Copaiba Oleoresin Improves Weight Gain and IL-10 Concentration, with No Impact on Hepatic Histology, in Liver Cirrhosis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Model
2.2. Serum Biochemical Parameters
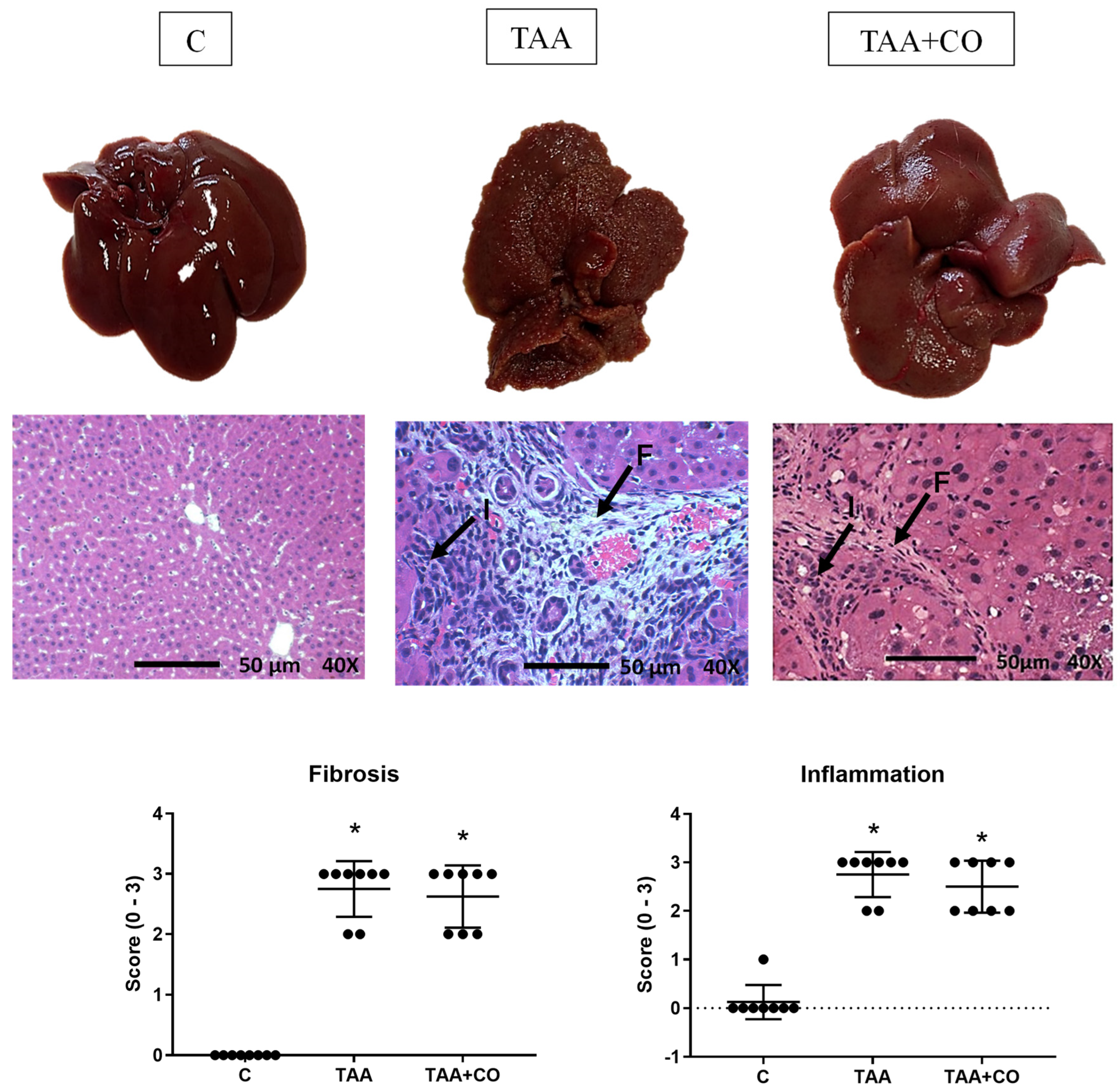
2.3. Liver Histology
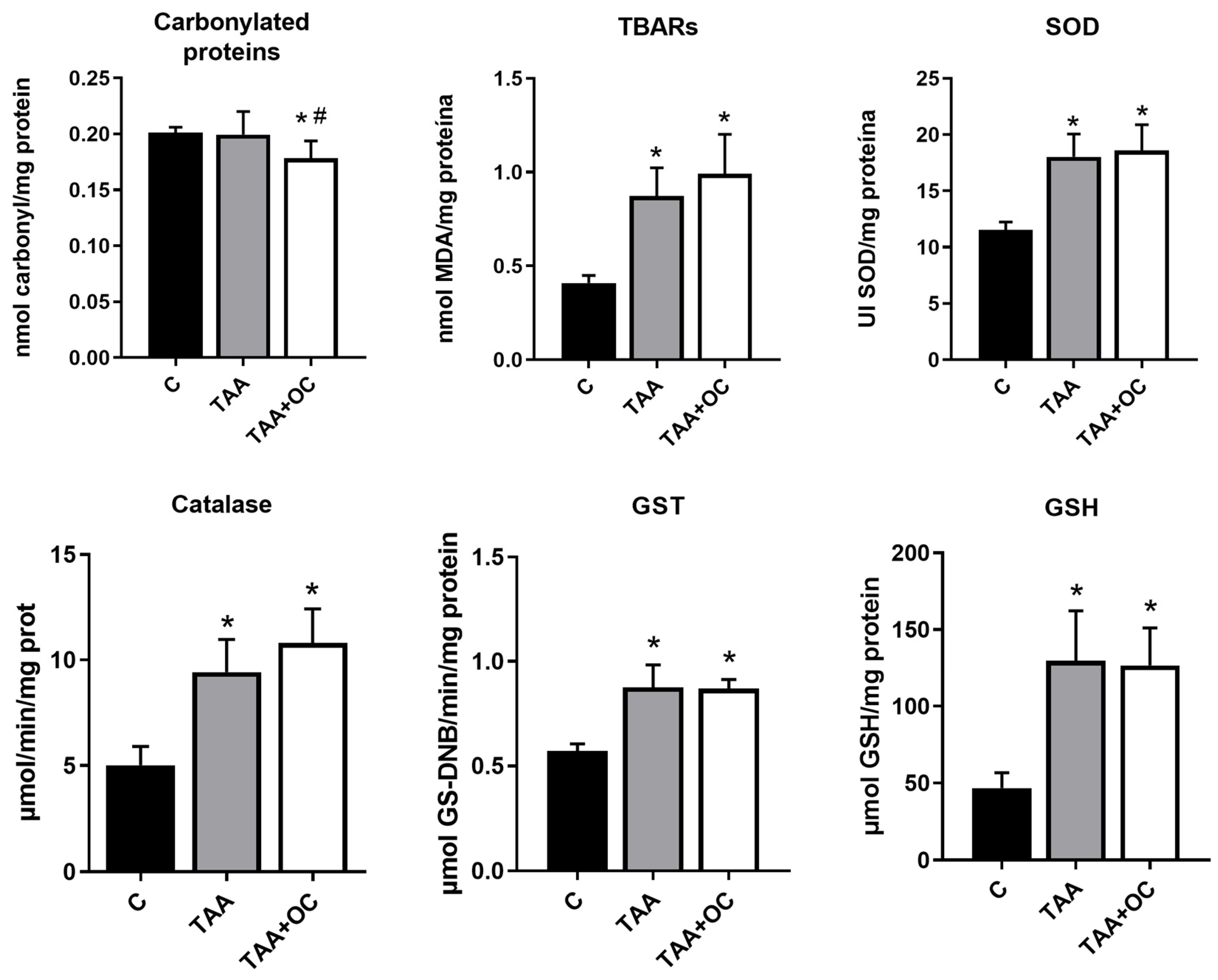
2.4. Evaluation of Markers of Hepatic Oxidative Damage
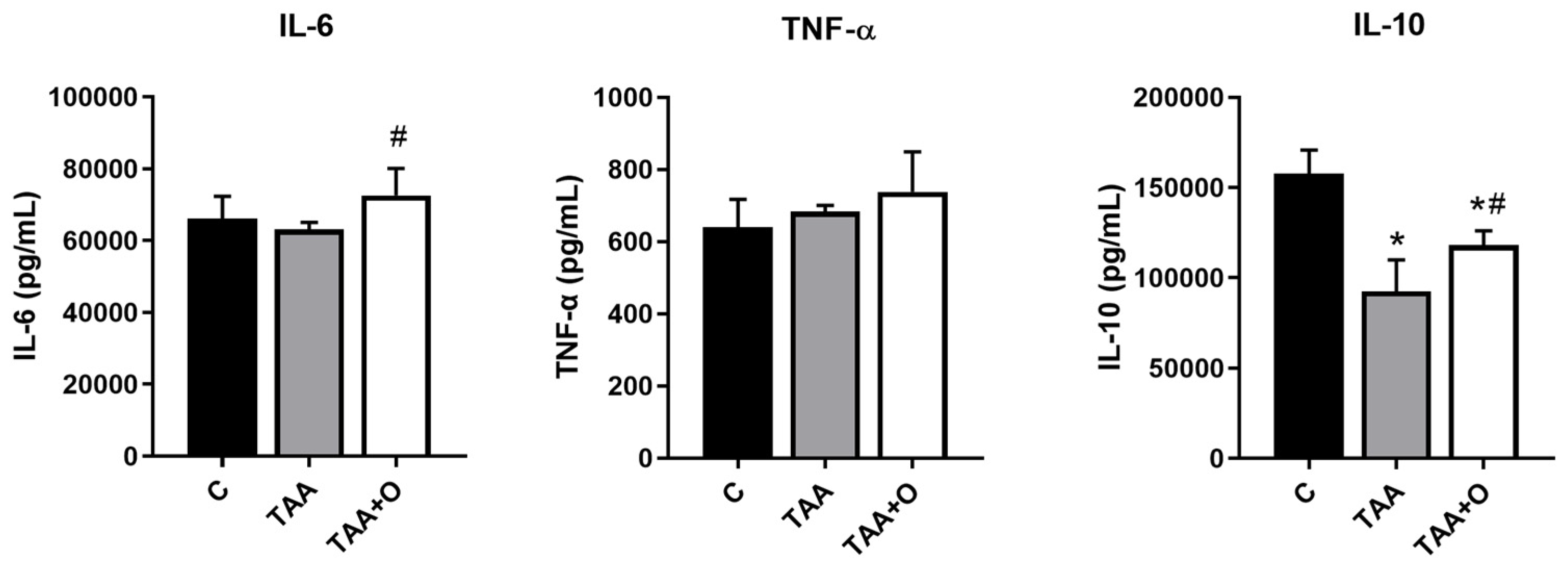
2.5. Hepatic Cytokines
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Veiga, V.F.; Rosas, E.C.; Carvalho, M.V.; Henriques, M.G.M.O.; Pinto, A.C. Chemical composition and anti-inflammatory activity of copaiba oils from Copaifera cearensis Huber ex Ducke, Copaifera reticulata Ducke and Copaifera multijuga Hayne—A comparative study. J. Ethnopharmacol. 2007, 112, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Aldana Mejía, J.A.; Ribeiro, V.P.; Gambeta Borges, C.H.; Martins, C.H.G.; Sola Veneziani, R.C.; Ambrósio, S.R.; Bastos, J.K. Occurrence, chemical composition, biological activities and analytical methods on Copaifera genus—A review. Biomed. Pharmacother. 2019, 109, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Santana, V.; Dini, Q.; Da Conceição Furtado, S.; Fernando, J.; Barcellos, M.; Ferreira Da Costa, O.T. Ação Anti-Inflamatória do Óleo de Copaíba em Artrite Induzida em Modelo Animal: Uma Revisão Sistemática. Sci. Amazon. 2019, 8, CB1–CB12. [Google Scholar]
- Gomes, N.d.M.; Rezende, C.M.d.; Fontes, S.P.; Matheus, M.E.; Pinto, A.d.C.; Fernandes, P.D. Characterization of the antinociceptive and anti-inflammatory activities of fractions obtained from Copaifera multijuga Hayne. J. Ethnopharmacol. 2010, 128, 177–183. [Google Scholar] [CrossRef]
- Pieri, F.A.; Mussi, M.C.; Moreira, M.A.S. Óleo de copaíba (Copaifera sp.): Histórico, extração, aplicações industriais e propriedades medicinais. Rev. Bras. Plantas Med. 2009, 11, 465–472. [Google Scholar] [CrossRef]
- Veiga, V.F., Jr.; Pinto, A.C. O gênero Copaifera L. Quim. Nova 2002, 25, 273–286. [Google Scholar] [CrossRef]
