Biomarkers of Type IV Collagen Turnover Reflect Disease Activity in Patients with Early-Stage Non-Alcoholic Fatty Liver (NAFL)
Abstract
Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Participants
2.2. Histological Assessement
2.3. Scores Applied in This Study
2.4. Basement-Membrane-Turnover Biomarkers
2.5. Statistics
3. Results
3.1. Patient Characteristics
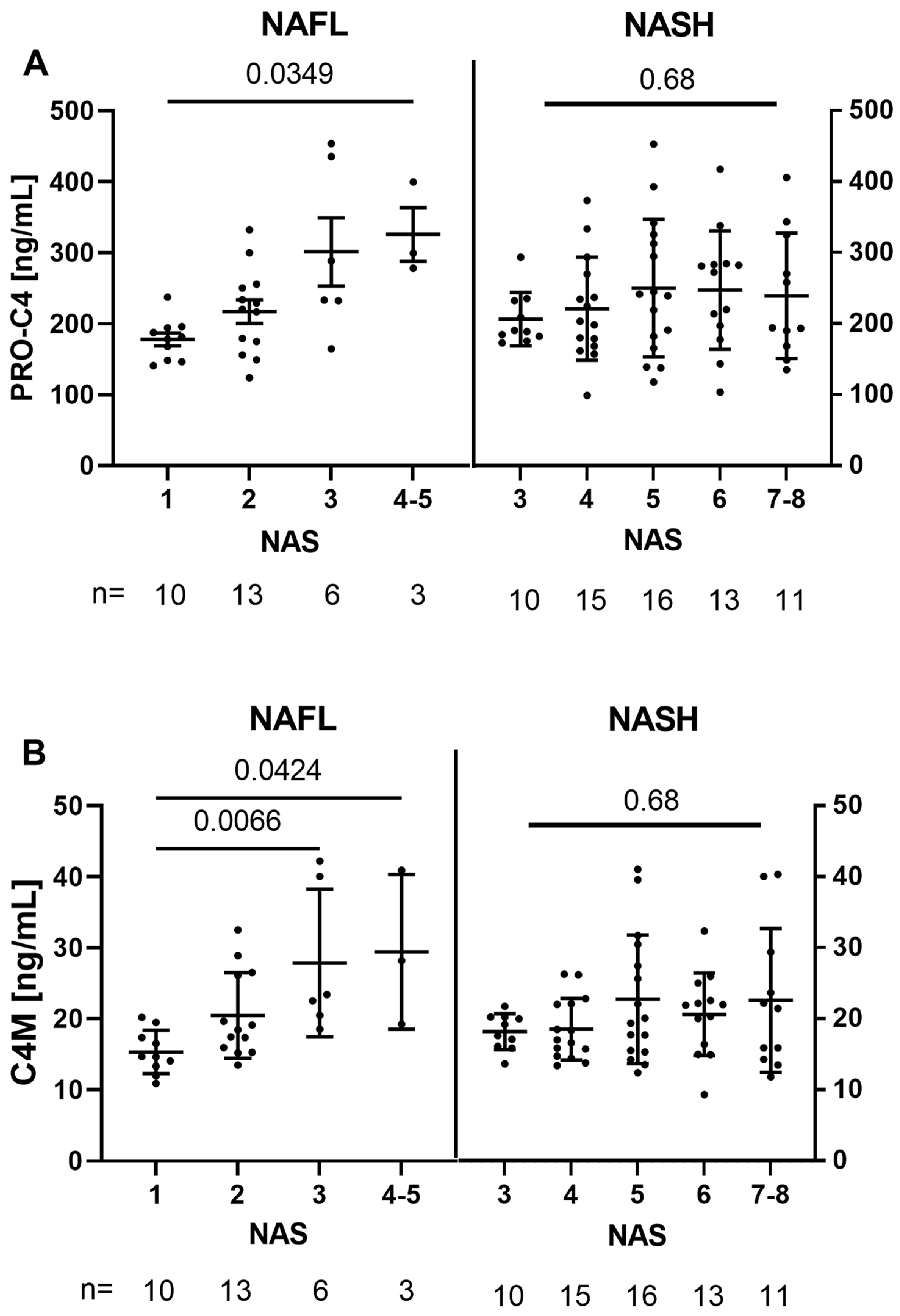
3.2. Basement-Membrane Turnover Is Associated with NAS in NAFL, but Not within NASH
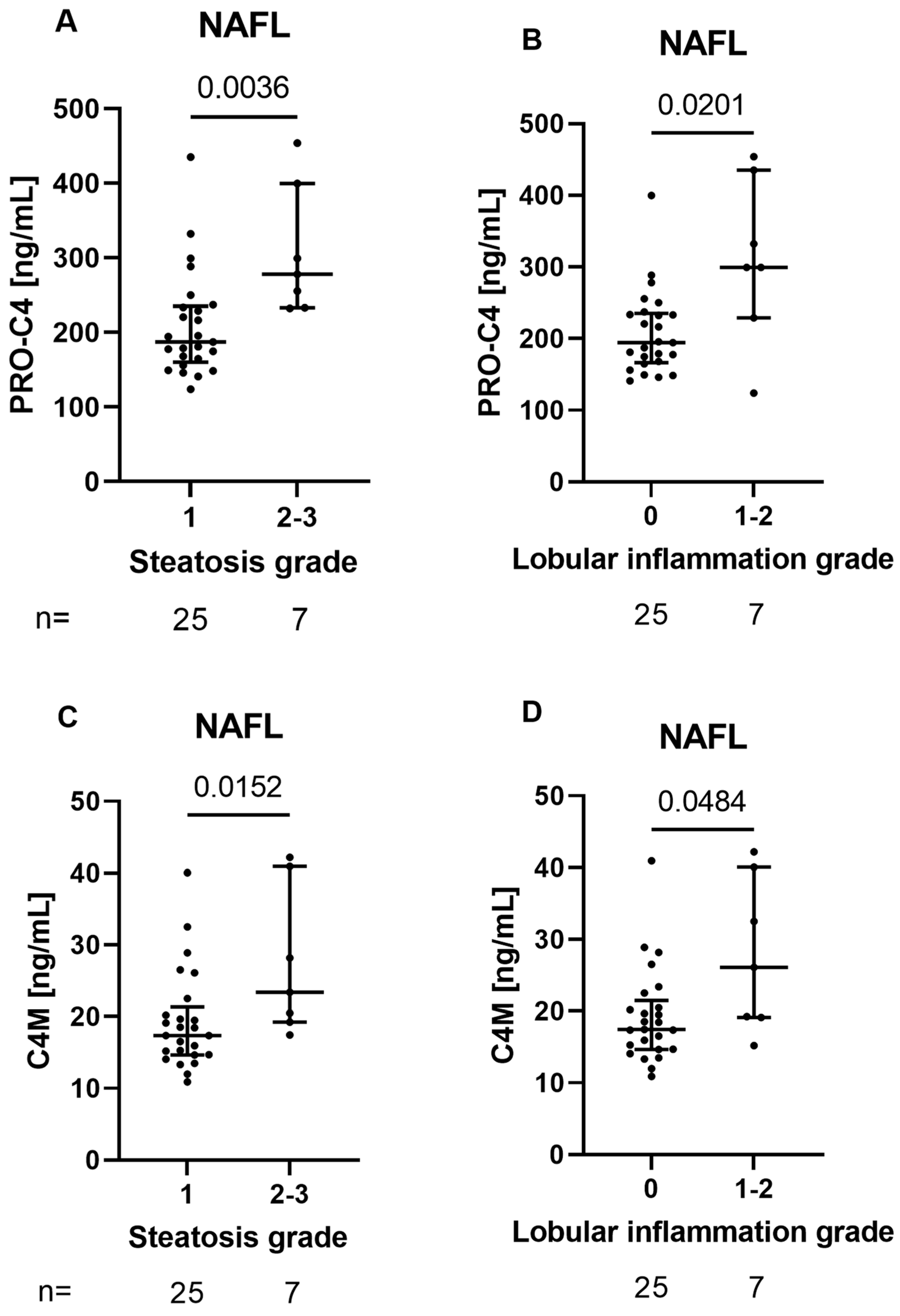
3.3. BM Biomarkers Reflect Histological Features of Early Liver Damage in Patients with NAFL
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karlsen, T.H.; Sheron, N.; Zelber-Sagi, S.; Carrieri, P.; Dusheiko, G.; Bugianesi, E.; Manns, M.P. The EASL–Lancet Liver Commission: Protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022, 399, 61–116. [Google Scholar] [CrossRef]
- Nielsen, M.J.; Nedergaard, A.F.; Sun, S.; Veidal, S.S.; Larsen, L.; Zheng, Q.; Leeming, D.J. The neo-epitope specific PRO-C3 ELISA measures true formation of type III collagen associated with liver and muscle parameters. Am. J. Transl. Res. 2013, 5, 303–315. [Google Scholar]
- Mak, A.L.; Lee, J.; van Dijk, A.-M.; Vali, Y.; Aithal, G.P.; Schattenberg, J.M.; Holleboom, A.G. Systematic Review with Meta-Analysis: Diagnostic Accuracy of Pro-C3 for Hepatic Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Biomedicines 2021, 9, 1920. [Google Scholar] [CrossRef]
- Taylor, R.S.; Taylor, R.J.; Bayliss, S.; Hagström, H.; Nasr, P.; Schattenberg, J.M.; Ewsome, P.N. Association Between Fibrosis Stage and Outcomes of Patients With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterology 2020, 158, 1611–1625.e12. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Loomba, R. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- Hernandez-Gea, V.; Friedman, S.L. Pathogenesis of liver fibrosis. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 425–456. [Google Scholar] [CrossRef]
