Barnyard Grass Stress Triggers Changes in Root Traits and Phytohormone Levels in Allelopathic and Non-Allelopathic Rice
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Growth and Sampling
2.2. Root Traits Calculation
2.3. Extraction, Purification, and Determination of Plant Hormones in Rice
2.4. Statistical Analysis
3. Results
3.1. Different Reactions of Allelopathic Rice and Non-Allelopathic Rice on Shoot and Root Biomass under Barnyard Grass Stress
3.2. Analysis of Root Length, Root Surface Area, Root Volume, and Root Diameter of Allelopathic and Non-Allelopathic Rice under Barnyard Grass Stress
3.3. Changes in Root Tip Number and Root Tip Length of Allelopathic and Non-Allelopathic Rice under Barnyard Grass Stress
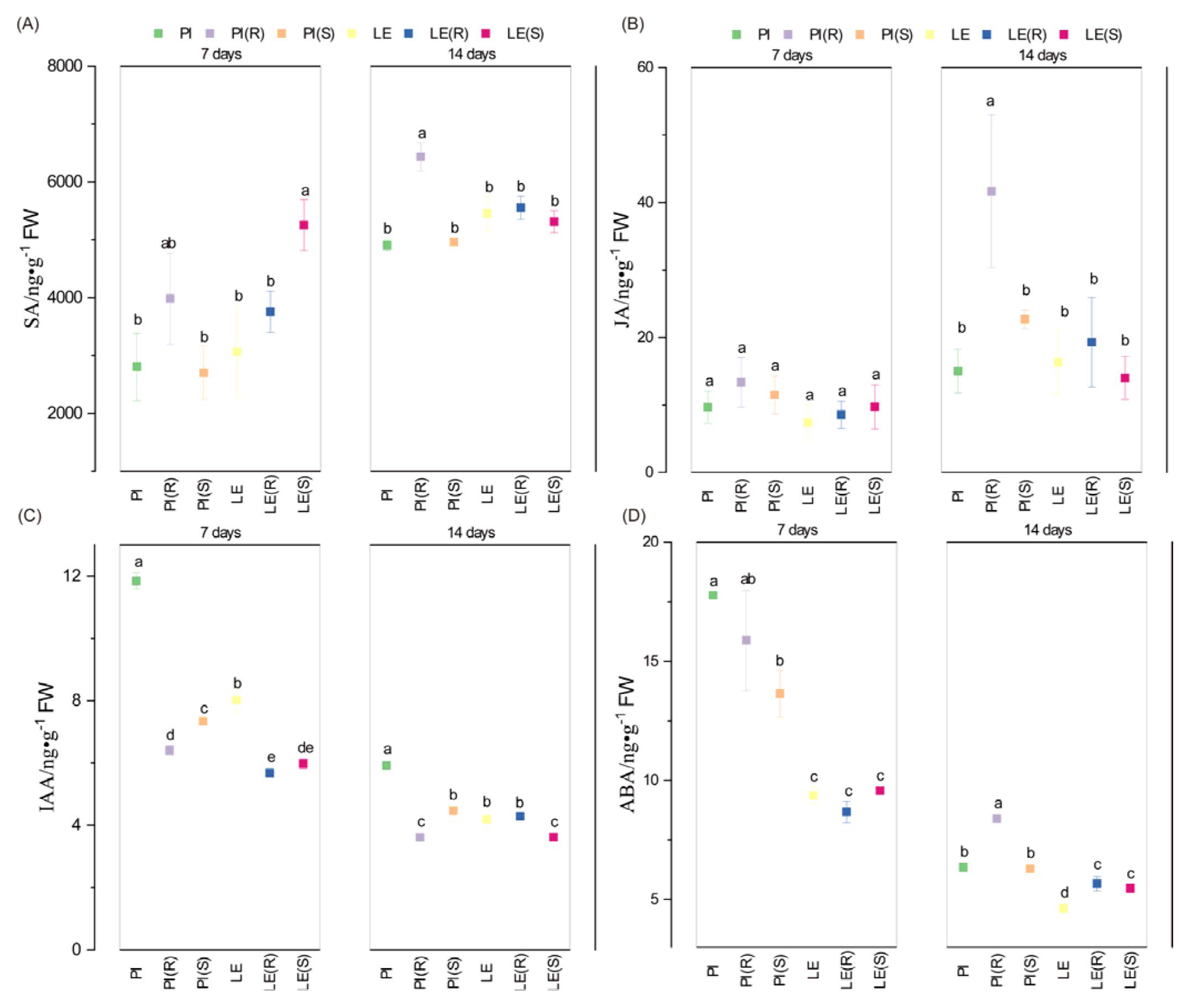
3.4. Impact of Barnyard Grass Stress on Phytohormone Levels
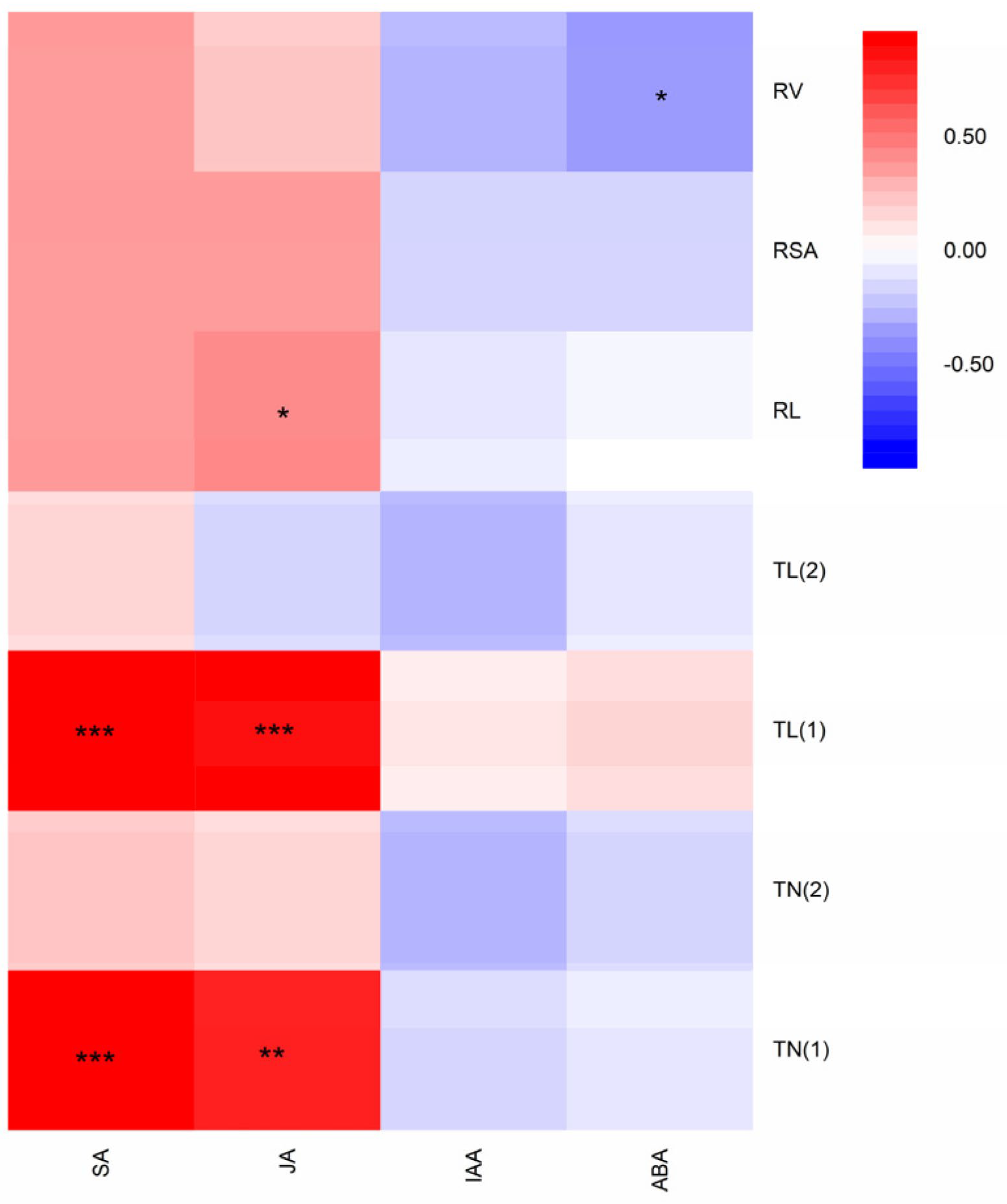
3.5. Correlation between Root Traits and Phytohormone Contents
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aoki, D.; Yamaguchi, H. Genetic relationship between Echinochloa crus-galli and Echinochloa oryzicola accessions inferred from internal transcribed spacer and chloroplast DNA sequences. Weed Biol. Manag. 2008, 8, 233–242. [Google Scholar] [CrossRef]
- Zhang, Z.C.; Cao, J.J.; Gu, T.; Yang, X.; Peng, Q.; Bai, L.Y.; Li, Y.F. Co-planted barnyardgrass reduces rice yield by inhibiting plant above-and belowground-growth during post-heading stages. Crop J. 2021, 9, 1198–1207. [Google Scholar] [CrossRef]
- Cui, H.C. Challenges and approaches to crop improvement through C3-to-C4 engineering. Front. Plant. Sci. 2021, 12, 715391. [Google Scholar]
- Zhang, Z.C.; Gu, T.; Zhao, B.; Yang, X.; Peng, Q.; Li, Y.; Bai, L.Y. Effects of common echinochloa varieties on grain yield and grain quality of rice. Field Crops Res. 2017, 203, 163–172. [Google Scholar]
- Oerke, E.C.; Dehne, H.W. Safeguarding production-losses in major crops and the role of crop protection. Crop Prot. 2004, 23, 275–285. [Google Scholar] [CrossRef]
