Acute Myocardial Infarction and Risk of Cognitive Impairment and Dementia: A Review
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
3. Results
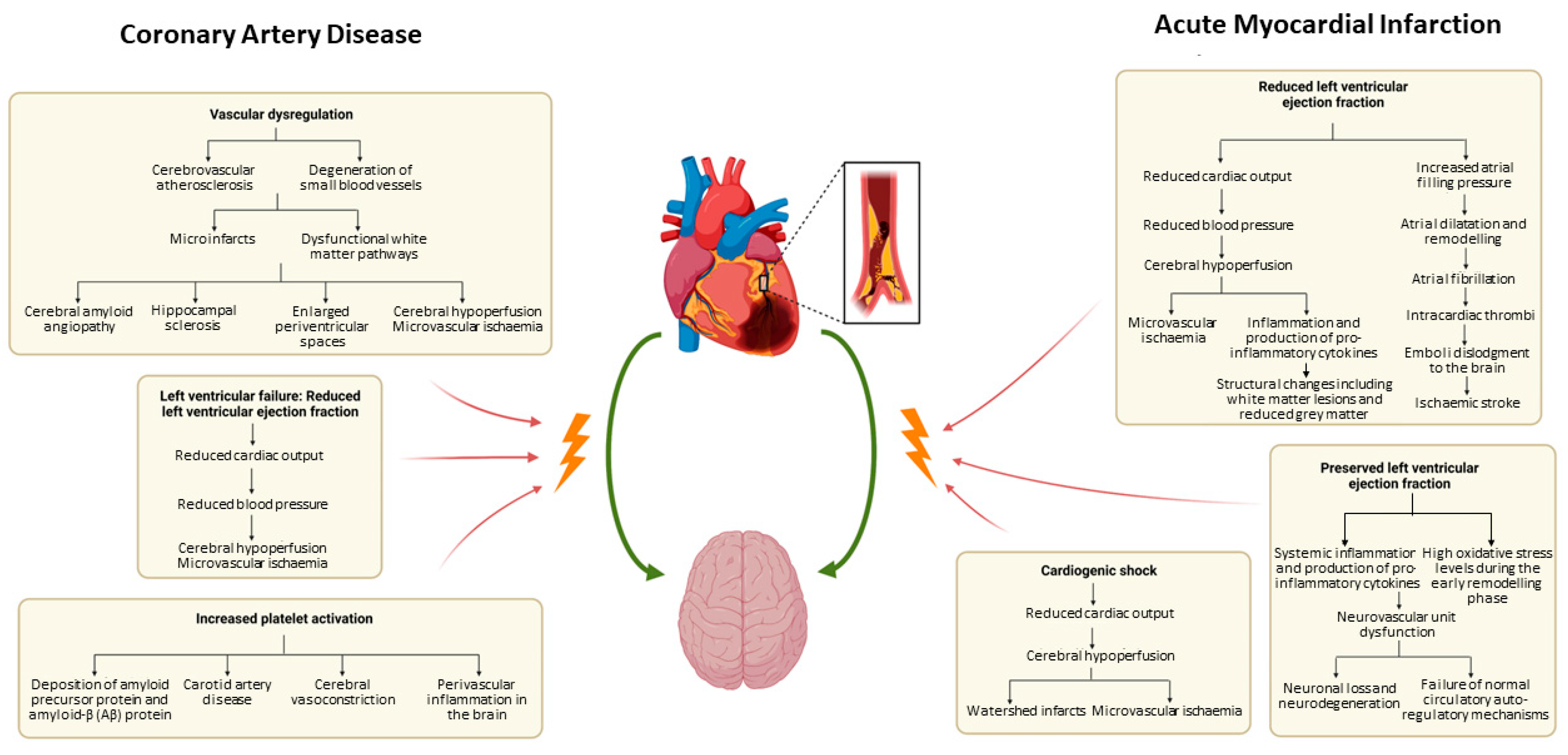
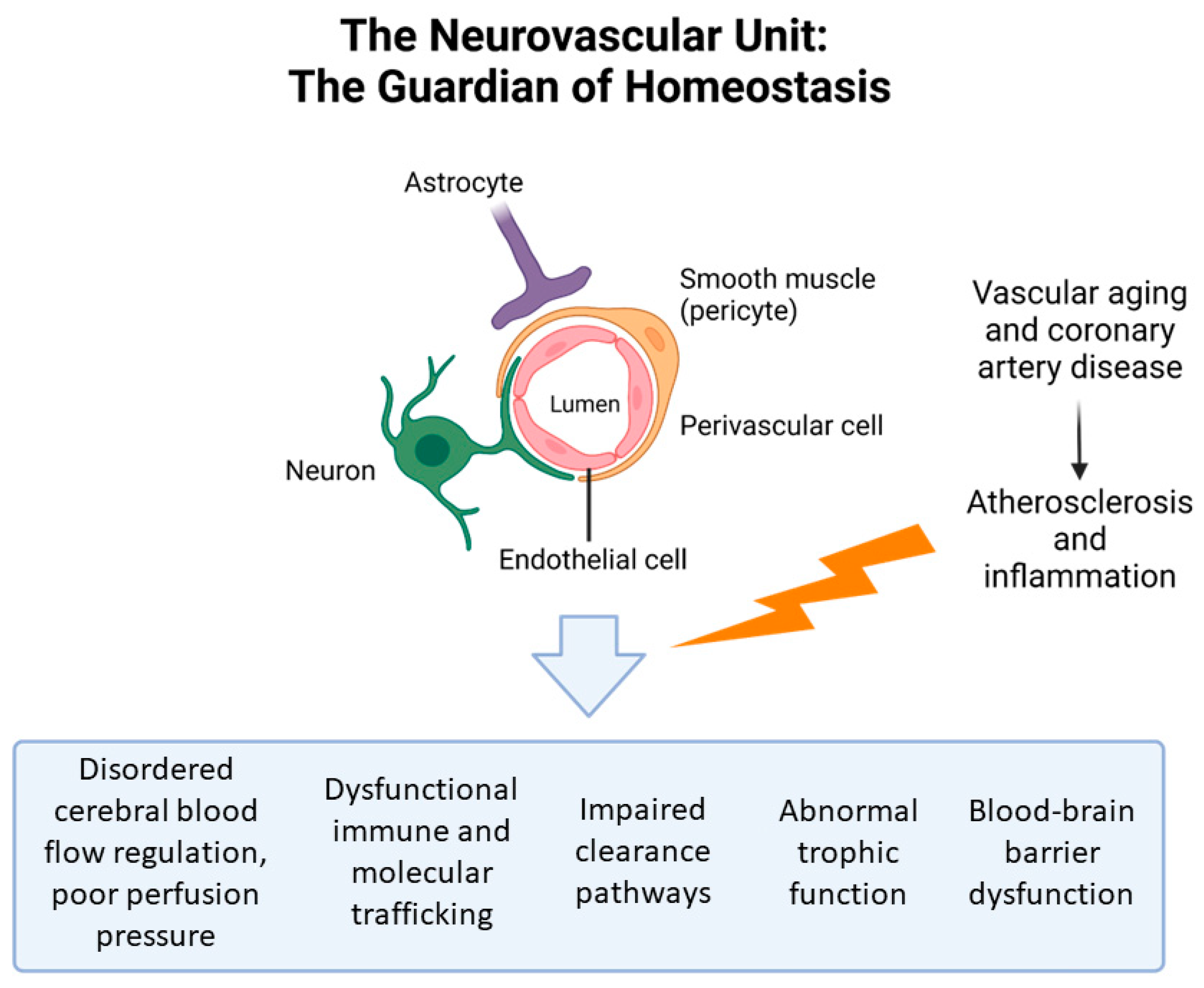
3.1. Pathophysiology
3.1.1. Cognitive Impairment and CAD: Pathophysiology
3.1.2. Cognitive Impairment and AMI: Pathophysiology
3.2. Cognitive Impairment Post-AMI
3.3. Impact of Cardiovascular Risk Factors on Dementia Subtypes
3.3.1. Cardiovascular Risk Factors and Alzheimer’s Dementia
3.3.2. Cardiovascular Risk Factors and Vascular Dementia
3.3.3. Cardiovascular Risk Factors and Dementia with Lewy Bodies
3.3.4. Cardiovascular Risk Factors and Frontal-Temporal Dementia
3.4. Cognitive Impairment Post-Intervention for AMI
3.5. The Impact of Age on CI Post-AMI
3.6. Sex Differences in Post-AMI CI
3.7. The Impact of Post-AMI Heart Failure on CI
3.8. Effect of Post-AMI CI on Prognosis
3.9. Neuroprotective Strategies Post-AMI
3.9.1. Antiplatelet Therapy
3.9.2. Statins
3.9.3. Renin-Angiotensin System Inhibitors
3.9.4. Beta-Blockers
3.9.5. Cardiac Rehabilitation
4. Limitations
5. Implications
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carro, A.; Kaski, J.C. Myocardial infarction in the elderly. Aging Dis. 2011, 2, 116–137. [Google Scholar]
- Schwarzinger, M.; Dufouil, C. Forecasting the prevalence of dementia. Lancet Public Health 2022, 7, e94–e95. [Google Scholar] [CrossRef] [PubMed]
- Geldmacher, D.S.; Whitehouse, P.J. Evaluation of Dementia. N. Engl. J. Med. 1996, 335, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Mahaffey, K.W.; Sun, L.J.; Pieper, K.S.; White, H.D.; Aylward, P.E.; Ferguson, J.J.; Califf, R.M.; Roe, M.T. Prevalence, predictors, and impact of conservative medical management for patients with non-ST-segment elevation acute coronary syndromes who have angiographically documented significant coronary disease. JACC Cardiovasc. Interv. 2008, 1, 369–378. [Google Scholar] [CrossRef]
- Chodosh, J.; Petitti, D.B.; Elliott, M.; Hays, R.D.; Crooks, V.C.; Reuben, D.B.; Galen Buckwalter, J.; Wenger, N. Physician recognition of cognitive impairment: Evaluating the need for improvement. J. Am. Geriatr. Soc. 2004, 52, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Hachinski, V. Stroke and potentially preventable dementias proclamation: Updated world stroke day proclamation. Stroke 2015, 46, 3039–3040. [Google Scholar] [CrossRef][Green Version]
- Redfield, M.M.; Jacobsen, S.J.; Burnett, J.C.J.; Mahoney, D.W.; Bailey, K.R.; Rodeheffer, R.J. Burden of systolic and diastolic ventricular dysfunction in the community: Appreciating the scope of the heart failure epidemic. JAMA 2003, 289, 194–202. [Google Scholar] [CrossRef]
- Wolf, P.A. Contributions of the Framingham Heart Study to stroke and dementia epidemiologic research at 60 years. Arch. Neurol. 2012, 69, 567–571. [Google Scholar] [CrossRef]
- Schievink, S.H.J.; van Boxtel, M.P.J.; Deckers, K.; van Oostenbrugge, R.J.; Verhey, F.R.J.; Köhler, S. Cognitive changes in prevalent and incident cardiovascular disease: A 12-year follow-up in the Maastricht Aging Study (MAAS). Eur. Heart J. 2022, 43, e2–e9. [Google Scholar] [CrossRef]
- Deckers, K.; Schievink, S.H.J.; Rodriquez, M.M.F.; van Oostenbrugge, R.J.; van Boxtel, M.P.J.; Verhey, F.R.J.; Köhler, S. Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0184244. [Google Scholar] [CrossRef]
- Newman, A.B.; Fitzpatrick, A.L.; Lopez, O.; Jackson, S.; Lyketsos, C.; Jagust, W.; Ives, D.; DeKosky, S.T.; Kuller, L.H. Dementia and Alzheimer’s disease incidence in relationship to cardiovascular disease in the Cardiovascular Health Study cohort. J. Am. Geriatr. Soc. 2005, 53, 1101–1107. [Google Scholar] [CrossRef]
- Breteler, M.M.B.; Claus, J.J.; Grobbee, D.E.; Hofman, A. Cardiovascular disease and distribution of cognitive function in elderly people: The Rotterdam Study. BMJ 1994, 308, 1604–1608. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Morris, R.G.; Huckstep, B.; Jones, D.K.; Williams, S.C.R.; Markus, H.S. Diffusion tensor MRI correlates with executive dysfunction in patients with ischaemic leukoaraiosis. J. Neurol. Neurosurg. Psychiatry 2004, 75, 441–447. [Google Scholar] [CrossRef]
- Di Legge, S.; Hachinski, V. Prospects for prevention and treatment of vascular cognitive impairment. Curr. Opin. Investig. Drugs 2003, 4, 1082–1087. [Google Scholar] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of cognitive impairment without dementia in the United States. Ann. Intern. Med. 2008, 148, 427–434. [Google Scholar] [CrossRef]
- Silbert, B.S.; Scott, D.A.; Evered, L.A.; Lewis, M.S.; Maruff, P.T. Preexisting cognitive impairment in patients scheduled for elective coronary artery bypass graft surgery. Obstet. Anesthesia Dig. 2007, 104, 1023–1028. [Google Scholar] [CrossRef]
- Gu, S.Z.; Beska, B.; Chan, D.; Neely, D.; Batty, J.A.; Adams-Hall, J.; Mossop, H.; Qiu, W.; Kunadian, V. Cognitive Decline in Older Patients with Non- ST Elevation Acute Coronary Syndrome. J. Am. Heart Assoc. 2019, 8, e011218. [Google Scholar] [CrossRef]
- Toledo, J.B.; Arnold, S.E.; Raible, K.; Brettschneider, J.; Xie, S.X.; Grossman, M.; Monsell, S.E.; Kukull, W.A.; Trojanowski, J.Q. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 2013, 136, 2697–2706. [Google Scholar] [CrossRef]
- Pendlebury, S.T.; Rothwell, P.M. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: Analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019, 18, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.Y.; Snyder, P.J.; Wu, W.-C.; Zhang, M.; Echeverria, A.; Alber, J. Pathophysiologic relationship between Alzheimer’s disease, cerebrovascular disease, and cardiovascular risk: A review and synthesis. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2017, 7, 69–87. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B.; Scuteri, A.; Black, S.E.; DeCarli, C.; Greenberg, S.M.; Iadecola, C.; Launer, L.J.; Laurent, S.; Lopez, O.L.; Nyenhuis, D. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 2672–2713. [Google Scholar] [PubMed]
- Fowkes, R.; Byrne, M.; Sinclair, H.; Tang, E.; Kunadian, V. Coronary artery disease in patients with dementia. Coron. Artery Dis. 2016, 27, 511–520. [Google Scholar] [PubMed]
- Love, S. Neuropathological investigation of dementia: A guide for neurologists. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. S5), v8–v14. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, S.E.; Longstreth, W.T.; Koudstaal, P.J. Silent brain infarcts: A systematic review. Lancet Neurol. 2007, 6, 611–619. [Google Scholar] [PubMed]
- Debette, S.; Beiser, A.; DeCarli, C.; Au, R.; Himali, J.J.; Kelly-Hayes, M.; Romero, J.R.; Kase, C.S.; Wolf, P.A.; Seshadri, S. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham Offspring Study. Stroke 2010, 41, 600–606. [Google Scholar] [CrossRef]
- DeCarli, C. Clinically asymptomatic vascular brain injury, a potent cause of cognitive impairment amongst older individuals. J. Alzheimer’s Dis. JAD 2013, 33, S417. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, S.E.; Prins, N.D.; den Heijer, T.; Hofman, A.; Koudstaal, P.J.; Breteler, M.M.B. Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 2003, 348, 1215–1222. [Google Scholar] [CrossRef]
- Leto, L.; Feola, M. Cognitive impairment in heart failure patients. J. Geriatr. Cardiol. 2014, 11, 316–328. [Google Scholar] [CrossRef]
- Abete, P.; Della-Morte, D.; Gargiulo, G.; Basile, C.; Langellotto, A.; Galizia, G.; Testa, G.; Canonico, V.; Bonaduce, D.; Cacciatore, F. Cognitive impairment and cardiovascular diseases in the elderly. A heart–brain continuum hypothesis. Ageing Res. Rev. 2014, 18, 41–52. [Google Scholar]
