Simple Summary
Bacterial involvement in cancer can be grouped into three categories: (i) direct association of bacteria in the processes of cancer development, (ii) secondary infection as a consequence of a patient’s weakened immune system, and (iii) utilization of bacteria in cancer therapeutics. Regarding a bacterial etiological or cancer-causing role, effective prevention strategies could be formulated after obtaining a precise understanding of the pathological mechanisms. Therefore, looking into the virulence factors of relevant bacterial species is critical in support of altering treatment plans with a proactive approach. This review has attempted to analyze both clinical/epidemiological and laboratory studies to understand bacterial mechanisms in cancer formation, particularly the virulence factors of Helicobacter pylori and Chlamydia. From secondary infections in cancer patients, microorganisms such as Pseudomonas aeruginosa, Escherichia coli, and Klebsiella species are commonly isolated. In general, these are highly diverse bacterial species and they have an exceptional ability to adapt and develop resistance against antimicrobial agents. Using an improved understanding of the pathogenic mechanisms of bacteria, it is necessary to review the topic of antimicrobial stewardship to optimize patient outcomes. Analysis of prophylactic antibiotic usage must also be addressed. Recent findings certainly support further examination of bacterial products that can be evaluated for their utilization as anti-cancer therapeutic agents.
Abstract
In cancer development and its clinical course, bacteria can be involved in etiology and secondary infection. Regarding etiology, various epidemiological studies have revealed that Helicobacter pylori can directly impact gastric carcinogenesis. The Helicobacter pylori-associated virulence factor cytotoxin-associated gene A perhaps plays an important role through different mechanisms such as aberrant DNA methylation, activation of nuclear factor kappa B, and modulation of the Wnt/β-catenin signaling pathway. Many other bacteria, including Salmonella and Pseudomonas, can also affect Wnt/β-catenin signaling. Although Helicobacter pylori is involved in both gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma, its role in the latter disease is more complicated. Among other bacterial species, Chlamydia is linked with a diverse range of diseases including cancers of different sites. The cellular organizations of Chlamydia are highly complex. Interestingly, Escherichia coli is believed to be associated with colon cancer development. Microorganisms such as Escherichia coli and Pseudomonas aeruginosa are frequently isolated from secondary infections in cancer patients. In these patients, the common sites of infection are the respiratory, gastrointestinal, and urinary tracts. There is an alarming rise in infections with multidrug-resistant bacteria and the scarcity of suitable antimicrobial agents adversely influences prognosis. Therefore, effective implementation of antimicrobial stewardship strategies is important in cancer patients.
1. Introduction
Pathogenic bacteria possess a large number of molecules and diverse mechanisms, which act as virulence factors and avert the host defense systems in order to initiate the disease processes. Apart from cell surface structures and metabolic arrangements, bacteria secrete several substances such as enzymes, toxins, and exopolysaccharides—many of these secreted biomolecules can enter the host cells and are able to control or damage the intracellular signaling/structures [1]. All of these bacterial biomolecules ultimately contribute to virulence and may lead to treatment complexity in the clinical setting.
One of the most common infectious agents and also one of the most studied organisms is Escherichia coli (E. coli), a Gram-negative, non-spore-forming, flagellated bacillus belonging to the family Enterobacteriaceae. Pathogenic E. coli strains are subdivided into different categories according to their clinical manifestations such as enteropathogenic (EPEC), necrotoxigenic (NTEC), and uropathogenic E. coli (UPEC). The prevalence of urinary tract infections (UTIs) is common in society and hospital settings, and UPEC is the leading pathogen in both uncomplicated and complicated UTIs [2]. Besides cell surface virulence factors (like fimbriae and capsular lipopolysaccharide/LPS) that help adhesion to epithelium/urothelium, the invasion of host cells, and biofilm formation, UPEC releases a number of virulence factors, e.g., α-haemolysin (HlyA), cytotoxic necrotising factor 1 (CNF1), secreted autotransporter toxin (SAT), cytolethal distending toxin (CDT), and toll/interleukin-1 receptor domain-containing protein (Tcp) [3]. These virulence factors play a significant role in bacterial colonization and disease propagation.
An intriguing aspect of certain bacterial virulence factors is their link with carcinogenesis. It is thought that chronic inflammation induced by E. coli in inflammatory bowel disease could predispose colon cancer development [4]. Of note, from Enterobacteriaceae family—E. coli and Klebsiella spp., and another Gram-negative bacillus—Pseudomonas aeruginosa have been commonly isolated from cancer patients [5,6,7]. In addition, patients with cancer are more vulnerable to secondary bacterial infections, which are a major cause of morbidity and mortality among these patients. However, evidence also shows a connection between certain bacterial persistent infections and carcinogenesis, e.g., Helicobacter pylori in gastric cancer, Salmonella typhi in gallbladder cancer, and Chlamydia pneumoniae in lung cancer [8,9]. Bacterial products such as colibactin (from Enterobacteriaceae) and cytotoxin-associated gene A (cagA, from H. pylori) can induce neoplasia by a number of mechanisms such as modulation of Wnt signaling pathway or maintaining an inflammatory state [8]. Interestingly, on the one hand Salmonella spp. (of the Enterobacteriaceae family) are believed to promote colon cancer, but on the other hand these bacteria may be used as anti-cancer agents. The Salmonella genome encodes a wide range of virulence factors, which can alter various intracellular signaling pathways including the Wnt/β-catenin and transforming growth factor-β (TGF-β), thus contributing to tumorigenesis [10]. In contrast, it has been demonstrated in experimental models that different attenuated Salmonella strains (such as A1-R and Χ9241) can preferentially colonize solid tumors and prevent tumor growth [11]. Similarly, virulence factors of P. aeruginosa such as mannose-sensitive hemagglutinin (PA-MSHA), cyclodipeptides, and exotoxin are being evaluated to be used in anti-cancer management [12,13,14]. A comprehensive understanding of these virulence factors at the cellular and molecular levels is greatly helpful to approach various pathological issues as well as their potential therapeutic aspects.
2. Helicobacter pylori and Gastric Adenocarcinomas
H. pylori (formerly called Campylobacter pylori) is a Gram-negative, flagellated, motile, and spiral-shaped/helical bacillus that grows in the gastric mucosa layer of more than 50% of the human population [15]. This bacterium is microaerophilic, i.e., it is aerobic but requires lower environmental oxygen levels, and it generally creates an asymptomatic chronic state of inflammation, which could lead to gastritis, peptic ulcer, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT) lymphoma of the stomach. After the discovery of H. pylori in 1982 by Barry J. Marshall and John R. Warren [16], it took about 6 years to realize that this bacterium might cause neoplasia [17]. Subsequently, some investigators showed an increased risk of gastric cancer among H. pylori seropositive subjects [18,19].
In an interesting review, Chiba et al. described two major pathways for the development of gastric adenocarcinoma due to H. pylori chronic infection [20]. One of the most important virulence factors in the direct pathway is the cagA protein. Of note, H. pylori can be divided into two strains, cagA-positive and cagA-negative; the former has a strong association with gastric cancer development. Furthermore, H. pylori’s direct action on gastric epithelial cells can cause the activation of nuclear factor kappa B (NF-κB), induction of mutations of tumor suppressor p53, and aberrant DNA methylation.
A key pro-inflammatory NF-κB pathway has been shown to promote the neoplastic processes of gastric mucosa. A study on AGS, SNU-1, and HGC-27 gastric cancer cell lines revealed that the activation of NF-κB occurred in H. pylori infection, which again triggered the activation of another pro-inflammatory transcription factor—signal transducer and activator of transcription 3 (STAT3) [21]. Similarly, after analyzing 255 human gastric cancer specimens immunohistochemically, one study noticed a significantly positive correlation between NF-κB and STAT3 activation [22]. Another study that examined gastric antral biopsies from 35 H. pylori-infected gastritis cases with 15 H. pylori-negative controls observed a distinctly enhanced NF-κB expression in the H. pylori-infected cases [23]. A number of in vivo and in vitro studies also recorded that, in the stomach, NF-κB activation was induced by H. pylori infection [24,25,26]. Furthermore, many studies documented that H. pylori disrupted the p53 tumor suppressor pathway, and p53 alterations can be observed in lesions ranging from gastritis to gastric cancer [27]. In a study on patients with chronic gastritis, it was detected that H. pylori-related lesions expressed the mutant-type p53 [28]. In addition, a lower expression of p53 was noticed in gastric epithelium of H. pylori-infected cases [29]. In the same way, a study on gastric cancer patients found that 80% of H. pylori-positive cases had p53 mutation [30]. Studies on different gastric cancer cell lines, e.g., STKM2, AGS, SNU1, and HFE145 cells, also showed that H. pylori infection resulted in p53 inhibition [31]. On the other hand, epigenetic modifications such as aberrant DNA methylation in the promoter regions of genes result in the alteration of different cancer-related genes and inactivation of tumor suppressor genes. In gastric cancer, aberrant methylation occurs in genes associated with DNA repair (such as hMLH1 and MGMT), transcriptional regulation (such as HLTF), cell growth/differentiation (such as HoxD10 and NDRG2), cell cycle (such as p16), and apoptosis (such as BNIP3) [32]. After analyzing gastric mucosa in H. pylori-linked chronic gastritis, gastric cancer, and cases with pre-neoplastic lesions followed up for 10 years, Compare et al. concluded that global DNA hypomethylation is an initial feature in H. pylori-linked gastric tumorigenesis [33]. Interestingly, Zhang et al. revealed hypermethylation of the tumor suppressor MGMT in H. pylori-induced gastric cancer development [34]. Their study showed that cagA enhanced the hypermethylation of tumor suppressor genes by stimulating DNA-methyltransferase 1 (involved in DNA methylation) via activation of AKT―NF-κB.
It is known that H. pylori-associated gastric cancer has a strong connection with gastritis, especially chronic atrophic gastritis of the corpus or type AB gastritis, where Th1-type CD4 cells and their product interferon-gamma (IFN-γ) may play a critical role, along with other pro-inflammatory cytokines such as interleukin-1β (IL-1β), IL-6, and tumor necrosis factor-α (TNFα). Apart from cagA, the bacterium also has a number of virulence factors, including vacuolating cytotoxin A (vacA), blood group antigen-binding adhesin (babA), and sialic acid-binding adhesin (sabA) [35,36]. These virulence factors trigger different cell-proliferation-related signaling pathways, e.g., phosphatidylinositol 3-kinases (PI3K)–Akt, Janus kinase (JAK)–STAT, and extracellular signal-regulated kinases (ERK), which could promote carcinogenesis in uncontrolled circumstances. It is notable that virulence factors such as babA and sabA bind to different blood group antigens (ABO and Lewis) on the gastric epithelial surface. This correlates to Wang and colleagues’ observation that persons with blood group A were more susceptible to H. pylori infection [37]. Overall, several investigators have recorded a higher risk of gastric cancer in blood group A-positive individuals [38].
3. Gastric MALT Lymphomas
Among H. pylori-infected people, approximately 2–3% and 0.1% of individuals develop gastric adenocarcinoma and gastric MALT lymphoma, respectively [35]. MALT lymphomas in the stomach are extranodal B-cell marginal-zone lymphomas with an indolent disease course [39]. When considered separately, probably none of the virulence factors that are commonly associated with gastritis, peptic ulcers, and gastric carcinoma has any significant role in gastric MALT lymphomas [40,41]. However, the gene cluster encompassing iceA1 allele, sabA, and hopZ, along with ORF JHP950, has been found to be associated with the risk of gastric MALT lymphomas. Nevertheless, gastric MALT lymphoma-related H. pylori strains are perhaps less virulent than those involved in gastric carcinoma [41]. On the other hand, it has been hypothesized that chronic inflammatory/antigenic stimulation by persistent H. pylori infection could result in continued immune cell activation that might favor malignant transformation [42,43]. Fortunately, H. pylori eradication therapy at the initial stage and for low-grade lymphoma is associated with satisfactory outcomes (Table 1) [44,45,46,47].

Table 1.
Commonly followed Helicobacter pylori eradication therapy in gastric MALT lymphomas.
