Immune Suppression and Rapid Invasion of Nile Tilapia Gills Following an Acute Challenge by Flavobacterium davisii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Husbandry, Infection, and Sample Collection
2.2. RNA Extraction, Library Preparation, and Sequencing
2.3. Quality Control and Reads Mapping
2.4. Differential Expression and Enrichment Analysis
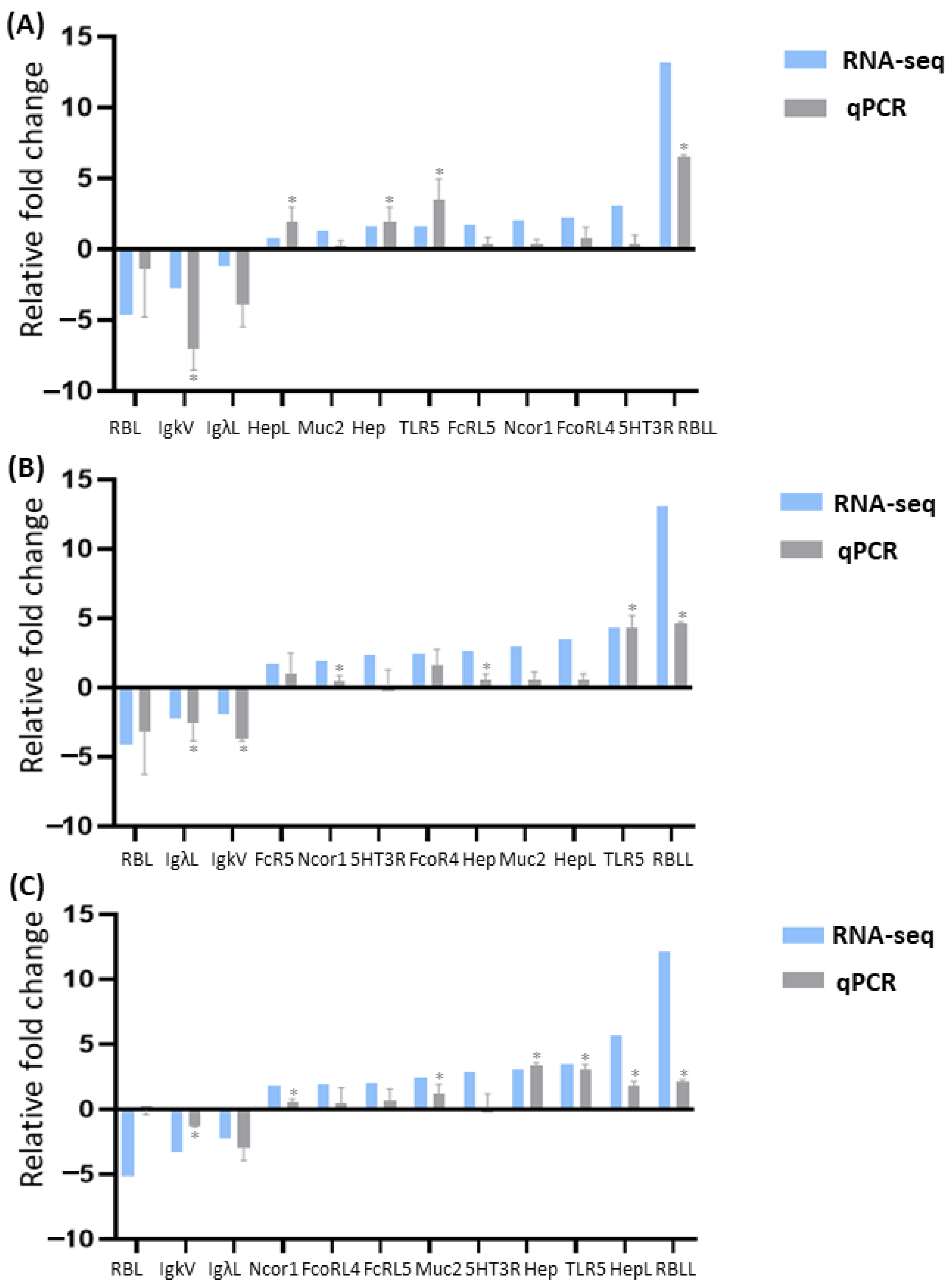
2.5. qPCR Validation
3. Results
3.1. F. davisii Infection
3.2. Sequencing Summary
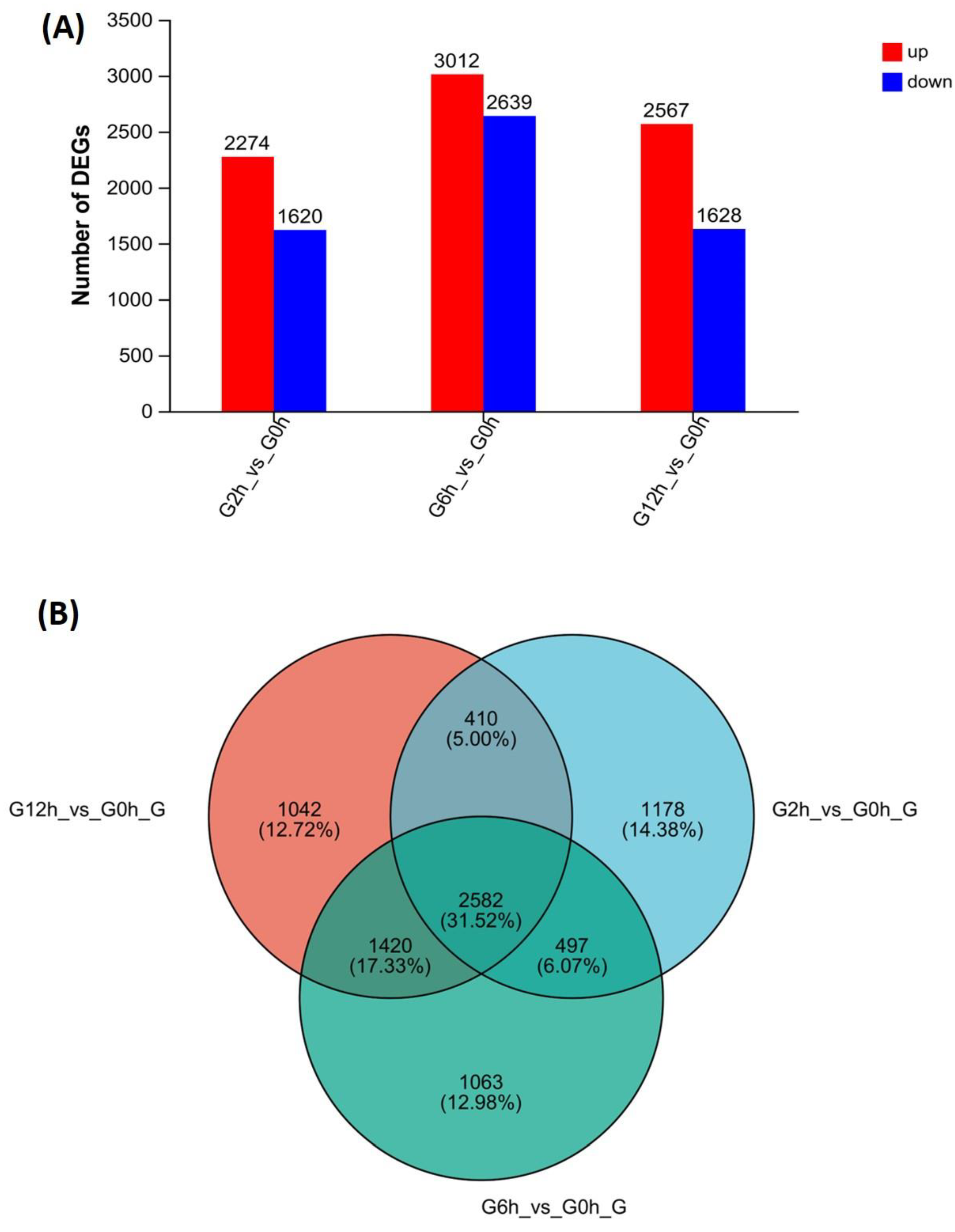
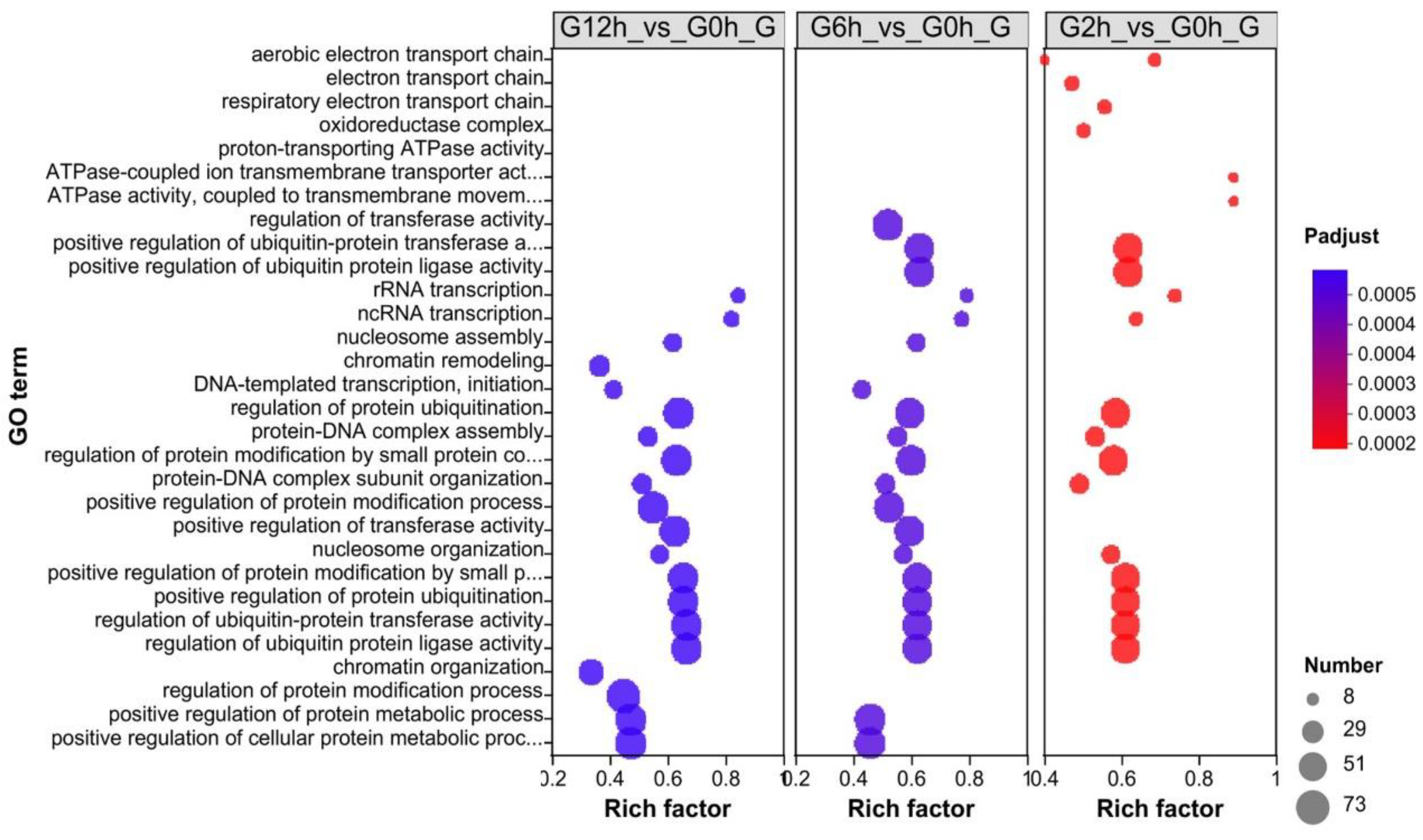
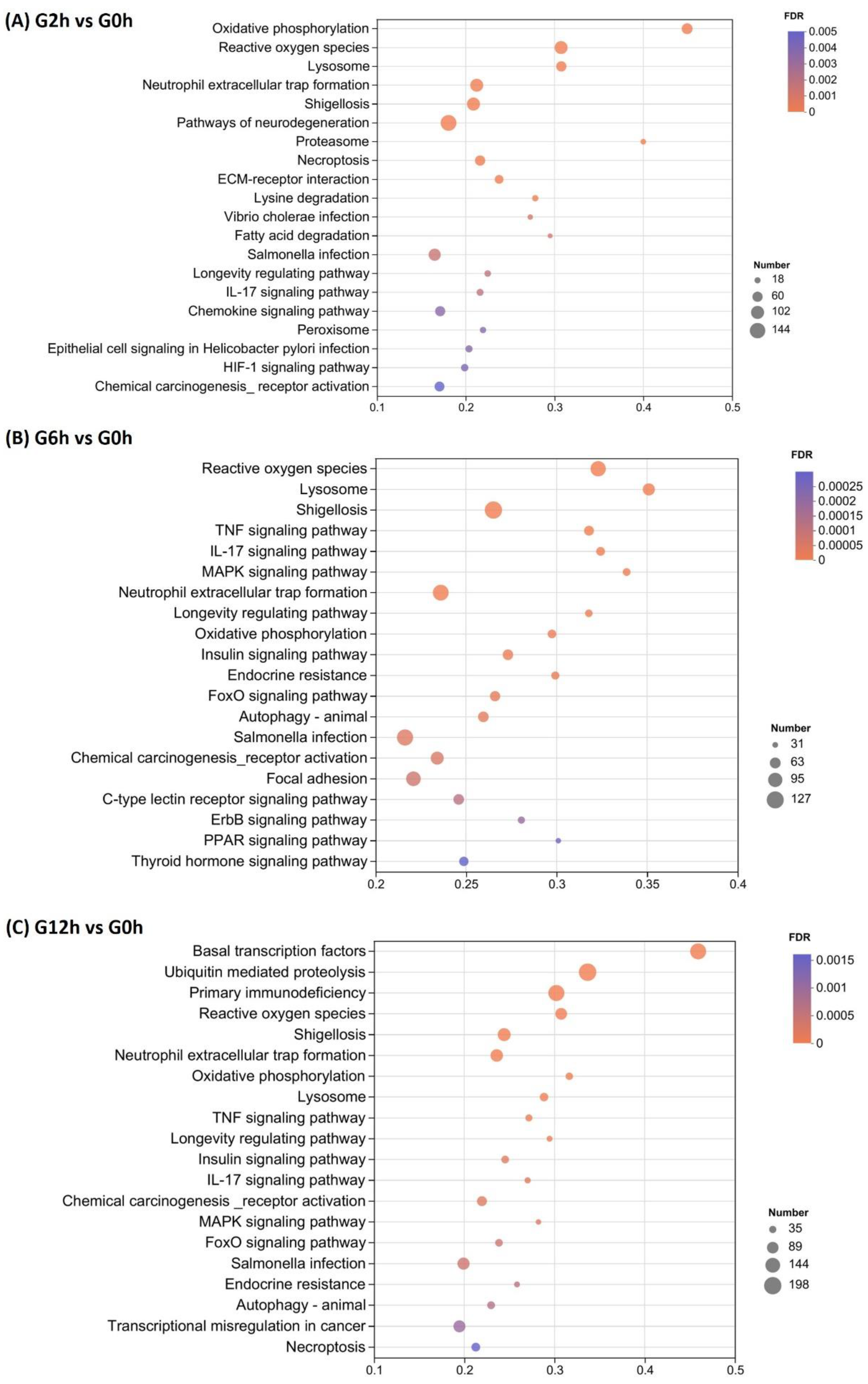
