Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
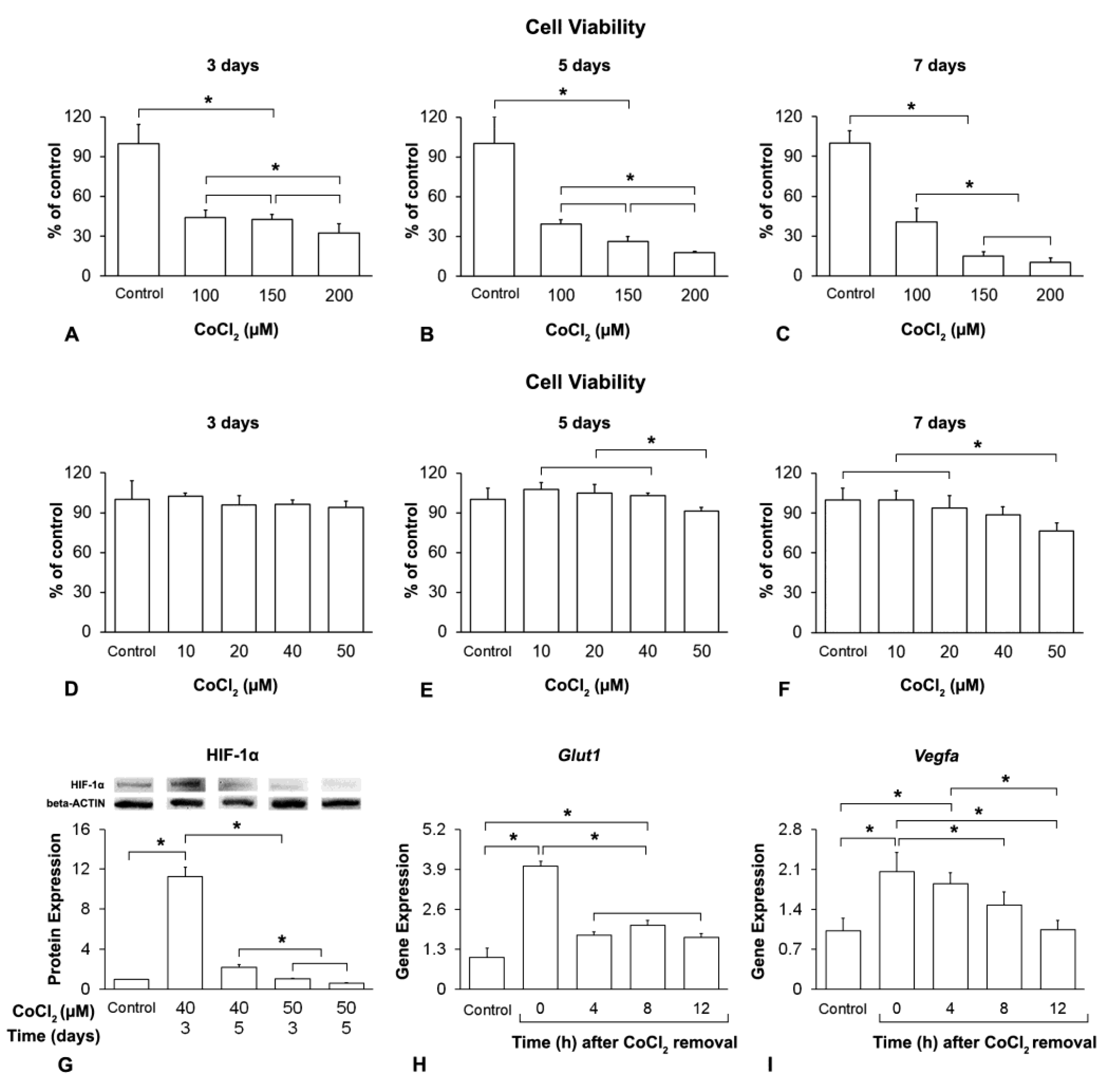
2.1. Hypoxia Induction and Duration of Its Effect on MSCs+BMP-9
2.1.1. MSCs+BMP-9
2.1.2. Analysis of the Cell Viability by Colorimetric Assay
2.1.3. Analysis of the HIF-1α Protein Expression by Western Blot
2.1.4. Analysis of the Glut1 and Vegfa Gene Expression by Real-Time Polymerase Chain Reaction (RT-qPCR)
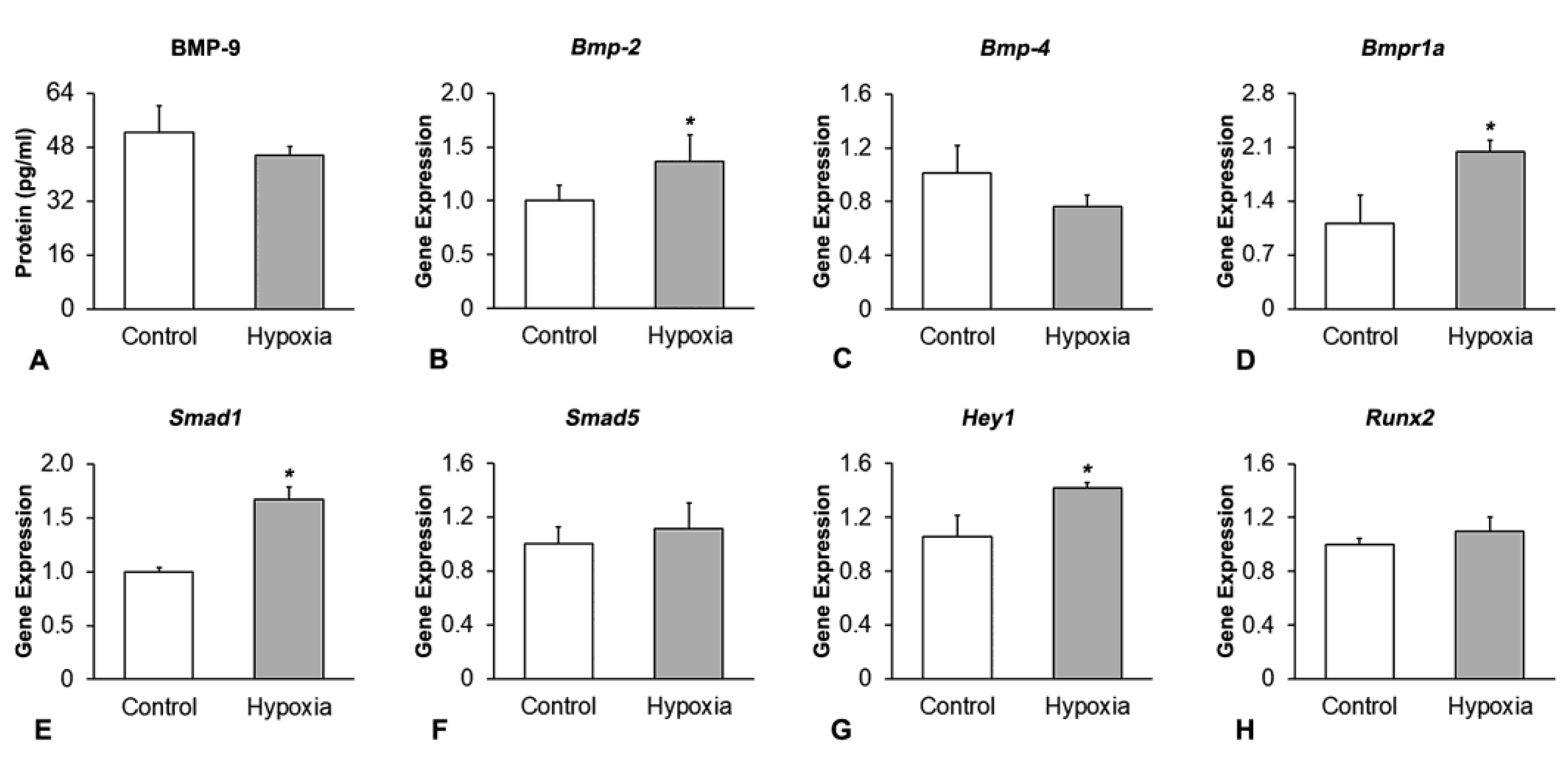
2.2. Effect of Hypoxia on the Expression of Components and Targets of the BMP Signaling Pathway in MSCs+BMP-9
2.2.1. Analysis of the BMP-9 Protein Expression by Enzyme-Linked Immunosorbent Assay (ELISA)
2.2.2. Analysis of the Gene Expression of Components and Targets of the BMP Signaling Pathway by RT-qPCR
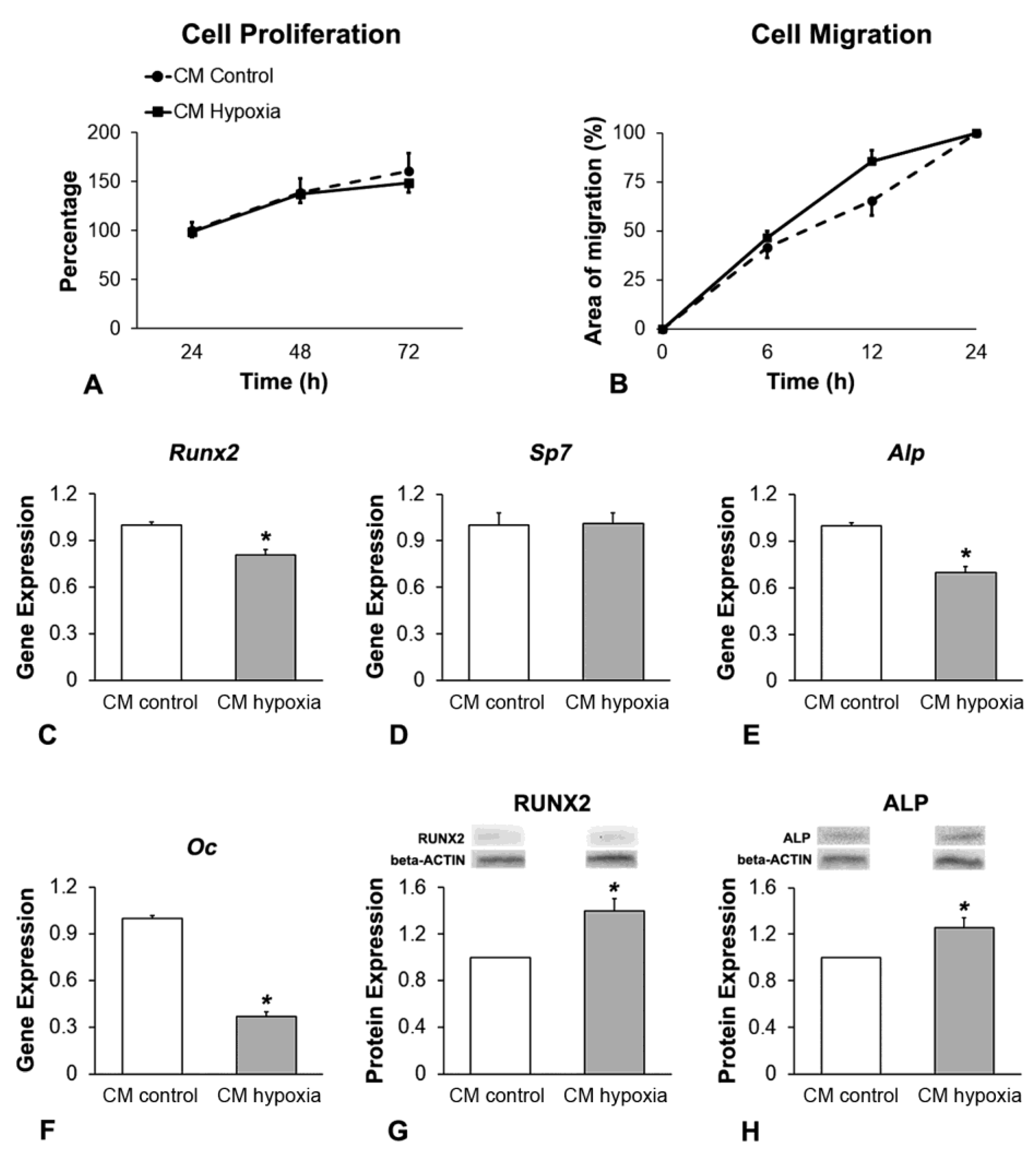
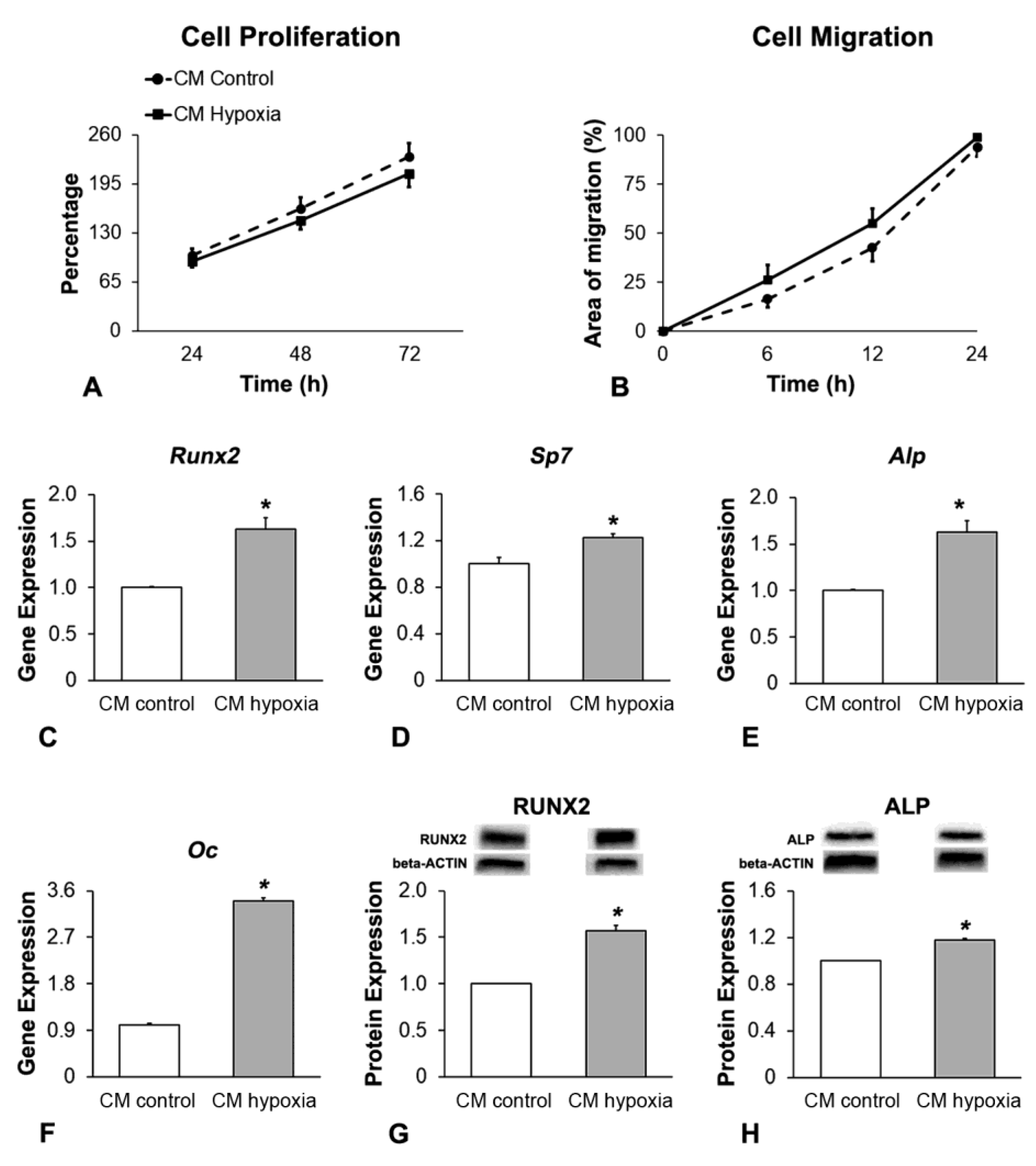
2.3. Effect of CM of MSCs+BMP-9 Primed with Hypoxia on Cell Proliferation, Migration and Osteoblast Differentiation of MSCs and MC3T3-E1 Cells
2.3.1. CM Production
2.3.2. MSCs
2.3.3. MC3T3-E1 Cells
2.3.4. Analysis of the Cell Proliferation by Colorimetric Assay
2.3.5. Analysis of the Cell Migration by Scratch Method
2.3.6. Analysis of the Gene Expression of Osteoblast Markers by RT-qPCR
2.3.7. Analysis of the RUNX2 and ALP Protein Expression by Western Blot
2.4. Effect of MSCs+BMP-9 Primed with Hypoxia on Bone Repair of Rat Calvarial Defects
2.4.1. Surgical Procedure to Create and Treatment of Calvarial Defects
2.4.2. Microtomographic (µCT) Analysis
2.4.3. Histological Analysis
2.5. Statistical Analyses
3. Results
3.1. Hypoxia Induction and Duration of Its Effect on MSCs+BMP-9
3.2. Effect of Hypoxia on the Expression of Components and Targets of the BMP Signaling Pathway in MSCs+BMP-9
3.3. Effect of CM of MSCs+BMP-9 Primed with Hypoxia on Cell Proliferation, Migration and Osteoblast Differentiation of MSCs