- Stellos, K.; Panagiota, V.; Kögel, A.; Leyhe, T.; Gawaz, M.; Laske, C. Predictive value of platelet activation for the rate of cognitive decline in Alzheimer’s disease patients. J. Cereb. Blood Flow Metab. 2010, 30, 1817–1820. [Google Scholar] [CrossRef]
- Barnes, J.M.; Barnes, N.M.; Costall, B.; Horovitz, Z.P.; Ironside, J.W.; Naylor, R.J.; Williams, T.J. Angiotensin II inhibits cortical cholinergic function: Implications for cognition. J. Cardiovasc. Pharmacol. 1990, 16, 234–238. [Google Scholar] [CrossRef]
- Savaskan, E.; Hock, C.; Olivieri, G.; Bruttel, S.; Rosenberg, C.; Hulette, C.; Müller-Spahn, F. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer’s dementia. Neurobiol. Aging 2001, 22, 541–546. [Google Scholar] [CrossRef]
- Tahsili-Fahadan, P.; Geocadin, R.G. Heart–Brain Axis. Circ. Res. 2017, 120, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekhar, Y.; Narula, J. Imaging Dynamic Heart–Brain Interactions. J. Am. Coll. Cardiol. 2018, 71, 276–278. [Google Scholar] [CrossRef] [PubMed]
- Lecrux, C.; Hamel, E. The neurovascular unit in brain function and disease. Acta Physiol. 2011, 203, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zlokovic, B. V Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat. Rev. Neurosci. 2011, 12, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Tooze, J.A.; Gaussoin, S.A.; Resnick, S.M.; Fischbein, N.J.; Robinson, J.G.; Bryan, R.N.; An, Y.; Espeland, M.A. A uniform approach to modeling risk factor relationships for ischemic lesion prevalence and extent: The Women’s Health Initiative magnetic resonance imaging study. Neuroepidemiology 2010, 34, 55–62. [Google Scholar] [CrossRef]
- Kuller, L.H.; Margolis, K.L.; Gaussoin, S.A.; Bryan, N.R.; Kerwin, D.; Limacher, M.; Wassertheil-Smoller, S.; Williamson, J.; Robinson, J.G.; Group, W.H.I.M.S.R. Relationship of hypertension, blood pressure, and blood pressure control with white matter abnormalities in the Women’s Health Initiative Memory Study (WHIMS)—MRI trial. J. Clin. Hypertens. 2010, 12, 203–212. [Google Scholar] [CrossRef]
- Iadecola, C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010, 120, 287–296. [Google Scholar] [CrossRef]
- Tan, Z.S.; Beiser, A.S.; Vasan, R.S.; Roubenoff, R.; Dinarello, C.A.; Harris, T.B.; Benjamin, E.J.; Au, R.; Kiel, D.P.; Wolf, P.A. Inflammatory markers and the risk of Alzheimer disease: The Framingham Study. Neurology 2007, 68, 1902–1908. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.S.; Beiser, A.S.; Fox, C.S.; Au, R.; Himali, J.J.; Debette, S.; DeCarli, C.; Vasan, R.S.; Wolf, P.A.; Seshadri, S. Association of metabolic dysregulation with volumetric brain magnetic resonance imaging and cognitive markers of subclinical brain aging in middle-aged adults: The Framingham Offspring Study. Diabetes Care 2011, 34, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Sundbøll, J.; Horváth-Puhó, E.; Adelborg, K.; Schmidt, M.; Pedersen, L.; Bøtker, H.E.; Henderson, V.W.; Sørensen, H.T. Higher Risk of Vascular Dementia in Myocardial Infarction Survivors. Circulation 2018, 137, 567–577. [Google Scholar] [CrossRef]
- Alosco, M.L.; Hayes, S.M. Structural brain alterations in heart failure: A review of the literature and implications for risk of Alzheimer’s disease. Heart Fail. Rev. 2015, 20, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Alves, T.C.T.F.; Rays, J.; Fráguas, R.J.; Wajngarten, M.; Meneghetti, J.C.; Prando, S.; Busatto, G.F. Localized cerebral blood flow reductions in patients with heart failure: A study using 99mTc-HMPAO SPECT. J. Neuroimaging Off. J. Am. Soc. Neuroimaging 2005, 15, 150–156. [Google Scholar] [CrossRef]
- Almeida, J.R.C.; Alves, T.C.T.F.; Wajngarten, M.; Rays, J.; Castro, C.C.; Cordeiro, Q.; Telles, R.M.S.; Fraguas, R.J.; Busatto, G.F. Late-life depression, heart failure and frontal white matter hyperintensity: A structural magnetic resonance imaging study. Braz. J. Med. Biol. Res. 2005, 38, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, T.; Chu, M.L.; Witte, V.A.; Villringer, A.; Kumral, D.; Riedel-Heller, S.G.; Roehr, S.; Hagendorff, A.; Laufs, U.; Loeffler, M.; et al. Heart failure is independently associated with white matter lesions: Insights from the population-based LIFE-Adult Study. ESC Heart Fail. 2021, 8, 697–704. [Google Scholar] [CrossRef]
- Sundbøll, J.; Horváth-Puhó, E.; Schmidt, M.; Pedersen, L.; Henderson, V.W.; Bøtker, H.E.; Sørensen, H.T. Long-term risk of stroke in myocardial infarction survivors: Thirty-year population-based cohort study. Stroke 2016, 47, 1727–1733. [Google Scholar] [CrossRef]
- Howard, R.; Trend, P.; Russell, R.W.R. Clinical features of ischemia in cerebral arterial border zones after periods of reduced cerebral blood flow. Arch. Neurol. 1987, 44, 934–940. [Google Scholar] [CrossRef]
- Cacciatore, F.; Testa, G.; Langellotto, A.; Galizia, G.; Della-Morte, D.; Gargiulo, G.; Bevilacqua, A.; Del Genio, M.T.; Canonico, V.; Rengo, F. Role of ventricular rate response on dementia in cognitively impaired elderly subjects with atrial fibrillation: A 10-year study. Dement. Geriatr. Cogn. Disord. 2012, 34, 143–148. [Google Scholar] [CrossRef]
- Cacciatore, F.; Abete, P.; De Santis, D.; Longobardi, G.; Ferrara, N.; Rengo, F. Mortality and blood pressure in elderly people with and without cognitive impairment. Gerontology 2005, 51, 53–61. [Google Scholar] [CrossRef]
- Bennett, S.J.; Sauvé, M.J. Cognitive deficits in patients with heart failure: A review of the literature. J. Cardiovasc. Nurs. 2003, 18, 219–242. [Google Scholar] [CrossRef]
- Arangalage, D.; Ederhy, S.; Dufour, L.; Joffre, J.; Van der Vynckt, C.; Lang, S.; Tzourio, C.; Cohen, A. Relationship between cognitive impairment and echocardiographic parameters: A review. J. Am. Soc. Echocardiogr. Off. Publ. Am. Soc. Echocardiogr. 2015, 28, 264–274. [Google Scholar] [CrossRef]
- Burkauskas, J.; Brozaitiene, J.; Bunevicius, A.; Neverauskas, J.; Zaliunaite, V.; Bunevicius, R. Association of Depression, Anxiety, and Type D Personality with Cognitive Function in Patients with Coronary Artery Disease. Cogn. Behav. Neurol. Off. J. Soc. Behav. Cogn. Neurol. 2016, 29, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Rauch, B.; Davos, C.H.; Doherty, P.; Saure, D.; Metzendorf, M.-I.; Salzwedel, A.; Völler, H.; Jensen, K.; Schmid, J.-P. The prognostic effect of cardiac rehabilitation in the era of acute revascularisation and statin therapy: A systematic review and meta-analysis of randomized and non-randomized studies—The Cardiac Rehabilitation Outcome Study (CROS). Eur. J. Prev. Cardiol. 2016, 23, 1914–1939. [Google Scholar] [CrossRef]
- Jinawong, K.; Apaijai, N.; Piamsiri, C.; Maneechote, C.; Arunsak, B.; Chunchai, T.; Pintana, H.; Nawara, W.; Chattipakorn, N.; Chattipakorn, S.C. Mild Cognitive impairment Occurs in Rats During the Early Remodeling Phase of Myocardial Infarction. Neuroscience 2022, 493, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; van Oijen, M.; de Jong, F.J.; Kors, J.A.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008, 39, 1421–1426. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Hupe, H.C.; Wang, Y.; Bankstahl, J.P.; Berding, G.; Ross, T.L.; Bauersachs, J.; Wollert, K.C.; Bengel, F.M. Myocardial Inflammation Predicts Remodeling and Neuroinflammation After Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 71, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.Q.; Kong, W.K.F.; Wong, R.C.C.; Chong, Y.F.; Chew, N.W.S.; Yeo, T.-C.; Sharma, V.K.; Poh, K.K.; Sia, C.-H. Cognitive Impairment in Heart Failure—A Review. Biology 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Georgiadis, D.; Sievert, M.; Cencetti, S.; Uhlmann, F.; Krivokuca, M.; Zierz, S.; Werdan, K. Cerebrovascular reactivity is impaired in patients with cardiac failure. Eur. Heart J. 2000, 21, 407–413. [Google Scholar] [CrossRef]