- Peterson, M.A.; Collavo, A.; Ovejero, R.; Shivrain, V.; Walsh, M.J. The challenge of herbicide resistance around the world: A current summary. Pest. Manag. Sci. 2018, 74, 2246–2259. [Google Scholar] [CrossRef]
- Heap, I. Global perspective of herbicide-resistant weeds. Pest. Manag. Sci. 2014, 70, 1306–1315. [Google Scholar] [CrossRef]
- Baucom, R.S. Evolutionary and ecological insights from herbicide-resistant weeds: What have we learned about plant adaptation, and what is left to uncover? New Phytol. 2019, 223, 68–82. [Google Scholar] [PubMed]
- Reiss, A.; Fomsgaard, I.S.; Mathiassen, S.K.; Stuart, R.M.; Kudsk, P. Weed suppression by winter cereals: Relative contribution of competition for resources and allelopathy. Chemoecology 2018, 28, 109–121. [Google Scholar] [CrossRef]
- Gealy, D.R.; Moldenhauer, K.A.K. Use of 13C isotope discrimination analysis to quantify distribution of barnyardgrass and rice roots in a four-year study of weed suppressive rice. Weed Sci. 2012, 60, 133–142. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Prot. 2015, 72, 57–65. [Google Scholar]
- Farooq, M.; Jabran, K.; Cheema, Z.A.; Wahid, A.; Siddique, K.H.M. The role of allelopathy in agricultural pest management. Pest. Manag. Sci. 2011, 67, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1221–1227. [Google Scholar] [CrossRef]
- Kong, C.H.; Chen, X.H.; Hu, F.; Zhang, S.Z. Breeding of commercially acceptable allelopathic rice cultivars in China. Pest. Manag. Sci. 2011, 67, 1100–1106. [Google Scholar] [CrossRef]
- Kong, C.H.; Zhang, S.Z.; Li, Y.H.; Xia, Z.H.; Yang, X.F.; Meiners, S.J.; Wang, P. Plant neighbor detection and allelochemical response are driven by root-secreted signaling chemicals. Nat. Commun. 2018, 9, 3867. [Google Scholar] [CrossRef]
- Rietveld, W.J. Allelopathic effects of juglone on germination and growth of several herbaceous and woody species. J. Chem. Ecol. 1983, 9, 295–308. [Google Scholar]
- Dilday, R.H.; Nastasi, P.; Smith, J.R. Allelopathic observations in rice (Oryza sativa L.) to ducksalad (Heteranthera limosa (Sw.) Willd). JAAS 1989, 43, 21–22. [Google Scholar]