3.4. Effect of CM of MSCs+BMP-9 Primed with Hypoxia on Cell Proliferation, Migration and Osteoblast Differentiation of MC3T3-E1 Cells
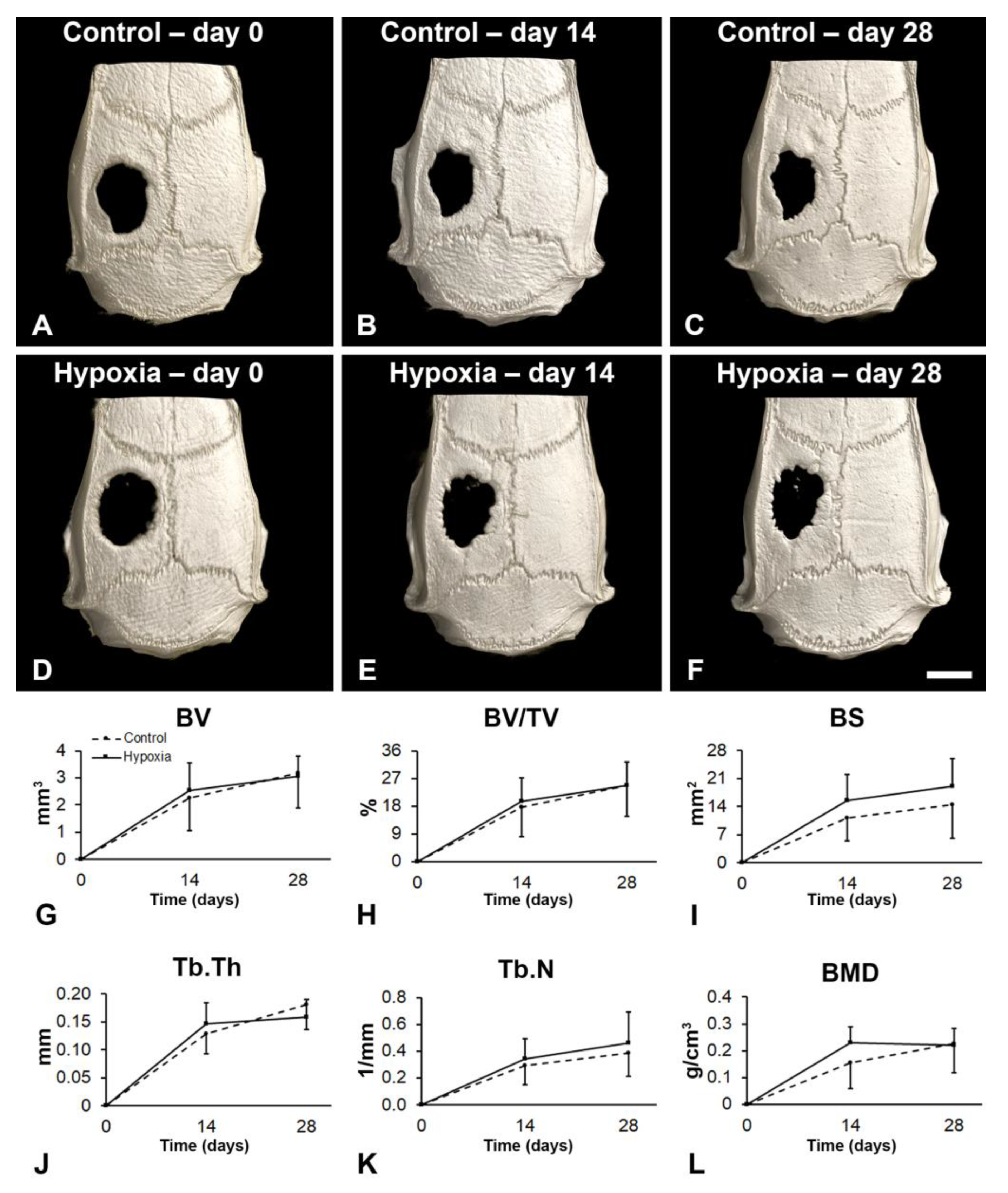
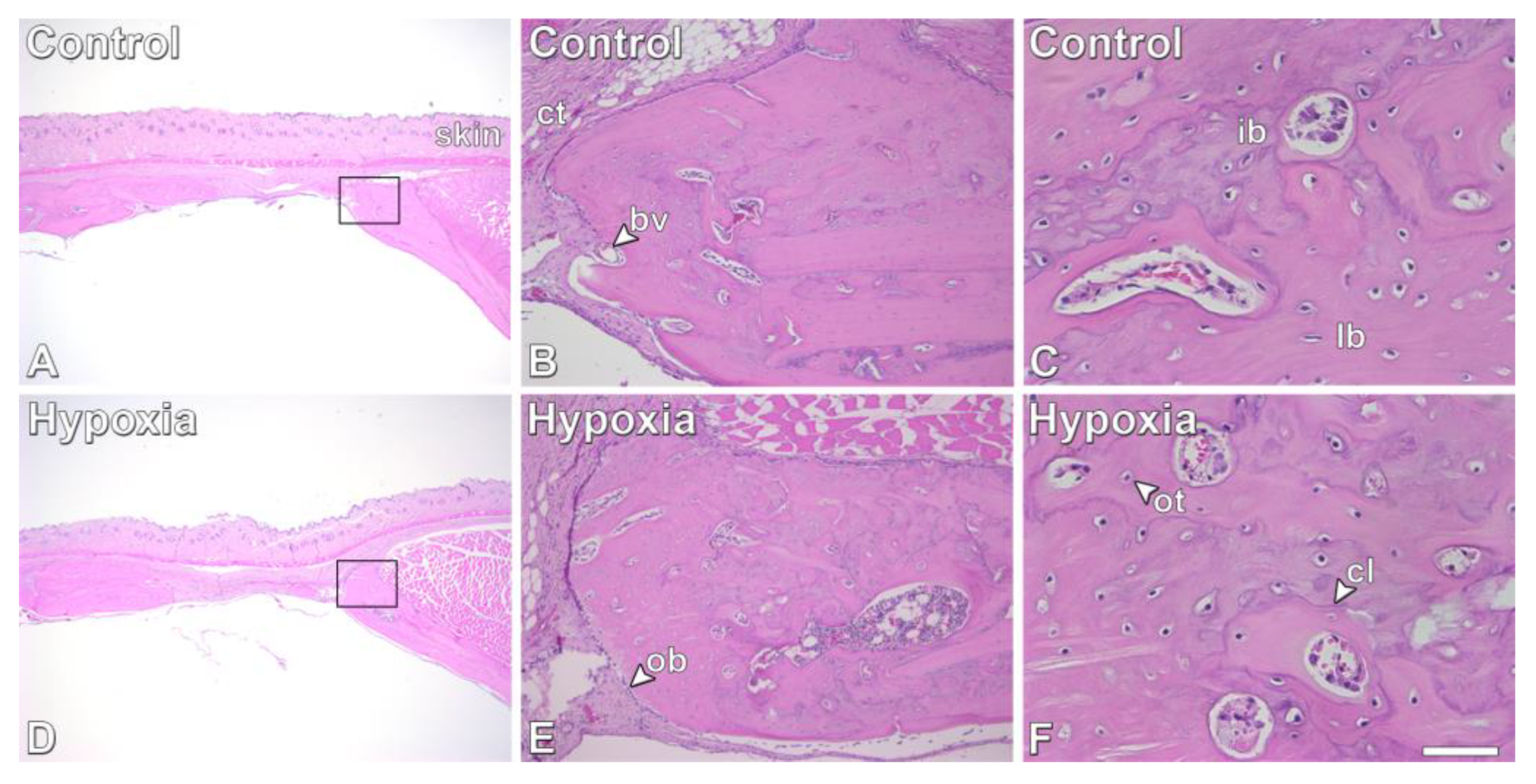
3.5. Effect of MSCs+BMP-9 Primed with Hypoxia on Bone Repair of Rat Calvarial Defects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotente mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Freitas, G.P.; Lopes, H.B.; Souza, A.T.P.; Oliveira, P.G.F.P.; Almeida, A.L.G.; Coelho, P.G.; Ferreira, F.U.; Covas, D.T.; Beloti, M.M.; Rosa, A.L. Effect of cell therapy with osteoblasts differentiated from bone marrow or adipose tissue stromal cells on bone repair. Regen. Med. 2019, 14, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Teotia, A.K.; Qayoom, I.; Singh, P.; Mishra, A.; Jaiman, D.; Seppälä, J.; Lidgren, L.; Kumar, A. Exosome-functionalized ceramic bone substitute promotes critical-sized bone defect repair in rats. ACS Appl. Bio Mater. 2021, 4, 3716–3726. [Google Scholar] [CrossRef] [PubMed]
- Venkataiah, V.S.; Yahata, Y.; Kitagawa, A.; Inagaki, M.; Kakiuchi, Y.; Nakano, M.; Suzuki, S.; Handa, K.; Saito, M. Clinical applications of cell-scaffold constructs for bone regeneration therapy. Cells 2021, 10, 2687. [Google Scholar] [CrossRef]
- Chan, N.T.; Lee, M.-S.; Wang, Y.; Galipeau, J.; Li, W.-J.; Xu, W. CTR9 drives osteochondral lineage differentiation of human mesenchymal stem cells via epigenetic regulation of BMP-2 signaling. Sci. Adv. 2022, 8, eadc9222. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, Q.; Gong, X.; Wang, C.; Bai, Y.; Wang, H.; Zhou, J.; Rong, X. Transmembrane anterior posterior transformation 1 regulates BMP signaling and modulates the protein stability of SMAD1/5. J. Biol. Chem. 2022, 298, 102684. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, C.; Liu, Y.; Li, J.; Wang, Y.; Zheng, S. RUNX2 regulates osteoblast differentiation via the BMP4 signaling pathway. J. Dent. Res. 2022, 101, 1227–1237. [Google Scholar] [CrossRef]
