The Effect of Skeletal Muscle Oxygenation on Hemodynamics, Cerebral Oxygenation and Activation, and Exercise Performance during Incremental Exercise to Exhaustion in Male Cyclists
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedures
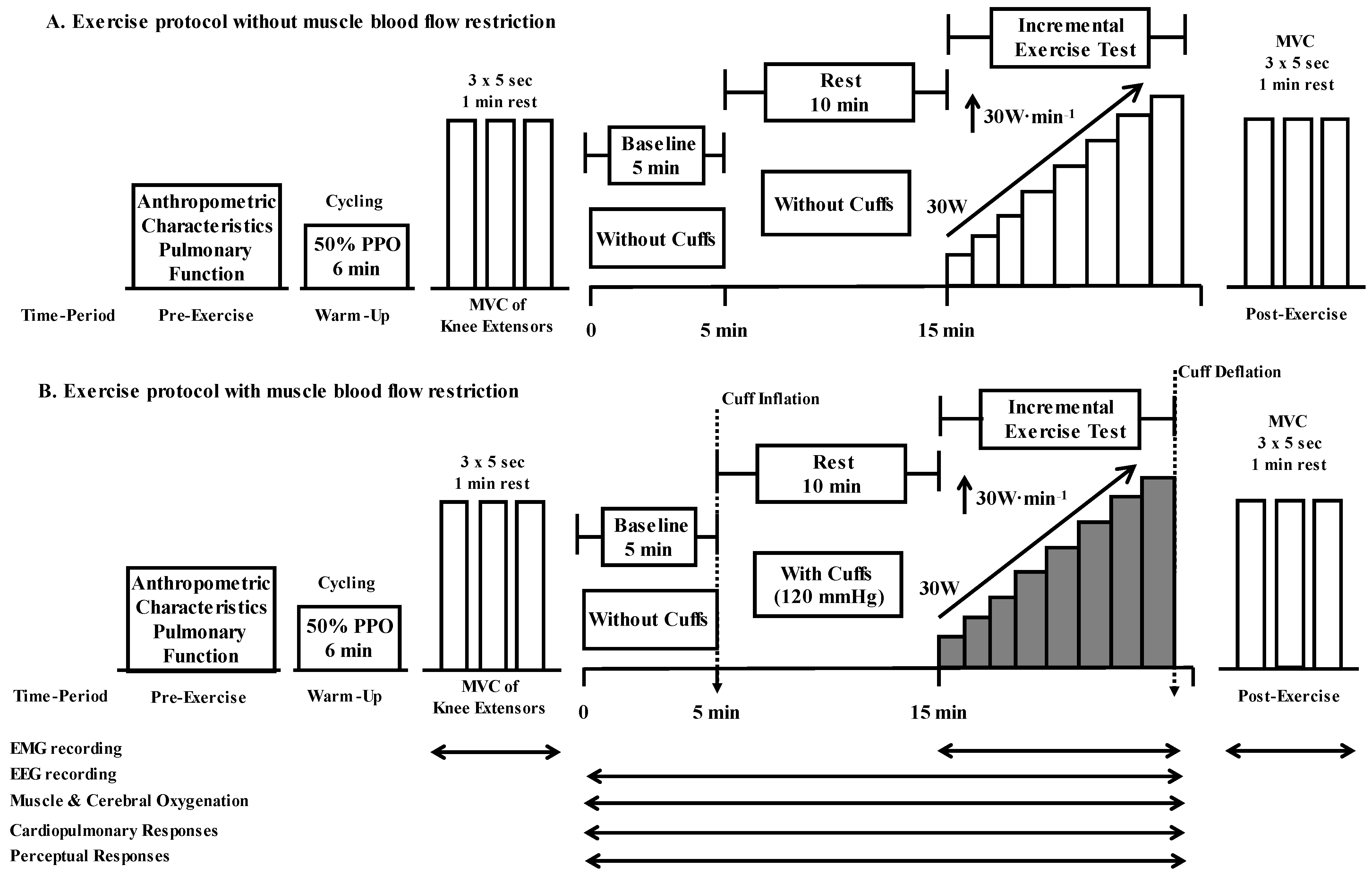
2.3. Experimental Protocol
2.4. Measurements
2.4.1. Muscle and Cerebral Oxygenation
2.4.2. Cardiopulmonary Measurements
2.4.3. Cerebral Activation
2.4.4. Maximal Voluntary Contraction (MVC) and Electromyography (EMG)
2.4.5. Perceptual Responses
2.4.6. Data Analysis and Statistics
3. Results
3.1. Exercise Tolerance
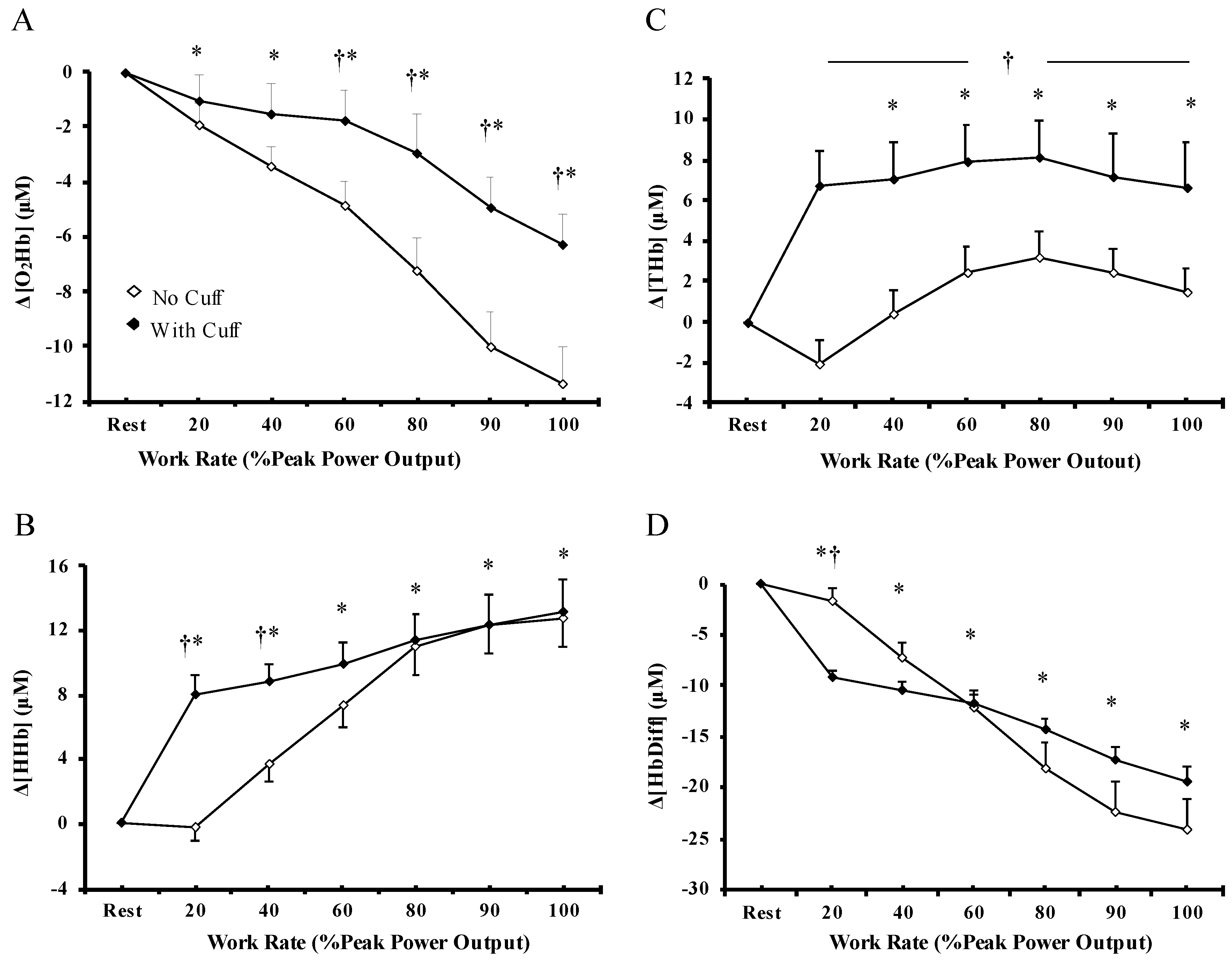
3.2. Muscle Oxygenation
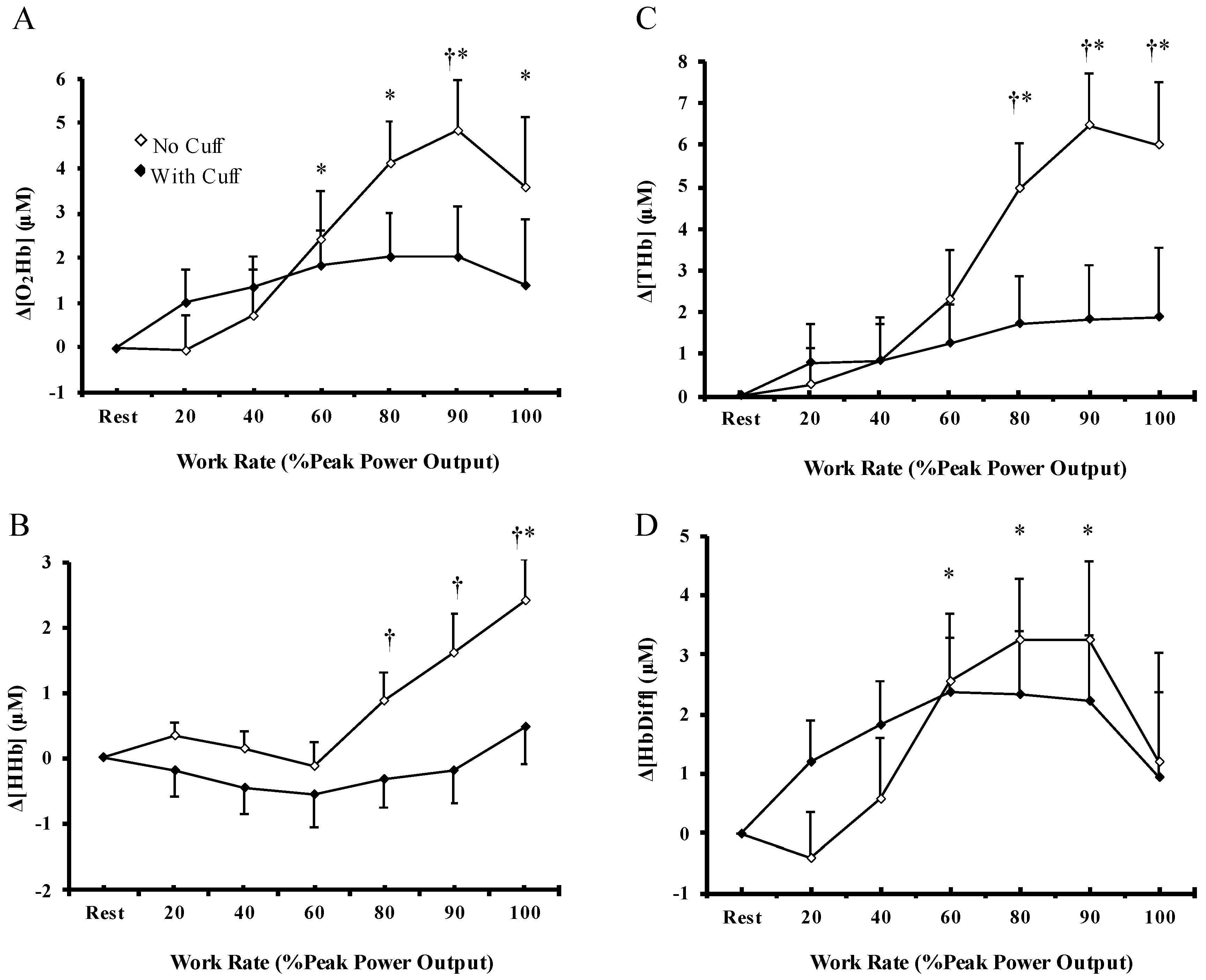
3.3. Cerebral Oxygenation
3.4. Cardiovascular Response
3.5. EEG Activity
3.6. EMG Activity and MVC Force
3.7. Rate of Perceived Exertion
4. Discussion
4.1. Skeletal Muscle Oxygenation and Exercise Tolerance
4.2. Muscle Blood Flow Restriction and Skeletal Muscle Oxygenation during Exercise
4.3. Skeletal Muscle Oxygenation and Cerebral Oxygenation during Exercise
4.4. Skeletal Muscle Oxygenation and Cerebral Activation during Exercise
4.5. Skeletal Muscle Oxygenation and Cardiovascular Response during Exercise
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rowell, L.B. Human cardiovascular adjustments to exercise and thermal stress. Physiol. Rev. 1974, 54, 75–159. [Google Scholar] [CrossRef] [PubMed]
- Bosquet, L.; Léger, L.; Legros, P. Methods to determine aerobic endurance. Sports Med. 2002, 32, 675–700. [Google Scholar] [CrossRef]
- di Prampero, P.E. Factors limiting maximal performance in humans. Eur. J. Appl. Physiol. 2003, 90, 420–429. [Google Scholar] [CrossRef]
- Norton, K.; Norton, L.; Sadgrove, D. Position statement on physical activity and exercise intensity terminology. J. Sci. Med. Sport 2009, 13, 496–502. [Google Scholar] [CrossRef]
- Laursen, P.B.; Shing, C.M.; Peake, J.M.; Coombes, J.S.; Jenkins, D.G. Interval training program optimization in highly trained endurance cyclists. Med. Sci. Sports Exerc. 2002, 34, 1801–1807. [Google Scholar] [CrossRef]
- Weber, K.T.; Janicki, J.S.; McElroy, P.A. Determination of aerobic capacity and the severity of chronic and circulatory failure. Circulation 1987, 76 (Suppl. SVI), V1–V40. [Google Scholar]
- Gibbons, L.W.; Mitchell, T.L.; Wei, M.; Blair, S.N.; Cooper, K.H. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am. J. Cardiol. 2000, 86, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kemps, H.M.; de Vries, W.R.; Schmikli, S.L.; Zonderland, M.L.; Hoogeveen, A.R.; Thijssen, E.J.; Schep, G. Assessment of the effects of physical training in patients with chronic heart failure: The utility of effort-independent exercise variables. Eur. J. Appl. Physiol. 2010, 108, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, B.; Goldbarg, A.N.; Gullbring, B. Response to exercise after blood loss and reinfusion. J. Appl. Physiol. 1972, 33, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.R.; McKenzie, D.C.; Schoene, R.B.; Glenny, R.W.; Robertson, T. Pulmonary gas exchange during exercise in athletes I. Ventilation—Perfusion mismatch and diffusion limitation. J. Appl. Physiol. 1994, 77, 912–917. [Google Scholar] [CrossRef]
