Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases
Abstract
Simple Summary
Abstract
1. Introduction
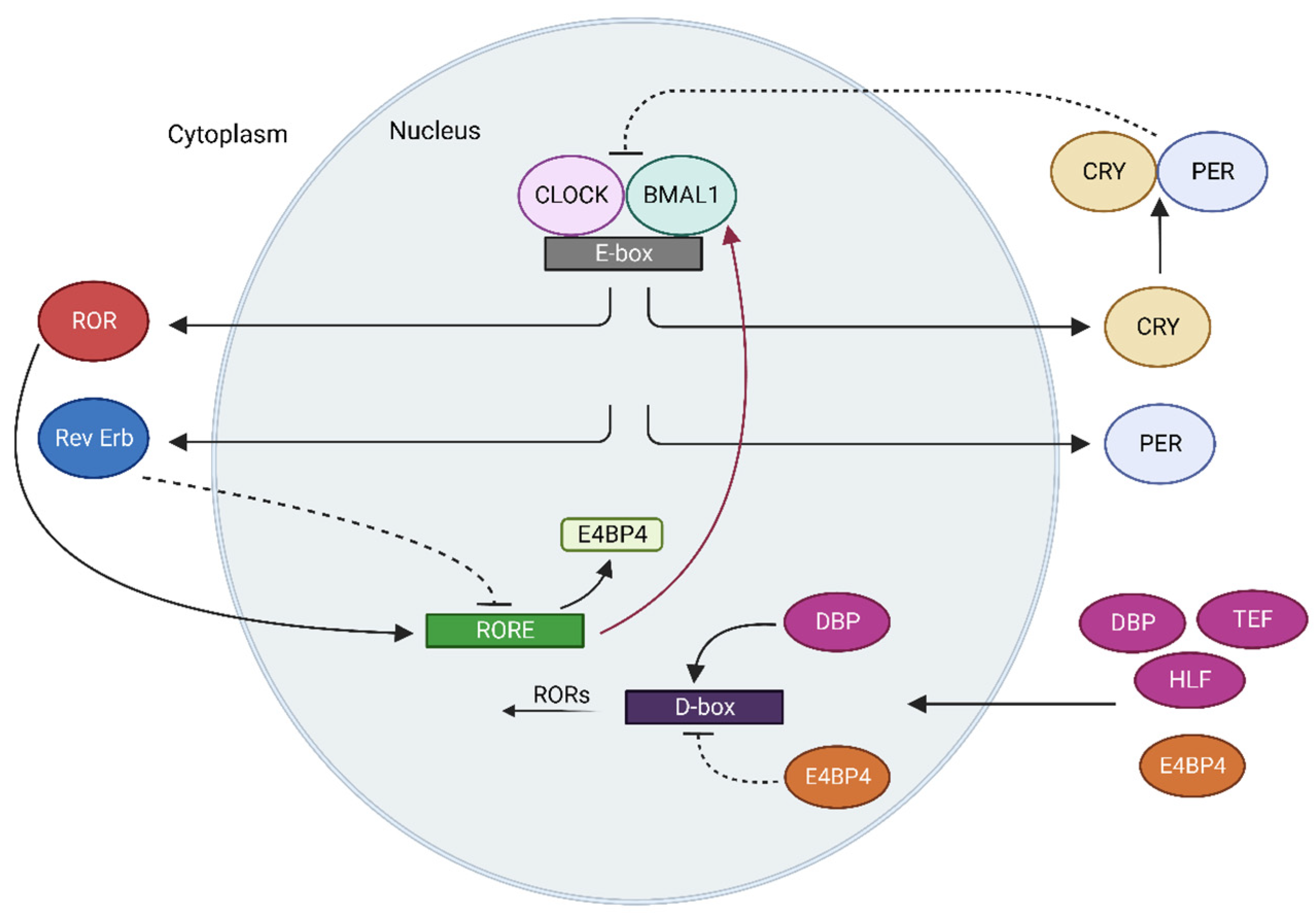
2. Circadian Rhythm
Molecular Mechanisms of Clock Machinery
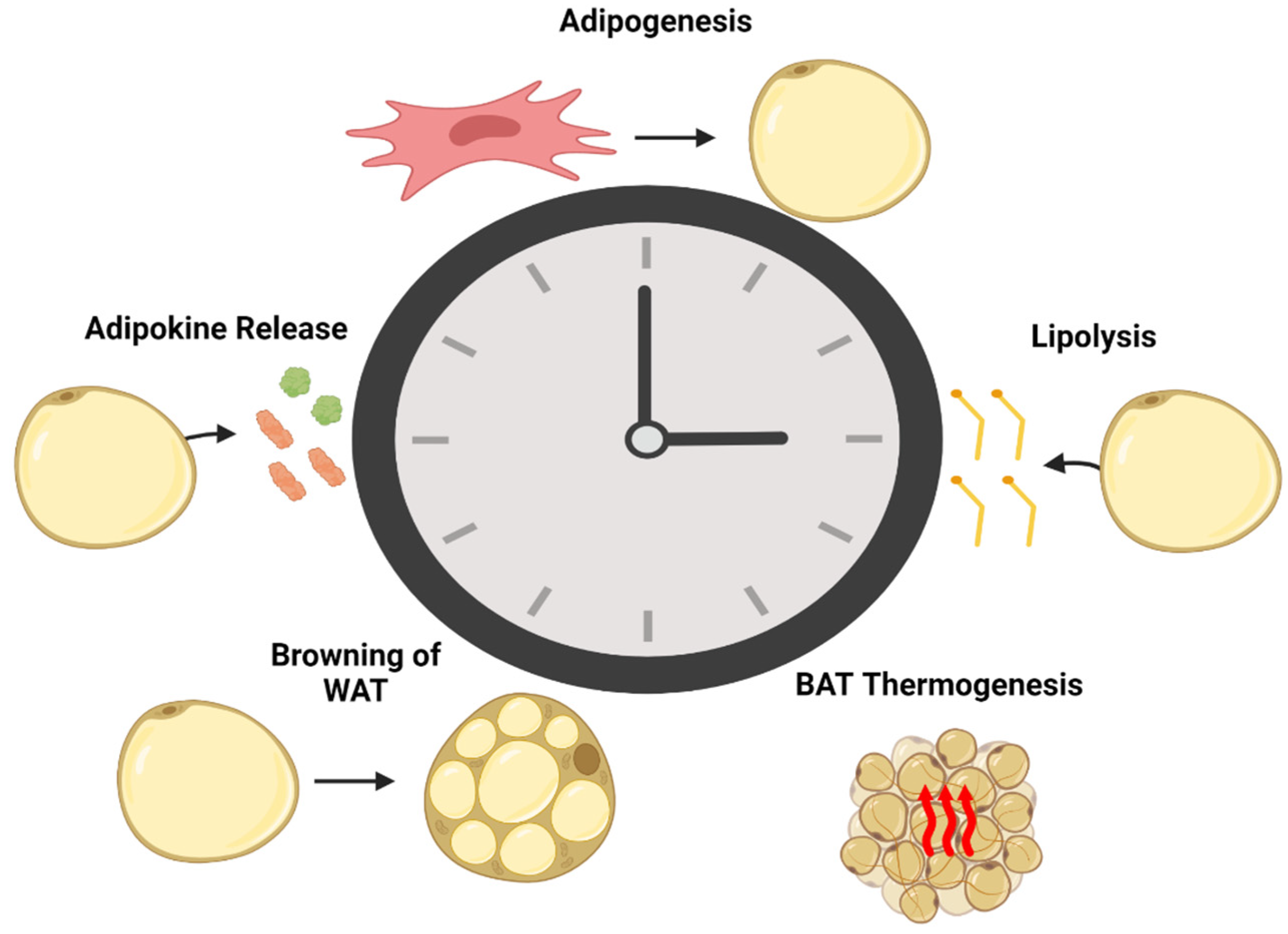
3. The Role of Circadian Rhythm in Adipose Tissue Function
3.1. The Role of Circadian Rhythm in Adipogenesis
3.2. The Role of the Circadian Rhythm in Adipose Tissue Lipolysis
3.3. Adipokine Release
3.4. Brown Adipose Tissue Non-Shivering Thermogenesis
3.5. Browning of Adipose Tissue
4. Circadian Rhythm Dysfunction in Obesity and Type 2 Diabetes
5. Chronotherapy Approaches in Metabolic Disorders
5.1. Chronotherapy of Antidiabetic and Anti-Obesity Drugs
5.2. Targeting the Circadian Clock in Metabolic Diseases
6. Non-Pharmacological Approaches: Time-Restricted Feeding and Light Therapy
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roenneberg, T.; Merrow, T. The Circadian Clock and Human Health. Curr. Biol. 2016, 26, R432–R443. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, U. The circadian clock, metabolism and obesity. Obes. Rev. 2017, 18, 25–33. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The Genetics of Mammalian Circadian Order and Disorder: Implications for Physiology and Disease. Nat. Rev. Genet. 2008, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F.; Okyar, A.; Dulong, S.; Innominato, P.F.; Clairambault, J. Circadian timing in cancer treatments. Annu. Rev. Pharmacol. Toxicol. 2010, 50, 377–421. [Google Scholar] [CrossRef]
- Levi, F.; Schibler, U. Circadian rhythms: Mechanisms and therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 593–628. [Google Scholar] [CrossRef] [PubMed]
- Kiehn, J.T.; Tsang, A.H.; Heyde, I.; Leinweber, B.; Kolbe, I.; Leliavski, A.; Oster, H. Circadian rhythms in adipose tissue physiology. Compr. Physiol. 2017, 7, 383–427. [Google Scholar] [CrossRef]
- Kume, K.; Zylka, M.J.; Sriram, S.; Shearman, L.P.; Weaver, D.R.; Jin, X.; Maywood, E.S.; Hastings, M.H.; Reppert, S.M. mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop. Cell 1999, 98, 193–205. [Google Scholar] [CrossRef]
- Camacho, F.; Cilio, M.; Guo, Y.; Virshup, D.M.; Patel, K.; Khorkova, O.; Styren, S.; Morse, B.; Yao, Z.; Keesler, G.A. Human casein kinase Iδ phosphorylation of human circadian clock proteins period 1 and 2. FEBS Lett. 2001, 489, 159–165. [Google Scholar] [CrossRef]
- Eide, E.J.; Woolf, M.F.; Kang, H.; Woolf, P.; Hurst, W.; Camacho, F.; Vielhaber, E.L.; Giovanni, A.; Virshup, D.M. Control of Mammalian Circadian Rhythm by CKI -Regulated Proteasome-Mediated PER2 Degradation. Mol. Cell. Biol. 2005, 25, 2795–2807. [Google Scholar] [CrossRef]
- Ozturk, N.; Ozturk, D.; Kavakli, I.H.; Okyar, A. Molecular Aspects of Circadian Pharmacology and Relevance for Cancer Chronotherapy. Int. J. Mol. Sci. 2017, 18, 2168. [Google Scholar] [CrossRef]
- Gul, S.; Akyel, Y.K.; Gul, Z.M.; Isin, S.; Ozcan, O.; Korkmaz, T.; Selvi, S.; Danis, I.; Ipek, O.S.; Aygenli, F.; et al. Discovery of a small molecule that selectively destabilizes Cryptochrome 1 and enhances life span in p53 knockout mice. Nat. Commun. 2022, 13, 6742. [Google Scholar] [CrossRef]
- Harada, Y.; Sakai, M.; Kurabayashi, N.; Hirota, T.; Fukada, Y. Ser-557-phosphorylated mCRY2 is degraded upon synergistic phosphorylation by glycogen synthase kinase-3 beta. J. Biol. Chem. 2005, 280, 31714–31721. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 2009, 326, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Triqueneaux, G.; Thenot, S.; Kakizawa, T.; Antoch, M.P.; Safi, R.; Takahashi, J.S.; Delaunay, F.; Laudet, V. The orphan receptor Rev-erbalpha gene is a target of the circadian clock pacemaker. J. Mol. Endocrinol. 2004, 33, 585–608. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.K.; Panda, S.; Miraglia, L.J.; Reyes, T.M.; Rudic, R.D.; McNamara, P.; Naik, K.A.; FitzGerald, G.A.; Kay, S.A.; Hogenesch, J.B. A functional genomics strategy reveals Rora as a component of the mammalian circadian clock. Neuron 2004, 43, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Guillaumond, F.; Dardente, H.; Giguère, V.; Cermakian, N. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J. Biol. Rhythm. 2005, 20, 391–403. [Google Scholar] [CrossRef]
- Kawamoto, T.; Noshiro, M.; Sato, F.; Maemura, K.; Takeda, N.; Nagai, R.; Iwata, T.; Fujimoto, K.; Furukawa, M.; Miyazaki, K.; et al. A novel autofeedback loop of Dec1 transcription involved in circadian rhythm regulation. Biochem. Biophys. Res. Commun. 2004, 313, 117–124. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Mitsui, S.; Yan, L.; Yagita, K.; Miyake, S.; Okamura, H. Role of DBP in the Circadian Oscillatory Mechanism. Mol. Cell. Biol. 2000, 20, 4773–4781. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and metabolic health. Nat. Rev. Mol. Cell Biol. 2018, 20, 242–258. [Google Scholar] [CrossRef]
- Moseti, D.; Regassa, A.; Kim, W.K. Molecular Regulation of Adipogenesis and Potential Anti-Adipogenic Bioactive Molecules. Int. J. Mol. Sci. 2016, 17, 124. [Google Scholar] [CrossRef]
- Garaulet, M.; Ordovás, J.M.; Gómez-Abellán, P.; Martínez, J.A.; Madrid, J.A. An Approximation to the Temporal Order in Endogenous Circadian Rhythms of Genes Implicated in Human Adipose Tissue Metabolism. J. Cell. Physiol. 2011, 226, 2075. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Downes, M.; Yu, R.T.; Bookout, A.L.; He, W.; Straume, M.; Mangelsdorf, D.J.; Evans, R.M. Nuclear receptor expression links the circadian clock to metabolism. Cell 2006, 126, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Bahrami-Nejad, Z.; Zhao, M.L.; Tholen, S.; Hunerdosse, D.; Tkach, K.E.; van Schie, S.; Chung, M.; Teruel, M.N. A Transcriptional Circuit Filters Oscillating Circadian Hormonal Inputs to Regulate Fat Cell Differentiation. Cell Metab. 2018, 27, 854–868. [Google Scholar] [CrossRef] [PubMed]
- Shimba, S.; Ishii, N.; Ohta, Y.; Ohno, T.; Watabe, Y.; Hayashi, M.; Wada, T.; Aoyagi, T.; Tezuka, M. Brain and muscle Arnt-like protein-1 (BMAL1), a component of the molecular clock, regulates adipogenesis. Proc. Natl. Acad. Sci USA 2005, 102, 12071–12076. [Google Scholar] [CrossRef]
- Shimba, S.; Ogawa, T.; Hitosugi, S.; Ichihashi, Y.; Nakadaira, Y.; Kobayashi, M.; Tezuka, M.; Kosuge, Y.; Ishige, K.; Ito, Y.; et al. Deficient of a clock gene, brain and muscle Arnt-like protein-1 (BMAL1), induces dyslipidemia and ectopic fat formation. PLoS ONE 2011, 6, e25231. [Google Scholar] [CrossRef]
- Guo, B.; Chatterjee, S.; Li, L.; Kim, J.M.; Lee, J.; Yechoor, V.K.; Minze, L.J.; Hsueh, W.; Ma, K. The clock gene, brain and muscle Arnt-like 1, regulates adipogenesis via Wnt signaling pathway. FASEB J. 2012, 26, 3453. [Google Scholar] [CrossRef]
- Costa, M.J.; So, A.Y.L.; Kaasik, K.; Krueger, K.C.; Pillsbury, M.L.; Fu, Y.H.; Ptacek, L.J.; Yamamoto, K.R.; Feldman, B.J. Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J. Biol. Chem. 2011, 286, 9063–9070. [Google Scholar] [CrossRef]
- Aggarwal, A.; Costa, M.J.; Rivero-Gutiérrez, B.; Ji, L.; Morgan, S.L.; Feldman, B.J. The Circadian Clock Regulates Adipogenesis by a Per3 Crosstalk Pathway to Klf15. Cell Rep. 2017, 21, 2367–2375. [Google Scholar] [CrossRef]
- Mori, T.; Sakaue, H.; Iguchi, H.; Gomi, H.; Okada, Y.; Takashima, Y.; Nakamura, K.; Nakamura, T.; Yamauchi, T.; Kubota, N.; et al. Role of Krüppel-like factor 15 (KLF15) in transcriptional regulation of adipogenesis. J. Biol. Chem. 2005, 280, 12867–12875. [Google Scholar] [CrossRef]
- Knarr, M.; Nagaraj, A.B.; Kwiatkowski, L.J.; DiFeo, A. miR-181a modulates circadian rhythm in immortalized bone marrow and adipose derived stromal cells and promotes differentiation through the regulation of PER3. Sci. Rep. 2019, 9, 307. [Google Scholar] [CrossRef]
- Wang, J.; Lazar, M.A. Bifunctional Role of Rev-erbα in Adipocyte Differentiation. Mol. Cell. Biol. 2008, 28, 2213. [Google Scholar] [CrossRef]
