Highlights
What are the main findings?
- Vitamin D reduces renin synthesis, which lowers angiotensin II (Ang-II) levels, suppressing coagulation, oxidative stress, hyper-inflammation, and cytokine storms following SARS-CoV-2 infection.
- Vitamin D sufficiency suppresses the RAS axis, increases the molecular expression of ACE-2, and converts Ang-II to Ang(1–7), which facilitates overcoming some harmful effects of corona-virus infections.
- Excess ACE-2 is released into the bloodstream as a soluble receptor, binding and neutralizing circulating SARS-CoV-2 and other coronaviruses.
- ACE inhibitors and angiotensin receptor blockers (ARBs) reduce Ang-II production and its in-teraction with the Ang-II type-1 receptor, thereby diminishing some harmful effects of SARS-CoV-2.
Simple Summary
The SARS-CoV-2 virus that caused COVID-19 devastated families, social structures, and economies worldwide. This pandemic has overwhelmed healthcare systems, increased deaths and disabilities, and triggered a global socio-economic crisis. Although the COVID-19 vaccines were developed rapidly, their effectiveness significantly decreased by the end of 2021 due to mutated viruses evading the immune system. As a result, despite high vaccination rates in industrialized countries, significant outbreaks occurred due to immune evasion associated with viral mutations. Over 300 clinical studies have shown that vitamin D (and ivermectin) are widely available and economical agents that promote immune system function. Proper doses of vitamin D effectively prevent and treat SARS-CoV-2, reducing complications, hospitalizations, and deaths by approximately 50%. Those with vitamin D deficiency fare the worse. SARS-CoV-2 activates the renin-angiotensin system by increasing renin expression, leading to elevated levels of the inflammatogenic and vasoconstrictor peptide angiotensin-II. SARS-CoV-2 viruses cause widespread inflammation, blood clots, and lung damage through multiple mechanisms, leading to impaired tissue oxygenation and death. In addition to enhancing the immune system, vitamin D increases ACE-2 enzyme levels, which breaks down angiotensin-II and reduces SARS-CoV-2-induced inflammation. It also lowers blood pressure and mitigates abnormal clotting. While the virus enters human cells through ACE-2 receptors, excess ACE-2 spills into the bloodstream and neutralizes viruses. This manuscript discusses how vitamin D mitigates the harmful effects of COVID-19.
Abstract
The interaction of the SARS-CoV-2 spike protein with membrane-bound angiotensin-converting enzyme-2 (ACE-2) receptors in epithelial cells facilitates viral entry into human cells. Despite this, ACE-2 exerts significant protective effects against coronaviruses by neutralizing viruses in circulation and mitigating inflammation. While SARS-CoV-2 reduces ACE-2 expression, vitamin D increases it, counteracting the virus’s harmful effects. Vitamin D’s beneficial actions are mediated through complex molecular mechanisms involving innate and adaptive immune systems. Meanwhile, vitamin D status [25(OH)D concentration] is inversely correlated with severity, complications, and mortality rates from COVID-19. This study explores mechanisms through which vitamin D inhibits SARS-CoV-2 replication, including the suppression of transcription enzymes, reduced inflammation and oxidative stress, and increased expression of neutralizing antibodies and antimicrobial peptides. Both hypovitaminosis D and SARS-CoV-2 elevate renin levels, the rate-limiting step in the renin-angiotensin-aldosterone system (RAS); it increases ACE-1 but reduces ACE-2 expression. This imbalance leads to elevated levels of the pro-inflammatory, pro-coagulatory, and vasoconstricting peptide angiotensin-II (Ang-II), leading to widespread inflammation. It also causes increased membrane permeability, allowing fluid and viruses to infiltrate soft tissues, lungs, and the vascular system. In contrast, sufficient vitamin D levels suppress renin expression, reducing RAS activity, lowering ACE-1, and increasing ACE-2 levels. ACE-2 cleaves Ang-II to generate Ang(1–7), a vasodilatory, anti-inflammatory, and anti-thrombotic peptide that mitigates oxidative stress and counteracts the harmful effects of SARS-CoV-2. Excess ACE-2 molecules spill into the bloodstream as soluble receptors, neutralizing and facilitating the destruction of the virus. These combined mechanisms reduce viral replication, load, and spread. Hence, vitamin D facilitates rapid recovery and minimizes transmission to others. Overall, vitamin D enhances the immune response and counteracts the pathological effects of SARS-CoV-2. Additionally, data suggests that widely used anti-hypertensive agents—angiotensin receptor blockers and ACE inhibitors—may lessen the adverse impacts of SARS-CoV-2, although they are less potent than vitamin D.
1. Introduction
The COVID-19 pandemic, triggered by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), caused havoc worldwide, leading to socio-economic crises and widespread negative repercussions on the global populace [1,2]. Vitamin D is a secosteroid molecule that undergoes two steps of hydroxylation to generate its most active form, calcitriol [1,25-dihydroxyvitamin D; 1,25(OH)2D] [3]. Beyond its pivotal role in calcium regulation, vitamin D engages in various biological functions affecting all tissues, especially the modulation of innate and adaptive immunity [4]. Upon binding to the vitamin (calcitriol) D receptor (VDR, CTR), it forms a complex with cofactors, translocates into the nucleus, and attaches to relevant portions of DNA [5]. While vitamin D and its receptor polymorphisms affect disease vulnerability and responses [6,7], they regulate over 1700 human genes, up- or down-regulating target genes [8].
The human immune system is operated by a complex network of mechanisms that respond to signals generated by membrane-bound signaling molecules, including toll-like receptors (TLR) [9,10,11]. In humans, many overlapping mechanisms effectively regulate the innate and adaptive immune systems [12]. Over 75% of the immune system functions rely on having sufficient calcitriol synthesized within immune cells. Consequently, vitamin D modulates immune cell functions and helps maintain a robust immune system [5,13]. Moreover, immune cells themselves express both vitamin D/calcitriol receptor (VDR/CTR) [14] and the 1α-hydroxylase enzyme (from the CYP27B1 gene) responsible for converting 25(OH)D into 1,25(OH)2D intracellularly [11,15,16]. Beyond its classical genomic functions, vitamin D exerts membrane-based and non-genomic actions, swiftly modulating various physiological pathways. These rapid actions and their autocrine and paracrine functions are particularly prominent within immune cells [17].
Vitamin D modulates the immune response, potentially reducing inflammation, and is associated with cytokine storm in severe COVID-19 [18]. Vitamin D up-regulates angiotensin-converting enzyme-2 (ACE-2) expression and generates angiotensin(1–7) (Ang(1–7)), a potent vasodilatory peptide that counteracts angiotensin-II (Ang-II). Ang(1–7) protects the cardiovascular system and mitigates lung injury caused by coronaviruses. Understanding the molecular interactions of these actions will provide insights into novel therapeutic strategies and public health interventions to reduce the burden of COVID-19. This review explores the interactions of vitamin D and ACE-2 on the backdrop of COVID-19 that affect clinical outcomes by critically evaluating ongoing research and recently published studies in this critical area.
This review article highlights the complex relationship between vitamin D and the ACE-2 receptor, focusing on their roles in mitigating the severity and mortality of COVID-19. We hypothesized that ACE-2 has a vital beneficial role in mitigating complications and infections of COVID-19 despite its role as the primary entry site for coronaviruses into epithelial cells. This study also investigated the vitamin D-dependent mechanisms of ACE-2 in inhibiting SARS-CoV-2 replication and subduing inflammation and oxidative stress reduction. This review highlights scientific data on the interactions of vitamin D on the expression and function of ACE-2 related to SARS-CoV-2 entering human cells and mitigating its harmful effects [19].
1.1. Benefits of Vitamin D
Research has shown that vitamin D is essential for more than just bone health. It plays a role in (A) the musculoskeletal system—it prevents rickets in children and osteomalacia in adults) [20,21,22], and (B) immune functions—vitamin D is crucial for maintaining a robust immune system [23,24] and reducing the risk of infections and autoimmune diseases [4,25], modulates metabolism [26] and energy generation [27,28], prevents acute infections [4,22], and minimizes chronic diseases [29]. Adequate vitamin D levels are linked to a lower risk of several chronic conditions, such as cardiovascular disease [30,31] and certain cancers [32,33], but others disagree [34,35]. In addition, it minimizes pregnancy-related complications and disorders [36,37,38,39] and mental health conditions [40,41], potentially including bipolar disorder [42] and the prevention of depression [43,44].
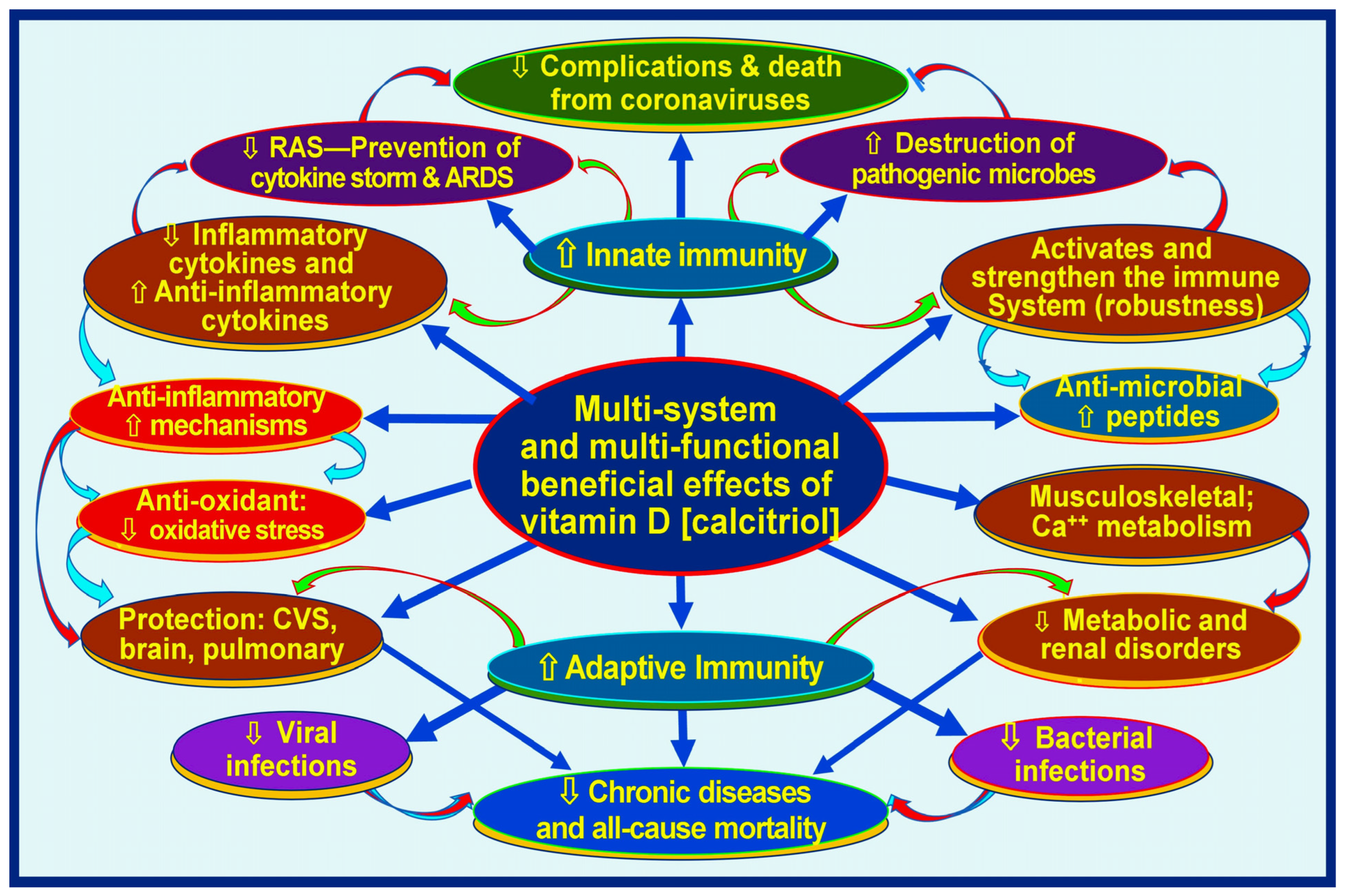
Meta-analyses and other clinical studies have consistently shown that sufficient vitamin D supplementation protects against acute respiratory tract infections, particularly in individuals with significant vitamin D deficiencies [45,46]. Further clinical research, including randomized controlled trials (RCTs), has revealed that vitamin D supplementation plays a crucial role in achieving positive health outcomes in cases of infectious diseases [13,47]. There are more than 310 peer-reviewed publications on the beneficial effects of prophylactic vitamin D in preventing SARS-CoV-2 [48] and as an adjunct in treatment [43,49,50]. Figure 1 illustrates the broader benefits of vitamin D in human health related to the immune system.
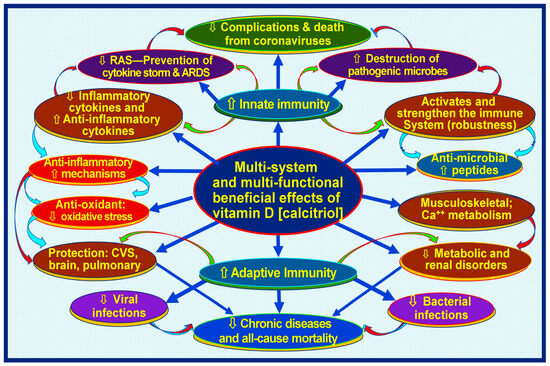
Figure 1.
Infections and immune-related broader functions of vitamin D (calcitriol, 1,25(OH)2D). The figure illustrates muti-system-wide functions of vitamin D related through the modulation of innate and adaptive immune systems, resulting in lowering complications from infections and chronic disease burdens [⇧ = increased; ⇩ = reduced; RAS: renin-angiotensin-system; CVS: cardiovascular system] (after Wimalawansa, Nutrients, 2022) [51].
Vitamin D is a threshold nutrient. Consequently, there will be little additional benefit from over-supplementation or intake by those with sufficient vitamin D [5]. However, supplementing deficient individuals provides a significant benefit [52]. In parallel, over-exposure to sunlight does not lead to excess vitamin D entering the blood [53]. Notably, for those infected with SARS-CoV-2 (and other viruses), vitamin D and its metabolites are consumed rapidly during infections and conditions associated with immune system activation [51,54]. Therefore, unless supplemented, even those with reasonable serum 25(OH)D levels (e.g., on hospitalization) are likely to develop a deficiency from an acute severe infection like SARS-CoV-2 [5].
Notably, over half of the world’s population is vitamin D deficient at any given time [5]. Therefore, with little or no adverse effects, it is beneficial to consume vitamin D supplements or be exposed to safe levels of daily sunlight [55,56,57]. However, even in the sunniest regions in the world, the majority spend time indoors. Thus, most people need supplemental vitamin D to prevent deficiency to stay healthy.
1.2. The Entry of SARS-CoV-2 into Human Cells
SARS-CoV-2, the virus responsible for COVID-19, enters human cells primarily by its spike (S) protein interaction with the angiotensin-converting enzyme 2 (ACE-2) receptor on the surface of human cells [57]. The spike protein has two subunits: S1, which contains the receptor-binding domain (RBD) that specifically binds to ACE-2, and S2, which facilitates membrane fusion [58,59]. Upon binding to ACE-2, the spike protein undergoes a conformational change, enabling the virus to attach more firmly to the host cell. Transmembrane protease serine 2 (TMPRSS2) in the host cell cleaves the S-protein at specific sites, triggering the fusion of the viral and cellular membranes and allowing the viral RNA to enter the cytoplasm [60].
Once inside the host cell, the host’s ribosomes translate the viral RNA into viral proteins. The viral RNA genome serves as a template for replication and transcription to produce more viral RNA genomes and sub-genomic RNAs [61], which encode structural and accessory proteins [60,62]. These newly synthesized viral components are assembled into new virions in the host cell’s endoplasmic reticulum-Golgi intermediate compartment [63]. The mature virions are transported to the cell surface in vesicles and then released into the extra-cellular space via exocytosis, including the tissue fluid and circulation. These virions are primed to infect new local and distant host cells. This viral entry, replication, and release cycle leads to the spread of SARS-CoV-2 within the host and contributes to the pathology of COVID-19 [64].
Meanwhile, the expression of ACE-2 is regulated by vitamin D metabolites [65]. Vitamin D sufficiency increases the expression of ACE-2 (including the soluble ACE-2 in the circulation) and dampens the renin-angiotensin system/axis (RAS) [59]. Despite cell membrane-bound ACE-2 receptors serving as the primary entry point for the SARS-CoV-2 virus, numerous benefits of vitamin D interaction have been reported, initiating ACE-2-related anti-viral effects against SARS-CoV-2 [19]. This review investigates the impact of vitamin D on the immune system, focusing on its effects on the renin-angiotensin system (RAS) and ACE-2 [66].
1.3. Functions of the Renin-Angiotensin System (RAS)
The renin-angiotensin-aldosterone axis (RAS) is a crucial endocrine axis that controls critical physiological parameters, such as blood pressure homeostasis, immune functions, and metabolism [66]. It is also known as the renin-angiotensin-aldosterone system (RAAS). The RAS is an organized complex hormonal cascade that plays a vital role in controlling critical functions in many organs. Traditionally, RAS plays a crucial role in the cardiovascular system, metabolism, cell growth, and homeostasis.
However, over the past 15 years, additional functions of the RAS, such as ACE-1 and ACE-2) were identified. These include inflammatory processes that lead to lung and other epithelial cell injury, even causing acute respiratory distress syndrome (ARDS), sepsis, cardiac hypertrophy, pulmonary hypertension, acute pancreatitis, and glomerulonephritis [67]. The RAS is crucial in regulating several physiological parameters related to cardiovascular and renal function [66]. Details of these are illustrated in Table 1.

Table 1.
Highlighting the critical functions of the RAS #.
The primary active peptide of the RAS is Ang-II. However, there are other minor components, like Ang-III and Ang-IV, as well as a counter-regulatory peptide, Ang(1–7) [66]. From a physiological point of view, the most important and widely studied two peptides are Ang-II and Ang(1–7) [68], which are involved in human health maintenance and disease statuses. Investigations are ongoing to understand its role in inflammation [18]. The RAAS is integral to the homeostatic regulation of cardiovascular, renal, and fluid balances (homeostasis) and is essential in physiological stability [67]. A sustained imbalance of the RAS can lead to a lowering of ACE-2 (with loss of its protective effects) [59], causing acute lung injury, like acute respiratory distress syndrome (ARDS), with high mortality.
1.4. Vitamin D on the Renin-Angiotensin-Aldosterone Hormonal (RAS) Axis
Activating the RAS, including coronaviruses, leads to the excess production of the enzyme renin, which cleaves angiotensinogen into Ang-I. ACE -1 then cleaves Ang-1 to form Ang-II [69]. This over-activation of the RAS leads to the excess production of Ang-II, which enhances the production of inflammatory cytokines [70], worsening the morbidity and mortality associated with infections/sepsis and leading to a cytokine storm that causes lung damage [71]. Excessive production of inflammatory cytokines leads to uncontrolled inflammation and is linked to immune dysfunction, as seen with excess Ang-II, which causes enhanced inflammation and oxidative stress [70]. In contrast, sufficient vitamin D suppresses the expression of renin, thus damping the RAS while increasing ACE-2 and reducing Ang-II. Consequently, it reduces inflammation and oxidative stress, improving clinical outcomes from infection and septic consequences [51,72,73].
As aforementioned, calcitriol is a crucial regulator of the RAS axis and suppresses the expression of the renin gene—a rate-limiting step of the synthesis of Ang-II via cyclic AMP (cAMP)-dependent PKA signaling [74]. Calcitriol independently increases the expression of ACE-2, reducing Ang-II and increasing the generation of angiotensin(1–7), suppressing the formation of the CRE-CREB-CBP complex [70], and keeping RAS activity under control. Meanwhile, certain viral infections like SARS-CoV-2 stimulate RAS activity, aggravating the situation in those with vitamin D deficiency and making them more vulnerable to developing cytokine storms [19,70].
1.5. Functions of the ACE-2/Ang(1–7)/MasR Axis (Counter-Regulatory Pathway)
Vitamin D inhibits renin, induces ACE-2/Ang(1–7)/MasR axis activity, and modulates the ACE/Ang II/AT1R axis. Through these mechanisms, vitamin D increases the expression of ACE-2, MasR, and Ang(1–7) and elicits a protective role against acute lung injury/ARDS and abnormal clotting activities [75]. Vitamin D can stimulate these beneficial targets like ACE-2 [65,76]. The RAS is regulated mainly by vitamin D (calcitriol) suppressing renin and increasing the expression of ACE-2 [65]—the latter also acts as a primary receptor for SARS-CoV-2 entry into the cells [76].