- de Almeida Júnior, J.; da Silva, É.; Moraes, T.; Kasper, A.; Sartoratto, A.; Baratto, L.C.; de Oliveira, E.C.P.; Oliveira, E.; Barata, L.E.S.; Minervino, A.H.H.; et al. Anti-Inflammatory Potential of the Oleoresin from the Amazonian Tree Copaifera reticulata with an Unusual Chemical Composition in Rats. Vet. Sci. 2021, 8, 320. [Google Scholar] [CrossRef]
- Carvalho, J.C.T.; Cascon, V.; Possebon, L.S.; Morimoto, M.S.S.; Cardoso, L.G.V.; Kaplan, M.A.C.; Gilbert, B. Topical antiinflammatory and analgesic activities of Copaifera duckei dwyer. Phytother. Res. 2005, 19, 946–950. [Google Scholar] [CrossRef]
- Castro Ghizoni, C.V.; Arssufi Ames, A.P.; Lameira, O.A.; Bersani Amado, C.A.; Sá Nakanishi, A.B.; Bracht, L.; Marcal Natali, M.R.; Peralta, R.M.; Bracht, A.; Comar, J.F. Anti-Inflammatory and Antioxidant Actions of Copaiba Oil Are Related to Liver Cell Modifications in Arthritic Rats. J. Cell Biochem. 2017, 118, 3409–3423. [Google Scholar] [CrossRef]
- Paiva, L.A.F.; Gurgel, L.A.; Silva, R.M.; Tomé, A.R.; Gramosa, N.V.; Silveira, E.R.; Santos, F.; Rao, V. Anti-inflammatory effect of kaurenoic acid, a diterpene from Copaifera langsdorffii on acetic acid-induced colitis in rats. Vasc. Pharmacol. 2002, 39, 303–307. [Google Scholar] [CrossRef]
- Li, S.; Tan, H.Y.; Wang, N.; Zhang, Z.J.; Lao, L.; Wong, C.W.; Feng, Y. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef] [PubMed]
- Pratim Das, P.; Medhi, S. Role of inflammasomes and cytokines in immune dysfunction of liver cirrhosis. Cytokine 2023, 170, 156347. [Google Scholar] [CrossRef] [PubMed]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.H.; Wan, Q.S.; Wang, T.; Zhang, K.H. Fluid Biomarkers for Predicting the Prognosis of Liver Cirrhosis. Biomed. Res. Int. 2020, 2020, 1–10. [Google Scholar] [CrossRef]
- Angeli, P.; Bernardi, M.; Villanueva, C.; Francoz, C.; Mookerjee, R.P.; Trebicka, J.; Krag, A.; Laleman, W.; Gines, P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M.; et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J. Gastroenterol. 2021, 56, 593–619. [Google Scholar] [CrossRef]
