Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
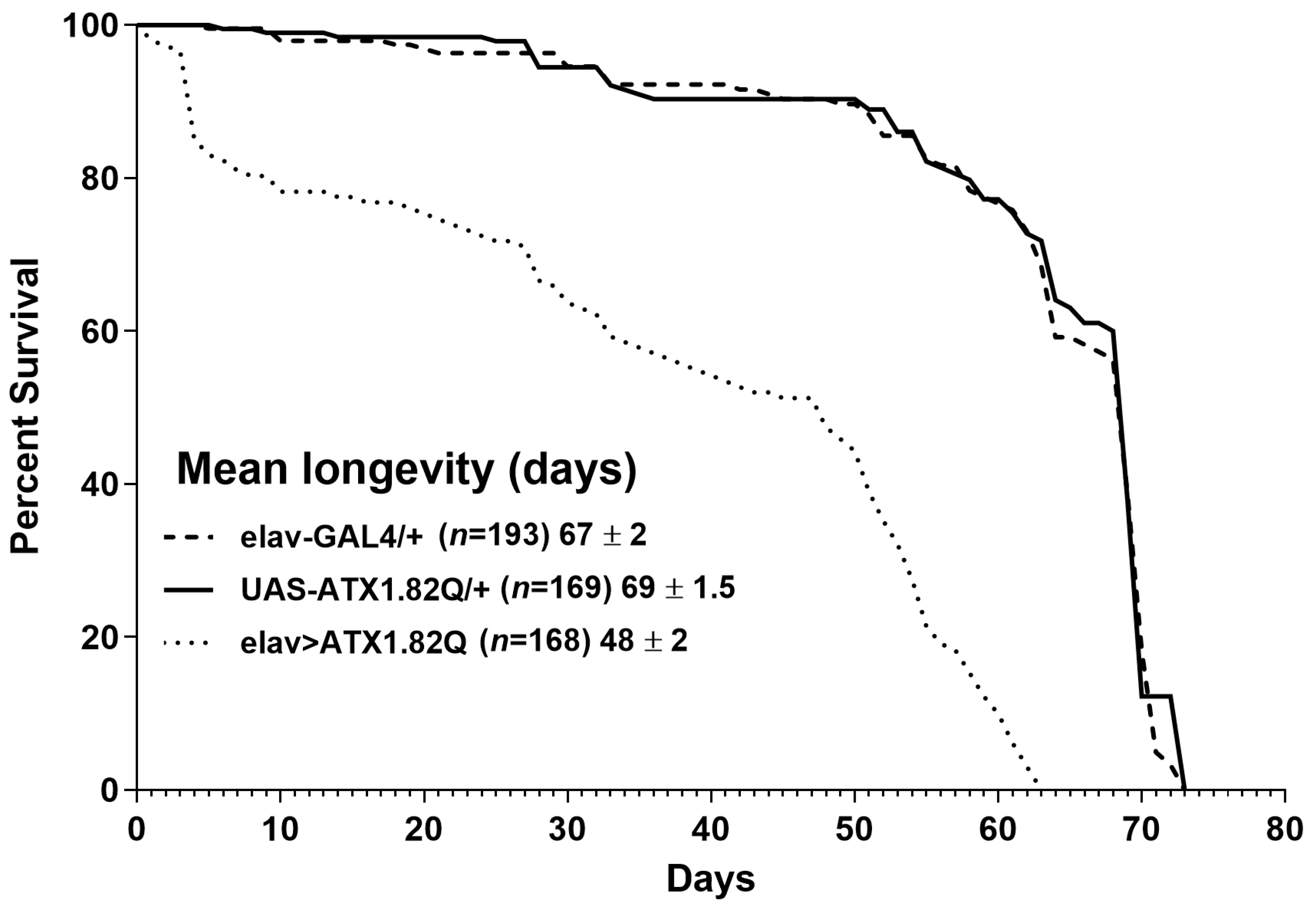
3.1. Pan-Neuronal Expression of Human ATX1.82Q Shortens Lifespan
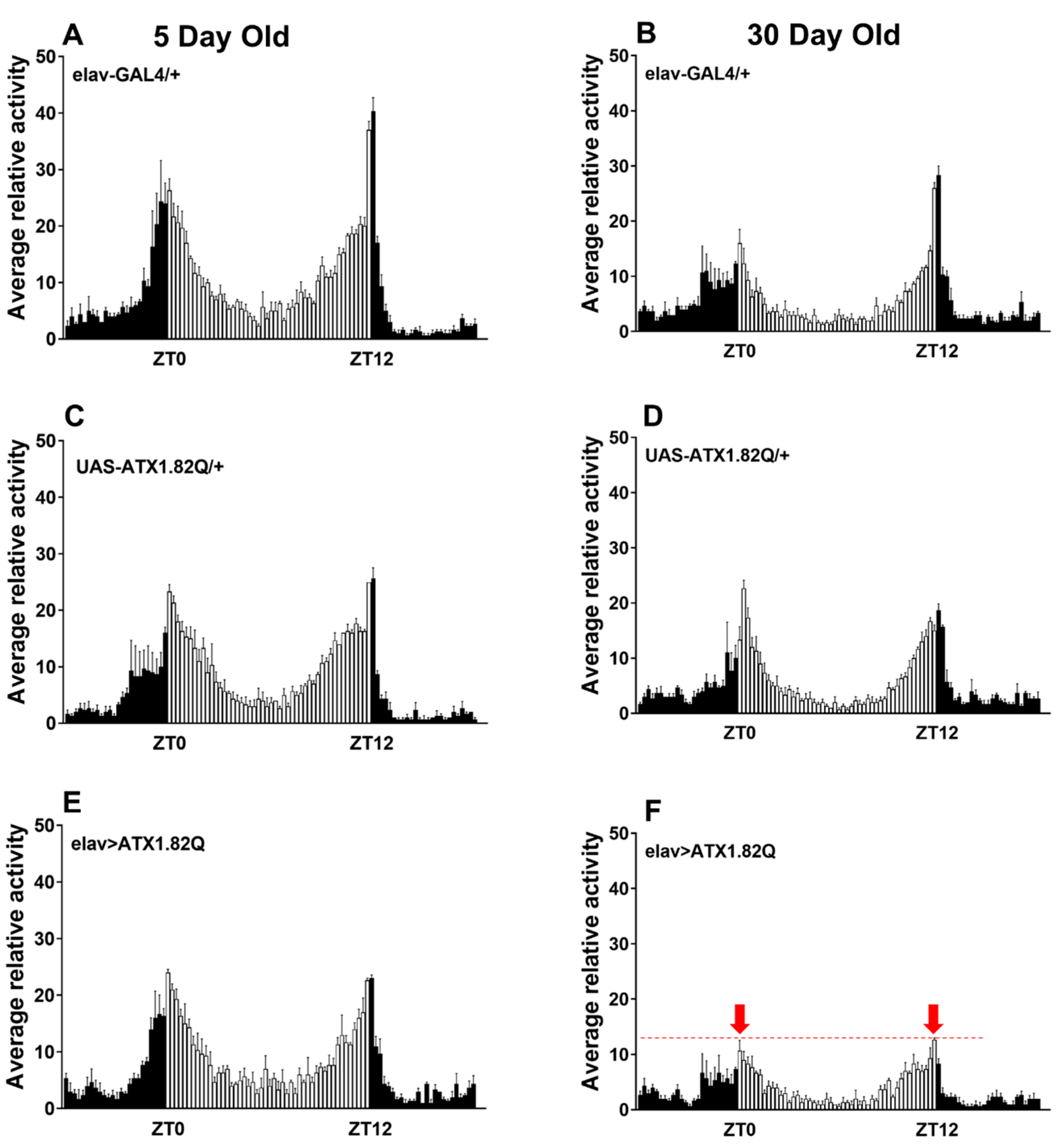
3.2. Pan-Neuronal Expression of Human ATX1.82Q Does Not Impact Diurnal Locomotor Rhythms in Young Flies but Dampens Diurnal Anticipatory Behavior in Old Flies
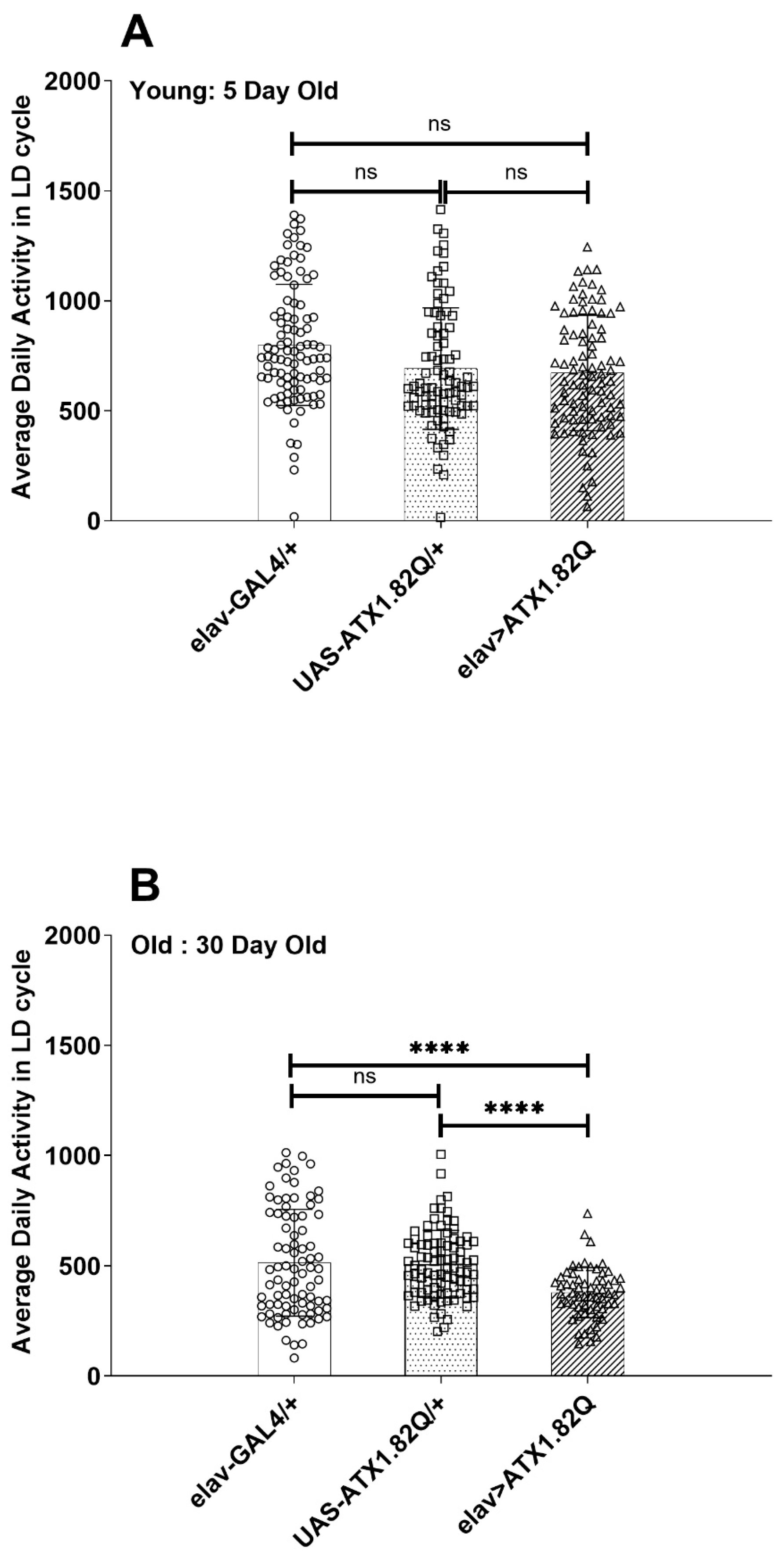
3.3. Expression of Human ATX1.82Q Results in Decreased Horizontal Daily Diurnal Locomotor Activity in Old Age
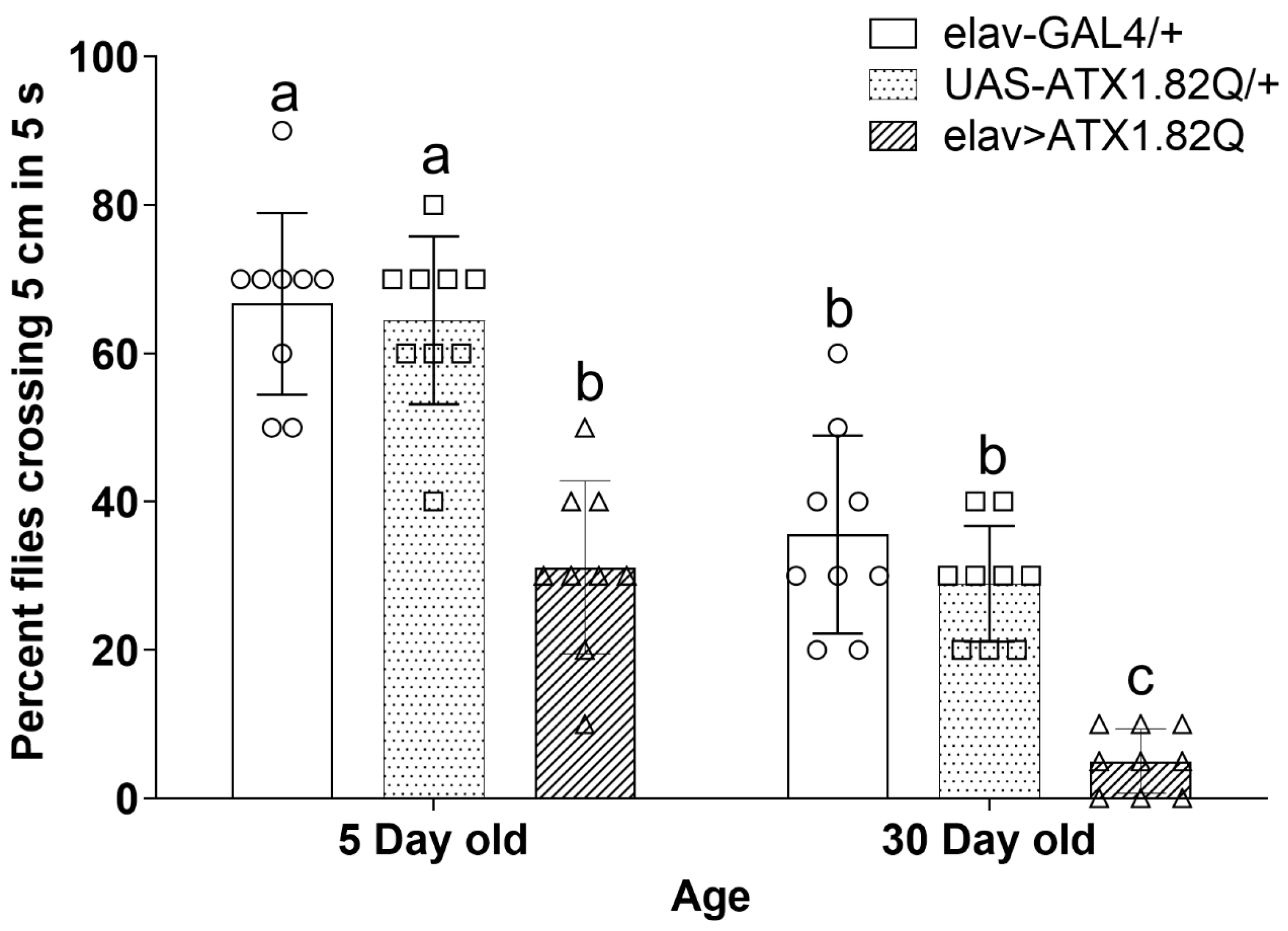
3.4. Negative Geotaxis Is Significantly Impaired in Young and Old Flies Expressing Human ATX1.82
3.5. Flies Expressing Human ATX1.82Q Exhibit a Significant Upregulation in Expression of dMMP1 in Young and Old Flies and a Significant Decline in Expression of Extracellular Matrix Fibroblast Growth Factors and Survival Motor Neuron Gene in Old Flies
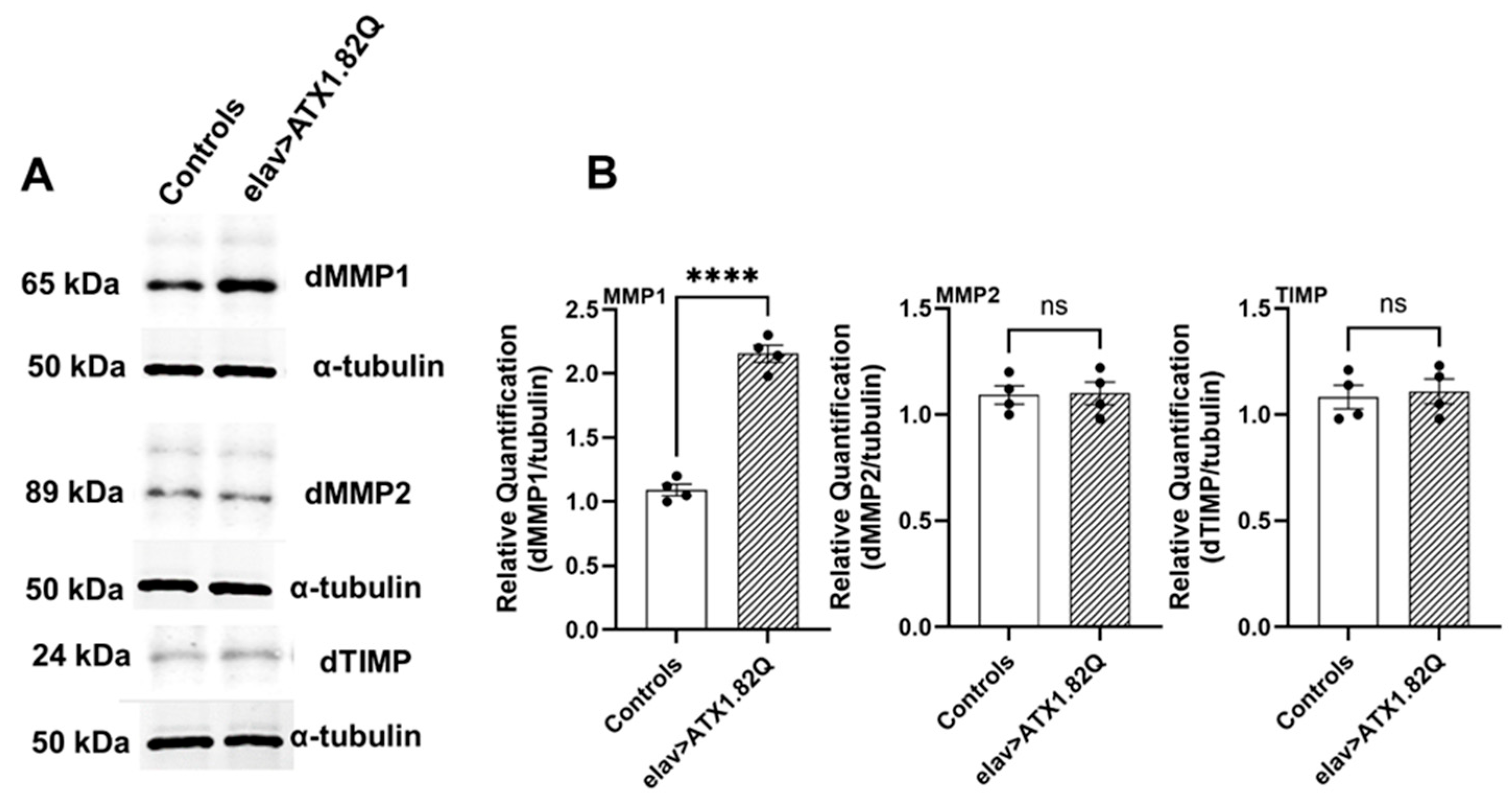
3.6. dMMP1 Protein Levels Were Markedly Increased in 30-Day-Old Flies Expressing Human ATX1.82Q Compared to Controls but Not dMMP2 and TIMP
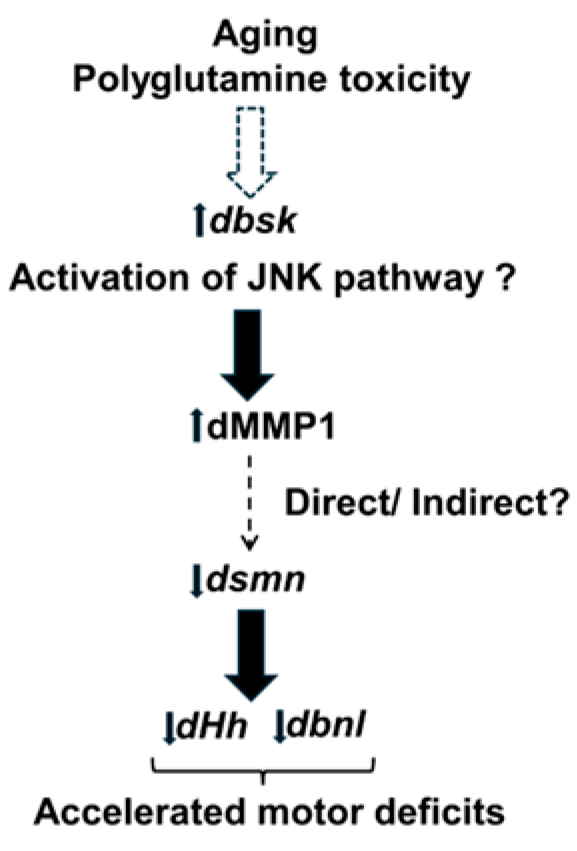
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dugger, B.N.; Dickson, D.W. Pathology of neurodegenerative diseases. CSH Perspect. Biol. 2017, 9, a028035. [Google Scholar] [CrossRef] [PubMed]
- Klockgether, T.; Mariotti, C.; Paulson, H.L. Spinocerebellar ataxia. Nat. Rev. Dis. Primers 2019, 5, 1–21. [Google Scholar] [CrossRef]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal dominant cerebellar ataxias: Clinical features, genetics, and pathogenesis. Lancet Neurol. 2004, 3, 291–304. [Google Scholar] [CrossRef]
- Soong, B.-W.; Morrison, P.J. Spinocerebellar ataxias. In Handbook of Clinical Neurology; Manto, M., Huisman, T.A.G.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 155, pp. 143–174. [Google Scholar] [CrossRef]
