Unveiling MHC-DAB Polymorphism Within the Western Balkan Salmonid Hotspot: Preliminary Outcomes from Native Trouts of Ohrid Lake and the Drin-Skadar Drainage (Albania)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
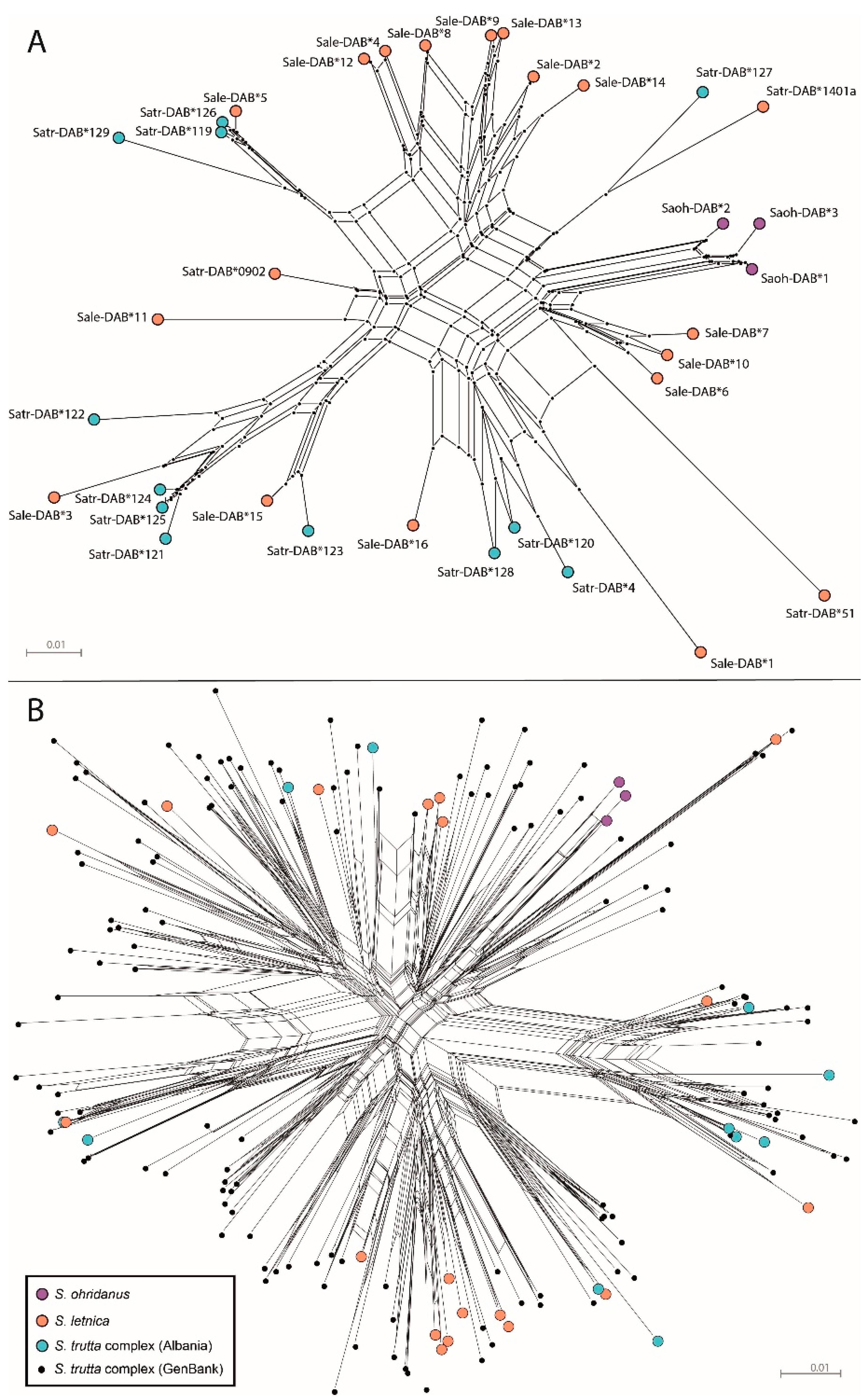
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobón-Cerviá, J.; Sanz, N. Brown Trout: Biology, Ecology and Management; John Wiley & Sons: Hoboken, NJ, USA, 2018; ISBN 978-1-119-26831-4. [Google Scholar]
- Tougard, C. Will the Genomics Revolution Finally Solve the Salmo Systematics? Hydrobiologia 2022, 849, 2209–2224. [Google Scholar] [CrossRef]
- Snoj, A.; Marić, S.; Berrebi, P.; Crivelli, A.J.; Shumka, S.; Sušnik, S. Genetic Architecture of Trout from Albania as Revealed by mtDNA Control Region Variation. Genet. Sel. Evol. 2009, 41, 22. [Google Scholar] [CrossRef]
- Sušnik, S.; Knizhin, I.; Snoj, A.; Weiss, S. Genetic and Morphological Characterization of a Lake Ohrid Endemic, Salmo (Acantholingua) ohridanus with a Comparison to Sympatric Salmo trutta. J. Fish Biol. 2006, 68, 2–23. [Google Scholar] [CrossRef]
- Sušnik, S.; Snoj, A.; Wilson, I.F.; Mrdak, D.; Weiss, S. Historical Demography of Brown Trout (Salmo trutta) in the Adriatic Drainage Including the Putative S. letnica Endemic to Lake Ohrid. Mol. Phylogenet. Evol. 2007, 44, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Schöffmann, J.; Marić, S. Salmonid Fish Species: Opportunities for Sustainable Use under Multiple Pressures and Current Climatic Change. In Ecological Sustainability of Fish Resources of Inland Waters of the Western Balkans; Simić, V., Simić, S., Pešić, V., Eds.; Fish & Fisheries Series; Springer International Publishing: Cham, Switzerland, 2023; Volume 43, pp. 375–410. ISBN 978-3-031-36925-4. [Google Scholar]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A Guide to Antigen Processing and Presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Radwan, J.; Babik, W.; Kaufman, J.; Lenz, T.L.; Winternitz, J. Advances in the Evolutionary Understanding of MHC Polymorphism. Trends Genet. 2020, 36, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Spurgin, L.G.; Richardson, D.S. How Pathogens Drive Genetic Diversity: MHC, Mechanisms and Misunderstandings. Proc. R. Soc. Lond. B Biol. Sci. 2010, 277, 979–988. [Google Scholar] [CrossRef]
- Sommer, S. The Importance of Immune Gene Variability (MHC) in Evolutionary Ecology and Conservation. Front. Zool. 2005, 2, 16. [Google Scholar] [CrossRef] [PubMed]