- Kopp, R.; Billecke, N.; Legradi, J.; Den Broeder, M.; Parekh, S.H.; Legler, J. Bringing obesity to light: Rev-erbα, a central player in light-induced adipogenesis in the zebrafish? Int. J. Obes. 2016, 40, 824–832. [Google Scholar] [CrossRef]
- Ozaki, N.; Noshiro, M.; Kawamoto, T.; Nakashima, A.; Honda, K.; Fukuzaki-Dohi, U.; Honma, S.; Fujimoto, K.; Tanimoto, K.; Tanne, K.; et al. Regulation of basic helix-loop-helix transcription factors Dec1 and Dec2 by RORα and their roles in adipogenesis†. Genes Cells 2012, 17, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Ohoka, N.; Kato, S.; Takahashi, Y.; Hayashi, H.; Sato, R. The orphan nuclear receptor RORalpha restrains adipocyte differentiation through a reduction of C/EBPbeta activity and perilipin gene expression. Mol. Endocrinol. 2009, 23, 759–771. [Google Scholar] [CrossRef]
- Lau, P.; Fitzsimmons, R.L.; Raichur, S.; Wang, S.C.M.; Lechtken, A.; Muscat, G.E.O. The Orphan Nuclear Receptor, RORα, Regulates Gene Expression That Controls Lipid Metabolism: Staggerer (SG/SG) mıce are resıstant to dıet-ınduced obesıty. J. Biol. Chem. 2008, 283, 18411–18421. [Google Scholar] [CrossRef]
- Wang, Q.; Tang, J.; Jiang, S.; Huang, Z.; Song, A.; Hou, S.; Gao, X.; Ruan, H. Bin Inhibition of PPARγ, adipogenesis and insulin sensitivity by MAGED1. J. Endocrinol. 2018, 239, 167–180. [Google Scholar] [CrossRef]
- Suzuki, C.; Ushijima, K.; Ando, H.; Kitamura, H.; Horiguchi, M.; Akita, T.; Yamashita, C.; Fujimura, A. Induction of Dbp by a histone deacetylase inhibitor is involved in amelioration of insulin sensitivity via adipocyte differentiation in ob/ob mice. Chronobiol. Int. 2019, 36, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Green, C.B.; Horowitz, M.; Ackert-Bicknell, C.; Lecka-Czernik, B.; Rosen, C.J. Nocturnin: A Circadian Target of Pparg- Induced Adipogenesis. Ann. N. Y. Acad. Sci. 2010, 1192, 131. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Green, C.B.; Lecka-Czernik, B.; Douris, N.; Gilbert, M.R.; Kojima, S.; Ackert-Bicknell, C.; Garg, N.; Horowitz, M.C.; Adamo, M.L.; et al. A circadian-regulated gene, Nocturnin, promotes adipogenesis by stimulating PPAR-gamma nuclear translocation. Proc. Natl. Acad. Sci. USA 2010, 107, 10508–10513. [Google Scholar] [CrossRef]
- Kitazawa, M.; Nagano, M.; Masumoto, K.H.; Shigeyoshi, Y.; Natsume, T.; Hashimoto, S. Angiopoietin-like 2, a circadian gene, improves type 2 diabetes through potentiation of insulin sensitivity in mice adipocytes. Endocrinology 2011, 152, 2558–2567. [Google Scholar] [CrossRef][Green Version]
- Kye, W.P.; Waki, H.; Villanueva, C.J.; Monticelli, L.A.; Hong, C.; Kang, S.; MacDougald, O.A.; Goldrath, A.W.; Tontonoz, P. Inhibitor of DNA Binding 2 Is a Small Molecule-Inducible Modulator of Peroxisome Proliferator-Activated Receptor-γ Expression and Adipocyte Differentiation. Mol. Endocrinol. 2008, 22, 2038. [Google Scholar] [CrossRef]
- Nam, D.; Guo, B.; Chatterjee, S.; Chen, M.H.; Nelson, D.; Yechoor, V.K.; Ma, K. The adipocyte clock controls brown adipogenesis through the TGF-β and BMP signaling pathways. J. Cell Sci. 2015, 128, 1835–1847. [Google Scholar] [CrossRef] [PubMed]
- Grabner, G.F.; Xie, H.; Schweiger, M.; ZechnerGrabner, G.F.; Xie, H.; Schweiger, M.; Zechner, R. Lipolysis: Cellular mechanisms for lipid mobilization from fat stores. Nat. Metab. 2021, 3, 1445–1465. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Shimomura, Y.; Satoh, Y. Diurnal changes in lipolytic activity of isolated fat cells and their increased responsiveness to epinephrine and theophylline with meal feeding in rats. J. Nutr. Sci. Vitaminol. 1983, 29, 399–411. [Google Scholar] [CrossRef]
- Shostak, A.; Meyer-Kovac, J.; Oster, H. Circadian regulation of lipid mobilization in white adipose tissues. Diabetes 2013, 62, 2195–2203. [Google Scholar] [CrossRef]
- Hagström-Toft, E.; Bolinder, J.; Ungerstedt, U.; ArnerHagström-Toft, E.; Bolinder, J.; Ungerstedt, U.; Arner, P. A circadian rhythm in lipid mobilization which is altered in IDDM. Diabetologia 1997, 40, 1070–1078. [Google Scholar] [CrossRef]
- Cincotta, A.H.; MeierCincotta, A.H.; Meier, A.H. Bromocriptine inhibits in vivo free fatty acid oxidation and hepatic glucose output in seasonally obese hamsters (Mesocricetus auratus). Metabolism 1995, 44, 1349–1355. [Google Scholar] [CrossRef] [PubMed]
- van Eenige, R.; In het Panhuis, W.; Schönke, M.; Jouffe, C.; Devilee, T.H.; Siebeler, R.; Streefland, T.C.M.; Sips, H.C.M.; Pronk, A.C.M.; Vorderman, R.H.P.; et al. Angiopoietin-like 4 governs diurnal lipoprotein lipase activity in brown adipose tissue. Mol. Metab. 2022, 60, 101497. [Google Scholar] [CrossRef]
- Lee, P.; Brychta, R.J.; Linderman, J.; Smith, S.; Chen, K.Y.; Celi, F.S. Mild cold exposure modulates fibroblast growth factor 21 (FGF21) diurnal rhythm in humans: Relationship between FGF21 levels, lipolysis, and cold-induced thermogenesis. J. Clin. Endocrinol. Metab. 2013, 98, E98–E102. [Google Scholar] [CrossRef]
- Smith, J.; Fahrenkrug, J.; Jørgensen, H.L.; Christoffersen, C.; Goetze, J.P. Diurnal gene expression of lipolytic natriuretic peptide receptors in white adipose tissue. Endocr. Connect. 2015, 4, 206–214. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Douris, N.; Tongjai, S.; Green, C.B. Nocturnin expression is induced by fasting in the white adipose tissue of restricted fed mice. PLoS ONE 2011, 6, e17051. [Google Scholar] [CrossRef]
- Samra, J.S.; Clark, M.L.; Humphreys, S.M.; Macdonald, I.A.; Matthews, D.R.; Frayn, K.N. Effects of morning rise in cortisol concentration on regulation of lipolysis in subcutaneous adipose tissue. Am. J. Physiol. 1996, 271, E996–E1002. [Google Scholar] [CrossRef]
- Boyle, P.J.; Avogaro, A.; Smith, L.; Bier, D.M.; Pappu, A.S.; Illingworth, D.R.; Cryer, P.E. Role of GH in regulating nocturnal rates of lipolysis and plasma mevalonate levels in normal and diabetic humans. Am. J. Physiol. 1992, 263, E168–E172. [Google Scholar] [CrossRef] [PubMed]
- BartnessBartness, T.J. Short day-induced depletion of lipid stores is fat pad- and gender-specific in Siberian hamsters. Physiol. Behav. 1995, 58, 539–550. [Google Scholar] [CrossRef] [PubMed]
- Vujović, N.; Piron, M.J.; Qian, J.; Chellappa, S.L.; Nedeltcheva, A.; Barr, D.; Heng, S.W.; Kerlin, K.; Srivastav, S.; Wang, W.; et al. Late isocaloric eating increases hunger, decreases energy expenditure, and modifies metabolic pathways in adults with overweight and obesity. Cell Metab. 2022, 34, 1486–1498. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ogasawara, J.; Takakura, H.; Shirato, K.; Sakurai, T.; Kizaki, T.; Izawa, T. Exercise Training-Enhanced Lipolytic Potency to Catecholamine Depends on the Time of the Day. Int. J. Mol. Sci. 2020, 21, 6920. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, M.; Kawamoto, T.; Nakashima, A.; Ozaki, N.; Saeki, M.; Honda, K.; Fujimoto, K.; Kato, Y. DEC1 regulates the rhythmic expression of PPARγ target genes involved in lipid metabolism in white adipose tissue. Genes Cells 2020, 25, 232–241. [Google Scholar] [CrossRef]
- Khazaal, A.Q.; Haque, N.; Krager, C.R.; Krager, S.L.; Chambers, C.; Wilber, A.; Tischkau, S.A. Aryl hydrocarbon receptor affects circadian-regulated lipolysis through an E-Box-dependent mechanism. Mol. Cell. Endocrinol. 2023, 559, 111809. [Google Scholar] [CrossRef]
- Meriin, A.B.; Zaarur, N.; Roy, D.; KandrorMeriin, A.B.; Zaarur, N.; Roy, D.; Kandror, K.V. Egr1 plays a major role in the transcriptional response of white adipocytes to insulin and environmental cues. Front. Cell Dev. Biol. 2022, 10, 1003030. [Google Scholar] [CrossRef]
- Markussen, L.K.; Rondini, E.A.; Johansen, O.S.; Madsen, J.G.S.; Sustarsic, E.G.; Marcher, A.B.; Hansen, J.B.; Gerhart-Hines, Z.; Granneman, J.G.; Mandrup, S. Lipolysis regulates major transcriptional programs in brown adipocytes. Nat. Commun. 2022, 13, 3956. [Google Scholar] [CrossRef]