- Bataller, R.; Brenner, D.A. Liver fibrosis. J. Clin. Investig. 2005, 115, 209–218. [Google Scholar] [CrossRef]
- Jalan, R.; D’amico, G.; Trebicka, J.; Moreau, R.; Angeli, P.; Arroyo, V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J. Hepatol. 2021, 75, S14–S26. [Google Scholar] [CrossRef]
- Nascimento, M.; Piran, R.; Da Costa, R.M.; Giordani, M.A.; Carneiro, F.S.; Aguiar, D.; Dias, M.; Sugizaki, M.; Luvizotto, R.; Nascimento, A.; et al. Hepatic injury induced by thioacetamide causes aortic endothelial dysfunction by a cyclooxygenase-dependent mechanism. Life Sci. 2018, 212, 168–175. [Google Scholar] [CrossRef]
- Chen, J.; Guo, Q.; Chen, Q.; Chen, Y.; Chen, D.; Chen, Z.; Wang, X.; Huang, Y. Interleukin 10 inhibits oxidative stress-induced autophagosome formation in hepatic stellate cells by activating the mTOR-STAT3 pathway. Exp. Cell Res. 2022, 411, 113001. [Google Scholar] [CrossRef]
- da Silva, B.S.; Paulino, A.M.B.; Taffarel, M.; Borba, I.G.; Telles, L.O.; Lima, V.V.; Aguiar, D.H.; Dias, M.C.; Nascimento, A.F.; Sinhorin, V.D.G.; et al. High sucrose diet attenuates oxidative stress, inflammation and liver injury in thioacetamide-induced liver cirrhosis. Life Sci. 2021, 15, 267. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Anastácio, L.R.; Lima, A.S.; Correia, M.I.T.D. Desnutrição e inadequação alimentar de pacientes aguardando transplante hepático. Rev. Assoc. Med. Bras. 2009, 55, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Poordad, F.F. Presentation and complications associated with cirrhosis of the liver. Curr. Med. Res. Opin. 2015, 31, 925–937. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, R.A.; Lima, V.V.; Bomfim, G.F.; Giachini, F.R.C. Interleukin-10 in the Vasculature: Pathophysiological Implications. Curr. Vasc. Pharmacol. 2021, 20, 230–243. [Google Scholar] [CrossRef]