- Saltin, B.; Blomqvist, G.; Mitchell, J.H.; Johnson, R.L., Jr.; Wildenthal, K.; Chapman, B.B. Response to exercise after bed rest and after training. Circulation 1968, 38 (Suppl. S5), VII1–VII78. [Google Scholar] [PubMed]
- Boushel, R.; Calbet, J.A.L.; Radegran, G.; Sondergaard, H.; Wagner, P.D.; Saltin, B. Parasympathetic neural activity accounts for the lowering of exercise heart rate at high altitude. Circulation 2001, 104, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; Boushel, R.; Radiogram, G.; Sondergaard, H.; Wagner, P.D.; Saltin, B. Why is VO2max after altitude acclimatization still reduced despite normalization of arterial O2 content? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R304–R316. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.; Saltin, B. Maximal perfusion of skeletal muscle in man. J. Physiol. 1985, 366, 233–249. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, S.P.; Damsgaard, R.; Dawson, E.A.; Secher, N.H.; Gonzalez-Alonso, J. Restrictions in systemic and locomotor skeletal muscle perfusion, oxygen supply and VO2 during high-intensity whole-body exercise in humans. J. Physiol. 2008, 586, 2621–2635. [Google Scholar] [CrossRef]
- Nybo, L.; Rasmussen, P. Inadequate Cerebral Oxygen Delivery and Central Fatigue during Strenuous Exercise. Exerc. Sport Sci. Rev. 2007, 35, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, A.W.; Miramon, B.R.; Granger, M.E.; Roach, R.C. Frontal and motor cortex oxygenation during maximal exercise in normoxia and hypoxia. J. Appl. Physiol. 2009, 106, 1153–1158. [Google Scholar] [CrossRef]
- Peltonen, J.E.; Paterson, D.H.; Shoemaker, J.K.; DeLorey, D.S.; duManoir, G.R.; Petrella, R.J.; Kowalchuk, J.M. Cerebral and muscle deoxygenation, hypoxic ventilatory chemosensitivity and cerebrovascular responsiveness during incremental exercise. Respir. Physiol. Neurobiol. 2009, 169, 24–35. [Google Scholar] [CrossRef]
- Richardson, R.S.; Duteil, S.; Wary, C.; Wray, D.W.; Hoff, J.; Carlier, P.G. Human skeletal muscle intracellular oxygenation: The impact of ambient oxygen availability. J. Physiol. 2006, 571, 415–424. [Google Scholar] [CrossRef]
- Burgomaster, A.K.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; MacDonald, M.J.; McGee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef]
- Daussin, F.N.; Zoll, J.; Dufour, S.P.; Ponsot, E.; Lonsdorfer-Wolf, E.; Doutreleau, S.; Mettauer, B.; Piquard, F.; Geny, B.; Richard, R. Effect of interval versus continuous training on cardiorespiratory and mitochondrial functions: Relationship to aerobic performance improvements in sedentary subjects. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R264–R272. [Google Scholar] [CrossRef] [PubMed]
- Marcora, S.M.; Staiano, W. The limit to exercise tolerance in humans: Mind over muscle? Eur. J. Appl. Physiol. 2010, 109, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.R.; Joyner, M.J.; Curry, T.B.; Borlaug, B.A.; Keller-Ross, M.L.; Van Iterson, E.H.; Olson, T.P. Locomotor muscle group III/IV afferents constrain stroke volume and contribute to exercise intolerance in human heart failure. J. Physiol. 2020, 598, 5379–5390. [Google Scholar] [CrossRef] [PubMed]