- Andreotti, A.C.; Lanzi, R.; Manzoni, M.F.; Caumo, A.; Moreschi, A.; Pontiroli, A.E. Acute pharmacologic blockade of lipolysis normalizes nocturnal growth hormone levels and pulsatility in obese subjects. Metabolism 1994, 43, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Almeda-Valdes, P.; Patterson, B.W.; Okunade, A.L.; Imai, S.I.; Mittendorfer, B.; Klein, S. Diurnal variation in insulin sensitivity of glucose metabolism is associated with diurnal variations in whole-body and cellular fatty acid metabolism in metabolically normal women. J. Clin. Endocrinol. Metab. 2014, 99, E1666–E1670. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Jongejan, A.; Atiqi, S.; Vreijling, J.P.; Limonard, E.J.; Endert, E.; Baas, F.; Moerland, P.D.; Fliers, E.; Kalsbeek, A.; et al. Diurnal rhythms in the white adipose tissue transcriptome are disturbed in obese individuals with type 2 diabetes compared with lean control individuals. Diabetologia 2019, 62, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Arredondo-Amador, M.; Zambrano, C.; Kulyté, A.; Luján, J.; Hu, K.; de Medina, F.S.; Scheer, F.A.J.L.; Arner, P.; Ryden, M.; Martínez-Augustin, O.; et al. Circadian Rhythms in Hormone-sensitive Lipase in Human Adipose Tissue: Relationship to Meal Timing and Fasting Duration. J. Clin. Endocrinol. Metab. 2020, 105, e4407–e4416. [Google Scholar] [CrossRef] [PubMed]
- Tsoli, M.; Schweiger, M.; Vanniasinghe, A.S.; Painter, A.; Zechner, R.; Clarke, S.; Robertson, G. Depletion of white adipose tissue in cancer cachexia syndrome is associated with inflammatory signaling and disrupted circadian regulation. PLoS ONE 2014, 9, e92966. [Google Scholar] [CrossRef]
- Xiao, T.; Langston, P.K.; Muñoz-Rojas, A.R.; Jayewickreme, T.; Lazar, M.A.; Benoist, C.; Mathis, D. Tregs in visceral adipose tissue up-regulate circadian-clock expression to promote fitness and enforce a diurnal rhythm of lipolysis. Sci. Immunol. 2022, 7, eabl7641. [Google Scholar] [CrossRef]
- Onuma, S.; Kinoshita, S.; Shimba, S.; Ozono, K.; Michigami, T.; Kawai, M. The Lack of Bmal1, a Core Clock Gene, in the Intestine Decreases Glucose Absorption in Mice. Endocrinology 2022, 163, bqac119. [Google Scholar] [CrossRef]
- Boden, G.; Chen, X.; Kolaczynski, J.W.; PolanskyBoden, G.; Chen, X.; Kolaczynski, J.W.; Polansky, M. Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J. Clin. Investig. 1997, 100, 1107–1113. [Google Scholar] [CrossRef]
- Licinio, J.; Negrão, A.B.; Mantzoros, C.; Kaklamani, V.; Wong, M.L.; Bongiorno, P.B.; Negro, P.P.; Mulla, A.; Veldhuis, J.D.; Cearnal, L.; et al. Sex differences in circulating human leptin pulse amplitude: Clinical implications. J. Clin. Endocrinol. Metab. 1998, 83, 4140–4147. [Google Scholar] [CrossRef]
- Zimmermann, R.C.; Krahn, L.; Rahmanie, N.; Sauer, M.V. Prolonged inhibition of presynaptic catecholamine synthesis does not alter leptin secretion in normal-weight men and women. Hum. Reprod. 1998, 13, 822–825. [Google Scholar] [CrossRef]
- Kalsbeek, A.; Ruiter, M.; la Fleur, S.E.; Van Heijningen, C.; Buijs, R.M. The diurnal modulation of hormonal responses in the rat varies with different stimuli. J. Neuroendocrinol. 2003, 15, 1144–1155. [Google Scholar] [CrossRef] [PubMed]
- Tolle, V.; Kadem, M.; Bluet-Pajot, M.T.; Frere, D.; Foulon, C.; Bossu, C.; Dardennes, R.; Mounier, C.; Zizzari, P.; Lang, F.; et al. Balance in ghrelin and leptin plasma levels in anorexia nervosa patients and constitutionally thin women. J. Clin. Endocrinol. Metab. 2003, 88, 109–116. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Suchard, M.A.; Wong, M.L.; McCann, S.M.; Licinio, J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. USA 2004, 101, 10434–10439. [Google Scholar] [CrossRef] [PubMed]
- Karakas, A.; Gündüz, B. Suprachiasmatic nuclei may regulate the rhythm of leptin hormone release in Syrian hamsters (Mesocricetus auratus). Chronobiol. Int. 2006, 23, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.; Oberste-Berghaus, C.; Herpertz, S.; Blum, W.F.; Pelz, B.; Hebebrand, J.; Senf, W.; Mann, K.; Albers, N. Time relationship between circadian variation of serum levels of leptin, insulin and cortisol in healthy subjects. Horm. Res. 2000, 54, 174–180. [Google Scholar] [CrossRef]
- Vujovic, P.; Lakic, I.; Laketa, D.; Jasnic, N.; Djurasevic, S.F.; Cvijic, G.; Djordjevic, J. Time-dependent effects of starvation on serum, pituitary and hypothalamic leptin levels in rats. Physiol. Res. 2011, 60, S165. [Google Scholar] [CrossRef]
- Herrmann, T.S.; Bean, M.L.; Black, T.M.; Wang, P.; Coleman, R.A. High glycemic index carbohydrate di; et alters the diurnal rhythm of leptin but not insulin concentrations. Exp. Biol. Med. 2001, 226, 1037–1044. [Google Scholar] [CrossRef]
- Dube, M.G.; Xu, B.; Kalra, P.S.; Sninsky, C.A.; Kalra, S.P. Disruption in neuropeptide Y and leptin signaling in obese ventromedial hypothalamic-lesioned rats. Brain Res. 1999, 816, 38–46. [Google Scholar] [CrossRef]
- Recabarren, S.E.; Lobos, A.; Vilches, C.; Munoz, P.; Sir-Petermann, T. Pulsatile leptin secretion is independent of luteinizing hormone secretion in prepubertal sheep. Endocrine 2002, 17, 175–184. [Google Scholar] [CrossRef]
- Kousta, E.; Chrisoulidou, A.; Lawrence, N.J.; Al-Shoumer, K.A.S.; Parker, K.H.; McCarthy, M.I.; Johnston, D.G. The circadian rhythm of leptin is preserved in growth hormone deficient hypopituitary adults. Clin. Endocrinol. 1998, 48, 685–690. [Google Scholar] [CrossRef]
- Mastronardi, C.A.; Walczewska, A.; Yu, W.H.; Karanth, S.; Parlow, A.F.; McCann, S.M. The possible role of prolactin in the circadian rhythm of leptin secretion in male rats. Proc. Soc. Exp. Biol. Med. 2000, 224, 152–158. [Google Scholar] [CrossRef]
- Torpy, D.J.; Bornstein, S.R.; Chrousos, G.P. Leptin and interleukin-6 in sepsis. Horm. Metab. Res. 1998, 30, 726–729. [Google Scholar] [CrossRef]
- Antonijevic, I.A.; Murck, H.; Frieboes, R.M.; Horn, R.; Brabant, G.; Steiger, A. Elevated nocturnal profiles of serum leptin in patients with depression. J. Psychiatr. Res. 1998, 32, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Li, S.X.; Shi, J.; Epstein, D.H.; Wang, X.; Zhang, X.; Bao, Y.; Zhang, D.; Zhang, X.; Kosten, T.R.; Lu, L. Circadian alteration in neurobiology during 30 days of abstinence in heroin users. Biol. Psychiatry 2009, 65, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, R.; Corsini, G.; Cataldi, A.; Fiorucci, A.; Tenerelli, P.; Rolandi, E.; Barreca, T. Twenty-four-hour variation in serum leptin in the elderly. Metabolism 1999, 48, 1011–1014. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Fu, Y.R.; Li, X.H.; Li, Y.Y.; Bogdan, A.; Touitou, Y. Age-related modifications of circadian rhythm of serum leptin in healthy men. Gerontology 2002, 48, 309–314. [Google Scholar] [CrossRef]
- Weise, M.; Abad, V.; Considine, R.V.; Nieman, L.; Rother, K.I. Leptin secretion in Cushing’s syndrome: Preservation of diurnal rhythm and absent response to corticotropin-releasing hormone. J. Clin. Endocrinol. Metab. 1999, 84, 2075–2079. [Google Scholar] [CrossRef][Green Version]
- Mantovani, G.; Macciò, A.; Mura, L.; Massa, E.; Mudu, M.C.; Mulas, C.; Lusso, M.R.; Madeddu, C.; Dessì, A. Serum levels of leptin and proinflammatory cytokines in patients with advanced-stage cancer at different sites. J. Mol. Med. 2000, 78, 554–561. [Google Scholar] [CrossRef]
- De Oliveira, C.; Scarabelot, V.L.; De Souza, A.; De Oliveira, C.M.; Medeiros, L.F.; De Macedo, I.C.; Marques Filho, P.R.; Cioato, S.G.; Caumo, W.; Torres, I.L.S. Obesity and chronic stress are able to desynchronize the temporal pattern of serum levels of leptin and triglycerides. Peptides 2014, 51, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Perelló, M.; Chacon, F.; Cardinali, D.P.; Esquifino, A.I.; Spinedi, E. Effect of social isolation on 24-h pattern of stress hormones and leptin in rats. Life Sci. 2006, 78, 1857–1862. [Google Scholar] [CrossRef]
- Cano, P.; Cardinali, D.P.; Spinedi, E.; Esquifino, A.I. Effect of aging on 24-hour pattern of stress hormones and leptin in rats. Life Sci. 2008, 83, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Vu, J.P.; Larauche, M.; Flores, M.; Luong, L.; Norris, J.; Oh, S.; Liang, L.J.; Waschek, J.; Pisegna, J.R.; Germano, P.M. Regulation of Appetite, Body Composition, and Metabolic Hormones by Vasoactive Intestinal Polypeptide (VIP). J. Mol. Neurosci. 2015, 56, 377–387. [Google Scholar] [CrossRef]
