Reduced Carbon Dioxide by Overexpressing EPSPS Transgene in Arabidopsis and Rice: Implications in Carbon Neutrality through Genetically Engineered Plants
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Gas-Exchange Measurements
2.3. RNA-Sequencing and Analysis
2.4. Confirmation of the RNA-Seq Data by Real Time Quantitative PCR (RT-qPCR)
2.5. Statistical Analysis
3. Results
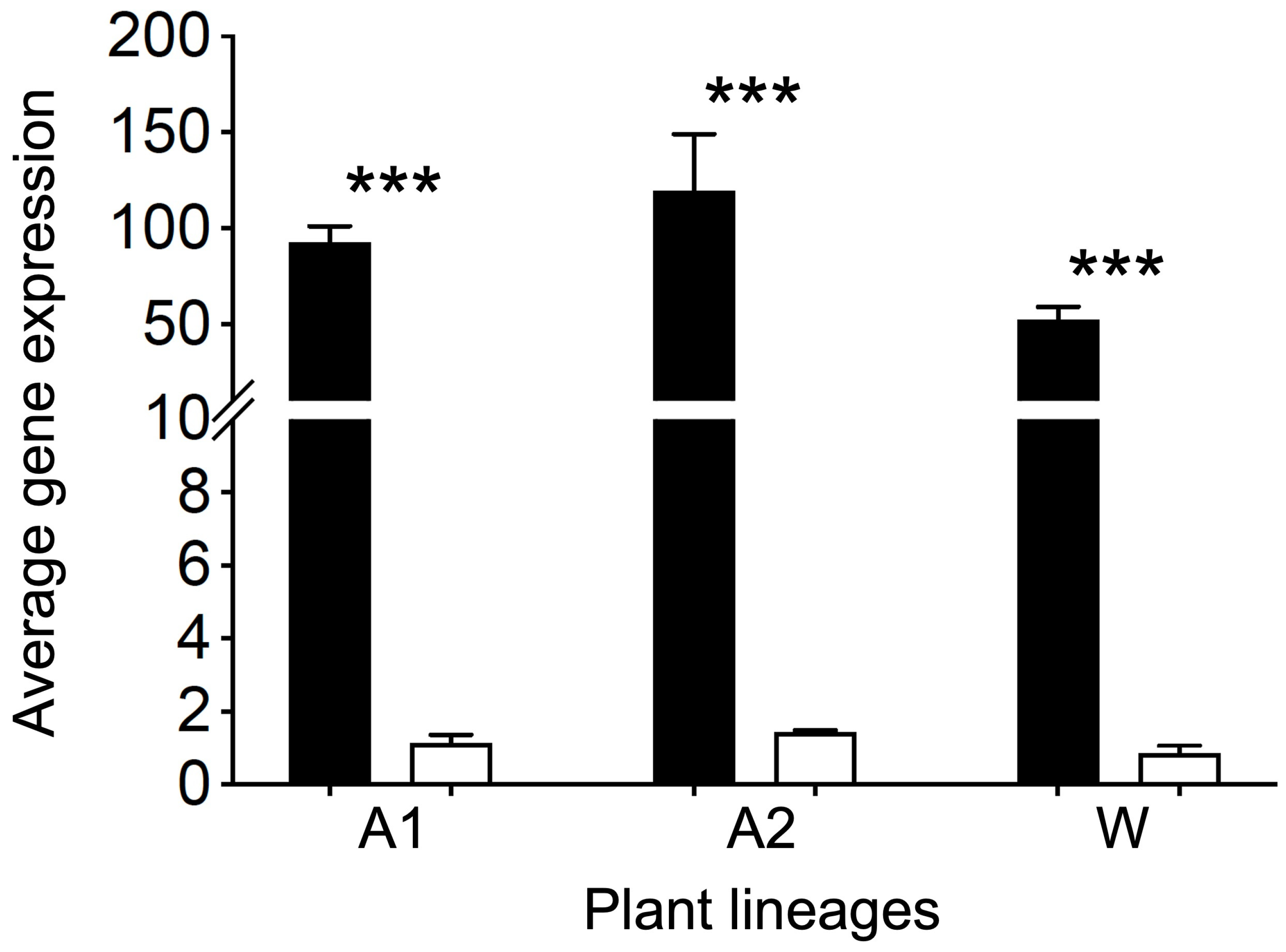
3.1. Increases in EPSPS Gene Expression and Carbon Consumption in EPSPS Tansgenic Arabidopsis and Rice Lineages
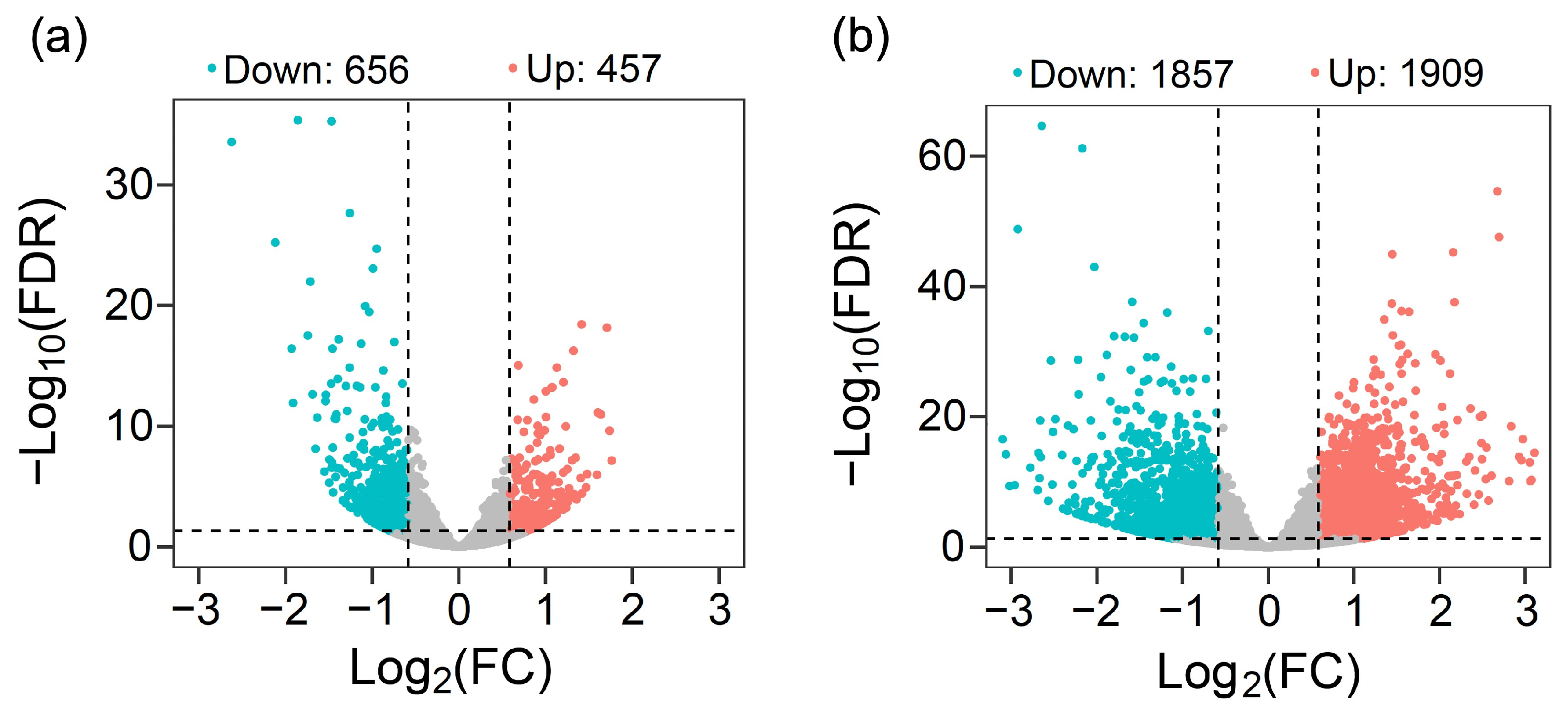
3.2. Pathways with Differential Gene Expression Caused by Overexpressing the EPSPS Gene between GE and Non-GE Arabidopsis Lineages
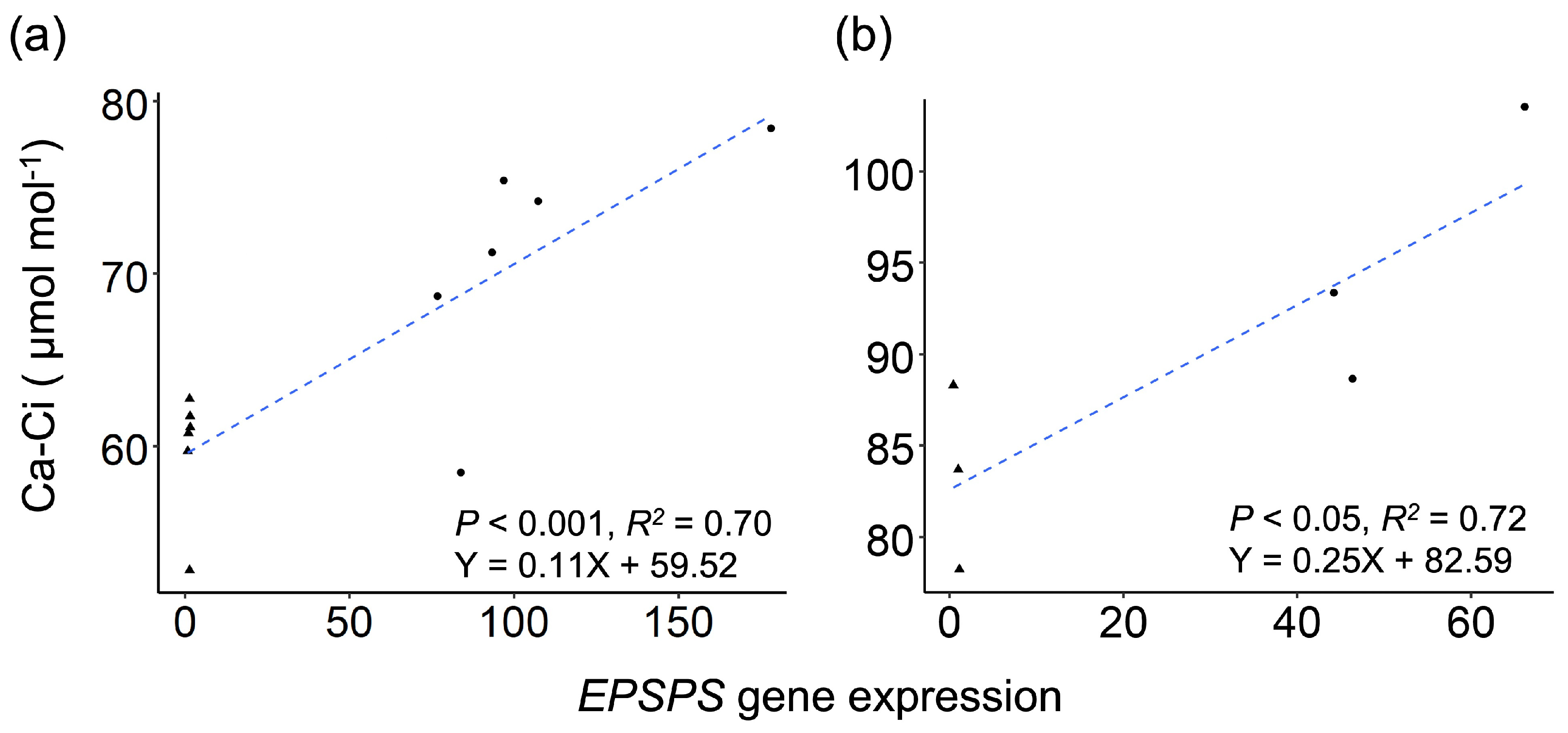
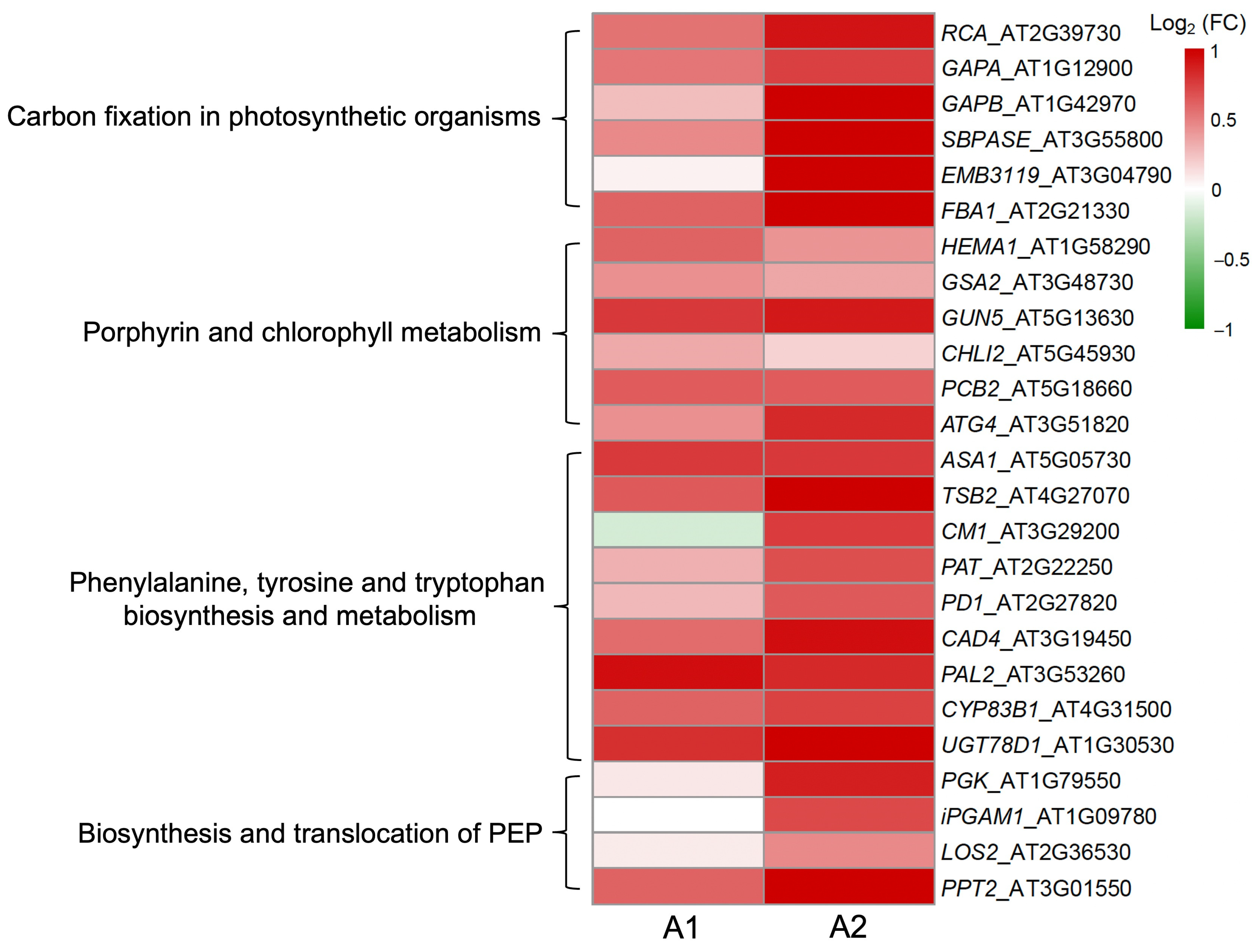
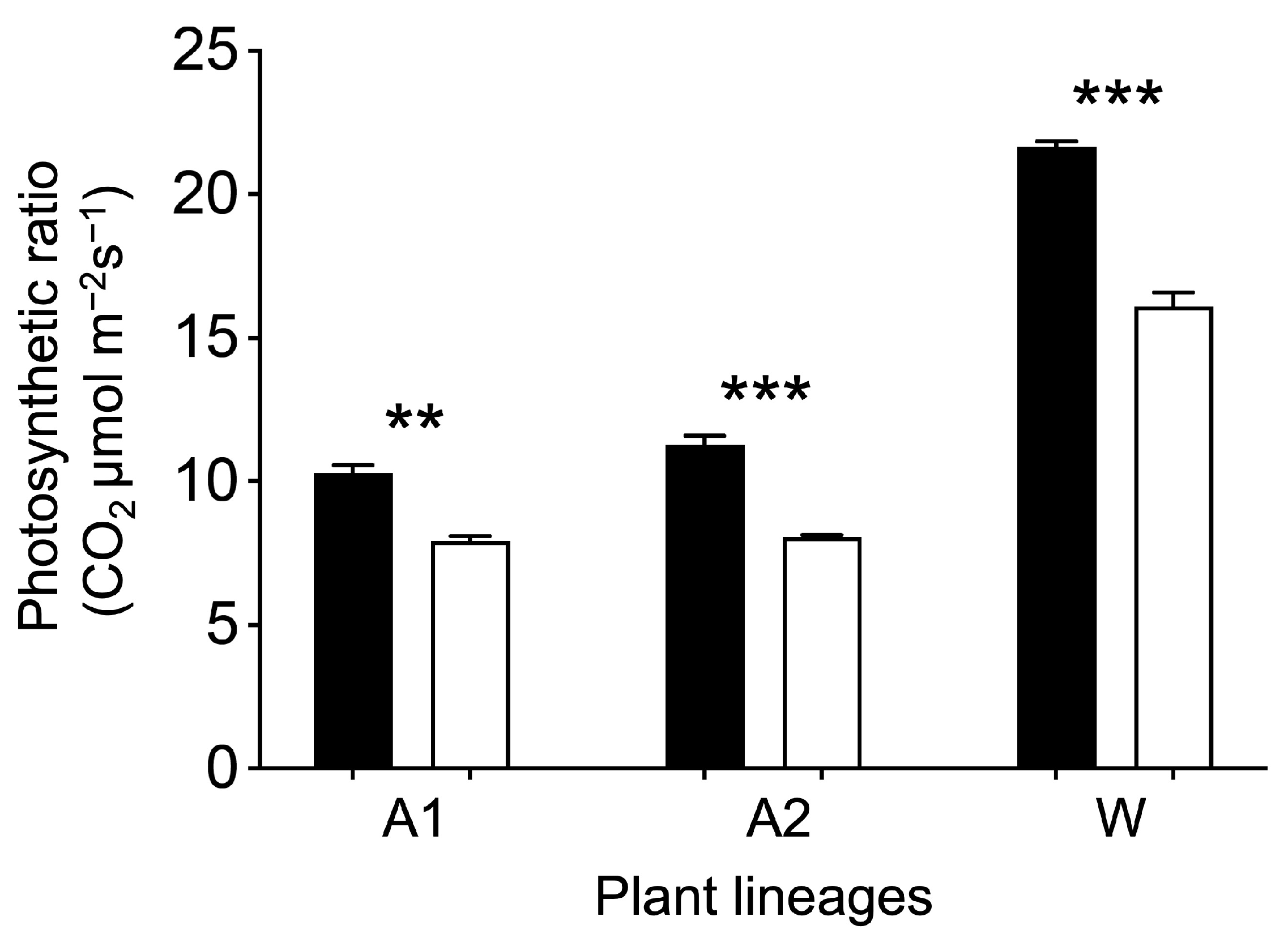
3.3. Increases in Expression of the Key Carbon-Fixation Genes and Photosynthetic Ratios of GE Arabidopsis and Rice Lineages
4. Discussion
4.1. Overexpressing the EPSPS Transgene Substantially Increased CO2 Consumption in Plants
4.2. Possible Mechanisms for Increased CO2 Consumption in Genetically Engineered Plants Overexpressing EPSPS Gene
4.3. Implications of Overexpressing EPSPS in Genetically Engineered Plants for Carbon Neutrality
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Landman, W.A. Climate change 2007: The physical science basis. S Afr. Geogr. J. 2010, 92, 86–87. [Google Scholar] [CrossRef]
- Glemarec, Y. Aligning national interests and global climate justice: The role of human rights in enhancing the ambition of nationally determined contributions to combat climate change. Fudan J. Hum. Soc. Sci. 2019, 12, 309–327. [Google Scholar] [CrossRef]
- Keohane, R.O. Institutions for a world of climate injustice. Fudan J. Hum. Soc. Sci. 2019, 12, 293–307. [Google Scholar] [CrossRef]
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef]
- Li, Y.; Yu, L.; Chen, L.; Han, C.; Jiang, H.; Liu, Z.; Zheng, N.; Wang, J.; Sun, M.; Yang, R.; et al. Subtle side chain triggers unexpected two-channel charge transport property enabling 80% fill factors and efficient thick-film organic photovoltaics. Innovation 2021, 2, 100090. [Google Scholar] [CrossRef]
