Simple Summary
Our study successfully cloned and obtained an 1866 bp segment of the full-length coding sequence (CDS) region of the estrogen receptor gene 1 (ESR1) in Sichuan white geese. We compared the involvement of ESR1 in lipid metabolism between pre-hierarchical granulosa cells and hierarchical granulosa cells in geese. Our findings indicate that ESR1 plays a more significant role in the lipid metabolism of hierarchical granulosa cells. When ESR1 is overexpressed in hierarchical granulosa cells, it leads to a reduction in lipid droplet deposition, cholesterol, and triglycerides, primarily regulated by APOB and PPARα, which control lipoprotein synthesis. Conversely, interfering with ESR1 in hierarchical granulosa cells results in increased lipid droplet deposition, cholesterol, and triglycerides. This increase is jointly mediated by ACCα, DGAT1, and SCD, which regulate fatty acid synthesis, as well as CPT1 and ATGL, which are involved in fatty acid degradation.
Abstract
(1) Background: The role of estrogen receptor gene 1 (ESR1) in female reproduction and lipid metabolism has been extensively investigated. However, its contribution to lipid metabolism during the development of poultry follicles remains unclear. (2) Methods: This study aimed to explore the function of ESR1 via overexpressing (ESR1ov) and interfering (ESR1si) with its expression in pre-hierarchical granulosa cells (phGCs) and hierarchical granulosa cells (poGCs). (3) Results: We successfully cloned and obtained an 1866 bp segment of the full-length CDS region of the Sichuan white goose ESR1 gene. In phGCs of the ESR1ov and ESR1si groups, there were no significant changes compared to the control group. However, in poGCs, the ESR1ov group exhibited decreased lipid deposition, triglycerides, and cholesterol compared to the control group, while the ESR1si group showed increased lipid deposition, triglycerides, and cholesterol. The expression of APOB and PPARα was significantly reduced in the ESR1ov group compared to the ESR1ov-NC group. Moreover, significant changes in the expression of ACCα, DGAT1, SCD, CPT1, and ATGL were observed between the ESR1si and ESR1si-NC group. (4) Conclusions: These findings shed light on the function and molecular mechanism of ESR1 in lipid metabolism in goose poGCs, providing a better understanding of the physiological process of goose follicular development.
1. Introduction
The annual egg production of geese ranges from 20 to 60, and enhancing egg production is a major focus of breeding research. The egg laying performance of poultry relies on the proper maintenance of follicle selection, development, and maturation. Granulosa cells, being the primary component of the follicle wall [1], have been increasingly recognized for their critical role in various stages of follicle development. During follicle selection, development, and maturation, there is a significant accumulation of lipids, and lipid metabolism has been identified as a vital regulatory mechanism [2,3]. These studies indicate that regulating lipid metabolism in granulosa cells is a key step in follicle selection, development, and maturation. Therefore, gaining insights into the molecular mechanism that govern lipid metabolism in granulosa cells during the development of goose egg follicles can provide a valuable theoretical reference for enhancing goose egg production.
As a well-established regulator of female reproduction, the estrogen receptor gene ESR1 plays a crucial role in this process. Within the first intron of ESR1, there exists a single nucleotide mutation site known as ESR1-PvuII, which directly affects the transcription of estrogen response elements and subsequently the estrogen pathway [4,5]. Numerous mouse experiments utilizing gene editing technology have provided substantial evidence highlighting the significance of ESR1 in the development and maintenance of ovarian function [6,7,8]. More recently, investigations have emerged establishing a connection between ESR1 and follicle development, along with its impact on poultry egg production [9,10]. Furthermore, the disruption of ESR1 function in humans [11,12] and mutant rodent models [13,14] has been observed to result in obesity and metabolic dysfunction.
Although the role of ESR1 in lipid metabolism and reproduction has been extensively studied, there is a lack of research confirming its involvement in lipid deposition during poultry follicle development. Therefore, the objective of this study was to clone and obtain the sequence of goose ESR1 and investigate its expression in granulosa cells at different stages of follicle development. Additionally, we aimed to elucidate the impact and molecular mechanism of ESR1 on lipid metabolism during follicle development stage by manipulating ESR1 expression through overexpression and interference in goose granulosa cells (both pre-hierarchical and hierarchical). The findings from this study have the potential to provide valuable theoretical insights for enhancing goose egg production.