- Gruhn, N.; Larsen, F.S.; Boesgaard, S.; Knudsen, G.M.; Mortensen, S.A.; Thomsen, G.; Aldershvile, J. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke 2001, 32, 2530–2533. [Google Scholar] [CrossRef]
- De Jong, G.I.; Farkas, E.; Stienstra, C.M.; Plass, J.R.; Keijser, J.N.; de la Torre, J.C.; Luiten, P.G. Cerebral hypoperfusion yields capillary damage in the hippocampal CA1 area that correlates with spatial memory impairment. Neuroscience 1999, 91, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Fazekas, F.; Offenbacher, H.; Dusleag, J.; Lechner, H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke 1991, 22, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, V.G.; Passannante, M.R.; Shah, T.; Modi, K.; Weisse, A.B. Effect of mitral regurgitation on left ventricular thrombus formation in dilated cardiomyopathy. Am. Heart J. 1998, 135, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Kure, C.E.; Rosenfeldt, F.L.; Scholey, A.B.; Pipingas, A.; Kaye, D.M.; Bergin, P.J.; Croft, K.D.; Wesnes, K.A.; Myers, S.P.; Stough, C. Relationships among cognitive function and cerebral blood flow, oxidative stress, and inflammation in older heart failure patients. J. Card. Fail. 2016, 22, 548–559. [Google Scholar] [CrossRef]
- Ogren, J.A.; Fonarow, G.C.; Woo, M.A. Cerebral impairment in heart failure. Curr. Heart Fail. Rep. 2014, 11, 321–329. [Google Scholar]
- Hammond, C.A.; Blades, N.J.; Chaudhry, S.I.; Dodson, J.A.; Longstreth, W.T.; Heckbert, S.R.; Psaty, B.M.; Arnold, A.M.; Dublin, S.; Sitlani, C.M.; et al. Long-Term Cognitive Decline After Newly Diagnosed Heart Failure. Circ. Heart Fail. 2018, 11, e004476. [Google Scholar] [CrossRef]
- Jefferson, A.L.; Himali, J.J.; Beiser, A.S.; Au, R.; Massaro, J.M.; Seshadri, S.; Gona, P.; Salton, C.J.; DeCarli, C.; O’Donnell, C.J.; et al. Cardiac Index Is Associated With Brain Aging. Circulation 2010, 122, 690–697. [Google Scholar] [CrossRef]
- Gharacholou, S.M.; Reid, K.J.; Arnold, S.V.; Spertus, J.; Rich, M.W.; Pellikka, P.A.; Singh, M.; Holsinger, T.; Krumholz, H.M.; Peterson, E.D.; et al. Cognitive impairment and outcomes in older adult survivors of acute myocardial infarction: Findings from the translational research investigating underlying disparities in acute myocardial infarction patients’ health status registry. Am. Heart J. 2011, 162, 860–869.e1. [Google Scholar] [CrossRef] [PubMed]
- Volonghi, I.; Pendlebury, S.T.; Welch, S.J.V.; Mehta, Z.; Rothwell, P.M. Cognitive outcomes after acute coronary syndrome: A population based comparison with transient ischaemic attack and minor stroke. Heart 2013, 99, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.A.; Hollander, M.; Bos, M.J.; Kors, J.A.; Koudstaal, P.J.; Hofman, A.; Witteman, J.C.M.; Breteler, M.M.B. Unrecognized myocardial infarction and the risk of stroke: The Rotterdam Study. Neurology 2006, 67, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Bursi, F.; Rocca, W.A.; Killian, J.M.; Weston, S.A.; Knopman, D.S.; Jacobsen, S.J.; Roger, V.L. Heart Disease and Dementia: A Population-based Study. Am. J. Epidemiol. 2006, 163, 135–141. [Google Scholar] [CrossRef]
- Petrovitch, H.; Lon White, M.D.; Masaki, K.H.; Ross, G.W.; Rodriguez, B.L.; Lu, G.; Blanchette, P.L.; Curb, J.D. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. Am. J. Cardiol. 1998, 81, 1017–1021. [Google Scholar] [CrossRef] [PubMed]
- Roberts, R.O.; Knopman, D.S.; Geda, Y.E.; Cha, R.H.; Roger, V.L.; Petersen, R.C. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol. Aging 2010, 31, 1894–1902. [Google Scholar] [CrossRef] [PubMed]
- Massaia, M.; Pallavicino Di Ceva, A.; Bo, M.; Cappa, G.; Zannella, P.; Persico, D.; Ferrario, E.; Fabris, F. Risk factors for dementia of Alzheimer’s type: A case-control, retrospective evaluation. Arch. Gerontol. Geriatr. 2001, 33, 253–259. [Google Scholar] [CrossRef]
- Takahashi, P.Y.; Caldwell, C.R.; Targonski, P.V. Effect of vascular burden as measured by vascular indexes upon vascular dementia: A matched case-control study. Clin. Interv. Aging 2012, 7, 27–33. [Google Scholar] [CrossRef][Green Version]
- Hayden, K.M.; Zandi, P.P.; Lyketsos, C.G.; Khachaturian, A.S.; Bastian, L.A.; Charoonruk, G.; Tschanz, J.T.; Norton, M.C.; Pieper, C.F.; Munger, R.G.; et al. Vascular Risk Factors for Incident Alzheimer Disease and Vascular Dementia: The Cache County Study. Alzheimer Dis. Assoc. Disord. 2006, 20, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.A.; Beckett, L.A.; Albert, M.S.; Hebert, L.E.; Scherr, P.A.; Funkenstein, H.H.; Taylor, J.O. Level of education and change in cognitive function in a community population of older persons. Ann. Epidemiol. 1993, 3, 71–77. [Google Scholar] [CrossRef]
- Deary, I.J.; Corley, J.; Gow, A.J.; Harris, S.E.; Houlihan, L.M.; Marioni, R.E.; Penke, L.; Rafnsson, S.B.; Starr, J.M. Age-associated cognitive decline. Br. Med. Bull. 2009, 92, 135–152. [Google Scholar] [CrossRef]
- Brunnström, H.; Gustafson, L.; Passant, U.; Englund, E. Prevalence of dementia subtypes: A 30-year retrospective survey of neuropathological reports. Arch. Gerontol. Geriatr. 2009, 49, 146–149. [Google Scholar] [CrossRef]
- Ritchie, K.; Lovestone, S. The dementias. Lancet 2002, 360, 1759–1766. [Google Scholar] [CrossRef]
- Schneider, J.A.; Wilson, R.S.; Bienias, J.L.; Evans, D.A.; Bennett, D.A. Cerebral infarctions and the likelihood of dementia from Alzheimer disease pathology. Neurology 2004, 62, 1148–1155. [Google Scholar] [CrossRef]
- Petrovitch, H.; Ross, G.W.; Steinhorn, S.C.; Abbott, R.D.; Markesbery, W.; Davis, D.; Nelson, J.; Hardman, J.; Masaki, K.; Vogt, M.R.; et al. AD lesions and infarcts in demented and non-demented Japanese-American men. Ann. Neurol. 2005, 57, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Snowdon, D.A.; Greiner, L.H.; Mortimer, J.A.; Riley, K.P.; Greiner, P.A.; Markesbery, W.R. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997, 277, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Gorelick, P.B. Risk Factors for Vascular Dementia and Alzheimer Disease. Stroke 2004, 35, 2620–2622. [Google Scholar] [CrossRef]
- Cribbs, D.H.; Berchtold, N.C.; Perreau, V.; Coleman, P.D.; Rogers, J.; Tenner, A.J.; Cotman, C.W. Extensive innate immune gene activation accompanies brain aging, increasing vulnerability to cognitive decline and neurodegeneration: A microarray study. J. Neuroinflammation 2012, 9, 179. [Google Scholar]
- Casolini, P.; Catalani, A.; Zuena, A.R.; Angelucci, L. Inhibition of COX-2 reduces the age-dependent increase of hippocampal inflammatory markers, corticosterone secretion, and behavioral impairments in the rat. J. Neurosci. Res. 2002, 68, 337–343. [Google Scholar] [CrossRef]
- Zotova, E.; Nicoll, J.A.; Kalaria, R.; Holmes, C.; Boche, D. Inflammation in Alzheimer’s disease: Relevance to pathogenesis and therapy. Alzheimer’s Res. Ther. 2010, 2, 1. [Google Scholar] [CrossRef]
- Noble, J.M.; Manly, J.J.; Schupf, N.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Association of C-reactive protein with cognitive impairment. Arch. Neurol. 2010, 67, 87–92. [Google Scholar] [CrossRef]
- Simen, A.A.; Bordner, K.A.; Martin, M.P.; Moy, L.A.; Barry, L.C. Cognitive dysfunction with aging and the role of inflammation. Ther. Adv. Chronic Dis. 2011, 2, 175–195. [Google Scholar] [CrossRef]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C.; Weiner, M.W.; Aisen, P.; Petersen, R.; Jack, C.R.; Jagust, W.; et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef] [PubMed]
- Lolatte, K.; Brackhan, M.; Hess, A.; Almarcha, P.B.; Willmann, M.; Ross, T.; Bengel, F.; Pahnke, J.; Thackeray, J. Impact of Acute Myocardial Infarction on Neuroinflammation and Alzheimer’s Disease Pathology in a Transgenic Mouse Model. J. Nucl. Med. 2022, 63 (Suppl. S2), 2299. Available online: http://jnm.snmjournals.org/content/63/supplement_2/2299.abstract (accessed on 1 July 2023).