In health, there is a balance between the RAS, the ACE-Ang–II-AT1R regulatory axis, and the counter-regulatory axis (a pathway) of ACE-2-Ang–1–7-MasR. They are crucial in maintaining human cardiovascular, inflammatory, and immune homeostasis [66]. The wide distribution of ACE-2 in the heart, kidneys, lungs, colon, testis, etc., reflects this. The activation of ACE-2 antagonizes the over-active RAS system, protecting against organ damage and mitigating hypertension and cardiovascular diseases [65]. Once infected, SARS-CoV-2 causes a major imbalance in the RAS, including the down-regulation of ACE-2 receptors when they invade human epithelial cells [75,77].
1.6. Molecular Aspects of the RAS and ACE
Angiotensin-converting enzyme-2 (ACE-2) is a peptidase expressed on epithelial cell membranes and plays a crucial role in catabolizing Ang-II, thereby regulating the RAS [78]. Furthermore, in humans, ACE-2 polymorphisms can influence susceptibility to diseases such as hypertension and coronaviruses, including SARS-CoV-2 [78,79]. ACE-2 counterbalances the enzymatic actions of ACE-1 and Ang-II synthesis by converting Ang-II into the peptide Ang(1–7), reducing Ang-II molecular concentration. Furthermore, Ang(1–7) acts via the G-protein-coupled Mas receptor (MasR) to induce vasodilation [80] and attenuate the expression of pro-inflammatory cytokines [68], like TNF-α and IL-6 in LPS-induced macrophages, thereby promoting anti-inflammatory effects [81]. Despite these benefits, functional membrane ACE-2 receptors have been identified as the primary entry site of coronavirus into human cells [82,83].
In addition, Ang II interacts with the adrenogenic axis—endothelin and neuro-adrenergic systems—enhancing the local expression of noradrenaline and endothelin. This pathway leads to trophic as well as adverse effects on the cardiac myocyte [84,85]. Ang II also stimulates aldosterone secretion from the adrenal glands, contributing to salt balances and cardiac and vascular remodeling [84]; these compensatory mechanisms readjust vascular homeostasis. However, continuously raised Ang-II could lead to a loss of compensatory capacity and negatively affect the vascular system. In these situations, ACE inhibitors (ACEi) and higher doses of angiotensin receptor blockers (ARBs) are vital in stabilizing the condition and preventing cardiac failure [85,86].
Additionally, Ang(1–7) has direct anti-inflammatory effects on microglia [87] and contributes to reducing inflammation in adipose tissue [88], as demonstrated in arthritis models [89]. Ang(1–7) also elicits anti-thrombotic actions through the Mas-receptor-mediated release of nitric oxide (NO) from platelets [90]. Therefore, Ang(1–7) also effectively counters and neutralizes the detrimental effects of Ang-II [91]. Ang(1–7) also provides benefits to several major organs. Ang(1–7) attenuates myocyte hypertrophy and cardiac interstitial fibrosis in cardiac tissue [92,93], leading to the higher expression of the ACE-2 gene [85]. In individuals with type-2 diabetes mellitus, Ang(1–7) improves insulin sensitivity, reverses hyperglycemia, and reduces diabetic nephropathy [94].
In the kidney, Ang(1–7) facilitates vasodilation, enhancing renal blood flow and mitigating renal hypertension, preventing further production of the vasoconstrictive Ang-II [95]. Additionally, Ang(1–7) increases the glomerular filtration rate (GFR) and water and electrolyte molecular excretion in a dose-dependent manner [68,96]. Following closed traumatic brain injury, both Ang(1–7) and ACE-2 have improved cognitive and neurological functions [97]. Furthermore, they exhibit protective effects in cerebral ischemia [98] and hemorrhagic stroke in animal models [99,100,101,102].
Moreover, the modulation of the ACE-2/MAS pathway holds promise in preventing pulmonary injuries and represents a potential target for drug development to reduce viral entry [103]. ACE-2 exists in membrane-bound and soluble forms [59]. It is a double-edged sword, as SARS-CoV-2 utilizes membrane-bound ACE-2 to invade epithelial cells [67]. Meanwhile, soluble ACE-2 in extra-cellular fluid, particularly in circulation, binds to coronaviruses like SARS-CoV-2, facilitating their neutralization [104].
2. Regulation of the Immune System
Calcitriol, 1,25-dihydroxycholecalciferol, the most active vitamin D metabolite, is crucial for immune cell functions. It is a potent immune modulator. It has been estimated that two-thirds of immune cells’ physiological activation and functions rely on generating sufficient calcitriol within them [13,105]. Because circulatory concentrations are too low, the capacity of calcitriol to diffuse into immune cells is minimal [106]. In contrast, vitamin D and calcifediol 25(OH)D (in nmol) circulate approximately 900 times higher than the hormonal form of calcitriol (pmol). This allows them to diffuse into peripheral target cells and is utilized for the intracellular generation of calcitriol [5,13]. This intracellular calcitriol is essential for maintaining immune cell activities, including preventing autoimmunity and combating invading pathogens [5,107,108,109]. Once generated adequately within the immune cells, calcitriol activates the cytosol’s vitamin D (calcitriol) receptors (VDR/CTR) and provides autocrine and paracrine signaling as well as genomic modulations [5].
Calcitriol concentrations in the circulation are controlled by parathyroid hormone (PTH) and ionized calcium in the blood but not by tissue 24-hydroxylase. In contrast, in the target tissues, the production of calcitriol is mainly regulated by a combination of the serum 25(OH)D concentration (and D3) in a concentration-dependent manner (for diffusion). It is subjected to the feedback catabolic activity of tissue 24-hydroxylases and not by PTH. Except for membrane effects, vitamin D has minimal actions; therefore, the “physiological concentrations of vitamin D status” level refers to D3 and 25(OH)D concentrations in the circulation, of which only the latter is measured routinely to assess vitamin D status. Peripheral target cells, like immune cells, primarily depend on the diffusion of vitamin D and 25(OH)D from the circulation to generate higher concentrations of non-hormonal calcitriol, intracellularly [13,51].
2.1. Mechanisms of Adequate Vitamin D Supplementation in Infections
The crucial role of vitamin D adequacy in combating acute infections was confirmed a decade ago [47,110,111]. Serum 25(OH)D concentration thresholds needed for robust immune systems to overcome infections [112,113,114] and to reduce health risks were clarified in recent years in adults [13,115,116,117] and children [113,118,119,120]. Studies have affirmed the mechanisms of action of how ultraviolet-B (UVB) rays and vitamin D supplements help individuals with infections recover faster [121,122]. Subsequently, many studies have consolidated these findings [47,123,124,125].
The converging data indicate that the minimum effective serum 25(OH)D concentration to reduce infections and their severity is 40 ng/mL (100 nmol/L) [4,108,117,126], with the optimum being above 50 ng/mL [13,47,51,111,125,127,128]. Regarding SARS-CoV-2 infection, numerous studies reported that effective use of vitamin D3 and calcifediol significantly improves clinical outcomes from those with hypovitaminosis D, including reduced hospitalizations and deaths [129,130,131,132,133,134,135]. The primary mechanism is stimulating the immune system, supported by other mechanisms discussed previously, including subduing the RAS and increasing the expression of ACE-2 [66].
Meta-analyses encompassing a variety of heterogeneous studies concluded that vitamin D reduces the incidence of acute respiratory illnesses [45,114,136,137] and significantly reduces the severity and mortality from COVID-19 [45,46,125,130,138,139,140,141,142]. In total, ten clinical trials out of 321 peer-reviewed clinical studies that used vitamin D as the primary intervention to investigate the effects on clinical outcomes in COVID-19 (from May 2020 to June 2024) reported a significant reduction in hospitalizations, ICU admissions, or deaths [117,131,132,135,143,144,145,146,147,148,149,150,151,152,153,154,155,156] [early therapies for COVID-19 (https://c19early.org, accessed on 25 January 2024) and publications specifically related to vitamin D (https://c19early.org/d, accessed on 25 January 2024) [48].
2.2. Mechanisms Lowering the Severity of Infections
Vitamin D supplementation has been shown to reduce the severity and complications of COVID-19 [157]. Hundreds of peer-reviewed publications have confirmed that serum 25(OH)D concentrations below 12 ng/mL (indicating severe vitamin D deficiency) pose a significant risk for vulnerability to SARS-CoV-2 infection [158,159,160,161], its complications, and mortality [135,162,163,164]. Supplementation with cholecalciferol (vitamin D3) or calcifediol [25(OH)D] rapidly elevates serum 25(OH)D concentrations and decreases the risk of complications and deaths from SARS-CoV-2 infection [52,165,166,167,168,169,170,171].
Moreover, adequate vitamin D supplementation in COVID-19 patients with co-morbidities has been observed to reduce complications, length of hospital stays, and disease severity, leading to lower mortality rates [47,150,151,152,155,172,173,174,175,176]. Given the evidence, vitamin D should be considered a crucial component of the physician’s arsenal in the fight against COVID-19 [135].
2.3. Vitamin D Is Essential for Activating Immune Cells
Calcitriol is the most active vitamin D metabolite, crucial for combating invading pathogens and preventing autoimmunity and chronic diseases [29,107,108]. Through multiple mechanisms, calcitriol modulates the immune system [12]. When secreted from renal tubular cells into the bloodstream, calcitriol functions as a hormone [5]. Circulatory calcitriol alters the behavior of cells involved in calcium–phosphate–bone metabolism and intestinal, bone, and parathyroid cells.
The average circulatory concentration of calcitriol in the circulation is about 0.045 ng/mL, while the concentration of its free, diffusible form is far below the threshold needed to diffuse into immune cells and initiate intracellular signaling [4,29]. Moreover, vitamin D and calcifediol [25(OH)D] concentrations in the circulation are about 900-fold higher than circulating calcitriol (ng vs. pg/L in the blood); thus, only these two compounds serve as the substrate for intracellular calcitriol generation. Consequently, circulating calcitriol has no evident impact outside the muscular-skeletal, parathyroid, and fat cells.
However, the higher nmol-range concentrations of calcitriol generated intracellularly in response to TLR signaling provide (physiological) intracellular autocrine/intracrine signaling crucial for immune functions to overcome threats like infections [177]. Consequently, a holding mechanism increases serum levels, i.e., beyond a threat like detecting unfamiliar proteins or antigens in the circulation or local tissues [178,179]. The sporadic increases in the synthesis of calcitriol and VDR in response to TLR-4 signaling ensure the formation of sufficient calcitriol–VDR complexes to modulate transcriptions and intra-cellular autocrine signaling and genomic modulation, as and when needed [178].
The mechanisms mentioned above regulate inflammation and oxidative stresses through the abovementioned mechanisms, primarily by suppressing inflammatory cytokines and enhancing the synthesis of anti-inflammatory cytokines [177]. The immunomodulatory effects of vitamin D include the activation of immune cells such as T and B cells, macrophage and dendritic cells, and the enhanced production of several antimicrobial peptides and neutralizing antibodies [108,180].
3. Vitamin D Deficiency and Vulnerability to Infections
Vitamin D modulates the innate and adaptive immune systems [178,181]. It enhances innate immunity via complex mechanisms [182,183], including the expression of antimicrobial peptides like cathelicidin in various cells, including keratinocytes, epithelial cells, and monocytes [184]. Vitamin D also down-regulates inflammatory responses through multiple mechanisms, including dampening the RAS [183], switching Th1 cells to Th2 and Th17 to Treg cells [185,186], and increasing the expression of MAPK phosphatase-1 (MKP-1). Consequently, the latter inhibits p38 activation and reduces pro-inflammatory cytokine production in human monocytes/macrophages, as demonstrated when stimulated with lipopolysaccharides (LPS) [187].
3.1. Vitamin D Deficiency Increases Infection Vulnerability
Individuals with hypovitaminosis D are at a heightened risk of developing COVID-19 (and other infections) and experiencing unfavorable clinical outcomes [157,158,188,189]. In addition to vitamin D’s robust anti-inflammatory, anti-oxidant, and antimicrobial properties [148,176], intracellularly-generated calcitriol offers other beneficial effects. These include enhanced cell repair, reduced apoptosis [190], and the protection of epithelial and vascular endothelial cells [191]. In addition, it mitigates pregnancy-associated complications [179,180] and reduces all-cause mortality [192]. As a result, maintaining vitamin D sufficiency helps in rapid recovery and reduces complications and death from SARS-CoV-2 infection [157,193].
Studies reported that calcitriol inhibits the production of pro-inflammatory cytokines via T cells. Moreover, when combined with IL-2, 1,25(OH)2D3, it promotes the regulatory function of T cells, thereby contributing to immune regulation and homeostasis [194]. Numerous studies, including meta-analyses, have shown an inverse correlation between serum vitamin D levels and the severity of COVID-19 [125,129,157,158,189,195]. Therefore, it is unsurprising that vitamin D deficiency aggravates complications from COVID-19 [157,158,188,189].
Hypovitaminosis D and SARS-CoV-2 infections weaken epithelial cell gap junctions, potentially facilitating the passage of substances, fluids, and microbes across membranes and allowing viral dissemination [196]; when combined with other micronutrients like selenium and zinc, vitamin D provides a protective effect and enhances the physiological functions of tight cell gap junctions in epithelial cells [197]. This synergy improves the effectiveness of the tissue barrier, particularly in preventing the entry and propagation of microbes, particularly viruses [198,199].
3.2. Hypovitaminosis D Causes Immune Cell Dysfunction
Vitamin D deficiency leads to immune dysfunction, heightening susceptibility to infections and autoimmune disorders [4]. Low vitamin D reduces ACE-2 expression and increases viral loads, replication, and dissemination [200]. It also increases the potential of mutations (also after COVID-19 vaccines) [201,202,203] and could promote the naturally evolving gain of functions in viruses. For instance, dominant mutations enhance the affinity between the spike protein receptor-binding domain (RBD) and the ACE-2 receptor molecules [203], as seen in variants like Delta and Omicron, resulting in heightened infectivity (R0) [204,205], as illustrated in recent dominant mutants such as Omicron BA.2 [204].
Natural and vaccine-mediated neutralizing antibodies bind either to or near the ACE-2 binding region of the RBD [206]. Consequently, critical mutations in the viral RBD interfere with their recognition by neutralizing antibodies, leading to immune evasion. Moreover, Omicron variants BA.4 and BA.5 and other newly described dominant mutations notably occur in the L452R and F486V regions of the RBD, impairing the immune neutralization of viruses [197]. These mechanisms have been documented using sera from individuals who have received triple vaccination [205].
3.3. Vitamin D Insufficiency and Chronic Diseases
Epidemiological and case-control studies reported strong associations between vitamin D deficiency and several immune-related chronic diseases, such as type-1 diabetes [207,208], type-2 diabetes [209], connective tissue disorders [210], inflammatory bowel disorders [211], chronic hepatitis [212], asthma [213], respiratory infections [45], and cancer [214,215]. However, interventional studies have yielded weak data primarily due to poor study designs [5,216,217,218]. With a dysfunctional immune system and low ACE-2 expression, this is unsurprising.
Aging and chronic diseases [219,220] have a higher prevalence of hypovitaminosis D, and most of these conditions have reduced ACE-2 expression [29,221]. Since ACE-2 has protective functions, it is predictable that its lower expression in aging and co-morbidities increases SARS-CoV-2-associated complications and deaths [220]. Additionally, the lower expression of ACE-2 is reported in chronic conditions such as hypertension, obesity, and diabetes [197], which are also associated with an increased risk of complications from SARS-CoV-2 infection [157,158,188,189,222,223].
Therefore, the fundamental vulnerability in aging and co-morbidities may lie in hypovitaminosis and the low expression of ACE-2, illustrating the interlink between the mentioned chronic conditions and increased infection vulnerability. These data support that the synergistic adverse effects of hypovitaminosis D and reduced ACE-2 expression in patients with chronic diseases heighten risks for symptomatic COVID-19, complications, and deaths [220,221].
3.4. Co-Morbidities and Disease Vulnerability
Over the last 20 years, over 8000 clinical research articles have been published illustrating a robust inverse association between serum 25(OH)D concentrations and disease vulnerability, severity, and deaths from various diseases [224,225], especially from infections [4,226,227]. Despite the country’s location, gross national products, healthcare expenditure, or access to healthcare, older people with co-morbidities [18] and institutionalized people have shown to have the highest prevalence of severe vitamin D deficiency (e.g., serum 25(OH)D concentrations less than 12 ng/mL) [162,228,229].
Co-morbidities are strongly associated with low serum 25(OH)D and ACE-2 levels [230]. These groups include nursing home residents, developmental disability centers, group homes, incarcerated people, and routine night shift workers [29]. They have the highest rates of hypovitaminosis D and experience complications [231], ICU admissions, and deaths from SARS-CoV-2—this was observed vividly during the early part of the COVID-19 pandemic [125,232,233,234,235,236,237]. One common factor in the groups mentioned above is the low expression of ACE-2 [220,231]. Therefore, supplementing them with sufficient vitamin D daily (or once a week) dampens the RAS system (thus subduing inflammation) and enhances the ACE-2 expression, reducing disease severity and co-morbidity [220,231].
Such an approach has significantly reduced symptomatic SARS-CoV-2 infections, complications, and deaths [13,234,236,238,239]. However, this expectation failed to materialize during the pandemic because health agencies neglected this important natural defense mechanism [240]. This failure to boost the immune systems with simple remedies like vitamin D, the denial of natural immunity, disallowing the use of early repurposed therapies, and reliance on vaccination alone to control the pandemic [241,242] have increased hospitalizations and deaths from COVID-19 [193,231,243].
4. Effects of SARS-CoV-2 on the Immune System
Having hypovitaminosis D pre-pandemic or pre-infection increases the risks and vulnerability of SARS-CoV-2 [130]. Moreover, vitamin D deficiency at the time of diagnosis of SARS-CoV-2 infection significantly increased disease severity and mortality [13,43,55,56]. In contrast, vitamin D sufficiency is protective against severe COVID-19 disease and death [13,46,57,58]. These data support the satisfaction of Bradford Hill’s criteria for establishing that hypovitaminosis D increases the risks of complications from SARS-CoV-2 [18,59,244,245]. Evidence also supports that vitamin D deficiency is a cause of vulnerability, severity, and mortality from the SARS-CoV-2 virus [13,43,46,47,48,49,50].
The RBD of the SARS-CoV-2 spike protein interacts with membrane-bound ACE-2 receptors [246,247,248]. Subsequently, the serine protease TMPRSS2 cleaves the spike protein into two functional subunits: S1 and S2. Following this cleavage, the S2 subunit undergoes a conformational change, enabling fusion with the host cell membrane and facilitating endocytotic internalization [103,249,250]. The Two-Pore Segment Channel 2 (TPC2) molecules in the endo-lysosomal system also facilitate the entry of SARS-CoV-2 into human cells [249]. This process is activated by ionized calcium (Ca2+), subsequently activating nicotinic acid adenine-dinucleotide phosphate (NAADP) intracellular messengers [251].
