Iodine Promotes Glucose Uptake through Akt Phosphorylation and Glut-4 in Adipocytes, but Higher Doses Induce Cytotoxic Effects in Pancreatic Beta Cells
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Culture Cells and Adipocytes Cell Differentiation
2.2. Viability MTT Assay, LD50 Calculation, Alpha-Amylase, and Caspase-3 Activities
2.3. Oxidative Stress and Acridine Orange/Ethidium Bromide Fluorescent Staining
2.4. Measurement of Glucose Uptake and Insulin Levels
2.5. Western Blotting
2.6. Determination of mRNA by RT-PCR
2.7. Statistical Analysis
3. Results
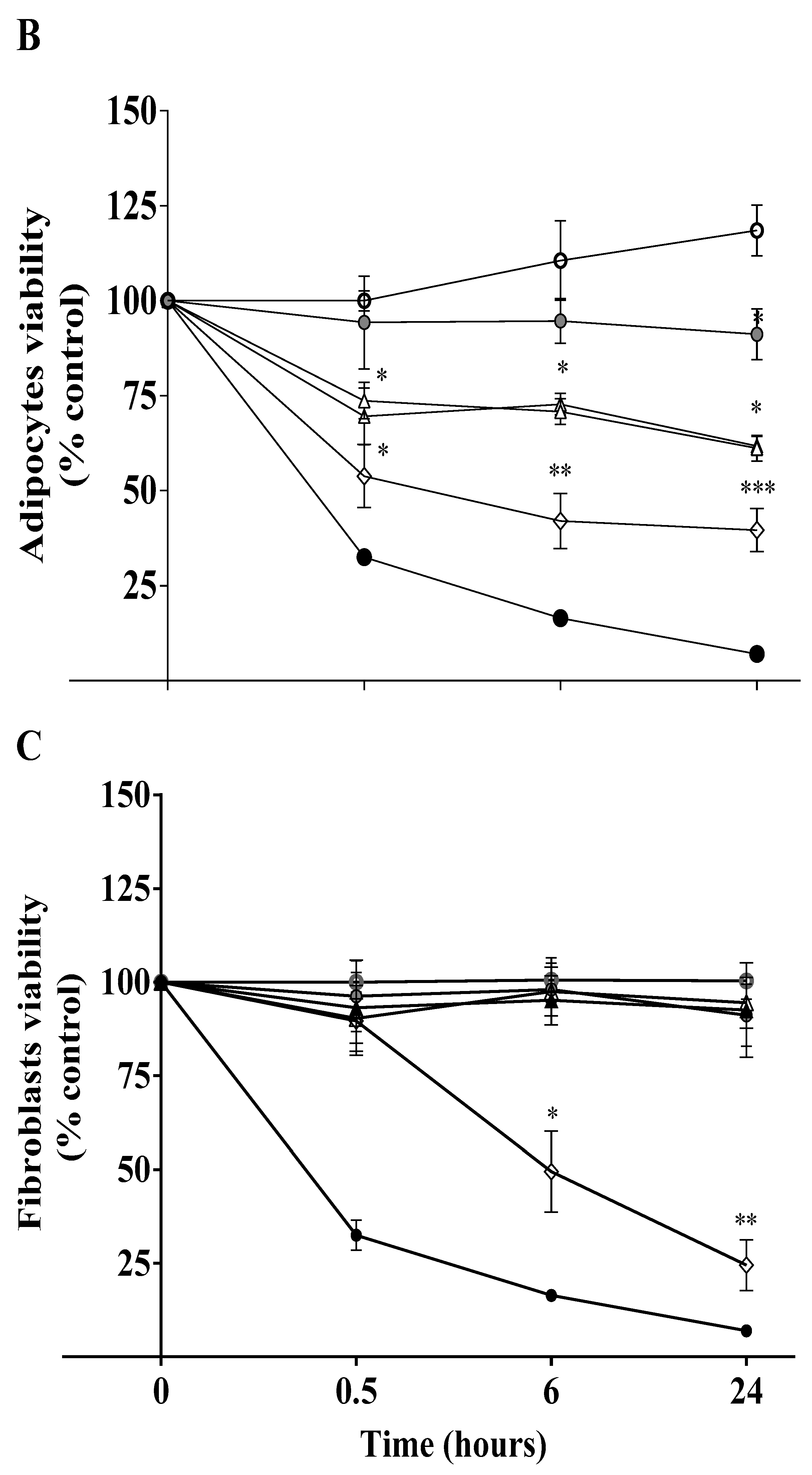
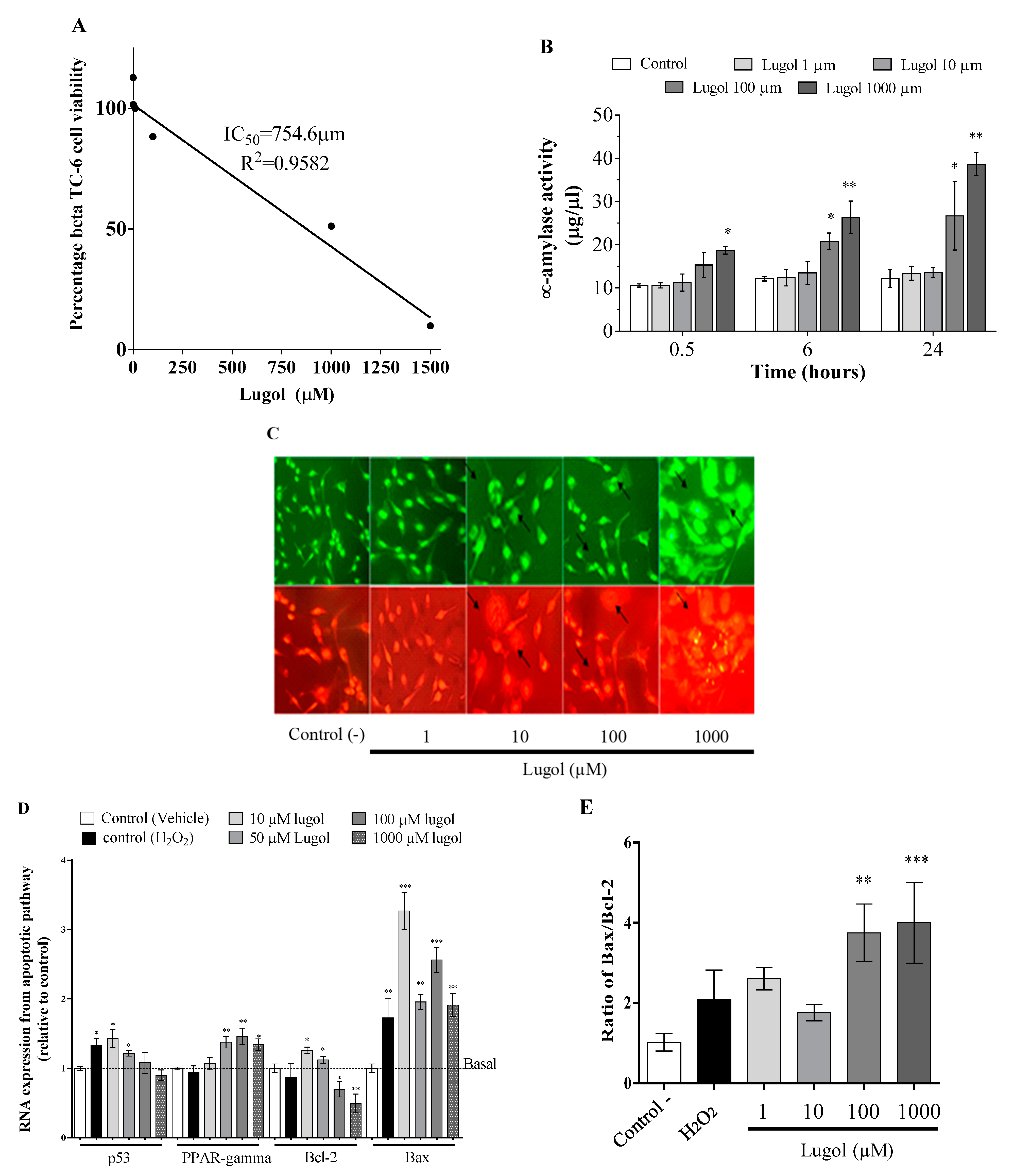