- Gure, T.R.; Blaum, C.S.; Giordani, B.; Koelling, T.M.; Galecki, A.; Pressler, S.J.; Hummel, S.L.; Langa, K.M. Prevalence of cognitive impairment in older adults with heart failure. J. Am. Geriatr. Soc. 2012, 60, 1724–1729. [Google Scholar] [CrossRef]
- Eggermont, L.H.P.; de Boer, K.; Muller, M.; Jaschke, A.C.; Kamp, O.; Scherder, E.J.A. Cardiac disease and cognitive impairment: A systematic review. Heart 2012, 98, 1334–1340. [Google Scholar] [CrossRef]
- Muqtadar, H.; Testai, F.D.; Gorelick, P.B. The dementia of cardiac disease. Curr. Cardiol. Rep. 2012, 14, 732–740. [Google Scholar] [CrossRef]
- Kivipelto, M.; Helkala, E.-L.; Laakso, M.P.; Hänninen, T.; Hallikainen, M.; Alhainen, K.; Iivonen, S.; Mannermaa, A.; Tuomilehto, J.; Nissinen, A.; et al. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann. Intern. Med. 2002, 137, 149–155. [Google Scholar] [CrossRef]
- Schmitz, F.; Mevissen, V.; Krantz, C.; Kimmel, M.; Erdmann, J.; Hoffmann, R.; Zerres, K.; Ortlepp, J.R. Robust association of the APOE epsilon4 allele with premature myocardial infarction especially in patients without hypercholesterolaemia: The Aachen study. Eur. J. Clin. Investig. 2007, 37, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Rothwell, P.M.; Salimi-Khorshidi, G.; Kiran, A.; Conrad, N.; Callender, T.; Mehta, Z.; Pendlebury, S.T.; Anderson, S.G.; Mohseni, H.; et al. Blood Pressure and Risk of Vascular Dementia: Evidence from a Primary Care Registry and a Cohort Study of Transient Ischemic Attack and Stroke. Stroke 2016, 47, 1429–1435. [Google Scholar] [CrossRef] [PubMed]
- Ho, Y.-S.; Yang, X.; Yeung, S.-C.; Chiu, K.; Lau, C.-F.; Tsang, A.W.-T.; Mak, J.C.-W.; Chang, R.C.-C. Cigarette smoking accelerated brain aging and induced pre-Alzheimer-like neuropathology in rats. PLoS ONE 2012, 7, e36752. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.; Poulter, R.; Warner, J.; Beckett, N.; Burch, L.; Bulpitt, C. Smoking, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Thorp, E.B.; Flanagan, M.E.; Popko, B.; DeBerge, M. Resolving inflammatory links between myocardial infarction and vascular dementia. Semin. Immunol. 2022, 59, 101600. [Google Scholar] [CrossRef]
- Francis, J.; Chu, Y.; Johnson, A.K.; Weiss, R.M.; Felder, R.B. Acute myocardial infarction induces hypothalamic cytokine synthesis. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H2264–H2271. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Mei, Y.; Hu, Z.; Xing, W.; Lv, K.; Hu, N.; Zhang, T.; Wang, D. Ghrelin attenuates depressive-like behavior, heart failure, and neuroinflammation in postmyocardial infarction rat model. Eur. J. Pharmacol. 2021, 901, 174096. [Google Scholar] [CrossRef]
- Wang, H.-W.; Ahmad, M.; Jadayel, R.; Najjar, F.; Lagace, D.; Leenen, F.H.H. Inhibition of inflammation by minocycline improves heart failure and depression-like behaviour in rats after myocardial infarction. PLoS ONE 2019, 14, e0217437. [Google Scholar] [CrossRef]
- Yang, T.; Lu, Z.; Wang, L.; Zhao, Y.; Nie, B.; Xu, Q.; Han, X.; Li, T.; Zhao, J.; Cheng, W.; et al. Dynamic Changes in Brain Glucose Metabolism and Neuronal Structure in Rats with Heart Failure. Neuroscience 2020, 424, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Meissner, A.; Visanji, N.P.; Momen, M.A.; Feng, R.; Francis, B.M.; Bolz, S.; Hazrati, L. Tumor Necrosis Factor-α Underlies Loss of Cortical Dendritic Spine Density in a Mouse Model of Congestive Heart Failure. J. Am. Heart Assoc. 2015, 4, e001920. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems: Alphabetical Index; World Health Organization: Geneva, Switzerland, 2004; Volume 3, ISBN 9241546549. [Google Scholar]
- Corraini, P.; Henderson, V.W.; Ording, A.G.; Pedersen, L.; Horváth-Puhó, E.; Sørensen, H.T. Long-term risk of dementia among survivors of ischemic or hemorrhagic stroke. Stroke 2017, 48, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Alonso, A.; Arenas de Larriva, A.P. Atrial Fibrillation, Cognitive Decline And Dementia. Eur. Cardiol. 2016, 11, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Bath, P.M.; Woodhouse, L.J.; Appleton, J.P.; Beridze, M.; Christensen, H.; Dineen, R.A.; Flaherty, K.; Duley, L.; England, T.J.; Havard, D.; et al. Triple versus guideline antiplatelet therapy to prevent recurrence after acute ischaemic stroke or transient ischaemic attack: The TARDIS RCT. Health Technol. Assess. 2018, 22, 48. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, N.A.; Algra, A.; Kappelle, L.J.; Bath, P.M.; Csiba, L.; Rothwell, P.M.; Greving, J.P. Early time course of major bleeding on antiplatelet therapy after TIA or ischemic stroke. Neurology 2018, 90, e683–e689. [Google Scholar] [CrossRef]
- Shoamanesh, A.; Pearce, L.A.; Bazan, C.; Catanese, L.; McClure, L.A.; Sharma, M.; Marti-Fabregas, J.; Anderson, D.C.; Kase, C.S.; Hart, R.G.; et al. Microbleeds in the Secondary Prevention of Small Subcortical Strokes Trial: Stroke, mortality, and treatment interactions. Ann. Neurol. 2017, 82, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.K.; Mintzer, M.; Himmelfarb, C.R.D.; Hayat, M.J.; Rotman, S.; Allen, J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. Eur. J. Heart Fail. 2012, 14, 1041–1049. [Google Scholar] [CrossRef]
- Vogels, R.L.C.; Scheltens, P.; Schroeder-Tanka, J.M.; Weinstein, H.C. Cognitive impairment in heart failure: A systematic review of the literature. Eur. J. Heart Fail. 2007, 9, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Grossardt, B.R.; Bower, J.H.; Boeve, B.F.; Ahlskog, J.E.; Rocca, W.A. Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol. 2013, 70, 1396–1402. [Google Scholar] [CrossRef]
- Papapetropoulos, S.; Ellul, J.; Argyriou, A.A.; Talelli, P.; Chroni, E.; Papapetropoulos, T. The effect of vascular disease on late onset Parkinson’s disease. Eur. J. Neurol. 2004, 11, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Scigliano, G.; Musicco, M.; Soliveri, P.; Piccolo, I.; Ronchetti, G.; Girotti, F. Reduced Risk Factors for Vascular Disorders in Parkinson Disease Patients. Stroke 2006, 37, 1184–1188. [Google Scholar] [CrossRef] [PubMed]
- Haugarvoll, K.; Aarsland, D.; Wentzel-Larsen, T.; Larsen, J.P. The influence of cerebrovascular risk factors on incident dementia in patients with Parkinson’s disease. Acta Neurol. Scand. 2005, 112, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.-C.; Wei, C.-Y.; Hung, G.-U.; Chiu, P.-Y. Reduced vascular risk factors in Parkinson’s disease dementia and dementia with Lewy bodies compared to Alzheimer’s disease. Brain Behav. 2018, 8, e00916. [Google Scholar] [CrossRef]
- Javanshiri, K.; Haglund, M.; Englund, E. Cardiovascular Disease, Diabetes Mellitus, and Hypertension in Lewy Body Disease: A Comparison with Other Dementia Disorders. J. Alzheimer’s Dis. 2019, 71, 851–859. [Google Scholar] [CrossRef]
- Karageorgiou, E.; Miller, B.L. Frontotemporal lobar degeneration: A clinical approach. Semin. Neurol. 2014, 34, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Golimstok, A.; Cámpora, N.; Rojas, J.I.; Fernandez, M.C.; Elizondo, C.; Soriano, E.; Cristiano, E. Cardiovascular risk factors and frontotemporal dementia: A case-control study. Transl. Neurodegener. 2014, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Soppela, H.; Katisko, K.; Gadola, Y.; Krüger, J.; Hartikainen, P.; Alberici, A.; Benussi, A.; Koivisto, A.; Haapasalo, A.; Remes, A.M.; et al. Modifiable potential risk factors in familial and sporadic frontotemporal dementia. Ann. Clin. Transl. Neurol. 2022, 9, 1195–1205. [Google Scholar] [CrossRef]
- Kalkonde, Y.V.; Jawaid, A.; Qureshi, S.U.; Shirani, P.; Wheaton, M.; Pinto-Patarroyo, G.P.; Schulz, P.E. Medical and environmental risk factors associated with frontotemporal dementia: A case-control study in a veteran population. Alzheimer’s Dement. 2012, 8, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Roach, G.W.; Kanchuger, M.; Mangano, C.M.; Newman, M.; Nussmeier, N.; Wolman, R.; Aggarwal, A.; Marschall, K.; Graham, S.H.; Ley, C. Adverse cerebral outcomes after coronary bypass surgery. N. Engl. J. Med. 1996, 335, 1857–1864. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, E.; Airdrie, J.; Littlejohns, T.J.; Lourida, I.; Thompson-Coon, J.; Lang, I.A.; Scrobotovici, M.; Thacker, E.L.; Fitzpatrick, A.; Kuller, L.H.; et al. Coronary Artery Bypass Graft Surgery and Dementia Risk in the Cardiovascular Health Study. Alzheimer Dis. Assoc. Disord. 2017, 31, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Greaves, D.; Psaltis, P.J.; Ross, T.J.; Davis, D.; Smith, A.E.; Boord, M.S.; Keage, H.A.D. Cognitive outcomes following coronary artery bypass grafting: A systematic review and meta-analysis of 91,829 patients. Int. J. Cardiol. 2019, 289, 43–49. [Google Scholar] [CrossRef]
- Tomioka, T.; Takahashi, R.; Ikumi, Y.; Tanaka, S.; Ito, Y.; Shioiri, H.; Koyama, J.; Inoue, K. Influence of cognitive impairment on cardiac mortality after percutaneous coronary intervention in very elderly patients: A retrospective observational study. J. Geriatr. Cardiol. 2019, 16, 733–740. [Google Scholar] [CrossRef]
- Giang, K.W.; Jeppsson, A.; Karlsson, M.; Hansson, E.C.; Pivodic, A.; Skoog, I.; Lindgren, M.; Nielsen, S.J. The risk of dementia after coronary artery bypass grafting in relation to age and sex. Alzheimer’s Dement. 2021, 17, 1042–1050. [Google Scholar] [CrossRef] [PubMed]
