Feasibility and Reliability of Quadriceps Muscle Power and Optimal Movement Velocity Measurements in Different Populations of Subjects
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Protocol
2.3. Maximum Power (Pmax) and Optimal Movement Velocity (υopt) of the Knee Extensor Muscles
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Muscle Strength and Functional Tests
4.2. Power Measurements
4.3. Power Measurements in Older Subjects and Those with Diseases
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petermann-Rocha, F.; Balntzi, V.; Gray, S.R.; Lara, J.; Ho, F.K.; Pell, J.P.; Celis-Morales, C. Global prevalence of sarcopenia and severe sarcopenia: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 86–99. [Google Scholar] [CrossRef]
- Veen, J.; Montiel-Rojas, D.; Kadi, F.; Nilsson, A. Effects of Reallocating Time Spent in Different Physical Activity Intensities on Sarcopenia Risk in Older Adults: An Isotemporal Substitution Analysis. Biology 2022, 11, 111. [Google Scholar] [CrossRef]
- Alcazar, J.; Rodriguez-Lopez, C.; Delecluse, C.; Thomis, M.; Van Roie, E. Ten-year longitudinal changes in muscle power, force, and velocity in young, middle-aged, and older adults. J. Cachexia Sarcopenia Muscle 2023, 14, 1019–1032. [Google Scholar] [CrossRef]
- Fukuoka, A.H.; de Oliveira, N.M.; Matias, C.N.; Teixeira, F.J.; Monteiro, C.P.; Valamatos, M.J.; Reis, J.F.; Gonçalves, E.M. Association between Phase Angle from Bioelectric Impedance and Muscular Strength and Power in Physically Active Adults. Biology 2022, 11, 1255. [Google Scholar] [CrossRef]