Overall, the gastrointestinal tract is a common extranodal site for the development of lymphomas, although the incidence rate of primary lymphomas in the gastrointestinal tract is low and the majority of these lymphomas originate in the stomach (Figure 1). It has been already stated that gastric MALT lymphoma is a slow-growing non-Hodgkin lymphoma, which is commonly associated with H. pylori infection. Without appropriate therapeutic management, H. pylori infection causes chronic inflammation that leads to the proliferation of B-cells and T-cells in the gastric mucosa. Finally, such a long-established inflammation stimulates the formation of aberrant mucosa-associated lymphoid tissue that can turn into malignancy [48]. In general, the eradication of H. pylori rectifies this lymphoid tissue problem. On the other hand, genetic aberrations such as chromosomal translocations in gastric MALT lymphomas can cause resistance to H. pylori eradication treatment. In this context, the most common translocation is t(11;18)(q21;q21) and the fusion product is a powerful NF-κB activator [49]. In addition, t(1;14)(p22;q32) is associated with the advanced stage. It may be worth mentioning that proton pump inhibitors are primarily metabolized through cytochrome P450 (CYP) 2C19, so polymorphisms in CYP 2C19 can affect the treatment [50]. Furthermore, bacterial factors for treatment resistance include mutations in the V domain of the 23S rRNA (most commonly A2143G) that reduce the affinity for clarithromycin, and mutations in the PBP1A in cases with amoxicillin resistance. Patients who are refractory to H. pylori eradication therapy should undergo alternative regimens such as chemotherapy and radiotherapy [47]. Regarding the prognostic markers, studies have revealed different predictors for poor patient outcomes such as older age, advanced staging, elevated lactate dehydrogenase levels, and high-grade histological subtype [51,52].
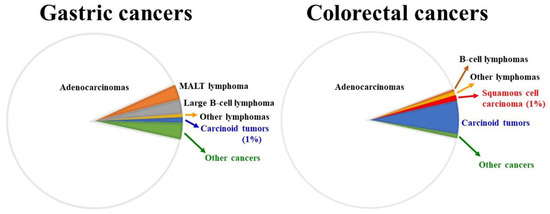
Figure 1.
Comparison between adenocarcinomas and lymphomas for the two most common gastrointestinal sites for cancer development. Roughly 2 million cases of colorectal cancers and 1.1 million cases of gastric cancers were diagnosed in 2020 (source: World Health Organization). Both gastric and colorectal adenocarcinomas: ~90%; gastric lymphomas: ~6% (generally, MALT lymphomas are low-grade lesions, whereas large B-cell lymphomas are high-grade malignancy; both make up around 90% of all gastric lymphomas); colorectal lymphomas: ~1.2% (B-cell lymphomas: ~0.5%).
Histological features of gastric MALT lymphomas include lymphoepithelial lesions and the presence of centrocyte-like cells, monocytoid cells, immunoblast-like cells, and plasma cells [53,54]. Moreover, reactive germinal centers (which might be infiltrated by lymphoma cells) with mantle zone obliteration could be observed. The immunophenotype is not specific—the neoplastic cells express CD19, CD20, CD22, and CD79a (i.e., pan B-cell markers) [55]. Interestingly, MALT lymphomas originate from mature post-germinal center B-cells, which are similar to the plasma cells [56]. Therefore, a number of patients displayed excess biosynthesis of monoclonal immunoglobulins (IgG or IgM), which may mimic conditions such as Waldenström macroglobulinemia [56,57]. On the other hand, gastric MALT lymphomas can increase the risk for the development of intestinal metaplasia (precancerous lesions) and subsequent gastric adenocarcinomas [58,59].
4. Colon Cancer—Connection with Bacterial Pathology
Gastrointestinal tract cancers represent more than one-fourth of the overall cancer incidence and the two major sites are the stomach and colon, which comprise nearly 60% of gastrointestinal tract cancers [60] (Figure 1). According to the World Health Organization, the most commonly diagnosed gastrointestinal malignancy in 2020 was colon cancer (almost 2 million cases). The risk factors for colon cancers are primarily associated with lifestyle and dietary habits such as obesity and the intake of processed/red meat and alcohol, apart from hereditary and chronic inflammatory conditions. However, a growing body of evidence suggests an important role of gut microorganisms and their imbalance (dysbiosis) in the development of colon cancers. In this connection, many studies have recorded the pathogenic effects of specific bacterial species such as E. coli, Fusobacterium nucleatum, Bacteroides fragilis, and Streptococcus gallolyticus (Streptococcus bovis) (Table 2) [61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83]. By appropriate assessment of these bacteria and their interactions in the pathological processes, suitable prevention strategies could be designed.

Table 2.
Recent reports on commonly studied bacteria that may have a pathological role in the development of human colorectal cancer.
5. Chlamydia Species: Their Virulence Factors and Involvement in Different Diseases Including Cancer
The life cycle of Chlamydia is unique. Chlamydiae are non-motile, Gram-negative, obligate intracellular bacteria, and exist in two morphologically distinct forms: elementary body (EB) and reticulate body (RB). The chlamydial cell wall consists of an outer membrane (containing a single major outer membrane protein/MOMP, and LPS, but no detectable peptidoglycan) and an inner cytoplasmic membrane [84]. The EB is the metabolically inert, infectious, environmentally resistant extracellular form, which has some similarities with a spore. It is responsible for spreading the infection within the host and transmission to other susceptible persons/hosts. The EB is capable of binding and invading primarily the mucosal epithelium, particularly columnar cells. For instance, in the case of Chlamydia trachomatis infection, the target areas are usually the single-layer columnar cells or their transformation zone with the non-keratinizing squamous epithelium, which is typically found in the genital tract [85]. The EB can bind with various cell surface receptors/molecules, e.g., cystic fibrosis transmembrane conductance regulator (CFTR), β1 integrin, epidermal growth factor receptor (EGFR), 3′sulfogalactolipid, ephrin receptor A2, apolipoprotein E4, and platelet-derived growth factor receptor (PDGFR) [86]. On the other hand, RB is the non-infectious replicative form. The surface membrane of the target cell forms a vacuole around the EB after it enters that transforms into a metabolically active RB. As a result, the characteristic inclusion bodies, which contain replicating organisms/RB, are formed near the nucleus. Finally, the condensation of RB (or intermediate body) results in the formation of infectious EB and the death of the host cell.
Three pathogenic species for humans are Chlamydia trachomatis, Chlamydia pneumoniae, and Chlamydia psittaci (zoonotic transmission). The latter two species can cause pneumonia. However, C. trachomatis is divided into 3 variant strains (biovars), which are again subdivided into several serotypes (serovars). Serovars A–C are responsible for trachoma (trachoma biovar), whereas the sexually transmitted serovars D–K (genital tract biovar) can cause genital tract infections and serious complications such as pelvic inflammatory disease, infertility, and ectopic pregnancy [87]. In addition, C. trachomatis promotes human immunodeficiency virus infection and cervical cancer pathogenesis. On the other hand, the serovars L1–L3 (LGV biovar) causes lymphogranuloma venereum [87]. According to the Centers for Disease Control and Prevention (CDC), Chlamydia infections have chronically been the most frequent notifiable sexually transmitted disease in the United States, with over 1.5 million cases reported in 2020 alone. More than 60% of all reported cases were among persons aged 15–24 and with most hosts being asymptomatic. Thus, understanding the long-term effects of this bacterium in terms of virulence, and the pathological consequences such as cancer development, is imperative.
A vital step in chlamydial pathogenesis involves the mechanisms by which Chlamydiae acquire the necessary nutrients. The bacteria are unable to produce the essential components of energy transduction and nucleic acid biosynthesis, as well as a number of amino acid biosynthesis pathways. So, they acquire these vital nutrients (including ATP) by selectively redirecting transport vesicles and capturing intracellular organelles [88]. Regarding bacterial virulence properties, several factors/components may play an important role in disease severity [89]. These factors include MOMP, polymorphic outer membrane proteins (Pmp), type III secretion systems (TTSS), putative chlamydial cytotoxin, stress-response proteins, LPS and other glycolipids, plasmid (cryptic) gene product, macrophage infectivity potentiator protein, chlamydial adhesins/invasins, and metabolic processes such as iron sequestration and modulation of tryptophan availability. Obviously, in order to understand the pathological processes of Chlamydia precisely, it is necessary to identify the relevant chlamydial virulence factors and their specific roles in disease severity.
One of the most studied chlamydial virulence factors is plasmid glycoprotein 3 (Pgp3). Generally, a number of chlamydial species and strains carry a 7.5 kb plasmid that encodes 8 Pgps. However, it is assumed that the abovementioned plasmid protein is a contributing factor at least for the pathogenesis of C. trachomatis species [90]. Moreover, Sturdevant et al. have commented that the plasmid and inclusion membrane protein CT135 (chromosomal gene product) are important virulence factors [91]. Their experiments with plasmid-deficient and CT135-null C. trachomatis serovar D strains in C3H/HeJ mice showed a reduced infectious capability compared to wild-type bacteria. Interestingly, Borges et al. revealed that CT135 impacted the expression of many proteins which are supposed to play a key role in bacterial virulence, including CT456/Tarp [92]. Of note, CT456 is an effector protein delivered to the host cell by a TTSS, which is a feature of many Gram-negative pathogens, to alter cytoskeletal processes [93]. Chlamydia spp. can manipulate the host cytoskeleton component actin to facilitate their invasion, replication, and disease spread. By restructuring the host actin cytoskeleton, the chlamydial type III-secreted ‘translocated actin recruiting phosphoprotein’ (Tarp) effector possibly supports bacterial entry into host cells [94,95].
For adhesion to host cells, the infectious chlamydial EB requires adhesins that include various polymorphic membrane proteins/Pmp. It has been suggested that C. pneumoniae utilizes Pmp6, Pmp20, and Pmp21 [96]. On the other hand, Favaroni et al. found Pmp22D, Pmp8G, and outer membrane complex protein B (OmcB) as necessary adhesins during C. psittaci infection [97]. In a study on human endothelial cells, Niessner et al. observed that C. pneumoniae Pmp 20 and Pmp 21 increased pro-inflammatory IL-6 and monocyte chemoattractant protein-1 via NF-κB pathway [98]. Interestingly, C. pneumoniae Pmp21 can bind to EGFR and induce EGFR activation [96]. In addition, C. pneumoniae infection has been shown to be associated with activation of different signaling molecules such as PI3K, mitogen-activated protein kinase, or ERK (Table 3).

Table 3.
Selected cell-signaling pathways that are linked with bacterial infections and neoplastic processes.
Iron is an essential nutrient for different pathogens as well as for the innate immune response [99]. However, chlamydial iron acquisition mechanisms are not clearly understood and may be affected by the host’s fluctuating iron status. For example, in female genital tract infections with C. trachomatis, variations in the levels of lactoferrin due to estrogenic alterations may change chlamydial iron availability [100]. On the other hand, iron chelator 2,2-bipyridyl can cause iron starvation in C. trachomatis [101]. Nevertheless, Pokorzynski et al. have hypothesized that iron is transported to chlamydial cells by the YtgABCD ABC-type metal permease complex, and YtgR is an iron-dependent transcriptional repressor, regulated by tryptophan availability [100,102,103].
Chlamydiae have a highly complex cell structure and hence they were originally considered to be viruses. Of note, poxviruses replicate in the host cytoplasm like non-viral intracellular pathogens such as Chlamydia or Rickettsia. Unlike Rickettsia spp., that are broadly divided only into a few categories such as the spotted fever and typhus groups, Chlamydia spp. can cause a diverse array of diseases. For example, C. trachomatis can cause eye diseases such as trachoma, inclusion conjunctivitis, and trichiasis [104]. It has been suggested that C. pneumoniae may contribute to a number of vascular disorders such as endothelial damage, angiogenesis, and atherosclerosis [105,106]. Interestingly, both C. pneumoniae and C. trachomatis have been shown to be involved in otitis media [107,108]. Furthermore, studies have documented that Chlamydia infection can increase the risk of cancer for several sites, e.g., lung, ovary, uterine cervix, vulva, and ocular adnexa lymphoma (Table 4) [109,110,111,112,113,114,115,116,117,118,119,120,121,122,123]. Perhaps, routine Chlamydia infection screening in susceptible populations is useful to prevent various complications.

Table 4.
Chlamydia infection and cancers of different sites.