- Sherman, P.; Chen, X.; McElroy, M. Offshore wind: An opportunity for cost-competitive decarbonization of China’s energy economy. Sci. Adv. 2020, 6, eaax9571. [Google Scholar] [CrossRef]
- Li, C.; Chen, Z.; Hu, Y.; Cai, C.; Zuo, X.; Shang, G.; Lin, H. The energy conservation and emission reduction co-benefits of China’s emission trading system. Sci. Rep. 2023, 13, 13758. [Google Scholar] [CrossRef]
- Kang, Z.; Liao, Q.; Zhang, Z.; Zhang, Y. Carbon neutrality orientates the reform of the steel industry. Nat. Mater. 2022, 21, 1094–1098. [Google Scholar] [CrossRef]
- Jiao, N.; Herndl, G.J.; Hansell, D.A.; Benner, R.; Kattner, G.; Wilhelm, S.W.; Kirchman, D.L.; Weinbauer, M.G.; Luo, T.; Chen, F.; et al. Microbial production of recalcitrant dissolved organic matter: Long-term carbon storage in the global ocean. Nat. Rev. Microbiol. 2010, 8, 593–599. [Google Scholar] [CrossRef]
- Bolan, N.S.; Kunhikrishnan, A.; Choppala, G.K.; Thangarajan, R.; Chung, J.W. Stabilization of carbon in composts and biochars in relation to carbon sequestration and soil fertility. Sci. Total Environ. 2012, 424, 264–270. [Google Scholar] [CrossRef]
- Lu, X.; Vitousek, P.M.; Mao, Q.; Gilliam, F.S.; Luo, Y.; Turner, B.L.; Zhou, G.; Mo, J. Nitrogen deposition accelerates soil carbon sequestration in tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2020790118. [Google Scholar] [CrossRef] [PubMed]
- Beerling, D.J.; Kantzas, E.P.; Lomas, M.R.; Wade, P.; Eufrasio, R.M.; Renforth, P.; Sarkar, B.; Andrews, M.G.; James, R.H.; Pearce, C.R.; et al. Potential for large-scale CO2 removal via enhanced rock weathering with croplands. Nature 2020, 583, 242–248. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Tian, H.; Wu, X.; Gao, Z.; Feng, Y.; Piao, S.; Lv, N.; Pan, N.; Fu, B. Accelerated increase in vegetation carbon sequestration in China after 2010: A turning point resulting from climate and human interaction. Glob. Chang. Biol. 2021, 27, 5848–5864. [Google Scholar] [CrossRef] [PubMed]