- Huang, Y.H.; Chen, M.H.; Guo, Q.L.; Chen, Z.X.; Chen, Q.D.; Wang, X.Z. Interleukin-10 induces senescence of activated hepatic stellate cells via STAT3-p53 pathway to attenuate liver fibrosis. Cell Signal. 2020, 66, 109445. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Huang, R.; Chen, Z.; Wang, X.; Chen, F.; Huang, Y. Interleukin-10 gene intervention ameliorates liver fibrosis by enhancing the immune function of natural killer cells in liver tissue. Int. Immunopharmacol. 2024, 127, 111341. [Google Scholar] [CrossRef]
- Caputo, L.d.S.; Alves, C.d.L.; Laranjeira, I.M.; Fonseca-Rodrigues, D.; da Silva Filho, A.A.; Dias, A.C.P.; Pinto-Ribeiro, F.; Pereira Junior, O.d.S.; de Paula, A.C.C.; Nagato, A.C.; et al. Copaiba oil minimizes inflammation and promotes parenchyma re-epithelization in acute allergic asthma model induced by ovalbumin in BALB/c mice. Front. Pharmacol. 2024, 15, 1356598. [Google Scholar] [CrossRef]
- Chilakapati, J. Saturation Toxicokinetics of Thioacetamide: Role in Initiation of Liver Injury. Drug Metab. Dispos. 2005, 33, 1877–1885. [Google Scholar] [CrossRef]
- Túnez, I.; Muñoz, M.C.; Villavicencio, M.A.; Medina, F.J.; Deprado, E.; Espejo, I.; Barcos, M.; Salcedo, M.; Feijóo, M.; Montilla, P. Hepato- and neurotoxicity induced by thioacetamide: Protective effects of melatonin and dimethylsulfoxide. Pharmacol. Res. 2005, 52, 223–228. [Google Scholar] [CrossRef]
- Telles, L.O.; Silva, B.S.D.; Paulino, A.M.B.; Mendonca, S.T.; Sinhorin, V.D.G.; Lima, M.C.F.D.; Veiga, V.F.; Andrighetti, C.R.; Nascimento, A.F.D.; Bomfim, G.F.; et al. Copaiba oleoresin presents anti-obesogenic effect and mitigates inflammation and redox imbalance in adipose tissue. Acta Amazon. 2022, 52, 331–338. [Google Scholar] [CrossRef]
- Lucca, L.G.; de Matos, S.P.; Kreutz, T.; Teixeira, H.F.; Veiga, V.F.; de Araújo, B.V.; Limberger, R.P.; Koester, L.S. Anti-inflammatory Effect from a Hydrogel Containing Nanoemulsified Copaiba oil (Copaifera multijuga Hayne). AAPS PharmSciTech. 2018, 19, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.A.; de Paula, M.G.; Telles, L.O.; da Silva, T.P.; Paulino, A.M.B.; Mendonça, S.T.; Nascimento, A.F.D.; Bomfim, G.F.; Luvizotto, R.d.A.M. Copaiba Oil-Resin Improves Ponderal Development and Attenuates Inflammation in Adipose Tissue in a Model of Hepatic Cirrhosis. Int. J. Health Sci. 2022, 2, 2–9. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic Steatohepatitis: A Proposal for Grading and Staging The Histological Lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef] [PubMed]