- Devapalasundarum, A.N.; Silbert, B.S.; Evered, L.A.; Scott, D.A.; MacIsaac, A.I.; Maruff, P.T. Cognitive function in patients undergoing coronary angiography. Heart Asia 2010, 2, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Özbek, C.; Bay, W.; Hamann, G.F. Cerebral microemboli during left heart catheterization. Am. Heart J. 1999, 137, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, F.; Kassnasrallah, S.; Cesari, J.-B.; Blard, J.-M.; Macia, J.-C.; Messner-Pellenc, P.; Mariottini, C.-J.; Grolleau-Raoux, R. Transcranial Doppler detection of cerebral microemboli during left heart catheterization. Cerebrovasc. Dis. 2001, 12, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Duijndam, S.; Denollet, J.; Nyklíček, I.; Kupper, N. Perceived Cognition after Percutaneous Coronary Intervention: Association with Quality of Life, Mood and Fatigue in the THORESCI Study. Int. J. Behav. Med. 2017, 24, 552–562. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV; American Psychiatric Association: Washington, DC, USA, 1994; Volume 4. [Google Scholar]
- Nekouei, Z.K.; Yousefy, A.; Doost, H.T.N.; Manshaee, G.; Sadeghei, M. Structural Model of psychological risk and protective factors affecting on quality of life in patients with coronary heart disease: A psychocardiology model. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 90. [Google Scholar]
- Lespérance, F.; Frasure-Smith, N.; Juneau, M.; Théroux, P. Depression and 1-year prognosis in unstable angina. Arch. Intern. Med. 2000, 160, 1354–1360. [Google Scholar] [CrossRef]
- Eller, T.; Vasar, V.; Shlik, J.; Maron, E. Pro-inflammatory cytokines and treatment response to escitalopram in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 445–450. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Jung, H.-G.; Myint, A.-M.; Kim, H.; Park, S.-H. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J. Affect. Disord. 2007, 104, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Freedland, K.E.; Eisen, S.A.; Rich, M.W.; Jaffe, A.S. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol. 1995, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Markovitz, J.H.; Matthews, K.A. Platelets and coronary heart disease: Potential psychophysiologic mechanisms. Psychosom. Med. 1991, 53, 643–668. [Google Scholar] [CrossRef] [PubMed]
- Carney, R.M.; Saunders, R.D.; Freedland, K.E.; Stein, P.; Rich, M.W.; Jaffe, A.S. Association of depression witk reduced heart rate variability in coronary artery disease. Am. J. Cardiol. 1995, 76, 562–564. [Google Scholar] [CrossRef]
- Gottesman, R.F.; Johansen, M.C. Coronary Revascularization and Cognitive Decline: The Patient or the Procedure? JAMA 2021, 325, 1941–1942. [Google Scholar] [CrossRef]
- Whitlock, E.L.; Diaz-Ramirez, L.G.; Smith, A.K.; Boscardin, W.J.; Covinsky, K.E.; Avidan, M.S.; Glymour, M.M. Association of Coronary Artery Bypass Grafting vs Percutaneous Coronary Intervention With Memory Decline in Older Adults Undergoing Coronary Revascularization. JAMA 2021, 325, 1955–1964. [Google Scholar] [CrossRef] [PubMed]
- Selnes, O.A.; Grega, M.A.; Bailey, M.M.; Pham, L.D.; Zeger, S.L.; Baumgartner, W.A.; McKhann, G.M. Do management strategies for coronary artery disease influence 6-year cognitive outcomes? Ann. Thorac. Surg. 2009, 88, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef]
- Whitson, H.E.; Hajduk, A.M.; Song, X.; Geda, M.; Tsang, S.; Brush, J.; Chaudhry, S.I. Comorbid vision and cognitive impairments in older adults hospitalized for acute myocardial infarction. J. Comorbidity 2020, 10, 2235042X20940493. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef]
- Salzwedel, A.; Heidler, M.-D.; Haubold, K.; Schikora, M.; Reibis, R.; Wegscheider, K.; Jöbges, M.; Völler, H. Prevalence of mild cognitive impairment in employable patients after acute coronary event in cardiac rehabilitation. Vasc. Health Risk Manag. 2017, 13, 55–60. [Google Scholar] [CrossRef]
- Lee, Y.J.; Gonzales, E.; Andel, R. Multifaceted Demands of Work and Cognitive Functioning: Findings From the Health and Retirement Study. J. Gerontol. B Psychol. Sci. Soc. Sci. 2022, 77, 351–361. [Google Scholar] [CrossRef]
- Choi, E.; Kim, S.-G.; Zahodne, L.B.; Albert, S.M. Older Workers with Physically Demanding Jobs and their Cognitive Functioning. Ageing Int. 2022, 47, 55–71. [Google Scholar] [CrossRef]
- Mosca, L.; Barrett-Connor, E.; Wenger, N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation 2011, 124, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Chen, Z.; Sun, A.; Deng, X. Gender differences in cardiovascular disease. Med. Nov. Technol. Devices 2019, 4, 100025. [Google Scholar] [CrossRef]
- Kannel, W.B.; Abbott, R.D. Incidence and prognosis of unrecognized myocardial infarction: An update on the Framingham study. N. Engl. J. Med. 1984, 311, 1144–1147. [Google Scholar] [CrossRef] [PubMed]
- Aronson, M.K.; Ooi, W.L.; Morgenstern, H.; Hafner, A.; Masur, D.; Crystal, H.; Frishman, W.H.; Fisher, D.; Katzman, R. Women, myocardial infarction, and dementia in the very old. Neurology 1990, 40, 1102–1106. [Google Scholar] [CrossRef]
- Haring, B.; Leng, X.; Robinson, J.; Johnson, K.C.; Jackson, R.D.; Beyth, R.; Wactawski-Wende, J.; von Ballmoos, M.W.; Goveas, J.S.; Kuller, L.H.; et al. Cardiovascular Disease and Cognitive Decline in Postmenopausal Women: Results From the Women’s Health Initiative Memory Study. J. Am. Heart Assoc. 2023, 2, e000369. [Google Scholar] [CrossRef] [PubMed]
- Dominika, K.; Janusz, R.; Teresa, G.; Tomasz, Ł.; Marek, S.; Jarosław, H.; Paweł, B. COGNITIVE IMPAIRMENT AFTER MYOCARDIAL INFARCTION. J. Am. Coll. Cardiol. 2022, 79 (Suppl. S9), 979. [Google Scholar] [CrossRef]
- Tilvis, R.S.; Kähönen-Väre, M.H.; Jolkkonen, J.; Valvanne, J.; Pitkala, K.H.; Strandberg, T.E. Predictors of cognitive decline and mortality of aged people over a 10-year period. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, M268–M274. [Google Scholar] [CrossRef] [PubMed]
- Murad, K.; Goff, D.C.; Morgan, T.M.; Burke, G.L.; Bartz, T.M.; Kizer, J.R.; Chaudhry, S.I.; Gottdiener, J.S.; Kitzman, D.W. Burden of comorbidities and functional and cognitive impairments in elderly patients at the initial diagnosis of heart failure and their impact on total mortality: The Cardiovascular Health Study. JACC Heart Fail. 2015, 3, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Dodson, J.A.; Truong, T.-T.N.; Towle, V.R.; Kerins, G.; Chaudhry, S.I. Cognitive impairment in older adults with heart failure: Prevalence, documentation, and impact on outcomes. Am. J. Med. 2013, 126, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Ampadu, J.; Morley, J.E. Heart failure and cognitive dysfunction. Int. J. Cardiol. 2015, 178, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Warraich, H.J.; Kitzman, D.W.; Whellan, D.J.; Duncan, P.W.; Mentz, R.J.; Pastva, A.M.; Nelson, M.B.; Upadhya, B.; Reeves, G.R. Physical Function, Frailty, Cognition, Depression, and Quality of Life in Hospitalized Adults ≥60 Years With Acute Decompensated Heart Failure With Preserved Versus Reduced Ejection Fraction. Circ. Heart Fail. 2018, 11, e005254. [Google Scholar] [CrossRef]
- Faulkner, K.M.; Dickson, V.V.; Fletcher, J.; Katz, S.D.; Chang, P.P.; Gottesman, R.F.; Witt, L.S.; Shah, A.M.; D’Eramo Melkus, G. Factors Associated With Cognitive Impairment in Heart Failure With Preserved Ejection Fraction. J. Cardiovasc. Nurs. 2022, 37, 17–30. [Google Scholar] [CrossRef]
- Sloan, F.A.; Trogdon, J.G.; Curtis, L.H.; Schulman, K.A. The Effect of Dementia on Outcomes and Process of Care for Medicare Beneficiaries Admitted with Acute Myocardial Infarction. J. Am. Geriatr. Soc. 2004, 52, 173–181. [Google Scholar] [CrossRef]
- Hajduk, A.M.; Saczynski, J.S.; Tsang, S.; Geda, M.E.; Dodson, J.A.; Ouellet, G.M.; Goldberg, R.J.; Chaudhry, S.I. Presentation, Treatment, and Outcomes of Older Adults Hospitalized for Acute Myocardial Infarction According to Cognitive Status: The SILVER-AMI Study. Am. J. Med. 2021, 134, 910–917. [Google Scholar] [CrossRef]
- Wharton, W.; Goldstein, F.C.; Zhao, L.; Steenland, K.; Levey, A.I.; Hajjar, I. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer’s disease. J. Am. Geriatr. Soc. 2015, 63, 1749–1756. [Google Scholar] [CrossRef]
- Deng, Z.; Jiang, J.; Wang, J.; Pan, D.; Zhu, Y.; Li, H.; Zhang, X.; Liu, X.; Xu, Y.; Li, Y.; et al. Angiotensin Receptor Blockers Are Associated With a Lower Risk of Progression From Mild Cognitive Impairment to Dementia. Hypertension 2022, 79, 2159–2169. [Google Scholar] [CrossRef]
- Lithell, H.; Hansson, L.; Skoog, I.; Elmfeldt, D.; Hofman, A.; Olofsson, B.; Trenkwalder, P.; Zanchetti, A. The Study on Cognition and Prognosis in the Elderly (SCOPE): Principal results of a randomized double-blind intervention trial. J. Hypertens. 2003, 21, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Soto, M.E.; van Kan, G.A.; Nourhashemi, F.; Gillette-Guyonnet, S.; Cesari, M.; Cantet, C.; Rolland, Y.; Vellas, B. Angiotensin-converting enzyme inhibitors and Alzheimer’s disease progression in older adults: Results from the Réseau sur la Maladie d’Alzheimer Français cohort. J. Am. Geriatr. Soc. 2013, 61, 1482–1488. [Google Scholar] [CrossRef] [PubMed]
- O’Caoimh, R.; Healy, L.; Gao, Y.; Svendrovski, A.; Kerins, D.M.; Eustace, J.; Kehoe, P.G.; Guyatt, G.; Molloy, D.W. Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 595–603. [Google Scholar] [CrossRef]
- Latini, R.; Maggioni, A.P.; Flather, M.; Sleight, P.; Tognoni, G. ACE inhibitor use in patients with myocardial infarction. Summary of evidence from clinical trials. Circulation 1995, 92, 3132–3137. [Google Scholar] [CrossRef] [PubMed]