- Harstad, H.; Lukacs, M.F.; Bakke, H.G.; Grimholt, U. Multiple Expressed MHC Class II Loci in Salmonids; Details of One Non-Classical Region in Atlantic Salmon (Salmo salar). BMC Genom. 2008, 9, 193. [Google Scholar] [CrossRef]
- Talarico, L.; Marta, S.; Rossi, A.R.; Crescenzo, S.; Petrosino, G.; Martinoli, M.; Tancioni, L. Balancing Selection, Genetic Drift, and Human Mediated-Introgression Interplay to Shape MHC (Functional) Diversity in Mediterranean Brown Trout. Ecol. Evol. 2021, 11, 10026–10041. [Google Scholar] [CrossRef]
- Campos, J.L.; Posada, D.; Morán, P. Genetic Variation at MHC, Mitochondrial and Microsatellite Loci in Isolated Populations of Brown Trout (Salmo trutta). Conserv. Genet. 2006, 7, 515–530. [Google Scholar] [CrossRef]
- Schenekar, T.; Weiss, S. Selection and Genetic Drift in Captive versus Wild Populations: An Assessment of Neutral and Adaptive (MHC-Linked) Genetic Variation in Wild and Hatchery Brown Trout (Salmo trutta) Populations. Conserv. Genet. 2017, 18, 1011–1022. [Google Scholar] [CrossRef]
- Rossi, A.R.; Talarico, L.; Petrosino, G.; Crescenzo, S.; Tancioni, L. Conservation Genetics of Mediterranean Brown Trout in Central Italy (Latium): A Multi-Marker Approach. Water 2022, 14, 937. [Google Scholar] [CrossRef]
- Sebastian, A.; Herdegen, M.; Migalska, M.; Radwan, J. AMPLISAS: A Web Server for Multilocus Genotyping Using next-Generation Amplicon Sequencing Data. Mol. Ecol. Resour. 2016, 16, 498–510. [Google Scholar] [CrossRef]
- Klein, J.; Bontrop, R.; Dawkins, R.; Erlich, H.; Gyllensten, U.; Heise, E.; Jones, P.; Parham, P.; Wakeland, E.; Watkins, D. Nomenclature for the Major Histocompatibility Complexes of Different Species: A Proposal. Immunogenetics 1990, 31, 217–219. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Huson, D.H.; Bryant, D. Application of Phylogenetic Networks in Evolutionary Studies. Mol. Biol. Evol. 2006, 23, 254–267. [Google Scholar] [CrossRef] [PubMed]
- Kosakovsky Pond, S.L.; Posada, D.; Gravenor, M.B.; Woelk, C.H.; Frost, S.D.W. Automated Phylogenetic Detection of Recombination Using a Genetic Algorithm. Mol. Biol. Evol. 2006, 23, 1891–1901. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Jardetzky, T.S.; Gorga, J.C.; Stern, L.J.; Urban, R.G.; Strominger, J.L.; Wiley, D.C. Three-Dimensional Structure of the Human Class II Histocompatibility Antigen HLA-DR1. Nature 1993, 364, 33–39. [Google Scholar] [CrossRef]
- Kosakovsky Pond, S.L.; Frost, S.D.W. Not so Different after All: A Comparison of Methods for Detecting Amino Acid Sites under Selection. Mol. Biol. Evol. 2005, 22, 1208–1222. [Google Scholar] [CrossRef]