6. Pseudomonas aeruginosa and Cancer
Studies from different geographical locations have documented that P. aeruginosa infections frequently occur among cancer patients [124,125,126]. In addition, the evidence shows a substantially higher mortality rate in cancer patients with P. aeruginosa infections [126,127]. Although it is generally believed that cancer patients with neutropenia are more susceptible to pseudomonas infections [128,129], after reviewing a considerable number of reports on cancer patients, Maschmeyer and Braveny did not find any distinct differences between neutropenic and non-neutropenic cases, or between cases with solid tumors and hematologic malignancies, in connection with the involvement of P. aeruginosa infections [126]. Apart from oncological cases, this opportunistic bacterium is commonly detected in other clinical situations such as immunodeficiency, burn, conditions linked with the use of medical instruments/devices, and cystic fibrosis. It may be worth mentioning that in cystic fibrosis approximately 60–80% of adults finally develop chronic P. aeruginosa infection, and usually this pulmonary infection persists indefinitely [130]. In addition, various poor clinical features such as worsening symptoms (such as cough), nutritional status, lung function, radiographic scores, and poor prognosis (such as end-stage lung disease) are more commonly noticed in patients with chronic P. aeruginosa infection than in those without.
Regarding P. aeruginosa bacteria-related conditions, it is known that several pathological states under primary immunodeficiency, such as common variable immunodeficiency and ataxia-telangiectasia, and secondary immunodeficiency, such as acquired immunodeficiency syndrome, are associated with an increased risk of cancer [131,132,133]. In general, the incidence of lymphomas is relatively higher in these patients. On the other hand, many studies have observed an increased risk of cancers in the gastrointestinal tract and other sites such as the thyroid and kidney among patients with cystic fibrosis [134,135,136,137]. Of note, cystic fibrosis is an autosomal recessive disease, which is caused by mutations in the CFTR gene, located on chromosome 7. The CFTR protein may act as a tumor suppressor, and its deficiency perhaps causes disruption of several cellular processes that could be linked with neoplastic processes, e.g., influence on immune reactions, inflammatory responses, and Wnt/β-catenin signaling [138]. Interestingly, P. aeruginosa can also affect the Wnt/β-catenin signaling pathway [139].
P. aeruginosa is a Gram-negative opportunistic bacillus which can survive in diverse environments and is considered an important pathogen for nosocomial infections. Bacterial virulence factors include several components, e.g., motility (which is associated with surface appendages such as flagella and pili), LPS (a component of the outer membrane), secretion systems (which render toxins and enzymes into the host’s intracellular or extracellular space), secondary metabolites (such as phenazines like pyocyanin—responsible for greenish color), quorum sensing (i.e., cell-to-cell communication system), and biofilm formation [140,141]. Pathological phenomena such as biofilm formation and chronic infection are associated with bacterial capabilities of adherence and motility. Furthermore, LPS can induce immune responses and CFTR. On the other hand, there are five secretion pathways: the type 1 secretion system (T1SS), T2SS, T3SS, T5SS, and T6SS. Exotoxin A belongs to the T2SS and is responsible for host cell death, while the T3SS is linked with the pathogenicity/severity of infections.
The management of P. aeruginosa infections has become a serious problem because of the bacteria’s very high capability to develop resistance against several antibiotics. Furthermore, indiscriminate use of antibiotics favors the emergence of multidrug-resistant P. aeruginosa strains. In general, the principal mechanisms by which P. aeruginosa can resist antibiotic molecules may be categorized into three groups, i.e., intrinsic, acquired, and adaptive resistance [142]. Examples of intrinsic resistance are reducing the permeability of the outer membrane, the development of efflux pumps that eject antibiotic molecules out of the bacterial cell, and the biosynthesis of inactivating enzymes for antibiotics. The acquired resistance can be attained by suitable mutations and/or horizontal transfer of antibiotic resistance genes such as plasmid-mediated conjugation and natural transformation, i.e., obtaining foreign genetic material. On the other hand, adaptive resistance includes biofilm formation in the involved tissue. The biofilm (i.e., matrix of extracellular polymeric substances comprising embedded bacteria, extracellular DNA, proteins, and polysaccharides) prevents antibiotic diffusion. By means of the protection of biofilm, P. aeruginosa can survive and cause recurrent infections.
Interestingly, accumulating evidence indicates a potential role for P. aeruginosa in cancer therapy. Since the initial observation of a bacterial anti-neoplastic role by German physician W. Busch in 1868 [143], the exploration of bacterial products/relevant mechanisms against cancer has been reported by many investigating groups throughout the world (Table 5) [144,145,146,147,148,149,150,151,152,153,154,155,156]. Different studies have identified a number of P. aeruginosa-released anti-cancer substances such as exotoxin A, mono-rhamnolipids, and azurin [157,158,159]. Overall, P. aeruginosa-linked anti-tumor mechanisms include the growth of bacteria in hypoxic regions of tumors, the production of toxins, modulation of host immune responses, and delivery of therapeutic genes that encode cytotoxic peptides or prodrug-converting enzymes [160] (Figure 2). In addition, it may be worth mentioning that P. aeruginosa toxins are favorable biomolecules for the construction of recombinant immunotoxins or chimeric proteins (such as monoclonal antibody plus bacterial toxin) for anti-cancer therapeutic strategy. Nevertheless, a greater understanding of the interactions between cancer cells and bacteria will definitely be useful for the development of novel anti-cancer management.

Table 5.
Anti-neoplastic effects of different microbial products and immune checkpoint inhibitors.
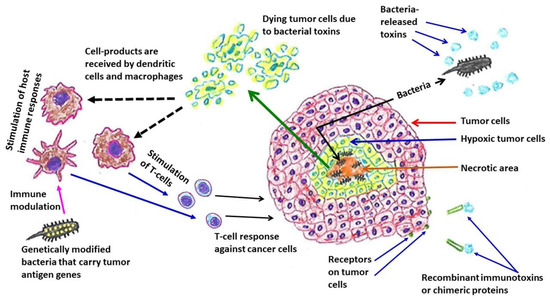
Figure 2.
Bacteria-linked anti-tumor therapeutic strategies. Many bacteria, such as Escherichia coli and Pseudomonas aeruginosa, colonize in hypoxic regions of tumors. Immunotoxins or chimeric proteins are produced by combining bacterial toxins (such as Pseudomonas aeruginosa exotoxin A) with monoclonal antibodies or cytokines or growth factors, which can bind with the specific tumor cell surface molecules or receptors that are over-expressed. An example of genetically modified bacteria is Pseudomonas aeruginosa—mannose-sensitive hemagglutinin (PA-MSHA), a less virulent/attenuated strain.
7. Systemic Cancer Therapy—Immunity and Infection
It is believed that after the transformation of a normal cell to malignancy, there is a need for the initial cancer cell(s) to evade the barriers/attacks from the host’s immunosurveillance mechanisms in order to survive and grow. In this process, a dynamic tumor microenvironment is created wherein active interactions occur between different biological constituents, e.g., cancer cells, immune cells, various stromal cells, extracellular matrix, blood vessels, and even the nervous system [161]. However, according to the circumstances, cancer cells may switch to a quiescent phase or state of dormancy [162]. On the other hand, cancers can progress to the advanced state, metastasize to different body systems, and may cause cachexia. Cancer cells spreading into the bone marrow clearly weakens the immune system. Similarly, various cancer chemotherapeutic agents can cause bone marrow suppression, neutropenia, immunosuppression, and an increased risk of infection [163]. In cancer-associated cachexia, the pathological processes affect different cytokines (e.g., TNFα, IFN-γ, and IL-6) including adipokines and myokines, their signaling pathways, gut microbiota and gut hormones, immune cells, immune checkpoints, the immune–metabolic axis, and the nervous system [164]. In a study on colon cancer patients, Zhang et al. found that cachectic patients had a significantly higher rate of bacterial DNA fragments in serum than non-cachectic patients and healthy controls. Furthermore, the former group had higher levels of IL-1α, IL-6, IL-8, and TNFα [165]. In an interesting review, Herremans et al. discussed the alterations of microbiota (dysbiosis) and their links with gut barrier dysfunction, pathways of systemic inflammation, and muscle wasting in cancer cachexia [166].
Regarding immunomodulatory therapy against cancer, vaccines are currently available for protection against human papillomavirus (HPV) and hepatitis B virus (HBV); HPV is primarily linked with cervical cancer and HBV infection can initiate liver cancer. Different reports have documented that these two vaccines are generally well tolerated among populations throughout the world [167,168,169,170,171]. In systemic therapy against cancer, there is some overlap between immunotherapy and targeted therapy. Clinically, monoclonal antibodies against the human epidermal growth factor receptor-2 (HER2), e.g., trastuzumab, are commonly used in cancers that overexpress HER2, particularly in breast cancer [172]. Of note, HER2 belongs to the EGFR family and frequently overexpresses in many cancers [173]. In a meta-analysis on 8669 breast cancer patients receiving trastuzumab, Jackson et al. noticed several adverse effects including increased susceptibility to infections (particularly respiratory tract infections) [174]. On the other hand, in treatment with immune checkpoint inhibitors, toxicities can affect any body systems, which includes inflammation of different organs such as pneumonitis, hepatitis, vasculitis, as well as neutropenia, and anemia [175]. In this context, reports have documented different opportunistic bacterial infections such as P. aeruginosa and Clostridium difficile, apart from fungal and viral infections [176,177].
8. Antimicrobial Stewardship in Cancer Patients
As mentioned before, patients with solid tumors or hematological malignancies frequently develop infections. Of note, roughly more than 90% of all cancers are solid tumors. Nevertheless, the increased risk of infections in cancer patients could be due to tumor-related factors or therapy-associated complications, which include neutropenia, damage to biological barriers (such as integument and mucosa), obstruction of physiological passages, patient’s age, nutritional status, as well as surgical procedures and instrumentations for diagnosis or therapy [178]. In general, neutropenia is common in hematological malignancies, but this problem can also happen as a consequence of chemotherapy, radiotherapy, and bone marrow metastasis. On the other hand, disruption of mucosal surfaces can occur due to medical devices such as catheters or treatment-linked mucositis (chemotherapy or radiotherapy), and the common sites of infection are the respiratory tract, gastrointestinal tract, and urinary tract. It is noteworthy that several risk factors for infection commonly exist in the same patient.
Although the use of antibiotics is intimately connected with cancer care, an alarming rise in infections with multidrug-resistant bacteria and the scarcity of suitable antimicrobial agents are important problems in the treatment of cancer patients. Among patients with cancer, isolated drug-resistant bacteria include E. coli, Klebsiella spp., P. aeruginosa, viridans group streptococci, and coagulase-negative staphylococci [179]. It may be worth mentioning that in immunocompromised patients with neoplastic diseases, the most common complications are secondary bacterial infections, particularly bloodstream infections, which are also responsible for significant morbidity, mortality, and economic burden [180]. Considering the emergence of multidrug-resistant organisms, there is a need to formulate better strategies in order to prevent the development and spread of multidrug-resistant bacteria, as well as to carefully use the currently available antimicrobial drugs (which is referred to as antimicrobial stewardship) [181].
In a recent report from Japan, the investigators had studied the effectiveness of antimicrobial stewardship on 32,202 patients during 2018–2021 at a cancer hospital [182]. They observed a declining trend of methicillin-resistant Staphylococcus aureus and multidrug-resistant P. aeruginosa infections. In addition, the integration of the antimicrobial stewardship program (ASP) and facility of infectious disease consultations (IDC) decreased the use of carbapenem (a β-lactam class antibiotics) without negative patient outcomes. Finally, they concluded that the application of their method might support appropriate cancer management. In another study that was conducted during 2009–2017 in a cancer department in Spain, the investigators noticed that the combination of IDC and ASP benefited antibiotic use in cancer patients, and this improvement was associated with a decrease in mortality due to bacterial infections [183]. Likewise, a prospective cohort study in Brazil during 2009–2011 analyzed 307 episodes of chemotherapy-induced febrile neutropenia in 169 patients [184]. Interestingly, the study revealed that adherence to the ASP was correlated with decreased mortality rates.