4.1. Physiology and Pathological Pathways of the RAS Axis
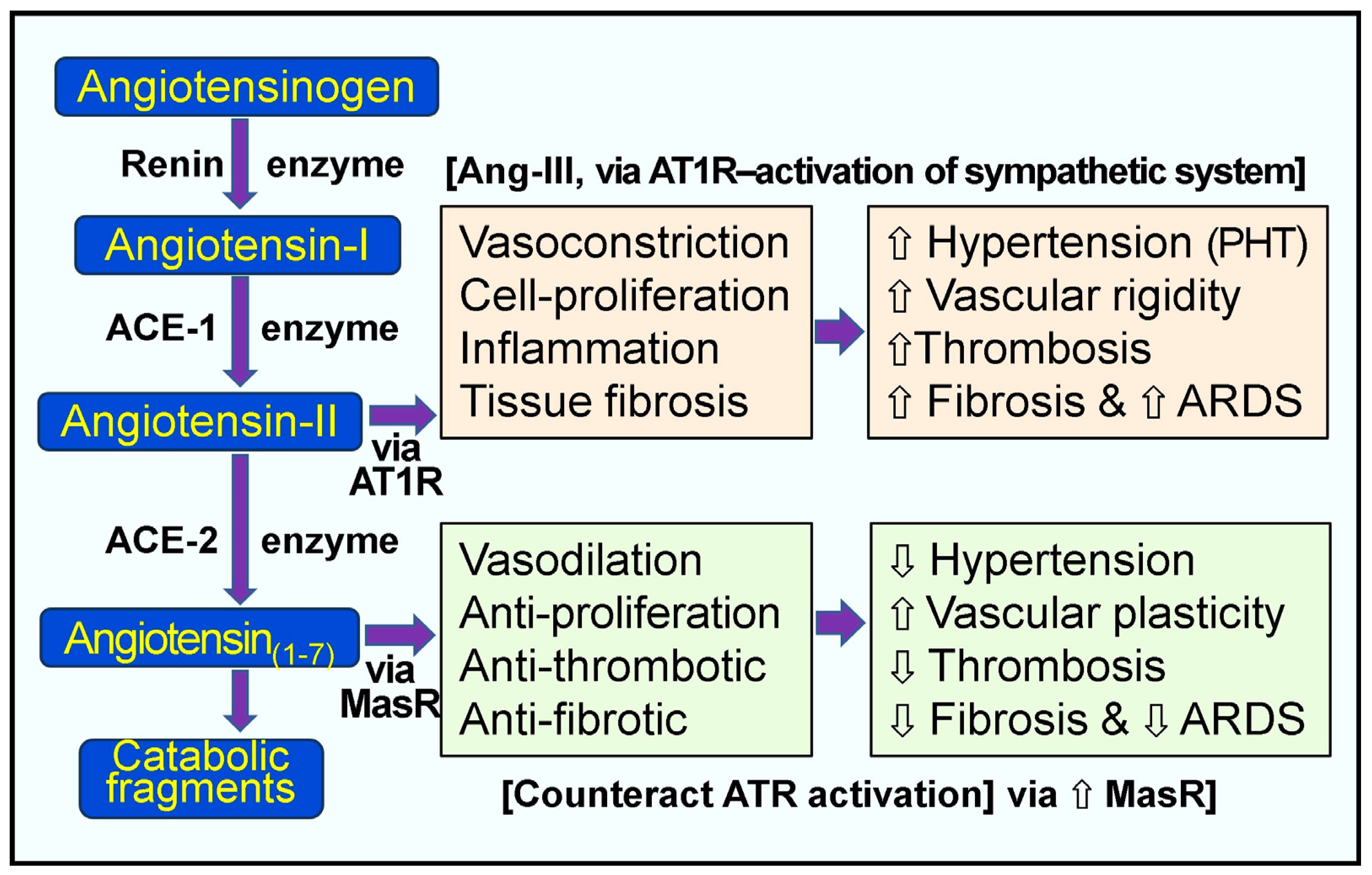
In the RAAS system, the enzyme renin (the rate-limiting step of RAS) activates angiotensinogen into angiotensin-I, and ACE-1 generates Ang-II (Figure 2). Renin catalyzes the formation of pro-peptide Ang-I and promotes the expression of ACE-1. Increased Ang-1 (relatively inactive molecules) leads to the increased synthesis of Ang-II via ACE-1—a potent vasoconstrictor peptide (Figure 2). Ang-II directly elevates peripheral vascular resistance and hypertension (especially pulmonary). Moreover, it also activates pro-coagulatory pathways, inflammatory cytokines, and interstitial fibrosis via the Ang-II receptor type 1 (AT1) receptors [93,100]. Figure 2 illustrates the normal, physiological, and alternative pathological pathways of the RAS.
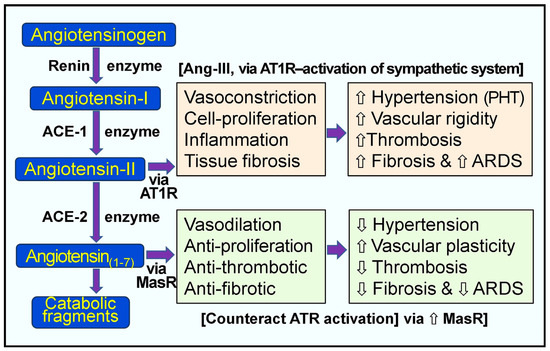
Figure 2.
Pathological and physiological responses of the renin-angiotensin system. Peach and green boxes illustrate the renin-angiotensin system’s regulatory and counter-regulatory physiologic pathways. When excess angiotensin-II (Ang-II) is synthesized, as in the case of hypovitaminosis D and SARS-CoV-2 infection, this leads to the over-activation of the AT1 receptors (AT1-R) with pathological manifestations, as indicated in the peach colored boxes [⇧ = increased; ⇩ = reduced; ARDS = acute respiratory distress syndrome; RAS, renin-angiotensin system; ACE, angiotensin-converting enzyme; ACE-2, angiotensin-converting enzyme 2; Ang 1–7, angiotensin 1–7; Ang-I, angiotensin-I; Ang-II, angiotensin-II; AT1R, type 1 angiotensin-II receptor; MasR, MAS proto-oncogene receptor. PHT, pulmonary hypertension].
The risk of the dysregulation of the RAS is high in hypovitaminosis D, which has less control over the enzyme renin. Severe coronaviral infections can lead to a cytokine storm, causing lung injury with ARDS and initiating coagulatory abnormalities [77]. SARS-CoV-2 primarily affects lung tissues, pneumocytes, alveolar interstitium, and capillaries. The alveolar epithelial cells, the port of entry of SARS-CoV-2, have high concentrations of ACE-2 receptors on their membranes [252]. ACE-2 expression is down-regulated with the viral infection, and the ACE-2/Ang(1–7)/Mas receptor (MasR) axis is suppressed [77], which augments the classic RAS, leading to diffuse inflammations and associated adverse effects. This cascade of events can cause severe inflammation, oxidative stress, and lung damage, leading to fluid extravasation into soft tissues, causing pulmonary edema and fibrosis [93,252].
4.2. Renin-Angiotensin System Related to SARS-CoV-2
As mentioned above, calcitriol is a crucial regulator of the RAS axis, mainly by suppressing renin gene expression, a rate-limiting step of synthesis of Ang-II via cyclic AMP (cAMP)-dependent PKA signaling [74]. Calcitriol independently increases the expression of ACE-2 [253], reducing Ang-II and increasing the generation of Ang(1–7), suppressing the formation of the CRE-CREB-CBP complex [70] and keeping RAS activity under control [197]. Meanwhile, certain viral infections like SARS-CoV-2 stimulate RAS activity, aggravating the situation in those with vitamin D deficiency and making them more vulnerable to developing cytokine storms [19,70].
SARS-CoV-2 infection in individuals with severe vitamin D deficiency markedly increases the vulnerability to severe complications, including ARDS and death [254,255,256]. Low ACE-2 concentrations in such persons [256] significantly increase the susceptibility to contract viruses, especially coronaviruses, and develop complications [117,146]. Once infected, coronaviruses indirectly increase the renin and, reduce ACE-2 and soluble ACE-2 concentrations. The latter is partly caused by the consumption (i.e., internalization and destruction) of membrane-bound ACE-2 receptors [59]. Low ACE-2, in conjunction with the over-activation of the RAS [55], leads to the excess and uncontrolled production of Ang-II, leading to severe adverse effects, increasing complications, the risk for cytokine storms, and death [19,197].
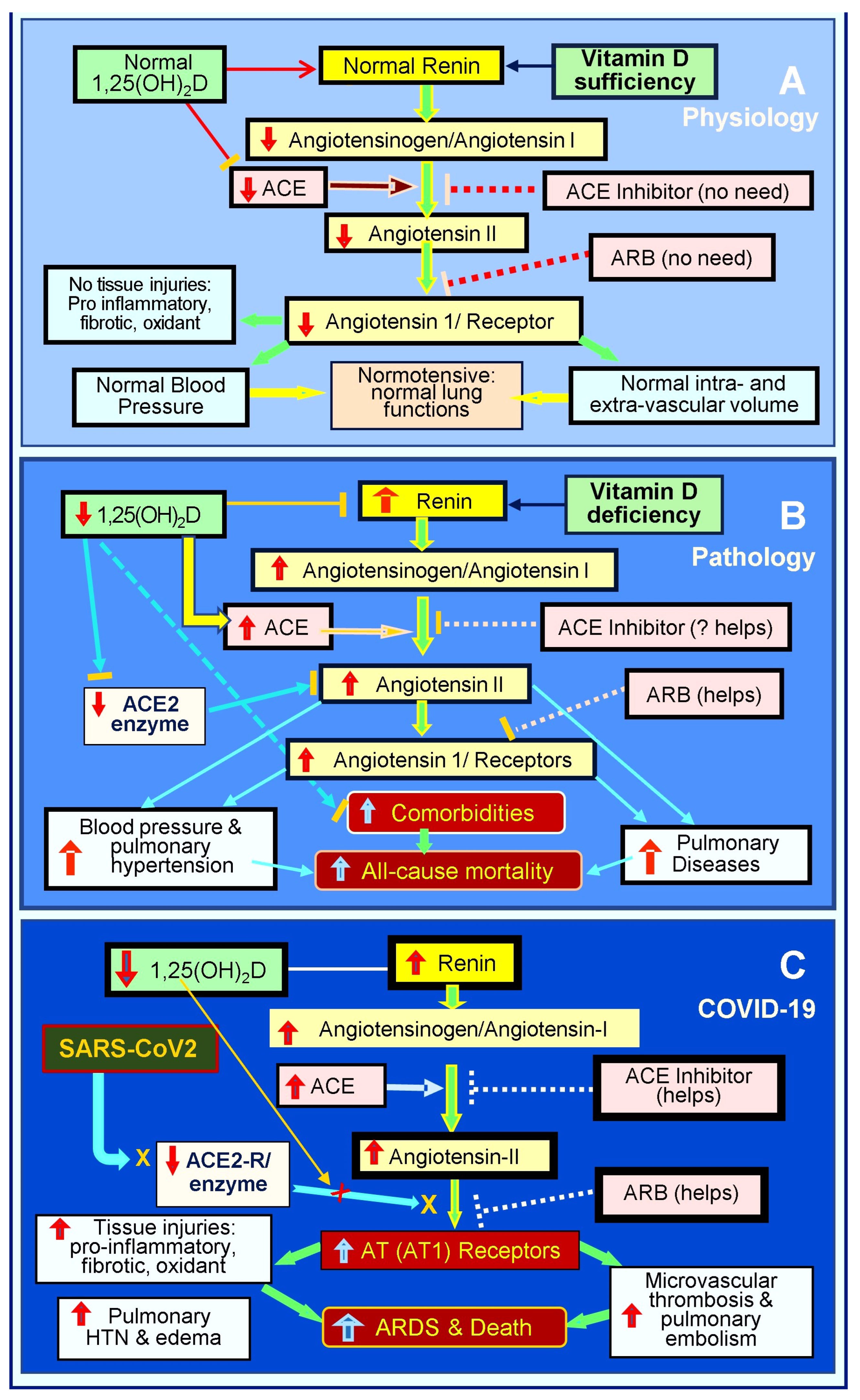
Also, widespread inflammation and oxidative stress injure the pulmonary epithelial and vascular endothelial cells and their basement membranes [257], impairing tight junction, with virus dissemination and fluid leakage into soft tissues [257], including the lungs, pericardium, intestine, and brain [29,196,258]. Pulmonary epithelial damage causes hypoxia and increases the risk of pneumonia and ARDS [69,70]. The endothelial abnormalities lead to micro-thrombosis, embolization, and intravascular thrombosis [259,260]. Figure 3 illustrates the RAS axis in a normal physiological state, in an activated state with hypovitaminosis D, and in the presence of SARS-CoV-2 infection [19].
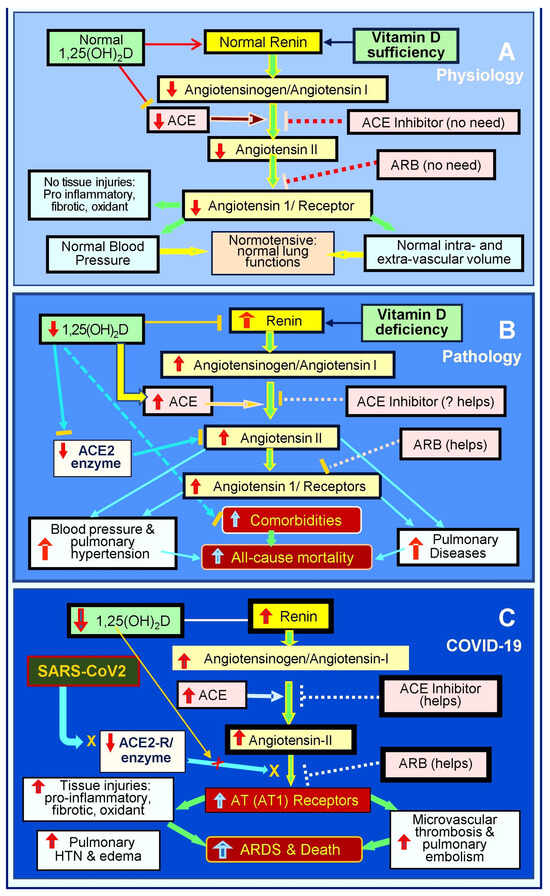
Figure 3.
This diagram outlines the status of the renin-angiotensin axis (RAS) axis: (A) physiological status, (B) pathological/activated status in the presence of vitamin D deficiency, and (C) following SARS-CoV-2 infection. RAS axis homeostasis is disrupted by hypovitaminosis D. SARS-CoV-2 or other coronal viral infections markedly activate the RAS, leading to pathologically elevated levels of angiotensin -II and the suppression of ACE-2. This hyperactivation of the RAS leads to increased complications and mortality (⇧ = increased; ⇩ = reduced; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers; AT1R: type 1 angiotensin-II receptor; ARDS: acute respiratory distress syndrome).
4.3. Regulation of Inflammation by Vitamin D via the RAS
Vitamin D is a potent negative endocrine regulator in the RAS through a canonical pathway [197]. It achieves this by inhibiting the cAMP response element-binding protein (CREB), a key transcription factor for renin gene regulation [74]. Vitamin D-driven suppression of renin [261] reduces RAS activity, such as lowering the molecular expression of ACE-1 and Ang-II while increasing the expression of ACE-2 [74,253]. These effects have been observed both in vitro and in vivo [253]. Notably, low serum levels of 25(OH)D concentrations are inversely correlated with higher RAS activity, elevated plasma renin activity, and Ang-II levels, contributing to increased blood pressure [262,263,264].
In studies using LPS-exposed rat lung tissue, calcitriol increased ACE-2 expression while reducing renin, angiotensin II, ACE, and AT1 receptor expression. Additionally, calcitriol exhibited a dose-dependent reduction in the permeability of blood vessels in rat lungs, mitigating damage induced by LPS [253]. Activating the ACE-2/Ang(1–7)/MAS axis by vitamin D further enhances the production of ACE-2 and Ang(1–7) concentrations. Ang(1–7)’s vasodilatory, anti-inflammatory, and anti-thrombotic effects help counteract hypertension, inflammation, and coagulatory abnormalities [265,266,267] (Figure 2), mitigating the adverse effects caused by SARS-CoV-2 and its spike protein.
As previously mentioned, vitamin D also blocks viral entry, strengthens immunity by tightening epithelial gap junctions, and inhibits SARS-CoV-2 transcription enzymes [60], thereby combating the viral infection. Vitamin D deficiency over-activates the RAS with the inefficient counter-regulatory activation of ACE-2/angiotensin(1–7)/Mas axis [100,268], increasing the risks for ARDS [197]. Consequently, vitamin D deficiency is a critical component that exacerbates COVID-19 via the over-activation of RAS with an excess generation of Ang-II [197].
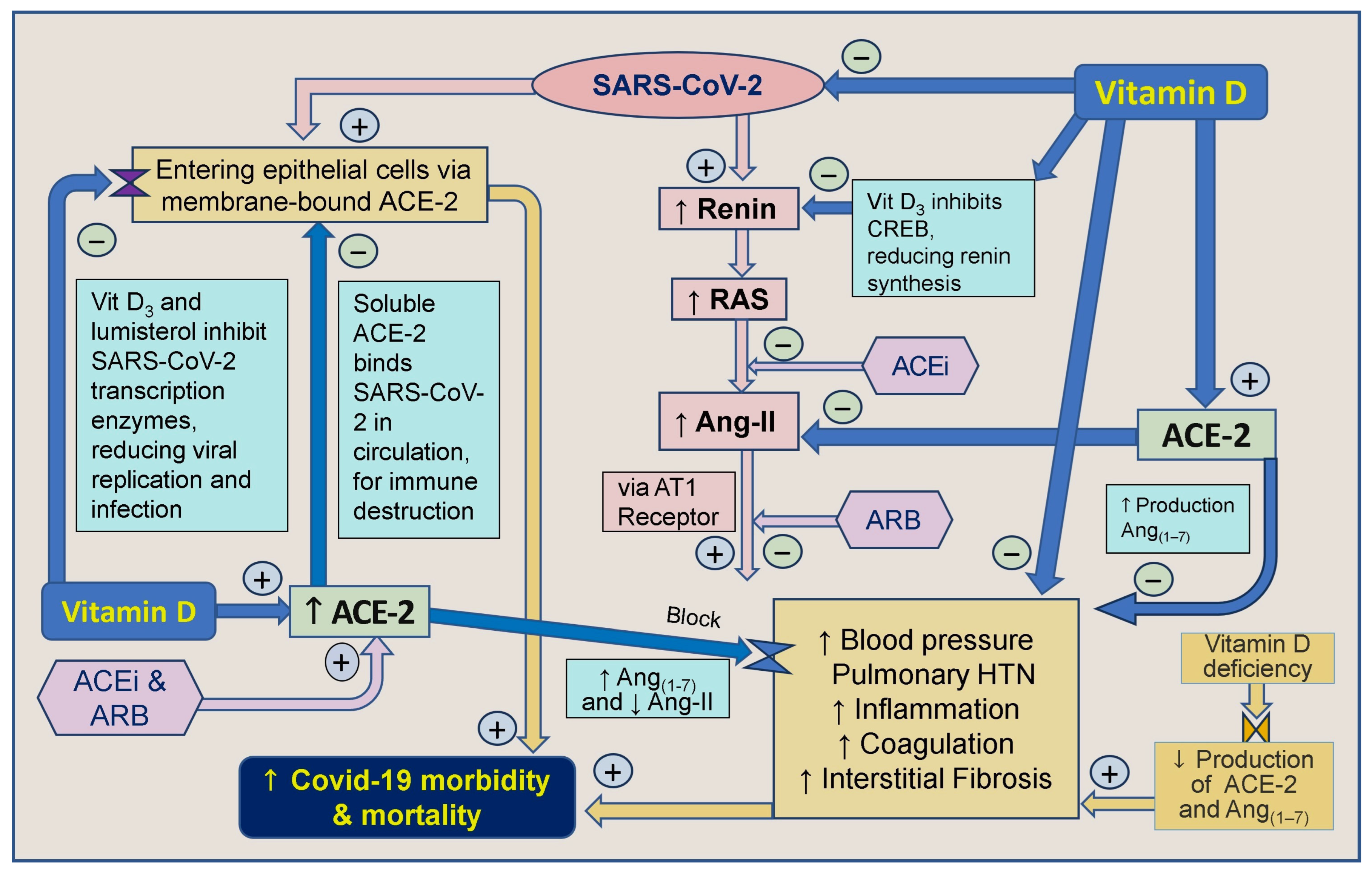
Vitamin D and inhibitors of ACE-1 and Ang receptor blockers work together indirectly to decrease renin synthesis, thereby reducing the activity of the RAS and the synthesis of Ang-I and Ang-II [66]. Angiotensin receptor blockers (ARBs) also inhibit the generation of Ang-II and decrease the stimulation of the angiotensin-1 (AT1) receptor. It has also been proposed that CD147/Basigin receptors bind to epitopes on the spike protein-S of the SARS-CoV virus, facilitating viral entry through endocytosis [269]. Therefore, blocking CD147/Basigin could be a target for drug development against COVID-19 [270]. Moreover, vitamin D indirectly regulates SARS-CoV-2 by modulating ACE-2 and CD147 receptor molecules [271,272]. The involvement of vitamin D and ACE-2 in modulating the SARS-CoV-2 virus [197] and its effects on the RAS are illustrated in Figure 4.
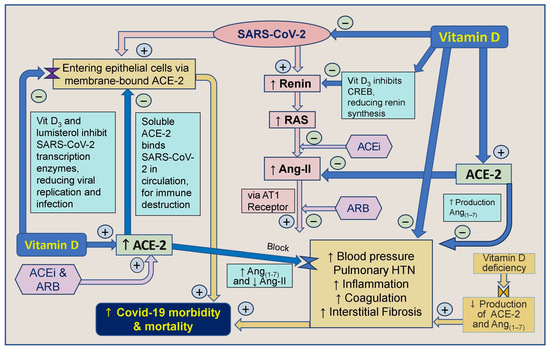
Figure 4.
