previous research done by Sun and colleagues that showed deletion of HDAC3 in the liver of C57BL/6 mice, resulting in hepatosteatosis and enhanced insulin responsiveness [97]. HDAC3 is also known to take part in modulating PPARγ function in adipocytes [98]. These studies have shown the role of HDAC3 in regulating IR. Moreover, fibroblast growth factors (FGFs) comprise a set of small polypeptide growth factors that contribute to the development of diseases. They comprise twenty-two structurally related proteins classified as endocrine, paracrine, and intracrine FGFs [99]. Li and colleagues explored the functions of FGF in high-fat diet HFD (HFD)-fed mice treated with HDAC inhibitors. They produced FGF21 KO mice that displayed glucose intolerance and obesity. This shows the significance of FGF21 in metabolic disorders [100]. FGF21 hormone has been shown to promote glucose homeostasis and lipid homeostasis. Various studies have also shown improvements in sensitizing insulin [101,102,103]. Thus, the induction of FGF21 can be a possible therapeutic strategy for treating IR and obesity, while inhibiting HDAC is one way to increase the expression of FGF21 [104].
Myocyte enhancer factor-2(MEF-2) binding domain and domain 1 mainly govern the GLUT4 gene promoter. The transcriptional activity is advanced when MEF2 attaches to the binding domain of MEF2 and GLUT4 enhancer factor (GEF) attaches to Domain 1 [105,106]. A reduction in MEF2 results in reduced expression of GLUT4 [107]. Class Ⅱ HDACs, HDAC4 and 5, inhibit the MEF2 transcriptional activity and inhibit GLUT4 expression leading to IR [108,109,110].
HDAC6 KO mice have improved insulin signaling and glucose intolerance in dexamethasone-induced diabetes and insulin-induced models. This is a clear indication of HDAC6 as an important regulator of insulin signaling [111]. The most widely used models for diabetes are the genetically modified db/db mouse and ob/ob mouse. HDAC 4/5/7 is required for the hyperglycemic state in diabetic mouse models mentioned above [112]. Mihaylova and co-workers demonstrated the role of HDACs in maintaining glucose levels in the liver of HFD-induced diabetic mice. They reported a reduction in the presence of class Ⅱa HDAC in diabetic mouse models and a reduction in blood glucose during fasting. The HFD mouse model also showed decreased fasting glycemia levels and enhanced glucose tolerance when class Ⅱa HDAC levels were reduced [112]. Wang and co-workers demonstrated an elevated expression of HDAC5 and HDAC9 in the brain of HFD-fed C57BL/6J mice. Compared to the control mice, these mice demonstrate reduced insulin sensitivity and increased levels of fasting blood glucose [113]. These studies showed links between HDAC and IR.
5. Linking Peripheral IR with Impaired Brain Insulin Signaling and AD
During normal conditions, insulin traverses the rigid blood–brain barrier (BBB) easily via a receptor-mediated transport mechanism. It is still not clear whether insulin is synthesized and secreted in the CNS. However, both rodent and human studies have confirmed the presence of insulin mRNA in the brain and the secretion of insulin from GABAergic interneurons and choroid plexus epithelial cells [114].
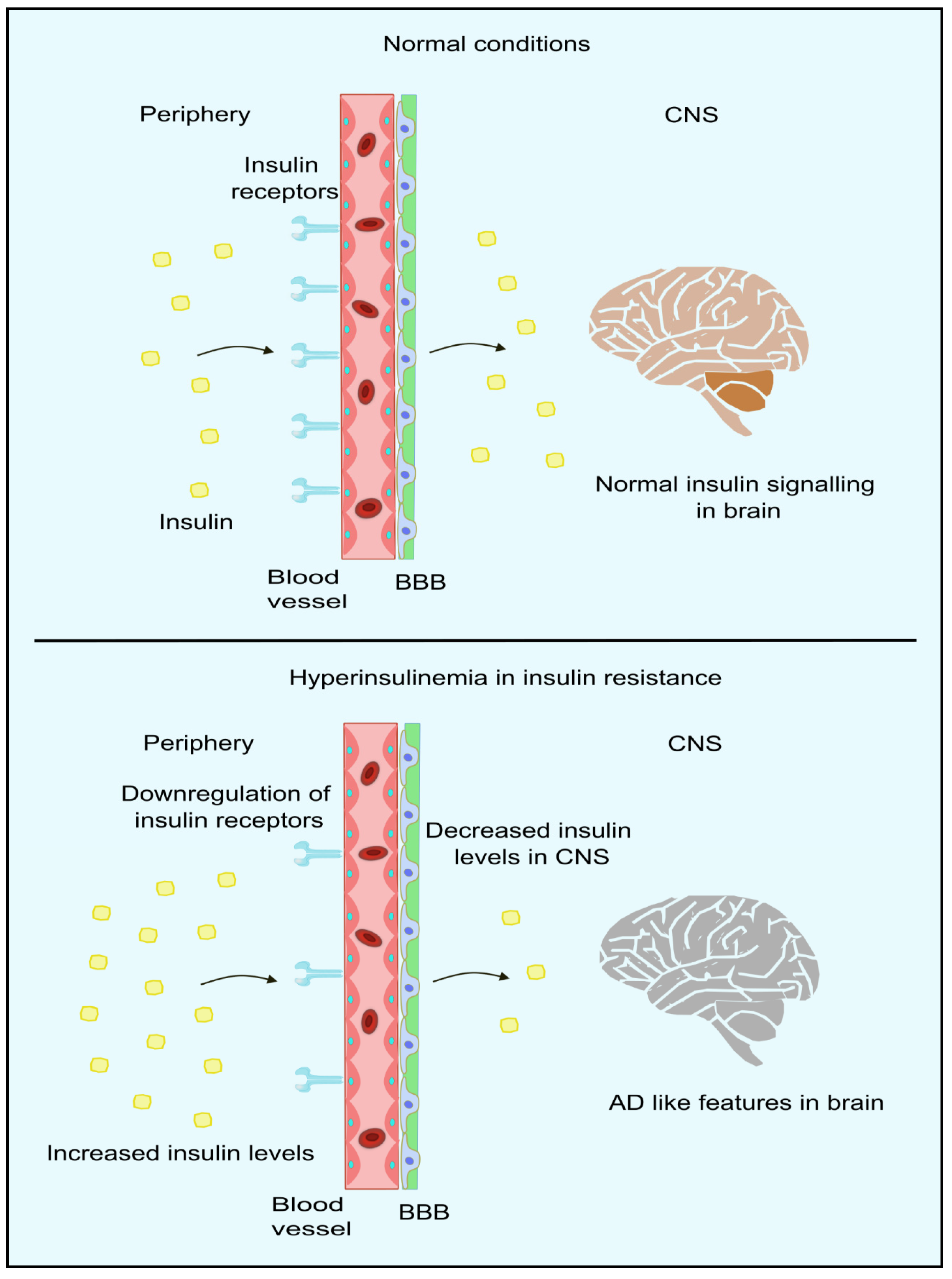
IR can be defined as the unresponsiveness of target tissues towards the action of insulin [114]. Peripheral IR is commonly assessed by the gold-standard assessment method of a HOMA-IR [115]. Accumulating studies have shown that IR in the periphery can result in negative impacts on the brain, which include reduced insulin uptake, rising amyloid β, pro-inflammatory cytokines, tau protein phosphorylation, oxidative stress, advanced glycation consequence, and finally, apoptosis [116,117,118,119,120,121]. It has also been proposed that the brain becomes resistant to insulin in AD with/without comorbid T2DM. This acts as a critical trigger in other pathophysiological events in this disorder [122,123,124,125]. Moreover, this is consistent with studies that report alterations in those molecules responsible for insulin signal transduction, present in the forebrain of individuals with AD. Furthermore, some studies have reported improvement in the memory of AD patients after administration of intranasal insulin [125,126,127,128,129]. The insulin receptors and insulin-dependent-GLUT is compromised in AD patients [130,131]. Moreover, peripheral IR also alters the proper functioning of CNS, such as the down-regulation of insulin transport to the brain, making the neuropathological features more evident in AD patients [132,133]. Insulin transport across BBB occurs mainly through carrier-mediated [134] and saturable processes [135]. Thus, an increase in the peripheral insulin levels, as in the case of hyperinsulinemia (mainly during IR), results in a higher amount of insulin in the cerebrospinal fluid, whereas in chronic cases, down-regulation of insulin receptors occurs in the BBB, leading to impaired insulin signaling as indicated in Figure 4 [136]. In studies where systemic insulin infusion [137,138,139,140] or intranasal insulin [141,142] is administered, a possible link between peripheral IR and brain IR was observed. However, CNS insulin sensitivity is restored through caloric restrictions for diabetic and obese patients. This improves the peripheral metabolism in diabetics and obese patients [143]. Thus, we can conclude that peripheral insulin signaling relates to brain insulin. However, it is not clear whether the resistance exists in the periphery and CNS. In the liver, insulin contributes to a significant role in inhibiting glucose synthesis as well as its release by blocking major enzymes in the gluconeogenesis and glycogenolysis pathway. Phosphoenolpyruvate carboxylase (PEPCK) is the major gene encoding the hepatic enzymes for the regulation of the pathways mentioned above. The blockage of the expression of PEPCK blocks the synthesis of glucose [144,145,146].