- Hureau, T.J.; Weavil, J.C.; Thurston, T.S.; Wan, H.Y.; Gifford, J.R.; Jessop, J.E.; Buys, M.J.; Richardson, R.S.; Amann, M. Pharmacological attenuation of group III/IV muscle afferents improves endurance performance when oxygen delivery to locomotor muscles is preserved. J. Appl. Physiol. 2019, 127, 1257–1266. [Google Scholar] [CrossRef]
- Amann, M.; Venturelli, M.; Ives, S.J.; McDaniel, J.; Mayec, G.; Rossman, M.J.; Richardson, R.S. Peripheral fatigue limits endurance exercise via a sensory feedback-mediated reduction in spinal motoneuronal output. J. Appl. Physiol. 2013, 115, 355–364. [Google Scholar] [CrossRef]
- Hill, A.V.; Lupton, H. Muscular exercise, lactic acid and the supply and utilisation of oxygen. QJM 1923, 16, 135–171. [Google Scholar] [CrossRef]
- Bassett, D.R.; Howley, E.T. Maximal oxygen uptake: ‘classical’ versus ‘contemporary’ viewpoints. Med. Sci. Sports Exerc. 1997, 29, 591–603. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Gonzalez-Alonso, J.; Helge, J.W.; Sondergaard, H.; Andersen, T.M.; Boushel, R.; Saltin, B. Cardiac output and leg and arm blood flow during incremental exercise to exhaustion on the cycle ergometer. J. Appl. Physiol. 2007, 103, 969–978. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Wang, J. Effect of blood flow restriction combined with low-intensity training on the lower limbs muscle strength and function in older adults: A meta-analysis. Exp. Gerontol. 2022, 164, 111827. [Google Scholar] [CrossRef]
- Freitas, E.D.S.; Karabulut, M.; Benben, M.G. The evolution of blood flow restricted exercise. Front. Physiol. 2021, 12, 747759. [Google Scholar] [CrossRef]
- Flocco, P.; Bermabei, L. Effects of blood flow restriction training on aerobic capacity: A systematic review and meta-analysis. Sport Sci. Health 2023, 19, 389–403. [Google Scholar] [CrossRef]
- Keramidas, M.E.; Kounalakis, S.N.; Geladas, N.D. The effect of interval training combined with thigh cuffs pressure on maximal and submaximal exercise performance. Clin. Physiol. Funct. Imaging 2012, 32, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Morita, T.; Iida, H.; Asada, K.; Kato, M.; Uno, K.; Hirose, K.; Matsumoto, A.; Takenaka, K.; Hirata, Y.; et al. Hemodynamic and hormonal responses to a short-term low-intensity resistance exercise with the reduction of muscle blood flow. Eur. J. Appl. Physiol. 2005, 95, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Abe, T.; Brechue, W.F.; Iida, H.; Takano, H.; Meguro, K.; Kurano, M.; Fujita, S.; Nakajima, T. Venous blood gas and metabolite response to low-intensity muscle contractions with external limb compression. Metabolism 2010, 59, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, M.; Mccarrson, J.; Abe, T.; Sato, Y.; Bemben, M. The effects of different initial restrictive pressures used to reduce blood flow and thigh composition on tissue oxygenation of the quadriceps. J. Sports Sci. 2011, 29, 951–958. [Google Scholar] [CrossRef]
- Geladas, N.D.; Anastassopoulos, S.; Keramidas, M.E.; Koskolou, M.D. Maximal oxygen uptake may be limited by sensation of muscle oxygenation. Open Sports Med. J. 2010, 4, 9–16. [Google Scholar] [CrossRef]
- Cherouveim, E.D.; Miliotis, P.; Dipla, K.; Koskolou, M.D.; Vrabas, I.S.; Geladas, N.D. The effect of muscle blood flow restriction on hemodynamics, cerebral oxygenation and activation at rest. Appl. Physiol. Nutr. Metab. 2021, 46, 1216–1225. [Google Scholar] [CrossRef]