- Miyazono, K.; Maeda, S.; Imamura, T. BMP receptor signaling: Transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth Factor Rev. 2005, 16, 251–263. [Google Scholar] [CrossRef]
- Sachse, A.; Hasenbein, I.; Hortschansky, P.; Schmuck, K.D.; Maenz, S.; Illerhaus, B.; Kuehmstedt, P.; Ramm, R.; Huber, R.; Kunisch, E.; et al. BMP-2 (and partially GDF-5) coating significantly accelerates and augments bone formation close to hydroxyapatite/tricalcium-phosphate/brushite implant cylinders for tibial bone defects in senile, osteopenic sheep. J. Mater. Sci. Mater. Med. 2023, 34, 31. [Google Scholar] [CrossRef]
- Wozney, J.M. Bone Morphogenetic Proteins. Prog. Growth Factor Res. 1989, 1, 267–280. [Google Scholar] [CrossRef]
- Chen, G.; Deng, C.; Li, Y.-P. TGF-β and BMP Signaling in Osteoblast Differentiation and Bone Formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Bi, D.; Cao, G.; Cheng, C.; Ma, S.; Liu, Y.; Cheng, K. Bone morphogenetic protein 7-transduced human dermal-derived fibroblast cells differentiate into osteoblasts and form bone in vivo. Connect. Tissue Res. 2018, 59, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Rico-Llanos, G.A.; Becerra, J.; Visser, R. Insulin-like growth factor-1 (IGF-1) enhances the osteogenic activity of bone morphogenetic protein-6 (BMP-6) in vitro and in vivo, and together have a stronger osteogenic effect than when IGF-1 is combined with BMP-2. J. Biomed. Mater. Res. Part A 2017, 105, 1867–1875. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhu, S.; Hu, J.; Geng, N.; Zou, S. Recombinant human bone morphogenetic protein-4 (BMP-4)-stimulated cell differentiation and bone formation within the expanding calvarial suture in rats. J. Craniofacial Surg. 2009, 20, 1561–1565. [Google Scholar] [CrossRef]