- Orr, H.T.; Zoghbi, H.Y. SCA1 molecular genetics: A history of a 13 year collaboration against glutamines. Hum. Mol. Genet. 2001, 10, 2307–2311. [Google Scholar] [CrossRef]
- Orr, H.T.; Chung, M.Y.; Banfi, S.; Kwiatkowski, T.J., Jr.; Servadio, A.; Beaudet, A.L.; McCall, A.E.; Duvick, L.A.; Ranum, L.P.; Zoghbi, H.Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat. Genet. 1993, 4, 221–226. [Google Scholar] [CrossRef]
- Nagase, H.; Visse, R.; Murphy, G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc. Res. 2006, 69, 562–573. [Google Scholar] [CrossRef]
- Sinha, S.K.; Asotra, K.; Uzui, H.; Nagwani, S.; Mishra, V.; Rajavashisth, T.B. Nuclear localization of catalytically active MMP-2 in endothelial cells and neurons. Am. J. Transl. Res. 2014, 6, 155–162. [Google Scholar]
- Bonneh-Barkay, D.; Wiley, C.A. Brain extracellular matrix in neurodegeneration. Brain Pathol. 2009, 19, 573–585. [Google Scholar] [CrossRef]
- Kim, Y.S.; Joh, T.H. Matrix metalloproteinases, new insights into the understanding of neurodegenerative disorders. Biomol. Ther. 2012, 20, 133–143. [Google Scholar] [CrossRef]
- Brkic, M.; Balusu, S.; Libert, C.; Vandenbroucke, R.E. Friends or foes: Matrix metalloproteinases and their multifaceted roles in neurodegenerative diseases. Mediat. Inflamm. 2015, 2015, 620581. [Google Scholar] [CrossRef] [PubMed]
- Lukaszewicz-Zajac, M.; Mroczko, B.; Slowik, A. Matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in amyotrophic lateral sclerosis (ALS). J. Neural Transm. 2014, 121, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Muneer, P.M.; Conte, A.A.; Haldar, D.; Long, M.; Patel, R.K.; Santhakumar, V.; Overall, C.M.; Pfister, B.J. Traumatic brain injury induced matrix metalloproteinase2 cleaves CXCL12α (stromal cell derived factor 1α) and causes neurodegeneration. Brain Behav. Immun. 