Vit D strengthens innate and adaptive immune systems. This summary outlines the correlation between vitamin D, angiotensin-converting enzyme-2 (ACE-2), angiotensin-converting enzyme inhibitors (ACEi), and angiotensin II receptor blockers (ARBs) concerning severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and their impact on COVID-19 morbidity and mortality ([↑ = increased; ↓ = reduced; RAS: renin-angiotensin-system; CVS: cardiovascular system; ACE: angiotensin-converting enzyme; ARB: angiotensin receptor blockers; AT1R: type 1 angiotensin-II receptor; ARDS: acute respiratory distress syndrome; HTN: hypertension).
Figure 3 and Figure 4 illustrate multiple mechanisms of SARS-CoV-2 that dysregulated the RAS, reducing ACE-2 and increasing Ang-II levels. Vitamin D, ARBs, and ACEi can intervene positively in these cycles initiated by the virus, particularly in individuals with severe vitamin D deficiency. Vitamin D plays a crucial role by modulating the RAS, inhibiting Ang-II synthesis, and up-regulating ACE-2 expression, which mitigates the harmful effects of SARS-CoV-2 [19].
Ang-II type 1 receptor blockers (ARBs) and ACEi medications influence RAS, potentially mitigating the dysregulation caused by SARS-CoV-2 [19]. Together, these interventions aim to dampen the vicious cycles initiated by the virus, potentially reducing the morbidity and mortality associated with COVID-19, especially in individuals with severe vitamin D deficiency. ACEi and ARBs are indicated clinically in hypertension and other cardiovascular diseases [197]. These agents have been shown to mitigate acute lung injury by restoring the balance between two regulatory processes. Preclinical and clinical studies support the evidence of RAS disequilibrium in COVID-19 and the beneficial role of RAS modulation [252].
5. ACE-2 Receptor, Vitamin D, and SARS-CoV-2
Following the internalization of the ACE-2 receptor complex, the SARS-CoV-2 virus replicates using the host’s cell machinery [273]. The increased infectivity (R0) of SARS-CoV-2 and its mutants is attributed partly to the heightened affinity of their spike proteins to cell surface ACE-2 receptors [204,274]. Therefore, ACE-2 receptors are potential targets for drug development to prevent SARS-CoV-2 from entering human cells [249,251,274]. Respiratory tract epithelial cells co-expressing ACE-2 and TMPRSS2 molecules are the primary targets for SARS-CoV-2 entry [275]. The blockage of TMPRSS2 could serve as a molecular target for new drug development to prevent cellular access by COVID-19 [276].
5.1. Reduction in Viral Load through Soluble ACE-2
In the circulation, soluble ACE-2 molecules bind with SARS-CoV-2, facilitating the transport of these complexes to natural killer (NK) cells and macrophages for subsequent destruction [59]. This neutralization of viral particles prevents them from reaching membrane-bound ACE-2 receptors in lung and vascular epithelial cells. Since viruses cannot replicate outside of host cells, the binding of SARS-CoV-2 to soluble ACE-2 in the extra-cellular fluid could inhibit the replication of SARS-CoV-2 [37]. Contrary to earlier publications, the in vivo up-regulation of ACE-2 does not exacerbate but mitigates the effects of SARS-CoV-2 [277,278].
Studies have demonstrated that soluble ACE-2 can reduce viral load in vitro and protect against infection in preclinical models. For instance, Monteil et al. (2020) showed that human recombinant soluble ACE-2 (hrsACE-2) significantly blocked the initial stages of SARS-CoV-2 infections in engineered human blood vessels and kidney organoids, highlighting its potential as a therapeutic agent [104]. In contrast, some research indicated pharmacological strategies to reduce ACE-2 expression, such as using ursodeoxycholic acid (UDCA) to reduce the infection rate of SARS-CoV-2 by targeting the farnesoid X receptor (FXR) [279]. Further research is ongoing to evaluate the efficacy and safety of soluble ACE-2 in clinical settings, aiming to offer a novel approach to mitigate the impact of COVID-19 [59].
Nevertheless, it is notable that the levels of soluble ACE-2 required to inhibit SARS-CoV-2 infection may be above physiological levels. Soluble ACE-2 could enhance SARS-CoV-2 [19] infection at physiological levels by forming a complex with the virus that enters cells via endocytosis through the AT-1 surface receptor [280]. Overall evidence suggests that while increased ACE-1 activity may be harmful, the increased expression of ACE-2 is beneficial in controlling coronaviruses [281,282]. Therefore, discontinuing ACE inhibitors (ACEi) and angiotensin receptor blockers (ARBs) solely due to COVID-19 infection is not recommended [19].
5.2. Reduction Consequences of the Lower Expression of ACE-2
SARS-CoV-2 also reduces ACE-2 expression, further hampering protective physiological functions of the ACE-2/angiotensin(1–7)/Mas axis [100,268] and increasing the risk for pulmonary and cardiac complications [278]. Despite using the ACE-2 receptor by SARS-CoV-2 S-protein to enter human cells, up-regulation does not increase infection vulnerability [19,278]. Meanwhile, hypovitaminosis D not only reduces ACE-2 synthesis but also exacerbates the over-production of Ang-II [19,283]. These increase the risks for abnormal coagulation and interstitial fibrosis [93]. The increased Ang-II creates pathologic vasoconstriction, causing pulmonary hypertension (Figure 4), and liberates excessive amounts of harmful cytokines, causing systemic inflammation that could lead to cytokine storms [18].
Young children have been observed to have lower expression levels of ACE-2 in their nasal respiratory tract epithelia than adults [197]. Except for those who are immuno-suppressed or have hypovitaminosis D, children have stronger innate immunity. Consequently, they are less susceptible to severe COVID-19 complications [284]. The binding process of SARS-CoV-2 also involves cell-membrane-bound heparan sulfate proteoglycans (HSPG). Lactoferrin, a nutrient found in mammalian milk, interferes with SARS-CoV-2 binding to HSPG and membrane-bound ACE-2 receptors, potentially reducing coronaviruses’ entry into human host cells [285].
SARS-CoV-2 infection significantly reduces ACE-2 concentrations [197] and disrupts the balance of ACE-2/ACE-1 ratio. It, initiating a pathological cycle, worsens in those with hypovitaminosis D. Besides, SARS-CoV-2 up-regulates metallopeptidase ADAM17 molecules, enhancing the RAS activity and increasing the production of the inflammatogenic Ang-II [286,287,288,289,290].
Unlike epithelial and immune cells, erythrocytes and platelets lack membrane-bound ACE-2 receptors [291]. However, the interaction of the spike protein with CD147 receptors on platelets and erythrocyte membranes can lead to platelet aggregation and erythrocyte abnormalities [271,292]. SARS-CoV-2-induced damage to gap junctions and epithelial barriers results in the loss of epithelial cell integrity, which is aggravated in the presence of hypovitaminosis D. This damage also affects endothelin function, impairing gas exchange in pulmonary epithelia [100,248,276,277,278,284,285,293].
5.3. Restriction of Generic Medication Use and Conflicts of Interest
Despite the availability of over 200 independent RCTs and extensive data on vitamin D related to preventing and treating COVID-19 [200,294], regulatory agencies withheld approvals for the use of vitamin D [240,295]. Preventing access and use of widely available, cost-effective, generic agents, such as vitamin D and ivermectin [296,297], may have proven detrimental to patient welfare [193,242,295], increasing hospitalizations and deaths [48,132,240].
The actions mentioned above may have been driven by the desire to maintain Emergency Use Authorization (EUA) for vaccines and anti-viral agents [298]. However, this created a scenario for the approval and use of COVID-19 vaccines and anti-viral agents under (EUA), but not widely available, cost-effective generic agents like vitamin D and ivermectin [48,193,242,298]. The lack of approvals for repurposed early therapies may have harmed people [48,132,240,294,296,297,299].
5.4. ACE-2—A Double-Edged Sword in SARS-CoV-2 Infection
Whether ACEi and ARBs play a harmful or helpful role in COVID-19 remains controversial [300,301]. Theoretically, it has been suggested that increased ACE-2 membrane receptors on epithelial cells could potentially increase the cellular entry of SARS-CoV-2 [274,302]. Consequently, it has been hypothesized that the increased ACE-2 receptors resulting from ARBs, ACEi, or vitamin D up-regulation might enhance SARS-CoV-2 entry via epithelial cells [278], thereby increasing cellular infection [248]. However, subsequent data did not support the view that ACEi and ARBs increase the risk of SARS-CoV-2 infection or worsen COVID-19 outcomes [19,277,281].
Soluble ACE-2 can effectively mimic the membrane-bound ACE-2, to which the SARS-CoV-2 spike protein binds, sequestering the virus and inhibiting its ability to infect host cells. The excess synthesis of ACE-2 soluble receptor spills into the bloodstream, potentially decoying and removing the SARS-CoV-2 virus and reducing viral load. Because of the mechanisms of action, soluble ACE-2 has emerged as a potential therapeutic strategy to neutralize SARS-CoV-2 (and other coronaviruses) in the bloodstream by acting as a decoy that binds the virus and prevents it from entering human cells [19]. These findings collectively highlight the potential of soluble ACE-2 and other ACE-2-targeted therapies in mitigating the impact of COVID-19 [197].
5.5. Importance of Strengthening the Immune System to Overcome Infections
The body’s initial defense against invading pathogens is the innate immune system that relies on adequate vitamin D levels to function effectively [13,51]. Ensuring vitamin D sufficiency in the population is a quick and cost-effective approach to promoting a robust immune system. Additionally, optimal immune function requires other micronutrients, such as magnesium, zinc, omega-3 fatty acids, vitamin K2, resveratrol, and quercetin [67], and comprehensive mental and physical health support. By addressing these nutritional and lifestyle factors, individuals can enhance their immune response and overall well-being.
The mentioned approach would ensure the maintenance of a robust innate immune system by preserving sufficient vitamin D, calcitriol receptors (CTR/VDR), and CYP27B1 expression through the production of calcitriol within immune cells [5,105]. The combined deficiencies of vitamins D and K2 could elevate the risk of cardiovascular events, adverse cardiac remodeling [303], and mortality and add to all-cause mortality compared to individuals with adequate levels of both vitamins. Therefore, maintaining sufficient levels of both vitamins D and K is crucial for overall health and reducing the risk of adverse outcomes [304].
Strengthening the immune system is crucial for protecting against SARS-CoV-2 infections and reducing the risk of high viral loads, which can lead to viral mutations with greater infectivity and immune evasion capabilities [193,242]. Numerous RCTs and meta-analyses have concluded that vitamin D supplementation protects against acute respiratory tract infections, particularly in individuals with profound hypovitaminosis D [305]. Maintaining adequate physiological levels of 25(OH)D supports a robust immune function that reduces the risk of respiratory infections, including those caused by SARS-CoV-2 [189].
6. Discussion
Numerous studies have explored the complex interactions between vitamin D and the immune system. However, it is essential to note that this article has focused on highlighting selected critically relevant studies. As previously mentioned, vitamin D is crucial in modulating and strengthening innate and adaptive immune systems. It achieves this by up-regulating or down-regulating the transcription of target genes [12] through the calcitriol receptor (CTR). Additionally, vitamin D prevents the weakening of epithelial cell barriers [190], induces the expression of antimicrobial peptides such as cathelicidin, and up-regulates MKP-1 to inhibit inflammatory cytokines [306]. Furthermore, in conjunction with interleukin-2, vitamin D promotes T cell regulation, further contributing to immune system modulation [194].
Strengthening the immune system protects against SARS-CoV-2 infections and greater viral loads, preventing the survival of viral mutations with greater infectivity and immune evasion capabilities. In addition to these general immune benefits, vitamin D and lumisterol inhibit SARS-CoV-2 transcription enzymes, reducing viral replication and infection [307]. Studies, including meta-analyses, have concluded that vitamin D supplementation protects from acute respiratory tract infection, especially in those with profound hypovitaminosis D [307].
Vitamin D counteracts the vasoconstriction, pulmonary hypertension, coagulation, and interstitial fibrosis caused by SARS-CoV-2 by inhibiting the transcription factor CREB to down-regulate renin [93], the rate-controlling step of the RAS system, resulting in reduced Ang-II production [66]. Secondly, vitamin D up-regulates ACE-2 [253], which counteracts Ang II. Converting Ang II into Ang(1–7) by ACE-2 makes less Ang-II available. This reduces Ang-II levels and causes the production of Ang(1–7), which, via its MAS receptor, exhibits vasodilatory, anti-inflammatory, and anti-thrombotic effects to prevent pulmonary injuries.
Vitamin D enhances the expression of ACE-2, but some have raised concerns that this could exacerbate the disease caused by SARS-CoV-2, as the virus exploits membrane-bound ACE-2 to invade epithelial cells [274,308,309,310]. However, as described above, this potential risk is mitigated by the excess synthesis of ACE-2 molecules, which spill over into the bloodstream as ACE-2-soluble decoy receptors. These soluble ACE-2 receptors bind to SARS-CoV-2 viruses and escort them to natural killer cells and macrophages for destruction [256]. This process has been confirmed in in vitro systems [104]. If this also occurs in vivo, this process will help reduce infection and viral load, minimizing complications and deaths from SARS-CoV-2 [19,286,311,312].
Therefore, rather than the associated risk of facilitating an increased entry of SARS-CoV-2 viruses, the up-regulation of ACE-2 by vitamin D sufficiency may reduce viral load, thus mitigating the effects of SARS-CoV-2 and decreasing the risks of complications from SARS-CoV-2 [259,313,314]. However, further investigation is required to gain an in-depth understanding and confirm if soluble ACE-2 is inhibitory at physiological levels [280]. These anti-viral processes are enhanced by having sufficient cofactors like magnesium, iodine, and selenium, which help maintain tight cell junctions in epithelial cells [198]. Vitamin D-mediated tight gap-junctions help to preserve cellular function and prevent excessive fluid diffusion and viral spread across cell membranes [59].
ACE-inhibiting enzymes and ARBs can prove beneficial by reducing Ang-II production and AT1 receptor activation and up-regulating soluble ACE-2 [277,281]. However, we note limited studies on the direct relationship between vitamin D and SARS-CoV-2 replication. In conclusion, overall data support that vitamin D sufficiency, ACE inhibitors, and ARBs reduce the risk of COVID-19, associated complications, and deaths [19,259,286,313].
7. Conclusions
The authors recommend ensuring the vitamin D sufficiency of patients with supplementation to rapidly raise and maintain serum 25(OH)D concentrations above 50 ng/mL for a robust immune system to protect against coronavirus [51], especially COVID-19, and other viral diseases (e.g., dengue) to mitigate morbidity/complications and mortality. Vitamin D is required for effective innate and adaptive immune system function and counteracts the pathological effects of over-stimulated RAS by SARS-CoV-2. Its beneficial actions include lowering renin and up-regulating ACE-2, which lowers Ang II and increases vasodilator Ang(1–7). The latter also has anti-inflammatory and anti-coagulant properties to mitigate the harmful effects of SARS-CoV-2, especially in the lungs and vascular system—vitamin D’s upregulation of soluble ACE-2 assists in the elimination of SARS-CoV-2, thus reducing viremia. ACEi and ARBs appear to contribute to partly relieving the adverse effects of SARS-CoV-2.
Funding
This research received no grant or aid from funding agencies or commercial or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The author has no conflicts of interest.