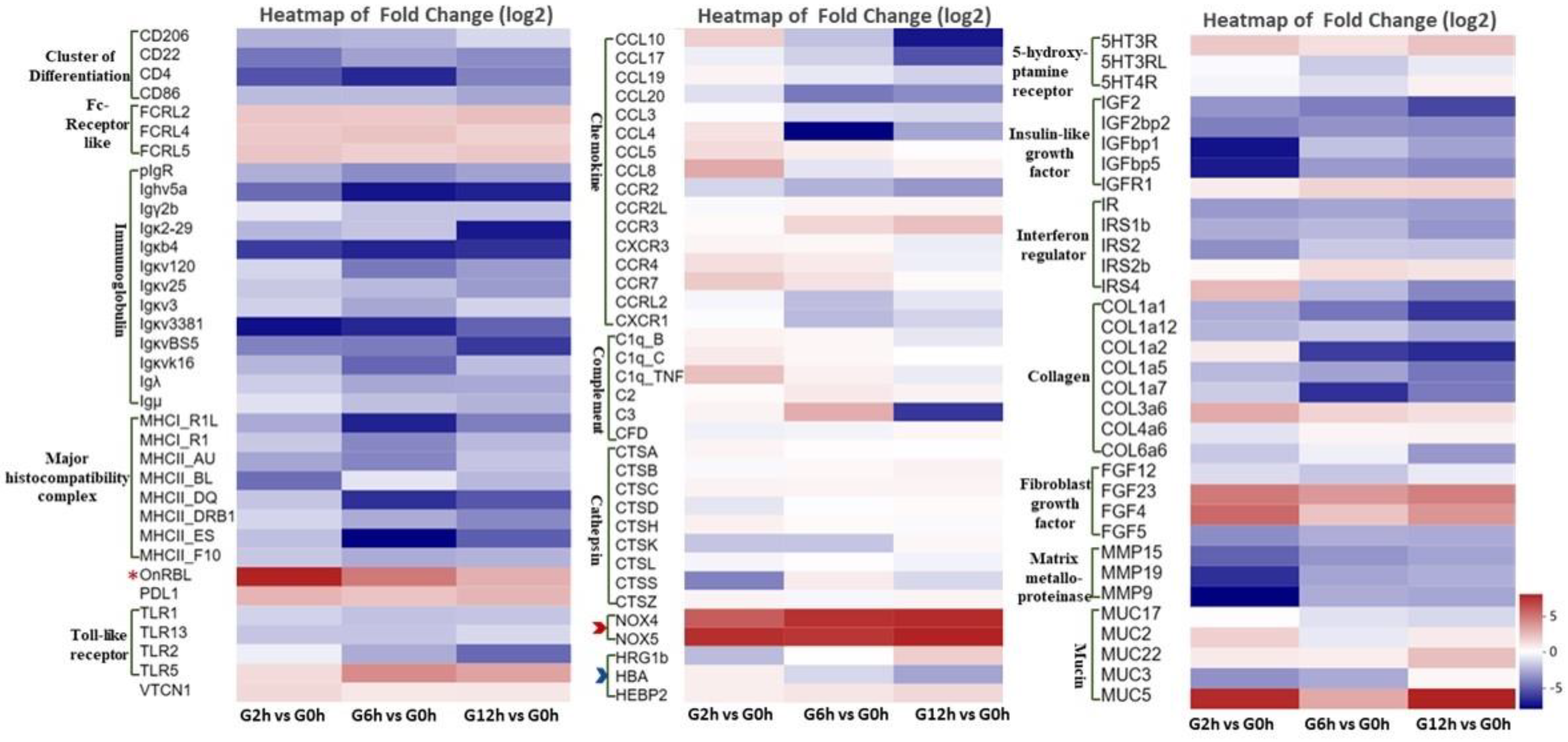
3.3. Identification of Differentially Expressed Genes (DEGs) and Enrichment Analysis
3.4. qPCR Validation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benhamed, S.; Guardiola, F.A.; Mars, M.; Esteban, M.Á. Pathogen bacteria adhesion to skin mucus of fishes. Vet. Microbiol. 2014, 171, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.B.; Ranzani-Paiva, M.J.T.; Tachibana, L.; de Carla Dias, D.; Ishikawa, C.M.; Esteban, M.A. Fish pathogen bacteria: Adhesion, parameters influencing virulence and interaction with host cells. Fish Shellfish Immunol. 2018, 80, 550–562. [Google Scholar] [CrossRef] [PubMed]