- Metter, E.J.; Conwit, R.; Tobin, J.; Fozard, J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, B267–B276. [Google Scholar] [CrossRef]
- Guralnik, J.M.; Ferrucci, L.; Simonsick, E.M.; Salive, M.E.; Wallace, R.B. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N. Engl. J. Med. 1995, 332, 556–561. [Google Scholar] [CrossRef]
- Hautier, C.A.; Linossier, M.T.; Belli, A.; Lacour, J.R.; Arsac, L.M. Optimal velocity for maximal power production in non-isokinetic cycling is related to muscle fibre type composition. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 74, 114–118. [Google Scholar] [CrossRef]
- Arsac, L.M.; Belli, A.; Lacour, J.R. Muscle function during brief maximal exercise: Accurate measurements on a friction-loaded cycle ergometer. Eur. J. Appl. Physiol. 1996, 74, 100–106. [Google Scholar] [CrossRef]
- Kozicka, I.; Kostka, T. Handgrip strength, quadriceps muscle power, and optimal shortening velocity roles in maintaining functional abilities in older adults living in a long-term care home: A 1-year follow-up study. Clin. Interv. Aging 2016, 26, 739–747. [Google Scholar] [CrossRef]
- Kostka, J.S.; Czernicki, J.W.; Kostka, T.J. Association of muscle strength, power, and optimal shortening velocity with functional abilities of women with chronic osteoarthritis participating in a multi-modal exercise program. J. Aging Phys. Act. 2014, 22, 564–570. [Google Scholar] [CrossRef]
- Driss, T.; Vandewalle, H. The measurement of maximal (anaerobic) power output on a cycle ergometer: A critical review. Biomed. Res. Int. 2013, 2013, 589361. [Google Scholar] [CrossRef]