- Mbalaviele, G.; Sheikh, S.; Stains, J.P.; Salazar, V.S.; Cheng, S.-L.; Chen, D.; Civitelli, R. Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J. Cell. Biochem. 2005, 94, 403–418. [Google Scholar] [CrossRef]
- Kang, Q.; Sun, M.H.; Cheng, H.; Peng, Y.; Montag, A.G.; Deyrup, A.T.; Jiang, W.; Luu, H.H.; Luo, J.; Szatkowski, J.P.; et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004, 11, 1312–1320. [Google Scholar] [CrossRef]
- Yang, Y.-Y.; Luo, H.-H.; Deng, Y.-X.; Yao, X.-T.; Zhang, J.; Su, Y.-X.; He, B.-C. Pyruvate dehydrogenase kinase 4 promotes osteoblastic potential of BMP9 by boosting Wnt/β-catenin signaling in mesenchymal stem cells. Int. J. Biochem. Cell Biol. 2023, 154, 106341. [Google Scholar] [CrossRef]
- Yao, X.-T.; Li, P.-P.; Liu, J.; Yang, Y.-Y.; Luo, Z.-L.; Jiang, H.-T.; He, W.-G.; Luo, H.-H.; Deng, Y.-X.; He, B.-C. Wnt/β-catenin promotes the osteoblastic potential of BMP9 through down-regulating Cyp26b1 in mesenchymal stem cells. Tissue Eng. Regen. Med. 2023, 20, 705–723. [Google Scholar] [CrossRef]
- Gaihre, B.; Bharadwaz, A.; Unagolla, J.M.; Jayasuriya, A.C. Evaluation of the optimal dosage of BMP-9 through the comparison of bone regeneration induced by BMP-9 versus BMP-2 using an injectable microparticle embedded thermosensitive polymeric carrier in a rat cranial defect model. Mater. Sci. Eng. C 2021, 127, 112252. [Google Scholar] [CrossRef]
- Souza, A.T.; Bezerra, B.L.; Oliveira, F.S.; Freitas, G.P.; Trevisan, R.L.B.; Oliveira, P.T.; Rosa, A.L.; Beloti, M.M. Effect of bone morphogenetic protein 9 on osteoblast differentiation of cells grown on titanium with nanotopography. J. Cell. Biochem. 2018, 119, 8441–8449. [Google Scholar] [CrossRef]