- Martin-Rincon, M.; Gonzalez-Henriquez, J.J.; Losa-Reyna, J.; Perez-Suarez, I.; Ponce-Gonzalez, J.G.; Calle-Herrero, J.; Perez-Valera, M.; Perez-Lopez, A.; Curtelin, D.; Cherouveim, E.D.; et al. Impact of data averaging strategies on VO2max assessment: Mathematical modeling and reliability. Scand. J. Med. Sci. Sport. 2019, 29, 1473–1488. [Google Scholar] [CrossRef]
- Gibbons, R.J.; Balady, G.J.; Bricker, J.T.; Chaitman, B.R.; Fletcher, G.F.; Froelicher, V.F.; Mark, D.B.; McCallister, B.D.; Mooss, A.N.; O’Reilly, M.G.; et al. ACC/AHA 2002 guideline update for exercise testing: Summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines). J. Am. Coll. Cardiol. 2002, 40, 1531–1540. [Google Scholar] [CrossRef]
- Schabort, E.J.; Killian, S.C.; Gibson, A.C.; Hawley, J.A.; Noakes, T.D. Prediction of triathlon race time from laboratory testing in national triathletes. Med. Sci. Sports Exerc. 2000, 32, 844–849. [Google Scholar] [CrossRef]
- Hunt, J.E.A.; Stodart, C.; Ferguson, R.A. The influence of participant characteristics on the relationship between cuff pressure and level of blood flow restriction. Eur. J. Appl. Physiol. 2016, 116, 1421–1432. [Google Scholar] [CrossRef]
- Subudhi, A.W.; Dimmen, A.C.; Roach, C. Effects of acute hypoxia on cerebral and muscle oxygenation during incremental exercise. J. Appl. Physiol. 2007, 103, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Barstow, T.J. Understanding near-infrared spectroscopy and its application to skeletal muscle research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.; Mottola, L.; Quaresima, V. Principles, techniques, and limitations of near infrared spectroscopy. Can. J. Appl. Physiol. 2004, 29, 463–487. [Google Scholar] [CrossRef] [PubMed]
- Van Beekvelt, M.C.; Colier, W.N.; Wevers, R.A.; Van Engelen, B.G. Performance of near-infrared spectroscopy in measuring local O2 consumption and blood flow in skeletal muscle. J. Appl. Physiol. 2001, 90, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, M.; Ferrari, M.; Quaresima, V. Gastrocnemius medialis and vastus lateralis oxygenation during whole-body vibration exercise. Med. Sci. Sports Exerc. 2007, 39, 694–700. [Google Scholar] [CrossRef]
- Mancini, D.M.; Bolinger, L.; Li, H.; Kandrick, K.; Chance, B.; Wilson, J.R. Validation of near-infrared spectroscopy in humans. J. Appl. Physiol. 1994, 77, 2740–2747. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pepelow, D.F.; Dempsey, J.A. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J. Physiol. 2011, 589, 5299–5309. [Google Scholar] [CrossRef]
- Cherouveim, E.D.; Botonis, P.G.; Tsakiris, T.; Koskolou, M.D.; Geladas, N.D. The effect of menstrual cycle on maximal breath-hold time. Respir. Physiol. Neurobiol. 2020, 274, 103381. [Google Scholar] [CrossRef]
- Bogert, L.W.J.; van Lieshout, J.J. Non-invasive pulsatile arterial pressure and stroke volume changes form the human finger. Exp. Physiol. 2005, 90, 437–446. [Google Scholar] [CrossRef]
- American Clinical Neurophysiology Society. Guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 2006, 23, 107–110. [Google Scholar] [CrossRef]