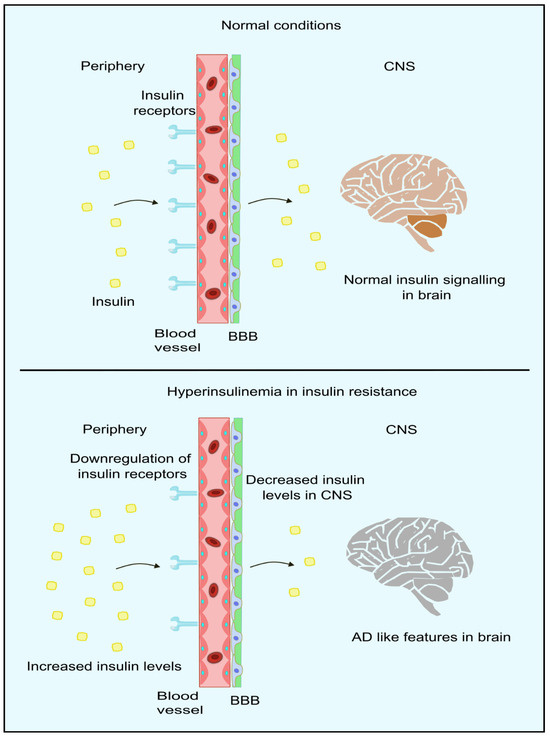
Figure 4.
Insulin signaling in periphery and brain. In normal conditions, insulin crosses the BBB through the insulin receptors present in the BBB. However, in hyperinsulinemic conditions, insulin receptors are downregulated, resulting in lower insulin levels in CNS, thus leading to AD-like features in the brain. Created using inkscape.org (Accessed on 17 November 2023).
When insulin attaches to the α-subunit, which is present extracellularly in the insulin receptor, autophosphorylation of the tyrosine residues occurs in the cytoplasmic segment of the β-subunit, resulting in the initiation of intrinsic tyrosine kinases, catalyzing the intracellular substrate phosphorylation on tyrosine which includes the insulin substrate family, the src homology and collagen (shc) adaptor protein, Gab-1, and Cbl. Following the phosphorylation process, various src homology 2 (SH2) domain proteins, along with the other adapter molecules like Grb-2, Crk, and Nck, bind to the IRS proteins upon phosphorylation. One of the most vital SH2 proteins is the phosphatidylinositol 3-kinase (PI-3 kinase). PI3-kinase contributes to the translocation of insulin-dependent GLUT-4. PI3-kinase, once activated, generates phosphatidyl inositol phosphate-3,4,5-trisphosphate (PIP3). An increment in the levels of PIP3 results in the initiation of protein kinase cascade, with PDK being the first protein kinase to be activated. PDK phosphorylates and initiates serine/threonine kinases, including the Akt (protein kinase B), protein kinase C-PKCζ and λ. Both isoforms and Akt help mediate insulin-mediated glucose transport in muscle and adipose tissue. However, Akt appears to take part in a more critical function in glycogen production and storing glucose in muscle, adipose tissue, and liver [147].
Researchers have reported the existence of insulin receptors in certain areas of the hippocampus, cortex, hypothalamus, and the olfactory bulb, which have been shown to take part in brain insulin signaling. In the brain, insulin receptors are more widespread in the neurons and synapses compared to glial cells. Brain insulin receptors are accountable for various functions, such as maintaining synaptic plasticity, neurotransmission, homeostatic regulation, and age-related neurodegeneration [4]. In a study conducted on AD patients by Frolich et al., reduced sensitivity of insulin signaling was reported in the brain tissues, even in those patients not having T2DM [148]. A reduction in the levels of IGF 1 and 2 was observed, along with a decrease in insulin receptors, insulin-associated PI3-kinase, and the activated Akt-PkB kinase in a few studies [3]. Brain IR is a state in which the brain cells do not respond to normal levels of insulin [5]. This condition causes an increased production of amyloid β and phosphorylation of tau protein, which are the two main hallmarks of AD. Additional hallmarks such as protein misfolding, oxidative stress, and mitochondrial and cognitive dysfunction are also associated with brain IR. IR further leads to elevation in insulin levels, thus competing with amyloid β for insulin-degrading enzyme (IDE). IDE is accountable for the degrading insulin and amyloid β [149].
6. Advanced Drug Delivery Systems in Epigenetics
The epigenome is very complex and highly regulated in nature. The delivery and kinetics of epigenetic drugs are crucial as these are highly potent medications that can produce several adverse drug reactions when the delivery is not targeted [150]. An ideal delivery system for epigenetics drugs should have the ability to retain the stability of the drug as well as provide a targeted delivery in a controlled manner. The pharmacokinetic studies of epigenetic drugs can be utilized for the design of an effective delivery system. For instance, the presence of enzymes can result in alteration in the ADME processes [151]. As these drugs generally do not bind to plasma proteins, a higher risk of renal clearance is present, which can result in the need for a delivery system that can continuously deliver the drug only to the desired location of action at a predetermined rate. Thus, there is a significant need for a drug delivery system for the delivery of epigenetic drugs.
Various types of delivery systems can be used for targeted delivery, such as nanoparticle-based technologies, vesicular systems, network systems, biological vectors, etc. Strategies such as stimuli-based delivery systems (heat, light, enzymes, mechanical force, magnetic field, etc.), synergistic drug combinations, and prodrug approaches can also be utilized to enhance drug delivery [152]. The selection of a vehicle or system is very important for the success of delivery. It will depend greatly upon the physiochemical properties of the drug and the pathological conditions of the patient [153]. The various types of drug delivery systems used for the delivery of epigenetic drugs mainly include nanoparticle-based drug delivery systems, vesicular systems, engineered nano-carriers, network systems, hydrogels, as well as biological vectors.
6.1. Nanoparticle-Based Systems
Use of nanoparticles (NPs) provides a versatile delivery system for brain delivery of epigenetic drugs due to their characteristic properties, such as smaller particle size, surface morphology, zeta potential, chemical composition, solubility, and functionalization [154]. Various types of polymers, polysaccharides, and proteins can be used for the synthesis of NPs, such as poly lactic-co-glycolic acid (PLGA), dextran, albumin, polyethylene glycol (PEG), gelatin, chitosan, and inorganic NPs such as silica, gold, zinc oxide, and silver particles. These particles are used for the delivery of both chemical moieties and peptides in chronic neurodegenerative diseases such as AD, autoimmune diseases such as multiple sclerosis and rheumatoid arthritis, as well as Parkinson’s disease [155,156,157]. NPs can be chemically modified and functionalized to traverse the BBB and effectively deliver the drug to the CNS [158]. PLGA NPs are regarded as a promising system for AD, with their property to produce a site-specific delivery [159]. Studies have proved that curcumin-loaded PLGA-based NPs conjugated with peptides can decrease the disease progression in AD [160]. It was also found that PLGA NPs when loaded with vitamin D binding proteins, suppress the symptoms of AD [161]. For uniform brain distribution, albumin-loaded NPs are preferred. Bovine serum albumin NPs combined with cyclodextrins are used for the intranasal delivery of tacrine in AD [162]. Mesoporous silica-based systems are used to produce a stimulus-based controlled delivery in AD [163]. Apart from all this, NPs can be utilized in theranostic applications [164,165,166]. For instance, chitosan–hyaluronic acid NPs are used as targeting agents in AD with additional theranostic properties [167]. NP-based systems are highly biocompatible and biodegradable, making them a versatile system for targeted drug delivery in neurodegenerative diseases.
6.2. Vesicular Systems
Vesicular systems widely utilized in the case of drug delivery include liposomes, niosomes, cubosomes, pharmacosomes, aquasomes, phytosomes, transferosomes, and electrosomes. These are self-assembling systems that are mainly composed of lipid bilayers. They can form a core and an outer layer, which enables them to encapsulate and deliver both hydrophilic and hydrophobic drugs. The release of drugs from these systems can be controlled by engineering the system and by using varying concentrations of lipids for the synthesis of the outer lipid layer. Surface functionalization combined with structural modifications of vesicular systems is extensively used for localized delivery and drug-targeting applications [168].
Liposomal systems are the frequently used delivery system for neurological disorders due to their potential to traverse through the BBB. In the past few decades, intranasal delivery of liposomes was explored for the treatment of AD [169]. It was reported that surface-functionalized liposomes can reduce the β-amyloid burden and reduce the progression of AD [170]. Liposomes can also be synthesized with the same lipid composition as that of exosomes, thereby, reducing the heterogenicity of naturally occurring exosomes, making it an innovative carrier that has high productivity [171]. Bifunctional niosomes have also been developed for AD that can be delivered intranasally to reduce drug wastage by first-pass metabolism [172]. Cubosome is a novel nano-carrier used for brain delivery that can cross the BBB and control the delivery rate. Nanostructured in situ gels of cubosomes are used for the delivery of AD medications. These systems have been utilized in other neurological diseases, such as epilepsy and dementia, for effective treatment [173,174]. Oral nanomedicines using cubosomes have been developed with piperine and tween for the treatment of AD. These systems use bioactive excipients for the oral delivery of AD medications [173].
6.3. Network Systems and Dendrimers
Interpenetrating polymeric network systems is one of the widely used strategies adopted for the delivery of epigenetic drugs [175]. Over the past few decades, scientists focused on the swelling properties, stability, and biocompatibility of these polymers to produce a biodegradable delivery device [175]. The advanced form of this network system is dendrimers with well-defined structures and shapes. The multivalency of these nanostructures is extensively utilized for the delivery of drugs, proteins, peptides, and genes [150]. They are made up of functionalized monomers that form the nano-architecture for the conjugation of molecules and their delivery [176]. Studies have proved that poly(amidoamine) (PAMAM)-based dendrimers not only deliver drugs in a controlled manner but also provide neuroprotective action [177]. It has also been reported that cationic phosphorus dendrimers can act synergistically with AD medications [178].