Among cancer patients receiving chemotherapy, febrile neutropenia is a serious complication and is negatively associated with overall prognosis, including mortality [185]. It is worth mentioning that the major threats to neutropenic patients are infections with multidrug-resistant Gram-negative bacteria, particularly with pathogens such as Klebsiella spp., E. coli, and P. aeruginosa [186]. In addition, about one-third of cases of febrile episodes in patients with neutropenia are associated with bacteremia (i.e., presence of bacteria in the circulation). Interestingly, in developed countries, the predominant pathogens are Gram-positive bacteria, whereas mortality is higher in Gram-negative bacteremia [187].
Kouranos and his colleagues reviewed lung cancer cases where the patients were treated with prophylactic antibiotics and they noticed that febrile neutropenia, infections, and the duration of hospitalization were significantly reduced [188]. The authors concluded that prophylactic antibiotics use appears to be efficacious and may be used as a prevention strategy for chemotherapy-induced neutropenia in patients with lung cancer. In general, quinolones (such as levofloxacin and ciprofloxacin) and trimethoprim-sulfamethoxazole are the commonly used prophylaxis, although their prophylactic use is debatable. In another study, 113 primary randomized or quasi-randomized trials on patients with cancer receiving chemotherapy or undergoing hematopoietic stem cell transplantation with expected neutropenia were reviewed [189]. The authors observed that the prophylactic use of a quinolone, trimethoprim-sulfamethoxazole, or a cephalosporin regimen decreased bacteremia. Furthermore, they noted that the use of quinolone/fluoroquinolone was not significantly related to higher infection rates of C. difficile or invasive fungal pathogens. Overall, different studies have documented that antibiotic prophylaxis can decrease the episodes of febrile neutropenia and infection-linked deaths in patients receiving chemotherapy [190]. Additionally, a study conducted by Itoh et al. found that an oral switch from intravenous antimicrobial therapy was beneficial in preventing catheter-related bloodstream infection with methicillin-sensitive S. aureus in cancer patients [191].
Like antibacterial management, the antifungal prophylaxis strategy has been employed in several places to control invasive fungal infections in high-risk hematologic cases such as acute myeloid leukemia and myelodysplastic syndrome [192,193,194]. Of note, Candida, Aspergillus, and Cryptococcus species are common fungal pathogens that cause invasive infections in immunocompromised conditions, including cancer [195]. Furthermore, after reviewing the data of a number of trials, Robenshtok et al. revealed that antifungal prophylaxis significantly reduced the mortality in patients after chemotherapy [196]. Nevertheless, in the prophylactic use of antibacterial and antifungal agents, reasonable clinical practice guidelines are required for appropriate decision making, optimization, and selection of adequately targeted patients to achieve the maximum benefit [189,197]. Therefore, antimicrobial stewardship is vital in antibacterial and antifungal prophylaxis among cancer patients.
Interestingly, Aitken and his colleagues mentioned a number of antimicrobial stewardship strategies that have been effectively implemented in cancer patients [198]. Overall, important measures such as the improvement of clinical outcomes, the reduction of antimicrobial drug-related adverse effects/consequences, and a decrease in antimicrobial overuse are the primary goals of an ASP [198,199]. Effective collaboration between oncologists and the institutional ASP can establish successful antimicrobial stewardship for cancer patients and support substantial progress in cancer management.
9. Conclusions
Many bacteria are linked with the pathological processes of cancer in diverse ways. Apart from H. pylori infection, which clearly displays a direct etiological connection to tumorigenesis, the neoplastic role of other bacteria cannot be established so decisively. Moreover, the intricate interactions between specific bacteria and the target cells of our body, as well as the exact nature of different virulence factors, are chiefly unidentified. Oppositely, a number of bacterial products are being evaluated for their utilization as anti-cancer therapeutic agents. Therefore, on the one hand certain virulence factors are pathognomonic for the disease course, but on the other hand these biomolecules could play a key role in treatment strategies. While research continues, clinicians should be ever diligent in having patients participate in screening testing, especially those who are at risk for high-virulence pathogens. By eliminating these pathogens early in a disease process, cancer management can be improved. Secondary bacterial infections among cancer patients have a major impact on the overall prognosis and patients’ survival due to the immunosuppressive nature of malignancy and related chemotherapy/radiotherapy. Moreover, along with bacterial infections, an alarming increase in antibiotic resistance is a great challenge for patients’ wellness. Currently, the careful use of antibiotics is the sole method to provide the relief of cancer patients.
Author Contributions
Conceptualization, A.R.; Methodology, R.P.; Resources, R.P.; Data Curation, R.P. and A.D.B.; Writing—Original Draft Preparation, A.R. and A.D.B.; Writing—Review and Editing, T.F.M., R.P. and D.M.B.; Visualization, R.P.; Supervision, A.R. and D.M.B.; Project Administration, A.R. and D.M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Leitão, J.H. Microbial virulence factors. Int. J. Mol. Sci. 2020, 21, 5320. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Castillo-Pino, E. An introduction to the epidemiology and burden of urinary tract infections. Ther. Adv. Urol. 2019, 11, 1756287219832172. [Google Scholar] [CrossRef] [PubMed]
- Bien, J.; Sokolova, O.; Bozko, P. Role of uropathogenic Escherichia coli virulence factors in development of urinary tract infection and kidney damage. Int. J. Nephrol. 2012, 2012, 681473. [Google Scholar]
- Khan, A.A.; Khan, Z.; Malik, A.; Kalam, M.A.; Cash, P.; Ashraf, M.T.; Alshamsan, A. Colorectal cancer-inflammatory bowel disease nexus and felony of Escherichia coli. Life Sci. 2017, 180, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Muthunatarajan, S.; Mulki, S.S.; Archana Bhat, K.; Kotian, K.H. Bacterial infection among cancer patients: Analysis of isolates and antibiotic sensitivity pattern. Int. J. Microbiol. 2021, 2021, 8883700. [Google Scholar] [CrossRef] [PubMed]
- Islas-Muñoz, B.; Volkow-Fernández, P.; Ibanes-Gutiérrez, C.; Villamar-Ramírez, A.; Vilar-Compte, D.; Cornejo-Juárez, P. Bloodstream infections in cancer patients. Risk factors associated with mortality. Int. J. Infect. Dis. 2018, 71, 59–64. [Google Scholar] [CrossRef]
- Perez, F.; Adachi, J.; Bonomo, R.A. Antibiotic-resistant gram-negative bacterial infections in patients with cancer. Clin. Infect. Dis. 2014, 59 (Suppl. 5), S335–S339. [Google Scholar] [CrossRef]
- Gagnaire, A.; Nadel, B.; Raoult, D.; Neefjes, J.; Gorvel, J.P. Collateral damage: Insights into bacterial mechanisms that predispose host cells to cancer. Nat. Rev. Microbiol. 2017, 15, 109–128. [Google Scholar] [CrossRef]
- Mager, D.L. Bacteria and cancer: Cause, coincidence or cure? A review. J. Transl. Med. 2006, 4, 14. [Google Scholar] [CrossRef]
- Sun, J. Impact of bacterial infection and intestinal microbiome on colorectal cancer development. Chin. Med. J. 2022, 135, 400–408. [Google Scholar] [CrossRef]
- Chorobik, P.; Czaplicki, D.; Ossysek, K.; Bereta, J. Salmonella and cancer: From pathogens to therapeutics. Acta Biochim. Pol. 2013, 60, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Xiao, W.; Yang, Y.; Li, H.; Xia, D.; Yu, G.; Guo, X.; Guan, W.; Hu, Z.; Xu, H.; et al. Pseudomonas aeruginosa-mannose-sensitive hemagglutinin inhibits epidermal growth factor receptor signaling pathway activation and induces apoptosis in bladder cancer cells in vitro and in vivo. Urol. Oncol. 2014, 32, 36.e11-8. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Rivera, D.; González, O.; Guzmán-Rodríguez, J.; Díaz-Pérez, A.L.; Ochoa-Zarzosa, A.; López-Bucio, J.; Meza-Carmen, V.; Campos-García, J. Cytotoxicity of cyclodipeptides from Pseudomonas aeruginosa PAO1 leads to apoptosis in human cancer cell lines. Biomed. Res. Int. 2015, 2015, 197608. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhu, J. Recent development and optimization of Pseudomonas aeruginosa exotoxin immunotoxins in cancer therapeutic applications. Int. Immunopharmacol. 2021, 96, 107759. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, P. Helicobacter pylori and inflammation. Curr. Pharm. Des. 2010, 16, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Marshall, B.J. The Campylobacter pylori story. Scand. J. Gastroenterol. Suppl. 1988, 146, 58–66. [Google Scholar] [CrossRef]
- Rathbone, B.; Wyatt, J. Campylobacter pylori and precancerous lesions. Cancer Lett. 1988, 39, S14. [Google Scholar] [CrossRef]
- Correa, P.; Fox, J.; Fontham, E.; Ruiz, B.; Lin, Y.P.; Zavala, D.; Taylor, N.; Mackinley, D.; de Lima, E.; Portilla, H.; et al. Helicobacter pylori and gastric carcinoma. Serum antibody prevalence in populations with contrasting cancer risks. Cancer 1990, 66, 2569–2574. [Google Scholar]
- Loffeld, R.J.; Willems, I.; Flendrig, J.A.; Arends, J.W. Helicobacter pylori and gastric carcinoma. Histopathology 1990, 17, 537–541. [Google Scholar] [CrossRef]
- Chiba, T.; Marusawa, H.; Seno, H.; Watanabe, N. Mechanism for gastric cancer development by Helicobacter pylori infection. J. Gastroenterol. Hepatol. 2008, 23, 1175–1181. [Google Scholar]
- Soutto, M.; Bhat, N.; Khalafi, S.; Zhu, S.; Poveda, J.; Garcia-Buitrago, M.; Zaika, A.; El-Rifai, W. NF-kB-dependent activation of STAT3 by H. pylori is suppressed by TFF1. Cancer Cell Int. 2021, 21, 444. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, S.J.; Ko, Y.S.; Park, J.; Shin, D.H.; Hwang, I.C.; Han, S.Y.; Nam, S.Y.; Kim, M.A.; Chang, M.S.; et al. A synergistic interaction between transcription factors nuclear factor-κB and signal transducers and activators of transcription 3 promotes gastric cancer cell migration and invasion. BMC Gastroenterol. 2013, 13, 29. [Google Scholar] [CrossRef]