2017, 59, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Annese, V.; Herrero, M.T.; Di Pentima, M.; Gomez, A.; Lombardi, L.; Ros, C.M.; De Pablos, V.; Fernandez-Villalba, E.; De Stefano, M.E. Metalloproteinase-9 contributes to inflammatory glia activation and nigro-striatal pathway degeneration in both mouse and monkey models of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced Parkinsonism. Brain Struct. Funct. 2015, 220, 703–727. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Models Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef]
- Slanzi, A.; Iannoto, G.; Rossi, B.; Zenaro, E.; Constantin, G. In vitro Models of Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020, 8, 328. [Google Scholar] [CrossRef]
- Pereira, I.; Lopez-Martinez, M.J.; Samitier, J. Advances in current in vitro models on neurodegenerative diseases. Front. Bioeng. Biotechnol. 2023, 11, 1260397. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Yoshida, H. Drosophila as a model organism. Adv. Exp. Med. Biol. 2018, 1076, 1–10. [Google Scholar] [CrossRef]
- Mark, M.; Rijli, F.M.; Chambon, P. Homeobox genes in embryogenesis and pathogenesis. Pediatr. Res. 1997, 42, 421–429. [Google Scholar] [CrossRef]
- McGurk, L.; Berson, A.; Bonini, N.M. Drosophila as an in vivo model for human neurodegenerative disease. Genetics 2015, 201, 377–402. [Google Scholar] [CrossRef]
- O’Kane, C.J. Modelling human diseases in Drosophila and Caenorhabditis. Semin. Cell Dev. Biol. 2003, 14, 3–10. [Google Scholar] [CrossRef]
- Lu, B.; Vogel, H. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. 2009, 4, 315–342. [Google Scholar] [CrossRef] [PubMed]
- Bolus, H.; Crocker, K.; Boekhoff-Falk, G.; Chtarbanova, S. Modeling neurodegenerative disorders in Drosophila melanogaster. Int. J. Mol. Sci. 2020, 21, 3055. [Google Scholar] [CrossRef] [PubMed]