- Sørensen, M.D.; Thiele, M.; Krag, A.; Daniels, S.J.; Leeming, D.J.; Karsdal, M.; Detlefsen, S. Stage-dependent expression of fibrogenic markers in alcohol-related liver disease. Pathol.-Res. Pract. 2022, 231, 153798. [Google Scholar] [CrossRef]
- Hammoutene, A.; Rautou, P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019, 70, 1278–1291. [Google Scholar] [CrossRef]
- Arteel, G.E.; Naba, A. The liver matrisome-looking beyond collagens. JHEP Rep. Innov. Hepatol. 2020, 2, 100115. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Linda, D. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Bedossa, P. FLIP Pathology Consortium. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef]
- Guha, I.N.; Parkes, J.; Roderick, P.; Chattopadhyay, D.; Cross, R.; Harris, S.; Rosenberg, W.M. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: Validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology 2007, 47, 455–460. [Google Scholar] [CrossRef]
- Daniels, S.J.; Leeming, D.J.; Eslam, M.; Hashem, A.M.; Nielsen, M.J.; Krag, A.; George, J. ADAPT: An Algorithm Incorporating PRO-C3 Accurately Identifies Patients With NAFLD and Advanced Fibrosis. Hepatology 2019, 69, 1075–1086. [Google Scholar] [CrossRef]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Leeming, D.J.; Nielsen, M.J.; Dai, Y.; Veidal, S.S.; Vassiliadis, E.; Zhang, C.; Karsdal, M.A. Enzyme-linked immunosorbent serum assay specific for the 7S domain of collagen type IV (P4NP 7S): A marker related to the extracellular matrix remodeling during liver fibrogenesis. Hepatol. Res. 2012, 42, 482–493. [Google Scholar] [CrossRef]