- Kruth, H.S.; Jones, N.L.; Huang, W.; Zhao, B.; Ishii, I.; Chang, J.; Combs, C.A.; Malide, D.; Zhang, W.-Y. Macropinocytosis is the endocytic pathway that mediates macrophage foam cell formation with native low density lipoprotein. J. Biol. Chem. 2005, 280, 2352–2360. [Google Scholar] [CrossRef]
- Ahmed, A. Use of angiotensin-converting enzyme inhibitors in patients with heart failure and renal insufficiency: How concerned should we be by the rise in serum creatinine? J. Am. Geriatr. Soc. 2002, 50, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Schoolwerth, A.C.; Sica, D.A.; Ballermann, B.J.; Wilcox, C.S. Renal Considerations in Angiotensin Converting Enzyme Inhibitor Therapy. Circulation 2001, 104, 1985–1991. [Google Scholar] [CrossRef] [PubMed]
- Barnes, N.M.; Cheng, C.H.; Costall, B.; Naylor, R.J.; Williams, T.J.; Wischik, C.M. Angiotensin converting enzyme density is increased in temporal cortex from patients with Alzheimer’s disease. Eur. J. Pharmacol. 1991, 200, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Staessen, J.A.; Fagard, R.; Thijs, L.; Celis, H.; Arabidze, G.G.; Birkenhäger, W.H.; Bulpitt, C.J.; de Leeuw, P.W.; Dollery, C.T.; Fletcher, A.E.; et al. Randomised double-blind comparison of placebo and active treatment for older patients with isolated systolic hypertension. The Systolic Hypertension in Europe (Syst-Eur) Trial Investigators. Lancet 1997, 350, 757–764. [Google Scholar] [CrossRef]
- PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet 2001, 358, 1033–1041. [Google Scholar] [CrossRef]
- Logsdon, R.G.; Gibbons, L.E.; McCurry, S.M.; Teri, L. Assessing Quality of Life in Older Adults With Cognitive Impairment. Psychosom. Med. 2002, 64, 510–519. Available online: https://journals.lww.com/psychosomaticmedicine/Fulltext/2002/05000/Assessing_Quality_of_Life_in_Older_Adults_With.16.aspx (accessed on 1 July 2023). [CrossRef]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.-T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef]
- Grosser, T.; Fries, S.; FitzGerald, G.A. Biological basis for the cardiovascular consequences of COX-2 inhibition: Therapeutic challenges and opportunities. J. Clin. Investig. 2006, 116, 4–15. [Google Scholar] [CrossRef]
- Choi, S.-H.; Bosetti, F. Cyclooxygenase-1 null mice show reduced neuroinflammation in response to β-amyloid. Aging 2009, 1, 234. [Google Scholar] [CrossRef]
- Choi, S.; Langenbach, R.; Bosetti, F. Genetic deletion or pharmacological inhibition of cyclooxygenase-1 attenuate lipopolysaccharide-induced inflammatory response and brain injury. FASEB J. 2008, 22, 1491–1501. [Google Scholar] [CrossRef]
- Hoozemans, J.J.M.; Rozemuller, A.J.M.; Janssen, I.; De Groot, C.J.A.; Veerhuis, R.; Eikelenboom, P. Cyclooxygenase expression in microglia and neurons in Alzheimer’s disease and control brain. Acta Neuropathol. 2001, 101, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Yermakova, A.V.; Rollins, J.; Callahan, L.M.; Rogers, J.; O’Banion, M.K. Cyclooxygenase-1 in human Alzheimer and control brain: Quantitative analysis of expression by microglia and CA3 hippocampal neurons. J. Neuropathol. Exp. Neurol. 1999, 58, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.-H.; Aid, S.; Bosetti, F. The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: Implications for translational research. Trends Pharmacol. Sci. 2009, 30, 174–181. [Google Scholar] [CrossRef]
- Raum, E.; Rothenbacher, D.; Löw, M.; Stegmaier, C.; Ziegler, H.; Brenner, H. Changes of cardiovascular risk factors and their implications in subsequent birth cohorts of older adults in Germany: A life course approach. Eur. J. Prev. Cardiol. 2007, 14, 809–814. [Google Scholar] [CrossRef]
- Mai Nguyen, T.N.; Chen, L.-J.; Trares, K.; Stocker, H.; Holleczek, B.; Beyreuther, K.; Brenner, H.; Schöttker, B. Long-term low-dose acetylsalicylic use shows protective potential for the development of both vascular dementia and Alzheimer’s disease in patients with coronary heart disease but not in other individuals from the general population: Results from two large cohort studies. medRxiv 2021. medRxiv:2021.09.20.21263830. [Google Scholar] [CrossRef]
- McNeil, J.J.; Woods, R.L.; Nelson, M.R.; Reid, C.M.; Kirpach, B.; Wolfe, R.; Storey, E.; Shah, R.C.; Lockery, J.E.; Tonkin, A.M.; et al. Effect of Aspirin on Disability-free Survival in the Healthy Elderly. N. Engl. J. Med. 2018, 379, 1499–1508. [Google Scholar] [CrossRef]
- Parish, S.; Mafham, M.; Offer, A.; Barton, J.; Wallendszus, K.; Stevens, W.; Buck, G.; Haynes, R.; Collins, R.; Bowman, L.; et al. Effects of aspirin on dementia and cognitive function in diabetic patients: The ASCEND trial. Eur. Heart J. 2022, 43, 2010–2019. [Google Scholar] [CrossRef]
- Ryan, J.; Storey, E.; Murray, A.M.; Woods, R.L.; Wolfe, R.; Reid, C.M.; Nelson, M.R.; Chong, T.T.J.; Williamson, J.D.; Ward, S.A.; et al. Randomized placebo-controlled trial of the effects of aspirin on dementia and cognitive decline. Neurology 2020, 95, e320–e331. [Google Scholar] [CrossRef] [PubMed]
- Jordan, F.; Quinn, T.J.; McGuinness, B.; Passmore, P.; Kelly, J.P.; Tudur Smith, C.; Murphy, K.; Devane, D. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst. Rev. 2020, 4, CD011459. [Google Scholar] [CrossRef] [PubMed]
- Davis, K.A.S.; Bishara, D.; Molokhia, M.; Mueller, C.; Perera, G.; Stewart, R.J. Aspirin in people with dementia, long-term benefits, and harms: A systematic review. Eur. J. Clin. Pharmacol. 2021, 77, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kaida-Yip, F.; Zabel, M. NSAID Use and the Prevention of Alzheimer’s Disease: A Meta-Analysis (P6.184). Neurology 2018, 90 (Suppl. S15), P6.184. [Google Scholar]
- Szekely, C.A.; Breitner, J.C.S.; Fitzpatrick, A.L.; Rea, T.D.; Psaty, B.M.; Kuller, L.H.; Zandi, P.P. NSAID use and dementia risk in the Cardiovascular Health Study: Role of APOE and NSAID type. Neurology 2008, 70, 17–24. [Google Scholar] [CrossRef]
- Douiri, A.; McKevitt, C.; Emmett, E.S.; Rudd, A.G.; Wolfe, C.D.A. Long-term effects of secondary prevention on cognitive function in stroke patients. Circulation 2013, 128, 1341–1348. [Google Scholar] [CrossRef]
- Nilsson, S.E.; Johansson, B.; Takkinen, S.; Berg, S.; Zarit, S.; McClearn, G.; Melander, A. Does aspirin protect against Alzheimer’s dementia? A study in a Swedish population-based sample aged > or =80 years. Eur. J. Clin. Pharmacol. 2003, 59, 313–319. [Google Scholar] [CrossRef]
- Dregan, A.; Chowienczyk, P.; Armstrong, D. Patterns of anti-inflammatory drug use and risk of dementia: A matched case-control study. Eur. J. Neurol. 2015, 22, 1421–1428. [Google Scholar] [CrossRef]
- Zandi, P.P.; Anthony, J.C.; Hayden, K.M.; Mehta, K.; Mayer, L.; Breitner, J.C.S. Reduced incidence of AD with NSAID but not H2 receptor antagonists: The Cache County Study. Neurology 2002, 59, 880–886. [Google Scholar] [CrossRef]
- Breitner, J.C.; Gau, B.A.; Welsh, K.A.; Plassman, B.L.; McDonald, W.M.; Helms, M.J.; Anthony, J.C. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: Initial results of a co-twin control study. Neurology 1994, 44, 227–232. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Grodstein, F.; Bienias, J.L.; Schneider, J.A.; Wilson, R.S.; Kelly, J.F.; Evans, D.A.; Bennett, D.A. Relation of NSAIDs to incident AD, change in cognitive function, and AD pathology. Neurology 2008, 70, 2219–2225. [Google Scholar] [CrossRef] [PubMed]
- Cornelius, C.; Fastbom, J.; Winblad, B.; Viitanen, M. Aspirin, NSAIDs, risk of dementia, and influence of the apolipoprotein E epsilon 4 allele in an elderly population. Neuroepidemiology 2004, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Gill, S.; Samii, A. Effect of non-steroidal anti-inflammatory drugs on risk of Alzheimer’s disease: Systematic review and meta-analysis of observational studies. BMJ 2003, 327, 128. [Google Scholar] [CrossRef]
- Alexander, P.; Visagan, S.; Jawhar, S.; Kare, A.; Issa, N.; Issa, R.; Jawhar, A.; Thomas, S.; Gorantla, V. Antiplatelets and Vascular Dementia: A Systematic Review. J. Aging Res. 2022, 2022, 9780067. [Google Scholar] [CrossRef]
- Wang, C.; Yi, X.; Zhang, B.; Liao, D.; Lin, J.; Chi, L. Clopidogrel Plus Aspirin Prevents Early Neurologic Deterioration and Improves 6-Month Outcome in Patients With Acute Large Artery Atherosclerosis Stroke. Clin. Appl. Thromb. 2014, 21, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Stewart, W.F.; Kawas, C.; Corrada, M.; Metter, E.J. Risk of Alzheimer’s disease and duration of NSAID use. Neurology 1997, 48, 626–632. [Google Scholar] [CrossRef]
- Vermond, R.A.; Van Gelder, I.C.; Crijns, H.J.; Rienstra, M. Does myocardial infarction beget atrial fibrillation and atrial fibrillation beget myocardial infarction? Circulation 2015, 131, 1824–1826. [Google Scholar] [CrossRef]
- Garg, L.; Agrawal, S.; Agarwal, M.; Shah, M.; Garg, A.; Patel, B.; Agarwal, N.; Nanda, S.; Sharma, A.; Cox, D. Influence of atrial fibrillation on outcomes in patients who underwent primary percutaneous coronary intervention for ST-segment elevation myocardial infarction. Am. J. Cardiol. 2018, 121, 684–689. [Google Scholar] [CrossRef]
- Jabre, P.; Roger, V.L.; Murad, M.H.; Chamberlain, A.M.; Prokop, L.; Adnet, F.; Jouven, X. Mortality associated with atrial fibrillation in patients with myocardial infarction: A systematic review and meta-analysis. Circulation 2011, 123, 1587–1593. [Google Scholar] [CrossRef]
- Siu, C.-W.; Jim, M.-H.; Ho, H.-H.; Miu, R.; Lee, S.W.L.; Lau, C.-P.; Tse, H.-F. Transient atrial fibrillation complicating acute inferior myocardial infarction: Implications for future risk of ischemic stroke. Chest 2007, 132, 44–49. [Google Scholar] [CrossRef]