- Nitta, Y.; Sugie, A. Studies of neurodegenerative diseases using Drosophila and the development of novel approaches for their analysis. Fly 2022, 16, 275–298. [Google Scholar] [CrossRef]
- Xu, Z.; Tito, A.J.; Rui, Y.N.; Zhang, S. Studying polyglutamine diseases in Drosophila. Exp. Neurol. 2015, 274, 25–41. [Google Scholar] [CrossRef]
- Long, Z.; Tang, B.; Jiang, H. Alleviating neurodegeneration in Drosophila models of PolyQ diseases. Cerebellum Ataxias 2014, 1, 9. [Google Scholar] [CrossRef]
- Page-McCaw, A.; Serano, J.; Sante, J.M.; Rubin, G.M. Drosophila matrix metalloproteinases are required for tissue remodeling, but not embryonic development. Dev. Cell 2003, 4, 95–106. [Google Scholar] [CrossRef]
- LaFever, K.S.; Wang, X.; Page-McCaw, P.; Bhave, G.; Page-McCaw, A. Both Drosophila matrix metalloproteinases have released and membrane-tethered forms but have different substrates. Sci. Rep. 2017, 7, 44560. [Google Scholar] [CrossRef]
- Bednářová, S.H.A.; Draughn, B.; Johnson, K.; Mills, D.; Thomas, C.; Scales, J.; Keenan, E.T.; Welcher, J.V.; Krishnan, N. Expression of Drosophila matrix metalloproteinases in cultured cell lines alters neural and glial cell morphology. Front. Cell. Dev. Biol. 2021, 9, 610887. [Google Scholar] [CrossRef]
- Azpurua, J.; Mahoney, R.E.; Eaton, B.A. Transcriptomics of aged Drosophila motor neurons reveals a matrix metalloproteinase that impairs motor function. Aging Cell. 2018, 7, e12729. [Google Scholar] [CrossRef]
- Grotewiel, M.S.; Martin, I.; Bhandari, P.; Cook-Wiens, E. Functional senescence in Drosophila melanogaster. Ageing Res. Rev. 2005, 4, 372–397. [Google Scholar] [CrossRef]
- Brand, A.H.; Perrimon, N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 1993, 118, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, A.; Tomčala, A.; Mochanová, M.; Kodrík, D.; Krishnan, N. Disruption of Adipokinetic hormone mediated energy homeostasis has subtle effects on physiology, behavior and lipid status during aging in Drosophila. Front. Physiol. 2018, 9, 949. [Google Scholar] [CrossRef] [PubMed]