3.1. High Iodine Levels Induce Cytotoxic Effects by Increasing Oxidative Stress and Apoptosis in Mature Adipocytes and Pancreatic Beta-TC-6 Cells
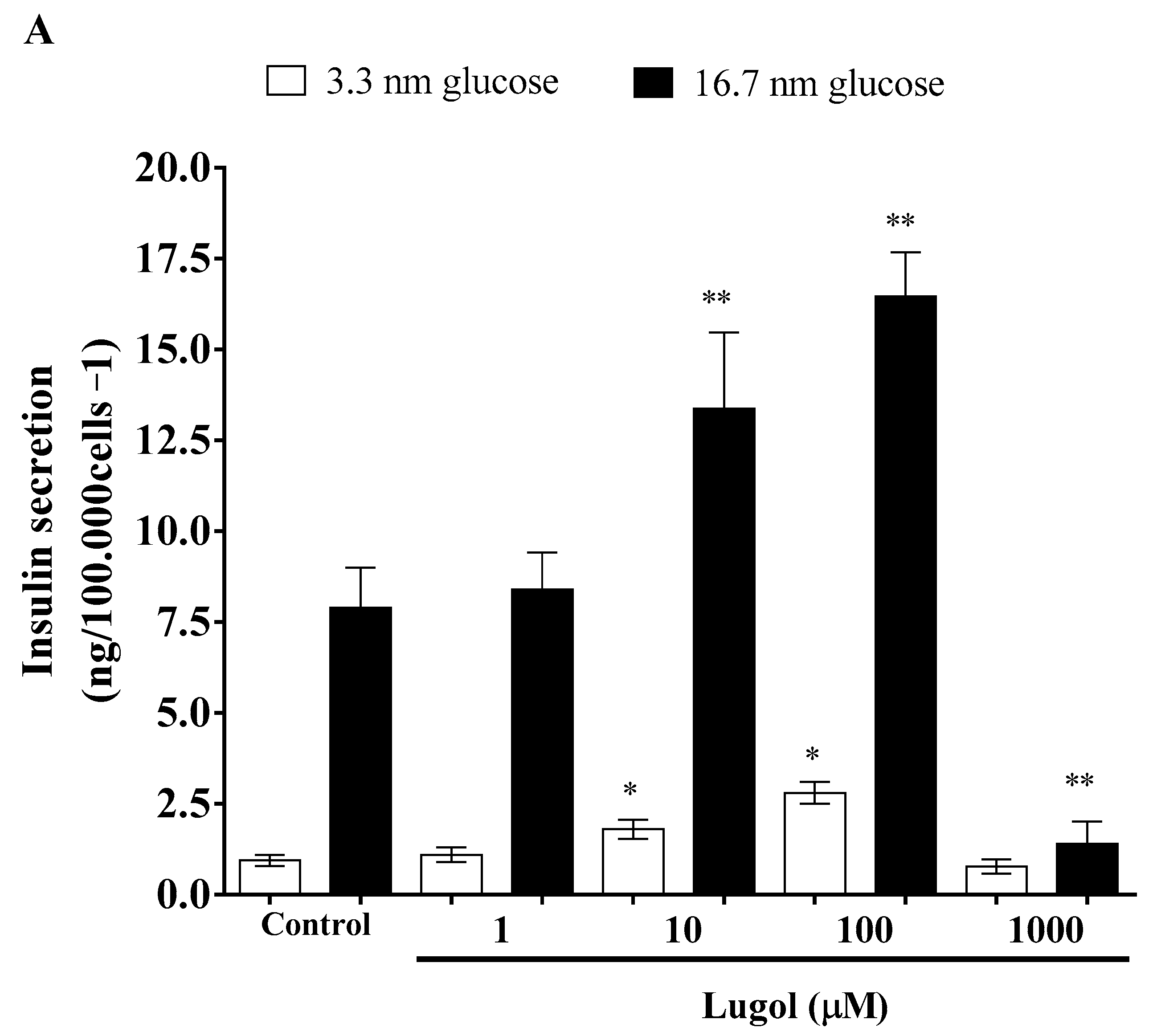
3.2. Effect of Low Doses of Lugol on Insulin Secretion in Pancreatic Beta-TC-6 Cells and Glucose Transport in Mature 3T3-L1 Adipocytes