- Terrer, C.; Phillips, R.P.; Hungate, B.A.; Rosende, J.; Pett-Ridge, J.; Craig, M.E.; van Groenigen, K.J.; Keenan, T.F.; Sulman, B.N.; Stocker, B.D.; et al. A trade-off between plant and soil carbon storage under elevated CO2. Nature 2021, 591, 599–603. [Google Scholar] [CrossRef]
- Keenan, T.F.; Williams, C.A. The terrestrial carbon sink. Annu. Rev. Environ. Resour. 2018, 43, 219–243. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Pugh, T.A.M.; Lindeskog, M.; Smith, B.; Poulter, B.; Arneth, A.; Haverd, V.; Calle, L. Role of forest regrowth in global carbon sink dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 4382–4387. [Google Scholar] [CrossRef]
- Yang, Y.; Shi, Y.; Sun, W.; Chang, J.; Zhu, J.; Chen, L.; Wang, X.; Guo, Y.; Zhang, H.; Yu, L.; et al. Terrestrial carbon sinks in China and around the world and their contribution to carbon neutrality. Sci. China Life Sci. 2022, 65, 861–895. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Wu, Y.S. The increase in the karstification-photosynthesis coupled carbon sink and its implication for carbon neutrality. Agronomy 2022, 12, 2147. [Google Scholar] [CrossRef]
- van Bel, A.J.E.; Offler, C.E.; Patrick, J.W. Photosynthesis and partitioning|sources and sinks. In Encyclopedia of Applied Plant Sciences; Thomas, B., Ed.; Elsevier: Oxford, UK, 2003; pp. 724–734. [Google Scholar]
- Vicca, S. Global vegetation’s CO2 uptake. Nat. Ecol. Evol. 2018, 2, 1840–1841. [Google Scholar] [CrossRef]
- Wang, W.; Xia, H.; Yang, X.; Xu, T.; Si, H.J.; Cai, X.X.; Wang, F.; Su, J.; Snow, A.A.; Lu, B.R. A novel 5-enolpyruvoylshikimate-3-phosphate (EPSP) synthase transgene for glyphosate resistance stimulates growth and fecundity in weedy rice (Oryza sativa) without herbicide. New Phytol. 2014, 202, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Achary, V.M.M.; Sheri, V.; Manna, M.; Panditi, V.; Borphukan, B.; Ram, B.; Agarwal, A.; Fartyal, D.; Teotia, D.; Masakapalli, S.K.; et al. Overexpression of improved EPSPS gene results in field level glyphosate tolerance and higher grain yield in rice. Plant Biotechnol. J. 2020, 18, 2504–2519. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, L.; Jiang, X.Q.; Wang, W.; Cai, X.X.; Su, J.; Wang, F.; Lu, B.R. Genetically engineered rice endogenous 5-enolpyruvoylshikimate-3-phosphate synthase (epsps) transgene alters phenology and fitness of crop-wild hybrid offspring. Sci. Rep. 2017, 7, 6834. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nan, P.; Gu, Z.Y.; Ge, X.C.; Feng, Y.Q.; Lu, B.R. Overexpressing exogenous 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) genes increases fecundity and auxin content of transgenic Arabidopsis plants. Front. Plant Sci. 2018, 9, 233. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Beres, Z.T.; Jin, L.; Parrish, J.T.; Zhao, W.; Mackey, D.; Snow, A.A. Effects of overexpressing a native gene encoding 5-enolpyruvylshikimate-3-phosphate synthase (EPSPS) on glyphosate resistance in Arabidopsis thaliana. PLoS ONE 2017, 12, e0175820. [Google Scholar]
- Herrmann, K.M. The shikimate pathway: Early steps in the biosynthesis of aromatic compounds. Plant Cell 1995, 7, 907–919. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jiang, X.Q.; Yang, X.; Lu, B.R. Increased longevity and dormancy of soil-buried seeds from advanced crop-wild rice hybrids overexpressing the EPSPS transgene. Biology 2021, 10, 562. [Google Scholar] [CrossRef]
- Xu, J.W.; Feng, D.J.; Li, X.G.; Chang, T.J.; Zhu, Z. Cloning of genomic DNA of rice 5-enolpyruvylshikimate 3-phosphate synthase gene and chromosomal localization of the gene. Sci. China Ser. C 2002, 45, 251–259. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Wang, X.H.; Chen, Y.; Xu, D.Q. Determinant of photosynthetic capacity in rice leaves under ambient air conditions. Photosynthetica 2005, 43, 273–276. [Google Scholar] [CrossRef]
- Wu, J.; Fang, J.; Cai, X.X.; Lu, B.R. Overexpressing 5-enolpyruvylshikimate-3-phosphate synthase gene increase lignin content of transgenic progeny derived from hybrids of EPSPS transgenic rice with weedy and wild rice. J. Fudan Univ. Nat. Sci. 2020, 59, 666–676. [Google Scholar]