- Bruck, R.; Ashkenazi, M.; Weiss, S.; Goldiner, I.; Shapiro, H.; Aeed, H.; Genina, O.; Helpern, Z.; Pines, M. Prevention of liver cirrhosis in rats by curcumin. Liver International. 2007, 27, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Müller, A.; Machnik, F.; Zimmermann, T.; Schubert, H. Thioacetamide-induced cirrhosis-like liver lesions in rats usefulness and reliability of this animal model. Exp. Pathol. 1988, 34, 229–236. [Google Scholar] [CrossRef]
- Furtado, K.S.; Prado, M.G.; e Silva, M.A.A.; Dias, M.C.; Rivelli, D.P.; Rodrigues, M.A.M.; Barbisan, L.F. Coffee and Caffeine Protect against Liver Injury Induced by Thioacetamide in Male Wistar Rats. Basic Clin. Pharmacol. Toxicol. 2012, 111, 339–347. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Garavaglia, M.E.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A step-by-step protocol for assaying protein carbonylation in biological samples. J. Chromatogr. B 2016, 1019, 178–190. [Google Scholar] [CrossRef]
- Misra, H.P.; Fridovich, I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972, 247, 3170–3175. [Google Scholar] [CrossRef]
- Nelson, D.P.; Kiesow, L.A. Enthalpy of decomposition of hydrogen peroxide by catalase at 25 °C (with molar extinction coefficients of H2O2 solutions in the UV). Anal. Biochem. 1972, 49, 474–478. [Google Scholar] [CrossRef]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-Transferases. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Sedlak, J.; Lindsay, R.H. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal. Biochem. 1968, 25, 192–205. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Anastácio, L.R.; Ferreira, L.G.; Ribeiro, H.d.S.; Lima, A.S.; Vilela, E.G.; Correia, M.I.T.D. Weight loss during cirrhosis is related to the etiology of liver disease. Arq. Gastroenterol. 2012, 49, 195–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Henkel, A.S.; Buchman, A.L. Nutritional support in patients with chronic liver disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2006, 3, 202–209. [Google Scholar] [CrossRef]