- Gealy, D.R.; Moldenhauer, K.A.K.; Duke, S. Root distribution and potential interactions between allelopathic rice, sprangletop (Leptochloa spp.), and barnyardgrass (Echinochloa crus-galli) based on 13C isotope discrimination analysis. J. Chem. Ecol. 2013, 39, 186–203. [Google Scholar]
- Yang, X.F.; Kong, C.H. Interference of allelopathic rice with paddy weeds at the root level. Plant Biol. 2017, 19, 584–591. [Google Scholar] [CrossRef]
- Yang, X.F.; Kong, C.H.; Yang, X.; Li, Y.F. Interference of allelopathic rice with penoxsulam-resistant barnyardgrass. Pest. Manag. Sci. 2017, 73, 2310–2317. [Google Scholar] [CrossRef]
- Rahaman, F.; Juraimi, A.S.; Rafii, M.Y.; Uddin, M.K.; Hassan, L.; Chowdhury, A.K.; Bashar, K.H.M. Allelopathic effect of selected rice (Oryza sativa) varieties against barnyard grass (Echinochloa cruss-gulli). Plants 2021, 10, 2017. [Google Scholar] [CrossRef]
- Zhao, S.; Li, Y. Current understanding of the interplays between host hormones and plant viral infections. PLoS Pathog. 2021, 17, e1009242. [Google Scholar]
- Aerts, N.; Pereira, M.M.; VanWees, S.C.M. Multiple levels of crosstalk in hormone networks regulating plant defense. Plant J. 2021, 105, 489–504. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yang, J.F.; Li, X.; Zhang, Y.L. Salicylic acid: Biosynthesis and signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791. [Google Scholar]
- Liang, B.; Wang, H.; Yang, C.; Wang, L.Y.; Qi, L.L.; Guo, Z.J.; Chen, X.J. Salicylic acid is required for broad-spectrum disease resistance in rice. Int. J. Mol. Sci. 2022, 23, 1354. [Google Scholar]
- Ku, Y.S.; Sintaha, M.; Cheung, M.Y.; Lam, H.M. Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Harun-Or-Rashid, M.; Kim, H.J.; Yeom, S.I.; Yu, H.A.; Manir, M.M.; Moon, S.S.; Kang, Y.J.; Chung, Y.R. Bacillus velezensis YC7010 enhances plant defenses against brown planthopper through transcriptomic and metabolic changes in rice. Front. Plant Sci. 2018, 9, 1904. [Google Scholar] [PubMed]
- You, L.X.; Wang, P.; Kong, C.H. The levels of jasmonic acid and salicylic acid in a rice-barnyardgrass coexistence system and their relation to rice allelochemicals. Biochem. Syst. Ecol. 2011, 39, 491–497. [Google Scholar] [CrossRef]