- Suzuki, H.; Conwit, R.A.; Stashuk, D.; Santarsiero, L.; Metter, E.J. Relationships between surface-detected EMG signals and motor unit activation. Med. Sci. Sports Exerc. 2002, 34, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Perceived exertion: A note on history and methods. Med. Sci. Sports Exerc. 1973, 5, 90–93. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Eiken, O.; Bjurstedt, H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol. Scand. 1987, 131, 339–345. [Google Scholar] [CrossRef]
- Williamson, J.W.; Grandall, C.G.; Potts, J.T.; Raven, P.B. Blood pressure responses to dynamic exercise with lower body positive pressure. Med. Sci. Sports Exerc. 1994, 26, 701–708. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Fadel, P.J.; Smith, S.A.; Norton, K.H.; Querry, R.G.; Olivencia-Yurvati, A.; Raven, P.B. Increases in intramuscular pressure raise arterial blood pressure during dynamic exercise. J. Appl. Physiol. 2001, 91, 2351–2358. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, A.W.; Olin, J.D.; Dimmen, A.C.; Polaner, D.M.; Kayser, B.; Robert, C. Does cerebral oxygen delivery limit incremental exercise performance? J. Appl. Physiol. 2011, 111, 1727–1734. [Google Scholar] [CrossRef]
- Vogiatzis, I.; Louvaris, Z.; Habazettl, H.; Athanasopoulos, D.; Andrianopoulos, V.; Cherouveim, E.; Wagner, H.; Roussos, C.; Wagner, P.D.; Zakynthinos, S. Frontal cerebral cortex blood flow, oxygen delivery and oxygenation during normoxic and hypoxic exercise in athletes. J. Physiol. 2011, 589, 4027–4039. [Google Scholar] [CrossRef]
- Joyner, M.J.; Dominelli, P. Central cardiovascular system limits to aerobic capacity. Exp. Physiol. 2021, 106, 2299–2303. [Google Scholar] [CrossRef]
- Ferretti, G. Maximal oxygen consumption in healthy humans: Theories and facts. Eur. J. Appl. Physiol. 2014, 114, 2007–2036. [Google Scholar] [CrossRef] [PubMed]
- Subudhi, A.W.; Lorenz, M.C.; Fulco, C.S.; Roach, R.C. Cerebrovascular responses to incremental exercise during hypobaric hypoxia: Effect of oxygenation on maximal performance. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H164–H171. [Google Scholar] [CrossRef] [PubMed]
- Rupp, T.; Perrey, S. Prefrontal cortex oxygenation and neuromuscular responses to exhaustive exercise. Eur. J. Appl. Physiol. 2008, 102, 153–163. [Google Scholar] [CrossRef]
- Olin, J.T.; Dimmen, A.C.; Subudhi, A.W.; Roach, R.C. Cerebral blood flow and oxygenation at maximal exercise: The effect of clamping carbon dioxide. Respir. Physiol. Neurobiol. 2011, 175, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, H.B.; Boushel, P.M.; Secher, N.H. Cerebral desaturation during exercise reversed by O2 supplementation. Am. J. Physiol. 1999, 277, H1045–H1052. [Google Scholar] [CrossRef]
- Takarada, Y.; Takazawa, H.; Sato, Y.; Takebayashi, S.; Tanaka, Y. Effects of resistance exercise combined with moderate vascular occlusion on muscular function in humans. J. Appl. Physiol. 2000, 88, 2097–2106. [Google Scholar] [CrossRef]
- Wei, J.; Nassis, G.P.; Gu, Z.; Zou, Y.; Wang, X.; Yongming Li, Y. Acute physiological and perceptual responses to moderate intensity cycling with different levels of blood flow restriction. Biol. Sport. 2021, 38, 437–443. [Google Scholar] [CrossRef]
- Pearson, S.J.; Hussain, S.R. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015, 45, 187–200. [Google Scholar] [CrossRef]