- Calvani, M.; Scarfone, A.; Granato, L.; Mora, E.V.; Nanni, G.; Castagneto, M.; Greco, A.V.; Manco, M.; Mingrone, G. Restoration of adiponectin pulsatility in severely obese subjects after weight loss. Diabetes 2004, 53, 939–947. [Google Scholar] [CrossRef]
- Tan, B.K.; Adya, R.; Lewandowski, K.C.; O’Hare, J.P.; Randeva, H.S. Diurnal variation and effect of insulin on circulating high molecular weight (HMW) adiponectin and NF-κB activity in human endothelial cells. Atherosclerosis 2011, 214, 174–177. [Google Scholar] [CrossRef]
- Kennaway, D.J.; Varcoe, T.J.; Voultsios, A.; Boden, M.J. Global loss of bmal1 expression alters adipose tissue hormones, gene expression and glucose metabolism. PLoS ONE 2013, 8, e65255. [Google Scholar] [CrossRef] [PubMed]
- Otway, D.T.; Frost, G.; Johnston, J.D. Circadian rhythmicity in murine pre-adipocyte and adipocyte cells. Chronobiol. Int. 2009, 26, 1340–1354. [Google Scholar] [CrossRef]
- Sukumaran, S.; Jusko, W.J.; DuBois, D.C.; Almon, R.R. Mechanistic modeling of the effects of glucocorticoids and circadian rhythms on adipokine expression. J. Pharmacol. Exp. Ther. 2011, 337, 734–746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Li, M.; Zhu, X.; Shi, Y. Effect of a high-calorie diet and constant light exposure on female reproduction, metabolism and immune inflammation: A comparative study of different mouse models. Am. J. Reprod. Immunol. 2021, 86, e13479. [Google Scholar] [CrossRef]
- Shea, S.A.; Hilton, M.F.; Orlova, C.; Ayers, R.T.; Mantzoros, C.S. Independent circadian and sleep/wake regulation of adipokines and glucose in humans. J. Clin. Endocrinol. Metab. 2005, 90, 2537–2544. [Google Scholar] [CrossRef]
- Crispim, C.A.; Padilha, H.G.; Zimberg, I.Z.; Waterhouse, J.; Dattilo, M.; Tufik, S.; De Mello, M.T. Adipokine levels are altered by shiftwork: A preliminary study. Chronobiol. Int. 2012, 29, 587–594. [Google Scholar] [CrossRef]
- Ramsey, K.M.; Yoshino, J.; Brace, C.S.; Abrassart, D.; Kobayashi, Y.; Marcheva, B.; Hong, H.K.; Chong, J.L.; Buhr, E.D.; Lee, C.; et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 2009, 324, 651–654. [Google Scholar] [CrossRef] [PubMed]
- Benedict, C.; Shostak, A.; Lange, T.; Brooks, S.J.; Schiöth, H.B.; Schultes, B.; Born, J.; Oster, H.; Hallschmid, M. Diurnal rhythm of circulating nicotinamide phosphoribosyltransferase (Nampt/visfatin/PBEF): Impact of sleep loss and relation to glucose metabolism. J. Clin. Endocrinol. Metab. 2012, 97, E218–E222. [Google Scholar] [CrossRef] [PubMed]
- Brøns, C.; Saltbæk, P.N.; Friedrichsen, M.; Chen, Y.; Vaag, A. Endocrine and metabolic diurnal rhythms in young adult men born small vs. appropriate for gestational age. Eur. J. Endocrinol. 2016, 175, 29–40. [Google Scholar] [CrossRef]
- Jeong, E.; Youn, B.S.; Kim, D.W.; Kim, E.H.; Park, J.W.; Namkoong, C.; Jeong, J.Y.; Yoon, S.Y.; Park, J.Y.; Lee, K.U.; et al. Circadian rhythm of serum vaspin in healthy male volunteers: Relation to meals. J. Clin. Endocrinol. Metab. 2010, 95, 1869–1875. [Google Scholar] [CrossRef]
- Ma, X.; Zhou, Z.; Chen, Y.; Wu, Y.; Liu, Y. RBP4 functions as a hepatokine in the regulation of glucose metabolism by the circadian clock in mice. Diabetologia 2016, 59, 354–362. [Google Scholar] [CrossRef]
- Duparc, T.; Colom, A.; Cani, P.D.; Massaly, N.; Rastrelli, S.; Drougard, A.; Le Gonidec, S.; Moulédous, L.; Frances, B.; Leclercq, I.; et al. Central apelin controls glucose homeostasis via a nitric oxide-dependent pathway in mice. Antioxid. Redox Signal. 2011, 15, 1477–1496. [Google Scholar] [CrossRef]
- Oliver, P.; Ribot, J.; Rodríguez, A.M.; Sánchez, J.; Picó, C.; Palou, A. Resistin as a putative modulator of insulin action in the daily feeding/fasting rhythm. Pflug. Arch. 2006, 452, 260–267. [Google Scholar] [CrossRef]
- Burgueño, A.; Gemma, C.; Gianotti, T.F.; Sookoian, S.; Pirola, C.J. Increased levels of resistin in rotating shift workers: A potential mediator of cardiovascular risk associated with circadian misalignment. Atherosclerosis 2010, 210, 625–629. [Google Scholar] [CrossRef] [PubMed]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A.J.L. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Hamnvik, O.P.R.; Thakkar, B.; Chamberland, J.; Aronis, K.; Schneider, B.; Mantzoros, C.S. Omentin-1 levels are reduced by pharmacologic doses of leptin, but remain unaffected by energy deprivation and display no day-night variation. Int. J. Obes. 2015, 39, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Chamberland, J.P.; Berman, R.L.; Aronis, K.N.; Mantzoros, C.S. Chemerin is expressed mainly in pancreas and liver, is regulated by energy deprivation, and lacks day/night variation in humans. Eur. J. Endocrinol. 2013, 169, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Brown adipose tissue: Updates in cellular and molecular biology. Tissue Cell 2016, 48, 452–460. [Google Scholar] [CrossRef]
- Eley, J.; Himms-Hagen, J. Brown adipose tissue of mice with GTG-induced obesity: Altered circadian control. Am. J. Physiol. 1989, 256, E773–E779. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.; Bova, R.; Schofield, L.; Bryant, W.; Dieckmann, W.; Slattery, A.; Govendir, M.A.; Emmett, L.; Greenfield, J.R. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab. 2016, 23, 602–609. [Google Scholar] [CrossRef]
- Matsushita, M.; Nirengi, S.; Hibi, M.; Wakabayashi, H.; Lee, S.İ.; Domichi, M.; Sakane, N.; Saito, M. Diurnal variations of brown fat thermogenesis and fat oxidation in humans. Int. J. Obes. 2021, 45, 2499–2505. [Google Scholar] [CrossRef] [PubMed]
- Moraes, M.N.; de Assis, L.V.M.; Henriques, F.D.S.; Batista, M.L.; Güler, A.D.; de Lauro Castrucci, A.M. Cold-sensing TRPM8 channel participates in circadian control of the brown adipose tissue. Biochim. Biophys. Acta. Mol. Cell Res. 2017, 1864, 2415–2427. [Google Scholar] [CrossRef] [PubMed]
- Razzoli, M.; Emmett, M.J.; Lazar, M.A.; Bartolomucci, A. β-Adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. FASEB J. 2018, 32, 5640–5646. [Google Scholar] [CrossRef]
- Orozco-Solis, R.; Aguilar-Arnal, L.; Murakami, M.; Peruquetti, R.; Ramadori, G.; Coppari, R.; Sassone-Corsi, P. The Circadian Clock in the Ventromedial Hypothalamus Controls Cyclic Energy Expenditure. Cell Metab. 2016, 23, 467–478. [Google Scholar] [CrossRef]
- Felipe, A.; Lopez-Soriano, F.J. Circadian changes in glycogen content in rat interscapular brown adipose tissue: Effect of cold exposure and food deprivation. Biochem. Int. 1990, 21, 537–543. [Google Scholar]
- Angers, M.; Uldry, M.; Kong, D.; Gimble, J.M.; Jetten, A.M. Mfsd2a encodes a novel major facilitator superfamily domain-containing protein highly induced in brown adipose tissue during fasting and adaptive thermogenesis. Biochem. J. 2008, 416, 347–355. [Google Scholar] [CrossRef]
- Hasan, N.; Nagata, N.; Morishige, J.İ.; Islam, M.T.; Jing, Z.; Harada, K.; Mieda, M.; Ono, M.; Fujiwara, H.; Daikoku, T.; et al. Brown adipocyte-specific knockout of Bmal1 causes mild but significant thermogenesis impairment in mice. Mol. Metab. 2021, 49, 101202. [Google Scholar] [CrossRef] [PubMed]
- Acosta, F.M.; Sanchez-Delgado, G.; Martinez-Tellez, B.; Alcantara, J.M.A.; Llamas-Elvira, J.M.; Ruiz, J.R. Diurnal variations of cold-induced thermogenesis in young, healthy adults: A randomized crossover trial. Clin. Nutr. 2021, 40, 5311–5321. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.M.; Zhang, Z.; Su, Y.; de Goede, P.; Jansen, R.; Foppen, E.; Coimbra, C.C.; Kalsbeek, A. Time-of-Day Effects on Metabolic and Clock-Related Adjustments to Cold. Front. Endocrinol. 2018, 9, 199. [Google Scholar] [CrossRef]
- Straat, M.E.; Martinez-Tellez, B.; Sardjoe Mishre, A.; Verkleij, M.M.A.; Kemmeren, M.; Pelsma, I.C.M.; Alcantara, J.M.A.; Mendez-Gutierrez, A.; Kooijman, S.; Boon, M.R.; et al. Cold-Induced Thermogenesis Shows a Diurnal Variation That Unfolds Differently in Males and Females. J. Clin. Endocrinol. Metab. 2022, 107, 1626–1635. [Google Scholar] [CrossRef]