Glossary/Abbreviations
| 1,25(OH)2D | 1,25-dihydroxyvitamin D |
| 25(OH)D | 25-hydroxy vitamin D |
| ACE-1 | Angiotensin-converting enzyme-1 |
| ACE-2 | Angiotensin-converting enzyme-2; |
| Ang(1–7) | Angiotensin(1–7) |
| Ang-I | Angiotensin-I |
| Ang-II | Angiotensin-II |
| AT1R | Type 1 angiotensin-II receptor |
| BMI | Body mass index |
| CVD | Cardiovascular disease |
| IU | International Units |
| MA | Meta-analysis |
| MasR | MAS proto-oncogene receptor |
| NIH | National Institutes of Health |
| PTH | Parathyroid hormone |
| RCTs | Randomized controlled clinical trials |
| RAS | Renin-angiotensin system |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus-2 |
| SR | Systematic reviews |
| T2D | Type 2 diabetes mellitus |
| TMPRSS2 | Transmembrane protease serine-2 |
| UVB | Ultraviolet-B |
| VDBP | Vitamin D binding protein |
| VDR | Vitamin D (calcitriol) receptor |
References
- Santaolalla, A.; Beckmann, K.; Kibaru, J.; Josephs, D.; Van Hemelrijck, M.; Irshad, S. Association between vitamin D and novel SARS-CoV-2 respiratory dysfunction—A scoping review of current evidence and Its implication for COVID-19 pandemic. Front. Physiol. 2020, 11, 564387. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Global epidemic of coronavirus—COVID-19: What can we do to minimize risks? Eur. J. Biomed. Pharma Sci. 2020, 7, 432–438. [Google Scholar]
- Vieth, R. Why “Vitamin D” is not a hormone, and not a synonym for 1,25-dihydroxy-vitamin D, its analogs or deltanoids. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 571–573. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Infections and autoimmunity-The immune system and vitamin D: A Systematic Review. Nutrients 2023, 15, 3842. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Physiological basis for using vitamin D to improve health. Biomedicines 2023, 11, 1542. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Zhang, M.; Dong, X.; Long, J.; Li, Y.; Tan, P.; He, T.; Giovannucci, E.L.; Zhang, X.; Zhou, Z.; et al. Vitamin D receptor gene polymorphisms, bioavailable 25-hydroxyvitamin D, and hepatocellular carcinoma survival. J. Natl. Cancer Inst. 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Ciocarlie, T.; Motofelea, A.C.; Motofelea, N.; Dutu, A.G.; Craciun, A.; Costachescu, D.; Roi, C.I.; Silaghi, C.N.; Crintea, A. Exploring the role of vitamin D, vitamin D-dependent proteins, and vitamin D receptor gene variation in lung cancer risk. Int. J. Mol. Sci. 2024, 25, 6664. [Google Scholar] [CrossRef]
- Voltan, G.; Cannito, M.; Ferrarese, M.; Ceccato, F.; Camozzi, V. Vitamin D: An overview of gene regulation, ranging from metabolism to genomic effects. Genes 2023, 14, 1691. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, D.M. The role of Toll-Like receptors and vitamin D in cardiovascular diseases-A review. Int. J. Mol. Sci. 2017, 18, 2252. [Google Scholar] [CrossRef]
- Kim, D.; Kim, M.A.; Cho, I.H.; Kim, M.S.; Lee, S.; Jo, E.K.; Choi, S.Y.; Park, K.; Kim, J.S.; Akira, S.; et al. A critical role of toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J. Biol. Chem. 2007, 282, 14975–14983. [Google Scholar] [CrossRef]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, O.; Hanel, A.; Carlberg, C. Key vitamin D target genes with functions in the immune system. Nutrients 2020, 12, 1140. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S. Overcoming infections including COVID-19, by maintaining circulating 25(OH)D concentrations above 50 ng/mL. Pathol. Lab. Med. Int. 2022, 14, 37–60. [Google Scholar] [CrossRef]
- Veldman, C.M.; Cantorna, M.T.; DeLuca, H.F. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch. Biochem. Biophys. 2000, 374, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Sharma, O.P.; Gacad, M.A.; Singer, F.R. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J. Clin. Investig. 1983, 72, 1856–1860. [Google Scholar] [CrossRef]
- Stoffels, K.; Overbergh, L.; Giulietti, A.; Verlinden, L.; Bouillon, R.; Mathieu, C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J. Bone Miner. Res. 2006, 21, 37–47. [Google Scholar] [CrossRef]
- Trochoutsou, A.I.; Kloukina, V.; Samitas, K.; Xanthou, G. Vitamin-D in the immune system: Genomic and non-genomic actions. Mini Rev. Med. Chem. 2015, 15, 953–963. [Google Scholar] [CrossRef]
- Daneshkhah, A.; Agrawal, V.; Eshein, A.; Subramanian, H.; Roy, H.K.; Backman, V. Evidence for possible association of vitamin D status with cytokine storm and unregulated inflammation in COVID-19 patients. Aging Clin. Exp. Res. 2020, 32, 2141–2158. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. ACE inhibitors and angiotensin receptor blockers reduce the complications associated with COVID-19 infection. World J. Pharma Res. 2021, 10, 2579–2600. [Google Scholar] [CrossRef]
- Michigami, T. Rickets/osteomalacia. consensus on vitamin D deficiency and insufficiency in children. Clin. Calcium 2018, 28, 1307–1311. [Google Scholar]
- Gogulothu, R.; Nagar, D.; Gopalakrishnan, S.; Garlapati, V.R.; Kallamadi, P.R.; Ismail, A. Disrupted expression of genes essential for skeletal muscle fibre integrity and energy metabolism in vitamin D deficient rats. J. Steroid Biochem. Mol. Biol. 2020, 197, 105525. [Google Scholar] [CrossRef] [PubMed]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Perspective: Vitamin D supplementation prevents rickets and acute respiratory infections when given as daily maintenance but not as intermittent bolus: Implications for COVID-19. Clin. Med. 2021, 21, e144–e149. [Google Scholar] [CrossRef] [PubMed]
- Schlumpf, M.; Reichrath, J.; Lehmann, B.; Sigmundsdottir, H.; Feldmeyer, L.; Hofbauer, G.F.; Lichtensteiger, W. Fundamental questions to sun protection: A continuous education symposium on vitamin D, immune system and sun protection at the University of Zurich. Dermatoendocrinol 2010, 2, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Borges, M.C.; Martini, L.A.; Rogero, M.M. Current perspectives on vitamin D, immune system, and chronic diseases. Nutrition 2011, 27, 399–404. [Google Scholar] [CrossRef]
- Himani, K.R.; Haq, A.; Wimalawansa, S.J.; Sharma, A. Putative roles of vitamin D in modulating immune response and immunopathology associated with COVID-19. Virus Res. 2020, 292, 198235. [Google Scholar] [CrossRef]
- Dzik, K.P.; Kaczor, J.J. Mechanisms of vitamin D on skeletal muscle function: Oxidative stress, energy metabolism and anabolic state. Eur. J. Appl. Physiol. 2019, 119, 825–839. [Google Scholar] [CrossRef]
- Ogata, M.; Iwasaki, N.; Ide, R.; Takizawa, M.; Tanaka, M.; Tetsuo, T.; Sato, A.; Uchigata, Y. Role of vitamin D in energy and bone metabolism in postmenopausal women with type 2 diabetes mellitus: A 6-month follow-up evaluation. J. Diabetes Investig. 2018, 9, 211–222. [Google Scholar] [CrossRef]
- Gupta, R.; Sharma, U.; Gupta, N.; Kalaivani, M.; Singh, U.; Guleria, R.; Jagannathan, N.R.; Goswami, R. Effect of cholecalciferol and calcium supplementation on muscle strength and energy metabolism in vitamin D-deficient Asian Indians: A randomized, controlled trial. Clin. Endocrinol. 2010, 73, 445–451. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Controlling chronic diseases and acute infections with vitamin D sufficiency. Nutrients 2023, 15, 3623. [Google Scholar] [CrossRef]
- Pilz, S.; Tomaschitz, A.; Marz, W.; Drechsler, C.; Ritz, E.; Zittermann, A.; Cavalier, E.; Pieber, T.R.; Lappe, J.M.; Grant, W.B.; et al. Vitamin D, cardiovascular disease and mortality. Clin. Endocrinol. 2011, 75, 575–584. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D and cardiovascular diseases: Causality. J. Steroid Biochem. Mol. Biol. 2018, 175, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Vieth, R. How to optimize vitamin D supplementation to prevent cancer, based on cellular adaptation and hydroxylase enzymology. Anticancer. Res. 2009, 29, 3675–3684. [Google Scholar] [PubMed]
- Chen, X.; Zhou, M.; Yan, H.; Chen, J.; Wang, Y.; Mo, X. Association of serum total 25-hydroxy-vitamin D concentration and risk of all-cause, cardiovascular and malignancies-specific mortality in patients with hyperlipidemia in the United States. Front. Nutr. 2022, 9, 971720. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, R.H. Calcium plus vitamin D did not prevent hip fracture or colorectal cancer in postmenopausal women. ACP J. Club 2006, 145, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Riddell, L. Calcium plus vitamin D did not prevent fractures or colorectal cancer in postmenopausal women. Evid. Based Nurs. 2006, 9, 114. [Google Scholar] [CrossRef]
- Boucher, B.J. Vitamin D deficiency in British South Asians, a persistent but avoidable problem associated with many health risks (including rickets, T2DM, CVD, COVID-19 and pregnancy complications): The case for correcting this deficiency. Endocr. Connect. 2022, 11, e220234. [Google Scholar] [CrossRef]
- Hollis, B.W.; Wagner, C.L. Vitamin D supplementation during pregnancy: Improvements in birth outcomes and complications through direct genomic alteration. Mol. Cell. Endocrinol. 2017, 453, 113–130. [Google Scholar] [CrossRef]
- Al-Musharaf, S.; Fouda, M.A.; Turkestani, I.Z.; Al-Ajlan, A.; Sabico, S.; Alnaami, A.M.; Wani, K.; Hussain, S.D.; Alraqebah, B.; Al-Serehi, A.; et al. Vitamin D deficiency prevalence and predictors in early pregnancy among Arab women. Nutrients 2018, 10, 489. [Google Scholar] [CrossRef]
- Heyden, E.L.; Wimalawansa, S.J. Vitamin D: Effects on human reproduction, pregnancy, and fetal well-being. J. Steroid Biochem. Mol. Biol. 2018, 180, 41–50. [Google Scholar] [CrossRef]
- Almuqbil, M.; Almadani, M.E.; Albraiki, S.A.; Alamri, A.M.; Alshehri, A.; Alghamdi, A.; Alshehri, S.; Asdaq, S.M.B. Impact of vitamin D deficiency on mental health in university students: A cross-sectionalstudy. Healthcare 2023, 11, 2097. [Google Scholar] [CrossRef]
- Jamilian, H.; Amirani, E.; Milajerdi, A.; Kolahdooz, F.; Mirzaei, H.; Zaroudi, M.; Ghaderi, A.; Asemi, Z. The effects of vitamin D supplementation on mental health, and biomarkers of inflammation and oxidative stress in patients with psychiatric disorders: A systematic review and meta-analysis of randomized controlled trials. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2019, 94, 109651. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, S.; Mirzaei, K.; Hossein-Nezhad, A.; Shariati, G. Vitamin D receptor FokI genotype may modify the susceptibility to schizophrenia and bipolar mood disorder by regulation of dopamine D1 receptor gene expression. Minerva Med. 2012, 103, 383–391. [Google Scholar]
- Jahan-Mihan, A.; Stevens, P.; Medero-Alfonso, S.; Brace, G.; Overby, L.K.; Berg, K.; Labyak, C. The role of water-soluble citamins and vitamin D in prevention and treatment of depression and seasonal affective disorder in adults. Nutrients 2024, 16, 1902. [Google Scholar] [CrossRef]
- de Koning, E.J.; van Schoor, N.M.; Penninx, B.W.; Elders, P.J.; Heijboer, A.C.; Smit, J.H.; Bet, P.M.; van Tulder, M.W.; den Heijer, M.; van Marwijk, H.W.; et al. Vitamin D supplementation to prevent depression and poor physical function in older adults: Study protocol of the D-Vitaal study, a randomized placebo-controlled clinical trial. BMC Geriatr. 2015, 15, 151. [Google Scholar] [CrossRef]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- Jolliffe, D.A.; Griffiths, C.J.; Martineau, A.R. Vitamin D in the prevention of acute respiratory infection: Systematic review of clinical studies. J. Steroid Biochem. Mol. Biol. 2013, 136, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; Hutter, M.M.; Camargo, C.A., Jr. Association between preoperative 25-hydroxyvitamin D level and hospital-acquired infections following Roux-en-Y gastric bypass surgery. JAMA Surg. 2014, 149, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Group-C19.com. Vitamin D for COVID-19: Real-Time Analysis of all 300 Studies. Available online: https://c19vitamind.com/ (accessed on 25 March 2024).
- Kawahara, T.; Suzuki, G.; Mizuno, S.; Tominaga, N.; Toda, M.; Toyama, N.; Inazu, T.; Kawahara, C.; Okada, Y.; Tanaka, Y. Active vitamin D treatment in the prevention of sarcopenia in adults with prediabetes (DPVD ancillary study): A randomised controlled trial. Lancet Healthy Longev. 2024, 5, e255–e263. [Google Scholar] [CrossRef] [PubMed]
- Chandra, H.; Rahman, A.; Yadav, P.; Maurya, G.; Kumar Shukla, S. Effect of adjunct Vitamin D treatment in vitamin D deficient pulmonary tuberculosis patients: A randomized, double blind, active controlled clinical trial. Indian J. Tuberc. 2024, 71, 170–178. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Rapidly increasing serum 25(OH)D boosts the immune system, against infections-sepsis and COVID-19. Nutrients 2022, 14, 2997. [Google Scholar] [CrossRef]
- Karonova, T.L.; Chernikova, A.T.; Golovatyuk, K.A.; Bykova, E.S.; Grant, W.B.; Kalinina, O.V.; Grineva, E.N.; Shlyakhto, E.V. Vitamin D intake may reduce SARS-CoV-2 infection morbidity in health care workers. Nutrients 2022, 14, 505. [Google Scholar] [CrossRef] [PubMed]
- Wimalawansa, S.J. Extra-skeletal and endocrine functions and toxicity of vitamin D. J. Endocrinol. Diabetes 2016, 3, 1–5. [Google Scholar] [CrossRef]
- Smaha, J.; Jackuliak, P.; Kuzma, M.; Max, F.; Binkley, N.; Payer, J. Vitamin D deficiency prevalence in hospitalized patients with COVID-19 significantly fecreased during the pandemic in Slovakia from 2020 to 2022 Which Was associated with decreasing mortality. Nutrients 2023, 15, 1132. [Google Scholar] [CrossRef]
- Wimalawansa, S. Commonsense approaches to minimizing risks from COVID-19. Open J. Pulmonol. Respir. Med. 2020, 2, 28–37. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Fighting against COVID-19: Boosting the immunity with micronutrients, stress reduction, physical activity, and vitamin D. Nutr. Food Sci. J. (Sci Lit.) 2020, 3, 126. [Google Scholar]
- Giordano, D.; De Masi, L.; Argenio, M.A.; Facchiano, A. Structural dissection of viral Spike-protein binding of SARS CoV-2 and SARS-CoV-1 to the human angiotensin-converting enzyme 2 (ACE2) as cellular receptor. Biomedicines 2021, 9, 1038. [Google Scholar] [CrossRef] [PubMed]
- Mancini, T.; Macis, S.; Mosetti, R.; Luchetti, N.; Minicozzi, V.; Notargiacomo, A.; Pea, M.; Marcelli, A.; Ventura, G.D.; Lupi, S.; et al. Infrared Spectroscopy of SARS-CoV-2 Viral Protein: From Receptor Binding Domain to Spike Protein. Adv. Sci. 2024, e2400823. [Google Scholar] [CrossRef]
- Lei, C.; Qian, K.; Li, T.; Zhang, S.; Fu, W.; Ding, M.; Hu, S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-Ig. Nat. Commun. 2020, 11, 2070. [Google Scholar] [CrossRef]
- Cui, D.; Liu, Y.; Jiang, X.; Ding, C.; Poon, L.C.; Wang, H.; Yang, H. Single-cell RNA expression profiling of SARS-CoV-2-related ACE2 and TMPRSS2 in human trophectoderm and placenta. Ultrasound Obstet. Gynecol. 2021, 57, 248–256. [Google Scholar] [CrossRef]
- Matsuyama, S.; Kawase, M.; Nao, N.; Shirato, K.; Ujike, M.; Kamitani, W.; Shimojima, M.; Fukushi, S. The inhaled steroid ciclesonide blocks SARS-CoV-2 RNA replication by targeting the viral replication-transcription complex in cultured cells. J. Virol. 2020, 95, 10-1128. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Reiss, K.; Allen, B.; Maschietto, F.; Lolis, E.; Konigsberg, W.H.; Lisi, G.P.; Batista, V.S. Insights into binding of single-stranded viral RNA template to the replication-transcription complex of SARS-CoV-2 for the priming reaction from molecular dynamics simulations. Biochemistry 2022, 61, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.; Barajas, D.; Qin, J.; Nagy, P.D. Inactivation of the host lipin gene accelerates RNA virus replication through viral exploitation of the expanded endoplasmic reticulum membrane. PLoS Pathog. 2014, 10, e1003944. [Google Scholar] [CrossRef]
- Sanyal, S. How SARS-CoV-2 (COVID-19) spreads within infected hosts—What we know so far. Emerg. Top. Life Sci. 2020, 4, 371–378. [Google Scholar] [CrossRef]
- Malek Mahdavi, A. A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19. Rev. Med. Virol. 2020, 30, e2119. [Google Scholar] [CrossRef]
- Vargas Vargas, R.A.; Varela Millan, J.M.; Fajardo Bonilla, E. Renin-angiotensin system: Basic and clinical aspects-A general perspective. Endocrinol. Diabetes Nutr. (Engl. Ed.) 2022, 69, 52–62. [Google Scholar] [CrossRef]
- Gaddam, R.R.; Chambers, S.; Bhatia, M. ACE and ACE2 in inflammation: A tale of two enzymes. Inflamm. Allergy Drug Targets 2014, 13, 224–234. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.A.; Simoes e Silva, A.C.; Maric, C.; Silva, D.M.; Machado, R.P.; de Buhr, I.; Heringer-Walther, S.; Pinheiro, S.V.; Lopes, M.T.; Bader, M.; et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. USA 2003, 100, 8258–8263. [Google Scholar] [CrossRef]
- Fedson, D.S. Treating the host response to emerging virus diseases: Lessons learned from sepsis, pneumonia, influenza and Ebola. Ann. Transl. Med. 2016, 4, 421. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.L.; Azemi, A.K.; Mokhtar, S.S.; Yahaya, S.; Yaacob, N.S.; Rasool, A.H.G. Vitamin D deficiency enhances vascular oxidative stress, inflammation, and angiotensin II levels in the microcirculation of diabetic patients. Microvasc. Res. 2023, 150, 104574. [Google Scholar] [CrossRef]
- Kong, J.; Zhu, X.; Shi, Y.; Liu, T.; Chen, Y.; Bhan, I.; Zhao, Q.; Thadhani, R.; Li, Y.C. VDR attenuates acute lung injury by blocking Ang-2-Tie-2 pathway and renin-angiotensin system. Mol. Endocrinol. 2013, 27, 2116–2125. [Google Scholar] [CrossRef]
- Beltran-Garcia, J.; Osca-Verdegal, R.; Pallardo, F.V.; Ferreres, J.; Rodriguez, M.; Mulet, S.; Sanchis-Gomar, F.; Carbonell, N.; Garcia-Gimenez, J.L. Oxidative stress and Inflammation in COVID-19-associated sepsis: The potential role of anti-oxidant therapy in avoiding disease progression. Antioxidants 2020, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.P.M.; Krishnananda, K.; Mohit Changani, M.; Patel, N.; Shenoy Belle, V. Vitamin D as a biomarker in predicting sepsis outcome at a tertiary care hospital. Asian J. Med. Sci. 2023, 14, 89–94. [Google Scholar] [CrossRef]
- Yuan, W.; Pan, W.; Kong, J.; Zheng, W.; Szeto, F.L.; Wong, K.E.; Cohen, R.; Klopot, A.; Zhang, Z.; Li, Y.C. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 2007, 282, 29821–29830. [Google Scholar] [CrossRef]
- Gibson, P.G.; Qin, L.; Puah, S.H. COVID-19 acute respiratory distress syndrome (ARDS): Clinical features and differences from typical pre-COVID-19 ARDS. Med. J. Aust. 2020, 213, 54–56.e51. [Google Scholar] [CrossRef]
- McLachlan, C.S. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin. Hypertens. 2020, 26, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Wang, Y.; Wang, G.Q. Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19. J. Med. Virol. 2020, 92, 726–730. [Google Scholar] [CrossRef]