- Murrell, B.; Wertheim, J.O.; Moola, S.; Weighill, T.; Scheffler, K.; Kosakovsky Pond, S.L. Detecting Individual Sites Subject to Episodic Diversifying Selection. PLoS Genet. 2012, 8, e1002764. [Google Scholar] [CrossRef] [PubMed]
- Murrell, B.; Moola, S.; Mabona, A.; Weighill, T.; Sheward, D.; Kosakovsky Pond, S.L.; Scheffler, K. FUBAR: A Fast, Unconstrained Bayesian Approximation for Inferring Selection. Mol. Biol. Evol. 2013, 30, 1196–1205. [Google Scholar] [CrossRef]
- Doytchinova, I.A.; Flower, D.R. In Silico Identification of Supertypes for Class II MHCs. J. Immunol. 2005, 174, 7085–7095. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Devillard, S.; Dufour, A.B.; Pontier, D. Revealing Cryptic Spatial Patterns in Genetic Variability by a New Multivariate Method. Heredity 2008, 101, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic Analysis in Excel. Population Genetic Software for Teaching and Research-an Update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef] [PubMed]
- Adamack, A.T.; Gruber, B. PopGenReport: Simplifying Basic Population Genetic Analyses in R. Methods Ecol. Evol. 2014, 5, 384–387. [Google Scholar] [CrossRef]
- Meirmans, P.G.; Hedrick, P.W. Assessing Population Structure: FST and Related Measures. Mol. Ecol. Resour. 2011, 11, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.L.; Meyer, A. Natural Hybridization in Primates: One Evolutionary Mechanism. Zoology 2006, 109, 261–276. [Google Scholar] [CrossRef]
| Control Region | MHC-DAB | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Taxon | Location | N | Lineage | Haplotypes (GenBank) | A | Ar | Ho | He | p | Supertypes |
| S. ohridanus | Ohrid Lake | 3 | ohridanus | Ohr-3 (AY926568) × 1; Ohr-6 (AY926559) × 1; Ohr-1 * (AY926564) × 1 | 3 | 2.55 | 0.67 | 0.60 | 0.861 | ST4 (100%) |
| S. letnica | Ohrid Lake | 22 | Adriatic (AD)-letnica | Let12 (AY926570) × 17; Let13 (AY926573) × 2; Let15 (AY926572) × 1; Let16 (DQ381568) × 2 | 19 | 5.11 | 0.82 | 0.94 | 0.154 | ST1 (25.0%); ST2 (27.3%); ST3 (38.6%); ST4 (9.1%) |
| S. trutta complex | Cem River | 6 | Adriatic (AD) | AD-cs11 * (AY836340) × 6 | 7 | 4.15 | 0.33 | 0.88 | 0.022 | ST1 (16.7%); ST2 (16.7%); ST3 (16.6%); ST4 (50.0%) |
| S. trutta complex | Skadar Lake | 5 | Adriatic (AD) | AD-cs11 * (AY836340) × 4 | 6 | 4.07 | 1.00 | 0.89 | 0.246 | ST1 (70.0%); ST3 (30.0%) |
| Taxon | Alleles | Partition | Nucleotide (±SE) | Amino Acid (±SE) | dN (±SE) | dS (±SE) | Z | p-Value |
|---|---|---|---|---|---|---|---|---|
| S. ohridanus | 3 | all sites | 0.019 (0.007) | 0.041 (0.018) | 0.021 (0.010) | 0.012 (0.012) | 0.763 | 0.224 |