- Teubner, B.J.W.; Leitner, C.; Thomas, M.A.; Ryu, V.; Bartness, T.J. An intact dorsomedial posterior arcuate nucleus is not necessary for photoperiodic responses in Siberian hamsters. Horm. Behav. 2015, 70, 22–29. [Google Scholar] [CrossRef][Green Version]
- Mercer, J.G.; Duncan, J.S.; Lawrence, C.B.; Trayhurn, P. Effect of photoperiod on mitochondrial GDP binding and adenylate cyclase activity in brown adipose tissue of Djungarian hamsters. Physiol. Behav. 1994, 56, 737–740. [Google Scholar] [CrossRef]
- McElroy, J.F.; Wade, G.N. Short photoperiod stimulates brown adipose tissue growth and thermogenesis but not norepinephrine turnover in Syrian hamsters. Physiol. Behav. 1986, 37, 307–311. [Google Scholar] [CrossRef]
- Jefimow, M.; Wojciechowski, M.S.; Tȩgowska, E. Effects of prolonged acclimation to intermediate photoperiod and photo-schedule reversal in photosensitive golden hamsters. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 987–997. [Google Scholar] [CrossRef]
- Himms-Hagen, J. Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am. J. Physiol. 1985, 248, E531–E539. [Google Scholar] [CrossRef] [PubMed]
- Chappuis, S.; Ripperger, J.A.; Schnell, A.; Rando, G.; Jud, C.; Wahli, W.; Albrecht, U. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol. Metab. 2013, 2, 184–193. [Google Scholar] [CrossRef]
- Gerhart-Hines, Z.; Feng, D.; Emmett, M.J.; Everett, L.J.; Loro, E.; Briggs, E.R.; Bugge, A.; Hou, C.; Ferrara, C.; Seale, P.; et al. The nuclear receptor Rev-erbα controls circadian thermogenic plasticity. Nature 2013, 503, 410–413. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Carpenter, B.J.; Remsberg, J.R.; Aubert, Y.; Peed, L.C.; Richter, H.J.; Lazar, M.A. Circadian lipid synthesis in brown fat maintains murine body temperature during chronic cold. Proc. Natl. Acad. Sci. USA 2019, 116, 18691–18699. [Google Scholar] [CrossRef]
- Li, S.; Yu, Q.; Wang, G.X.; Lin, J.D. The biological clock is regulated by adrenergic signaling in brown fat but is dispensable for cold-induced thermogenesis. PLoS ONE 2013, 8, e70109. [Google Scholar] [CrossRef] [PubMed]
- Noshiro, M.; Kawamoto, T.; Nakashima, A.; Ozaki, N.; Ueno, T.; Saeki, M.; Honda, K.; Fujimoto, K.; Kato, Y. Deficiency of the basic helix-loop-helix transcription factor DEC1 prevents obesity induced by a high-fat diet in mice. Genes Cells 2018, 23, 658–669. [Google Scholar] [CrossRef]
- Mathew, D.; Zhou, P.; Pywell, C.M.; van der Veen, D.R.; Shao, J.; Xi, Y.; Bonar, N.A.; Hummel, A.D.; Chapman, S.; Leevy, W.M.; et al. Ablation of the ID2 gene results in altered circadian feeding behavior, and sex-specific enhancement of insulin sensitivity and elevated glucose uptake in skeletal muscle and brown adipose tissue. PLoS ONE 2013, 8, e73064. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, M.; George, J.C. Pinealectomy has no effect on diet-induced thermogenesis and brown adipose tissue proliferation in rats. J. Pineal Res. 1984, 1, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- De Fano, M.; Bartolini, D.; Tortoioli, C.; Vermigli, C.; Malara, M.; Galli, F.; Murdolo, G. Adipose Tissue Plasticity in Response to Pathophysiological Cues: A Connecting Link between Obesity and Its Associated Comorbidities. Int. J. Mol. Sci. 2022, 23, 5511. [Google Scholar] [CrossRef]
- Machado, S.A.; Pasquarelli-do-Nascimento, G.; da Silva, D.S.S.; Farias, G.R.; de Oliveira Santos, I.; Baptista, L.B.; Magalhães, K.G. Browning of the white adipose tissue regulation: New insights into nutritional and metabolic relevance in health and diseases. Nutr. Metab. 2022, 19, 61. [Google Scholar] [CrossRef]
- Shao, M.; Wang, Q.A.; Song, A.; Vishvanath, L.; Busbuso, N.C.; Scherer, P.E.; Gupta, R.K. Cellular Origins of Beige Fat Cells Revisited. Diabetes 2019, 68, 1874–1885. [Google Scholar] [CrossRef]
- Grimaldi, B.; Bellet, M.M.; Katada, S.; Astarita, G.; Hirayama, J.; Amin, R.H.; Granneman, J.G.; Piomelli, D.; Leff, T.; Sassone-Corsi, P. PER2 controls lipid metabolism by direct regulation of PPARγ. Cell Metab. 2010, 12, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Monnier, C.; Auclair, M.; Le Cam, G.; Garcia, M.P.; Antoine, B. The nuclear retinoid-related orphan receptor RORα controls circadian thermogenic programming in white fat depots. Physiol. Rep. 2018, 6, e13678. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.F.; Kleiner, S.; Douris, N.; Fox, E.C.; Mepani, R.J.; Verdeguer, F.; Wu, J.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E.; et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012, 26, 271–281. [Google Scholar] [CrossRef]
- Lowden, A.; Moreno, C.; Holmbäck, U.; Lennernäs, M.; Tucker, P. Eating and shift work—Effects on habits, metabolism, and performance. In Scandinavian Journal of Work, Environment and Health; Nordic Association of Occupational Safety and Health: Helsinki, Finland, 2010; Volume 36, pp. 150–162. [Google Scholar] [CrossRef]
- Vetter, C.; Dashti, H.S.; Lane, J.M.; Anderson, S.G.; Schernhammer, E.S.; Rutter, M.K.; Saxena, R.; Scheer, F.A.J.L. Night shift work, genetic risk, and type 2 diabetes in the UK biobank. Diabetes Care 2018, 41, 762–769. [Google Scholar] [CrossRef]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of circadian disruption on glucose metabolism: Implications for type 2 diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef]
- Gottlieb, D.J.; Punjabi, N.M.; Newman, A.B.; Resnick, H.E.; Redline, S.; Baldwin, C.M.; Javier Nieto, F. Association of Sleep Time With Diabetes Mellitus and Impaired Glucose Tolerance. Arch. Intern. Med. 2005, 165, 863–868. [Google Scholar] [CrossRef]
- Ayas, N.T.; White, D.P.; Al-Delaimy, W.K.; Manson, J.E.; Stampfer, M.J.; Speizer, F.E.; Patel, S.; Hu, F.B. A Prospective Study of Self-Reported Sleep Duration and Incident Diabetes in Women. Diabetes Care 2003, 26, 380–384. [Google Scholar] [CrossRef]
- Gan, Y.; Yang, C.; Tong, X.; Sun, H.; Cong, Y.; Yin, X.; Li, L.; Cao, S.; Dong, X.; Gong, Y.; et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 2015, 72, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Schernhammer, E.S.; Sun, Q.; Hu, F.B. Rotating night shift work and risk of type 2 diabetes: Two prospective cohort studies in women. PLoS Med. 2011, 8, e1001141. [Google Scholar] [CrossRef] [PubMed]
- Manodpitipong, A.; Saetung, S.; Nimitphong, H.; Siwasaranond, N.; Wongphan, T.; Sornsiriwong, C.; Luckanajantachote, P.; Mangjit, P.; Keesukphan, P.; Crowley, S.J.; et al. Night-shift work is associated with poorer glycaemic control in patients with type 2 diabetes. J. Sleep Res. 2017, 26, 764–772. [Google Scholar] [CrossRef]
- Broussard, J.L.; Ehrmann, D.A.; van Cauter, E.; Tasali, E.; Brady, M.J. Impaired insulin signaling in human adipocytes after experimental sleep restriction: A randomized, crossover study. Ann. Intern. Med. 2012, 157, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; L’Hermite-Balériaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- Mullington, J.M.; Chan, J.L.; Van Dongen, H.P.A.; Szuba, M.P.; Samaras, J.; Price, N.J.; Meier-Ewert, H.K.; Dingesz, D.F.; Mantzoros, C.S.; Mullington, J.; et al. Sleep Loss Reduces Diurnal Rhythm Amplitude of Leptin in Healthy Men. J. Neuroendocrinol. 2003, 15, 851–854. [Google Scholar] [CrossRef]
- Dzaja, A.; Dalal, M.A.; Himmerich, H.; Uhr, M.; Pollmächer, T.; Schuld, A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. Am. J. Physiol. Endocrinol. Metab. 2004, 286, 963–967. [Google Scholar] [CrossRef]
- Pijut, S.S.; Corbett, D.E.; Wang, Y.; Li, J.; Charnigo, R.J.; Graf, G.A.; Ss, P.; De, C.; Li, W.Y.; Rj, C.; et al. Effect of peripheral circadian dysfunction on metabolic disease in response to a diabetogenic diet. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 900–911. [Google Scholar] [CrossRef][Green Version]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.R.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.W.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, H.; Ando, H.; Hayashi, Y.; Obi, Y.; Fujimura, A. High-fat feeding exerts minimal effects on rhythmic mRNA expression of clock genes in mouse peripheral tissues. Chronobiol. Int. 2006, 23, 905–914. [Google Scholar] [CrossRef]