- Lakomy, H.K.A. Measurements of work and power output using friction-loaded cycle ergometers. Ergonomics 1986, 29, 509–517. [Google Scholar] [CrossRef]
- Hartmann, A.; Knols, R.; Murer, K.; de Bruin, E.D. Reproducibility of an isokinetic strength-testing protocol of the knee and ankle in older adults. Gerontology 2009, 55, 259–268. [Google Scholar] [CrossRef]
- Buckinx, F.; Croisier, J.L.; Reginster, J.Y.; Dardenne, N.; Beaudart, C.; Slomian, J.; Leonard, S.; Bruyère, O. Reliability of muscle strength measures obtained with a hand-held dynamometer in an elderly population. Clin. Physiol. Funct. Imaging 2017, 37, 332–340. [Google Scholar] [CrossRef]
- Bohannon, R.W. Test-Retest Reliability of Measurements of Hand-Grip Strength Obtained by Dynamometry from Older Adults: A Systematic Review of Research in the PubMed Database. J. Frailty Aging 2017, 6, 83–87. [Google Scholar] [CrossRef]
- Bohannon, R.W. Test-retest reliability of the five-repetition sit-to-stand test: A systematic review of the literature involving adults. J. Strength Cond. Res. 2011, 25, 3205–3207. [Google Scholar] [CrossRef]
- Muñoz-Bermejo, L.; Adsuar, J.C.; Mendoza-Muñoz, M.; Barrios-Fernández, S.; Garcia-Gordillo, M.A.; Pérez-Gómez, J.; Carlos-Vivas, J. Test-Retest Reliability of Five Times Sit to Stand Test (FTSST) in Adults: A Systematic Review and Meta-Analysis. Biology 2021, 10, 510. [Google Scholar] [CrossRef]
- McAllister, L.S.; Palombaro, K.M. Modified 30-Second Sit-to-Stand Test: Reliability and Validity in Older Adults Unable to Complete Traditional Sit-to-Stand Testing. J. Geriatr. Phys. Ther. 2020, 43, 153–158. [Google Scholar] [CrossRef]