- Freitas, G.P.; Lopes, H.B.; Souza, A.T.P.; Gomes, M.P.O.; Quiles, G.K.; Gordon, J.; Tye, C.; Stein, J.L.; Stein, G.S.; Lian, J.B.; et al. Mesenchymal stem cells overexpressing BMP-9 by CRISPR-Cas9 present high in vitro osteogenic potential and enhance in vivo bone formation. Gene Ther. 2021, 28, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Usategui-Martín, R.; Rigual, R.; Ruiz-Mambrilla, M.; Fernández-Gómez, J.-M.; Dueñas, A.; Pérez-Castrillón, J.L. Molecular mechanisms involved in hypoxia-induced alterations in bone remodeling. Int. J. Mol. Sci. 2022, 23, 3233. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Miao, R.; Liu, G.; Qiu, X.; Yang, B.; Tan, X.; Liu, L.; Long, J.; Tang, W.; Jing, W. Spatiotemporal correlation between HIF -1α and bone regeneration. FASEB J. 2022, 36, e22520. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Li, Q.; Gao, H.; Yao, L.; Lin, Z.; Li, D.; Zhu, S.; Liu, C.; Yang, Z.; Wang, G.; et al. 3D printing of Cu-doped bioactive glass composite scaffolds promotes bone regeneration through activating the HIF-1α and TNF-α pathway of hUVECs. Biomater. Sci. 2021, 9, 5519–5532. [Google Scholar] [CrossRef] [PubMed]
- Towler, D.A. Vascular biology and bone formation: Hints from HIF. J. Clin. Investig. 2007, 117, 1477–1480. [Google Scholar] [CrossRef]
- Stegen, S.; Deprez, S.; Eelen, G.; Torrekens, S.; Van Looveren, R.; Goveia, J.; Ghesquière, B.; Carmeliet, P.; Carmeliet, G. Adequate hypoxia inducible factor 1α signaling is indispensable for bone regeneration. Bone 2016, 87, 176–186. [Google Scholar] [CrossRef]