- Kalas, M.A.; Chavez, L.; Leon, M.; Taweesedt, P.T.; Surani, S. Abnormal liver enzymes: A review for clinicians. World J. Hepatol. 2021, 13, 1688–1698. [Google Scholar] [CrossRef]
- Brito, M.V.H.; Costa, F.D.; de Vasconcelos, D.M.; Costa, L.A.V.; Yasojima, E.Y.; Teixeira, R.K.C.; Yamaki, V.N. Attenuation of copaiba oil in hepatic damage in rats. Acta Cir. Bras. 2014, 29, 776–780. [Google Scholar] [CrossRef]
- Brito, M.V.H.; Oliveira, R.V.B.d.; Silveira, E.L.; Reis, J.M.C.d.; Noguchi, A.; Epaminondas, W.A.; Moraes, M.R. Aspectos microscópicos do fígado de ratos após administração do óleo de copaíba. Acta Cir. Bras. 2000, 15, 102–106. [Google Scholar] [CrossRef]
- Pereira, D.L.; Cunha, A.P.S.D.; Cardoso, C.R.P.; Rocha, C.Q.D.; Vilegas, W.; Sinhorin, A.P.; Sinhorin, V.D.G. Antioxidant and hepatoprotective effects of ethanolic and ethyl acetate stem bark extracts of Copaifera multijuga (Fabaceae) in mice. Acta Amazon. 2018, 48, 347–357. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-Antioxidant Response Element Signaling Pathway and Its Activation by Oxidative Stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Ichikawa, T.; Machida, N.; Kaneko, H.; Oi, I.; Fujino, M.A. C-reactive protein can predict patients with cirrhosis at a high risk of early mortality after acute esophageal variceal bleeding. Intern. Med. 2019, 58, 487–495. [Google Scholar] [CrossRef]
- Zheng, M.; Fang, W.; Yu, M.; Ding, R.; Zeng, H.; Huang, Y.; Mi, Y.; Duan, C. IL-6 and IL-10 gene polymorphisms and cirrhosis of liver risk from a comprehensive analysis. BMC Endocr. Disord. 2021, 21, 242. [Google Scholar] [CrossRef]
| Parameters | Groups | ||
|---|---|---|---|
| C | TAA | TAA + CO | |
| FBW (g) | 436.0 ± 16.67 | 369.0 ± 21.03 * | 396.2 ± 19.13 *# |
| Weight Gain (g) | 136.3 ± 16.84 | 46.0 ± 13.64 * | 68.8 ± 9.10 *# |
| Epididymal Fat (g) | 9.3 ± 0.42 | 5.5 ± 0.55 * | 6.5 ± 0.75 *# |