- Bednářová, A.; Hanna, M.E.; Rakshit, K.; O’Donnell, J.M.; Krishnan, N. Disruption of dopamine homeostasis has sexually dimorphic effects on senescence characteristics of Drosophila melanogaster. Eur. J. Neurosci. 2017, 45, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffenberger, C.; Lear, B.C.; Keegan, K.P.; Allada, R. Locomotor activity level monitoring using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harb. Protoc. 2010, 2010, pdb.prot5518. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef]
- Krishnan, N.; Rakshit, K.; Chow, E.S.; Wentzell, J.S.; Kretzschmar, D.; Giebultowicz, J.M. Loss of circadian clock accelerates aging in neurodegeneration-prone mutants. Neurobiol. Dis. 2012, 45, 1129–1135. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Long, K.R.; Huttner, W.B. How the extracellular matrix shapes neural development. Open Biol. 2019, 9, 180216. [Google Scholar] [CrossRef]
- Grice, S.J.; Liu, J.L. Survival motor neuron protein regulates stem cell division, proliferation, and differentiation in Drosophila. PLoS Genet. 2011, 7, e1002030. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, R.; Tamura, T.; Sone, M.; Okazawa, H. Systematic analysis of fly models with multiple drivers reveals different effects of ataxin-1 and huntingtin in neuron subtype-specific expression. PLoS ONE 2014, 9, e116567. [Google Scholar] [CrossRef] [PubMed]
- Oswal, N.; Martin, O.M.F.; Stroustrup, S.; Bruckner, M.A.M.; Stroustrup, N. A hierarchical process model links behavioral aging and lifespan in C. elegans. PLoS Comput. Biol. 2022, 18, e1010415. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Van Remmen, H. Age-associated alterations of the neuromuscular junction. Exp. Gerontol. 2011, 46, 193–198. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef]