- Jarnlo, G.-B.; Nordell, E. Reliability of the modified figure of eight--a balance performance test for elderly women. Physiother. Theory Pract. 2003, 19, 35–43. [Google Scholar] [CrossRef]
- Holsgaard Larsen, A.; Caserotti, P.; Puggaard, L.; Aagaard, P. Reproducibility and relationship of single-joint strength vs. multi-joint strength and power in aging individuals. Scand. J. Med. Sci. Sports 2007, 17, 43–53. [Google Scholar] [CrossRef]
- Rockwood, K.; Awalt, E.; Carver, D.; MacKnight, C. Feasibility and measurement properties of the functional reach and the timed up and go tests in the Canadian study of health and aging. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M70-3. [Google Scholar] [CrossRef]
- Beauchamp, M.K.; Hao, Q.; Kuspinar, A.; D’Amore, C.; Scime, G.; Ma, J.; Mayhew, A.; Bassim, C.; Wolfson, C.; Kirkland, S.; et al. Reliability and Minimal Detectable Change Values for Performance-Based Measures of Physical Functioning in the Canadian Longitudinal Study on Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 2030–2038. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Schabort, E.J.; Hawley, J.A. Reliability of power in physical performance tests. Sports Med. 2001, 31, 211–234. [Google Scholar] [CrossRef]
- Bosco, C.; Belli, A.; Astrua, M.; Tihanyi, J.; Pozzo, R.; Kellis, S.; Tsarpela, O.; Foti, C.; Manno, R.; Tranquilli, C. A dynamometer for evaluation of dynamic muscle work. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 70, 379–386. [Google Scholar] [CrossRef]