- Flügge, U.I.; Hausler, R.E.; Ludewig, F.; Gierth, M. The role of transporters in supplying energy to plant plastids. J. Exp. Bot. 2011, 62, 2381–2392. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Voll, L.; Häusler, R.E.; Hecker, R.; Weber, A.; Weissenböck, G.; Fiene, G.; Waffenschmidt, S.; Flügge, U.I. The phenotype of the Arabidopsis cue1 mutant is not simply caused by a general restriction of the shikimate pathway. Plant J. 2003, 36, 301–317. [Google Scholar] [CrossRef]
- Heldt, H.; Piechulla, B. The Calvin cycle catalyzes photosynthetic CO2 assimilation. In Plant Biochemistry; Academic Press: London, UK, 2011; pp. 163–191. [Google Scholar]
- Spreitzer, R.J.; Salvucci, M.E. Rubisco: Structure, regulatory interactions, and possibilities for a better enzyme. Annu. Rev. Plant Biol. 2002, 53, 449–475. [Google Scholar] [CrossRef]
- Mate, C.J.; Hudson, G.S.; Voncaemmerer, S.; Evans, J.R.; Andrews, T.J. Reduction of ribulose bisphosphate carboxylase activase levels in tobacco (Nicotiana tabacum) by antisense RNA reduces ribulose bisphosphate carboxylase carbamylation and impairs photosynthesis. Plant Physiol. 1993, 102, 1119–1128. [Google Scholar] [CrossRef]
- Somerville, C.R.; Portis, A.R.; Ogren, W.L. A mutant of Arabidopsis thaliana which lacks activation of RuBP carboxylase In vivo. Plant Physiol. 1982, 70, 381–387. [Google Scholar] [CrossRef]
- Kurek, I.; Chang, T.K.; Bertain, S.M.; Madrigal, A.; Liu, L.; Lassner, M.W.; Zhu, G. Enhanced thermostability of Arabidopsis Rubisco activase improves photosynthesis and growth rates under moderate heat stress. Plant Cell 2007, 19, 3230–3241. [Google Scholar] [CrossRef]
- Suganami, M.; Suzuki, Y.; Tazoe, Y.; Yamori, W.; Makino, A. Co-overproducing Rubisco and Rubisco activase enhances photosynthesis in the optimal temperature range in rice. Plant Physiol. 2021, 185, 108–119. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ishiyama, K.; Sugawara, M.; Suzuki, Y.; Kondo, E.; Takegahara-Tamakawa, Y.; Yoon, D.K.; Suganami, M.; Wada, S.; Miyake, C.; et al. Overproduction of chloroplast glyceraldehyde-3-phosphate dehydrogenase improves photosynthesis alightly under elevated [CO2] conditions in rice. Plant Cell Physiol. 