- Amir, M.S.; Chiba, N.; Seong, C.H.; Kusuyama, J.; Eiraku, N.; Ohnishi, T.; Nakamura, N.; Matsuguchi, T. HIF-1α plays an essential role in BMP9-mediated osteoblast differentiation through the induction of a glycolytic enzyme, PDK1. J. Cell. Physiol. 2022, 237, 2183–2197. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Wang, H.-D.; Zhang, J.; Lin, M.; Yang, J.; Huang, J.; Yan, W.; Ao, Y. HIF1α promotes BMP9-mediated osteoblastic differentiation and vascularization by interacting with CBFA1. BioMed Res. Int. 2022, 2022, 2475169. [Google Scholar] [CrossRef]
- Hsu, S.-H.; Chen, C.-T.; Wei, Y.-H. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2013, 31, 2779–2788. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.-C.; Park, A.Y.; Guan, J.-L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef]
- Hu, N.; Jiang, D.; Huang, E.; Liu, X.; Li, R.; Liang, X.; Kim, S.H.; Chen, X.; Gao, J.-L.; Zhang, H.; et al. BMP9-regulated angiogenic signaling plays an important role in the osteogenic differentiation of mesenchymal progenitor cells. J. Cell Sci. 2013, 126, 532–541. [Google Scholar] [CrossRef]
- Rezaei, A.; Li, Y.; Turmaine, M.; Bertazzo, S.; Howard, C.A.; Arnett, T.R.; Shakib, K.; Jell, G. Hypoxia mimetics restore bone biomineralisation in hyperglycaemic environments. Sci. Rep. 2022, 12, 13944. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhu, X.; He, Y.; Hou, S.; Liu, T.; Zhi, K.; Hou, T.; Gao, L. CircCDK8 regulates osteogenic differentiation and apoptosis of PDLSCs by inducing ER stress/autophagy during hypoxia. Ann. N. Y. Acad. Sci. 2021, 1485, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wan, Q.; Ye, X.; Cheng, Y.; Pathak, J.L.; Li, Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell. Mol. Biol. Lett. 2019, 24, 64. [Google Scholar] [CrossRef]
- Yu, X.; Wan, Q.; Cheng, G.; Cheng, X.; Zhang, J.; Pathak, J.L.; Li, Z. CoCl2, a mimic of hypoxia, enhances bone marrow mesenchymal stem cells migration and osteogenic differentiation via STAT3 signaling pathway. Cell Biol. Int. 2018, 42, 1321–1329. [Google Scholar] [CrossRef]