- Ando, H.; Yanagihara, H.; Hayashi, Y.; Obi, Y.; Tsuruoka, S.; Takamura, T.; Kaneko, S.; Fujimura, A. Rhythmic messenger ribonucleic acid expression of clock genes and adipocytokines in mouse visceral adipose tissue. Endocrinology 2005, 146, 5631–5636. [Google Scholar] [CrossRef]
- Wang, S.; Lin, Y.; Gao, L.; Yang, Z.; Lin, J.; Ren, S.; Li, F.; Chen, J.; Wang, Z.; Dong, Z.; et al. PPAR-γ integrates obesity and adipocyte clock through epigenetic regulation of Bmal1. Theranostics 2022, 12, 1589–1606. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Abellán, P.; Hernández-Morante, J.J.; Luján, J.A.; Madrid, J.A.; Garaulet, M. Clock genes are implicated in the human metabolic syndrome. Int. J. Obes. 2008, 32, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Otway, D.T.; Mäntele, S.; Bretschneider, S.; Wright, J.; Trayhurn, P.; Skene, D.J.; Robertson, M.D.; Johnston, J.D. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and type 2 diabetic. Diabetes 2011, 60, 1577–1581. [Google Scholar] [CrossRef]
- Gómez-Santos, C.; Gómez-Abellán, P.; Madrid, J.A.; Hernández-Morante, J.J.; Lujan, J.A.; Ordovas, J.M.; Garaulet, M. Circadian rhythm of clock genes in human adipose explants. Obesity 2009, 17, 1481–1485. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, O.; Gögebakan, Ö.; Sucher, S.; Groth, J.; Murahovschi, V.; Kessler, K.; Osterhoff, M.; Rudovich, N.; Kramer, A.; Pfeiffer, A.F.H. Regulation of the clock gene expression in human adipose tissue by weight loss. Int. J. Obes. 2016, 40, 899–906. [Google Scholar] [CrossRef]
- Thomas, A.P.; Hoang, J.; Vongbunyong, K.; Nguyen, A.; Rakshit, K.; Matveyenko, A.V. Administration of melatonin and metformin prevents deleterious effects of circadian disruption and obesity in male rats. Endocrinology 2016, 157, 4720–4731. [Google Scholar] [CrossRef]
- Barnea, M.; Haviv, L.; Gutman, R.; Chapnik, N.; Madar, Z.; Froy, O. Metformin affects the circadian clock and metabolic rhythms in a tissue-specific manner. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 1796–1806. [Google Scholar] [CrossRef]
- Jee, H.U.; Yang, S.; Yamazaki, S.; Kang, H.; Viollet, B.; Foretz, M.; Chung, J.H. Activation of 5′-AMP-activated kinase with diabetes drug metformin induces casein kinase Iε (CKIε)-dependent degradation of clock protein mPer2. J. Biol. Chem. 2007, 282, 20794–20798. [Google Scholar] [CrossRef]
- Henriksson, E.; Huber, A.L.; Soto, E.K.; Kriebs, A.; Vaughan, M.E.; Duglan, D.; Chan, A.B.; Papp, S.J.; Nguyen, M.; Afetian, M.E.; et al. The Liver Circadian Clock Modulates Biochemical and Physiological Responses to Metformin. J. Biol. Rhythm. 2017, 32, 345–358. [Google Scholar] [CrossRef]
- Alex, A.; Luo, Q.; Mathew, D.; Di, R.; Bhatwadekar, A.D. Metformin Corrects Abnormal Circadian Rhythm and Kir4.1 Channels in Diabetes. Investig. Ophthalmol. Vis. Sci. 2020, 61, 46. [Google Scholar] [CrossRef]
- Caton, P.W.; Kieswich, J.; Yaqoob, M.M.; Holness, M.J.; Sugden, M.C. Metformin opposes impaired AMPK and SIRT1 function and deleterious changes in core clock protein expression in white adipose tissue of genetically-obese db/db mice. Diabetes Obes. Metab. 2011, 13, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Türk, D.; Scherer, N.; Selzer, D.; Dings, C.; Hanke, N.; Dallmann, R.; Schwab, M.; Timmins, P.; Nock, V.; Lehr, T. Significant impact of time-of-day variation on metformin pharmacokinetics. Diabetologia 2023, 66, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, G. PPARs Integrate the Mammalian Clock and Energy Metabolism. PPAR Res. 2014, 2014, 653017. [Google Scholar] [CrossRef]
- Oishi, K.; Shirai, H.; Ishida, N. PPARα is involved in photoentrainment of the circadian clock. Neuroreport 2008, 19, 487–489. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Miyazaki, K.; Kadota, K.; Kikuno, R.; Nagase, T.; Atsumi, G.I.; Ohkura, N.; Azama, T.; Mesaki, M.; Yukimasa, S.; et al. Genome-wide Expression Analysis of Mouse Liver Reveals CLOCK-regulated Circadian Output Genes. J. Biol. Chem. 2003, 278, 41519–41527. [Google Scholar] [CrossRef]
- Oishi, K.; Uchida, D.; Ishida, N. Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett. 2008, 582, 3639–3642. [Google Scholar] [CrossRef]
- Inagaki, T.; Dutchak, P.; Zhao, G.; Ding, X.; Gautron, L.; Parameswara, V.; Li, Y.; Goetz, R.; Mohammadi, M.; Esser, V.; et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell Metab. 2007, 5, 415–425. [Google Scholar] [CrossRef]
- Chikahisa, S.; Tominaga, K.; Kawai, T.; Kitaoka, K.; Oishi, K.; Ishida, N.; Rokutan, K.; Séi, H. Bezafibrate, a peroxisome proliferator-activated receptors agonist, decreases body temperature and enhances electroencephalogram delta-oscillation during sleep in mice. Endocrinology 2008, 149, 5262–5271. [Google Scholar] [CrossRef]
- Yang, S.C.; Tseng, H.L.; Shieh, K.R. Circadian-clock system in mouse liver affected by insulin resistance. Chronobiol. Int. 2013, 30, 796–810. [Google Scholar] [CrossRef]
- Tseng, H.L.; Yang, S.C.; Yang, S.H.; Shieh, K.R. Hepatic circadian-clock system altered by insulin resistance, diabetes and insulin sensitizer in mice. PLoS ONE 2015, 10, e0120380. [Google Scholar] [CrossRef][Green Version]
- Ribas-Latre, A.; Fekry, B.; Kwok, C.; Baumgartner, C.; Shivshankar, S.; Sun, K.; Chen, Z.; Eckel-Mahan, K. Rosiglitazone reverses high fat diet-induced changes in BMAL1 function in muscle, fat, and liver tissue in mice. Int. J. Obes. 2019, 43, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Fedchenko, T.; Izmailova, O.; Shynkevych, V.; Shlykova, O.; Kaidashev, I. PPAR- γ Agonist Pioglitazone Restored Mouse Liver mRNA Expression of Clock Genes and Inflammation-Related Genes Disrupted by Reversed Feeding. PPAR Res. 2022, 2022, 7537210. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, J.V.; Bustamante, M.A.; Teter, M.L.; Markert, R.J.; McDonald, S.D. Bedtime dosing of glyburide and the treatment of type II diabetes mellitus. Am. J. Med. Sci. 1994, 308, 234–238. [Google Scholar] [CrossRef]
- Yoshioka, H.; Hirose, Y.; Ohishi, R.; Tominaga, S.; Torii-Goto, A.; Park, S.J.; Miura, N.; Yoshikawa, M. Diurnal Variation of Sitagliptin-Induced Pharmacological Effects in C57BL/6J Mice. Biol. Pharm. Bull. 2019, 42, 1562–1568. [Google Scholar] [CrossRef]
- Chamarthi, B.; Cincotta, A.H. Effect of bromocriptine-QR therapy on glycemic control in subjects with type 2 diabetes mellitus whose dysglycemia is inadequately controlled on insulin. Postgrad. Med. 2017, 129, 446–455. [Google Scholar] [CrossRef]
- Raskin, P.; Cincotta, A.H. Bromocriptine-QR therapy for the management of type 2 diabetes mellitus: Developmental basis and therapeutic profile summary. Expert Rev. Endocrinol. Metab. 2016, 11, 113–1480. [Google Scholar] [CrossRef] [PubMed]
- Pijl, H.; Ohashi, S.; Matsuda, M.; Miyazaki, Y.; Mahankali, A.; Kumar, V.; Pipek, R.; Iozzo, P.; Lancaster, J.L.; Cincotta, A.H.; et al. Bromocriptine: A novel approach to the treatment of type 2 diabetes. Diabetes Care 2000, 23, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Gaziano, J.M.; Cincotta, A.H.; O’Connor, C.M.; Ezrokhi, M.; Rutty, D.; Ma, Z.J.; Scranton, R.E. Randomized clinical trial of quick-release bromocriptine among patients with type 2 diabetes on overall safety and cardiovascular outcomes. Diabetes Care 2010, 33, 1503–1508. [Google Scholar] [CrossRef]
- Stoelzel, C.R.; Zhang, Y.; Cincotta, A.H. Circadian-timed dopamine agonist treatment reverses high-fat diet-induced diabetogenic shift in ventromedial hypothalamic glucose sensing. Endocrinol. Diabetes Metab. 2020, 3, e00139. [Google Scholar] [CrossRef]