- Robert-Seilaniantz, A.; Grant, M.; Jones, J.D.G. Hormone crosstalk in plant disease and defense: More than just jasmonate-salicylate antagonism. Annu. Rev. Phytopathol. 2011, 49, 317–343. [Google Scholar]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 86. [Google Scholar] [PubMed]
- Dilday, R.H.; Mattice, J.; Moldenhauer, K. An overview of rice allelopathy in the USA. In Proceedings of the International Workshop in Rice Allelopathy, Daegu, Republic of Korea, 17–19 August 2000; Kyungpook National University: Daegu, Republic of Korea, 2000; pp. 15–26. [Google Scholar]
- Lupini, A.; Sorgonà, A.; Princi, M.P.; Sunseri, F.; Abenavoli, M.R. Morphological and physiological effects of trans-cinnamic acid and its hydroxylated derivatives on maize root types. Plant Growth Regul. 2016, 78, 263–273. [Google Scholar] [CrossRef]
- Zhou, L.J.; Xiao, L.T.; Xue, H.W. Dynamic cytology and transcriptional regulation of rice lamina joint development. Plant Physiol. 2017, 174, 1728–1746. [Google Scholar] [CrossRef]
- Alam, M.A.; Hakim, M.A.; Juraimi, A.S.; Rafii, M.Y.; Hasan, M.M.; Aslani, F. Potential allelopathic effects of rice plant aqueous extracts on germination and seedling growth of some rice field common weeds. Ital. J. Agron. 2018, 13, 134–140. [Google Scholar] [CrossRef]
- Sun, B.; Wang, P.; Kong, C.H. Plant-soil feedback in the interference of allelopathic rice with barnyardgrass. Plant Soil. 2014, 377, 309–321. [Google Scholar] [CrossRef]
- Wardle, D.A.; Bardgett, R.R.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Li, Y.H.; Kong, C.H.; Xu, X.H. Interference of allelopathic wheat with different weeds. Pest. Manag. Sci. 2016, 72, 172–178. [Google Scholar] [CrossRef]
- Li, J.Y.; Lin, S.X.; Zhang, Q.X.; Zhang, Q.; Hu, W.; He, H.B. Fine-root traits of allelopathic rice at the seedling stage and their relationship with allelopathic potential. PeerJ 2019, 7, e7006. [Google Scholar]