- Devaux, C.A.; Rolain, J.M.; Raoult, D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J. Microbiol. Immunol. Infect. 2020, 53, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Bosso, M.; Thanaraj, T.A.; Abu-Farha, M.; Alanbaei, M.; Abubaker, J.; Al-Mulla, F. The two faces of ACE2: The role of ACE2 receptor and Its polymorphisms in hypertension and COVID-19. Mol. Ther. Methods Clin. Dev. 2020, 18, 321–327. [Google Scholar] [CrossRef]
- Souza, A.P.; Sobrinho, D.B.; Almeida, J.F.; Alves, G.M.; Macedo, L.M.; Porto, J.E.; Vencio, E.F.; Colugnati, D.B.; Santos, R.A.; Ferreira, A.J.; et al. Angiotensin II type 1 receptor blockade restores angiotensin-(1–7)-induced coronary vasodilation in hypertrophic rat hearts. Clin. Sci. 2013, 125, 449–459. [Google Scholar] [CrossRef]
- Souza, L.L.; Costa-Neto, C.M. Angiotensin-(1–7) decreases LPS-induced inflammatory response in macrophages. J. Cell. Physiol. 2012, 227, 2117–2122. [Google Scholar] [CrossRef]
- Dong, W.Q.; Bai, B.; Lin, Y.P.; Gao, J.; Yu, N.S. Detection of the mRNA expression of human angiotensin-converting enzyme 2 as a SARS coronavirus functional receptor in human femoral head. Nan Fang Yi Ke Da Xue Xue Bao 2008, 28, 441–443. [Google Scholar] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef]
- Zisman, L.S. ACE and ACE2: A tale of two enzymes. Eur. Heart J. 2005, 26, 322–324. [Google Scholar] [CrossRef] [PubMed]
- Goulter, A.B.; Goddard, M.J.; Allen, J.C.; Clark, K.L. ACE2 gene expression is up-regulated in the human failing heart. BMC Med. 2004, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Keller, R.S.; Weaver, B.; Zisman, L.S. Interaction of ACE2 and integrin beta1 in failing human heart. Biochim. Biophys. Acta 2004, 1689, 175–178. [Google Scholar] [CrossRef][Green Version]
- Liu, M.; Shi, P.; Sumners, C. Direct anti-inflammatory effects of angiotensin-(1–7) on microglia. J. Neurochem. 2016, 136, 163–171. [Google Scholar] [CrossRef]
- Mori, J.; Patel, V.B.; Ramprasath, T.; Alrob, O.A.; DesAulniers, J.; Scholey, J.W.; Lopaschuk, G.D.; Oudit, G.Y. Angiotensin 1–7 mediates renoprotection against diabetic nephropathy by reducing oxidative stress, inflammation, and lipotoxicity. Am. J. Physiol. Renal Physiol. 2014, 306, F812–F821. [Google Scholar] [CrossRef]
- da Silveira, K.D.; Coelho, F.M.; Vieira, A.T.; Sachs, D.; Barroso, L.C.; Costa, V.V.; Bretas, T.L.; Bader, M.; de Sousa, L.P.; da Silva, T.A.; et al. Anti-inflammatory effects of the activation of the angiotensin-(1–7) receptor, MAS, in experimental models of arthritis. J. Immunol. 2010, 185, 5569–5576. [Google Scholar] [CrossRef]
- Fraga-Silva, R.A.; Pinheiro, S.V.; Goncalves, A.C.; Alenina, N.; Bader, M.; Santos, R.A. The antithrombotic effect of angiotensin-(1–7) involves mas-mediated NO release from platelets. Mol. Med. 2008, 14, 28–35. [Google Scholar] [CrossRef]
- Patel, V.B.; Zhong, J.C.; Grant, M.B.; Oudit, G.Y. Role of the ACE2/angiotensin 1–7 axis of the renin-angiotensin system in heart failure. Circ. Res. 2016, 118, 1313–1326. [Google Scholar] [CrossRef]
- Grobe, J.L.; Mecca, A.P.; Lingis, M.; Shenoy, V.; Bolton, T.A.; Machado, J.M.; Speth, R.C.; Raizada, M.K.; Katovich, M.J. Prevention of angiotensin II-induced cardiac remodeling by angiotensin-(1–7). Am. J. Physiology. Heart Circ. Physiol. 2007, 292, H736–H742. [Google Scholar] [CrossRef] [PubMed]
- Kurdi, M.; Booz, G.W. New take on the role of angiotensin II in cardiac hypertrophy and fibrosis. Hypertension 2011, 57, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.H.; Giani, J.F.; Burghi, V.; Miquet, J.G.; Qadri, F.; Braga, J.F.; Todiras, M.; Kotnik, K.; Alenina, N.; Dominici, F.P.; et al. Oral administration of angiotensin-(1–7) ameliorates type 2 diabetes in rats. J. Mol. Med. 2014, 92, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Maleki, M.; Nematbakhsh, M. Renal blood flow response to angiotensin 1–7 versus hypertonic sodium chloride 7.5% administration after acute hemorrhagic shock in rats. Int. J. Vasc. Med. 2016, 2016, 6562017. [Google Scholar] [CrossRef] [PubMed]
- DelliPizzi, A.M.; Hilchey, S.D.; Bell-Quilley, C.P. Natriuretic action of angiotensin(1–7). Br. J. Pharmacol. 1994, 111, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, R.P.; Sulaiman, M.I.; Gaub, M.; Bae, E.H.; Davidson Knapp, R.B.; Larson, A.R.; Smith, A.; Coleman, D.L.; Staatz, W.D.; Sandweiss, A.J.; et al. Angiotensin-(1–7) improves cognitive function and reduces inflammation in mice following mild traumatic brain injury. Front. Behav. Neurosci. 2022, 16, 903980. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Y.; Chen, S.; Wang, J.; Xiao, X.; Ma, X.; Penchikala, M.; Xia, H.; Lazartigues, E.; Zhao, B.; et al. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology 2014, 79, 550–558. [Google Scholar] [CrossRef]
- Regenhardt, R.W.; Mecca, A.P.; Desland, F.; Ritucci-Chinni, P.F.; Ludin, J.A.; Greenstein, D.; Banuelos, C.; Bizon, J.L.; Reinhard, M.K.; Sumners, C. Centrally administered angiotensin-(1–7) increases the survival of stroke-prone spontaneously hypertensive rats. Exp. Physiol. 2014, 99, 442–453. [Google Scholar] [CrossRef]
- Santos, R.A.S.; Sampaio, W.O.; Alzamora, A.C.; Motta-Santos, D.; Alenina, N.; Bader, M.; Campagnole-Santos, M.J. The ACE2/Angiotensin-(1–7)/MAS axis of the renin-angiotensin system: Focus on angiotensin-(1–7). Physiol. Rev. 2018, 98, 505–553. [Google Scholar] [CrossRef]
- Satou, R.; Penrose, H.; Navar, L.G. Inflammation as a regulator of the renin-angiotensin system and blood pressure. Curr. Hypertens. Rep. 2018, 20, 100. [Google Scholar] [CrossRef]
- Benigni, A.; Cassis, P.; Remuzzi, G. Angiotensin II revisited: New roles in inflammation, immunology and aging. EMBO Mol. Med. 2010, 2, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Uhal, B.D. ACE2, much more than just a receptor for SARS-COV-2. Front. Cell. Infect. Microbiol. 2020, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Monteil, V.; Kwon, H.; Prado, P.; Hagelkruys, A.; Wimmer, R.A.; Stahl, M.; Leopoldi, A.; Garreta, E.; Hurtado Del Pozo, C.; Prosper, F.; et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020, 181, 905–913.e907. [Google Scholar] [CrossRef] [PubMed]
- Hollis, B.W.; Wagner, C.L.; Drezner, M.K.; Binkley, N.C. Circulating vitamin D3 and 25-hydroxyvitamin D in humans: An important tool to define adequate nutritional vitamin D status. J. Steroid Biochem. Mol. Biol. 2007, 103, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Bold, A.; Gross, H.; Holzmann, E.; Smetak, M.; Birkmann, J.; Bertsch, T.; Triebel, J.; Sauer, K.; Wilhelm, M.; Hoeres, T. Immune activating and inhibiting effects of calcitriol on gammadelta T cells and NK cells. Immunobiology 2022, 227, 152286. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef]
- Gotelli, E.; Soldano, S.; Hysa, E.; Paolino, S.; Campitiello, R.; Pizzorni, C.; Sulli, A.; Smith, V.; Cutolo, M. Vitamin D and COVID-19: Narrative review after 3 years of pandemic. Nutrients 2022, 14, 4907. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Prophylactic use of vitamin D to maintain a robust immune system against infections like SARS-CoV-2. Glob. J. Endocrinol. Metab. GJEM 2023, 3, 000571. [Google Scholar] [CrossRef]
- Pender, M.P. CD8+ T-cell deficiency, Epstein-Barr virus infection, vitamin D deficiency, and steps to autoimmunity: A unifying hypothesis. Autoimmune Dis. 2012, 2012, 189096. [Google Scholar] [CrossRef]
- Quraishi, S.A.; Bittner, E.A.; Blum, L.; McCarthy, C.M.; Bhan, I.; Camargo, C.A., Jr. Prospective study of vitamin D status at initiation of care in critically ill surgical patients and risk of 90-day mortality. Crit. Care Med. 2014, 42, 1365–1371. [Google Scholar] [CrossRef]
- Fernandez, G.J.; Ramirez-Mejia, J.M.; Castillo, J.A.; Urcuqui-Inchima, S. Vitamin D modulates expression of antimicrobial peptides and proinflammatory cytokines to restrict Zika virus infection in macrophages. Int. Immunopharmacol. 2023, 119, 110232. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, N.; Sepidarkish, M.; Ghaffari, S.; Rostami-Mansoor, S. Does vitamin D reduce the mortality rate of Plasmodium infection?: A systematic review and meta-analysis. Malar. J. 2023, 22, 173. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, M.E.; Huynh, L.M.; Smith, C.M.; Mustad, V.A.; Duarte, M.O.; Cramer, J.T. Immunomodulatory effects of vitamin D and prevention of respiratory tract infections and COVID-19. Top. Clin. Nutr. 2022, 37, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Merzon, E.; Tworowski, D.; Gorohovski, A.; Vinker, S.; Golan Cohen, A.; Green, I.; Frenkel-Morgenstern, M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: An Israeli population-based study. FEBS J. 2020, 287, 3693–3702. [Google Scholar] [CrossRef] [PubMed]
- Sposito, F.; Pennington, S.H.; David, C.A.W.; Duggan, J.; Northey, S.; Biagini, G.A.; Liptrott, N.J.; Charras, A.; McNamara, P.S.; Hedrich, C.M. Age-differential CD13 and interferon expression in airway epithelia affect SARS-CoV-2 infection—Effects of vitamin D. Mucosal Immunol. 2023, 16, 776–787. [Google Scholar] [CrossRef] [PubMed]
- Dror, A.A.; Morozov, N.; Daoud, A.; Namir, Y.; Yakir, O.; Shachar, Y.; Lifshitz, M.; Segal, E.; Fisher, L.; Mizrachi, M.; et al. Pre-infection 25-hydroxyvitamin D3 levels and association with severity of COVID-19 illness. PLoS ONE 2022, 17, e0263069. [Google Scholar] [CrossRef]
- Gan, Y.; You, S.; Ying, J.; Mu, D. The association between serum vitamin D levels and urinary tract infection risk in children: A systematic review and meta-analysis. Nutrients 2023, 15, 2690. [Google Scholar] [CrossRef]
- Bayrak, H.; Ozturk, D.; Bolat, A.; Unay, B. Association between vitamin D levels and COVID-19 infection in children: A case-control study. Turk. Arch. Pediatr. 2023, 58, 250–255. [Google Scholar] [CrossRef]
- Raju, A.; Luthra, G.; Shahbaz, M.; Almatooq, H.; Foucambert, P.; Esbrand, F.D.; Zafar, S.; Panthangi, V.; Cyril Kurupp, A.R.; Khan, S. Role of vitamin D deficiency in increased susceptibility to respiratory infections among children: A systematic review. Cureus 2022, 14, e29205. [Google Scholar] [CrossRef]
- Bekele, A.; Gebreselassie, N.; Ashenafi, S.; Kassa, E.; Aseffa, G.; Amogne, W.; Getachew, M.; Aseffa, A.; Worku, A.; Raqib, R.; et al. Daily adjunctive therapy with vitamin D(3) and phenylbutyrate supports clinical recovery from pulmonary tuberculosis: A randomized controlled trial in Ethiopia. J. Intern. Med. 2018, 284, 292–306. [Google Scholar] [CrossRef]
- Salahuddin, N.; Ali, F.; Hasan, Z.; Rao, N.; Aqeel, M.; Mahmood, F. Vitamin D accelerates clinical recovery from tuberculosis: Results of the SUCCINCT Study [Supplementary Cholecalciferol in recovery from tuberculosis]. A randomized, placebo-controlled, clinical trial of vitamin D supplementation in patients with pulmonary tuberculosis’. BMC Infect. Dis. 2013, 13, 22. [Google Scholar] [CrossRef]
- Quraishi, S.A.; De Pascale, G.; Needleman, J.S.; Nakazawa, H.; Kaneki, M.; Bajwa, E.K.; Camargo, C.A., Jr.; Bhan, I. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 2015, 43, 1928–1937. [Google Scholar] [CrossRef] [PubMed]
- Fu, G.; Wu, R.; Zhang, R.; Chen, D.; Li, H.; Zheng, Q.; Ma, Y. Preoperative vitamin D deficiency is associated with increased one-year mortality in Chinese geriatric hip fracture patients—A propensity score matching study. Clin. Interv. Aging 2023, 18, 263–272. [Google Scholar] [CrossRef]
- Charoenngam, N.; Shirvani, A.; Reddy, N.; Vodopivec, D.M.; Apovian, C.M.; Holick, M.F. Association of vitamin D status with hospital morbidity and mortality in adult hospitalized patients wth COVID-19. Endocr. Pract. 2021, 27, 271–278. [Google Scholar] [CrossRef]
- Rustecka, A.; Maret, J.; Drab, A.; Leszczynska, M.; Tomaszewska, A.; Lipinska-Opalka, A.; Bedzichowska, A.; Kalicki, B.; Kubiak, J.Z. The Impact of COVID-19 pandemic during 2020-2021 on the vitamin D serum levels in the paediatric population in Warsaw, Poland. Nutrients 2021, 13, 1990. [Google Scholar] [CrossRef]
- Borsche, L.; Glauner, B.; von Mendel, J. COVID-19 mortality risk correlates inversely with vitamin D3 status, and a mortality rate close to zero could theoretically be achieved at 50 ng/mL 25(OH)D3: Results of a systematic review and meta-analysis. Nutrients 2021, 13, 3596. [Google Scholar] [CrossRef]
- Dudenkov, D.V.; Yawn, B.P.; Oberhelman, S.S.; Fischer, P.R.; Singh, R.J.; Cha, S.S.; Maxson, J.A.; Quigg, S.M.; Thacher, T.D. Changing incidence of serum 25-hydroxyvitamin D values above 50 ng/mL: A 10-year population-based study. Mayo Clin. Proc. 2015, 90, 577–586. [Google Scholar] [CrossRef]
- Radujkovic, A.; Hippchen, T.; Tiwari-Heckler, S.; Dreher, S.; Boxberger, M.; Merle, U. Vitamin D deficiency and outcome of COVID-19 patients. Nutrients 2020, 12, 2757. [Google Scholar] [CrossRef]
- Wimalawansa, S.J.; Polonowita, A. Boosting immunity with vitamin D for preventing complications and deaths from COVID-19. In Proceedings of the COVID 19: Impact, Mitigation, Opportunities and Building Resilience “From Adversity to Serendipity,” Perspectives of Global Relevance Based on Research, Experience and Successes in Combating COVID-19 in Sri Lanka, Colombo, Sri Lanka, January 2021; pp. 171–198. [Google Scholar]
- Baktash, V.; Hosack, T.; Patel, N.; Shah, S.; Kandiah, P.; Van Den Abbeele, K.; Mandal, A.K.J.; Missouris, C.G. Vitamin D status and outcomes for hospitalised older patients with COVID-19. Postgrad. Med. J. 2020, 97, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Argano, C.; Mallaci Bocchio, R.; Natoli, G.; Scibetta, S.; Lo Monaco, M.; Corrao, S. Protective effect of vitamin D supplementation on COVID-19-related intensive care hospitalization and mortality: Sefinitive evidence from meta-analysis and trial sequential analysis. Pharmaceuticals 2023, 16, 130. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Borghi, C. Vitamin D supplementation and COVID-19 outcomes: Mounting evidence and fewer doubts. Nutrients 2022, 14, 3584. [Google Scholar] [CrossRef]
- Gonen, M.S.; Alaylioglu, M.; Durcan, E.; Ozdemir, Y.; Sahin, S.; Konukoglu, D.; Nohut, O.K.; Urkmez, S.; Kucukece, B.; Balkan, İ.İ.; et al. Rapid and effective vitamin D supplementation may present better clinical outcomes in COVID-19 (SARS-CoV-2) patients by altering serum INOS1, IL1B, IFNg, cathelicidin-LL37, and ICAM1. Nutrients 2021, 13, 4047. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, D.A.; Camargo, C.A., Jr.; Sluyter, J.D.; Aglipay, M.; Aloia, J.F.; Ganmaa, D.; Bergman, P.; Bischoff-Ferrari, H.A.; Borzutzky, A.; Damsgaard, C.T.; et al. Vitamin D supplementation to prevent acute respiratory infections: A systematic review and meta-analysis of aggregate data from randomised controlled trials. Lancet Diabetes Endocrinol. 2021, 9, 276–292. [Google Scholar] [CrossRef]
- Hong, M.; Xiong, T.; Huang, J.; Wu, Y.; Lin, L.; Zhang, Z.; Huang, L.; Gao, D.; Wang, H.; Kang, C.; et al. Association of vitamin D supplementation with respiratory tract infection in infants. Matern. Child. Nutr. 2020, 16, e12987. [Google Scholar] [CrossRef]
- Shiravi, A.A.; Saadatkish, M.; Abdollahi, Z.; Miar, P.; Khanahmad, H.; Zeinalian, M. Vitamin D can be effective on the prevention of COVID-19 complications: A narrative review on molecular aspects. Int. J. Vitam. Nutr. Res. 2022, 92, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Molloy, E.J.; Murphy, N. Vitamin D, Covid-19 and Children. Ir. Med. J. 2020, 113, 64. [Google Scholar]
- Stohs, S.J.; Aruoma, O.I. Vitamin D and Wellbeing beyond Infections: COVID-19 and Future Pandemics. J. Am. Coll. Nutr. 2020, 40, 41–42. [Google Scholar] [CrossRef]
- Garg, M.; Al-Ani, A.; Mitchell, H.; Hendy, P.; Christensen, B. Editorial: Low population mortality from COVID-19 in countries south of latitude 35 degrees North-supports vitamin D as a factor determining severity. Authors’ reply. Aliment. Pharmacol. Ther. 2020, 51, 1438–1439. [Google Scholar] [CrossRef]
- Jaun, F.; Boesing, M.; Luthi-Corridori, G.; Abig, K.; Makhdoomi, A.; Bloch, N.; Lins, C.; Raess, A.; Grillmayr, V.; Haas, P.; et al. High-dose vitamin D substitution in patients with COVID-19: Study protocol for a randomized, double-blind, placebo-controlled, multi-center study-VitCov Trial. Trials 2022, 23, 114. [Google Scholar] [CrossRef]
- Quesada-Gomez, J.M.; Lopez-Miranda, J.; Entrenas-Castillo, M.; Casado-Diaz, A.; Nogues, Y.S.X.; Mansur, J.L.; Bouillon, R. Vitamin D endocrine system and COVID-19: Treatment with calcifediol. Nutrients 2022, 14, 2716. [Google Scholar] [CrossRef] [PubMed]
- Maghbooli, Z.; Sahraian, M.A.; Jamalimoghadamsiahkali, S.; Asadi, A.; Zarei, A.; Zendehdel, A.; Varzandi, T.; Mohammadnabi, S.; Alijani, N.; Karimi, M.; et al. Treatment With 25-Hydroxyvitamin D3 (Calcifediol) Is Associated With a Reduction in the Blood Neutrophil-to-Lymphocyte Ratio Marker of Disease Severity in Hospitalized Patients With COVID-19: A Pilot Multicenter, Randomized, Placebo-Controlled, Double-Blinded Clinical Trial. Endocr. Pract. 2021, 27, 1242–1251. [Google Scholar] [CrossRef]
- Ling, S.F.; Broad, E.; Murphy, R.; Pappachan, J.M.; Pardesi-Newton, S.; Kong, M.F.; Jude, E.B. High-dose cholecalciferol booster therapy is associated with a reduced risk of mortality in patients with COVID-19: A cross-sectional multi-centre observational study. Nutrients 2020, 12, 3799. [Google Scholar] [CrossRef]
- Entrenas Castillo, M.; Entrenas Costa, L.M.; Vaquero Barrios, J.M.; Alcala Diaz, J.F.; Lopez Miranda, J.; Bouillon, R.; Quesada Gomez, J.M. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study. J. Steroid Biochem. Mol. Biol. 2020, 203, 105751. [Google Scholar] [CrossRef]
- Ebrahimzadeh, A.; Mohseni, S.; Narimani, B.; Ebrahimzadeh, A.; Kazemi, S.; Keshavarz, F.; Yaghoubi, M.J.; Milajerdi, A. Association between vitamin D status and risk of covid-19 in-hospital mortality: A systematic review and meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2021, 63, 5033–5043. [Google Scholar] [CrossRef]
- AlSafar, H.; Grant, W.B.; Hijazi, R.; Uddin, M.; Alkaabi, N.; Tay, G.; Mahboub, B.; Al Anouti, F. COVID-19 disease severity and death in relation to vitamin D status among SARS-CoV-2-positive UAE residents. Nutrients 2021, 13, 1714. [Google Scholar] [CrossRef] [PubMed]
- Bianconi, V.; Mannarino, M.R.; Figorilli, F.; Cosentini, E.; Batori, G.; Marini, E.; Lombardini, R.; Gargaro, M.; Fallarino, F.; Scarponi, A.M.; et al. Prevalence of vitamin D deficiency and its prognostic impact on patients hospitalized with COVID-19. Nutrition 2021, 91–92, 111408. [Google Scholar] [CrossRef] [PubMed]
- Davies, G.; Mazess, R.B.; Benskin, L.L. Letter to the editor in response to the article: “Vitamin D concentrations and COVID-19 infection in UK biobank” (Hastie et al.). Diabetes Metab. Syndr. 2021, 15, 643–644. [Google Scholar] [CrossRef]