- Wańkowicz, P.; Staszewski, J.; Dębiec, A.; Nowakowska-Kotas, M.; Szylińska, A.; Turoń-Skrzypińska, A.; Rotter, I. Pre-Stroke Statin Therapy Improves In-Hospital Prognosis Following Acute Ischemic Stroke Associated with Well-Controlled Nonvalvular Atrial Fibrillation. J. Clin. Med. 2021, 10, 3036. [Google Scholar] [CrossRef]
- Choi, K.-H.; Seo, W.-K.; Park, M.-S.; Kim, J.-T.; Chung, J.-W.; Bang, O.Y.; Kim, G.-M.; Song, T.-J.; Kim, B.J.; Heo, S.H.; et al. Effect of Statin Therapy on Outcomes of Patients With Acute Ischemic Stroke and Atrial Fibrillation. J. Am. Heart Assoc. 2019, 8, e013941. [Google Scholar] [CrossRef] [PubMed]
- Tu, H.T.H.; Campbell, B.C.V.; Christensen, S.; Collins, M.; De Silva, D.A.; Butcher, K.S.; Parsons, M.W.; Desmond, P.M.; Barber, P.A.; Levi, C.R. Pathophysiological determinants of worse stroke outcome in atrial fibrillation. Cerebrovasc. Dis. 2010, 30, 389–395. [Google Scholar] [CrossRef]
- Horwich, T.B.; Middlekauff, H.R. Potential autonomic nervous system effects of statins in heart failure. Heart Fail. Clin. 2008, 4, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Loppnow, H.; Zhang, L.; Buerke, M.; Lautenschläger, M.; Chen, L.; Frister, A.; Schlitt, A.; Luther, T.; Song, N.; Hofmann, B. Statins potently reduce the cytokine-mediated IL-6 release in SMC/MNC cocultures. J. Cell. Mol. Med. 2011, 15, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Pliquett, R.U.; Cornish, K.G.; Peuler, J.D.; Zucker, I.H. Simvastatin normalizes autonomic neural control in experimental heart failure. Circulation 2003, 107, 2493–2498. [Google Scholar] [CrossRef]
- Dechend, R.; Fiebeler, A.; Park, J.-K.; Muller, D.N.; Theuer, J.; Mervaala, E.; Bieringer, M.; Gulba, D.; Dietz, R.; Luft, F.C. Amelioration of angiotensin II–induced cardiac injury by a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor. Circulation 2001, 104, 576–581. [Google Scholar] [CrossRef][Green Version]
- Lefer, D.J. Statins as potent antiinflammatory drugs. Circulation 2002, 106, 2041–2042. [Google Scholar] [CrossRef]
- von Haehling, S.; Anker, S.D.; Bassenge, E. Statins and the role of nitric oxide in chronic heart failure. Heart Fail. Rev. 2003, 8, 99–106. [Google Scholar] [CrossRef]
- Hayashidani, S.; Tsutsui, H.; Shiomi, T.; Suematsu, N.; Kinugawa, S.; Ide, T.; Wen, J.; Takeshita, A. Fluvastatin, a 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitor, attenuates left ventricular remodeling and failure after experimental myocardial infarction. Circulation 2002, 105, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Safieh, M.; Korczyn, A.D.; Michaelson, D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019, 17, 64. [Google Scholar] [CrossRef]
- Casserly, I.; Topol, E.J. Convergence of atherosclerosis and Alzheimer’s disease: Inflammation, cholesterol, and misfolded proteins. Lancet 2004, 363, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Lumsden, A.L.; Mulugeta, A.; Zhou, A.; Hyppönen, E. Apolipoprotein E (APOE) genotype-associated disease risks: A phenome-wide, registry-based, case-control study utilising the UK Biobank. EBioMedicine 2020, 59, 102954. [Google Scholar] [CrossRef]
- Joseph, S.B.; Castrillo, A.; Laffitte, B.A.; Mangelsdorf, D.J.; Tontonoz, P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat. Med. 2003, 9, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, R.K.; Singh, N. Liver X receptors: Emerging therapeutic targets for Alzheimer’s disease. Pharmacol. Res. 2013, 72, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wolozin, B.; Kellman, W.; Ruosseau, P.; Celesia, G.G.; Siegel, G. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methyglutaryl coenzyme A reductase inhibitors. Arch. Neurol. 2000, 57, 1439–1443. [Google Scholar] [CrossRef]
- Jick, H.; Zornberg, G.L.; Jick, S.S.; Seshadri, S.; Drachman, D.A. Statins and the risk of dementia. Lancet 2000, 356, 1627–1631. [Google Scholar] [CrossRef]
- Rockwood, K.; Kirkland, S.; Hogan, D.B.; MacKnight, C.; Merry, H.; Verreault, R.; Wolfson, C.; McDowell, I. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch. Neurol. 2002, 59, 223–227. [Google Scholar] [CrossRef]
- Santanello, N.C.; Barber, B.L.; Applegate, W.B.; Elam, J.; Curtis, C.; Hunninghake, D.B.; Gordon, D.J. Effect of pharmacologic lipid lowering on health-related quality of life in older persons: Results from the Cholesterol Reduction in Seniors Program (CRISP) Pilot Study. J. Am. Geriatr. Soc. 1997, 45, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Trompet, S.; van Vliet, P.; de Craen, A.J.M.; Jolles, J.; Buckley, B.M.; Murphy, M.B.; Ford, I.; Macfarlane, P.W.; Sattar, N.; Packard, C.J.; et al. Pravastatin and cognitive function in the elderly. Results of the PROSPER study. J. Neurol. 2010, 257, 85–90. [Google Scholar] [CrossRef]
- Zandi, P.P.; Sparks, D.L.; Khachaturian, A.S.; Tschanz, J.; Norton, M.; Steinberg, M.; Welsh-Bohmer, K.A.; Breitner, J.C.S. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch. Gen. Psychiatry 2005, 62, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Schatz, I.J.; Masaki, K.; Yano, K.; Chen, R.; Rodriguez, B.L.; Curb, J.D. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: A cohort study. Lancet 2001, 358, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Bryant-Thomas, T.K.; Herbert, D.; Pacheco, J.; Fabra Garcia, M.; Manjon, M.; Girones, X.; Henry, T.L.; Matsubara, E.; Zambon, D.; et al. Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Neurology 2003, 61, 199–205. [Google Scholar] [CrossRef]
- Olmastroni, E.; Molari, G.; De Beni, N.; Colpani, O.; Galimberti, F.; Gazzotti, M.; Zambon, A.; Catapano, A.L.; Casula, M. Statin use and risk of dementia or Alzheimer’s disease: A systematic review and meta-analysis of observational studies. Eur. J. Prev. Cardiol. 2022, 29, 804–814. [Google Scholar] [CrossRef]
- Zoungas, S.; Curtis, A.; Spark, S.; Wolfe, R.; McNeil, J.J.; Beilin, L.; Chong, T.T.-J.; Cloud, G.; Hopper, I.; Kost, A.; et al. Statins for extension of disability-free survival and primary prevention of cardiovascular events among older people: Protocol for a randomised controlled trial in primary care (STAREE trial). BMJ Open 2023, 13, e069915. [Google Scholar] [CrossRef]
- Joseph, J.; Pajewski, N.M.; Dolor, R.J.; Sellers, M.A.; Perdue, L.H.; Peeples, S.R.; Henrie, A.M.; Woolard, N.; Jones, W.S.; Benziger, C.P.; et al. Pragmatic evaluation of events and benefits of lipid lowering in older adults (PREVENTABLE): Trial design and rationale. J. Am. Geriatr. Soc. 2023, 71, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Ciobica, A.; Bild, W.; Hritcu, L.; Haulica, I. Brain renin-angiotensin system in cognitive function: Pre-clinical findings and implications for prevention and treatment of dementia. Acta Neurol. Belg. 2009, 109, 171–180. [Google Scholar]
- Kehoe, P.G. The Coming of Age of the Angiotensin Hypothesis in Alzheimer’s Disease: Progress Toward Disease Prevention and Treatment? J. Alzheimer’s Dis. 2018, 62, 1443–1466. [Google Scholar] [CrossRef]
- Brasier, A.R.; Recinos, A., 3rd; Eledrisi, M.S. Vascular inflammation and the renin-angiotensin system. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Hirawa, N.; Uehara, Y.; Kawabata, Y.; Numabe, A.; Gomi, T.; Ikeda, T.; Suzuki, T.; Goto, A.; Toyo-oka, T.; Omata, M. Long-Term Inhibition of Renin-Angiotensin System Sustains Memory Function in Aged Dahl Rats. Hypertension 1999, 34, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Michikawa, M. Angiotensin-converting enzyme as a potential target for treatment of Alzheimer’s disease: Inhibition or activation? Rev. Neurosci. 2008, 19, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.J.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Sink, K.M.; Leng, X.; Williamson, J.; Kritchevsky, S.B.; Yaffe, K.; Kuller, L.; Yasar, S.; Atkinson, H.; Robbins, M.; Psaty, B.; et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009, 169, 1195–1202. [Google Scholar] [CrossRef]
- Gao, Y.; O’Caoimh, R.; Healy, L.; Kerins, D.M.; Eustace, J.; Guyatt, G.; Sammon, D.; Molloy, D.W. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open 2013, 3, e002881. [Google Scholar] [CrossRef]
- Horiuchi, M.; Mogi, M.; Iwai, M. The angiotensin II type 2 receptor in the brain. J. Renin. Angiotensin. Aldosterone. Syst. 2010, 11, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Zhao, L.; Steenland, K.; Goldstein, F.C.; Schneider, J.A.; Barnes, L.L.; Gearing, M.; Yasar, S. Neurofibrillary tangles and conversion to mild cognitive impairment with certain antihypertensives. J. Alzheimer’s Dis. 2019, 70, 153–161. [Google Scholar] [CrossRef]
- Lehmann, D.J.; Cortina-Borja, M.; Warden, D.R.; Smith, A.D.; Sleegers, K.; Prince, J.A.; van Duijn, C.M.; Kehoe, P.G. Large meta-analysis establishes the ACE insertion-deletion polymorphism as a marker of Alzheimer’s disease. Am. J. Epidemiol. 2005, 162, 305–317. [Google Scholar] [CrossRef]
- Li, N.-C.; Lee, A.; Whitmer, R.A.; Kivipelto, M.; Lawler, E.; Kazis, L.E.; Wolozin, B. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: Prospective cohort analysis. BMJ 2010, 340, b5465. [Google Scholar] [CrossRef]
- Ye, R.; Hu, Y.; Yao, A.; Yang, Y.; Shi, Y.; Jiang, Y.; Zhang, J. Impact of renin-angiotensin system-targeting antihypertensive drugs on treatment of Alzheimer’s disease: A meta-analysis. Int. J. Clin. Pract. 2015, 69, 674–681. [Google Scholar] [CrossRef]
- Anderson, C.; Teo, K.; Gao, P.; Arima, H.; Dans, A.; Unger, T.; Commerford, P.; Dyal, L.; Schumacher, H.; Pogue, J.; et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: Analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011, 10, 43–53. [Google Scholar] [CrossRef]