- Kreko-Pierce, T.; Azpurua, J.; Mahoney, R.E.; Eaton, B.A. Extension of health span and life span in Drosophila by S107 requires the calstabin homologue FK506-BP2. J. Biol. Chem. 2016, 291, 26045–26055. [Google Scholar] [CrossRef]
- Tonoki, A.; Davis, R.L. Aging impairs intermediate-term behavioral memory by disrupting the dorsal paired medial neuron memory trace. Proc. Natl. Acad. Sci. USA 2012, 109, 6319–6324. [Google Scholar] [CrossRef]
- Yamazaki, D.; Horiuchi, J.; Ueno, K.; Ueno, T.; Saeki, S.; Matsuno, M.; Naganos, S.; Miyashita, T.; Hirano, Y.; Nishikawa, H.; et al. Glial dysfunction causes age-related memory impairment in Drosophila. Neuron 2014, 84, 753–763. [Google Scholar] [CrossRef]
- Liao, S.; Broughton, S.; Nässel, D.R. Behavioral senescence and aging-related changes in motor neurons and brain neuromodulator levels are ameliorated by lifespan-extending reproductive dormancy in Drosophila. Front. Cell. Neurosci. 2017, 11, 111. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Matrix metalloproteinases and their multiple roles in neurodegenerative diseases. Lancet Neurol. 2009, 8, 205–216. [Google Scholar] [CrossRef]
- Rosenberg, G.A. Metalloproteinases and neurodegenerative diseases: Pathophysiological and therapeutic perspectives. Met. Med. 2015, 2, 39–50. [Google Scholar] [CrossRef]
- Yong, V.W.; Power, C.; Forsyth, P.; Edwards, D.R. Metalloproteinases in biology and pathology of the nervous system. Nat. Rev. Neurosci. 2001, 2, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Yong, V.W. Metalloproteinases: Mediators of pathology and regeneration in the CNS. Nat. Rev. Neurosci. 2005, 6, 931–944. [Google Scholar] [CrossRef] [PubMed]
- Dear, M.L.; Dani, N.; Parkinson, W.; Broadie, S.Z.K. Two classes of matrix metalloproteinases reciprocally regulate synaptogenesis. Development 2016, 143, 75–87. [Google Scholar] [CrossRef]