- Hastie, C.E.; Pell, J.P.; Sattar, N. Vitamin D and COVID-19 infection and mortality in UK Biobank. Eur. J. Nutr. 2020, 60, 545–548. [Google Scholar] [CrossRef]
- Raisi-Estabragh, Z.; McCracken, C.; Bethell, M.S.; Cooper, J.; Cooper, C.; Caulfield, M.J.; Munroe, P.B.; Harvey, N.C.; Petersen, S.E. Greater risk of severe COVID-19 in Black, Asian and Minority Ethnic populations is not explained by cardiometabolic, socioeconomic or behavioural factors, or by 25(OH)-vitamin D status: Study of 1326 cases from the UK Biobank. J. Public Health 2020, 42, 451–460. [Google Scholar] [CrossRef]
- Kazemi, A.; Mohammadi, V.; Aghababaee, S.A.; Golzarand, M.; Clark, C.C.T.; Babajafari, S. Association of vitamin D status with SARS-CoV-2 infection or COVID-19 severity: A systematic review and meta-analysis. Adv. Nutr. 2021, 12, 1636–1658. [Google Scholar] [CrossRef] [PubMed]
- Hastie, C.E.; Mackay, D.F.; Ho, F.; Celis-Morales, C.A.; Katikireddi, S.V.; Niedzwiedz, C.L.; Jani, B.D.; Welsh, P.; Mair, F.S.; Gray, S.R.; et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metab. Syndr. 2020, 14, 561–565. [Google Scholar] [CrossRef]
- Kaufman, H.W.; Niles, J.K.; Kroll, M.H.; Bi, C.; Holick, M.F. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS ONE 2020, 15, e0239252. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.A. Preventing a COVID-19 pandemic-COVID-19. BMJ 2020, 368, m810. [Google Scholar] [CrossRef]
- Laird, E.; Rhodes, J.; Kenny, R.A. Vitamin D and inflammation: Potential implications for severity of Covid-19. Ir. Med. J. 2020, 113, 81. [Google Scholar]
- Annweiler, C.; Hanotte, B.; Grandin de l’Eprevier, C.; Sabatier, J.M.; Lafaie, L.; Celarier, T. Vitamin D and survival in COVID-19 patients: A quasi-experimental study. J. Steroid Biochem. Mol. Biol. 2020, 204, 105771. [Google Scholar] [CrossRef]
- Annweiler, G.; Corvaisier, M.; Gautier, J.; Dubee, V.; Legrand, E.; Sacco, G.; Annweiler, C. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: The GERIA-COVID Quasi-Experimental Study. Nutrients 2020, 12, 3377. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D deficiency and treatment with COVID-19 incidence. medRxiv 2020. [Google Scholar] [CrossRef]
- Meltzer, D.O.; Best, T.J.; Zhang, H.; Vokes, T.; Arora, V.; Solway, J. Association of vitamin D status and other clinical characteristics with COVID-19 Test resultt. JAMA Netw. Open 2020, 3, e2019722. [Google Scholar] [CrossRef]
- Smaha, J.; Kuzma, M.; Brazdilova, K.; Nachtmann, S.; Jankovsky, M.; Pastirova, K.; Gazova, A.; Jackuliak, P.; Killinger, Z.; Kyselovic, J.; et al. Patients with COVID-19 pneumonia with 25(OH)D levels lower than 12 ng/ml are at increased risk of death. Int. J. Infect. Dis. 2022, 116, 313–318. [Google Scholar] [CrossRef]
- Durazo-Arvizu, R.A.; Dawson-Hughes, B.; Kramer, H.; Cao, G.; Merkel, J.; Coates, P.M.; Sempos, C.T. The reverse J-shaped association between serum Total 25-hydroxyvitamin D concentration and all-cause mortality: The impact of assay standardization. Am. J. Epidemiol. 2017, 185, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Durup, D.; Jorgensen, H.L.; Christensen, J.; Schwarz, P.; Heegaard, A.M.; Lind, B. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice: The CopD study. J. Clin. Endocrinol. Metab. 2012, 97, 2644–2652. [Google Scholar] [CrossRef] [PubMed]
- Seal, K.H.; Bertenthal, D.; Carey, E.; Grunfeld, C.; Bikle, D.D.; Lu, C.M. Association of Vitamin D Status and COVID-19-Related Hospitalization and Mortality. J. Gen. Intern. Med. 2022, 37, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Bychinin, M.V.; Klypa, T.V.; Mandel, I.A.; Andreichenko, S.A.; Baklaushev, V.P.; Yusubalieva, G.M.; Kolyshkina, N.A.; Troitsky, A.V. Low Circulating Vitamin D in Intensive Care Unit-Admitted COVID-19 Patients as a Predictor of Negative Outcomes. J. Nutr. 2021, 151, 2199–2205. [Google Scholar] [CrossRef]
- Cervero, M.; Lopez-Wolf, D.; Casado, G.; Novella-Mena, M.; Ryan-Murua, P.; Taboada-Martinez, M.L.; Rodriguez-Mora, S.; Vigon, L.; Coiras, M.; Torres, M. Beneficial effect of short-term supplementation of high dose of vitamin D(3) in hospitalized patients with COVID-19: A multicenter, single-blinded, prospective randomized pilot clinical trial. Front. Pharmacol. 2022, 13, 863587. [Google Scholar] [CrossRef]
- Gavioli, E.M.; Miyashita, H.; Hassaneen, O.; Siau, E. An evaluation of serum 25-hydroxy vitamin D levels in patients with COVID-19 in New York City. J. Am. Nutr. Assoc. 2022, 41, 201–206. [Google Scholar] [CrossRef]
- Ben-Eltriki, M.; Hopefl, R.; Wright, J.M.; Deb, S. Association between vitamin D status and risk of developing severe COVID-19 infection: A meta-analysis of observational studies. J. Am. Coll. Nutr. 2021, 41, 679–689. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Raju, M.N.P.; da Graca, B.; Wang, D.; Mohamed, N.A.; Mutnal, M.B.; Rao, A.; Bennett, M.; Gokingco, M.; Pham, H.; et al. 25-hydroxyvitamin D is a predictor of COVID-19 severity of hospitalized patients. PLoS ONE 2022, 17, e0268038. [Google Scholar] [CrossRef]
- Takase, T.; Tsugawa, N.; Sugiyama, T.; Ikesue, H.; Eto, M.; Hashida, T.; Tomii, K.; Muroi, N. Association between 25-hydroxyvitamin D levels and COVID-19 severity. Clin. Nutr. ESPEN 2022, 49, 256–263. [Google Scholar] [CrossRef]
- Chauss, D.; Freiwald, T.; McGregor, R.; Yan, B.; Wang, L.; Nova-Lamperti, E.; Kumar, D.; Zhang, Z.; Teague, H.; West, E.E.; et al. Autocrine vitamin D signaling switches off pro-inflammatory programs of TH1 cells. Nat. Immunol. 2022, 23, 62–74. [Google Scholar] [CrossRef]
- Bilezikian, J.P.; Bikle, D.; Hewison, M.; Lazaretti-Castro, M.; Formenti, A.M.; Gupta, A.; Madhavan, M.V.; Nair, N.; Babalyan, V.; Hutchings, N.; et al. Mechanisums in Endocrinology: Vitamin D and COVID-19. Eur. J. Endocrinol. 2020, 183, R133–R147. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and innate and adaptive immunity. Vitam. Horm. 2011, 86, 23–62. [Google Scholar] [CrossRef]
- Walsh, J.B.; McCartney, D.M.; Laird, E.; McCarroll, K.; Byrne, D.G.; Healy, M.; O’Shea, P.M.; Kenny, R.A.; Faul, J.L. Understanding a low vitamin D state in the context of COVID-19. Front. Pharmacol. 2022, 13, 835480. [Google Scholar] [CrossRef]
- Werneke, U.; Gaughran, F.; Taylor, D.M. Vitamin D in the time of the coronavirus (COVID-19) pandemic—A clinical review from a public health and public mental health perspective. Ther. Adv. Psychopharmacol. 2021, 11, 20451253211027699. [Google Scholar] [CrossRef] [PubMed]
- Hewison, M. Vitamin D and immune function: Autocrine, paracrine or endocrine? Scand. J. Clin. Lab. Investig. Suppl. 2012, 243, 92–102. [Google Scholar] [CrossRef]
- Aranow, C. Vitamin D and the immune system. J. Investig. Med. 2011, 59, 881–886. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D and the immune system: Role in protection against bacterial infection. Curr. Opin. Nephrol. Hypertens. 2008, 17, 348–352. [Google Scholar] [CrossRef]
- Hollis, B.W.; Marshall, D.T.; Savage, S.J.; Garrett-Mayer, E.; Kindy, M.S.; Gattoni-Celli, S. Vitamin D3 supplementation, low-risk prostate cancer, and health disparities. J. Steroid Biochem. Mol. Biol. 2013, 136, 233–237. [Google Scholar] [CrossRef]
- Hewison, M. Vitamin D and the immune system: New perspectives on an old theme. Endocrinol. Metab. Clin. N. Am. 2010, 39, 365–379. [Google Scholar] [CrossRef]
- Giannini, S.; Giusti, A.; Minisola, S.; Napoli, N.; Passeri, G.; Rossini, M.; Sinigaglia, L. The immunologic profile of vitamin D and its role in different immune-mediated diseases: An expert opinion. Nutrients 2022, 14, 473. [Google Scholar] [CrossRef]
- Olliver, M.; Spelmink, L.; Hiew, J.; Meyer-Hoffert, U.; Henriques-Normark, B.; Bergman, P. Immunomodulatory effects of vitamin D on innate and adaptive immune responses to Streptococcus pneumoniae. J. Infect. Dis. 2013, 208, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Schauber, J.; Dorschner, R.A.; Yamasaki, K.; Brouha, B.; Gallo, R.L. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology 2006, 118, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Esmaeili Gouvarchin Ghaleh, H.; Aghamollaei, H.; Fasihi Ramandi, M.; Alishiri, G.; Shahriary, A.; Hassanpour, K.; Tat, M.; Farnoosh, G. Evaluation of Th1 and Th2 mediated cellular and humoral immunity in patients with COVID-19 following the use of melatonin as an adjunctive treatment. Eur. J. Pharmacol. 2021, 904, 174193. [Google Scholar] [CrossRef]
- Li, Q.; Wang, B.; Mu, K.; Zhang, J.A. The pathogenesis of thyroid autoimmune diseases: New T lymphocytes—Cytokines circuits beyond the Th1-Th2 paradigm. J. Cell. Physiol. 2019, 234, 2204–2216. [Google Scholar] [CrossRef]
- Zhang, Y.; Leung, D.Y.; Richers, B.N.; Liu, Y.; Remigio, L.K.; Riches, D.W.; Goleva, E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J. Immunol. 2012, 188, 2127–2135. [Google Scholar] [CrossRef]
- Panagiotou, G.; Tee, S.A.; Ihsan, Y.; Athar, W.; Marchitelli, G.; Kelly, D.; Boot, C.S.; Stock, N.; Macfarlane, J.; Martineau, A.R.; et al. Low serum 25-hydroxyvitamin D (25[OH]D) levels in patients hospitalized with COVID-19 are associated with greater disease severity. Clin. Endocrinol. 2020, 93, 508. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvao Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Yang, J.; Hu, X.; Li, M.; Wang, Q.; Dancer, R.C.A.; Parekh, D.; Gao-Smith, F.; Thickett, D.R.; Jin, S. Vitamin D attenuates lung injury via stimulating epithelial repair, reducing epithelial cell apoptosis and inhibits TGF-beta induced epithelial to mesenchymal transition. Biochem. Pharmacol. 2020, 177, 113955. [Google Scholar] [CrossRef]
- Martinez-Moreno, J.M.; Herencia, C.; Montes de Oca, A.; Munoz-Castaneda, J.R.; Rodriguez-Ortiz, M.E.; Diaz-Tocados, J.M.; Peralbo-Santaella, E.; Camargo, A.; Canalejo, A.; Rodriguez, M.; et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016, 30, 1367–1376. [Google Scholar] [CrossRef]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin. Exp. Res. 2020, 32, 1195–1198. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Unlocking insights: Navigating COVID-19 challenges and emulating future pandemic resilience strategies with strengthening natural immunity. Heliyon 2024, 10, e34691. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.E.; Burke, F.; Mura, M.; Zheng, Y.; Qureshi, O.S.; Hewison, M.; Walker, L.S.; Lammas, D.A.; Raza, K.; Sansom, D.M. 1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J. Immunol. 2009, 183, 5458–5467. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Tang, F.; Liao, X.; Shaw, B.A.; Deng, M.; Huang, G.; Qin, Z.; Peng, X.; Xiao, H.; Chen, C.; et al. Does serum vitamin D level affect COVID-19 infection and Its severity?-A case-control study. J. Am. Coll. Nutr. 2021, 40, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Wu, S.; Sun, J. Vitamin D, vitamin D receptor, and tissue barriers. Tissue Barriers 2013, 1. [Google Scholar] [CrossRef]
- Getachew, B.; Tizabi, Y. Vitamin D and COVID-19: Role of ACE2, age, gender, and ethnicity. J. Med. Virol. 2021, 93, 5285–5294. [Google Scholar] [CrossRef]
- Martin, T.A.; Das, T.; Mansel, R.E.; Jiang, W.G. Enhanced tight junction function in human breast cancer cells by antioxidant, selenium and polyunsaturated lipid. J. Cell. Biochem. 2007, 101, 155–166. [Google Scholar] [CrossRef]
- Alexander, J.; Tinkov, A.; Strand, T.A.; Alehagen, U.; Skalny, A.; Aaseth, J. Early nutritional interventions with zinc, selenium and vitamin D for raising anti-viral resistance against progressive COVID-19. Nutrients 2020, 12, 2358. [Google Scholar] [CrossRef]
- Ashique, S.; Gupta, K.; Gupta, G.; Mishra, N.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Dureja, H.; Zacconi, F.; Oliver, B.G.; et al. Vitamin D-A prominent immunomodulator to prevent COVID-19 infection. Int. J. Rheum. Dis. 2023, 26, 13–30. [Google Scholar] [CrossRef]
- Rouzine, I.M.; Rozhnova, G. Evolutionary implications of SARS-CoV-2 vaccination for the future design of vaccination strategies. Commun. Med. 2023, 3, 86. [Google Scholar] [CrossRef]
- Tenaillon, O.; Matic, I. The impact of neutral mutations on genome evolvability. Curr. Biol. 2020, 30, R527–R534. [Google Scholar] [CrossRef]
- Konishi, T. Mutations in SARS-CoV-2 are on the increase against the acquired immunity. PLoS ONE 2022, 17, e0271305. [Google Scholar] [CrossRef] [PubMed]
- Abeywardhana, S.; Premathilaka, M.; Bandaranayake, U.; Perera, D.; Peiris, L.D.C. In silico study of SARS-CoV-2 spike protein RBD and human ACE-2 affinity dynamics across variants and Omicron subvariants. J. Med. Virol. 2023, 95, e28406. [Google Scholar] [CrossRef] [PubMed]
- Tuekprakhon, A.; Nutalai, R.; Dijokaite-Guraliuc, A.; Zhou, D.; Ginn, H.M.; Selvaraj, M.; Liu, C.; Mentzer, A.J.; Supasa, P.; Duyvesteyn, H.M.E.; et al. Antibody escape of SARS-CoV-2 Omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell 2022, 185, 2422–2433.e2413. [Google Scholar] [CrossRef]
- Dejnirattisai, W.; Zhou, D.; Ginn, H.M.; Duyvesteyn, H.M.E.; Supasa, P.; Case, J.B.; Zhao, Y.; Walter, T.S.; Mentzer, A.J.; Liu, C.; et al. The antigenic anatomy of SARS-CoV-2 receptor binding domain. Cell 2021, 184, 2183–2200.e22. [Google Scholar] [CrossRef]
- Hypponen, E.; Laara, E.; Reunanen, A.; Jarvelin, M.R.; Virtanen, S.M. Intake of vitamin D and risk of type 1 diabetes: A birth-cohort study. Lancet 2001, 358, 1500–1503. [Google Scholar] [CrossRef]
- Littorin, B.; Blom, P.; Scholin, A.; Arnqvist, H.J.; Blohme, G.; Bolinder, J.; Ekbom-Schnell, A.; Eriksson, J.W.; Gudbjornsdottir, S.; Nystrom, L.; et al. Lower levels of plasma 25-hydroxyvitamin D among young adults at diagnosis of autoimmune type 1 diabetes compared with control subjects: Results from the nationwide Diabetes Incidence Study in Sweden (DISS). Diabetologia 2006, 49, 2847–2852. [Google Scholar] [CrossRef]
- Mitri, J.; Muraru, M.D.; Pittas, A.G. Vitamin D and type 2 diabetes: A systematic review. Eur. J. Clin. Nutr. 2011, 65, 1005–1015. [Google Scholar] [CrossRef] [PubMed]
- Zold, E.; Szodoray, P.; Gaal, J.; Kappelmayer, J.; Csathy, L.; Gyimesi, E.; Zeher, M.; Szegedi, G.; Bodolay, E. Vitamin D deficiency in undifferentiated connective tissue disease. Arthritis Res. Ther. 2008, 10, R123. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between inflammatory bowel disease and Vitamin D deficiency: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Hu, Y.C.; Wang, W.W.; Jiang, W.Y.; Li, C.Q.; Guo, J.C.; Xun, Y.H. Low vitamin D levels are associated with high viral loads in patients with chronic hepatitis B: A systematic review and meta-analysis. BMC Gastroenterol. 2019, 19, 84. [Google Scholar] [CrossRef]
- Bener, A.; Ehlayel, M.S.; Tulic, M.K.; Hamid, Q. Vitamin D deficiency as a strong predictor of asthma in children. Int. Arch. Allergy Immunol. 2012, 157, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Garland, F.C.; Gorham, E.D.; Lipkin, M.; Newmark, H.; Mohr, S.B.; Holick, M.F. The role of vitamin D in cancer prevention. Am. J. Public Health 2006, 96, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Garland, C.F.; Gorham, E.D.; Mohr, S.B.; Garland, F.C. Vitamin D for cancer prevention: Global perspective. Ann. Epidemiol. 2009, 19, 468–483. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J. Randomized controlled trials of vitamin D and cancer incidence: A modeling study. PLoS ONE 2017, 12, e0176448. [Google Scholar] [CrossRef]
- Grant, W.B.; Boucher, B.J.; Bhattoa, H.P.; Lahore, H. Why vitamin D clinical trials should be based on 25-hydroxyvitamin D concentrations. J. Steroid Biochem. Mol. Biol. 2018, 177, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, P.; Quacquarelli, L. Hypovitaminosis D and aging: Is there a role in muscle and brain health? Nutrients 2020, 12, 628. [Google Scholar] [CrossRef]
- Giustina, A.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Lazaretti-Castro, M.; Lips, P.; Marcocci, C.; Bilezikian, J.P. Vitamin D in the older population: A consensus statement. Endocrine 2023, 79, 31–44. [Google Scholar] [CrossRef]
- Caccamo, D.; Ricca, S.; Curro, M.; Ientile, R. Health risks of hypovitaminosis D: A review of new molecular insights. Int. J. Mol. Sci. 2018, 19, 892. [Google Scholar] [CrossRef]
- Tikellis, C.; Thomas, M.C. Angiotensin-converting enzyme 2 (ACE2) Is a key modulator of the renin angiotensin system in health and disease. Int. J. Pept. 2012, 2012, 256294. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Alloubani, A.; Akhu-Zaheya, L.; Samara, R.; Abdulhafiz, I.; Saleh, A.; Altowijri, A. Relationship between vitamin D deficiency, diabetes, and obesity. Diabetes Metab. Syndr. 2019, 13, 1457–1461. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Zhou, L. Vitamin D deficiency, obesity and diabetes. Cell. Mol. Biol. 2015, 61, 35–38. [Google Scholar] [PubMed]
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Sarau, O.S.; Rachabattuni, H.C.; Gadde, S.T.; Daruvuri, S.P.; Marusca, L.M.; Horhat, F.G.; Fildan, A.P.; Tanase, E.; Prodan-Barbulescu, C.; Horhat, D.I. Exploring the preventive potential of vitamin D against respiratory infections in preschool-age children: A cross-sectional study. Nutrients 2024, 16, 1595. [Google Scholar] [CrossRef]
- Stagi, S.; Rigante, D.; Lepri, G.; Matucci Cerinic, M.; Falcini, F. Severe vitamin D deficiency in patients with Kawasaki disease: A potential role in the risk to develop heart vascular abnormalities? Clin. Rheumatol. 2016, 35, 1865–1872. [Google Scholar] [CrossRef]
- Okazaki, N.; Ikeda, H.; Honda, T.; Tsuno, K.; Inoue, F.; Takahashi, S.; Sakurai, A.; Ueki, H.; Noguchi, Y.; Hamada, H.; et al. The impact of vitamin D on the onset and progress of Kawasaki disease. Pediatr. Int. 2022, 64, e15191. [Google Scholar] [CrossRef]
- Karonova, T.L.; Andreeva, A.T.; Golovatuk, K.A.; Bykova, E.S.; Simanenkova, A.V.; Vashukova, M.A.; Grant, W.B.; Shlyakhto, E.V. Low 25(OH)D level is associated with severe course and poor prognosis in COVID-19. Nutrients 2021, 13, 3021. [Google Scholar] [CrossRef]
- AlGhatrif, M.; Tanaka, T.; Moore, A.Z.; Bandinelli, S.; Lakatta, E.G.; Ferrucci, L. Age-associated difference in circulating ACE2, the gateway for SARS-COV-2, in humans: Results from the InCHIANTI study. Geroscience 2021, 43, 619–627. [Google Scholar] [CrossRef]
- Marik, P.E.; Kory, P.; Varon, J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med. Drug Discov. 2020, 6, 100041. [Google Scholar] [CrossRef]
- Zuo, L.; Miller Juve, A. Transitioning to a new era: Future directions for staff development during COVID-19. Med. Educ. 2020, 55, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Suvankar, S.; Panda, V.K.; Pati, A.; Panda, A.K. Lower levels of vitamin D are associated with SARS-CoV-2 infection and mortality in the Indian population: An observational study. Int. Immunopharmacol. 2020, 88, 107001. [Google Scholar] [CrossRef] [PubMed]