- Attiogbe, E.; Driss, T.; Rouis, M.; Vandewalle, H.; Le Pellec-Muller, A. Etude de la reproductibilite des indices de l’epreuve charge-vitesse sur ergocycle pour les membres inferieurs et les membres superieurs. In Proceedings of the 13ème Congrès International de l’ACAPS, Lyon, France, 28–30 October 2009. [Google Scholar]
- Doré, E.; Duché, P.; Rouffet, D.; Ratel, S.; Bedu, M.; Van Praagh, E. Measurement error in short-term power testing in young people. J. Sports Sci. 2003, 21, 135–142. [Google Scholar] [CrossRef]
- Bassey, E.J.; Short, A.H. A new method for measuring power output in a single leg extension: Feasibility, reliability and validity. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 60, 385–390. [Google Scholar] [CrossRef]
- Blackwell, T.; Cawthon, P.M.; Marshall, L.M.; Brand, R. Consistency of leg extension power assessments in older men: The Osteoporotic Fractures in Men (MrOS) Study. Am. J. Phys. Med. Rehabil. 2009, 88, 934–940. [Google Scholar] [CrossRef]
- Hurst, C.; Batterham, A.M.; Weston, K.L.; Weston, M. Short- and long-term reliability of leg extensor power measurement in middle-aged and older adults. J. Sports Sci. 2018, 36, 970–977. [Google Scholar] [CrossRef]
- Katsoulis, K.; Mathur, S.; Amara, C.E. Reliability of Lower Extremity Muscle Power and Functional Performance in Healthy, Older Women. J. Aging Res. 2021, 2021, 8817231. [Google Scholar] [CrossRef]
- Schroeder, E.T.; Wang, Y.; Castaneda-Sceppa, C.; Cloutier, G.; Vallejo, A.F.; Kawakubo, M.; Jensky, N.E.; Coomber, S.; Azen, S.P.; Sattler, F.R. Reliability of maximal voluntary muscle strength and power testing in older men. J. Gerontol. A Biol. Sci. Med. Sci. 2007, 62, 543–549. [Google Scholar] [CrossRef]