2021, 62, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Haake, V.; Zrenner, R.; Sonnewald, U.; Stitt, M. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the levels of sugars and starch, and inhibits growth of potato plants. Plant J. 1998, 14, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Haake, V.; Geiger, M.; Walch-Liu, P.; Engels, C.; Zrenner, R.; Stitt, M. Changes in aldolase activity in wild-type potato plants are important for acclimation to growth irradiance and carbon dioxide concentration, because plastid aldolase exerts control over the ambient rate of photosynthesis across a range of growth conditions. Plant J. 1999, 17, 479–489. [Google Scholar] [CrossRef]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Increased fructose 1,6-bisphosphate aldolase in plastids enhances growth and photosynthesis of tobacco plants. J. Exp. Bot. 2012, 63, 3001–3009. [Google Scholar] [CrossRef] [PubMed]
- Raines, C.A. The Calvin cycle revisited. Photosynth. Res. 2003, 75, 1–10. [Google Scholar] [CrossRef]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Davey, P.A.; Headland, L.R.; Lawson, T.; Timm, S.; Bauwe, H.; Raines, C.A. Simultaneous stimulation of sedoheptulose 1,7-bisphosphatase, fructose 1,6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol. J. 2017, 15, 805–816. [Google Scholar] [CrossRef]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.J.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef]
- Kobayashi, K.; Masuda, T. Transcriptional regulation of tetrapyrrole biosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1811. [Google Scholar] [CrossRef]
- Buttery, B.R.; Buzzell, R.I. The relationship between chlorophyll content and rate of photosynthesis in soybeans. Can. J. Plant Sci. 1977, 57, 1–5. [Google Scholar] [CrossRef]