- Niu, X.; Chen, Y.; Qi, L.; Liang, G.; Wang, Y.; Zhang, L.; Qu, Y.; Wang, W. Hypoxia regulates angeogenic-osteogenic coupling process via up-regulating IL-6 and IL-8 in human osteoblastic cells through hypoxia-inducible factor-1α pathway. Cytokine 2019, 113, 117–127. [Google Scholar] [CrossRef]
- Toberer, F.; Haenssle, H.A.; Heinzel-Gutenbrunner, M.; Enk, A.; Hartschuh, W.; Helmbold, P.; Kutzner, H. Metabolic reprogramming and angiogenesis in primary cutaneous Merkel cell carcinoma: Expression of hypoxia-inducible factor-1α and its central downstream factors. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 88–94. [Google Scholar] [CrossRef]
- Sharff, K.A.; Song, W.-X.; Luo, X.; Tang, N.; Luo, J.; Chen, J.; Bi, Y.; He, B.-C.; Huang, J.; Li, X.; et al. Hey1 basic helix-loop-helix protein plays an important role in mediating BMP9-induced osteogenic differentiation of mesenchymal progenitor cells. J. Biol. Chem. 2009, 284, 649–659. [Google Scholar] [CrossRef]
- de Jong, D.S.; Vaes, B.L.; Dechering, K.J.; Feijen, A.; Hendriks, J.M.; Wehrens, R.; Mummery, C.L.; van Zoelen, E.J.; Olijve, W.; Steegenga, W.T. Identification of novel regulators associated with early-phase osteoblast differentiation. J. Bone Miner. Res. 2004, 19, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Usas, A.; Proto, J.D.; Lu, A.; Cummins, J.H.; Proctor, A.; Huard, J.; Chen, C.-W. Role of donor and host cells in muscle-derived stem cell-mediated bone repair: Differentiation vs. paracrine effects. FASEB J. 2014, 28, 3792–3809. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, J.; Huang, Y.; Zhang, Y.; Liu, W.; Wang, G.; Zhang, Q.; Wang, G.; Yang, Y.; Li, H.; et al. NRF2 overexpression in mesenchymal stem cells induces stem-cell marker expression and enhances osteoblastic differentiation. Biochem. Biophys. Res. Commun. 