- Iversen, C.M.; McCormack, M.L.; Powell, A.S.; Blackwood, C.B.; Freschet, G.T.; Kattge, J.; Roumet, C.; Stover, D.B.; Soudzilovskaia, N.A.; Valverde-Barrantes, O.J.; et al. A global fine-root ecology database to address below-ground challenges in plant ecology. New Phytol. 2017, 215, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Fernandez, I.; Lok, G.B.; Pozo, M.J.; Pieterse, C.M.; Wees, V.S.C. Shifting from priming of salicylic acid- to jasmonic acid-regulated defences by Trichoderma protects tomato against the root knot nematode Meloidogyne incognita. New Phytol. 2017, 213, 1363–1377. [Google Scholar] [PubMed]
- Vo, K.T.X.; Rahman, M.M.; Rahman, M.M.; Trinh, K.T.T.; Kim, S.T.; Jeon, J.S. Proteomics and metabolomics studies on the biotic stress responses of rice: An update. Rice 2021, 14, 30. [Google Scholar]
- Kunkel, B.N.; Johnson, J.M.B. Auxin plays multiple roles during plant-pathogen interactions. CSH Perspect. Biol. 2021, 13, a040022. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Audenaert, K.; Hofte, M.; Vleesschauwer, D.D. Abscisic acid Promotes susceptibility to the rice leaf blight pathogen Xanthomonas oryzae pv. oryzae by suppressing salicylic acid-mediated defenses. PLoS ONE 2013, 8, e67413. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Q.; Tong, J.; Li, S.; Peng, Q. Barnyard Grass Stress Triggers Changes in Root Traits and Phytohormone Levels in Allelopathic and Non-Allelopathic Rice. Biology 2023, 12, 1074. https://doi.org/10.3390/biology12081074
Yan Q, Tong J, Li S, Peng Q. Barnyard Grass Stress Triggers Changes in Root Traits and Phytohormone Levels in Allelopathic and Non-Allelopathic Rice. Biology. 2023; 12(8):1074. https://doi.org/10.3390/biology12081074
Chicago/Turabian StyleYan, Qiling, Jianhua Tong, Shuyan Li, and Qiong Peng. 2023. "Barnyard Grass Stress Triggers Changes in Root Traits and Phytohormone Levels in Allelopathic and Non-Allelopathic Rice" Biology 12, no. 8: 1074. https://doi.org/10.3390/biology12081074
APA StyleYan, Q., Tong, J., Li, S., & Peng, Q. (2023). Barnyard Grass Stress Triggers Changes in Root Traits and Phytohormone Levels in Allelopathic and Non-Allelopathic Rice. Biology, 12(8), 1074. https://doi.org/10.3390/biology12081074