- Yamauchi, K.; Tsuchimochi, H.; Stone, A.J.; Stocker, S.D.; Kaufman, M.P. Increased dietary salt intake enhances the exercise pressor reflex. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H450–H454. [Google Scholar] [CrossRef]
- Kaufman, M.P.; Hayes, S.G.; Adreani, C.M.; Pickar, J.G. Discharge properties of group III and IV muscle afferents. Adv. Exp. Med. Biol. 2002, 508, 25–32. [Google Scholar] [CrossRef]
- Haouzi, P.; Chenuel, B.; Huszczuk, A. Sensing vascular distension in skeletal muscle by slow conducting afferent fibers: Neurophysiological basis and implication for respiratory control. J. Appl. Physiol. 2004, 96, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.G.; Weerakkody, N.; Gandevia, S.C.; Taylor, J.L. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J. Physiol. 2008, 586, 1277–1289. [Google Scholar] [CrossRef]
- Sidhu, S.K.; Weavil, J.C.; Thurston, T.S.; Rosenberger, D.; Jessop, J.E.; Wang, E.; Richardson, R.S.; McNeil, C.J.; Amann, M. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J. Physiol. 2018, 596, 4789–4801. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, J.A. New perspectives concerning feedback influences on cardiorespiratory control during rhythmic exercise and on exercise performance. J. Physiol. 2012, 590, 4129–4144. [Google Scholar] [CrossRef] [PubMed]
- Hilty, L.; Lutz, K.; Maurer, K.; Rodenkirch, T.; Spengler, C.M.; Boutellier, U.; Jancke, L.; Amann, M. Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp. Physiol. 2011, 96, 505–517. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, J.; Dalsgaard, M.K.; Osada, T.; Volianitis, S.; Dawson, E.A.; Yoshiga, C.C.; Secher, N.H. Brain and central haemodynamics and oxygenation during maximal exercise in humans. J. Physiol. 2004, 557, 331–342. [Google Scholar] [CrossRef]
- Rasmussen, P.; Nielsen, J.; Overgaard, M.; Krogh-Madsen, R.; Gjedde, A.; Secher, N.H.; Petersen, N.C. Reduced muscle activation during exercise related to brain oxygenation and metabolism in humans. J. Physiol. 2010, 588, 1985–1995. [Google Scholar] [CrossRef]
- Rasmussen, P.; Dawson, E.A.; Nybo, L.; Van Lieshout, J.J.; Secher, N.H.; Gjedde, A. Capillary-oxygenation-level-dependent near-infrared spectrometry in frontal lobe of humans. J. Cereb. Blood Flow. Metab. 2007, 27, 1082–1093. [Google Scholar] [CrossRef]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J. Appl. Physiol. 2010, 109, 966–976. [Google Scholar] [CrossRef]
- Morree, H.M.; Klein, C.; Marcora, S.M. Perception of effort reflects central motor command during movement execution. Psychophysiology 2012, 49, 1242–1253. [Google Scholar] [CrossRef]
- Ichinose, M.; Delliaux, S.; Watanabe, K.; Fujii, N.; Takeshi, N. Evaluation of muscle metaboreflex function through grated reduction in forearm blood flow during rhythmic handgrip exercise in humans. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H609–H616. [Google Scholar] [CrossRef]
- Gonzalez-Alonso, J.; Mortensen, S.P.; Jeppesen, T.D.; Ali, L.; Barker, H.; Damsgaard, R.; Secher, N.H.; Dawson, E.A.; Dufour, S.P. Haemodynamic responses to exercise, ATP infusion and thigh compression in humans: Insight into the role of muscle mechanisms on cardiovascular function. J. Physiol. 2008, 586, 2405–2417. [Google Scholar] [CrossRef] [PubMed]