| ABSs | 0.071 (0.026) | 0.157 (0.071) | 0.077 (0.034) | 0.050 (0.053) | 0.498 | 0.310 | ||
| non-ABSs | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 (0.000) | 0.000 | 1.000 | ||
| S. letnica | 19 | all sites | 0.097 (0.013) | 0.200 (0.033) | 0.110 (0.018) | 0.041 (0.014) | 3.211 | 0.001 |
| ABSs | 0.223 (0.041) | 0.492 (0.097) | 0.271 (0.056) | 0.073 (0.036) | 3.197 | 0.001 | ||
| non-ABSs | 0.056 (0.010) | 0.111 (0.028) | 0.057 (0.014) | 0.031 (0.014) | 1.358 | 0.088 | ||
| S. trutta complex | 12 | all sites | 0.094 (0.014) | 0.190 (0.031) | 0.104 (0.017) | 0.044 (0.019) | 2.347 | 0.010 |
| ABSs | 0.214 (0.040) | 0.457 (0.096) | 0.261 (0.058) | 0.065 (0.047) | 2.542 | 0.006 | ||
| non-ABSs | 0.055 (0.011) | 0.109 (0.028) | 0.053 (0.013) | 0.037 (0.021) | 0.684 | 0.248 | ||
| overall | 34 | all sites | 0.101 (0.014) | 0.206 (0.034) | 0.114 (0.019) | 0.046 (0.017) | 2.289 | 0.003 |
| ABSs | 0.229 (0.041) | 0.493 (0.099) | 0.276 (0.053) | 0.079 (0.044) | 2.928 | 0.002 | ||
| non-ABSs | 0.060 (0.011) | 0.119 (0.028) | 0.061 (0.015) | 0.035 (0.017) | 1.134 | 0.129 |
| S. ohridanus | S. letnica | S. trutta Complex (Cem) | S. trutta Complex (Skadar) | |
|---|---|---|---|---|
| S. ohridanus | - | 0.17 | 0.21 | 0.21 |
| S. letnica | 1.00 | - | 0.07 | 0.08 |
| S. trutta complex (Cem) | 1.00 | 1.00 | - | 0.11 |
| S. trutta complex (Skadar) | 1.00 | 1.00 | 0.91 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talarico, L.; Rakaj, A.; Tancioni, L. Unveiling MHC-DAB Polymorphism Within the Western Balkan Salmonid Hotspot: Preliminary Outcomes from Native Trouts of Ohrid Lake and the Drin-Skadar Drainage (Albania). Biology 2024, 13, 1060. https://doi.org/10.3390/biology13121060
Talarico L, Rakaj A, Tancioni L. Unveiling MHC-DAB Polymorphism Within the Western Balkan Salmonid Hotspot: Preliminary Outcomes from Native Trouts of Ohrid Lake and the Drin-Skadar Drainage (Albania). Biology. 2024; 13(12):1060. https://doi.org/10.3390/biology13121060
Chicago/Turabian StyleTalarico, Lorenzo, Arnold Rakaj, and Lorenzo Tancioni. 2024. "Unveiling MHC-DAB Polymorphism Within the Western Balkan Salmonid Hotspot: Preliminary Outcomes from Native Trouts of Ohrid Lake and the Drin-Skadar Drainage (Albania)" Biology 13, no. 12: 1060. https://doi.org/10.3390/biology13121060
APA StyleTalarico, L., Rakaj, A., & Tancioni, L. (2024). Unveiling MHC-DAB Polymorphism Within the Western Balkan Salmonid Hotspot: Preliminary Outcomes from Native Trouts of Ohrid Lake and the Drin-Skadar Drainage (Albania). Biology, 13(12), 1060. https://doi.org/10.3390/biology13121060