- Tongsri, P.; Meng, K.; Liu, X.; Wu, Z.; Yin, G.; Wang, Q.; Liu, M.; Xu, Z. The predominant role of mucosal immunoglobulin IgT in the gills of rainbow trout (Oncorhynchus mykiss) after infection with Flavobacterium columnare. Fish Shellfish Immunol. 2020, 99, 654–662. [Google Scholar] [CrossRef]
- Finlay, B.B.; McFadden, G. Anti-immunology: Evasion of the host immune system by bacterial and viral pathogens. Cell 2006, 124, 767–782. [Google Scholar] [CrossRef]
- Van Avondt, K.; Sorge, N.M.V.; Meyaard, L. Bacterial immune evasion through manipulation of host inhibitory immune signaling. PLoS Pathog. 2015, 11, e1004644. [Google Scholar] [CrossRef] [PubMed]
- Thongda, W.; Li, C.; Luo, Y.; Beck, B.H.; Peatman, E. L-Rhamnose-binding lectins (RBLs) in channel catfish, Ictalurus punctatus: Characterization and expression profiling in mucosal tissues. Dev. Comp. Immunol. 2014, 44, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Beck, B.H.; Farmer, B.D.; Straus, D.L.; Li, C.; Peatman, E. Putative roles for a rhamnose binding lectin in Flavobacterium columnare pathogenesis in channel catfish Ictalurus punctatus. Fish Shellfish Immunol. 