- Srahna, M.; Leyssen, M.; Choi, C.M.; Fradkin, L.G.; Noordermeer, J.N.; Hassan, B.A. A signaling network for patterning of neuronal connectivity in the Drosophila brain. PLoS Biol. 2006, 4, e348. [Google Scholar] [CrossRef]
- Sternlicht, M.D.; Werb, Z. How matrix metalloproteinases regulate cell behavior. Annu. Rev. Cell Dev. Biol. 2001, 17, 463–516. [Google Scholar] [CrossRef]
- Rallis, A.; Navarro, J.A.; Rass, M.; Hu, A.; Birman, S.; Schneuwly, S.; Thérond, P.P. Hedgehog signaling modulates glial proteostasis and lifespan. Cell Rep. 2020, 30, 2627–2643. [Google Scholar] [CrossRef]
- Seidel, K.; Siswanto, S.; Brunt, E.R.; den Dunnen, W.; Korf, H.W.; Rüb, U. Brain pathology of spinocerebellar ataxias. Acta Neuropathol. 2012, 124, 1–21. [Google Scholar] [CrossRef]
- McLoughlin, H.S.; Moore, L.R.; Paulson, H.L. Pathogenesis of SCA3 and implications for other polyglutamine diseases. Neurobiol. Dis. 2020, 134, 104635. [Google Scholar] [CrossRef]
- Ding, D.; Wang, C.; Chen, Z.; Xia, K.; Tang, B.; Qiu, R.; Jiang, H. Polyglutamine-expanded ataxin3 alter specific gene expressions through changing DNA methylation status in SCA3/MJD. Aging 2020, 13, 3680–3698. [Google Scholar] [CrossRef]
- Klämbt, C.; Glazer, L.; Shilo, B.Z. Breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 1992, 6, 1668–1678. [Google Scholar] [CrossRef] [PubMed]
- Barrett, A.L.; Krueger, S.; Datta, S. Branchless and Hedgehog operate in a positive feedback loop to regulate the initiation of neuroblast division in the Drosophila larval brain. Dev. Biol. 2008, 317, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Packard, M.; Koo, E.S.; Gorczyca, M.; Sharpe, J.; Cumberledge, S.; Budnik, V. The Drosophila Wnt, wingless, provides an essential signal for pre- and postsynaptic differentiation. Cell 2002, 111, 319–330. [Google Scholar] [CrossRef]