6.4. Hydrogel-Based Systems
Hydrogel-based nano-formulations are gaining interest due to their ability to control the degradability of the system and produce a desired drug-release pattern. One of the most advanced hydrogel systems is the use of nanogels, which are developed to release therapeutic drugs in response to a particular stimulus. One of the major advantages of a nanogel is its high biodegradability, biocompatibility, and lower toxicity in the in vivo system [179]. When the drug to be delivered is highly lipophilic, another nano-formulation known as the solid-lipid nano-carrier system is used [180]. The potential of these systems to traverse through the BBB helps the drug to reach the brain cells easily. These nanostructured lipid carriers can also be further engineered to receive an optimum delivery pattern near the targeted cells [181]. A hydrogel micro post-based system has been developed for the detection of microRNA (miRNA) that are associated with AD. These systems use PCR techniques for early diagnosis of the disease [182] and are highly recommended in AD drug delivery owing to their properties to induce neurite outgrowth and neuroprotective actions [183].
6.5. Biological Vectors
Nanocells have been recently used for the delivery of biological products. Chemical moieties can also be loaded into these cells for targeted delivery. These bacterial cells are devoid of genetic material and cannot replicate on their own, thus maintaining the dose that is initially administered. The inability of these cells to produce mutations increases the acceptance of these bacterial cells for human use [150]. They can also deliver genetic materials as they provide a stable environment for RNAs and DNAs. A major risk produced by nano-cells is the higher chances of immunogenicity caused due to the presence of lipopolysaccharide [184]. Further research should be carried out to optimize the use of biological vectors for epigenetic drug delivery. Organoids have gained much interest among scientists for the development of a qualified model for AD [185]. Cerebral organoids are mainly used in culturing and modeling of phenotypes like AD. Brain organoids are more advantageous as compared to other AD models due to their ability to form a next-generation humanized disease model [186]. Organoids have not only been developed for AD but have also been cultured for other neurological diseases such as amyotrophic lateral sclerosis, Parkinson’s disease, and Huntington’s disease. Various other types of therapies include the use of gene therapy [187], mRNA [188], siRNA [189], miRNA [190], and antisense oligonucleotides [191].
7. Conclusions
In this review, we attempted to explain how epigenetic modifications can impact the insulin levels in the periphery to cause peripheral insulin resistance. Long-term peripheral insulin resistance leads to type 2 diabetes mellitus and further complications. Type 2 diabetes mellitus is the trigger for most metabolic disorders like hypertension, metabolic dysfunction associated with steatotic liver disease, metabolic dysfunction associated with steatohepatitis, hyperlipidemia, etc. Studies have also shown that insulin resistance influences the brain insulin signaling and causes insulin resistance in the brain. Insulin in the brain competes with amyloid-β for insulin degrading enzyme, thus causing Alzheimer’s disease, also known as type 3 diabetes mellitus. We also discussed the various advanced drug delivery systems that can be used to counter the epigenetic changes leading to AD. The various drug delivery systems discussed in this paper (nanoparticles, vesicular systems, hydrogel-based systems, and biological vectors) possess unique characteristics that make them suitable and a potential option for targeting drugs to the brain. With the addition of biotechnology into these methods, the above-mentioned drug delivery systems pave the way for newer and technologically advanced systems for not only treating Alzheimer’s disease but also other neurological diseases such as Huntington’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis.
Author Contributions
M.N. developed the manuscript structure and conducted the supervision. A.G. and A.N. wrote the original draft with assistance from P.S. and A.C.F. S.S. carried out the proofreading and developed the figures. All authors have read and agreed to the published version of the manuscript.
Funding
This work received no external funding.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Aβ | Amyloid β |
| AD | Alzheimer’s Disease |
| ATF-2 | Activator Transcripton Factor-2 |
| BBB | Blood Brain Barrier |
| CRE | cAMP Responsive Element |
| CREB | cAMP Responsive Binding Protein-1 |
| CRB | CREB Binding Protein |
| Dnmts | DNA methyl transferases |
| FADS | Fatty Acid Desaturase |
| FGF | Fibroblast Growth Factor |
| GCK | Glucokinase |
| GLP-1 | Glucagon Like Peptide-1 |
| HAT | Histone Acetyl Transferases |
| HDAC | Histone Deacetylases |
| HFD | High Fat Diet |
| IDE | Insulin Degrading Enzyme |
| IGFBP | Insulin Like Growth Factor Binding Protein |
| IR | Insulin Resistance |
| KO | Knock Out |
| MEF-2 | Monocyte Enhancer Factor-2 |
| ncRNA | non-coding RNA |
| NP | Nano Particles |
| NT | Neurofibrillary Tangles |
| SiRNA | Short Interfering RNA |
| T2DM | Type 2 Diabetes Mellitus |
References
- Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 16 November 2023).
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Srivastav, S.; Yadav, A.K.; Srikrishna, S.; Perry, G. Overview of Alzheimer’s Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds. Oxid. Med. Cell. Longev. 2016, 2016, 7361613. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Hyman, B.T. The Intersection of Amyloid Beta and Tau at Synapses in Alzheimer’s Disease. Neuron 2014, 82, 756. [Google Scholar] [CrossRef] [PubMed]
- Schachter, A.S.; Davis, K.L. Alzheimer’s Disease. Dialogues Clin. Neurosci. 2000, 2, 91. [Google Scholar] [CrossRef] [PubMed]
- Jatoi, S.; Hafeez, D.A.; Riaz, S.U.; Ali, A.; Ghauri, M.I.; Zehra, M. Low Vitamin B12 Levels: An Underestimated Cause of Minimal Cognitive Impairment and Dementia. Cureus 2020, 12, e6976. [Google Scholar] [CrossRef] [PubMed]
- Diniz, L.P.; Tortelli, V.; Matias, I.; Morgado, J.; Araujo, A.P.B.; Melo, H.M.; da Silva, G.S.S.; Alves-Leon, S.V.; de Souza, J.M.; Ferreira, S.T.; et al. Astrocyte Transforming Growth Factor Beta 1 Protects Synapses against Aβ Oligomers in Alzheimer’s Disease Model. J. Neurosci. 2017, 37, 6797. [Google Scholar] [CrossRef] [PubMed]
- De-Paula, V.J.; Radanovic, M.; Diniz, B.S.; Forlenza, O.V. Alzheimer’s Disease. Subcell. Biochem. 2012, 65, 329–352. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s Disease: Definition, Natural History, and Diagnostic Criteria. Alzheimers Dement. 2016, 12, 292. [Google Scholar] [CrossRef]
- Kumar, A.; Sidhu, J.; Goyal, A.; Tsao, J.W. Alzheimer Disease. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2022; pp. 1–27. [Google Scholar]
- Wattmo, C.; Minthon, L.; Wallin, Å.K. Mild versus Moderate Stages of Alzheimer’s Disease: Three-Year Outcomes in a Routine Clinical Setting of Cholinesterase Inhibitor Therapy. Alzheimer’s Res. Ther. 2016, 8, 7. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Q.; Yao, H.; Tan, J.; Liu, Z.; Zhou, Y.; Zou, Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022, 14, 911635. [Google Scholar] [CrossRef] [PubMed]
- Waddington, C.H. The Epigenotype. Int. J. Epidemiol. 2012, 41, 10–13. [Google Scholar] [CrossRef] [PubMed]
- Henikoff, S. The Epigenetic Landscape: An Evolving Concept. Front. Epigenet. Epigenom. 2023, 1, 1176449. [Google Scholar] [CrossRef]
- Fyfe, I. Epigenetics Links Ageing with Alzheimer Disease. Nat. Rev. Neurol. 2018, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Nisticò, R.; Seyfried, N.T.; Levey, A.I.; Modeste, E.; Lemercier, P.; Baldacci, F.; Toschi, N.; Garaci, F.; Perry, G.; et al. Omics Sciences for Systems Biology in Alzheimer’s Disease: State-of-the-Art of the Evidence. Ageing Res. Rev. 2021, 69, 101346. [Google Scholar] [CrossRef] [PubMed]