- Hobbs, S.L.A.; Mahon, J.D. Inheritance of chlorophyll content, ribulose-l,5-bisphosphate carboxylase activity, and stomatal resistance in peas1. Crop Sci. 1985, 25, 1031–1034. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Chen, J.; Zheng, S.; Zhuang, C. Research Progress in Improving Photosynthetic Efficiency. Int. J. Mol. Sci. 2023, 24, 9286. [Google Scholar] [CrossRef] [PubMed]
- Kummari, D.; Palakolanu, S.R.; Kishor, P.B.K.; Bhatnagar-Mathur, P.; Singam, P.; Vadez, V.; Sharma, K.K. An update and perspectives on the use of promoters in plant genetic engineering. J. Biosci. 2020, 45, 119. [Google Scholar] [CrossRef]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016, 6, 32741. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Calcagno, P.E.; Brown, K.L.; Simkin, A.J.; Fisk, S.J.; Vialet-Chabrand, S.; Lawson, T.; Raines, C.A. Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat. Plants 2020, 6, 1054–1063. [Google Scholar] [CrossRef]
- Grogan, D.; Frolking, S.; Wisser, D.; Prusevich, A.; Glidden, S. Global gridded crop harvested area, production, yield, and monthly physical area data circa 2015. Sci Data 2022, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- Potapov, P.; Turubanova, S.; Hansen, M.C.; Tyukavina, A.; Zalles, V.; Khan, A.; Song, X.P.; Pickens, A.; Shen, Q.; Cortez, J. Global maps of cropland extent and change show accelerated cropland expansion in the twenty-first century. Nat. Food 2022, 3, 19–28. [Google Scholar] [CrossRef] [PubMed]
- ISAAA. ISAAA Brief 55-2019: Biotech Crops Drive Socio-Economic Development and Sustainable Environment in the New Frontier; International Service for the Acquisition of Agri-biotech Applications: Ithaca, NY, USA, 2019. [Google Scholar]
- Pickson, R.B.; He, G.; Boateng, E. Impacts of climate change on rice production: Evidence from 30 Chinese provinces. Environ. Dev. Sustain. 2021, 24, 3907–3925. [Google Scholar] [CrossRef]
- Yuan, S.; Linquist, B.A.; Wilson, L.T.; Cassman, K.G.; Stuart, A.M.; Pede, V.; Miro, B.; Saito, K.; Agustiani, N.; Aristya, V.E.; et al. Sustainable intensification for a larger global rice bowl. Nat. Commun. 2021, 12, 7163. [Google Scholar] [CrossRef]