- Bogaard, H.J.; Hopkins, S.R.; Yoshiki, Y.; Niizeki, K.; Ziegler, M.G.; Wagner, P.D. Role of the autonomic nervous system in the reduced maximal cardiac output at altitude. J. Appl. Physiol. 2002, 93, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, S.R.; Bogaard, H.J.; Niizeki, K.; Yamaya, Y.; Ziegler, M.G.; Wagner, P.D. Βeta-adrenergic or parasympathetic inhibition, heart rate and cardiac output during normoxic and acute hypoxic exercise in humans. J. Physiol. 2003, 550, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Romer, L.M.; Pigeon, D.F.; Jacques, A.J.; Hess, C.J.; Dempsey, J.A. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J. Appl. Physiol. 2006, 101, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Boushel, R. Muscle metaboreflex control of the circulation during exercise. Acta Physiol. 2010, 199, 367–383. [Google Scholar] [CrossRef]
- Amann, M.; Runnels, S.; Morgan, D.E.; Trinity, J.D.; Fjeldstad, A.S.; Wray, D.W.; Reese, V.R.; Richardson, R.S. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J. Physiol. 2011, 589, 3855–3866. [Google Scholar] [CrossRef]
- Luu, B.L.; Fitzpatrick, R.C. Blood pressure and the contractility of a human leg muscle. J. Physiol. 2013, 591, 5401–5412. [Google Scholar] [CrossRef]
- Ichinose, M.; Maeda, S.; Kondo, N.; Nishiyasu, T. Blood pressure regulation II: What happens one system must serve two masters—Oxygen delivery and pressure regulation? Eur. J. Appl. Physiol. 2014, 114, 451–465. [Google Scholar] [CrossRef]
- Sheriff, D.D.; O’Leary, D.S.; Scher, A.M.; Rowell, L.B. Baroreflex attenuates pressor response to graded muscle ischemia in exercising dogs. Am. J. Physiol. 1990, 258, 305–310. [Google Scholar] [CrossRef]
- Smith, S.A.; Mitchell, J.H.; Garry, M.G. The mammalian exercise pressor reflex in health and disease. Exp. Physiol. 2006, 91, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Angius, L.; Crisafulli, A. Exercise intolerance and fatigue in chronic heart failure: Is there a role for group III/IV afferent feedback? Eur. J. Prev. Cardiol. 2020, 27, 1862–1872. [Google Scholar] [CrossRef] [PubMed]


| No Cuff Condition | With Cuff Condition | |||||
|---|---|---|---|---|---|---|
| Variables | 60 W | 120 W | 180 W | 60 W | 120 W | 180 W |
| Muscle oxygenation | ||||||
| Δ[O2Hb]m (μΜ) | −1.76 ± 0.74 | −3.20 ± 0.70 | −4.48 ± 0.92 * | −1.06 ± 0.99 | −1.12 ± 1.10 | −2.83 ± 1.19 * |
| Δ[HHb]m (μΜ) | 0.06 ± 0.92 | 3.46 ± 1.22 * | 6.71 ± 1.46 * | 8.10 ± 1.14 † | 8.64 ± 1.42 † | 11.14 ± 1.63 †,* |
| Δ[THb]m (μΜ) | −1.69 ± 1.16 | 0.26 ± 1.24 * | 2.22 ± 1.26 * | 6.77 ± 1.90 † | 7.25 ± 1.96 † | 8.03 ± 2.00 † |
| Δ[HbDiff]m (μΜ) | −1.82 ± 1.21 | −6.67 ± 1.54 * | −11.19 ± 2.09 * | −9.15 ± 1.10 † | −9.76 ± 1.68 † | −13.97 ± 2.05 †,* |
| Cerebral oxygenation | ||||||
| Δ[O2Hb]c (μΜ) | −0.04 ± 0.68 | 0.61 ± 0.85 | 1.90 ± 0.93 | 0.73 ± 0.79 | 1.54 ± 0.91 | 1.87 ± 0.97 |
| Δ[HHb]c (μΜ) | 0.40 ± 0.25 | 0.25 ± 0.30 | −0.22 ± 0.30 | −0.12 ± 0.31 | −0.55 ± 0.37 | −0.28 ± 0.43 |
| Δ[THb]c (μΜ) | 0.36 ± 0.78 | 0.86 ± 0.90 | 1.68 ± 0.99 | 0.61 ± 0.91 | 1.00 ± 1.00 | 1.59 ± 1.10 |
| Δ[HbDiff]c (μΜ) | −0.45 ± 0.67 | 0.36 ± 0.91 | 2.12 ± 0.97 | 0.85 ± 0.78 | 2.09 ± 0.96 | 2.15 ± 1.03 |