- Castrillo, J.I.; Oliver, S.G. Alzheimer’s as a Systems-Level Disease Involving the Interplay of Multiple Cellular Networks. Methods Mol. Biol. 2016, 1303, 3–48. [Google Scholar] [CrossRef] [PubMed]
- Mehler, M.F.; Mattick, J.S. Non-coding RNAs and RNA Editing in Brain Development, Functional Diversification, and Neurological Disease. Physiol. Rev. 2007, 87, 799–823. [Google Scholar] [CrossRef]
- Vecsey, C.G.; Hawk, J.D.; Lattal, K.M.; Stein, J.M.; Fabian, S.A.; Attner, M.A.; Cabrera, S.M.; McDonough, C.B.; Brindle, P.K.; Abel, T.; et al. Histone Deacetylase Inhibitors Enhance Memory and Synaptic Plasticity via CREB: CBP-Dependent Transcriptional Activation. J. Neurosci. 2007, 27, 6128. [Google Scholar] [CrossRef]
- Guan, J.S.; Haggarty, S.J.; Giacometti, E.; Dannenberg, J.H.; Joseph, N.; Gao, J.; Nieland, T.J.F.; Zhou, Y.; Wang, X.; Mazitschek, R.; et al. HDAC2 Negatively Regulates Memory Formation and Synaptic Plasticity. Nature 2009, 459, 55–60. [Google Scholar] [CrossRef]
- Michán, S.; Li, Y.; Chou, M.M.H.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J. Neurosci. 2010, 30, 9695. [Google Scholar] [CrossRef]
- Nassar, A.; Satarker, S.; Gurram, P.C.; Upadhya, D.; Fayaz, S.; Nampoothiri, M. Repressor Element-1 Binding Transcription Factor (REST) as a Possible Epigenetic Regulator of Neurodegeneration and MicroRNA-Based Therapeutic Strategies. Mol. Neurobiol. 2023, 60, 5557–5577. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, M.; Kumar, N.; Ramalingayya, G.V.; Kutty, N.G.; Krishnadas, N.; Rao, C.M. Effect of Insulin on Spatial Memory in Aluminum Chloride-Induced Dementia in Rats. Neuroreport 2017, 28, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, M.; Ramalingayya, G.V.; Kutty, N.G.; Krishnadas, N.; Rao, C.M. Insulin Combined with Glucose Improves Spatial Learning and Memory in Aluminum Chloride & minus; Induced Dementia in Rats. J. Environ. Pathol. Toxicol. Oncol. 2017, 36, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Khan, S.; Wani, I.A.; Gupta, R.; Verma, S.; Alam, P.; Alaklabi, A. Unravelling the Role of Epigenetic Modifications in Development and Reproduction of Angiosperms: A Critical Appraisal. Front. Genet. 2022, 13, 819941. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.L.; Kouzarides, T.; Shiekhattar, R.; Shilatifard, A. An Operational Definition of Epigenetics. Genes Dev. 2009, 23, 781. [Google Scholar] [CrossRef] [PubMed]
- Al Aboud, N.M.; Tupper, C.; Jialal, I. Genetics, Epigenetic Mechanism. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Jirtle, R.L.; Skinner, M.K. Environmental Epigenomics and Disease Susceptibility. Nat. Rev. Genet. 2007, 8, 253. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Zhou, Y.; Campbell, S.L.; Le, T.; Li, E.; Sweatt, J.D.; Silva, A.J.; Fan, G. Dnmt1 and Dnmt3a Are Required for the Maintenance of DNA Methylation and Synaptic Function in Adult Forebrain Neurons. Nat. Neurosci. 2010, 13, 423. [Google Scholar] [CrossRef] [PubMed]
- Goto, K.; Numata, M.; Komura, J.I.; Ono, T.; Bestor, T.H.; Kondo, H. Expression of DNA Methyltransferase Gene in Mature and Immature Neurons as Well as Proliferating Cells in Mice. Differentiation 1994, 56, 39–44. [Google Scholar] [CrossRef]
- Volkov, P.; Bacos, K.; Ofori, J.K.; Esguerra, J.L.S.; Eliasson, L.; Rönn, T.; Ling, C. Whole-Genome Bisulfite Sequencing of Human Pancreatic Islets Reveals Novel Differentially Methylated Regions in Type 2 Diabetes Pathogenesis. Diabetes 2017, 66, 1074–1085. [Google Scholar] [CrossRef]
- Lister, R.; Pelizzola, M.; Dowen, R.H.; Hawkins, R.D.; Hon, G.; Tonti-Filippini, J.; Nery, J.R.; Lee, L.; Ye, Z.; Ngo, Q.-M.; et al. Human DNA Methylomes at Base Resolution Show Widespread Epigenomic Differences. Nature 2009, 462, 315–322. [Google Scholar] [CrossRef]
- Balaji, E.V.; Kumar, N.; Satarker, S.; Nampoothiri, M. Zinc as a Plausible Epigenetic Modulator of Glioblastoma Multiforme. Eur. J. Pharmacol. 2020, 887, 173549. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, M.A. Epigenetic Editing: How Cutting-Edge Targeted Epigenetic Modification Might Provide Novel Avenues for Autoimmune Disease Therapy. Clin. Immunol. 2018, 196, 49. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin Modifications and Their Function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Allis, C.D. The Language of Covalent Histone Modifications. Nature 2000, 403, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, E.; Zink, D. Nuclear Architecture and Gene Regulation. Biochim. Biophys. Acta Mol. Cell Res. 2008, 1783, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic Modifications: Basic Mechanisms and Role in Cardiovascular Disease. Circulation 2011, 123, 2145. [Google Scholar] [CrossRef] [PubMed]
- Berndsen, C.E.; Denu, J.M. Catalysis and Substrate Selection by Histone/Protein Lysine Acetyltransferases. Curr. Opin. Struct. Biol. 2008, 18, 682. [Google Scholar] [CrossRef]
- Frías-Lasserre, D.; Villagra, C.A. The Importance of NcRNAs as Epigenetic Mechanisms in Phenotypic Variation and Organic Evolution. Front. Microbiol. 2017, 8, 298939. [Google Scholar] [CrossRef]
- Parveen, N.; Dhawan, S. DNA Methylation Patterning and the Regulation of Beta Cell Homeostasis. Front. Endocrinol. 2021, 12, 651258. [Google Scholar] [CrossRef]
- Nassar, A.; Kodi, T.; Satarker, S.; Chowdari Gurram, P.; Upadhya, D.; SM, F.; Mudgal, J.; Nampoothiri, M. Astrocytic MicroRNAs and Transcription Factors in Alzheimer’s Disease and Therapeutic Interventions. Cells 2022, 11, 4111. [Google Scholar] [CrossRef]
- Non-Coding RNAs as Regulators in Epigenetics (Review). Available online: https://www.spandidos-publications.com/or/37/1/3 (accessed on 18 November 2023).
- Saltiel, A.R.; Kahn, C.R. Insulin Signalling and the Regulation of Glucose and Lipid Metabolism. Nature 2001, 414, 799–806. [Google Scholar] [CrossRef]
- Bogan, J.S. Regulation of Glucose Transporter Translocation in Health and Diabetes. Annu. Rev. Biochem. 2012, 81, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Mitron, P.; Lambadiari, V.; Maratou, E.; Raptis, S.A. Insulin Effects in Muscle and Adipose Tissue. Diabetes Res. Clin. Pract. 2011, 93, S52–S59. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.W.; Ferguson, L.A.; Docherty, K. ATF-2 Stimulates the Human Insulin Promoter through the Conserved CRE2 Sequence. Biochim. Biophys. Acta BBA-Gene Struct. Expr. 2007, 1769, 79–91. [Google Scholar] [CrossRef]
- Hay, C.W.; Docherty, K. Comparative Analysis of Insulin Gene Promoters Implications for Diabetes Research. Diabetes 2006, 55, 3201–3213. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Rauch, T.A.; Todorov, I.; Ku, H.T.; Al-Abdullah, I.H.; Kandeel, F.; Mullen, Y.; Pfeifer, G.P.; Ferreri, K. Insulin Gene Expression Is Regulated by DNA Methylation. PLoS ONE 2009, 4, e6953. [Google Scholar] [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23. [Google Scholar] [CrossRef]
- Rui, J.; Deng, S.; Lebastchi, J.; Clark, P.L.; Usmani-Brown, S.; Herold, K.C. Methylation of Insulin DNA in Response to Pro-inflammatory Cytokines during the Progression of Autoimmune Diabetes in NOD Mice. Diabetologia 2016, 59, 1021. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, G.; Han, Y.; Xie, J.; Li, J. Circulating Unmethylated Insulin DNA as a Potential Non-Invasive Biomarker of Beta Cell Death in Type 1 Diabetes: A Review and Future Prospect. Clin. Epigenet. 2017, 9, 44. [Google Scholar] [CrossRef]
- Yang, B.T.; Dayeh, T.A.; Kirkpatrick, C.L.; Taneera, J.; Kumar, R.; Groop, L.; Wollheim, C.B.; Nitert, M.D.; Ling, C. Insulin Promoter DNA Methylation Correlates Negatively with Insulin Gene Expression and Positively with HbA1c Levels in Human Pancreatic Islets. Diabetologia 2011, 54, 360. [Google Scholar] [CrossRef]
- Ishikawa, K.; Tsunekawa, S.; Ikeniwa, M.; Izumoto, T.; Iida, A.; Ogata, H.; Uenishi, E.; Seino, Y.; Ozaki, N.; Sugimura, Y.; et al. Long-Term Pancreatic Beta Cell Exposure to High Levels of Glucose but Not Palmitate Induces DNA Methylation within the Insulin Gene Promoter and Represses Transcriptional Activity. PLoS ONE 2015, 10, e0115350. [Google Scholar] [CrossRef]
- Smith, Z.D.; Meissner, A. DNA Methylation: Roles in Mammalian Development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Pinney, S.E. DNA Methylation and Its Role in the Pathogenesis of Diabetes. Pediatr. Diabetes 2017, 18, 167. [Google Scholar] [CrossRef] [PubMed]