- Jenkins, N.D.; Buckner, S.L.; Bergstrom, H.C.; Cochrane, K.C.; Goldsmith, J.A.; Housh, T.J.; Johnson, G.O.; Schmidt, R.J.; Cramer, J.T. Reliability and relationships among handgrip strength, leg extensor strength and power, and balance in older men. Exp. Gerontol. 2014, 58, 47–50. [Google Scholar] [CrossRef]
- Webber, S.C.; Porter, M.M. Reliability of Ankle Isometric, Isotonic, and Isokinetic Strength and Power Testing in Older Women. Physical. Therapy 2010, 90, 1165–1175. [Google Scholar] [CrossRef]
- Toshifumi, F.; Iwata, M.; Fukuda, M. Reliability and Validity of a New Test for Muscle Power Evaluation of Stroke Patients. J. Phys. Ther. Sci. 2011, 23, 259–263. [Google Scholar] [CrossRef]
- Bui, K.L.; Maia, N.; Saey, D.; Dechman, G.; Maltais, F.; Camp, P.G.; Mathur, S. Reliability of quadriceps muscle power and explosive force, and relationship to physical function in people with chronic obstructive pulmonary disease: An observational prospective multicenter study. Physiother. Theory Pract. 2021, 37, 945–953. [Google Scholar] [CrossRef]
- Clémençon, M.; Hautier, C.A.; Rahmani, A.; Cornu, C.; Bonnefoy, M. Potential role of optimal velocity as a qualitative factor of physical functional performance in women aged 72 to 96 years. Arch. Phys. Med. Rehabil. 2008, 89, 1594–1599. [Google Scholar] [CrossRef]
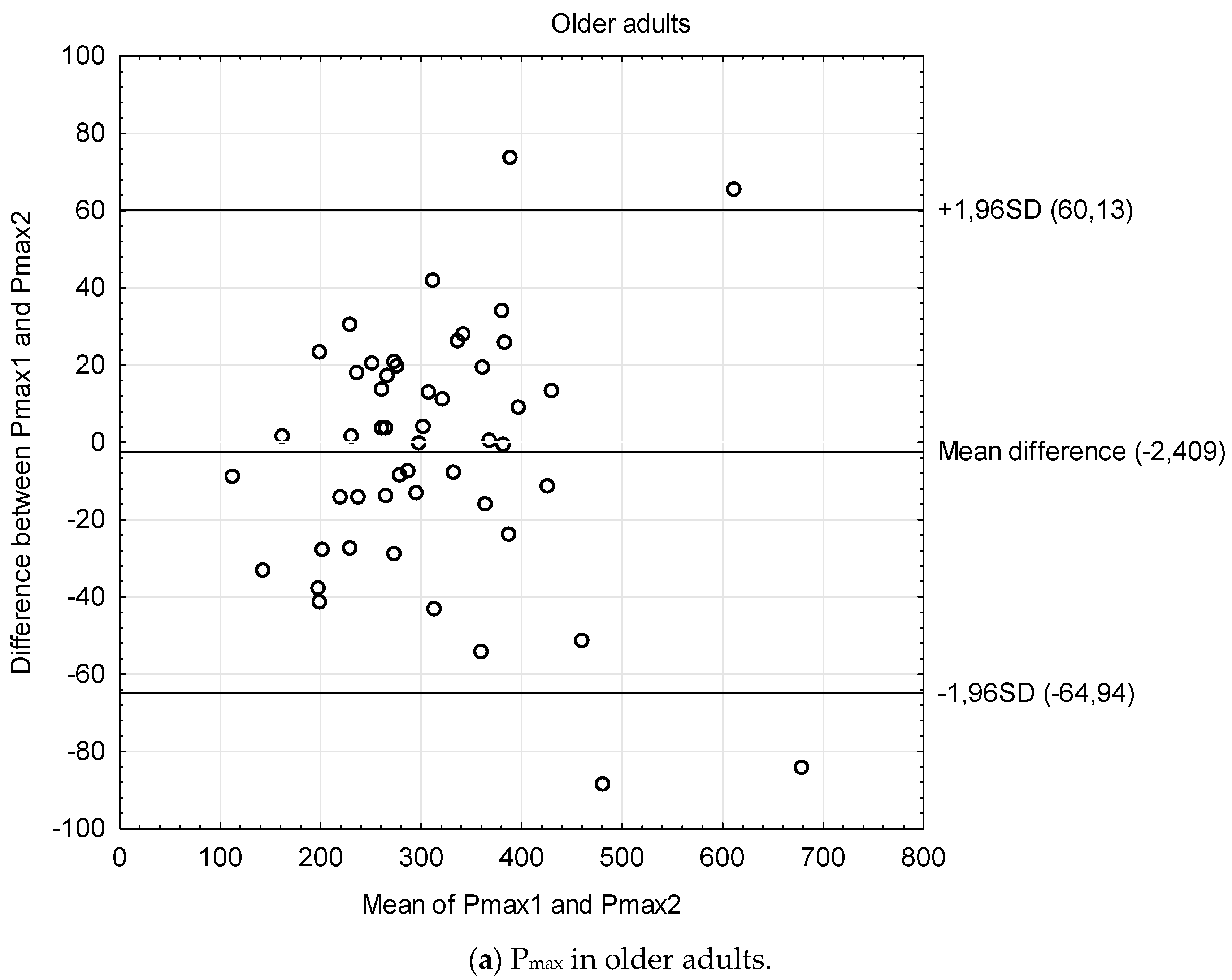
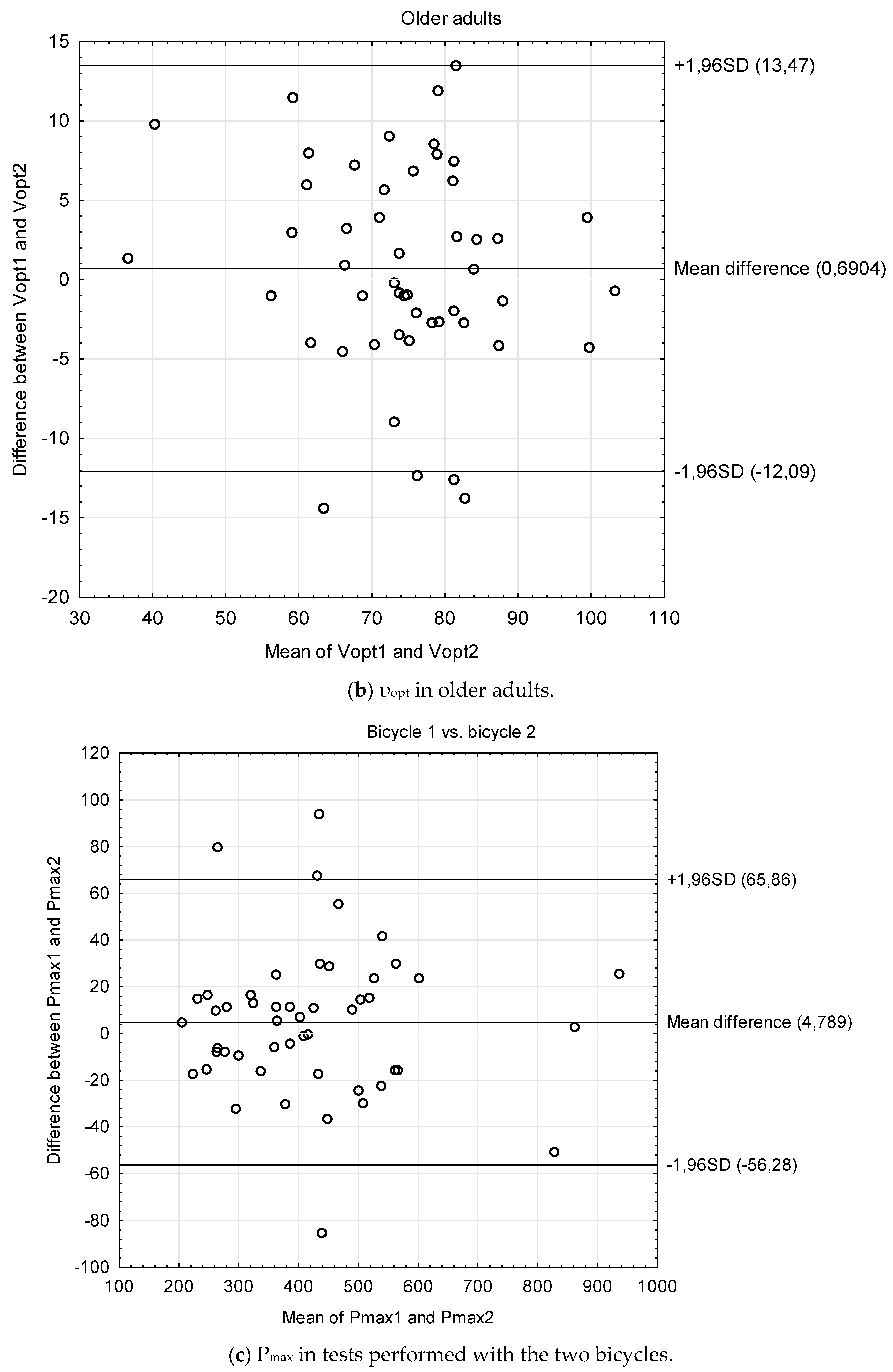
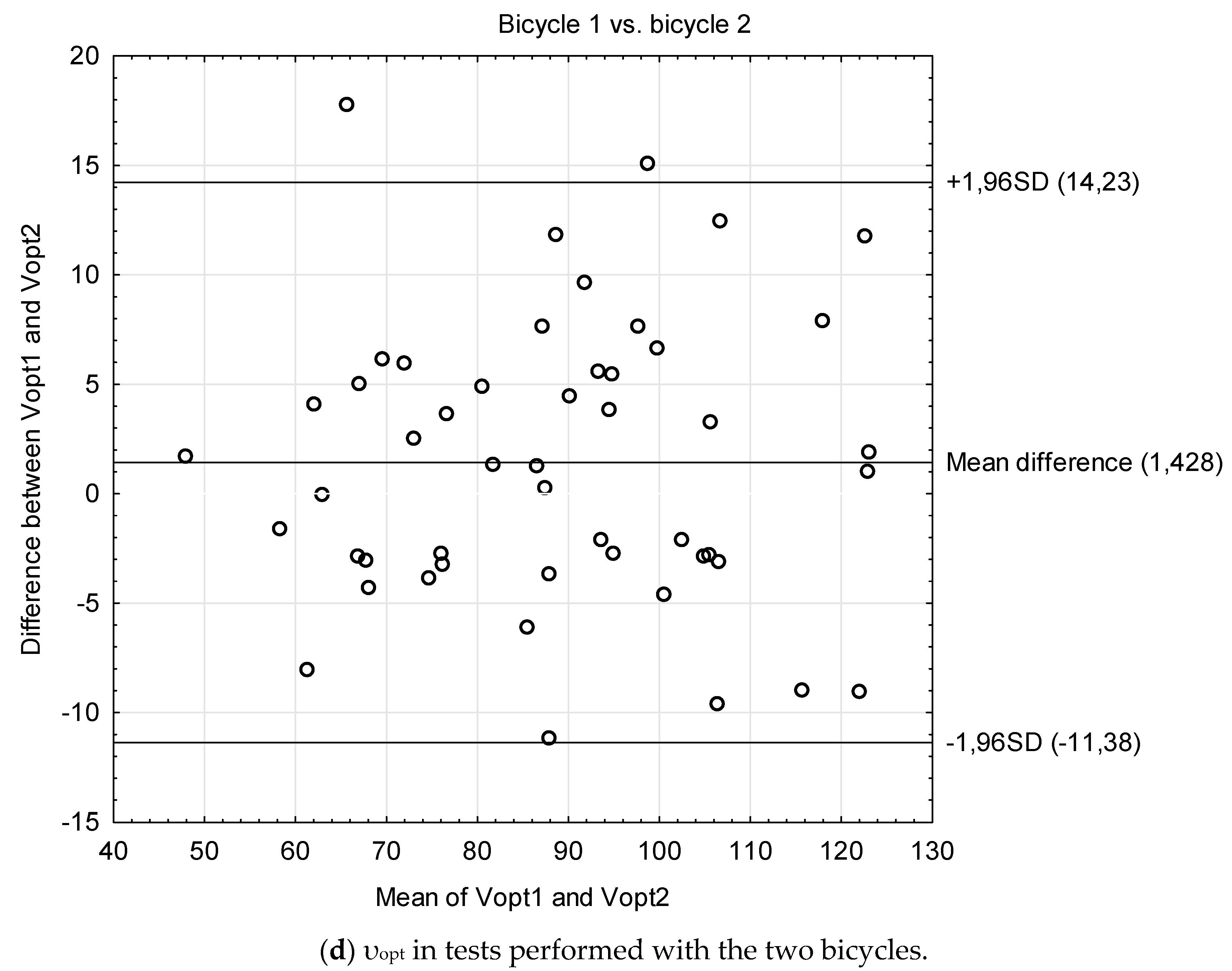