- Doger, F.K.; Meteoglu, I.; Ozkara, E.; Erkul, Z.K.; Okyay, P.; Yükselen, V. Expression of NF-κB in Helicobacter pylori infection. Dig. Dis. Sci. 2006, 51, 2306–2309. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, R.L.; Avé, P.; Ndiaye, D.; Bambou, J.C.; Huerre, M.R.; Philpott, D.J.; Mémet, S. NF-κB activation during acute Helicobacter pylori infection in mice. Infect. Immun. 2008, 76, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.; Yoshida, H.; Ogura, K.; Mitsuno, Y.; Hirata, Y.; Yamaji, Y.; Akanuma, M.; Shiratori, Y.; Omata, M.H. pylori activates NF-κB through a signaling pathway involving IκB kinases, NF-κB—Inducing kinase, TRAF2, and TRAF6 in gastric cancer cells. Gastroenterology 2000, 119, 97–108. [Google Scholar] [CrossRef]
- Lamb, A.; Chen, J.; Blanke, S.R.; Chen, L.F. Helicobacter pylori activates NF-κB by inducing Ubc13-mediated ubiquitination of lysine 158 of TAK1. J. Cell. Biochem. 2013, 114, 2284–2292. [Google Scholar] [CrossRef]
- Li, N.; Xie, C.; Lu, N.H. p53, a potential predictor of Helicobacter pylori infection-associated gastric carcinogenesis? Oncotarget. 2016, 7, 66276–66286. [Google Scholar] [CrossRef]
- Kodama, M.; Murakami, K.; Okimoto, T.; Sato, R.; Watanabe, K.; Fujioka, T. Expression of mutant type-p53 products in H. pylori-associated chronic gastritis. World J. Gastroenterol. 2007, 13, 1541–1546. [Google Scholar] [CrossRef][Green Version]
- Abu-Lubad, M.A.; Helaly, G.F.; Haddadin, W.J.; Jarajreh, D.A.K.; Aqel, A.A.; Al-Zeer, M.A. Loss of p53 expression in gastric epithelial cells of Helicobacter pylori-infected Jordanian patients. Int. J. Microbiol. 2022, 2022, 7779770. [Google Scholar] [CrossRef]
- Rahman, M.M.; Sarker, M.A.K.; Hossain, M.M.; Alam, M.S.; Islam, M.M.; Shirin, L.; Sultana, R.; Sultana, G.N.N. Association of p53 gene mutation with Helicobacter pylori infection in gastric cancer patients and its correlation with clinicopathological and environmental factors. World J. Oncol. 2019, 10, 46–54. [Google Scholar] [CrossRef]
- Bhardwaj, V.; Noto, J.M.; Wei, J.; Andl, C.; El-Rifai, W.; Peek, R.M.; Zaika, A.I. Helicobacter pylori bacteria alter the p53 stress response via ERK-HDM2 pathway. Oncotarget 2015, 6, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Dang, S.; Hou, P. Gene methylation in gastric cancer. Clin. Chim. Acta 2013, 424, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Compare, D.; Rocco, A.; Liguori, E.; D’Armiento, F.P.; Persico, G.; Masone, S.; Coppola-Bottazzi, E.; Suriani, R.; Romano, M.; Nardone, G. Global DNA hypomethylation is an early event in Helicobacter pylori-related gastric carcinogenesis. J. Clin. Pathol. 2011, 64, 677–682. [Google Scholar] [CrossRef]
- Zhang, B.G.; Hu, L.; Zang, M.D.; Wang, H.X.; Zhao, W.; Li, J.F.; Su, L.P.; Shao, Z.; Zhao, X.; Zhu, Z.G.; et al. Helicobacter pylori CagA induces tumor suppressor gene hypermethylation by upregulating DNMT1 via AKT-NFκB pathway in gastric cancer development. Oncotarget 2016, 7, 9788–9800. [Google Scholar] [CrossRef]
- Alipour, M. Molecular mechanism of Helicobacter pylori-induced gastric cancer. J. Gastrointest. Cancer 2021, 52, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.; Valenzuela Valderrama, M.; Bravo, J.; Quest, A.F.G. Helicobacter pylori and gastric cancer: Adaptive cellular mechanisms involved in disease progression. Front. Microbiol. 2018, 9, 5. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, L.; Ji, J.; Zhang, J.; Yan, M.; Zhang, J.; Liu, B.; Zhu, Z.; Yu, Y. ABO blood group system and gastric cancer: A case-control study and meta-analysis. Int. J. Mol. Sci. 2012, 13, 13308–13321. [Google Scholar] [CrossRef]
- Franchini, M.; Liumbruno, G.M.; Lippi, G. The prognostic value of ABO blood group in cancer patients. Blood Transfus. 2016, 14, 434–440. [Google Scholar]
- Salar, A. Gastric MALT lymphoma and Helicobacter pylori. Med. Clin. 2019, 152, 65–71. [Google Scholar] [CrossRef]
- Lehours, P.; Ménard, A.; Dupouy, S.; Bergey, B.; Richy, F.; Zerbib, F.; Ruskoné-Fourmestraux, A.; Delchier, J.C.; Mégraud, F. Evaluation of the association of nine Helicobacter pylori virulence factors with strains involved in low-grade gastric mucosa-associated lymphoid tissue lymphoma. Infect. Immun. 2004, 72, 880–888. [Google Scholar] [CrossRef]
- Floch, P.; Mégraud, F.; Lehours, P. Helicobacter pylori strains and gastric MALT lymphoma. Toxins 2017, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Suarez, F.; Lortholary, O.; Hermine, O.; Lecuit, M. Infection-associated lymphomas derived from marginal zone B cells: A model of antigen-driven lymphoproliferation. Blood 2006, 107, 3034–3044. [Google Scholar] [CrossRef] [PubMed]
- Marcelis, L.; Tousseyn, T.; Sagaert, X. MALT lymphoma as a model of chronic inflammation-induced gastric tumor development. Curr. Top. Microbiol. Immunol. 2019, 421, 77–106. [Google Scholar] [PubMed]
- Gong, E.J.; Ahn, J.Y.; Jung, H.Y.; Park, H.; Ko, Y.B.; Na, H.K.; Jung, K.W.; Kim, D.H.; Lee, J.H.; Choi, K.D.; et al. Helicobacter pylori eradication therapy is effective as the initial treatment for patients with H. pylori-negative and disseminated gastric mucosa-associated lymphoid tissue lymphoma. Gut Liver 2016, 10, 706–713. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, S.H.; Moon, H.S.; Sung, J.K.; Jeong, H.Y. Clinical outcome of eradication therapy for gastric mucosa-associated lymphoid tissue lymphoma according to H. pylori infection status. Gastroenterol. Res. Pract. 2016, 2016, 6794848. [Google Scholar] [CrossRef]
- Diaconu, S.; Predescu, A.; Moldoveanu, A.; Pop, C.S.; Fierbințeanu-Braticevici, C. Helicobacter pylori infection: Old and new. J. Med. Life 2017, 10, 112–117. [Google Scholar]
- Violeta Filip, P.; Cuciureanu, D.; Sorina Diaconu, L.; Maria Vladareanu, A.; Silvia Pop, C. MALT lymphoma: Epidemiology, clinical diagnosis and treatment. J. Med. Life 2018, 11, 187–193. [Google Scholar] [CrossRef]
- Herlevic, V.; Morris, J.D. Gastric Lymphoma; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Du, M.Q.; Atherton, J.C. Molecular subtyping of gastric MALT lymphomas: Implications for prognosis and management. Gut 2006, 55, 886–893. [Google Scholar] [CrossRef]
- Zhang, M. High antibiotic resistance rate: A difficult issue for Helicobacter pylori eradication treatment. World J. Gastroenterol. 2015, 21, 13432–13437. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhao, L.Y.; Liu, C.Q.; Pan, S.C.; Chen, X.L.; Liu, K.; Zhang, W.H.; Yang, K.; Chen, X.Z.; Zhang, B.; et al. Clinical characteristics and prognostic factors of primary gastric lymphoma: A retrospective study with 165 cases. Medicine 2016, 95, e4250. [Google Scholar] [CrossRef]
- Thieblemont, C.; Cascione, L.; Conconi, A.; Kiesewetter, B.; Raderer, M.; Gaidano, G.; Martelli, M.; Laszlo, D.; Coiffier, B.; Lopez Guillermo, A.; et al. A MALT lymphoma prognostic index. Blood 2017, 130, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.M.; Du, M.Q.; Dogan, A. Mucosa-associated lymphoid tissue (MALT) lymphoma: A practical guide for pathologists. J. Clin. Pathol. 2007, 60, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Quach, M.A.; Ake, C.D.; Chen, M.; Wang, J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J Gastrointest. Oncol. 2012, 3, 209–225. [Google Scholar] [PubMed]
- Wotherspoon, A. Pathology of extranodal marginal zone lymphoma at different anatomic sites. Ann. Lymphoma 2020, 4, 15. [Google Scholar] [CrossRef]
- Raderer, M.; Kiesewetter, B. What you always wanted to know about gastric MALT-lymphoma: A focus on recent developments. Ther. Adv. Med. Oncol. 2021, 13, 17588359211033825. [Google Scholar] [CrossRef]
- Akoum, R.; Serhal, W.; Farhat, H. Disseminated gastric MALT lymphoma with monoclonal gammopathy, t(11;18)(q21;q21), and subsequent development of T-large granular lymphocytic leukemia: A case report and review of the literature. Case Rep. Med. 2015, 2015, 953297. [Google Scholar] [CrossRef]
- Capelle, L.G.; de Vries, A.C.; Looman, C.W.; Casparie, M.K.; Boot, H.; Meijer, G.A.; Kuipers, E.J. Gastric MALT lymphoma: Epidemiology and high adenocarcinoma risk in a nation-wide study. Eur. J. Cancer 2008, 44, 2470–2476. [Google Scholar] [CrossRef]
- Zullo, A.; Rago, A.; Felici, S.; Licci, S.; Ridola, L.; Caravita di Toritto, T. Onset and progression of precancerous lesions on gastric mucosa of patients treated for gastric lymphoma. J. Gastrointestin. Liver Dis. 2020, 29, 27–31. [Google Scholar] [CrossRef]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global burden of 5 major types of gastrointestinal cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Mirzarazi, M.; Bashiri, S.; Hashemi, A.; Vahidi, M.; Kazemi, B.; Bandehpour, M. The OmpA of commensal Escherichia coli of CRC patients affects apoptosis of the HCT116 colon cancer cell line. BMC Microbiol. 2022, 22, 139. [Google Scholar] [CrossRef]
- Périchon, B.; Lichtl-Häfele, J.; Bergsten, E.; Delage, V.; Trieu-Cuot, P.; Sansonetti, P.; Sobhani, I.; Dramsi, S. Detection of Streptococcus gallolyticus and four other CRC-associated bacteria in patient stools reveals a potential “Driver” role for Enterotoxigenic Bacteroides fragilis. Front. Cell. Infect. Microbiol. 2022, 12, 794391. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jiao, X.; Zeng, M.; Fan, Z.; Li, X.; Yuan, Y.; Zhang, Q.; Xia, Y. Clinical significance of Fusobacterium nucleatum and microsatellite instability in evaluating colorectal cancer prognosis. Cancer Manag. Res. 2022, 14, 3021–3036. [Google Scholar] [CrossRef] [PubMed]
- Bertocchi, A.; Carloni, S.; Ravenda, P.S.; Bertalot, G.; Spadoni, I.; Lo Cascio, A.; Gandini, S.; Lizier, M.; Braga, D.; Asnicar, F.; et al. Gut vascular barrier impairment leads to intestinal bacteria dissemination and colorectal cancer metastasis to liver. Cancer Cell. 2021, 39, 708–724.e11. [Google Scholar] [CrossRef] [PubMed]
- Butt, J.; Jenab, M.; Werner, J.; Fedirko, V.; Weiderpass, E.; Dahm, C.C.; Tjønneland, A.; Olsen, A.; Boutron-Ruault, M.C.; Rothwell, J.A.; et al. Association of pre-diagnostic antibody responses to Escherichia coli and Bacteroides fragilis toxin proteins with colorectal cancer in a European cohort. Gut Microbes 2021, 13, 1903825. [Google Scholar] [CrossRef]
- Cuellar-Gómez, H.; Ocharán-Hernández, M.E.; Calzada-Mendoza, C.C.; Comoto-Santacruz, D.A. Association of Fusobacterium nucleatum infection and colorectal cancer: A Mexican study. Rev. Gastroenterol. Mex. 2022, 87, 277–284. [Google Scholar] [CrossRef]
- Khodaverdi, N.; Zeighami, H.; Jalilvand, A.; Haghi, F.; Hesami, N. High frequency of enterotoxigenic Bacteroides fragilis and Enterococcus faecalis in the paraffin-embedded tissues of Iranian colorectal cancer patients. BMC Cancer 2021, 21, 1353. [Google Scholar] [CrossRef]