- Sand, J.M.; Larsen, L.; Hogaboam, C.; Martinez, F.; Han, M.; Larsen, M.R.; Leeming, D.J. MMP mediated degradation of type IV collagen alpha 1 and alpha 3 chains reflects basement membrane remodeling in experimental and clinical fibrosis-Validation of two novel biomarker assays. PLoS ONE 2013, 8, e84934. [Google Scholar] [CrossRef]
- Yoshida, T.; Adachi, E.; Nigi, H.; Fujii, S.; Yanagi, M. Changes of sinusoidal basement membrane collagens in early hepatic fibrosis induced with CCl4 in cynomolgus monkeys. Pathology 1999, 31, 29–35. [Google Scholar] [CrossRef]
- Veidal, S.S.; Karsdal, M.A.; Nawrocki, A.; Larsen, M.R.; Dai, Y.; Zheng, Q.; Leeming, D.J. Assessment of proteolytic degradation of the basement membrane: A fragment of type IV collagen as a biochemical marker for liver fibrosis. Fibrogenesis Tissue Repair 2011, 4, 22. [Google Scholar] [CrossRef]
- Mak, K.M.; Chen, L.L.; Lee, T.-F. Codistribution of collagen type IV and laminin in liver fibrosis of elderly cadavers: Immunohistochemical marker of perisinusoidal basement membrane formation. Anat. Rec. 2013, 296, 953–964. [Google Scholar] [CrossRef]
- Chen, W.; Rock, J.B.; Yearsley, M.M.; Ferrell, L.D.; Frankel, W.L. Different collagen types show distinct rates of increase from early to late stages of hepatitis C-related liver fibrosis. Hum. Pathol. 2014, 45, 160–165. [Google Scholar] [CrossRef]
- Ratziu, V.; Francque, S.; Sanyal, A. Breakthroughs in therapies for NASH and remaining challenges. J. Hepatol. 2022, 76, 1263–1278. [Google Scholar] [CrossRef]
- Davison, B.A.; Harrison, S.A.; Cotter, G.; Alkhouri, N.; Sanyal, A.; Edwards, C.; Dittrich, H.C. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J. Hepatol. 2020, 73, 1322–1332. [Google Scholar] [CrossRef]