- Ponnaluri, V.K.C.; Ehrlich, K.C.; Zhang, G.; Lacey, M.; Johnston, D.; Pradhan, S.; Ehrlich, M. Association of 5-Hydroxymethylation and 5-Methylation of DNA Cytosine with Tissue-Specific Gene Expression. Epigenetics 2017, 12, 123–138. [Google Scholar] [CrossRef] [PubMed]
- De Mello, V.D.F.; Pulkkinen, L.; Lalli, M.; Kolehmainen, M.; Pihlajamäki, J.; Uusitupa, M. DNA Methylation in Obesity and Type 2 Diabetes. Ann. Med. 2014, 46, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Boucher, J.; Kleinridders, A.; Ronald Kahn, C. Insulin Receptor Signaling in Normal and Insulin-Resistant States. Cold Spring Harb. Perspect. Biol. 2014, 6, a009191. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Tobe, K.; Terauchi, Y.; Eto, K.; Yamauchi, T.; Suzuki, R.; Tsubamoto, Y.; Komeda, K.; Nakano, R.; Miki, H.; et al. Disruption of Insulin Receptor Substrate 2 Causes Type 2 Diabetes Because of Liver Insulin Resistance and Lack of Compensatory Beta-Cell Hyperplasia. Diabetes 2000, 49, 1880–1889. [Google Scholar] [CrossRef]
- Krause, C.; Geißler, C.; Tackenberg, H.; El Gammal, A.T.; Wolter, S.; Spranger, J.; Mann, O.; Lehnert, H.; Kirchner, H. Multi-Layered Epigenetic Regulation of IRS2 Expression in the Liver of Obese Individuals with Type 2 Diabetes. Diabetologia 2020, 63, 2182. [Google Scholar] [CrossRef]
- Ide, T.; Shimano, H.; Yahagi, N.; Matsuzaka, T.; Nakakuki, M.; Yamamoto, T.; Nakagawa, Y.; Takahashi, A.; Suzuki, H.; Sone, H.; et al. SREBPs Suppress IRS-2-Mediated Insulin Signalling in the Liver. Nat. Cell Biol. 2004, 6, 351–357. [Google Scholar] [CrossRef]
- Kelly, D.P.; Scarpulla, R.C. Transcriptional Regulatory Circuits Controlling Mitochondrial Biogenesis and Function. Genes Dev. 2004, 18, 357–368. [Google Scholar] [CrossRef]
- Westerbacka, J.; Kolak, M.; Kiviluoto, T.; Arkkila, P.; Sirén, J.; Hamsten, A.; Fisher, R.M.; Yki-Järvinen, H. Genes Involved in Fatty Acid Partitioning and Binding, Lipolysis, Monocyte/Macrophage Recruitment, and Inflammation Are Overexpressed in the Human Fatty Liver of Insulin-Resistant Subjects. Diabetes 2007, 56, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, M.; Ammerpohl, O.; Von Schönfels, W.; Kolarova, J.; Bens, S.; Itzel, T.; Teufel, A.; Herrmann, A.; Brosch, M.; Hinrichsen, H.; et al. DNA Methylation Analysis in Nonalcoholic Fatty Liver Disease Suggests Distinct Disease-Specific and Remodeling Signatures after Bariatric Surgery. Cell Metab. 2013, 18, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Rosselli, M.S.; Gemma, C.; Burgueñ, A.L.; Gianotti, T.F.; Castañ, G.O.; Pirola, C.J. Epigenetic Regulation of Insulin Resistance in Nonalcoholic Fatty Liver Disease: Impact of Liver Methylation of the Peroxisome Proliferator-Activated Receptor c Coactivator 1a Promoter. Hepatology 2010, 52, 1992–2000. [Google Scholar] [CrossRef] [PubMed]
- Gol, S.; Pena, R.N.; Rothschild, M.F.; Tor, M.; Estany, J. A Polymorphism in the Fatty Acid Desaturase-2 Gene Is Associated with the Arachidonic Acid Metabolism in Pigs. Sci. Rep. 2018, 8, 14336. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, R.; Jiang, F.; Zhang, H.; Zhao, A.; Xu, B.; Jin, L.; Wang, T.; Jia, W.; Jia, W.; et al. FADS1-FADS2 Genetic Polymorphisms Are Associated with Fatty Acid Metabolism through Changes in DNA Methylation and Gene Expression. Clin. Epigenet. 2018, 10, 113. [Google Scholar] [CrossRef] [PubMed]
- Glaser, C.; Heinrich, J.; Koletzko, B. Role of FADS1 and FADS2 Polymorphisms in Polyunsaturated Fatty Acid Metabolism. Metabolism 2010, 59, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Walle, P.; Takkunen, M.; Männistö, V.; Vaittinen, M.; Lankinen, M.; Kärjä, V.; Käkelä, P.; Ågren, J.; Tiainen, M.; Schwab, U.; et al. Fatty Acid Metabolism Is Altered in Non-Alcoholic Steatohepatitis Independent of Obesity. Metabolism 2016, 65, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Vujkovic, M.; Ramdas, S.; Lorenz, K.M.; Guo, X.; Darlay, R.; Cordell, H.J.; He, J.; Gindin, Y.; Chung, C.; Myers, R.P.; et al. A Multiancestry Genome-Wide Association Study of Unexplained Chronic ALT Elevation as a Proxy for Nonalcoholic Fatty Liver Disease with Histological and Radiological Validation. Nat. Genet. 2022, 54, 761. [Google Scholar] [CrossRef]
- Wang, X.; Sato, R.; Brown, M.S.; Hua, X.; Goldstein, J.L. SREBP-1, a Membrane-Bound Transcription Factor Released by Sterol-Regulated Proteolysis. Cell 1994, 77, 53–62. [Google Scholar] [CrossRef]
- Walle, P.; Männistö, V.; De Mello, V.D.; Vaittinen, M.; Perfilyev, A.; Hanhineva, K.; Ling, C.; Pihlajamäki, J. Liver DNA Methylation of FADS2 Associates with FADS2 Genotypex. Clin. Epigenet. 2019, 11, 10. [Google Scholar] [CrossRef]
- Ding, H.; Wu, T. Insulin-Like Growth Factor Binding Proteins in Autoimmune Diseases. Front. Endocrinol. 2018, 9, 499. [Google Scholar] [CrossRef] [PubMed]
- Rajpathak, S.N.; Gunter, M.J.; Wylie-Rosett, J.; Ho, G.Y.F.; Kaplan, R.C.; Muzumdar, R.; Rohan, T.E.; Strickler, H.D. The Role of Insulin-like Growth Factor-I and Its Binding Proteins in Glucose Homeostasis and Type 2 Diabetes. Diabetes Metab. Res. Rev. 2009, 25, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Gu, H.F.; Hilding, A.; Sjöholm, L.K.; Östenson, C.G.; Ekström, T.J.; Brismar, K. Increased DNA Methylation Levels of the Insulin-like Growth Factor Binding Protein 1 Gene Are Associated with Type 2 Diabetes in Swedish Men. Clin. Epigenet. 2013, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Shen, F.; Weinfeld, M.; Sergi, C. Insulin Growth Factor Binding Protein 7 (IGFBP7)-Related Cancer and IGFBP3 and IGFBP7 Crosstalk. Front. Oncol. 2020, 10, 727. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.F.; Gu, T.; Hilding, A.; Zhu, Y.; Kärvestedt, L.; Östenson, C.G.; Lai, M.; Kutsukake, M.; Frystyk, J.; Tamura, K.; et al. Evaluation of IGFBP-7 DNA Methylation Changes and Serum Protein Variation in Swedish Subjects with and without Type 2 Diabetes. Clin. Epigenet. 2013, 5, 20. [Google Scholar] [CrossRef]
- Robertson, K.D. DNA Methylation and Human Disease. Nat. Rev. Genet. 2005, 6, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.Y.; Park, Y.J.; Pan, X.; Shin, K.C.; Kwak, S.H.; Bassas, A.F.; Sallam, R.M.; Park, K.S.; Alfadda, A.A.; Xu, A.; et al. Obesity-Induced DNA Hypermethylation of the Adiponectin Gene Mediates Insulin Resistance. Nat. Commun. 2015, 6, 7585. [Google Scholar] [CrossRef]
- You, D.; Nilsson, E.; Tenen, D.E.; Lyubetskaya, A.; Lo, J.C.; Jiang, R.; Deng, J.; Dawes, B.A.; Vaag, A.; Ling, C.; et al. Dnmt3a Is an Epigenetic Mediator of Adipose Insulin Resistance. Elife 2017, 6, e30766. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a Novel Metabolic Regulator. J. Clin. Investig. 2005, 115, 1627. [Google Scholar] [CrossRef]
- Yang, M.L.; Horstman, S.; Gee, R.; Guyer, P.; Lam, T.K.T.; Kanyo, J.; Perdigoto, A.L.; Speake, C.; Greenbaum, C.J.; Callebaut, A.; et al. Citrullination of Glucokinase Is Linked to Autoimmune Diabetes. Nat. Commun. 2022, 13, 1870. [Google Scholar] [CrossRef]
- Ren, Y.; Li, L.; Wan, L.; Huang, Y.; Cao, S. Glucokinase as an Emerging Anti-Diabetes Target and Recent Progress in the Development of Its Agonists. J. Enzyme Inhib. Med. Chem. 2022, 37, 606. [Google Scholar] [CrossRef] [PubMed]