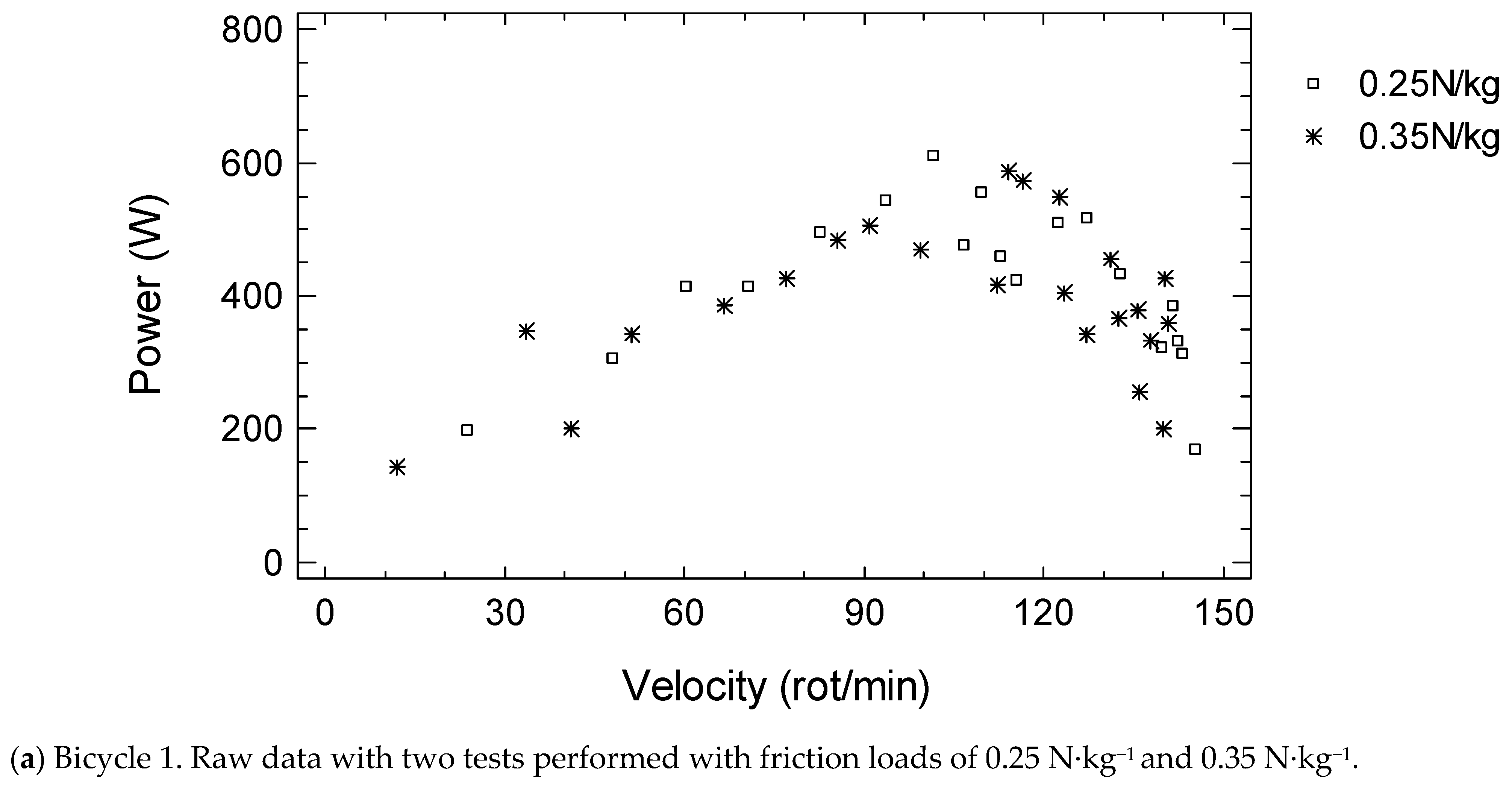
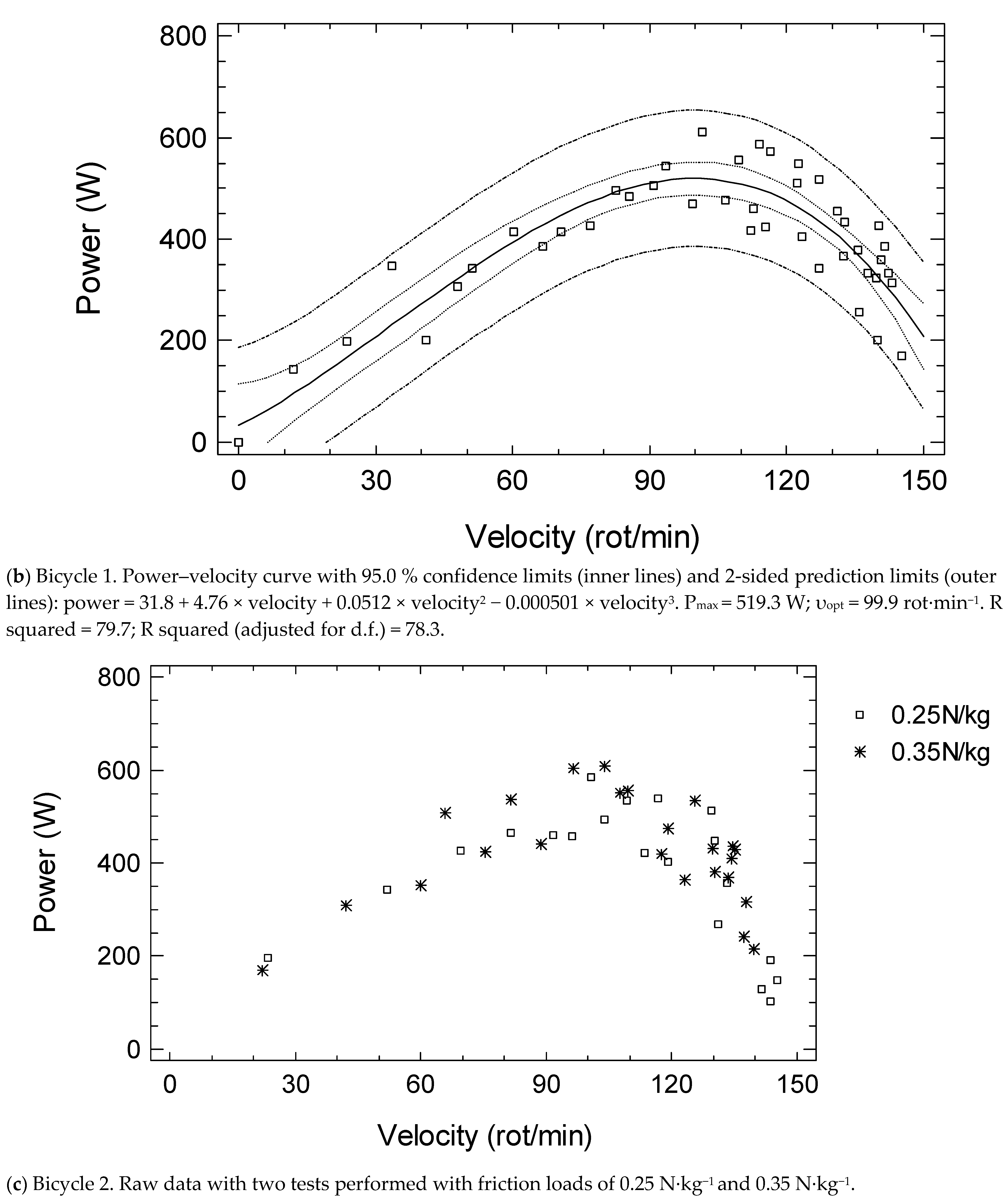
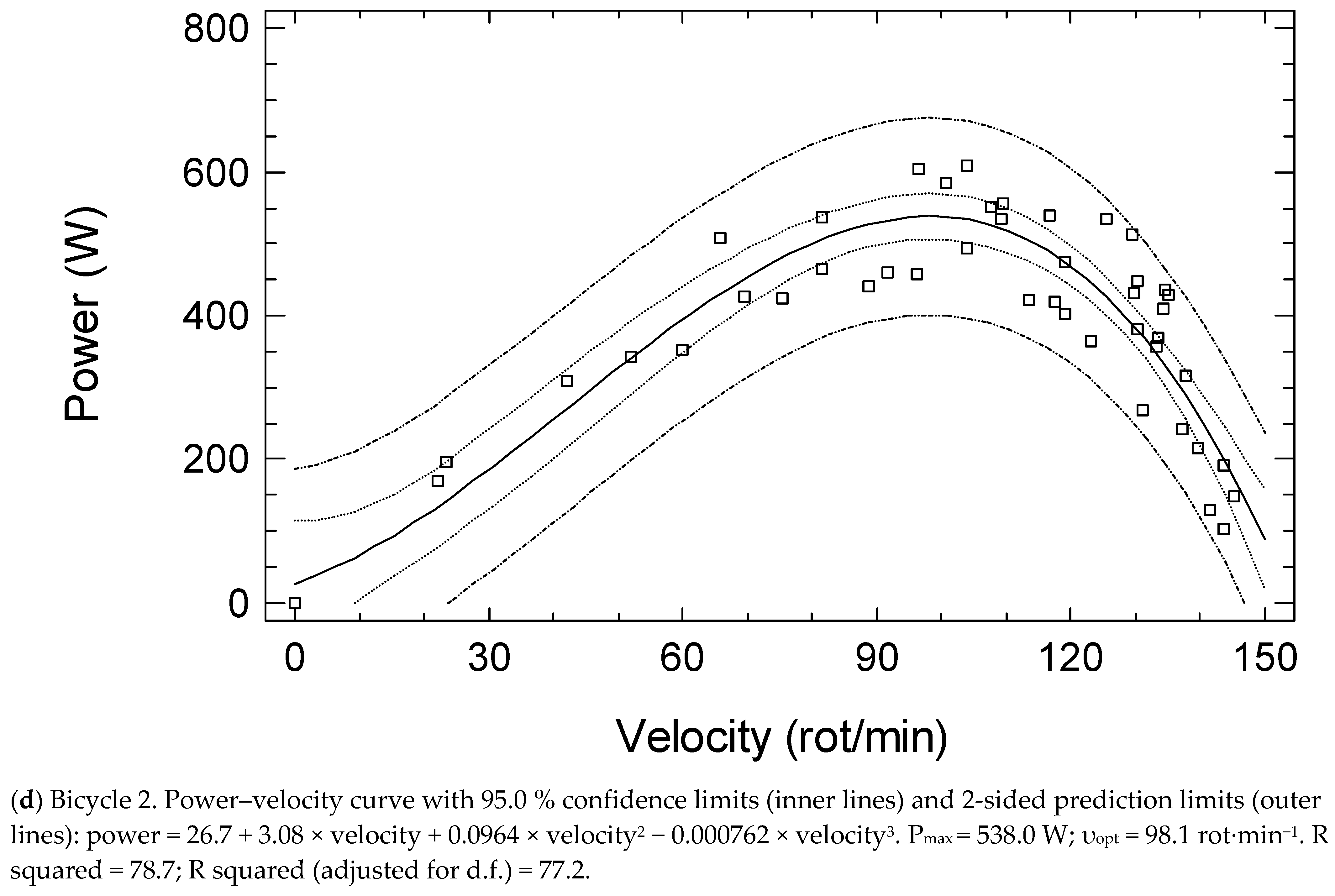
| Students | Cardiac Patients | Stroke Patients | Older Adults | Bicycle 1 vs. 2 | |
|---|---|---|---|---|---|
| Age (years) | 24.1 ± 1.1 | 54.9 ± 7.58 | 66.7 ± 8.6 | 72.6 ± 5.6 | 47.6 ± 19.8 |
| Body mass (kg) | 60.1 ± 10.4 W-57.5 ± 6.1 M-84.2 ± 10.5 | 82.6 ± 8.9 | 76.9 ± 14.7 W-70.6 ± 14.2 M-81.4 ± 13.5 | 72.4 ± 12.4 W-70.6 ± 12.7 M-76.9 ± 10.6 | 68.1 ± 10.9 W-59.5 ± 6.9 M-77.3 ± 8.7 |
| Sex [number (%)] | W-45(90%) M-5(10%) | W-0 M-50 (100%) | W-21(42%) M-29(58%) | W-36(72%) M-14(28%) | W-26 (52%) M-24 (48%) |
| Group | Students | Cardiac Patients | Stroke Patients | Older Adults | Bicycle 1 vs. 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | 50 | 50 | 50 | 50 | 50 | ||||||
| Measurement | Pmax (W) | υopt (rot/min) | Pmax (W) | υopt (rot/min) | Pmax (W) | υopt (rot/min) | Pmax (W) | υopt (rot/min) | Pmax (W) | υopt (rot/min) | |
| Test 1 | 460.0 | 101.7 | 570.5 | 98.8 | 157.2 | 48.8 | 310.5 | 74.8 | 417.6 | 89.4 | |
| SD | 139.7 | 10.5 | 113.7 | 13.5 | 60.7 | 15.6 | 106.2 | 12.4 | 134.9 | 19.2 | |
| Test 2 | 454.2 | 100.9 | 569.4 | 98.2 | 160.8 | 48.1 | 312.9 | 74.1 | 415.3 | 88.2 | |
| SD | 135.7 | 11.2 | 112.1 | 12.5 | 66.4 | 14.1 | 108.3 | 13.4 | 134.0 | 18.6 | |
| Test 1-test 2 (r) | 0.98 | 0.93 | 0.97 | 0.95 | 0.93 | 0.86 | 0.96 | 0.88 | 0.99 | 0.96 | |
| t-test | 1.35 | −1.19 | 0.29 | 1.0 | −1.05 | 0.57 | −0.53 | 0.75 | 0.83 | 1.45 | |
| P for t-test | 0.18 | 0.24 | 0.77 | 0.30 | 0.30 | 0.57 | 0.60 | 0.46 | 0.48 | 0.15 | |