- McCabe, B.D.; Marqués, G.; Haghighi, A.P.; Fetter, R.D.; Crotty, M.L.; Haerry, T.E.; Goodman, C.S.; O’Connor, M.B. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 2003, 39, 241–254. [Google Scholar] [CrossRef]
- Liu, Q.; Fischer, U.; Wang, F.; Dreyfuss, G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 1997, 90, 1013–1102. [Google Scholar] [CrossRef]
- Grice, S.J.; Liu, J.L. Motor defects in a Drosophila model for spinal muscular atrophy result from SMN depletion during early neurogenesis. PLoS Genet. 2022, 18, e1010325. [Google Scholar] [CrossRef]
- Sen, A.; Yokokura, T.; Kankel, M.W.; Dimlich, D.N.; Manent, J.; Sanyal, S.; Artavanis-Tsakonas, S. Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling. J. Cell Biol. 2011, 192, 481–495. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmer, E.M.; Snoddy, C.A.; York, P.M.; Davis, S.M.; Hunter, M.F.; Krishnan, N. Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase. Biology 2024, 13, 854. https://doi.org/10.3390/biology13110854
Palmer EM, Snoddy CA, York PM, Davis SM, Hunter MF, Krishnan N. Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase. Biology. 2024; 13(11):854. https://doi.org/10.3390/biology13110854
Chicago/Turabian StylePalmer, Emma M., Caleb A. Snoddy, Peyton M. York, Sydney M. Davis, Madelyn F. Hunter, and Natraj Krishnan. 2024. "Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase" Biology 13, no. 11: 854. https://doi.org/10.3390/biology13110854
APA StylePalmer, E. M., Snoddy, C. A., York, P. M., Davis, S. M., Hunter, M. F., & Krishnan, N. (2024). Enhanced Age-Dependent Motor Impairment in Males of Drosophila melanogaster Modeling Spinocerebellar Ataxia Type 1 Is Linked to Dysregulation of a Matrix Metalloproteinase. Biology, 13(11), 854. https://doi.org/10.3390/biology13110854