- Gouveia, F.; Camins, A.; Ettcheto, M.; Bicker, J.; Falcão, A.; Cruz, M.T.; Fortuna, A. Targeting brain Renin-Angiotensin System for the prevention and treatment of Alzheimer’s disease: Past, present and future. Ageing Res. Rev. 2022, 77, 101612. [Google Scholar] [CrossRef]
- den Brok, M.G.H.E.; van Dalen, J.W.; Abdulrahman, H.; Larson, E.B.; van Middelaar, T.; van Gool, W.A.; van Charante, E.P.M.; Richard, E. Antihypertensive Medication Classes and the Risk of Dementia: A Systematic Review and Network Meta-Analysis. J. Am. Med. Dir. Assoc. 2021, 22, 1386–1395.e15. [Google Scholar] [CrossRef] [PubMed]
- Andrade, C. β-Blockers and the Risk of New-Onset Depression: Meta-analysis Reassures, but the Jury Is Still Out. J. Clin. Psychiatry 2021, 82, 34035. [Google Scholar] [CrossRef] [PubMed]
- Luijendijk, H.J.; Koolman, X. The incentive to publish negative studies: How beta-blockers and depression got stuck in the publication cycle. J. Clin. Epidemiol. 2012, 65, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Waal, H.J. Propranolol-induced depression. Br. Med. J. 1967, 2, 50. [Google Scholar] [CrossRef]
- Kessing, L.V.; Rytgaard, H.C.; Ekstrøm, C.T.; Torp-Pedersen, C.; Berk, M.; Gerds, T.A. Antihypertensive Drugs and Risk of Depression: A Nationwide Population-Based Study. Hypertension 2020, 76, 1263–1279. [Google Scholar] [CrossRef]
- Gilstrap, L.; Cohen, A.; Ouellet, G.M.; Goyal, P.; Gladders, B.; Flint, D.; Skinner, J. The association between beta-blockers and outcomes in patients with heart failure and concurrent Alzheimer’s disease and related dementias. J. Am. Geriatr. Soc. 2023, 71, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Duprey, M.S.; Devlin, J.W.; Griffith, J.L.; Travison, T.G.; Briesacher, B.A.; Jones, R.; Saczynski, J.S.; Schmitt, E.M.; Gou, Y.; Marcantonio, E.R.; et al. Association between Perioperative Medication Use and Postoperative Delirium and Cognition in Older Adults Undergoing Elective Noncardiac Surgery. Anesth. Analg. 2022, 134, 1154–1163. [Google Scholar] [CrossRef]
- Evans, A.K.; Park, H.H.; Saw, N.L.; Singhal, K.; Ogawa, G.; Leib, R.D.; Shamloo, M. Age-related neuroinflammation and pathology in the locus coeruleus and hippocampus: Beta-adrenergic antagonists exacerbate impairment of learning and memory in aged mice. Neurobiol. Aging 2021, 106, 241–256. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.K.; Ardestani, P.M.; Yi, B.; Park, H.H.; Lam, R.K.; Shamloo, M. Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s Disease. Neurobiol. Dis. 2020, 146, 105089. [Google Scholar] [CrossRef]
- Holm, H.; Ricci, F.; Di Martino, G.; Bachus, E.; Nilsson, E.D.; Ballerini, P.; Melander, O.; Hansson, O.; Nägga, K.; Magnusson, M.; et al. Beta-blocker therapy and risk of vascular dementia: A population-based prospective study. Vascul. Pharmacol. 2020, 125–126, 106649. [Google Scholar] [CrossRef] [PubMed]
- Safarudin, F.; Iloabuchi, C.O.; Ladani, A.; Sambamoorthi, U. The Association of Beta-Blocker Use to Cognitive Impairment among Adults with Hypertension or Cardiovascular Diseases in the United States. Chronic pain Manag. 2020, 4, 125. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.-T.; Wang, N.-D.; Ma, T.; Jiang, H.; Guan, J.; Tan, L. Roles of β-adrenergic receptors in Alzheimer’s disease: Implications for novel therapeutics. Brain Res. Bull. 2011, 84, 111–117. [Google Scholar] [CrossRef]
- Steinman, M.A.; Zullo, A.R.; Lee, Y.; Daiello, L.A.; Boscardin, W.J.; Dore, D.D.; Gan, S.; Fung, K.; Lee, S.J.; Komaiko, K.D.R.; et al. Association of β-Blockers With Functional Outcomes, Death, and Rehospitalization in Older Nursing Home Residents After Acute Myocardial Infarction. JAMA Intern. Med. 2017, 177, 254–262. [Google Scholar] [CrossRef]
- Vitagliano, G.; Curtis, J.P.; Concato, J.; Feinstein, A.R.; Radford, M.J.; Krumholz, H.M. Association between functional status and use and effectiveness of beta-blocker prophylaxis in elderly survivors of acute myocardial infarction. J. Am. Geriatr. Soc. 2004, 52, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Lanham, D.; Ali, S.; Davis, D.; Rawle, M.J. Beta-Blockers for the Secondary Prevention of Myocardial Infarction in People with Dementia: A Systematic Review. J. Alzheimer’s Dis. 2019, 71, 1105–1114. [Google Scholar] [CrossRef]
- Pasquali, S.K.; Alexander, K.P.; Coombs, L.P.; Lytle, B.L.; Peterson, E.D. Effect of cardiac rehabilitation on functional outcomes after coronary revascularization. Am. Heart J. 2003, 145, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Suaya, J.A.; Stason, W.B.; Ades, P.A.; Normand, S.-L.T.; Shepard, D.S. Cardiac rehabilitation and survival in older coronary patients. J. Am. Coll. Cardiol. 2009, 54, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hammill, B.G.; Curtis, L.H.; Schulman, K.A.; Whellan, D.J. Relationship between cardiac rehabilitation and long-term risks of death and myocardial infarction among elderly Medicare beneficiaries. Circulation 2010, 121, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Landi, F.; Russo, A.; Barillaro, C.; Cesari, M.; Pahor, M.; Danese, P.; Bernabei, R.; Onder, G. Physical activity and risk of cognitive impairment among older persons living in the community. Aging Clin. Exp. Res. 2007, 19, 410–416. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Erickson, K.I.; Scalf, P.E.; Kim, J.S.; Prakash, R.; McAuley, E.; Elavsky, S.; Marquez, D.X.; Hu, L.; Kramer, A.F. Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A. Biol. Sci. Med. Sci. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Lopes, M.; Fregni, F. A systematic review and meta-analysis of clinical studies on major depression and BDNF levels: Implications for the role of neuroplasticity in depression. Int. J. Neuropsychopharmacol. 2008, 11, 1169–1180. [Google Scholar] [CrossRef]
- Weinberg, R.S.; Gould, D. Foundations of Sport and Exercise Psychology; Human Kinetics: Champaign, IL, USA, 2023; ISBN 171820759X. [Google Scholar]
- Taylor, R.S.; Brown, A.; Ebrahim, S.; Jolliffe, J.; Noorani, H.; Rees, K.; Skidmore, B.; Stone, J.A.; Thompson, D.R.; Oldridge, N. Exercise-based rehabilitation for patients with coronary heart disease: Systematic review and meta-analysis of randomized controlled trials. Am. J. Med. 2004, 116, 682–692. [Google Scholar] [CrossRef]
- Dabbaghipour, N.; Javaherian, M.; Moghadam, B.A. Effects of cardiac rehabilitation on cognitive impairments in patients with cardiovascular diseases: A systematic review. Int. J. Neurosci. 2021, 131, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Pendlebury, S.T.; Welch, S.J.V.; Cuthbertson, F.C.; Mariz, J.; Mehta, Z.; Rothwell, P.M. Telephone assessment of cognition after transient ischemic attack and stroke: Modified telephone interview of cognitive status and telephone Montreal Cognitive Assessment versus face-to-face Montreal Cognitive Assessment and neuropsychological battery. Stroke 2013, 44, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Reynish, E.L.; Hapca, S.M.; De Souza, N.; Cvoro, V.; Donnan, P.T.; Guthrie, B. Epidemiology and outcomes of people with dementia, delirium, and unspecified cognitive impairment in the general hospital: Prospective cohort study of 10,014 admissions. BMC Med. 2017, 15, 140. [Google Scholar] [CrossRef]
- Hapca, S.; Guthrie, B.; Cvoro, V.; Bu, F.; Rutherford, A.C.; Reynish, E.; Donnan, P.T. Mortality in people with dementia, delirium, and unspecified cognitive impairment in the general hospital: Prospective cohort study of 6724 patients with 2 years follow-up. Clin. Epidemiol. 2018, 10, 1743–1753. [Google Scholar] [CrossRef]
- Urcun, Y.S.; Altun, Y.; Pala, A.A. Early and late predictors of postoperative neurocognitive dysfunction in cardiac surgery. Clin. Neurosci. 2022, 75, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Ormseth, C.H.; LaHue, S.C.; Oldham, M.A.; Josephson, S.A.; Whitaker, E.; Douglas, V.C. Predisposing and Precipitating Factors Associated With Delirium: A Systematic Review. JAMA Netw. Open 2023, 6, e2249950. [Google Scholar] [CrossRef] [PubMed]
- Lowenstern, A.; Wang, T.Y. Rethinking Cognitive Impairment in the Management of Older Patients with Cardiovascular Disease. J. Am. Heart Assoc. 2019, 8, e011968. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thong, E.H.E.; Quek, E.J.W.; Loo, J.H.; Yun, C.-Y.; Teo, Y.N.; Teo, Y.H.; Leow, A.S.T.; Li, T.Y.W.; Sharma, V.K.; Tan, B.Y.Q.; et al. Acute Myocardial Infarction and Risk of Cognitive Impairment and Dementia: A Review. Biology 2023, 12, 1154. https://doi.org/10.3390/biology12081154
Thong EHE, Quek EJW, Loo JH, Yun C-Y, Teo YN, Teo YH, Leow AST, Li TYW, Sharma VK, Tan BYQ, et al. Acute Myocardial Infarction and Risk of Cognitive Impairment and Dementia: A Review. Biology. 2023; 12(8):1154. https://doi.org/10.3390/biology12081154
Chicago/Turabian StyleThong, Elizabeth Hui En, Ethan J. W. Quek, Jing Hong Loo, Choi-Ying Yun, Yao Neng Teo, Yao Hao Teo, Aloysius S. T. Leow, Tony Y. W. Li, Vijay K. Sharma, Benjamin Y. Q. Tan, and et al. 2023. "Acute Myocardial Infarction and Risk of Cognitive Impairment and Dementia: A Review" Biology 12, no. 8: 1154. https://doi.org/10.3390/biology12081154
APA StyleThong, E. H. E., Quek, E. J. W., Loo, J. H., Yun, C.-Y., Teo, Y. N., Teo, Y. H., Leow, A. S. T., Li, T. Y. W., Sharma, V. K., Tan, B. Y. Q., Yeo, L. L. L., Chong, Y. F., Chan, M. Y., & Sia, C.-H. (2023). Acute Myocardial Infarction and Risk of Cognitive Impairment and Dementia: A Review. Biology, 12(8), 1154. https://doi.org/10.3390/biology12081154