2012, 33, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Peatman, E.; Li, C.; Liu, S.; Jiang, Y.; Zhou, Z.; Liu, Z. Transcriptomic signatures of attachment, NF-κB suppression and IFN stimulation in the catfish gill following columnaris bacterial infection. Dev. Comp. Immunol. 2012, 38, 169–180. [Google Scholar] [CrossRef]
- Zhang, D.; Thongda, W.; Li, C.; Zhao, H.; Beck, B.H.; Mohammed, H.; Arias, C.R.; Peatman, E. More than just antibodies: Protective mechanisms of a mucosal vaccine against fish pathogen Flavobacterium columnare. Fish Shellfish Immunol. 2017, 71, 160–170. [Google Scholar] [CrossRef]
- Zhai, X.; Kong, W.-G.; Cheng, G.-F.; Cao, J.-F.; Dong, F.; Han, G.-K.; Song, Y.-L.; Qin, C.-J.; Xu, Z. Molecular characterization and expression analysis of intercellular adhesion molecule-1 (ICAM-1) genes in rainbow trout (Oncorhynchus mykiss) in response to viral, bacterial and parasitic challenge. Front. Immunol. 2021, 12, 704224. [Google Scholar] [CrossRef]
- LaFrentz, B.R.; Goodwin, A.E.; Shoemaker, C.A. 3 Columnaris Disease. 2012. Available online: https://units.fisheries.org/fhs/wp-content/uploads/sites/30/2017/08/1.2.3-Columnaris2014.pdf (accessed on 15 June 2012).