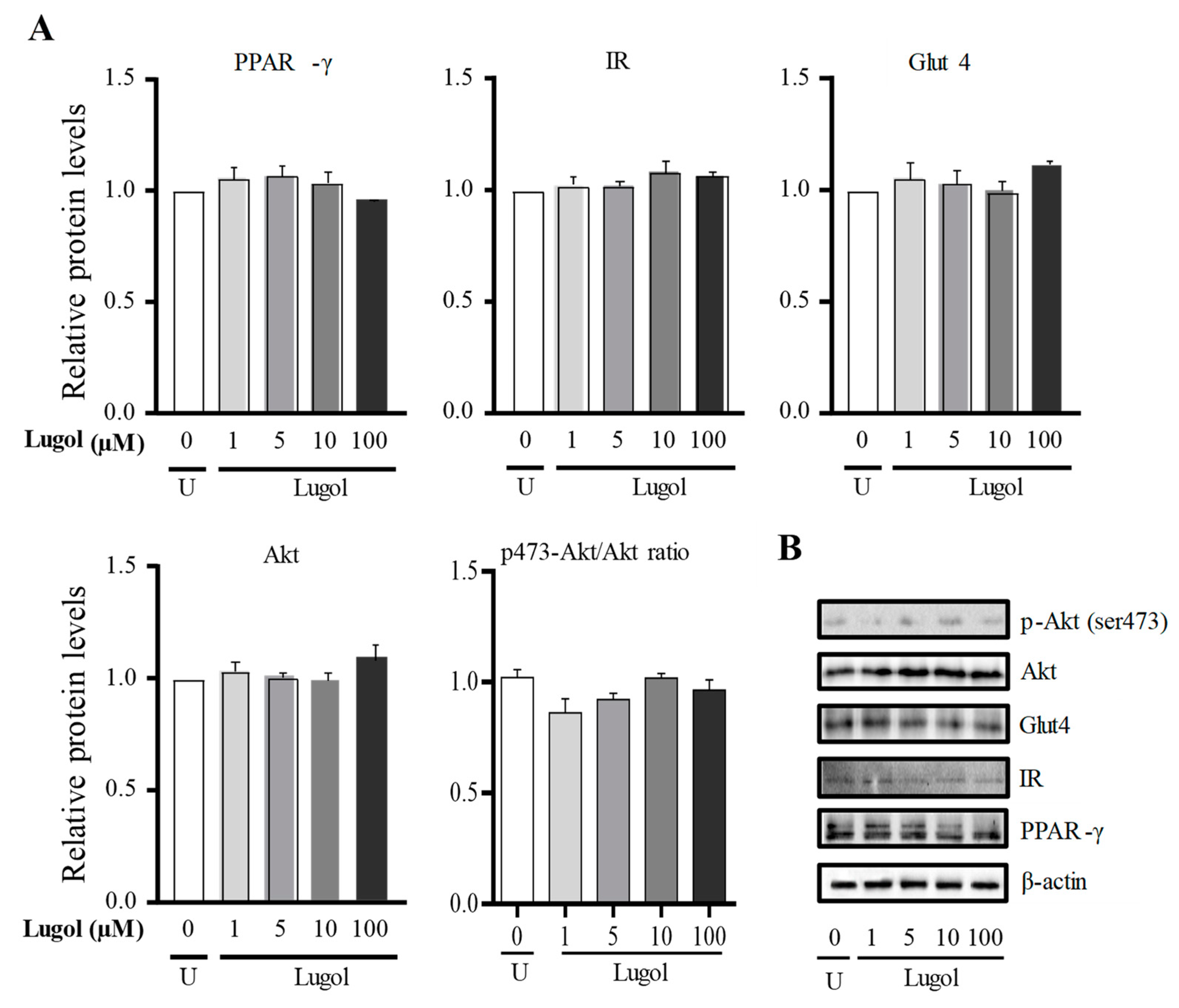
3.3. The Mechanism Involved in the Glucose Uptake Mediated by Lugol in Mature Adipocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sapra, A.; Bhandari, P. Diabetes Mellitus. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK551501/ (accessed on 13 November 2023).