| Retroperitoneal Fat (g) | 13.5 ± 1.35 | 7.6 ± 0.90 * | 9.2 ± 1.01 *# |
| Visceral Fat (g) | 5.8 ± 0.51 | 4.6 ± 0.47 * | 6.0 ± 0.93 # |
| Liver/BW (g) | 26.5 ± 1.04 | 38.1 ± 2.06 * | 40.8 ± 2.16 *# |
| ALT (U/L) | 77.1 ± 9.54 | 151.1 ± 15.01 * | 198.0 ± 67.05 * |
| AST (U/L) | 263.6 ± 37.14 | 455.1 ± 53.43 * | 400.8 ± 72.1 * |
| CRP (mg/dL) | 2.5 ± 0.52 | 3.6 ± 0.55 * | 1.8 ± 0.30 *# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taffarel, M.; Silva, B.S.d.; Paulino, A.M.B.; Telles, L.O.; Mendonça, S.T.; Santos, C.V.d.; Giordani, M.A.; Nascimento, A.F.; Aguiar, D.H.; Sinhorin, V.D.G.; et al. Copaiba Oleoresin Improves Weight Gain and IL-10 Concentration, with No Impact on Hepatic Histology, in Liver Cirrhosis. Biology 2024, 13, 853. https://doi.org/10.3390/biology13110853
Taffarel M, Silva BSd, Paulino AMB, Telles LO, Mendonça ST, Santos CVd, Giordani MA, Nascimento AF, Aguiar DH, Sinhorin VDG, et al. Copaiba Oleoresin Improves Weight Gain and IL-10 Concentration, with No Impact on Hepatic Histology, in Liver Cirrhosis. Biology. 2024; 13(11):853. https://doi.org/10.3390/biology13110853
Chicago/Turabian StyleTaffarel, Maiara, Bianca Sulzbacher da Silva, Angélica Macedo Borgês Paulino, Luciana Ortega Telles, Sabrina Trigueiro Mendonça, Cintia Vieira dos Santos, Morenna Alana Giordani, André Ferreira Nascimento, Danilo Henrique Aguiar, Valéria Dornelles Gindri Sinhorin, and et al. 2024. "Copaiba Oleoresin Improves Weight Gain and IL-10 Concentration, with No Impact on Hepatic Histology, in Liver Cirrhosis" Biology 13, no. 11: 853. https://doi.org/10.3390/biology13110853
APA StyleTaffarel, M., Silva, B. S. d., Paulino, A. M. B., Telles, L. O., Mendonça, S. T., Santos, C. V. d., Giordani, M. A., Nascimento, A. F., Aguiar, D. H., Sinhorin, V. D. G., Andrighetti, C. R., Luvizotto, R. d. A. M., & Bomfim, G. F. (2024). Copaiba Oleoresin Improves Weight Gain and IL-10 Concentration, with No Impact on Hepatic Histology, in Liver Cirrhosis. Biology, 13(11), 853. https://doi.org/10.3390/biology13110853