2017, 491, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Peng, J.; Cao, G.; Lu, S.; Liu, L.; Li, Z.; Zhou, M.; Feng, M.; Shen, H. Hypoxia-induced microRNA-429 promotes differentiation of MC3T3-E1 osteoblastic cells by mediating ZFPM2 expression. Cell. Physiol. Biochem. 2016, 39, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.S.; Hung, B.P.; Heyrani, N.; Lee, M.A.; Leach, J.K. Hypoxic preconditioning of mesenchymal stem cells with subsequent spheroid formation accelerates repair of segmental bone defects. Stem Cells 2018, 36, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paz, J.E.R.M.; Adolpho, L.F.; Ramos, J.I.R.; Bighetti-Trevisan, R.L.; Calixto, R.D.; Oliveira, F.S.; Almeida, A.L.G.; Beloti, M.M.; Rosa, A.L. Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair. Biology 2023, 12, 1147. https://doi.org/10.3390/biology12081147
Paz JERM, Adolpho LF, Ramos JIR, Bighetti-Trevisan RL, Calixto RD, Oliveira FS, Almeida ALG, Beloti MM, Rosa AL. Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair. Biology. 2023; 12(8):1147. https://doi.org/10.3390/biology12081147
Chicago/Turabian StylePaz, Jessica Emanuella Rocha Moura, Leticia Faustino Adolpho, Jaqueline Isadora Reis Ramos, Rayana Longo Bighetti-Trevisan, Robson Diego Calixto, Fabiola Singaretti Oliveira, Adriana Luisa Gonçalves Almeida, Marcio Mateus Beloti, and Adalberto Luiz Rosa. 2023. "Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair" Biology 12, no. 8: 1147. https://doi.org/10.3390/biology12081147
APA StylePaz, J. E. R. M., Adolpho, L. F., Ramos, J. I. R., Bighetti-Trevisan, R. L., Calixto, R. D., Oliveira, F. S., Almeida, A. L. G., Beloti, M. M., & Rosa, A. L. (2023). Effect of Mesenchymal Stem Cells Overexpressing BMP-9 Primed with Hypoxia on BMP Targets, Osteoblast Differentiation and Bone Repair. Biology, 12(8), 1147. https://doi.org/10.3390/biology12081147