- Kharroubi, A.T.; Darwish, H.M. Diabetes mellitus: The epidemic of the century. World J. Diabetes 2015, 6, 850–867. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; He, H.; Mandarino, L.J.; DeFronzo, R.A. Rosiglitazone improves downstream insulin receptor signaling in type 2 diabetic patients. Diabetes 2003, 52, 1943–1950. [Google Scholar] [CrossRef] [PubMed]
- Fazakerley, D.J.; Krycer, J.R.; Kearney, A.L.; Hocking, S.L.; James, D.E. Muscle and adipose tissue insulin resistance: Malady without mechanism? J. Lipid Res. 2019, 60, 1720–1732. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, K.L.; Hohnen-Behrens, C.; Cederberg, A.; Wu, L.E.; Turner, N.; Yuasa, T.; Ebina, Y.; James, D.E. IRS1-independent defects define major nodes of insulin resistance. Cell Metab. 2008, 7, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Motshakeri, M.; Ebrahimi, M.; Goh, Y.M.; Othman, H.H.; Hair-Bejo, M.; Mohamed, S. Effects of Brown Seaweed (Sargassum polycystum) Extracts on Kidney, Liver, and Pancreas of Type 2 Diabetic Rat Model. Evid. Based Complement. Alternat. Med. 2014, 2014, 379407. [Google Scholar] [CrossRef]
- Aakre, I.; Solli, D.D.; Markhus, M.W.; Mæhre, H.K.; Dahl, L.; Henjum, S.; Alexander, J.; Korneliussen, P.A.; Madsen, L.; Kjellevold, M. Commercially available kelp and seaweed products—Valuable iodine source or risk of excess intake? Food Nutr. Res. 2021, 65, 7584. [Google Scholar] [CrossRef]
- Arroyo-Helguera, O.; Anguiano, B.; Delgado, G.; Aceves, C. Uptake and antiproliferative effect of molecular iodine in the MCF-7 breast cancer cell line. Endocr. Relat. Cancer 2006, 13, 1147–1158. [Google Scholar] [CrossRef][Green Version]
- Arroyo-Helguera, O.; Rojas, E.; Delgado, G.; Aceves, C. Signaling pathways involved in the antiproliferative effect of molecular iodine in normal and tumoral breast cells: Evidence that 6-iodolactone mediates apoptotic effects. Endocr. Relat. Cancer 2008, 15, 1003–1011. [Google Scholar] [CrossRef]
- Cuellar-Rufino, S.; Zepeda, R.; Flores-Muñoz, M.; Roque-Santiago, I.; Arroyo-Helguera, O. Lugol Increases Lipolysis through Upregulation of PPAR-Gamma and Downregulation of C/EBP-Alpha in Mature 3T3-L1 Adipocytes. J. Nutr. Metab. 2020, 2020, 2302795. [Google Scholar] [CrossRef]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Hong, S.H.; Park, Y.-J.; Sung, J.-H.; Suh, W.; Lee, K.W.; Jung, K.; Lim, C.; Kim, J.-H.; Kim, H.; et al. MD001, a Novel Peroxisome Proliferator-activated Receptor α/γ Agonist, Improves Glucose and Lipid Metabolism. Sci. Rep. 2019, 9, 1656. [Google Scholar] [CrossRef] [PubMed]
- Nunez-Anita, R.E.; Arroyo-Helguera, O.; Cajero-Juarez, M.; Lopez-Bojorquez, L.; Aceves, C. A complex between 6-iodolactone and the peroxisome proliferator-activated receptor type gamma may mediate the antineoplastic effect of iodine in mammary cancer. Prostaglandins Other Lipid Mediat. 2009, 89, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Mancini, F.R.; Rajaobelina, K.; Dow, C.; Habbal, T.; Affret, A.; Balkau, B.; Bonnet, F.; Boutron-Ruault, M.C.; Fagherazzi, G. High iodine dietary intake is associated with type 2 diabetes among women of the E3N-EPIC cohort study. Clin. Nutr. 2019, 38, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, L.; Jia, Q.; Zhang, X.; Jin, X.; Shen, H. Effects of Excessive Iodine Intake on Blood Glucose, Blood Pressure, and Blood Lipids in Adults. Biol. Trace Elem. Res. 2019, 192, 136–144. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, Z.; Li, Y.; Teng, D.; Shi, X.; Ba, J.; Chen, B.; Du, J.; He, L.; Lai, X.; et al. U-Shaped Associations between Urinary Iodine Concentration and the Prevalence of Metabolic Disorders: A Cross-Sectional Study. Thyroid 2020, 30, 1053–1065. [Google Scholar] [CrossRef]
- Funahashi, H.; Imai, T.; Tanaka, Y.; Tobinaga, J.; Wada, M.; Morita, T.; Yamada, F.; Tsukamura, K.; Oiwa, M.; Kikumori, T.; et al. Suppressive effect of iodine on DMBA-induced breast tumor growth in the rat. J. Surg. Oncol. 1996, 61, 209–213. [Google Scholar] [CrossRef]
- Kciuk, M.I.; Davies, M.J.; Marciniak, B. P-254—Mechanistic view of iodide in oxidative stress. Free Radic. Biol. Med. 2018, 120 (Suppl. S1), S122. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Li, J.; Yang, X.; Sun, J.; Wu, Y.; Qiao, H. High iodine effects on the proliferation, apoptosis, and migration of papillary thyroid carcinoma cells as a result of autophagy induced by BRAF kinase. Biomed. Pharmacother. 2019, 120, 109476. [Google Scholar] [CrossRef]
- Ciaraldi, T.P.; Kong, A.P.; Chu, N.V.; Kim, D.D.; Baxi, S.; Loviscach, M.; Plodkowski, R.; Reitz, R.; Caulfield, M.; Mudaliar, S.; et al. Regulation of glucose transport and insulin signaling by troglitazone or metformin in adipose tissue of type 2 diabetic subjects. Diabetes 2002, 51, 30–36. [Google Scholar] [CrossRef]
- Festuccia, W.T.; Blanchard, P.G.; Turcotte, V.; Laplante, M.; Sariahmetoglu, M.; Brindley, D.N.; Deshaies, Y. Depot-specific effects of the PPARγ agonist rosiglitazone on adipose tissue glucose uptake and metabolism. J. Lipid Res. 2009, 50, 1185–1194. [Google Scholar] [CrossRef]
- Aceves, C.; Anguiano, B.; Delgado, G. The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. Thyroid 2013, 23, 938–946. [Google Scholar] [CrossRef]
- Leguisamo, N.M.; Lehnen, A.M.; Machado, U.F.; Okamoto, M.M.; Markoski, M.M.; Pinto, G.H.; Schaan, B.D. GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 2012, 11, 100. [Google Scholar] [CrossRef]
- Arakawa, K.; Ishihara, T.; Aoto, M.; Inamasu, M.; Saito, A.; Ikezawa, K. Actions of novel antidiabetic thiazolidinedione, T-174, in animal models of non-insulin-dependent diabetes mellitus (NIDDM) and in cultured muscle cells. Br. J. Pharmacol. 1998, 125, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Leonardini, A.; Laviola, L.; Perrini, S.; Natalicchio, A.; Giorgino, F. Cross-Talk between PPARγ and Insulin Signaling and Modulation of Insulin Sensitivity. PPAR Res. 2009, 2009, 818945. [Google Scholar] [CrossRef] [PubMed]
- Im, S.S.; Kwon, S.K.; Kim, T.H.; Kim, H.I.; Ahn, Y.H. Regulation of glucose transporter type 4 isoform gene expression in muscle and adipocytes. IUBMB Life 2007, 59, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Bailbe, D.; Philippe, E.; Gorbunov, E.; Tarasov, S.; Epstein, O.; Portha, B. The novel oral drug Subetta exerts an antidiabetic effect in the diabetic Goto-Kakizaki rat: Comparison with rosiglitazone. J. Diabetes Res. 2013, 2013, 763125. [Google Scholar] [CrossRef] [PubMed]