| Hemodynamics | ||||||
| HR (beats·min−1) | 97 ± 3 | 116 ± 3 * | 137 ± 3 * | 101 ± 3 † | 123 ± 4 †,* | 141 ± 4 †,* |
| SV (ml·beat−1) | 115.08 ± 3.32 | 124.65 ± 2.49 * | 127.98 ± 2.77 | 104.17 ± 4.44 † | 109.65 ± 4.98 †,* | 112.44 ± 3.75 † |
| (L·min−1) | 11.71 ± 0.42 | 14.54 ± 0.56 * | 17.35 ± 0.73 | 10.59 ± 0.63 † | 13.34 ± 0.88 †,* | 15.34 ± 0.72 † |
| SBP (mmHg) | 152± 3 | 164 ± 5 * | 186 ± 5 * | 169 ± 4 † | 193 ± 4 †,* | 206 ± 5 †,* |
| DBP (mmHg) | 82 ± 1 | 86 ± 2 | 95 ± 1 * | 95 ± 1 † | 105 ± 2 †,* | 111 ± 2 †,* |
| MAP (mmHg) | 107 ± 2 | 113 ± 4 * | 127 ± 2 * | 121 ± 2 † | 138 ± 3 †,* | 148 ± 3 †,* |
| TPR (mu) | 0.57 ± 0.02 | 0.52 ± 0.03 * | 0.51 ± 0.03 | 0.74 ± 0.05 † | 0.67 ± 0.05 †,* | 0.61 ± 0.03 †,* |
| Exercise capacity/ Metabolic parameters | ||||||
| O2 (ml·min−1) | 1104 ± 44 | 1802 ± 64 * | 2419 ± 63 * | 1186 ± 45 † | 1962 ± 57 †,* | 2602 ± 63 †,* |
| CO2 (ml·min−1) | 887 ± 45 | 1526 ± 66 * | 2280 ± 79 * | 940 ± 42 † | 1742 ± 60 †,* | 2624 ± 89 †,* |
| E (L·min−1) | 27.23 ± 1.31 | 41.54 ± 1.63 * | 58.07 ± 2.20 * | 28.77 ± 1.44 | 47.76 ± 1.75 †,* | 72.74 ± 4.23 †,* |
| RER | 0.80 ± 0.02 | 0.85 ± 0.02 * | 0.95 ± 0.02 * | 0.79 ± 0.02 | 0.89 ± 0.02 * | 1.01 ± 0.03 †,* |
| VT (ml·breath−1) | 1259 ± 52 | 1730 ± 59 * | 2204 ± 82 * | 1252 ± 55 | 1884 ± 96 * | 2311 ± 144 * |
| Bf (breaths·min−1) | 22 ± 1 | 24 ± 1 | 27 ± 1 * | 24 ± 2 | 26 ± 2 | 33 ± 3 †,* |
| Cerebral activation | ||||||
| iEMG (%MVC) | 11.38 ± 3.32 | 16.94 ± 4.87 * | 20.33 ± 5.91 * | 8.65 ± 1.39 | 13.49 ± 1.91 * | 18.04 ± 2.40 * |
| Fatigue perception | ||||||
| RPEdyspnea | 6.38 ± 0.14 | 8.92 ± 0.43 * | 10.77 ± 0.44 * | 6.77 ± 0.32 † | 10.23 ± 0.46 †,* | 12.23 ± 0.75 †,* |
| RPEleg | 8.08 ± 0.33 | 10.00 ± 0.67 * | 12.69 ± 0.33 * | 11.82 ± 0.52 † | 14.33 ± 0.38 †,* | 16.46 ± 0.54 †,* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cherouveim, E.D.; Miliotis, P.G.; Koskolou, M.D.; Dipla, K.; Vrabas, I.S.; Geladas, N.D. The Effect of Skeletal Muscle Oxygenation on Hemodynamics, Cerebral Oxygenation and Activation, and Exercise Performance during Incremental Exercise to Exhaustion in Male Cyclists. Biology 2023, 12, 981. https://doi.org/10.3390/biology12070981
Cherouveim ED, Miliotis PG, Koskolou MD, Dipla K, Vrabas IS, Geladas ND. The Effect of Skeletal Muscle Oxygenation on Hemodynamics, Cerebral Oxygenation and Activation, and Exercise Performance during Incremental Exercise to Exhaustion in Male Cyclists. Biology. 2023; 12(7):981. https://doi.org/10.3390/biology12070981
Chicago/Turabian StyleCherouveim, Evgenia D., Panagiotis G. Miliotis, Maria D. Koskolou, Konstantina Dipla, Ioannis S. Vrabas, and Nickos D. Geladas. 2023. "The Effect of Skeletal Muscle Oxygenation on Hemodynamics, Cerebral Oxygenation and Activation, and Exercise Performance during Incremental Exercise to Exhaustion in Male Cyclists" Biology 12, no. 7: 981. https://doi.org/10.3390/biology12070981
APA StyleCherouveim, E. D., Miliotis, P. G., Koskolou, M. D., Dipla, K., Vrabas, I. S., & Geladas, N. D. (2023). The Effect of Skeletal Muscle Oxygenation on Hemodynamics, Cerebral Oxygenation and Activation, and Exercise Performance during Incremental Exercise to Exhaustion in Male Cyclists. Biology, 12(7), 981. https://doi.org/10.3390/biology12070981