| ICC | 0.98 | 0.92 | 0.97 | 0.95 | 0.93 | 0.86 | 0.96 | 0.87 | 0.98 | 0.94 | |
| SEM | 19.5 | 3.08 | 19.6 | 2.91 | 16.9 | 5.54 | 21.4 | 4.65 | 19.0 | 4.63 | |
| CV (SEM%) | 4.27 | 3.04 | 3.44 | 2.95 | 10.6 | 11.4 | 6.87 | 6.24 | 4.56 | 5.21 | |
| MDC | 54.1 | 8.54 | 54.3 | 8.07 | 46.6 | 15.4 | 59.3 | 12.9 | 52.7 | 12.8 | |
| MDC% | 11.8 | 8.43 | 9.53 | 8.19 | 29.3 | 31.7 | 19.1 | 17.3 | 12.7 | 14.4 | |
| Heterosc edasticity | R | −0.13 | 0.17 | −0.06 | −0.24 | −0.24 | 0.20 | 0.07 | 0.15 | 0.027 | −0.006 |
| P | 0.36 | 0.24 | 0.66 | 0.09 | 0.09 | 0.16 | 0.64 | 0.30 | 0.85 | 0.97 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostka, T.; Kostka, J. Feasibility and Reliability of Quadriceps Muscle Power and Optimal Movement Velocity Measurements in Different Populations of Subjects. Biology 2024, 13, 140. https://doi.org/10.3390/biology13030140
Kostka T, Kostka J. Feasibility and Reliability of Quadriceps Muscle Power and Optimal Movement Velocity Measurements in Different Populations of Subjects. Biology. 2024; 13(3):140. https://doi.org/10.3390/biology13030140
Chicago/Turabian StyleKostka, Tomasz, and Joanna Kostka. 2024. "Feasibility and Reliability of Quadriceps Muscle Power and Optimal Movement Velocity Measurements in Different Populations of Subjects" Biology 13, no. 3: 140. https://doi.org/10.3390/biology13030140
APA StyleKostka, T., & Kostka, J. (2024). Feasibility and Reliability of Quadriceps Muscle Power and Optimal Movement Velocity Measurements in Different Populations of Subjects. Biology, 13(3), 140. https://doi.org/10.3390/biology13030140