- Luo, S.; Ezrokhi, M.; Cominos, N.; Tsai, T.H.; Stoelzel, C.R.; Trubitsyna, Y.; Cincotta, A.H. Experimental dopaminergic neuron lesion at the area of the biological clock pacemaker, suprachiasmatic nuclei (SCN) induces metabolic syndrome in rats. Diabetol. Metab. Syndr. 2021, 13, 11. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, Y.; Ezrokhi, M.; Li, Y.; Tsai, T.H.; Cincotta, A.H. Circadian peak dopaminergic activity response at the biological clock pacemaker (suprachiasmatic nucleus) area mediates the metabolic responsiveness to a high-fat diet. J. Neuroendocrinol. 2018, 30, e12563. [Google Scholar] [CrossRef]
- Ezrokhi, M.; Luo, S.; Trubitsyna, Y.; Cincotta, A.H. Neuroendocrine and metabolic components of dopamine agonist amelioration of metabolic syndrome in SHR rats. Diabetol. Metab. Syndr. 2014, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Ezrokhi, M.; Zhang, Y.; Luo, S.; Cincotta, A.H. Time-of-Day-Dependent Effects of Bromocriptine to Ameliorate Vascular Pathology and Metabolic Syndrome in SHR Rats Held on High Fat Diet. Int. J. Mol. Sci. 2021, 22, 6142. [Google Scholar] [CrossRef] [PubMed]
- Guagnano, M.T.; Di Vincenzo, M.; Sensi, S. Chronotherapy of obesity. I. Effect of fenfluramine on the decrease of adipose mass in the obese, in relation to the time of administration. Boll. Soc. Ital. Biol. Sper. 1982, 58, 736–739. Available online: https://pubmed.ncbi.nlm.nih.gov/7104096/ (accessed on 17 January 2023). [PubMed]
- Di Vincenzo, M.; Guagnano, M.T.; Della Loggia, F.; Sensi, S. Chronotherapy of Obesity. II. Variations in Eating Behavior in the Obese after Fenfluramine Treatment in Relation to Time of Administration. Boll. Soc. Ital. Biol. Sper. 1982, 58, 740–744. Available online: https://pubmed.ncbi.nlm.nih.gov/7104097/ (accessed on 19 January 2023).
- Zhang, G.; Cai, D. Circadian intervention of obesity development via resting-stage feeding manipulation or oxytocin treatment. Am. J. Physiol. Endocrinol. Metab. 2011, 301, E1004–E1012. [Google Scholar] [CrossRef]
- Lee, A.; Bray, G.A. Insulin secretion in hypothalamic obesity: Diurnal variation and the effect of naloxone. Obes. Res. 1993, 1, 449–458. [Google Scholar] [CrossRef]
- Tsuneki, H.; Nagata, T.; Fujita, M.; Kon, K.; Wu, N.; Takatsuki, M.; Yamaguchi, K.; Wada, T.; Nishijo, H.; Yanagisawa, M.; et al. Nighttime administration of nicotine improves hepatic glucose metabolism via the hypothalamic orexin system in mice. Endocrinology 2016, 157, 195–206. [Google Scholar] [CrossRef]
- Nakata, M.; Kumari, P.; Kita, R.; Katsui, N.; Takeuchi, Y.; Kawaguchi, T.; Yamazaki, T.; Zhang, B.; Shimba, S.; Yada, T. Circadian clock component bmal1 in the paraventricular nucleus regulates glucose metabolism. Nutrients 2021, 13, 4487. [Google Scholar] [CrossRef]
- Mandl, M.; Viertler, H.P.; Zopoglou, M.; Mitterberger-Vogt, M.C.; Gasser, J.; Hatzmann, F.M.; Rauchenwald, T.; Zwierzina, M.E.; Mattesich, M.; Weiss, A.K.H.; et al. The circadian transcription factor ARNTL2 is regulated by weight-loss interventions in human white adipose tissue and inhibits adipogenesis. Cell Death Discov. 2022, 8, 443. [Google Scholar] [CrossRef]
- Adlanmerini, M.; Nguyen, H.C.B.; Krusen, B.M.; Teng, C.W.; Geisler, C.E.; Peed, L.C.; Carpenter, B.J.; Hayes, M.R.; Lazar, M.A. Hypothalamic REV-ERB nuclear receptors control diurnal food intake and leptin sensitivity in diet-induced obese mice. J. Clin. Investig. 2021, 131, e140424. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Kojetin, D.J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Noel, R.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef]
- Vieira, E.; Marroquí, L.; Figueroa, A.L.C.; Merino, B.; Fernandez-Ruiz, R.; Nadal, A.; Burris, T.P.; Gomis, R.; Quesada, I. Involvement of the Clock Gene Rev-erb alpha in the Regulation of Glucagon Secretion in Pancreatic Alpha-Cells. PLoS ONE 2013, 8, e69939. [Google Scholar] [CrossRef]
- Garaulet, M.; Smith, C.E.; Gomez-Abellán, P.; Ordovás-Montañés, M.; Lee, Y.C.; Parnell, L.D.; Arnett, D.K.; Ordovás, J.M. REV-ERB-ALPHA circadian gene variant associates with obesity in two independent populations: Mediterranean and North American. Mol. Nutr. Food Res. 2014, 58, 821–829. [Google Scholar] [CrossRef] [PubMed]
- Lau, P.; Tuong, Z.K.; Wang, S.C.; Fitzsimmons, R.L.; Goode, J.M.; Thomas, G.P.; Cowin, G.J.; Pearen, M.A.; Mardon, K.; Stow, J.L.; et al. Rorα deficiency and decreased adiposity are associated with induction of thermogenic gene expression in subcutaneous white adipose and brown adipose tissue. Am. J. Physiol. Endocrinol. Metab. 2015, 308, E159–E171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Papazyan, R.; Damle, M.; Fang, B.; Jager, J.; Feng, D.; Peed, L.C.; Guan, D.; Sun, Z.; Lazar, M.A. The hepatic circadian clock fine-tunes the lipogenic response to feeding through RORα/γ. Genes Dev. 2017, 31, 1202–1211. [Google Scholar] [CrossRef]
- Ruiz-Lozano, T.; Vidal, J.; De Hollanda, A.; Canteras, M.; Garaulet, M.; Izquierdo-Pulido, M. Evening chronotype associates with obesity in severely obese subjects: Interaction with CLOCK 3111T/C. Int. J. Obes. 2016, 40, 1550–1557. [Google Scholar] [CrossRef]
- Espinosa-Salinas, I.; San-Cristobal, R.; Colmenarejo, G.; Loria-Kohen, V.; Molina, S.; Reglero, G.; de Molina, A.R.; Alfredo Martinez, J. Polymorphic appetite effects on waist circumference depend on RS3749474 clock gene variant. Nutrients 2020, 12, 1846. [Google Scholar] [CrossRef]
- Oishi, K.; Ohkura, N.; Wakabayashi, M.; Shirai, H.; Sato, K.; Matsuda, J.; Atsumi, G.; Ishida, N. CLOCK is involved in obesity-induced disordered fibrinolysis in ob/ob mice by regulating PAI-1 gene expression. J. Thromb. Haemost. 2006, 4, 1774–1780. [Google Scholar] [CrossRef]
- Barclay, J.L.; Shostak, A.; Leliavski, A.; Tsang, A.H.; Jöhren, O.; Müller-Fielitz, H.; Landgraf, D.; Naujokat, N.; van der Horst, G.T.J.; Oster, H. High-fat diet-induced hyperinsulinemia and tissue-specific insulin resistance in Cry-deficient mice. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E1053–E1063. [Google Scholar] [CrossRef]
- Griebel, G.; Ravinet-Trillou, C.; Beeské, S.; Avenet, P.; Pichat, P. Mice deficient in cryptochrome 1 (cry1 (-/-)) exhibit resistance to obesity induced by a high-fat diet. Front. Endocrinol. 2014, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.; Batista-Gonzalez, A.; Merheb, E.; Aoun, M.L.; Tarabra, E.; Feng, D.; Sarparanta, J.; Merlo, P.; Botrè, F.; Schwartz, G.J.; et al. Autophagy Regulates the Liver Clock and Glucose Metabolism by Degrading CRY1. Cell Metab. 2018, 28, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, I.H.; Gul, S.; Turkay, M. Identification of novel small molecules targeting core clock proteins to regulate circadian rhythm. Curr. Opin. Chem. Eng. 2022, 35, 100730. [Google Scholar] [CrossRef]
- Chen, Z.; Yoo, S.-H.; Takahashi, J.S. Annual Review of Pharmacology and Toxicology Development and Therapeutic Potential of Small-Molecule Modulators of Circadian Systems. Annu. Rev. Pharmacol. Toxicol. 2018, 58, 231–252. [Google Scholar] [CrossRef]
- Ribeiro, R.F.N.; Cavadas, C.; Silva, M.M.C. Small-molecule modulators of the circadian clock: Pharmacological potentials in circadian-related diseases. Drug Discov. Today 2021, 26, 1620–1641. [Google Scholar] [CrossRef]
- Hirota, T.; Lee, J.W.; St. John, P.C.; Sawa, M.; Iwaisako, K.; Noguchi, T.; Pongsawakul, P.Y.; Sonntag, T.; Welsh, D.K.; Brenner, D.A.; et al. Identification of Small Molecule Activators of Cryptochrome. Science 2012, 337, 1094–1097. [Google Scholar] [CrossRef]
- Lamia, K.A.; Papp, S.J.; Yu, R.T.; Barish, G.D.; Uhlenhaut, N.H.; Jonker, J.W.; Downes, M.; Evans, R.M. Cryptochromes Mediate Rhythmic Repression of the Glucocorticoid Receptor. Nature 2011, 480, 552–556. [Google Scholar] [CrossRef]
- Miller, S.; Son, Y.L.; Aikawa, Y.; Makino, E.; Nagai, Y.; Srivastava, A.; Oshima, T.; Sugiyama, A.; Hara, A.; Abe, K.; et al. Isoform-Selective Regulation of Mammalian Cryptochromes. Nat. Chem. Biol. 2020, 16, 676–685. [Google Scholar] [CrossRef]