- Bogdarina, I.; Murphy, H.C.; Burns, S.P.; Clark, A.J.L. Investigation of the Role of Epigenetic Modification of the Rat Glucokinase Gene in Fetal Programming. Life Sci. 2004, 74, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.H.; Fei, J.; Lan, M.S.; Lu, Z.P.; Liu, M.; Fan, W.W.; Gao, X.; Lu, D.R. Hypermethylation of Hepatic Gck Promoter in Ageing Rats Contributes to Diabetogenic Potential. Diabetologia 2008, 51, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, C.; Donnelly, D.; Wootten, D.; Lau, J.; Sexton, P.M.; Miller, L.J.; Ahn, J.M.; Liao, J.; Fletcher, M.M.; Yang, D.; et al. Glucagon-Like Peptide-1 and Its Class B G Protein–Coupled Receptors: A Long March to Therapeutic Successes. Pharmacol. Rev. 2016, 68, 954. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, M.; Wen, Z.; Lu, Z.; Cui, L.; Fu, C.; Xue, H.; Liu, Y.; Zhang, Y. GLP-1 Receptor Agonists: Beyond Their Pancreatic Effects. Front. Endocrinol. 2021, 12, 1040. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.; Dayeh, T.; Kirkpatrick, C.L.; Wollheim, C.B.; Dekker Nitert, M.; Ling, C. DNA Methylation of the Glucagon-like Peptide 1 Receptor (GLP1R) in Human Pancreatic Islets. BMC Med. Genet. 2013, 14, 76. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.Y.; Denu, J.M.; Allis, C.D. Histone Acetyltransferases. Annu. Rev. Biochem. 2003, 70, 81–120. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, X.; Sun, X.; Hu, W.; Miao, Q.R. The Role of Histone Protein Acetylation in Regulating Endothelial Function. Front. Cell Dev. Biol. 2021, 9, 672447. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Kaiser, C.; James, S.R. Acetylation of Insulin Receptor Substrate-1 Is Permissive for Tyrosine Phosphorylation. BMC Biol. 2004, 2, 23. [Google Scholar] [CrossRef]
- Beljanski, V.; Trichostatin, A. xPharm: The Comprehensive Pharmacology Reference; Elsevier: Amsterdam, The Netherlands, 2009; pp. 1–4. [Google Scholar] [CrossRef]
- Zeng, Z.; Liao, R.; Yao, Z.; Zhou, W.; Ye, P.; Zheng, X.; Li, X.; Huang, Y.; Chen, S.; Chen, Q. Three Single Nucleotide Variants of the HDAC Gene Are Associated with Type 2 Diabetes Mellitus in a Chinese Population: A Community-Based Case–Control Study. Gene 2014, 533, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Miller, R.A.; Patel, R.T.; Chen, J.; Dhir, R.; Wang, H.; Zhang, D.; Graham, M.J.; Unterman, T.G.; Shulman, G.I.; et al. Hepatic Hdac3 Promotes Gluconeogenesis by Repressing Lipid Synthesis and Sequestration. Nat. Med. 2012, 18, 934. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ye, X.; Guo, W.; Lu, H.; Gao, Z. Inhibition of HDAC3 Promotes Ligand-Independent PPARγ Activation by Protein Acetylation. J. Mol. Endocrinol. 2014, 53, 191. [Google Scholar] [CrossRef] [PubMed]
- Ornitz, D.M.; Itoh, N. Fibroblast Growth Factors. Genome Biol. 2001, 2, reviews3005.1. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gao, Z.; Zhang, J.; Ye, X.; Xu, A.; Ye, J.; Jia, W. Sodium Butyrate Stimulates Expression of Fibroblast Growth Factor 21 in Liver by Inhibition of Histone Deacetylase 3. Diabetes 2012, 61, 797–806. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, N.; Guo, X.; Li, J.; Zhang, T.; Ren, G.; Li, D. Fibroblast Growth Factor 21 Regulates Glucose Metabolism in Part by Reducing Renal Glucose Reabsorption. Biomed. Pharmacother. 2018, 108, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.G.; Jornayvaz, F.R.; Petersen, M.C.; Pesta, D.; Guigni, B.A.; Serr, J.; Zhang, D.; Kahn, M.; Samuel, V.T.; Jurczak, M.J.; et al. Cellular Mechanisms by Which FGF21 Improves Insulin Sensitivity in Male Mice. Endocrinology 2013, 154, 3099. [Google Scholar] [CrossRef] [PubMed]
- Szczepańska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Gu, J.; Jiang, S.; Liu, Q.; Zheng, Y.; Freedman, J.H.; Sun, J.; Cai, L. HDAC3 Inhibition in Diabetic Mice May Activate Nrf2 Preventing Diabetes-Induced Liver Damage and FGF21 Synthesis and Secretion Leading to Aortic Protection. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E150–E162. [Google Scholar] [CrossRef]
- Thai, M.V.; Guruswamy, S.; Cao, K.T.; Pessin, J.E.; Olson, A.L. Myocyte Enhancer Factor 2 (MEF2)-Binding Site Is Required ForGLUT4 Gene Expression in Transgenic Mice. J. Biol. Chem. 1998, 273, 14285–14292. [Google Scholar] [CrossRef]
- Sparrow, D.B.; Miska, E.A.; Langley, E.; Reynaud-Deonauth, S.; Kotecha, S.; Towers, N.; Spohr, G.; Kouzarides, T.; Mohun, T.J. MEF-2 Function Is Modified by a Novel Co-Repressor, MITR. EMBO J. 1999, 18, 5085. [Google Scholar] [CrossRef] [PubMed]
- Nikzamir, A.; Palangi, A.; Kheirollaha, A.; Tabar, H.; Malakaskar, A.; Shahbazian, H.; Fathi, M. Expression of Glucose Transporter 4 (GLUT4) Is Increased by Cinnamaldehyde in C2C12 Mouse Muscle Cells. Iran. Red Crescent Med. J. 2014, 16, 13426. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Rhee, J.; Lin, J.; Tarr, P.T.; Spiegelman, B.M. An Autoregulatory Loop Controls Peroxisome Proliferator-Activated Receptor γ Coactivator 1α Expression in Muscle. Proc. Natl. Acad. Sci. USA 2003, 100, 7111. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; McKinsey, T.A.; Nicol, R.L.; Olson, E.N. Signal-Dependent Activation of the MEF2 Transcription Factor by Dissociation from Histone Deacetylases. Proc. Natl. Acad. Sci. USA 2000, 97, 4070. [Google Scholar] [CrossRef] [PubMed]
- McKinsey, T.A.; Zhang, C.L.; Lu, J.; Olson, E.N. Signal-Dependent Nuclear Export of a Histone Deacetylase Regulates Muscle Differentiation. Nature 2000, 408, 106. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Benz, V.; Clemenz, M.; Bloch, M.; Foryst-Ludwig, A.; Wardat, S.; Witte, N.; Trappiel, M.; Namsolleck, P.; Mai, K.; et al. Histone Deacetylase 6 (HDAC6) Is an Essential Modifier of Glucocorticoid-Induced Hepatic Gluconeogenesis. Diabetes 2012, 61, 513. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Vasquez, D.S.; Ravnskjaer, K.; Denechaud, P.D.; Yu, R.T.; Alvarez, J.G.; Downes, M.; Evans, R.M.; Montminy, M.; Shaw, R.J. Class IIa Histone Deacetylases Are Hormone-Activated Regulators of FOXO and Mammalian Glucose Homeostasis. Cell 2011, 145, 607. [Google Scholar] [CrossRef]
- Wang, J.; Gong, B.; Zhao, W.; Tang, C.; Varghese, M.; Nguyen, T.; Bi, W.; Bilski, A.; Begum, S.; Vempati, P.; et al. Epigenetic Mechanisms Linking Diabetes and Synaptic Impairments. Diabetes 2014, 63, 645–654. [Google Scholar] [CrossRef]
- Kellar, D.; Craft, S. Brain Insulin Resistance in Alzheimer’s Disease and Related Disorders: Mechanisms and Therapeutic Approaches. Lancet Neurol. 2020, 19, 758. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and Fl-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef]
- Baker, L.D.; Cross, D.J.; Minoshima, S.; Belongia, D.; Stennis Watson, G.; Craft, S. Insulin Resistance Is Associated with Alzheimer-Like Reductions in Regional Cerebral Glucose Metabolism for Cognitively Normal Adults with Pre-Diabetes or Early Type 2 Diabetes. Arch. Neurol. 2011, 68, 51. [Google Scholar] [CrossRef]
- Craft, S. Insulin Resistance Syndrome and Alzheimer’s Disease: Age- and Obesity-Related Effects on Memory, Amyloid, and Inflammation. Neurobiol. Aging 2005, 26, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.R.; Astell, A.; Green, C.; Sutherland, C. Molecular Connexions between Dementia and Diabetes. Neurosci. Biobehav. Rev. 2007, 31, 1046–1063. [Google Scholar] [CrossRef] [PubMed]
- Neumann, K.; Rojo, L.; Navarrete, L.; Farias, G.; Reyes, P.; Maccioni, R. Insulin Resistance and Alzheimers Disease: Molecular Links & Clinical Implications. Curr. Alzheimer Res. 2008, 5, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Sims-Robinson, C.; Kim, B.; Rosko, A.; Feldman, E.L. How Does Diabetes Accelerate Alzheimer Disease Pathology? Nat. Rev. Neurol. 2010, 6, 551. [Google Scholar] [CrossRef] [PubMed]
- Cholerton, B.; Baker, L.D.; Craft, S. Insulin Resistance and Pathological Brain Ageing. Diabet. Med. 2011, 28, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.Q.; Townsend, M. Insulin Resistance and Amyloidogenesis as Common Molecular Foundation for Type 2 Diabetes and Alzheimer’s Disease. Biochim. Biophys. Acta Mol. Basis Dis. 2009, 1792, 482–496. [Google Scholar] [CrossRef]
- Correia, S.C.; Santos, R.X.; Perry, G.; Zhu, X.; IMoreira, P.I.; Smith, M.A. Insulin-Resistant Brain State: The Culprit in Sporadic Alzheimer’s Disease? Ageing Res. Rev. 2011, 10, 264. [Google Scholar] [CrossRef]
- Hoyer, S. Sporadic Alzheimer Disease: A Challenging Hypothesis Is Sporadic Alzheimer Disease the Brain Type of Non-Insulin Dependent Diabetes Mellitus? A Challenging Hypothesis. J. Neural Transm. 1998, 105, 415–422. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, F.; Grundke-Iqbal, I.; Iqbal, K.; Gong, C.X. Deficient Brain Insulin Signalling Pathway in Alzheimer’s Disease and Diabetes. J. Pathol. 2011, 225, 54. [Google Scholar] [CrossRef]