- Kong, C.; Yan, X.; Zhu, Y.; Zhu, H.; Luo, Y.; Liu, P.; Ferrandon, S.; Kalady, M.F.; Gao, R.; He, J.; et al. Fusobacterium nucleatum promotes the development of colorectal cancer by activating a cytochrome P450/epoxyoctadecenoic acid axis via TLR4/Keap1/NRF2 signaling. Cancer Res. 2021, 81, 4485–4498. [Google Scholar] [CrossRef]
- Nardelli, C.; Granata, I.; Nunziato, M.; Setaro, M.; Carbone, F.; Zulli, C.; Pilone, V.; Capoluongo, E.D.; De Palma, G.D.; Corcione, F.; et al. 16S rRNA of mucosal colon microbiome and CCL2 circulating levels are potential biomarkers in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 10747. [Google Scholar] [CrossRef]
- Pignatelli, P.; Iezzi, L.; Pennese, M.; Raimondi, P.; Cichella, A.; Bondi, D.; Grande, R.; Cotellese, R.; Di Bartolomeo, N.; Innocenti, P.; et al. The potential of colonic tumor tissue Fusobacterium nucleatum to predict staging and its interplay with oral abundance in colon cancer patients. Cancers 2021, 13, 1032. [Google Scholar] [CrossRef]
- Iyadorai, T.; Mariappan, V.; Vellasamy, K.M.; Wanyiri, J.W.; Roslani, A.C.; Lee, G.K.; Sears, C.; Vadivelu, J. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS ONE 2020, 15, e0228217. [Google Scholar] [CrossRef]
- Zamani, S.; Taslimi, R.; Sarabi, A.; Jasemi, S.; Sechi, L.A.; Feizabadi, M.M. Enterotoxigenic Bacteroides fragilis: A possible etiological candidate for bacterially-induced colorectal precancerous and cancerous lesions. Front. Cell. Infect. Microbiol. 2020, 9, 449. [Google Scholar] [CrossRef]
- Alomair, A.O.; Masoodi, I.; Alyamani, E.J.; Allehibi, A.A.; Qutub, A.N.; Alsayari, K.N.; Altammami, M.A.; Alshanqeeti, A.S. Colonic mucosal microbiota in colorectal cancer: A single-center metagenomic study in Saudi Arabia. Gastroenterol. Res. Pract. 2018, 2018, 5284754. [Google Scholar] [CrossRef]
- Kwong, T.N.Y.; Wang, X.; Nakatsu, G.; Chow, T.C.; Tipoe, T.; Dai, R.Z.W.; Tsoi, K.K.K.; Wong, M.C.S.; Tse, G.; Chan, M.T.V.; et al. Association between bacteremia from specific microbes and subsequent diagnosis of colorectal cancer. Gastroenterology 2018, 155, 383–390.e8. [Google Scholar] [CrossRef]
- Proença, M.A.; Biselli, J.M.; Succi, M.; Severino, F.E.; Berardinelli, G.N.; Caetano, A.; Reis, R.M.; Hughes, D.J.; Silva, A.E. Relationship between Fusobacterium nucleatum, inflammatory mediators and microRNAs in colorectal carcinogenesis. World J. Gastroenterol. 2018, 24, 5351–5365. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.S.; Nishihara, R.; Cao, Y.; Song, M.; Mima, K.; Qian, Z.R.; Nowak, J.A.; Kosumi, K.; Hamada, T.; Masugi, Y.; et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by Fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017, 3, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Jiang, B. Analysis of mucosa-associated microbiota in colorectal cancer. Med. Sci. Monit. 2017, 23, 4422–4430. [Google Scholar] [CrossRef]
- Mima, K.; Nishihara, R.; Qian, Z.R.; Cao, Y.; Sukawa, Y.; Nowak, J.A.; Yang, J.; Dou, R.; Masugi, Y.; Song, M.; et al. Fusobacterium nucleatum in colorectal carcinoma tissue and patient prognosis. Gut 2016, 65, 1973–1980. [Google Scholar] [CrossRef] [PubMed]
- Magdy, A.; Elhadidy, M.; Abd Ellatif, M.E.; El Nakeeb, A.; Abdallah, E.; Thabet, W.; Youssef, M.; Khafagy, W.; Morshed, M.; Farid, M. Enteropathogenic Escherichia coli (EPEC): Does it have a role in colorectal tumourigenesis? A Prospective Cohort Study. Int. J. Surg. 2015, 18, 169–173. [Google Scholar] [CrossRef]
- Bonnet, M.; Buc, E.; Sauvanet, P.; Darcha, C.; Dubois, D.; Pereira, B.; Déchelotte, P.; Bonnet, R.; Pezet, D.; Darfeuille-Michaud, A. Colonization of the human gut by E. coli and colorectal cancer risk. Clin. Cancer Res. 2014, 20, 859–867. [Google Scholar] [CrossRef]
- Kohoutova, D.; Smajs, D.; Moravkova, P.; Cyrany, J.; Moravkova, M.; Forstlova, M.; Cihak, M.; Rejchrt, S.; Bures, J. Escherichia coli strains of phylogenetic group B2 and D and bacteriocin production are associated with advanced colorectal neoplasia. BMC Infect. Dis. 2014, 14, 733. [Google Scholar] [CrossRef]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Brunham, R.C.; McClarty, G. Chlamydia. In Sexually Transmitted Diseases; Stanberry, L.R., Bernstein, D.I., Eds.; Academic Press: London, UK, 2000; pp. 339–367. [Google Scholar]
- Nogueira, A.T.; Braun, K.M.; Carabeo, R.A. Characterization of the growth of Chlamydia trachomatis in in vitro-generated stratified epithelium. Front. Cell. Infect. Microbiol. 2017, 7, 438. [Google Scholar] [CrossRef] [PubMed]
- Gitsels, A.; Sanders, N.; Vanrompay, D. Chlamydial infection from outside to inside. Front. Microbiol. 2019, 10, 2329. [Google Scholar] [CrossRef] [PubMed]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Microbiol. 2016, 14, 385–400. [Google Scholar] [CrossRef] [PubMed]
- Saka, H.A.; Valdivia, R.H. Acquisition of nutrients by Chlamydiae: Unique challenges of living in an intracellular compartment. Curr. Opin. Microbiol. 2010, 13, 4–10. [Google Scholar] [CrossRef]
- Byrne, G.I. Chlamydia trachomatis strains and virulence: Rethinking links to infection prevalence and disease severity. J. Infect. Dis. 2010, 201 (Suppl. 2), S126–S133. [Google Scholar] [CrossRef]
- Zhong, G. Chlamydial plasmid-dependent pathogenicity. Trends Microbiol. 2017, 25, 141–152. [Google Scholar] [CrossRef]
- Sturdevant, G.L.; Zhou, B.; Carlson, J.H.; Whitmire, W.M.; Song, L.; Caldwell, H.D. Infectivity of urogenital Chlamydia trachomatis plasmid-deficient, CT135-null, and double-deficient strains in female mice. Pathog. Dis. 2014, 71, 90–92. [Google Scholar] [CrossRef]
- Borges, V.; Pinheiro, M.; Antelo, M.; Sampaio, D.A.; Vieira, L.; Ferreira, R.; Nunes, A.; Almeida, F.; Mota, L.J.; Borrego, M.J.; et al. Chlamydia trachomatis in vivo to in vitro transition reveals mechanisms of phase variation and down-regulation of virulence factors. PLoS ONE 2015, 10, e0133420. [Google Scholar] [CrossRef]
- Clifton, D.R.; Fields, K.A.; Grieshaber, S.S.; Dooley, C.A.; Fischer, E.R.; Mead, D.J.; Carabeo, R.A.; Hackstadt, T. A chlamydial type III translocated protein is tyrosine-phosphorylated at the site of entry and associated with recruitment of actin. Proc. Natl. Acad. Sci. USA 2004, 101, 10166–10171. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Park, J.; Thomas, M.; Cruz, E.; Cardona, O.; Kang, H.; Jewett, T. Biophysical characterization of actin bundles generated by the Chlamydia trachomatis Tarp effector. Biochem. Biophys. Res. Commun. 2018, 500, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Caven, L.; Carabeo, R.A. Pathogenic puppetry: Manipulation of the host actin cytoskeleton by Chlamydia trachomatis. Int. J. Mol. Sci. 2019, 21, 90. [Google Scholar] [CrossRef] [PubMed]
- Mölleken, K.; Becker, E.; Hegemann, J.H. The Chlamydia pneumoniae invasin protein Pmp21 recruits the EGF receptor for host cell entry. PLoS Pathog. 2013, 9, e1003325. [Google Scholar] [CrossRef]
- Favaroni, A.; Trinks, A.; Weber, M.; Hegemann, J.H.; Schnee, C. Pmp repertoires influence the different infectious potential of avian and mammalian Chlamydia psittaci strains. Front. Microbiol. 2021, 12, 656209. [Google Scholar] [CrossRef]
- Niessner, A.; Kaun, C.; Zorn, G.; Speidl, W.; Türel, Z.; Christiansen, G.; Pedersen, A.S.; Birkelund, S.; Simon, S.; Georgopoulos, A.; et al. Polymorphic membrane protein (PMP) 20 and PMP 21 of Chlamydia pneumoniae induce proinflammatory mediators in human endothelial cells in vitro by activation of the nuclear factor-kappaB pathway. J. Infect. Dis. 2003, 188, 108–113. [Google Scholar] [CrossRef]
- Nairz, M.; Dichtl, S.; Schroll, A.; Haschka, D.; Tymoszuk, P.; Theurl, I.; Weiss, G. Iron and innate antimicrobial immunity-depriving the pathogen, defending the host. J. Trace Elem. Med. Biol. 2018, 48, 118–133. [Google Scholar] [CrossRef]
- Pokorzynski, N.D.; Thompson, C.C.; Carabeo, R.A. Ironing out the unconventional mechanisms of iron acquisition and gene regulation in Chlamydia. Front. Cell. Infect. Microbiol. 2017, 7, 394. [Google Scholar] [CrossRef]
- Thompson, C.C.; Carabeo, R.A. An optimal method of iron starvation of the obligate intracellular pathogen, Chlamydia trachomatis. Front. Microbiol. 2011, 2, 20. [Google Scholar] [CrossRef]
- Pokorzynski, N.D.; Brinkworth, A.J.; Carabeo, R. A bipartite iron-dependent transcriptional regulation of the tryptophan salvage pathway in Chlamydia trachomatis. eLife 2019, 8, e42295. [Google Scholar] [CrossRef]
- Pokorzynski, N.D.; Hatch, N.D.; Ouellette, S.P.; Carabeo, R.A. The iron-dependent repressor YtgR is a tryptophan-dependent attenuator of the trpRBA operon in Chlamydia trachomatis. Nat. Commun. 2020, 11, 6430. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef] [PubMed]
- Sessa, R.; Nicoletti, M.; Di Pietro, M.; Schiavoni, G.; Santino, I.; Zagaglia, C.; Del Piano, M.; Cipriani, P. Chlamydia pneumoniae and atherosclerosis: Current state and future prospectives. Int. J. Immunopathol. Pharmacol. 2009, 22, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, L.; Liu, J.; Ma, L.; Wang, H.; Zheng, N.; Chen, X.; Shen, B.; Xu, Z.; Zhang, L. Chlamydia pneumoniae infection promotes vascular endothelial cell angiogenesis through an IQGAP1-related signaling pathway. Int. J. Med. Microbiol. 2017, 307, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ning, B.; Li, Y. Study on the relationship between Chlamydia infection and otitis media with effusion. Zhonghua Er Bi Yan Hou Ke Za Zhi (Chin. J. Otorhinolaryngol.) 1999, 34, 92–94. [Google Scholar]
- Keles, E.; Bulut, Y.; Kaygusuz, I.; Karlidag, T.; Yalçin, S.; Ozdarendeli, A.; Alpay, H.C. Identification of Chlamydia trachomatis with polymerase chain reaction in middle ear fluid in otitis media with effusion. Indian Pediatr. 2005, 42, 686–691. [Google Scholar]
- Xu, X.; Liu, Z.; Xiong, W.; Qiu, M.; Kang, S.; Xu, Q.; Cai, L.; He, F. Combined and interaction effect of Chlamydia pneumoniae infection and smoking on lung cancer: A case-control study in Southeast China. BMC Cancer 2020, 20, 903. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Gaydos, C.A.; Agreda, P.; Holden, J.P.; Chatterjee, N.; Goedert, J.J.; Caporaso, N.E.; Engels, E.A. Chlamydia pneumoniae infection and risk for lung cancer. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1498–1505. [Google Scholar] [CrossRef]
- Liu, Z.; Su, M.; Yu, S.C.; Yin, Z.H.; Zhou, B.S. Association of Chlamydia pneumoniae immunoglobulin G antibodies with the risk of lung cancer among non-smoking women in Liaoning, China. Thorac. Cancer 2010, 1, 126–129. [Google Scholar] [CrossRef]