- Shoemaker, C.; LaFrentz, B. Lack of association between Flavobacterium columnare genomovar and virulence in hybrid tilapia Oreochromis niloticus (L.) × Oreochromis aureus (Steindachner). J. Fish Dis. 2015, 38, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Wonmongkol, P.; Sukhavachana, S.; Ampolsak, K.; Srisapoome, P.; Suwanasopee, T.; Poompuang, S. Genetic parameters for resistance against Flavobacterium columnare in Nile tilapia Oreochromis niloticus (Linnaeus, 1758). J. Fish Dis. 2018, 41, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Cai, Y.; Zhou, Y.; Zhao, Z.; Wang, Y.; Zhang, D.; Wang, S. Pathogen identification, risk factor and preventive measure of a columnaris disease outbreak in Tilapia (Oreochromis niloticus) eggs and larvae from a tilapia hatchery. Aquaculture 2022, 561, 738718. [Google Scholar] [CrossRef]
- Elgendy, M.Y.; Abdelsalam, M.; Mohamed, S.A.; Ali, S.E. Molecular characterization, virulence profiling, antibiotic susceptibility, and scanning electron microscopy of Flavobacterium columnare isolates retrieved from Nile tilapia (Oreochromis niloticus). Aquac. Int. 2022, 30, 845–862. [Google Scholar] [CrossRef]
- Declercq, A.M.; Haesebrouck, F.; Van den Broeck, W.; Bossier, P.; Decostere, A. Columnaris disease in fish: A review with emphasis on bacterium-host interactions. Vet. Res. 2013, 44, 27. [Google Scholar] [CrossRef]
- Olivares-Fuster, S.; Bullard, S.A.; McElwain, A.; Llosa, M.J.; Arias, C.R. Adhesion dynamics of Flavobacterium columnare to channel catfish Ictalurus punctatus and zebrafish Danio rerio after immersion challenge. Dis. Aquat. Organ. 2011, 96, 221–227. [Google Scholar] [CrossRef]
- Declercq, A.M.; Chiers, K.; Van den Broeck, W.; Dewulf, J.; Eeckhaut, V.; Cornelissen, M.; Bossier, P.; Haesebrouck, F.; Decostere, A. Interactions of highly and low virulent Flavobacterium columnare isolates with gill tissue in carp and rainbow trout. Vet. Res. 2015, 46, 25. [Google Scholar] [CrossRef]
- Zhang, D.; Beck, B.H.; Lange, M.; Zhao, H.; Thongda, W.; Ye, Z.; Li, C.; Peatman, E. Impact of oral and waterborne administration of rhamnolipids on the susceptibility of channel catfish (Ictalurus punctatus) to Flavobacterium columnare infection. Fish Shellfish Immunol. 2017, 60, 44–49. [Google Scholar] [CrossRef]
- Mohammed, H.H.; Arias, C.R. Epidemiology of columnaris disease affecting fishes within the same watershed. Dis. Aquat. Organ. 2014, 109, 201–211. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Rostamzadeh, D.; Kazemi, T.; Amirghofran, Z.; Shabani, M. Update on Fc receptor-like (FCRL) family: New immunoregulatory players in health and diseases. Expert Opin. Ther. Targets 2018, 22, 487–502. [Google Scholar] [CrossRef]
- Peatman, E.; Lange, M.; Zhao, H.; Beck, B.H. Physiology and immunology of mucosal barriers in catfish (Ictalurus spp.). Tissue Barriers 2015, 3, e1068907. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, G.; Thongda, W.; Li, C.; Ye, Z.; Zhao, H.; Beck, B.H.; Mohammed, H.; Peatman, E. Early divergent responses to virulent and attenuated vaccine isolates of Flavobacterium covae sp. nov. in channel catfish, Ictalurus punctatus. Fish Shellfish Immunol. 2024, 144, 109248. [Google Scholar] [CrossRef]
- Lee, R.D.; Knutson, T.P.; Munro, S.A.; Miller, J.T.; Heltemes-Harris, L.M.; Mullighan, C.G.; Jepsen, K.; Farrar, M.A. Nuclear corepressors NCOR1/NCOR2 regulate B cell development, maintain genomic integrity and prevent transformation. Nat. Immunol. 2022, 23, 1763–1776. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K. Nuclear receptor transrepression pathways that regulate inflammation in macrophages and T cells. Nat. Rev. Immunol. 