- Rivera, E.J.; Goldin, A.; Fulmer, N.; Tavares, R.; Wands, J.R.; De La Monte, S.M. Insulin and Insulin-like Growth Factor Expression and Function Deteriorate with Progression of Alzheimer’s Disease: Link to Brain Reductions in Acetylcholine. J. Alzheimer’s Dis. 2005, 8, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Talbot, K.; Han, L.; Schneider, J.A.; Wilson, R.S.; Bennett, D.A.; Arnold, S.E. O3–02–02: Expression of PIRS–1 (S312 and S616) Is Elevated in MCI and AD and Correlates with Cognitive Impairment and Neurofibrillary Pathology. Alzheimer’s Dement. 2006, 2, S54. [Google Scholar] [CrossRef]
- Moloney, A.M.; Griffin, R.J.; Timmons, S.; O’Connor, R.; Ravid, R.; O’Neill, C. Defects in IGF-1 Receptor, Insulin Receptor and IRS-1/2 in Alzheimer’s Disease Indicate Possible Resistance to IGF-1 and Insulin Signalling. Neurobiol. Aging 2010, 31, 224–243. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.L.; Yang, F.; Rosario, E.R.; Ubeda, O.J.; Beech, W.; Gant, D.J.; Ping, P.C.; Hudspeth, B.; Chen, C.; Zhao, Y.; et al. β-Amyloid Oligomers Induce Phosphorylation of Tau and Inactivation of Insulin Receptor Substrate via c-Jun N-Terminal Kinase Signaling: Suppression by Omega-3 Fatty Acids and Curcumin. J. Neurosci. 2009, 29, 9078. [Google Scholar] [CrossRef] [PubMed]
- Apelt, J.; Mehlhorn, G.; Schliebs, R. Insulin-Sensitive GLUT4 Glucose Transporters Are Colocalized with GLUT3-Expressing Cells and Demonstrate a Chemically Distinct Neuron-Specific Localization in Rat Brain. J. Neurosci. Res. 1999, 57, 693–705. [Google Scholar] [CrossRef]
- Ibberson, M.; Uldry, M.; Thorens, B. GLUTX1, a Novel Mammalian Glucose Transporter Expressed in the Central Nervous System and Insulin-Sensitive Tissues. J. Biol. Chem. 2000, 275, 4607–4612. [Google Scholar] [CrossRef]
- Craft, S.; Peskind, E.; Schwartz, M.W.; Schellenberg, G.D.; Raskind, M.; Porte, D. Cerebrospinal Fluid and Plasma Insulin Levels in Alzheimer’s Disease. Neurology 1998, 50, 164–168. [Google Scholar] [CrossRef] [PubMed]
- de la Monte, S.M.; Wands, J.R. Molecular Indices of Oxidative Stress and Mitochondrial Dysfunction Occur Early and Often Progress with Severity of Alzheimer’s Disease. J. Alzheimer’s Dis. 2006, 9, 167–181. [Google Scholar] [CrossRef]
- Erol, A. An Integrated and Unifying Hypothesis for the Metabolic Basis of Sporadic Alzheimer’s Disease. J. Alzheimer’s Dis. 2008, 13, 241–253. [Google Scholar] [CrossRef]
- Choeiri, C.; Staines, W.; Messier, C. Immunohistochemical Localization and Quantification of Glucose Transporters in the Mouse Brain. Neuroscience 2002, 111, 19–34. [Google Scholar] [CrossRef]
- Moreira, P.I.; Duarte, A.I.; Santos, M.S.; Rego, A.C.; Oliveira, C.R. An Integrative View of the Role of Oxidative Stress, Mitochondria and Insulin in Alzheimer’s Disease. J. Alzheimer’s Dis. 2009, 16, 741–761. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Preissl, H.; Hennige, A.M.; Stumvoll, M.; Porubska, K.; Frost, R.; Marx, H.; Klösel, B.; Lutzenberger, W.; Birbaumer, N.; et al. The Cerebrocortical Response to Hyperinsulinemia Is Reduced in Overweight Humans: A Magnetoencephalographic Study. Proc. Natl. Acad. Sci. USA 2006, 103, 12103. [Google Scholar] [CrossRef] [PubMed]
- Anthony, K.; Reed, L.J.; Dunn, J.T.; Bingham, E.; Hopkins, D.; Marsden, P.K.; Amiel, S.A. Attenuation of Insulin-Evoked Responses in Brain Networks Controlling Appetite and Reward in Insulin Resistance the Cerebral Basis for Impaired Control of Food Intake in Metabolic Syndrome? Diabetes 2006, 55, 2986–2992. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Hennige, A.M.; Preissl, H.; Porubska, K.; Schäfer, S.A.; Lutzenberger, W.; Machicao, F.; Birbaumer, N.; Fritsche, A.; Häring, H.U. Cerebrocortical Beta Activity in Overweight Humans Responds to Insulin Detemir. PLoS ONE 2007, 2, 1196. [Google Scholar] [CrossRef] [PubMed]
- Hirvonen, J.; Virtanen, K.A.; Nummenmaa, L.; Hannukainen, J.C.; Honka, M.J.; Bucci, M.; Nesterov, S.V.; Parkkola, R.; Rinne, J.; Iozzo, P.; et al. Effects of Insulin on Brain Glucose Metabolism in Impaired Glucose Tolerance. Diabetes 2011, 60, 443. [Google Scholar] [CrossRef]
- Kullmann, S.; Frank, S.; Heni, M.; Ketterer, C.; Veit, R.; Häring, H.U.; Fritsche, A.; Preissl, H. Intranasal Insulin Modulates Intrinsic Reward and Prefrontal Circuitry of the Human Brain in Lean Women. Neuroendocrinology 2013, 97, 176–182. [Google Scholar] [CrossRef]
- Kullmann, S.; Heni, M.; Veit, R.; Scheffler, K.; Machann, J.; Häring, H.U.; Fritsche, A.; Preissl, H. Selective Insulin Resistance in Homeostatic and Cognitive Control Brain Areas in Overweight and Obese Adults. Diabetes Care 2015, 38, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Kullmann, S.; Heni, M.; Hallschmid, M.; Fritsche, A.; Preissl, H.; Häring, H.-U. Crossroads of Metabolic and Cognitive Disorders in Humans. Physiol. Rev. 2016, 96, 1169–1209. [Google Scholar] [CrossRef]
- DeFronzo, R.A. The Triumvirate: β-Cell, Muscle, Liver: A Collusion Responsible for NIDDM. Diabetes 1988, 37, 667–687. [Google Scholar] [CrossRef]
- Shulman, G.I. Cellular Mechanisms of Insulin Resistance. J. Clin. Investig. 2000, 106, 171. [Google Scholar] [CrossRef]
- Shulman, G.I.; Rothman, D.L.; Jue, T.; Stein, P.; DeFronzo, R.A.; Shulman, R.G. Quantitation of Muscle Glycogen Synthesis in Normal Subjects and Subjects with Non-Insulin-Dependent Diabetes by 13C Nuclear Magnetic Resonance Spectroscopy. N. Engl. J. Med. 2010, 322, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Sesti, G. Pathophysiology of Insulin Resistance. Best. Pract. Res. Clin. Endocrinol. Metab. 2006, 20, 665–679. [Google Scholar] [CrossRef] [PubMed]
- Frölich, L.; Blum-Degen, D.; Bernstein, H.G.; Engelsberger, S.; Humrich, J.; Laufer, S.; Muschner, D.; Thalheimer, A.; Türk, A.; Hoyer, S.; et al. Brain Insulin and Insulin Receptors in Aging and Sporadic Alzheimer’s Disease. J. Neural Transm. 1998, 105, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Teter, B.; Morihara, T.; Lim, G.P.; Ambegaokar, S.S.; Ubeda, O.J.; Frautschy, S.A.; Cole, G.M. Insulin-Degrading Enzyme as a Downstream Target of Insulin Receptor Signaling Cascade: Implications for Alzheimer’s Disease Intervention. J. Neurosci. 2004, 24, 11120. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.A.; Adjei, I.M.; Labhasetwar, V. Advancements in the Delivery of Epigenetic Drugs. Expert. Opin. Drug Deliv. 2015, 12, 1501. [Google Scholar] [CrossRef] [PubMed]
- Mayersohn, M. Pharmacokinetics in the Elderly. Environ. Health Perspect. 1994, 102, 119. [Google Scholar] [CrossRef] [PubMed]
- El Bahhaj, F.; Dekker, F.J.; Martinet, N.; Bertrand, P. Delivery of Epidrugs. Drug Discov. Today 2014, 19, 1337–1352. [Google Scholar] [CrossRef] [PubMed]
- Narang, A.S.; Boddu, S.H. Excipient Applications in Formulation Design and Drug Delivery. In Excipient Applications in Formulation Design and Drug Delivery; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–10. [Google Scholar] [CrossRef]
- Wang, Z.; Tiruppathi, C.; Minshall, R.D.; Malik, A.B. Size and Dynamics of Caveolae Studied Using Nanoparticles in Living Endothelial Cells. ACS Nano 2009, 3, 4110. [Google Scholar] [CrossRef]
- González-Maciel, A.; Reynoso-Robles, R.; Torres-Jardón, R.; Mukherjee, P.S.; Calderón-Garcidueñas, L. Combustion-Derived Nanoparticles in Key Brain Target Cells and Organelles in Young Urbanites: Culprit Hidden in Plain Sight in Alzheimer’s Disease Development. J. Alzheimer’s Dis. 2017, 59, 189–208. [Google Scholar] [CrossRef]
- Sadanandan, P.; Payne, N.L.; Sun, G.; Ashokan, A.; Gowd, S.G.; Lal, A.; Satheesh Kumar, M.K.; Pulakkat, S.; Nair, S.V.; Menon, K.N.; et al. Exploiting the Preferential Phagocytic Uptake of Nanoparticle-Antigen Conjugates for the Effective Treatment of Autoimmunity. Nanomedicine 2022, 40, 102481. [Google Scholar] [CrossRef]
- Yang, X.; He, C.; Li, J.; Chen, H.; Ma, Q.; Sui, X.; Tian, S.; Ying, M.; Zhang, Q.; Luo, Y.; et al. Uptake of Silica Nanoparticles: Neurotoxicity and Alzheimer-like Pathology in Human SK-N-SH and Mouse Neuro2a Neuroblastoma Cells. Toxicol. Lett. 2014, 229, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Fatima, M.T.; Islam, Z.; Khan, R.H.; Uversky, V.N.; Salahuddin, P. Nanoparticle Formulations in the Diagnosis and Therapy of Alzheimer’s Disease. Int. J. Biol. Macromol. 2019, 130, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, L.; Cano, A.; Ettcheto, M.; Souto, E.B.; Espina, M.; Camins, A.; García, M.L.; Sánchez-López, E. Surface Functionalization of PLGA Nanoparticles to Increase Transport across the BBB for Alzheimer’s Disease. Appl. Sci. 2021, 11, 4305. [Google Scholar] [CrossRef]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin Loaded-PLGA Nanoparticles Conjugated with Tet-1 Peptide for Potential Use in Alzheimer’s Disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef]