- Gorbunov, E.A.; Nicoll, J.; Kachaeva, E.V.; Tarasov, S.A.; Epstein, O.I. Subetta increases phosphorylation of insulin receptor beta-subunit alone and in the presence of insulin. Nutr. Diabetes 2015, 5, e169. [Google Scholar] [CrossRef]
- Hernandez, R.; Teruel, T.; Lorenzo, M. Rosiglitazone produces insulin sensitisation by increasing expression of the insulin receptor and its tyrosine kinase activity in brown adipocytes. Diabetologia 2003, 46, 1618–1628. [Google Scholar] [CrossRef]
- Kim, Y.B.; Ciaraldi, T.P.; Kong, A.; Kim, D.; Chu, N.; Mohideen, P.; Mudaliar, S.; Henry, R.R.; Kahn, B.B. Troglitazone but not metformin restores insulin-stimulated phosphoinositide 3-kinase activity and increases p110beta protein levels in skeletal muscle of type 2 diabetic subjects. Diabetes 2002, 51, 443–448. [Google Scholar] [CrossRef][Green Version]
- Park, K.S.; Ciaraldi, T.P.; Abrams-Carter, L.; Mudaliar, S.; Nikoulina, S.E.; Henry, R.R. Troglitazone regulation of glucose metabolism in human skeletal muscle cultures from obese type II diabetic subjects. J. Clin. Endocrinol. Metab. 1998, 83, 1636–1643. [Google Scholar] [CrossRef]
- Mîinea, C.P.; Sano, H.; Kane, S.; Sano, E.; Fukuda, M.; Peränen, J.; Lane, W.S.; Lienhard, G.E. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 2005, 391, 87–93. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, B.; Lu, X.; Miao, S.; Yang, F.; Zava, T.; Ding, Q.; Zhang, S.; Liu, J.; Zava, D.; et al. Iodine stimulates estrogen receptor singling and its systemic level is increased in surgical patients due to topical absorption. Oncotarget 2018, 9, 375–384. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arely, R.-J.; Cristian, A.-E.; Omar, A.-X.; Antonio, P.-J.J.; Isela, S.-R.; Yeimy Mar, D.L.-R.; Xcaret Alexa, H.-D.; Omar, A.-H. Iodine Promotes Glucose Uptake through Akt Phosphorylation and Glut-4 in Adipocytes, but Higher Doses Induce Cytotoxic Effects in Pancreatic Beta Cells. Biology 2024, 13, 26. https://doi.org/10.3390/biology13010026
Arely R-J, Cristian A-E, Omar A-X, Antonio P-JJ, Isela S-R, Yeimy Mar DL-R, Xcaret Alexa H-D, Omar A-H. Iodine Promotes Glucose Uptake through Akt Phosphorylation and Glut-4 in Adipocytes, but Higher Doses Induce Cytotoxic Effects in Pancreatic Beta Cells. Biology. 2024; 13(1):26. https://doi.org/10.3390/biology13010026
Chicago/Turabian StyleArely, Reséndiz-Jiménez, Arbez-Evangelista Cristian, Arroyo-Xochihua Omar, Palma-Jacinto José Antonio, Santiago-Roque Isela, De León-Ramírez Yeimy Mar, Hernández-Domínguez Xcaret Alexa, and Arroyo-Helguera Omar. 2024. "Iodine Promotes Glucose Uptake through Akt Phosphorylation and Glut-4 in Adipocytes, but Higher Doses Induce Cytotoxic Effects in Pancreatic Beta Cells" Biology 13, no. 1: 26. https://doi.org/10.3390/biology13010026
APA StyleArely, R.-J., Cristian, A.-E., Omar, A.-X., Antonio, P.-J. J., Isela, S.-R., Yeimy Mar, D. L.-R., Xcaret Alexa, H.-D., & Omar, A.-H. (2024). Iodine Promotes Glucose Uptake through Akt Phosphorylation and Glut-4 in Adipocytes, but Higher Doses Induce Cytotoxic Effects in Pancreatic Beta Cells. Biology, 13(1), 26. https://doi.org/10.3390/biology13010026