2010, 10, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Peatman, E.; Li, C.; Peterson, B.C.; Straus, D.L.; Farmer, B.D.; Beck, B.H. Basal polarization of the mucosal compartment in Flavobacterium columnare susceptible and resistant channel catfish (Ictalurus punctatus). Mol. Immunol. 2013, 56, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, C.; Beck, B.H.; Zhang, R.; Thongda, W.; Davis, D.A.; Peatman, E. Impact of feed additives on surface mucosal health and columnaris susceptibility in channel catfish fingerlings (Ictalurus punctatus). Fish Shellfish Immunol. 2015, 46, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Elaswad, A.; Khalil, K.; Ye, Z.; Liu, Z.; Liu, S.; Peatman, E.; Odin, R.; Vo, K.; Drescher, D.; Gosh, K. Effects of CRISPR/Cas9 dosage on TICAM1 and RBL gene mutation rate, embryonic development, hatchability and fry survival in channel catfish. Sci. Rep. 2018, 8, 16499. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Shangguan, X.; Wang, L.; Liu, X. Identification, annotation of Mucin genes in channel catfish (Ictalurus punctatus) and their expression after bacterial infections revealed by RNA-Seq analysis. Aquac. Res. 2020, 51, 2020–2028. [Google Scholar] [CrossRef]
- Herr, N.; Bode, C.; Duerschmied, D. The effects of serotonin in immune cells. Front. Cardiovasc. Med. 2017, 4, 48. [Google Scholar] [CrossRef]
- Duffy-Whritenour, J.; Zelikoff, J. Relationship between serotonin and the immune system in a teleost model. Brain Behav. Immun. 2008, 22, 257–264. [Google Scholar] [CrossRef]
- Quilapi, A.M.; Vargas-Lagos, C.; Martínez, D.; Muñoz, J.L.; Spies, J.; Esperguel, I.; Tapia, J.; Oyarzún-Salazar, R.; Vargas-Chacoff, L. Brain immunity response of fish Eleginops maclovinus to infection with Francisella noatunensis. Fish Shellfish Immunol. 2022, 120, 695–705. [Google Scholar] [CrossRef]
- Li, Q.; Jiang, B.; Zhang, Z.; Huang, Y.; Xu, Z.; Chen, X.; Hou, X.; Cai, J.; Huang, Y.; Jian, J. Serotonin system is partially involved in immunomodulation of Nile tilapia (Oreochromis niloticus) immune cells. Front. Immunol. 2022, 13, 944388. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, G.; Bern, H.A. Insulin-like growth factor signaling in fish. Int. Rev. Cytol. 2005, 243, 215–285. [Google Scholar]
- Yada, T. Effects of insulin-like growth factor-I on non-specific immune functions in rainbow trout. Zool. Sci. 2009, 26, 338–343. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Yang, X.; Cao, Z.; Chen, X.; Qin, Q.; Liu, C.; Sun, Y. Insulin-like growth factor binding protein 3 gene of golden pompano (TroIGFBP3) promotes antimicrobial immune defense. Fish Shellfish Immunol. 2020, 103, 47–57. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Wang, S.; Zhou, Y.; Xie, Z.; Wang, B.; Zhao, Z.; Cai, W.; Wang, P.; Guo, W.; Zhang, D.; et al. Immune Suppression and Rapid Invasion of Nile Tilapia Gills Following an Acute Challenge by Flavobacterium davisii. Biology 2024, 13, 894. https://doi.org/10.3390/biology13110894
Xu Y, Wang S, Zhou Y, Xie Z, Wang B, Zhao Z, Cai W, Wang P, Guo W, Zhang D, et al. Immune Suppression and Rapid Invasion of Nile Tilapia Gills Following an Acute Challenge by Flavobacterium davisii. Biology. 2024; 13(11):894. https://doi.org/10.3390/biology13110894
Chicago/Turabian StyleXu, Yingxuan, Shifeng Wang, Yongcan Zhou, Zhenyu Xie, Bei Wang, Zhangding Zhao, Wenlong Cai, Peibo Wang, Weiliang Guo, Dongdong Zhang, and et al. 2024. "Immune Suppression and Rapid Invasion of Nile Tilapia Gills Following an Acute Challenge by Flavobacterium davisii" Biology 13, no. 11: 894. https://doi.org/10.3390/biology13110894
APA StyleXu, Y., Wang, S., Zhou, Y., Xie, Z., Wang, B., Zhao, Z., Cai, W., Wang, P., Guo, W., Zhang, D., & Ye, Z. (2024). Immune Suppression and Rapid Invasion of Nile Tilapia Gills Following an Acute Challenge by Flavobacterium davisii. Biology, 13(11), 894. https://doi.org/10.3390/biology13110894