- Thiele, M.; Johansen, S.; Gudmann, N.S.; Madsen, B.; Kjaergaard, M.; Mette, J.; Trebicka, J. Progressive alcohol-related liver fibrosis is characterised by imbalanced collagen formation and degradation Correspondence. Aliment. Pharmacol. Ther. 2021, 54, 1070–1080. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
| PARAMETER | ALL (N = 97) | NAFL (N = 32) | NASH (N = 65) | p-VALUE |
|---|---|---|---|---|
| BMI, KG/M2 | 30.9 (±5.6) | 29.58 (±5.76) | 31.48 (±5.36) | 0.125 |
| AGE, YEARS | 53 (±13) | 50 (±16) | 54 (±11) | 0.181 |
| SEX, FEMALE | 38.1% | 34.4% | 40% | 0.754 |
| DIABETES, YES | 41.2% | 28.1% | 47.7% | 0.081 |
| PLATELETS, 109/L | 229.0 (185–283) | 254 (202.5–298) | 217 (178–268) | 0.038 |
| BILIRUBIN,µMOL/L | 10.0 (8.0–13.0) | 10.0 (7.0–13.0) | 11.0 (9.0–14.0) | 0.263 |
| ALBUMIN, G/L | 39.0 (38.0–42.0) | 38.0 (37.0–41.0) | 40.0 (38.0–43.0) | 0.102 |
| ALT, U/L | 56.0 (38.0–81.0) | 47.5 (30.5–69.0) | 57.0 (39.0–92.0) | 0.045 |
| AST, U/L | 39.0 (30.0–55.0) | 31.5 (25.75–37.5) | 40.0 (34.0–71.0) | 0.005 |
| GGT, U/L | 85.0 (57.2–165.0) | 96.5 (48.5–150.75) | 84.0 (60.0–169.25) | 0.729 |
| FERRITIN,µG/L | 168.0 (66.2–308.2) | 221 (97.5–306.5) | 152 (61.5–321.5) | 0.766 |
| FASTING GLUCOSE, MMOL/L | 5.3 (4.7–6.8) | 4.9 (4.5–6.0) | 5.5 (4.9–7.2) | 0.022 |
| HDL, MMOL/L | 1.3 (1.0–1.6) | 1.4 (1.1–1.8) | 1.1 (1.0–1.4) | 0.035 |
| LDL, MMOL/L | 2.7 (1.8–3.5) | 3.0 (2.1–3.3) | 2.7 (1.6–3.6) | 0.358 |
| TRIGLYCERIDES, MMOL/L | 1.8 (1.2–2.9) | 1.7 (1.1–2.2) | 2.2 (1.4–3.1) | 0.047 |
| ELF | 9.2 (8.5–10.1) | 8.6 (7.9–9.6) | 9.3 (8.8–10.4) | 0.002 |
| ADAPT | 6.7 (5.3–9.0) | 5.3 (4.5–6.8) | 7.6 (5.9–9.5) | <0.001 |
| FLI | 84.4 (70.3–94.2) | 77.8 (65.7–84.4) | 88.6 (73.1–96.7) | 0.038 |
| NAFLD ACTIVITY SCORE, N | ||||