- Anttila, T.; Koskela, P.; Leinonen, M.; Laukkanen, P.; Hakulinen, T.; Lehtinen, M.; Pukkala, E.; Paavonen, J.; Saikku, P. Chlamydia pneumoniae infection and the risk of female early-onset lung cancer. Int. J. Cancer 2003, 107, 681–682. [Google Scholar] [CrossRef]
- Bhardwaj, M.; Sharma, A.; Sen, S.; Kumar, L.; Satpathy, G.; Kashyap, S.; Pushker, N.; Singh, V.K.; Rai, A. Chlamydia and ocular adnexal lymphomas: An Indian experience. Exp. Mol. Pathol. 2016, 101, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Aigelsreiter, A.; Gerlza, T.; Deutsch, A.J.; Leitner, E.; Beham-Schmid, C.; Beham, A.; Popper, H.; Borel, N.; Pospischil, A.; Raderer, M.; et al. Chlamydia psittaci Infection in nongastrointestinal extranodal MALT lymphomas and their precursor lesions. Am. J. Clin. Pathol. 2011, 135, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Carugi, A.; Onnis, A.; Antonicelli, G.; Rossi, B.; Mannucci, S.; Luzzi, A.; Lazzi, S.; Bellan, C.; Tosi, G.M.; Sayed, S.; et al. Geographic variation and environmental conditions as cofactors in Chlamydia psittaci association with ocular adnexal lymphomas: A comparison between Italian and African samples. Hematol. Oncol. 2010, 28, 20–26. [Google Scholar] [PubMed]
- Ferreri, A.J.; Dolcetti, R.; Dognini, G.P.; Malabarba, L.; Vicari, N.; Pasini, E.; Ponzoni, M.; Cangi, M.G.; Pecciarini, L.; Resti, A.G.; et al. Chlamydophila psittaci is viable and infectious in the conjunctiva and peripheral blood of patients with ocular adnexal lymphoma: Results of a single-center prospective case-control study. Int. J. Cancer 2008, 123, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Idahl, A.; Le Cornet, C.; González Maldonado, S.; Waterboer, T.; Bender, N.; Tjønneland, A.; Hansen, L.; Boutron-Ruault, M.C.; Fournier, A.; Kvaskoff, M.; et al. Serologic markers of Chlamydia trachomatis and other sexually transmitted infections and subsequent ovarian cancer risk: Results from the EPIC cohort. Int. J. Cancer 2020, 147, 2042–2052. [Google Scholar] [CrossRef]
- Fortner, R.T.; Terry, K.L.; Bender, N.; Brenner, N.; Hufnagel, K.; Butt, J.; Waterboer, T.; Tworoger, S.S. Sexually transmitted infections and risk of epithelial ovarian cancer: Results from the Nurses’ Health Studies. Br. J. Cancer 2019, 120, 855–860. [Google Scholar] [CrossRef]
- Trabert, B.; Waterboer, T.; Idahl, A.; Brenner, N.; Brinton, L.A.; Butt, J.; Coburn, S.B.; Hartge, P.; Hufnagel, K.; Inturrisi, F.; et al. Antibodies against Chlamydia trachomatis and ovarian cancer risk in two independent populations. J. Natl. Cancer Inst. 2019, 111, 129–136. [Google Scholar] [CrossRef]
- Ness, R.B.; Goodman, M.T.; Shen, C.; Brunham, R.C. Serologic evidence of past infection with Chlamydia trachomatis, in relation to ovarian cancer. J. Infect. Dis. 2003, 187, 1147–1152. [Google Scholar] [CrossRef]
- Jensen, K.E.; Thomsen, L.T.; Schmiedel, S.; Frederiksen, K.; Norrild, B.; van den Brule, A.; Iftner, T.; Kjær, S.K. Chlamydia trachomatis and risk of cervical intraepithelial neoplasia grade 3 or worse in women with persistent human papillomavirus infection: A cohort study. Sex. Transm. Infect. 2014, 90, 550–555. [Google Scholar] [CrossRef]
- Luostarinen, T.; Namujju, P.B.; Merikukka, M.; Dillner, J.; Hakulinen, T.; Koskela, P.; Paavonen, J.; Surcel, H.M.; Lehtinen, M. Order of HPV/Chlamydia infections and cervical high-grade precancer risk: A case-cohort study. Int. J. Cancer. 2013, 133, 1756–1759. [Google Scholar] [CrossRef]
- Olejek, A.; Kozak-Darmas, I.; Kellas-Sleczka, S.; Jarek, A.; Wiczkowski, A.; Krol, W.; Stencel-Gabriel, K. Chlamydia trachomatis infection in women with lichen sclerosus vulvae and vulvar cancer. Neuro. Endocrinol. Lett. 2009, 30, 671–674. [Google Scholar] [PubMed]
- Alghamdi, S. Microbiological profile and antibiotic vulnerability of bacterial isolates from cancer patients. Cell. Mol. Biol. 2021, 67, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Bhat, V.; Gupta, S.; Kelkar, R.; Biswas, S.; Khattry, N.; Moiyadi, A.; Bhat, P.; Ambulkar, R.; Chavan, P.; Chiplunkar, S.; et al. Bacteriological profile and antibiotic susceptibility patterns of clinical isolates in a tertiary care cancer center. Indian J. Med. Paediatr. Oncol. 2016, 37, 20–24. [Google Scholar] [CrossRef]
- Maschmeyer, G.; Braveny, I. Review of the incidence and prognosis of Pseudomonas aeruginosa infections in cancer patients in the 1990s. Eur. J. Clin. Microbiol. Infect. Dis. 2000, 19, 915–925. [Google Scholar] [CrossRef]
- Samonis, G.; Vardakas, K.Z.; Kofteridis, D.P.; Dimopoulou, D.; Andrianaki, A.M.; Chatzinikolaou, I.; Katsanevaki, E.; Maraki, S.; Falagas, M.E. Characteristics, risk factors and outcomes of adult cancer patients with extensively drug-resistant Pseudomonas aeruginosa infections. Infection 2014, 42, 721–728. [Google Scholar] [CrossRef]
- Paprocka, P.; Durnaś, B.; Mańkowska, A.; Król, G.; Wollny, T.; Bucki, R. Pseudomonas aeruginosa infections in cancer patients. Pathogens. 2022, 11, 679. [Google Scholar] [CrossRef]
- Bodey, G.P. Pseudomonas aeruginosa infections in cancer patients: Have they gone away? Curr. Opin. Infect. Dis. 2001, 14, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Parkins, M.D.; Somayaji, R.; Waters, V.J. Epidemiology, biology, and impact of clonal Pseudomonas aeruginosa infections in cystic fibrosis. Clin. Microbiol. Rev. 2018, 31, e00019-18. [Google Scholar] [CrossRef]
- Mellemkjaer, L.; Hammarstrom, L.; Andersen, V.; Yuen, J.; Heilmann, C.; Barington, T.; Bjorkander, J.; Olsen, J.H. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: A combined Danish and Swedish study. Clin. Exp. Immunol. 2002, 130, 495–500. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Mansouri, D.; Khosravi, A.; Garssen, J.; Velayati, A.; Adcock, I.M. Cancers related to immunodeficiencies: Update and perspectives. Front. Immunol. 2016, 7, 365. [Google Scholar] [CrossRef]
- Yarchoan, R.; Uldrick, T.S. HIV-associated cancers and related diseases. N. Engl. J. Med. 2018, 378, 2145. [Google Scholar] [CrossRef] [PubMed]
- Appelt, D.; Fuchs, T.; Steinkamp, G.; Ellemunter, H. Malignancies in patients with cystic fibrosis: A case series. J. Med. Case Rep. 2022, 16, 27. [Google Scholar] [CrossRef] [PubMed]
- Maisonneuve, P.; Marshall, B.C.; Knapp, E.A.; Lowenfels, A.B. Cancer risk in cystic fibrosis: A 20-year nationwide study from the United States. J. Natl. Cancer Inst. 2013, 105, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Johannesson, M.; Askling, J.; Montgomery, S.M.; Ekbom, A.; Bahmanyar, S. Cancer risk among patients with cystic fibrosis and their first-degree relatives. Int. J. Cancer 2009, 125, 2953–2956. [Google Scholar] [CrossRef]
- Neglia, J.P.; FitzSimmons, S.C.; Maisonneuve, P.; Schöni, M.H.; Schöni-Affolter, F.; Corey, M.; Lowenfels, A.B. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N. Engl. J. Med. 1995, 332, 494–499. [Google Scholar] [CrossRef]
- Scott, P.; Anderson, K.; Singhania, M.; Cormier, R. Cystic fibrosis, CFTR, and colorectal cancer. Int. J. Mol. Sci. 2020, 21, 2891. [Google Scholar] [CrossRef]
- Silva-García, O.; Valdez-Alarcón, J.J.; Baizabal-Aguirre, V.M. Wnt/β-catenin signaling as a molecular target by pathogenic bacteria. Front. Immunol. 2019, 10, 2135. [Google Scholar] [CrossRef]
- de Sousa, T.; Hébraud, M.; Dapkevicius, M.L.N.E.; Maltez, L.; Pereira, J.E.; Capita, R.; Alonso-Calleja, C.; Igrejas, G.; Poeta, P. Genomic and metabolic characteristics of the pathogenicity in Pseudomonas aeruginosa. Int. J. Mol. Sci. 2021, 22, 12892. [Google Scholar] [CrossRef]
- Liao, C.; Huang, X.; Wang, Q.; Yao, D.; Lu, W. Virulence factors of Pseudomonas aeruginosa and antivirulence strategies to combat its drug resistance. Front. Cell. Infect. Microbiol. 2022, 12, 926758. [Google Scholar] [CrossRef]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanisms and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Busch, W. Aus der sitzung der medicinischen section vom (from the meeting of the medical section of) 13 November 1867. Berlin Klin Wochenschr (Berl. Clin. Wkly.) 1868, 5, 137. [Google Scholar]
- Yang, X.; Kessler, E.; Su, L.J.; Thorburn, A.; Frankel, A.E.; Li, Y.; La Rosa, F.G.; Shen, J.; Li, C.Y.; Varella-Garcia, M.; et al. Diphtheria toxin-epidermal growth factor fusion protein DAB389EGF for the treatment of bladder cancer. Clin. Cancer Res. 2013, 19, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Frankel, A.E.; Powell, B.L.; Hall, P.D.; Case, L.D.; Kreitman, R.J. Phase I trial of a novel diphtheria toxin/granulocyte macrophage colony-stimulating factor fusion protein (DT388GMCSF) for refractory or relapsed acute myeloid leukemia. Clin. Cancer Res. 2002, 8, 1004–1013. [Google Scholar] [PubMed]
- Kawai, H.; Ando, K.; Maruyama, D.; Yamamoto, K.; Kiyohara, E.; Terui, Y.; Fukuhara, N.; Miyagaki, T.; Tokura, Y.; Sakata-Yanagimoto, M.; et al. Phase II study of E7777 in Japanese patients with relapsed/refractory peripheral and cutaneous T-cell lymphoma. Cancer Sci. 2021, 112, 2426–2435. [Google Scholar] [CrossRef]
- Ray, T.; Chakrabarti, M.K.; Pal, A. Hemagglutinin protease secreted by V. cholerae induced apoptosis in breast cancer cells by ROS mediated intrinsic pathway and regresses tumor growth in mice model. Apoptosis 2016, 21, 143–154. [Google Scholar] [CrossRef]
- Liu-Chittenden, Y.; Jain, M.; Kumar, P.; Patel, D.; Aufforth, R.; Neychev, V.; Sadowski, S.; Gara, S.K.; Joshi, B.H.; Cottle-Delisle, C.; et al. Phase I trial of systemic intravenous infusion of interleukin-13-Pseudomonas exotoxin in patients with metastatic adrenocortical carcinoma. Cancer Med. 2015, 4, 1060–1068. [Google Scholar] [CrossRef]
- Hassan, R.; Alewine, C.; Mian, I.; Spreafico, A.; Siu, L.L.; Gomez-Roca, C.; Delord, J.P.; Italiano, A.; Lassen, U.; Soria, J.C.; et al. Phase 1 study of the immunotoxin LMB-100 in patients with mesothelioma and other solid tumors expressing mesothelin. Cancer 2020, 126, 4936–4947. [Google Scholar] [CrossRef]
- Hotz, B.; Backer, M.V.; Backer, J.M.; Buhr, H.J.; Hotz, H.G. Specific targeting of tumor endothelial cells by a shiga-like toxin-vascular endothelial growth factor fusion protein as a novel treatment strategy for pancreatic cancer. Neoplasia 2010, 12, 797–806. [Google Scholar] [CrossRef]
- Monteillier, A.; Allard, P.M.; Gindro, K.; Wolfender, J.L.; Cuendet, M. Lung cancer chemopreventive activity of patulin isolated from Penicillium vulpinum. Molecules 2018, 23, 636. [Google Scholar] [CrossRef]