- Abbasi, A.O.; Tang, X.; Harris, N.L.; Goldman, E.D.; Gamarra, J.G.P.; Herold, M.; Kim, H.S.; Luo, W.; Silva, C.A.; Tchebakova, N.M.; et al. Spatial database of planted forests in East Asia. Sci. Data 2023, 10, 480. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R. Carbon Sequestration in Grassland Soils. In Carbon Sequestration in Agricultural Ecosystems; Springer International Publishing: Cham, Switzerland, 2018; pp. 175–209. [Google Scholar]
- Hall, L.; Topinka, K.; Huffman, J.; Davis, L.; Good, A. Pollen flow between herbicide-resistant Brassica napus is the cause of multiple-resistant B. napus volunteers. Weed Sci. 2000, 48, 688–694. [Google Scholar] [CrossRef]
- Stewart, C.N.; Richards, H.A.; Halfhill, M.D. Transgenic plants and biosafety: Science, misconceptions and public perceptions. Biotechniques 2000, 29, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.R.; Snow, A.A. Gene flow from genetically modified rice and its environmental consequences. BioScience 2005, 55, 669–678. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, L.-X.; Li, N.; Yuan, Y.; Wang, Y.; Lu, B.-R. Reduced Carbon Dioxide by Overexpressing EPSPS Transgene in Arabidopsis and Rice: Implications in Carbon Neutrality through Genetically Engineered Plants. Biology 2024, 13, 25. https://doi.org/10.3390/biology13010025
Sun L-X, Li N, Yuan Y, Wang Y, Lu B-R. Reduced Carbon Dioxide by Overexpressing EPSPS Transgene in Arabidopsis and Rice: Implications in Carbon Neutrality through Genetically Engineered Plants. Biology. 2024; 13(1):25. https://doi.org/10.3390/biology13010025
Chicago/Turabian StyleSun, Li-Xue, Ning Li, Ye Yuan, Ying Wang, and Bao-Rong Lu. 2024. "Reduced Carbon Dioxide by Overexpressing EPSPS Transgene in Arabidopsis and Rice: Implications in Carbon Neutrality through Genetically Engineered Plants" Biology 13, no. 1: 25. https://doi.org/10.3390/biology13010025
APA StyleSun, L.-X., Li, N., Yuan, Y., Wang, Y., & Lu, B.-R. (2024). Reduced Carbon Dioxide by Overexpressing EPSPS Transgene in Arabidopsis and Rice: Implications in Carbon Neutrality through Genetically Engineered Plants. Biology, 13(1), 25. https://doi.org/10.3390/biology13010025