- Martin Gimenez, V.M.; Inserra, F.; Ferder, L.; Garcia, J.; Manucha, W. Vitamin D deficiency in African Americans is associated with a high risk of severe disease and mortality by SARS-CoV-2. J. Hum. Hypertens. 2021, 35, 378–380. [Google Scholar] [CrossRef]
- Udaya Kumar, V.; Pavan, G.; Murti, K.; Kumar, R.; Dhingra, S.; Haque, M.; Ravichandiran, V. Rays of immunity: Role of sunshine vitamin in management of COVID-19 infection and associated comorbidities. Clin. Nutr. ESPEN 2021, 46, 21–32. [Google Scholar] [CrossRef]
- Alguwaihes, A.M.; Sabico, S.; Hasanato, R.; Al-Sofiani, M.E.; Megdad, M.; Albader, S.S.; Alsari, M.H.; Alelayan, A.; Alyusuf, E.Y.; Alzahrani, S.H.; et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: A retrospective case-control study in an Arab Gulf country. Aging Clin. Exp. Res. 2021, 33, 1415–1422. [Google Scholar] [CrossRef]
- Mishra, P.; Parveen, R.; Bajpai, R.; Agarwal, N. Vitamin D deficiency and comorbidities as risk factors of COVID-19 Infection: A systematic review and meta-analysis. J. Prev. Med. Public Health 2022, 55, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Alberca, G.G.F.; Alberca, R.W. Role of vitamin D deficiency and comorbidities in COVID-19. World J. Virol. 2022, 11, 85–89. [Google Scholar] [CrossRef]
- Polonowita, A.; Wimalawansa, S. The impact of withholding cost-effective early treatments, such as vitamin D, on COVID-19: An analysis using an innovative logical paradigm. World J. Adv. Pharm. Life Sci. 2023, 5, 13–34. [Google Scholar] [CrossRef]
- Tenali, N.; Babu, G.R.M. A systematic literature review and future perspectives for handling big data analytics in COVID-19 diagnosis. New Gener. Comput. 2023, 41, 243–280. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Decoding the paradox: Understanding elevated hospitalization and reduced mortality in SARS-CoV-2 variants. Int. J. Front. in Sci. Technol. Res. 2024, 6, 1–20. [Google Scholar] [CrossRef]
- Nafilyan, V.; Bermingham, C.R.; Ward, I.L.; Morgan, J.; Zaccardi, F.; Khunti, K.; Stanborough, J.; Banerjee, A.; Doidge, J.C. Risk of death following COVID-19 vaccination or positive SARS-CoV-2 test in young people in England. Nat. Commun. 2023, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Israel, A.; Cicurel, A.; Feldhamer, I.; Stern, F.; Dror, Y.; Giveon, S.M.; Gillis, D.; Strich, D.; Lavie, G. Vitamin D deficiency is associated with higher risks for SARS-CoV-2 infection and COVID-19 severity: A retrospective case-control study. Intern. Emerg. Med. 2022, 17, 1053–1063. [Google Scholar] [CrossRef]
- Menshawey, E.; Menshawey, R.; Nabeh, O.A. Shedding light on vitamin D: The shared mechanistic and pathophysiological role between hypovitaminosis D and COVID-19 risk factors and complications. Inflammopharmacology 2021, 29, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef]
- Zeng, C.; Evans, J.P.; King, T.; Zheng, Y.M.; Oltz, E.M.; Whelan, S.P.J.; Saif, L.J.; Peeples, M.E.; Liu, S.L. SARS-CoV-2 spreads through cell-to-cell transmission. Proc. Natl. Acad. Sci. USA 2022, 119, e2111400119. [Google Scholar] [CrossRef]
- Pereira, G.J.; Hirata, H.; Fimia, G.M.; do Carmo, L.G.; Bincoletto, C.; Han, S.W.; Stilhano, R.S.; Ureshino, R.P.; Bloor-Young, D.; Churchill, G.; et al. Nicotinic acid adenine dinucleotide phosphate (NAADP) regulates autophagy in cultured astrocytes. J. Biol. Chem. 2011, 286, 27875–27881. [Google Scholar] [CrossRef]
- Sarzani, R.; Giulietti, F.; Di Pentima, C.; Giordano, P.; Spannella, F. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L325–L336. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef] [PubMed]
- Notz, Q.; Herrmann, J.; Schlesinger, T.; Kranke, P.; Sitter, M.; Helmer, P.; Stumpner, J.; Roeder, D.; Amrein, K.; Stoppe, C.; et al. Vitamin D deficiency in critically ill COVID-19 ARDS patients. Clin. Nutr. 2022, 41, 3089–3095. [Google Scholar] [CrossRef] [PubMed]
- Faul, J.L.; Kerley, C.P.; Love, B.; O’Neill, E.; Cody, C.; Tormey, W.; Hutchinson, K.; Cormican, L.J.; Burke, C.M. Vitamin D deficiency and ARDS after SARS-CoV-2 infection. Ir. Med. J. 2020, 113, 84. [Google Scholar]
- Dancer, R.C.; Parekh, D.; Lax, S.; D’Souza, V.; Zheng, S.; Bassford, C.R.; Park, D.; Bartis, D.G.; Mahida, R.; Turner, A.M.; et al. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax 2015, 70, 617–624. [Google Scholar] [CrossRef]
- Skultetyova, D.; Filipova, S.; Riecansky, I.; Skultety, J. The role of angiotensin type 1 receptor in inflammation and endothelial dysfunction. Recent. Pat. Cardiovasc. Drug Discov. 2007, 2, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, Y.G. Vitamin D receptor influences Intestinal barriers in health and disease. Cells 2022, 11, 1129. [Google Scholar] [CrossRef]
- Danser, A.H.J.; Epstein, M.; Batlle, D. Renin-angiotensin system blockers and the COVID-19 pandemic: At present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 2020, 75, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.X.; Xie, L.; Wu, H.J.; Jiang, J.; Zhao, L.; Dong, H.; Yang, L.Y.; Qiu, H. The clinical characteristics and influencing factors of patients with severe COVID-19. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2021, 29, 1295–1300. [Google Scholar] [CrossRef]
- Li, Y.C.; Kong, J.; Wei, M.; Chen, Z.F.; Liu, S.Q.; Cao, L.P. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J. Clin. Investig. 2002, 110, 229–238. [Google Scholar] [CrossRef]
- Forman, J.P.; Williams, J.S.; Fisher, N.D. Plasma 25-hydroxyvitamin D and regulation of the renin-angiotensin system in humans. Hypertension 2010, 55, 1283–1288. [Google Scholar] [CrossRef]
- Scragg, R.; Sowers, M.; Bell, C. Serum 25-hydroxyvitamin D, ethnicity, and blood pressure in the Third National Health and Nutrition Examination Survey. Am. J. Hypertens. 2007, 20, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Hintzpeter, B.; Mensink, G.B.; Thierfelder, W.; Muller, M.J.; Scheidt-Nave, C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008, 62, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Bessa, A.S.M.; Jesus, E.F.; Nunes, A.D.C.; Pontes, C.N.R.; Lacerda, I.S.; Costa, J.M.; Souza, E.J.; Lino-Junior, R.S.; Biancardi, M.F.; Dos Santos, F.C.A.; et al. Stimulation of the ACE2/Ang-(1–7)/Mas axis in hypertensive pregnant rats attenuates cardiovascular dysfunction in adult male offspring. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2019, 42, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, V.; Singh, J.; Tiwari, P.; Chaturvedi, S.; Gupta, S.; Mishra, A.; Singh, S.; Wahajuddin, M.; Hanif, K.; Shukla, S. ACE2/ANG-(1–7)/Mas receptor axis activation prevents inflammation and improves cognitive functions in streptozotocin induced rat model of Alzheimer’s disease-like phenotypes. Eur. J. Pharmacol. 2023, 946, 175623. [Google Scholar] [CrossRef]
- Lin, S.; Pan, H.; Wu, H.; Ren, D.; Lu, J. Role of the ACE2-Ang-(1–7)-Mas axis in blood pressure regulation and its potential as an antihypertensive in functional foods. Mol. Med. Rep. 2017, 16, 4403–4412. [Google Scholar] [CrossRef]
- Sahu, S.; Patil, C.R.; Kumar, S.; Apparsundaram, S.; Goyal, R.K. Role of ACE2-Ang (1–7)-Mas axis in post-COVID-19 complications and its dietary modulation. Mol. Cell. Biochem. 2022, 477, 225–240. [Google Scholar] [CrossRef]
- Wang, K.; Chen, W.; Zhang, Z.; Deng, Y.; Lian, J.Q.; Du, P.; Wei, D.; Zhang, Y.; Sun, X.X.; Gong, L.; et al. CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 2020, 5, 283. [Google Scholar] [CrossRef]
- Chambers, P. Basigin binds Spike S on SARS-CoV2. Sci. Res. 2021, 8, 1–3. [Google Scholar] [CrossRef]
- Fenizia, C.; Galbiati, S.; Vanetti, C.; Vago, R.; Clerici, M.; Tacchetti, C.; Daniele, T. SARS-CoV-2 entry: At the crossroads of CD147 and ACE2. Cells 2021, 10, 1434. [Google Scholar] [CrossRef]
- Song, X.; Hu, W.; Yu, H.; Zhao, L.; Zhao, Y.; Zhao, X.; Xue, H.H.; Zhao, Y. Little to no expression of angiotensin-converting enzyme-2 on most human peripheral blood immune cells but highly expressed on tissue macrophages. Cytom. A 2023, 103, 136–145. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for SARS-CoV-2. Nat. Rev. Microbiol. 2021, 19, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Alipoor, S.D.; Mirsaeidi, M. SARS-CoV-2 cell entry beyond the ACE2 receptor. Mol. Biol. Rep. 2022, 49, 10715–10727. [Google Scholar] [CrossRef] [PubMed]
- Neerukonda, S.N.; Vassell, R.; Herrup, R.; Liu, S.; Wang, T.; Takeda, K.; Yang, Y.; Lin, T.L.; Wang, W.; Weiss, C.D. Establishment of a well-characterized SARS-CoV-2 lentiviral pseudovirus neutralization assay using 293T cells with stable expression of ACE2 and TMPRSS2. PLoS ONE 2021, 16, e0248348. [Google Scholar] [CrossRef]
- Mahoney, M.; Damalanka, V.C.; Tartell, M.A.; Chung, D.H.; Lourenco, A.L.; Pwee, D.; Mayer Bridwell, A.E.; Hoffmann, M.; Voss, J.; Karmakar, P.; et al. A novel class of TMPRSS2 inhibitors potently block SARS-CoV-2 and MERS-CoV viral entry and protect human epithelial lung cells. Proc. Natl. Acad. Sci. USA 2021, 118, e2108728118. [Google Scholar] [CrossRef]
- Davidson, A.M.; Wysocki, J.; Batlle, D. Interaction of SARS-CoV-2 and other coronavirus with ACE (angiotensin-converting enzyme)-2 as their main receptor: Therapeutic implications. Hypertension 2020, 76, 1339–1349. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.; Shurbaji, S. Do changes in ACE-2 expression affect SARS-CoV-2 virulence and related complications: A closer Look into nembrane-bound and soluble forms. Int. J. Mol. Sci. 2021, 22, 6703. [Google Scholar] [CrossRef]
- Wei, L.; Liu, S.; Lu, S.; Luo, S.; An, X.; Fan, H.; Chen, W.; Li, E.; Tong, Y.; Song, L. Lethal infection of human ACE2-transgenic mice caused by SARS-CoV-2-related Pangolin coronavirus GX_P2V. BioRxiv 2024. [Google Scholar] [CrossRef]
- Yeung, M.L.; Teng, J.L.L.; Jia, L.; Zhang, C.; Huang, C.; Cai, J.P.; Zhou, R.; Chan, K.H.; Zhao, H.; Zhu, L.; et al. Soluble ACE2-mediated cell entry of SARS-CoV-2 via interaction with proteins related to the renin-angiotensin system. Cell 2021, 184, 2212–2228.e2212. [Google Scholar] [CrossRef] [PubMed]
- South, A.M.; Brady, T.M.; Flynn, J.T. ACE2 (angiotensin-converting enzyme 2), COVID-19, and ACE inhibitor and Ang II (angiotensin II) receptor blocker use during the pandemic: The pediatric perspective. Hypertension 2020, 76, 16–22. [Google Scholar] [CrossRef]
- Hou, Y.; Zhao, J.; Martin, W.; Kallianpur, A.; Chung, M.K.; Jehi, L.; Sharifi, N.; Erzurum, S.; Eng, C.; Cheng, F. New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020, 18, 216. [Google Scholar] [CrossRef]
- Buitrago, C.G.; Pardo, V.G.; de Boland, A.R.; Boland, R. Activation of RAF-1 through Ras and protein kinase Calpha mediates 1alpha,25(OH)2-vitamin D3 regulation of the mitogen-activated protein kinase pathway in muscle cells. J. Biol. Chem. 2003, 278, 2199–2205. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, R.; Votto, M.; Licari, A.; Brambilla, I.; Bruno, R.; Perlini, S.; Rovida, F.; Baldanti, F.; Marseglia, G.L. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review. JAMA Pediatr. 2020, 174, 882–889. [Google Scholar] [CrossRef] [PubMed]
- Bermejo-Jambrina, M.; Eder, J.; Kaptein, T.M.; van Hamme, J.L.; Helgers, L.C.; Vlaming, K.E.; Brouwer, P.J.M.; van Nuenen, A.C.; Spaargaren, M.; de Bree, G.J.; et al. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J. 2021, 40, e106765. [Google Scholar] [CrossRef]
- Mehrabadi, M.E.; Hemmati, R.; Tashakor, A.; Homaei, A.; Yousefzadeh, M.; Hemati, K.; Hosseinkhani, S. Induced dysregulation of ACE2 by SARS-CoV-2 plays a key role in COVID-19 severity. Biomed. Pharmacother. 2021, 137, 111363. [Google Scholar] [CrossRef]
- de Queiroz, T.M.; Lakkappa, N.; Lazartigues, E. ADAM17-mMediated shedding of inflammatory cytokines in hypertension. Front. Pharmacol. 2020, 11, 1154. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, J.; Schiavon, C.R.; He, M.; Chen, L.; Shen, H.; Zhang, Y.; Yin, Q.; Cho, Y.; Andrade, L.; et al. SARS-CoV-2 Spike Protein impairs endothelial function via downregulation of ACE 2. Circ. Res. 2021, 128, 1323–1326. [Google Scholar] [CrossRef]
- Hedges, J.F.; Snyder, D.T.; Robison, A.; Grifka-Walk, H.M.; Blackwell, K.; Shepardson, K.; Kominsky, D.; Rynda-Apple, A.; Walcheck, B.; Jutila, M.A. An ADAM17-neutralizing antibody reduces inflammation and mortality while increasing viral burden in a COVID-19 mouse model. Front. Immunol. 2022, 13, 918881. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Yamamoto, N.; Nakai-Murakami, C.; Osawa, Y.; Tokunaga, K.; Sata, T.; Yamamoto, N.; Sasazuki, T.; Ishizaka, Y. Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. USA 2008, 105, 7809–7814. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Aleya, L.; Sehgal, A.; Singh, S.; Sharma, N.; Bhatia, S.; Al-Harrasi, A.; Bungau, S. CD147-spike protein interaction in COVID-19: Get the ball rolling with a novel receptor and therapeutic target. Sci. Total Environ. 2022, 808, 152072. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kanno, H.; Zhou, Y.; Xiao, T.H.; Suzuki, T.; Ibayashi, Y.; Harmon, J.; Takizawa, S.; Hiramatsu, K.; Nitta, N.; et al. Massive image-based single-cell profiling reveals high levels of circulating platelet aggregates in patients with COVID-19. Nat. Commun. 2021, 12, 7135. [Google Scholar] [CrossRef]
- Bunyavanich, S.; Do, A.; Vicencio, A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020, 323, 2427–2429. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R. Notable developments for vitamin D Amid the COVID-19 pandemic, but caution warranted overall: A narrative review. Nutrients 2021, 13, 740. [Google Scholar] [CrossRef] [PubMed]
- Jude, E.B.; Tentolouris, N.; Rastogi, A.; Yap, M.H.; Pedrosa, H.C.; Ling, S.F. Vitamin D prescribing practices among clinical practitioners during the COVID-19 pandemic. Health Sci. Rep. 2022, 5, e691. [Google Scholar] [CrossRef]
- Bryant, A.; Lawrie, T.A.; Dowswell, T.; Fordham, E.J.; Mitchell, S.; Hill, S.R.; Tham, T.C. Ivermectin for prevention and treatment of COVID-19 infection: A systematic review, meta-analysis, and trial sequential analysis to inform clinical guidelines. Am. J. Ther. 2021, 28, e434–e460. [Google Scholar] [CrossRef]
- Chowdhury, A.; Sajid, M.; Jahan, N.; Adelusi, T.I.; Maitra, P.; Yin, G.; Wu, X.; Gao, Y.; Wang, S. A secondary approach with conventional medicines and supplements to recuperate current COVID-19 status. Biomed. Pharmacother. 2021, 142, 111956. [Google Scholar] [CrossRef]
- FDA. Emergency Use Authorization for Vaccines Explained. Available online: https://www.fda.gov/vaccines-blood-biologics/vaccines/emergency-use-authorization-vaccines-explained (accessed on 22 May 2023).
- Polonowita, A.; Wimalawansa, S.J. Molecular quantum and logic process of consciousness—Vitamin D big-data in COVID-19—A case for incorporating machine learning in medicine. Euro. J. Biomed. Pharma. Sci. 2023, 10, 24–43. [Google Scholar] [CrossRef]
- Viana, S.D.; Nunes, S.; Reis, F. ACE2 imbalance as a key player for the poor outcomes in COVID-19 patients with age-related comorbidities—Role of gut microbiota dysbiosis. Ageing Res. Rev. 2020, 62, 101123. [Google Scholar] [CrossRef]
- Sawalha, A.H.; Zhao, M.; Coit, P.; Lu, Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Beyerstedt, S.; Casaro, E.B.; Rangel, E.B. COVID-19: Angiotensin-converting enzyme 2 (ACE2) expression and tissue susceptibility to SARS-CoV-2 infection. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 905–919. [Google Scholar] [CrossRef]
- Dal Canto, E.; Beulens, J.W.J.; Elders, P.; Rutters, F.; Stehouwer, C.D.A.; van der Heijden, A.A.; van Ballegooijen, A.J. The association of vitamin D and vitamin K status with subclinical measures of cardiovascular health and all-cause mortality in older adults: The hoorn study. J. Nutr. 2020, 150, 3171–3179. [Google Scholar] [CrossRef]
- van Ballegooijen, A.J.; Beulens, J.W.J.; Kieneker, L.M.; de Borst, M.H.; Gansevoort, R.T.; Kema, I.P.; Schurgers, L.J.; Vervloet, M.G.; Bakker, S.J.L. Combined low vitamin D and K status amplifies mortality risk: A prospective study. Eur. J. Nutr. 2021, 60, 1645–1654. [Google Scholar] [CrossRef] [PubMed]
- Cariolou, M.; Cupp, M.A.; Evangelou, E.; Tzoulaki, I.; Berlanga-Taylor, A.J. Importance of vitamin D in acute and critically ill children with subgroup analyses of sepsis and respiratory tract infections: A systematic review and meta-analysis. BMJ Open 2019, 9, e027666. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, Z.; Tang, R.; Ouyang, S.; Li, S.; Wu, J. Vitamin D inhibits palmitate-induced macrophage pro-inflammatory cytokine production by targeting the MAPK pathway. Immunol. Lett. 2018, 202, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Qayyum, S.; Mohammad, T.; Slominski, R.M.; Hassan, M.I.; Tuckey, R.C.; Raman, C.; Slominski, A.T. Vitamin D and lumisterol novel metabolites can inhibit SARS-CoV-2 replication machinery enzymes. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E246–E251. [Google Scholar] [CrossRef]
- Diaz, J.H. Hypothesis: Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may increase the risk of severe COVID-19. J. Travel. Med. 2020, 27, taaa041. [Google Scholar] [CrossRef]
- Akhtar, S.; Benter, I.F.; Danjuma, M.I.; Doi, S.A.R.; Hasan, S.S.; Habib, A.M. Pharmacotherapy in COVID-19 patients: A review of ACE2-raising drugs and their clinical safety. J. Drug Target. 2020, 28, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Karnik, S.; Saef, J.; Bergmann, C.; Barnard, J.; Lederman, M.M.; Tilton, J.; Cheng, F.; Harding, C.V.; Young, J.B.; et al. SARS-CoV-2 and ACE2: The biology and clinical data settling the ARB and ACEI controversy. EBioMedicine 2020, 58, 102907. [Google Scholar] [CrossRef]
- Glasgow, A.; Glasgow, J.; Limonta, D.; Solomon, P.; Lui, I.; Zhang, Y.; Nix, M.A.; Rettko, N.J.; Zha, S.; Yamin, R.; et al. Engineered ACE2 receptor traps potently neutralize SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 28046–28055. [Google Scholar] [CrossRef]
- Bracken, C.J.; Lim, S.A.; Solomon, P.; Rettko, N.J.; Nguyen, D.P.; Zha, B.S.; Schaefer, K.; Byrnes, J.R.; Zhou, J.; Lui, I.; et al. Bi-paratopic and multivalent VH domains block ACE2 binding and neutralize SARS-CoV-2. Nat. Chem. Biol. 2021, 17, 113–121. [Google Scholar] [CrossRef]
- Aleksova, A.; Ferro, F.; Gagno, G.; Cappelletto, C.; Santon, D.; Rossi, M.; Ippolito, G.; Zumla, A.; Beltrami, A.P.; Sinagra, G. COVID-19 and renin-angiotensin system inhibition: Role of angiotensin converting enzyme 2 (ACE2)—Is there any scientific evidence for controversy? J. Intern. Med. 2020, 288, 410–421. [Google Scholar] [CrossRef]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).