- Jeon, S.G.; Cha, M.Y.; Kim, J.I.; Hwang, T.W.; Kim, K.A.; Kim, T.H.; Song, K.C.; Kim, J.J.; Moon, M. Vitamin D-Binding Protein-Loaded PLGA Nanoparticles Suppress Alzheimer’s Disease-Related Pathology in 5XFAD Mice. Nanomedicine 2019, 17, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.M.; Zecchi, V. Albumin Nanoparticles Carrying Cyclodextrins for Nasal Delivery of the Anti-Alzheimer Drug Tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Li, M.; Wu, L.; Chen, C.; Qu, X. Mesoporous Silica Nanoparticle-Based H2O2 Responsive Controlled-Release System Used for Alzheimer’s Disease Treatment. Adv. Healthc. Mater. 2012, 1, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Geetha, K.M. Neurotherapeutic Applications of Nanomedicine for Treating Alzheimer’s Disease. J. Control. Release 2020, 325, 25–37. [Google Scholar] [CrossRef]
- Ashokan, A.; Somasundaram, V.H.; Gowd, G.S.; Anna, I.M.; Malarvizhi, G.L.; Sridharan, B.; Jobanputra, R.B.; Peethambaran, R.; Unni, A.K.K.; Nair, S.; et al. Biomineral Nano-Theranostic Agent for Magnetic Resonance Image Guided, Augmented Radiofrequency Ablation of Liver Tumor. Sci. Rep. 2017, 7, 14481. [Google Scholar] [CrossRef]
- Ramachandran, R.; Junnuthula, V.R.; Gowd, G.S.; Ashokan, A.; Thomas, J.; Peethambaran, R.; Thomas, A.; Unni, A.K.K.; Panikar, D.; Nair, S.V.; et al. Theranostic 3-Dimensional Nano Brain-Implant for Prolonged and Localized Treatment of Recurrent Glioma. Sci. Rep. 2017, 7, 43271. [Google Scholar] [CrossRef]
- Wang, W.; Liu, M.; Gao, W.; Sun, Y.; Dong, X. Coassembled Chitosan-Hyaluronic Acid Nanoparticles as a Theranostic Agent Targeting Alzheimer’s β-Amyloid. ACS Appl. Mater. Interfaces 2021, 13, 55879–55889. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome Delivery Systems for the Treatment of Alzheimer’s Disease. Int. J. Nanomed. 2018, 13, 8507. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Shao, X.; Zhang, C.; Tan, Y.; Liu, Q.; Wan, X.; Zhang, Q.; Xu, S.; Jiang, X. Intranasal H102 Peptide-Loaded Liposomes for Brain Delivery to Treat Alzheimer’s Disease. Pharm. Res. 2015, 32, 3837–3849. [Google Scholar] [CrossRef] [PubMed]
- Balducci, C.; Mancini, S.; Minniti, S.; La Vitola, P.; Zotti, M.; Sancini, G.; Mauri, M.; Cagnotto, A.; Colombo, L.; Fiordaliso, F.; et al. Multifunctional Liposomes Reduce Brain β-Amyloid Burden and Ameliorate Memory Impairment in Alzheimer’s Disease Mouse Models. J. Neurosci. 2014, 34, 14022. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Lopes, I.; Magalhães, L.; Sárria, M.P.; Machado, R.; Sousa, J.C.; Botelho, C.; Teixeira, J.; Gomes, A.C. Novel Concept of Exosome-like Liposomes for the Treatment of Alzheimer’s Disease. J. Control. Release 2021, 336, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Rawtani, D.; Barot, T. Design, Development and in-Vitro/in-Vivo Evaluation of Intranasally Delivered Rivastigmine and N-Acetyl Cysteine Loaded Bifunctional Niosomes for Applications in Combinative Treatment of Alzheimer’s Disease. Eur. J. Pharm. Biopharm. 2021, 163, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Novel Piperine-Loaded Tween-Integrated Monoolein Cubosomes as Brain-Targeted Oral Nanomedicine in Alzheimer’s Disease: Pharmaceutical, Biological, and Toxicological Studies. Int. J. Nanomed. 2015, 10, 5459–5473. [Google Scholar] [CrossRef] [PubMed]
- Mohsen, A.M.; Salama, A.A.A.; Asfour, M.H. Cubosome-Based Thermosensitive in Situ Gelling System for Intranasal Administration of Lamotrigine with Enhanced Antiepileptic Efficacy. Pharm. Dev. Technol. 2023, 28, 520–534. [Google Scholar] [CrossRef]
- Raina, N.; Rani, R.; Khan, A.; Nagpal, K.; Gupta, M. Interpenetrating Polymer Network as a Pioneer Drug Delivery System: A Review. Polym. Bull. 2020, 77, 5027–5050. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, V.A.; Mahmood, T.; Ahsan, F.; Wasim, R. Dendrimers: A Neuroprotective Lead in Alzheimer Disease: A Review on Its Synthetic Approach and Applications. Drug Res. 2022, 72, 417–423. [Google Scholar] [CrossRef]
- Singh, A.; Ujjwal, R.R.; Naqvi, S.; Verma, R.K.; Tiwari, S.; Kesharwani, P.; Shukla, R. Formulation Development of Tocopherol Polyethylene Glycol Nanoengineered Polyamidoamine Dendrimer for Neuroprotection and Treatment of Alzheimer Disease. J. Drug Target. 2022, 30, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Wasiak, T.; Marcinkowska, M.; Pieszynski, I.; Zablocka, M.; Caminade, A.M.; Majoral, J.P.; Klajnert-Maculewicz, B. Cationic Phosphorus Dendrimers and Therapy for Alzheimer’s Disease. New J. Chem. 2015, 39, 4852–4859. [Google Scholar] [CrossRef]
- Zha, L.; Banik, B.; Alexis, F. Stimulus Responsive Nanogels for Drug Delivery. Soft Matter 2011, 7, 5908–5916. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-Based Drug Delivery Systems for the Treatment of Alzheimer’s Disease. Int. J. Nanomed. 2015, 10, 4981. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Radtke, M.; Wissing, S.A. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) in Cosmetic and Dermatological Preparations. Adv. Drug Deliv. Rev. 2002, 54, S131–S155. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.; Yeom, S.Y.; Kim, J.; Jung, S.; Jung, S.; Shim, T.S.; Kim, S.K.; Kang, J.Y.; Lee, S.H.; Cho, I.J.; et al. Hydrogel Micropost-Based QPCR for Multiplex Detection of MiRNAs Associated with Alzheimer’s Disease. Biosens. Bioelectron. 2018, 101, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Adak, A.; Das, G.; Barman, S.; Mohapatra, S.; Bhunia, D.; Jana, B.; Ghosh, S. Biodegradable Neuro-Compatible Peptide Hydrogel Promotes Neurite Outgrowth, Shows Significant Neuroprotection, and Delivers Anti-Alzheimer Drug. ACS Appl. Mater. Interfaces 2017, 9, 5067–5076. [Google Scholar] [CrossRef]
- Macdiarmid, J.A.; Mugridge, N.B.; Weiss, J.C.; Phillips, L.; Burn, A.L.; Paulin, R.P.; Haasdyk, J.E.; Dickson, K.-A.; Brahmbhatt, V.N.; Pattison, S.T.; et al. Article Bacterially Derived 400 Nm Particles for Encapsulation and Cancer Cell Targeting of Chemotherapeutics. Cancer Cell 2007, 11, 431–435. [Google Scholar] [CrossRef]
- Bi, F.C.; Yang, X.H.; Cheng, X.Y.; Deng, W.B.; Guo, X.L.; Yang, H.; Wang, Y.; Li, J.; Yao, Y. Optimization of Cerebral Organoids: A More Qualified Model for Alzheimer’s Disease Research. Transl. Neurodegener. 2021, 10, 27. [Google Scholar] [CrossRef]
- Gerakis, Y.; Hetz, C. Brain Organoids: A next Step for Humanized Alzheimer’s Disease Models? Mol. Psychiatry 2019, 24, 474–478. [Google Scholar] [CrossRef]
- Unnisa, A.; Greig, N.; Kamal, M. Nanotechnology-Based Gene Therapy as a Credible Tool in the Treatment of Alzheimer’s Disease. Neural Regen. Res. 2023, 18, 2127. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cao, Y.; Ye, J.; Yang, Z.; Chen, Q.; Liu, X.; Zhang, B.; Qiao, J.; Tang, Q.; Yang, H.; et al. Engineering Brain-Derived Neurotrophic Factor MRNA Delivery for the Treatment of Alzheimer’s Disease. Chem. Eng. J. 2023, 466, 143152. [Google Scholar] [CrossRef]
- Imran Sajid, M.; Sultan Sheikh, F.; Anis, F.; Nasim, N.; Sumbria, R.K.; Nauli, S.M.; Kumar Tiwari, R. SiRNA Drug Delivery across the Blood–Brain Barrier in Alzheimer’s Disease. Adv. Drug Deliv. Rev. 2023, 199, 114968. [Google Scholar] [CrossRef]
- Bhatnagar, D.; Ladhe, S.; Kumar, D. Discerning the Prospects of MiRNAs as a Multi-Target Therapeutic and Diagnostic for Alzheimer’s Disease. Mol. Neurobiol. 2023, 60, 5954–5974. [Google Scholar] [CrossRef]
- Mummery, C.J.; Börjesson-Hanson, A.; Blackburn, D.J.; Vijverberg, E.G.B.; De Deyn, P.P.; Ducharme, S.; Jonsson, M.; Schneider, A.; Rinne, J.O.; Ludolph, A.C.; et al. Tau-Targeting Antisense Oligonucleotide MAPTRx in Mild Alzheimer’s Disease: A Phase 1b, Randomized, Placebo-Controlled Trial. Nat. Med. 2023, 29, 1437. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).