| 1/2/3/4/5/6/7/8 | 10/13/16/17/17/13/9/2 | 10/13/6/2/1/0/0/0 | 0/0/10/15/16/13/9/2 | <0.001 |
| STEATOSIS, N | ||||
| 1/2/3 | 47/24/26 | 25/3/4 | 22/21/22 | <0.001 |
| LOBULAR INFLAMMATION, N | ||||
| 0/1/2/3 | 47/24/26 | 25/6/1 | 0/41/19 | <0.001 |
| HEPATOCYTE BALLOONING, N | ||||
| 0/1/2 | 19/37/41 | 19/10/3 | 0/27/38 | <0.001 |
| FIBROSIS, N | ||||
| 0/1/2/3/4 | 24/15/18/27/12 | 14/7/5/3/2 | 10/8/13/24/10 | 0.003 |
| PRO-C4, NG/ML | 219 (178–282) | 219 (173–261) | 219 (179–283) | 0.645 |
| C4M, NG/ML | 19 (16–23) | 19 (15–24) | 19 (16–23) | 0.933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lønsmann, I.; Grove, J.I.; Haider, A.; Kaye, P.; Karsdal, M.A.; Leeming, D.J.; Aithal, G.P. Biomarkers of Type IV Collagen Turnover Reflect Disease Activity in Patients with Early-Stage Non-Alcoholic Fatty Liver (NAFL). Biology 2023, 12, 1087. https://doi.org/10.3390/biology12081087
Lønsmann I, Grove JI, Haider A, Kaye P, Karsdal MA, Leeming DJ, Aithal GP. Biomarkers of Type IV Collagen Turnover Reflect Disease Activity in Patients with Early-Stage Non-Alcoholic Fatty Liver (NAFL). Biology. 2023; 12(8):1087. https://doi.org/10.3390/biology12081087
Chicago/Turabian StyleLønsmann, Ida, Jane I. Grove, Asma Haider, Philip Kaye, Morten A. Karsdal, Diana J. Leeming, and Guruprasad P. Aithal. 2023. "Biomarkers of Type IV Collagen Turnover Reflect Disease Activity in Patients with Early-Stage Non-Alcoholic Fatty Liver (NAFL)" Biology 12, no. 8: 1087. https://doi.org/10.3390/biology12081087
APA StyleLønsmann, I., Grove, J. I., Haider, A., Kaye, P., Karsdal, M. A., Leeming, D. J., & Aithal, G. P. (2023). Biomarkers of Type IV Collagen Turnover Reflect Disease Activity in Patients with Early-Stage Non-Alcoholic Fatty Liver (NAFL). Biology, 12(8), 1087. https://doi.org/10.3390/biology12081087