- Surme, S.; Ergun, C.; Gul, S.; Akyel, Y.K.; Gul, Z.M.; Ozcan, O.; Savglug Ipek, O.; Akarlar, B.A.; Ozlu, N.; Taskin, A.C.; et al. TW68, Cryptochromes stabilizer, regulates fasting blood glucose level in ob/ob and fat-induced diabetic mice. In Proceedings of the 8th International Bahçeşehir University (BAU) Drug Design Congress, Istanbul, Turkey, 15 December 2022. [Google Scholar]
- Grant, D.; Yin, L.; Collins, J.L.; Parks, D.J.; Orband-Miller, L.A.; Wisely, G.B.; Joshi, S.; Lazar, M.A.; Willson, T.M.; Zuercher, W.J. GSK4112, a Small Molecule Chemical Probe for the Cell Biology of the Nuclear Heme Receptor Rev-Erbα. ACS Chem. Biol. 2010, 5, 925–932. [Google Scholar] [CrossRef]
- Kojetin, D.; Wang, Y.; Kamenecka, T.M.; Burris, T.P. Identification of SR8278, a Synthetic Antagonist of the Nuclear Heme Receptor REV-ERB. ACS Chem. Biol. 2011, 6, 131–134. [Google Scholar] [CrossRef]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-Erb-α Modulates Skeletal Muscle Oxidative Capacity by Regulating Mitochondrial Biogenesis and Autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kumar, N.; Nuhant, P.; Cameron, M.D.; Istrate, M.A.; Roush, W.R.; Griffin, P.R.; Burris, T.P. Identification of SR1078, a Synthetic Agonist for the Orphan Nuclear Receptors RORα and RORγ. ACS Chem. Biol. 2010, 5, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.K.; Choi, Y.K.; Kang, Y.N.; Jang, B.K.; Kang, K.J.; Jeon, Y.H.; Lee, H.W.; Jeon, J.H.; Koo, S.H.; Jeong, W.I.; et al. Retinoic Acid-Related Orphan Receptor Alpha Reprograms Glucose Metabolism in Glutamine-Deficient Hepatoma Cells. Hepatology 2015, 61, 953–964. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Kojetin, D.J.; Solt, L.A.; Kumar, K.G.; Nuhant, P.; Duckett, D.R.; Cameron, M.D.; Butler, A.A.; Roush, W.R.; Griffin, P.R.; et al. Identification of SR3335 (ML-176): A Synthetic RORα Selective Inverse Agonist. ACS Chem. Biol. 2011, 6, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.R.; He, Y.; Khan, T.M.; Kuruvilla, D.S.; Garcia-Ordonez, R.; Corzo, C.A.; Unger, T.J.; White, D.W.; Khan, S.; Lin, L.; et al. Antiobesity Effect of a Small Molecule Repressor of RORγ. Mol. Pharmacol. 2015, 88, 48–56. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.S.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The Small Molecule Nobiletin Targets the Molecular Oscillator to Enhance Circadian Rhythms and Protect against Metabolic Syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin Fortifies Mitochondrial Respiration in Skeletal Muscle to Promote Healthy Aging against Metabolic Challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef]
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In Pancreatic Islets from Type 2 Diabetes Patients, the Dampened Circadian Oscillators Lead to Reduced Insulin and Glucagon Exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495. [Google Scholar] [CrossRef]
- De Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. In Ageing Research Reviews; Elsevier Ireland Ltd.: Dublin, Ireland, 2017; Volume 39, pp. 46–58. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef]
- Woodie, L.N.; Luo, Y.; Wayne, M.J.; Graff, E.C.; Ahmed, B.; O’Neill, A.M.; Greene, M.W. Restricted feeding for 9 h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism 2018, 82, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Zarrinpar, A.; Miu, P.; Panda, S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014, 20, 991–1005. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.J.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Duregon, E.; Pomatto-Watson, L.C.D.D.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent fasting: From calories to time restriction. GeroScience 2021, 43, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Jamshed, H.; Beyl, R.A.; Manna, D.L.D.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves 24-hour glucose levels and affects markers of the circadian clock, aging, and autophagy in humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef]
- Wirz-Justice, A.; Benedetti, F.; Berger, M.; Lam, R.W.; Martiny, K.; Terman, M.; Wu, J.C. Chronotherapeutics (light and wake therapy) in affective disorders. Psychol. Med. 2005, 35, 939–944. [Google Scholar] [CrossRef]
- Brouwer, A.; van Raalte, D.H.; Nguyen, H.T.; Rutters, F.; van de Ven, P.M.; Elders, P.J.M.; Moll, A.C.; Van Someren, E.J.W.; Snoek, F.J.; Beekman, A.T.F.; et al. Effects of light therapy on mood and insulin sensitivity in patients with type 2 diabetes and depression: Results from a randomized placebo-controlled trial. Diabetes Care 2019, 42, 529–538. [Google Scholar] [CrossRef]
- Rizza, S.; Luzi, A.; Mavilio, M.; Ballanti, M.; Massimi, A.; Porzio, O.; Magrini, A.; Hannemann, J.; Menghini, R.; Cridland, J.; et al. Impact of light therapy on rotating night shift workers: The EuRhythDia study. Acta Diabetol. 2022, 59, 589–1596. [Google Scholar] [CrossRef]
| Compound | Effects on the Circadian Clock | Mechanism of Action | Metabolic Effects | Refs. |
|---|---|---|---|---|
| KL001 | Period lengthening Amplitude dampening | CRY stabilizer | Represses the induction of gluconeogenesis by glucagon | [217,218] |
| KL101 | Period lengthening | CRY1-selective stabilizer | Increases brown adipocyte differentiation | [219] |
| TH301 | Period lengthening | CRY2-selective stabilizer | Increases brown adipocyte differentiation | [219] |
| TW68 | Period lengthening | CRY Stabilizer | Blood glucose lowering effect in ob/ob mice | [220] |
| GSK4112 | Altering circadian gene expression Represses transcription of Bmal1 | REV-ERBα agonist | Inhibits gluconeogenesis | [221] |
| SR8278 | Altering circadian gene expression Increases BMAL1 | REV-ERBα antagonist | Inhibits glucagon secretion | [204,222] |
| SR9009 and SR9011 | Altering circadian gene expression Represses transcription of Bmal1 | REV-ERBα agonists | Increases energy expenditure and decreases fat mass, plasma triglycerides, and cholesterol levels in diet-induced obesity mouse model. SR9009 inhibits de novo lipogenesis. | [203,223] |
| SR1078 | Activating ROR dependenttranscription | RORα agonist | Lowers aerobic glycolysis. Lowers expression of pyruvate dehydrogenase kinase 2. Inhibits phosphorylation of pyruvate dehydrogenase and promotes the full oxidation of pyruvate. | [224,225] |
| SR3335 | NA | RORα inverse agonist | Inhibits gluconeogenesis and reduces glucose plasma levels. | [226] |
| SR1555 | NA | RORc inverse agonist | Improves insulin sensitivity and decreases food intake in obese diabetic mice. Induces thermogenic gene expression in fat depots, inhibits hormone-sensitive lipase activation, and increases fatty acid oxidation. | [227] |
| Nobiletin | Amplitude enhancer Period lengthening | ROR agonist | Restores energy hemostasis and prevents metabolic syndrome in mice with diet-induced obesity and db/db mutations. Restores energy hemostasis, improves metabolic fitness, and increases energy expenditure, cold tolerance, exercise endurance, grip strength, and inflammatory markers in aged mice fed a high-fat diet. | [228,229] |
| Nobiletin | Amplitude enhancer | ROR agonist | Enhances basal and stimulated insulin secretion by T2D islets. | [230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Civelek, E.; Ozturk Civelek, D.; Akyel, Y.K.; Kaleli Durman, D.; Okyar, A. Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases. Biology 2023, 12, 1077. https://doi.org/10.3390/biology12081077
Civelek E, Ozturk Civelek D, Akyel YK, Kaleli Durman D, Okyar A. Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases. Biology. 2023; 12(8):1077. https://doi.org/10.3390/biology12081077
Chicago/Turabian StyleCivelek, Erkan, Dilek Ozturk Civelek, Yasemin Kubra Akyel, Deniz Kaleli Durman, and Alper Okyar. 2023. "Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases" Biology 12, no. 8: 1077. https://doi.org/10.3390/biology12081077
APA StyleCivelek, E., Ozturk Civelek, D., Akyel, Y. K., Kaleli Durman, D., & Okyar, A. (2023). Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases. Biology, 12(8), 1077. https://doi.org/10.3390/biology12081077