- Menon, K.; Alvarez, E.; Forler, P.; Phares, V.; Amsrud, T.; Shih, C.; Al-Awar, R.; Teicher, B.A. Antitumor activity of cryptophycins: Effect of infusion time and combination studies. Cancer Chemother. Pharmacol. 2000, 46, 142–149. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Makker, V.; Colombo, N.; Casado Herráez, A.; Santin, A.D.; Colomba, E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus pembrolizumab for advanced endometrial cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Hashimi, S.M.; Grant, B.; Alqurashi, N.; Alowaidi, F.; Wei, M.Q. EGF ligand fused to truncated Pseudomonas aeruginosa exotoxin A specifically targets and inhibits EGFR-positive cancer cells. Oncol. Rep. 2018, 40, 2690–2697. [Google Scholar] [CrossRef] [PubMed]
- Twigg, M.S.; Adu, S.A.; Sugiyama, S.; Marchant, R.; Banat, I.M. Mono-rhamnolipid biosurfactants synthesized by Pseudomonas aeruginosa detrimentally affect colorectal cancer cells. Pharmaceutics 2022, 14, 2799. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.K.; Naffouje, S.A.; Goto, M.; Wang, J.; Christov, K.; Rademacher, D.J.; Green, A.; Stecenko, A.A.; Chakrabarty, A.M.; Das Gupta, T.K.; et al. Cross-talk between cancer and Pseudomonas aeruginosa mediates tumor suppression. Commun. Biol. 2023, 6, 16. [Google Scholar] [CrossRef]
- Pang, Z.; Gu, M.D.; Tang, T. Pseudomonas aeruginosa in cancer therapy: Current knowledge, challenges and future perspectives. Front. Oncol. 2022, 12, 891187. [Google Scholar] [CrossRef]
- Ephraim, R.; Fraser, S.; Nurgali, K.; Apostolopoulos, V. Checkpoint markers and tumor microenvironment: What do we know? Cancers 2022, 14, 3788. [Google Scholar] [CrossRef]
- Gomatou, G.; Syrigos, N.; Vathiotis, I.A.; Kotteas, E.A. Tumor dormancy: Implications for invasion and metastasis. Int. J. Mol. Sci. 2021, 22, 4862. [Google Scholar] [CrossRef]
- Dropulic, L.K.; Lederman, H.M. Overview of infections in the immunocompromised host. Microbiol. Spectr. 2016, 4, 1–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liu, Z.; Li, B.; Liu, Y.E.; Wang, P. Immunoregulation in cancer-associated cachexia. J. Adv. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Mi, L.; Wang, Y.; Zhang, D. Detection of bacterial DNA in serum from colon cancer patients: Association with cytokine levels and cachexia. J. Cancer Ther. Res. 2012, 1, 19. [Google Scholar] [CrossRef][Green Version]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; Trevino, J.G. The microbiota and cancer cachexia. Int. J. Mol. Sci. 2019, 20, 6267. [Google Scholar] [CrossRef] [PubMed]
- Nicol, A.F.; Andrade, C.V.; Russomano, F.B.; Rodrigues, L.L.; Oliveira, N.S.; Provance, D.W., Jr. HPV vaccines: A controversial issue? Braz. J. Med. Biol. Res. 2016, 49, e5060. [Google Scholar] [CrossRef] [PubMed]
- Elwood, J.M.; Ameratunga, R. Autoimmune diseases after hepatitis B immunization in adults: Literature review and meta-analysis, with reference to ‘autoimmune/autoinflammatory syndrome induced by adjuvants’ (ASIA). Vaccine 2018, 36, 5796–5802. [Google Scholar] [CrossRef]
- Gidengil, C.; Goetz, M.B.; Newberry, S.; Maglione, M.; Hall, O.; Larkin, J.; Motala, A.; Hempel, S. Safety of vaccines used for routine immunization in the United States: An updated systematic review and meta-analysis. Vaccine 2021, 39, 3696–3716. [Google Scholar] [CrossRef]
- Eun, B.W.; Bahar, E.; Xavier, S.; Kim, H.; Borys, D. Post-marketing surveillance study of the safety of the HPV-16/18 vaccine in Korea (2017–2021). Hum. Vaccin. Immunother. 2023, 19, 2184756. [Google Scholar] [CrossRef]
- Seida, I.; Seida, R.; Elsalti, A.; Mahroum, N. Vaccines and autoimmunity-from side effects to ASIA syndrome. Medicina 2023, 59, 364. [Google Scholar] [CrossRef]
- Shuel, S.L. Targeted cancer therapies: Clinical pearls for primary care. Can. Fam. Physician 2022, 68, 515–518. [Google Scholar] [CrossRef]
- Ray, A. Tumor-linked HER2 expression: Association with obesity and lipid-related microenvironment. Horm. Mol. Biol. Clin. Investig. 2017, 32, 20170020. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.; Finikarides, L.; Freeman, A.L.J. The adverse effects of trastuzumab-containing regimes as a therapy in breast cancer: A piggy-back systematic review and meta-analysis. PLoS ONE 2022, 17, e0275321. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Sofiya, L.; Sykiotis, G.P.; Lamine, F.; Maillard, M.; Fraga, M.; Shabafrouz, K.; Ribi, C.; Cairoli, A.; Guex-Crosier, Y.; et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol. 2019, 16, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Babacan, N.A.; Tanvetyanon, T. Superimposed Clostridium difficile infection during checkpoint inhibitor immunotherapy-induced colitis. J. Immunother. 2019, 42, 350–353. [Google Scholar] [CrossRef]
- Morelli, T.; Fujita, K.; Redelman-Sidi, G.; Elkington, P.T. Infections due to dysregulated immunity: An emerging complication of cancer immunotherapy. Thorax 2022, 77, 304–311. [Google Scholar] [CrossRef]
- Rolston, K.V. Infections in cancer patients with solid tumors: A review. Infect. Dis. Ther. 2017, 6, 69–83. [Google Scholar] [CrossRef]
- Gudiol, C.; Carratalà, J. Antibiotic resistance in cancer patients. Expert Rev. Anti Infect. Ther. 2014, 12, 1003–1016. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Current trends in the epidemiology of nosocomial bloodstream infections in patients with hematological malignancies and solid neoplasms in hospitals in the United States. Clin. Infect. Dis. 2003, 36, 1103–1110. [Google Scholar] [CrossRef]
- Rolston, K.V. New antimicrobial agents for the treatment of bacterial infections in cancer patients. Hematol. Oncol. 2009, 27, 107–114. [Google Scholar] [CrossRef]
- Itoh, N.; Akazawa, N.; Kanawaku, E.; Murakami, H.; Ishibana, Y.; Kawamura, D.; Kawabata, T.; Mori, K.; Kodama, E.N.; Ohmagari, N. Effects of infectious disease consultation and antimicrobial stewardship program at a Japanese cancer center: An interrupted time-series analysis. PLoS ONE 2022, 17, e0263095. [Google Scholar] [CrossRef]
- Molina, J.; Noguer, M.; Lepe, J.A.; Pérez-Moreno, M.A.; Aguilar-Guisado, M.; Lasso de la Vega, R.; Peñalva, G.; Crespo-Rivas, J.C.; Gil-Navarro, M.V.; Salvador, J.; et al. Clinical impact of an educational antimicrobial stewardship program associated with infectious diseases consultation targeting patients with cancer: Results of a 9-year quasi-experimental study with an interrupted time-series analysis. J. Infect. 2019, 79, 206–211. [Google Scholar] [CrossRef]
- Rosa, R.G.; Goldani, L.Z.; dos Santos, R.P. Association between adherence to an antimicrobial stewardship program and mortality among hospitalised cancer patients with febrile neutropaenia: A prospective cohort study. BMC Infect. Dis. 2014, 14, 286. [Google Scholar] [CrossRef] [PubMed]
- Rolfes, N.; Lümmen, G. Management of febrile neutropenia. Urologe A 2011, 50, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Nouér, S.A.; Nucci, M.; Anaissie, E. Tackling antibiotic resistance in febrile neutropenia: Current challenges with and recommendations for managing infections with resistant Gram-negative organisms. Expert Rev. Hematol. 2015, 8, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Feld, R. Bloodstream infections in cancer patients with febrile neutropenia. Int. J. Antimicrob. Agents 2008, 32 (Suppl. 1), S30–S33. [Google Scholar] [CrossRef]
- Kouranos, V.; Dimopoulos, G.; Vassias, A.; Syrigos, K.N. Chemotherapy-induced neutropenia in lung cancer patients: The role of antibiotic prophylaxis. Cancer Lett. 2011, 313, 9–14. [Google Scholar] [CrossRef]
- Egan, G.; Robinson, P.D.; Martinez, J.P.D.; Alexander, S.; Ammann, R.A.; Dupuis, L.L.; Fisher, B.T.; Lehrnbecher, T.; Phillips, B.; Cabral, S.; et al. Efficacy of antibiotic prophylaxis in patients with cancer and hematopoietic stem cell transplantation recipients: A systematic review of randomized trials. Cancer Med. 2019, 8, 4536–4546. [Google Scholar] [CrossRef]
- Pascoe, J.; Steven, N. Antibiotics for the prevention of febrile neutropenia. Curr. Opin. Hematol. 2009, 16, 48–52. [Google Scholar] [CrossRef]
- Itoh, N.; Hadano, Y.; Saito, S.; Myokai, M.; Nakamura, Y.; Kurai, H. Intravenous to oral switch therapy in cancer patients with catheter-related bloodstream infection due to methicillin-sensitive Staphylococcus aureus: A single-center retrospective observational study. PLoS ONE 2018, 13, e0207413. [Google Scholar] [CrossRef]
- Coussement, J.; Lindsay, J.; Teh, B.W.; Slavin, M. Choice and duration of antifungal prophylaxis and treatment in high-risk haematology patients. Curr. Opin. Infect. Dis. 2021, 34, 297–306. [Google Scholar] [CrossRef]
- Stemler, J.; de Jonge, N.; Skoetz, N.; Sinkó, J.; Brüggemann, R.J.; Busca, A.; Ben-Ami, R.; Ráčil, Z.; Piechotta, V.; Lewis, R.; et al. Antifungal prophylaxis in adult patients with acute myeloid leukaemia treated with novel targeted therapies: A systematic review and expert consensus recommendation from the European Hematology Association. Lancet Haematol. 2022, 9, e361–e373. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.V.; Ullmann, A.J.; Almyroudis, N.G.; Segal, B.H. Broad-spectrum antifungal prophylaxis in patients with cancer at high risk for invasive mold infections: Point. J. Natl. Compr. Canc. Netw. 2008, 6, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Pathakumari, B.; Liang, G.; Liu, W. Immune defence to invasive fungal infections: A comprehensive review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef] [PubMed]
- Robenshtok, E.; Gafter-Gvili, A.; Goldberg, E.; Weinberger, M.; Yeshurun, M.; Leibovici, L.; Paul, M. Antifungal prophylaxis in cancer patients after chemotherapy or hematopoietic stem-cell transplantation: Systematic review and meta-analysis. J. Clin. Oncol. 2007, 25, 5471–5489. [Google Scholar] [CrossRef] [PubMed]
- Teh, B.W.; Yeoh, D.K.; Haeusler, G.M.; Yannakou, C.K.; Fleming, S.; Lindsay, J.; Slavin, M.A.; Australasian Antifungal Guidelines Steering Committee. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2021. Intern. Med. J. 2021, 51 (Suppl. 7), 67–88. [Google Scholar] [CrossRef]
- Aitken, S.L.; Nagel, J.L.; Abbo, L.; Alegria, W.; Barreto, J.N.; Dadwal, S.; Freifeld, A.G.; Jain, R.; Pergam, S.A.; Tverdek, F.P.; et al. Antimicrobial stewardship in patients with cancer: The time is now. J. Natl. Compr. Canc. Netw. 2019, 17, 772–775. [Google Scholar] [CrossRef]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an antibiotic stewardship program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).