2. Materials and Methods
2.1. Sample Collection
Ethical approval on animal survival was provided by the Animal Welfare and Ethics Committee of the Institute of Animal Sciences (IAS), Sichuan Agricultural University: Approval No. 20180034. The samples used in this study consisted of laying geese obtained from the Waterfowl Breeding Laboratory of Sichuan Agricultural University (Ya’an, Sichuan, China). These geese were healthy Sichuan white geese from the same incubation batch, reared in identical environmental conditions, and at the same peak of laying concerning weight and time. Euthanasia of the geese was performed by neck exsanguination, following which their entire ovaries were extracted and placed in preheated PBS buffer solution at 37 °C. The follicular granular layers were subsequently stripped using the method described by Gilbert et al. [15]. The granular layers from three goose were used to obtained the ESR1 expression in different follicle development (small yellow follicle (SYF), large yellow follicle (LYF), F5, F4–2, F1). In the cell experiment, the ovarian follicles were divided into two classes: pre-hierarchical (6 to 8 mm and 8 to 10 mm in diameter) and hierarchical (F5–F2, F2 > F3 > F4 > F5) based on their diameter.
2.2. Transcriptome Analysis
Transcriptome sequencing data of granular layers of follicles in Sichuan white goose and Tianfu meat goose at different developmental stages were downloaded from previous studies: NCBI PRJNA506334 [16] and PRJNA552525 [17]. The data underwent quality control using the FastaQC software, and low-quality reads were filtered out to obtain clean reads. These clean reads were then aligned to our assembled Sichuan white goose genome using HISAT2 software (version 2.2.1) [18]. The resulting SAM file was converted to a BAM file and sorted using SAMtools (version 1.10). Subsequently, the expression level of each transcript was calculated and the counts of ESR1 extracted through featureCounts (version 1.6.0) [19]. To visualize the expression level of ESR1 (in terms of read counts), Prism 9.0 Graphpad software was employed.
2.3. Cloning CDS Region of Goose ESR1
The ESR1 mRNA reference sequence was extracted from the reference genome of Sichuan white geese. Primers for amplifying the full-length CDS region were designed using Primer Premier 5.0 software: Forward primer: CCGAAGTAATGGCAACAACCT; Reverse primer: TCCCACTCAGGAAGATACCAATA. Total RNA was extracted from the granular layers using the TRIzol® Reagent (Plant RNA Purification Reagent for plant tissue) following the manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA), and genomic DNA was removed using DNase I (TaKaRa, Dalian, China). The quality of the extracted total RNA was assessed using the 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA), and the concentration of total RNA was determined using the ND-2000 (NanoDrop Technologies, Wilmington, DC, USA). Only RNA samples meeting the following criteria were used for cDNA synthesis: OD260/280 = 1.8~2.2, OD260/230 ≥ 2.0, RIN ≥ 6.5. cDNA was synthesized using the PrimeScript RTTM Reagent Kit (TaKaRa, Dalian, China). PCR amplification was performed using cDNA as template with the following reaction mixture: 1 µL forward primer (10 μmol/L), 1 µL reverse primer (10 μmol/L), 1 µL cDNA, 12.5 µL 2 × Rapid Taq Master Mix, 9.5 µL ddH2O. The procedure was as follows: 95 °C for 3 min; 35 cycle for “95 °C for 15 s, 60 °C for 15 s, 72 °C for 27 s”; 72 °C for 5 min. The amplified product was analyzed by agarose gel electrophoresis (1.5% agarose gel), and the target fragment was extracted and purified using the FastPure Gel DNA Extraction Mini Kit (Vazyme, Nanjing, China). The amplified product was ligated to a vector using the 5 min TA/Blunt-Zero Cloning Kit (Vazyme, Nanjing, China) and then transformed using DH5α competent cells. The transformed products were added to the liquid medium without ambenzyl and cultured in a gas bath thermostatic oscillator for 1 h, and then they were centrifuged at 4000× g speed for 10 min. The supernatant (700 µL) was discarded, and the remaining liquid and precipitates were mixed and transferred to solid LB medium plates. The uniformly coated plates were placed in an electrothermal incubator at 37 °C for 12 h. A single colony was selected and incubated in 1 mL LB medium containing 0.1% ampicillin for 5 h oscillating at 37 °C. The bacterial solution was used as a template for PCR amplification using M13F (GTTGTAAAACGACGGCCAG) and M13R (CAGGAAACAGCTATGAC) primers. The PCR system and procedure were the same as described above. The amplified products were sent to Sangong (Shanghai, China) for Sanger sequencing.
2.4. Sequence Analysis of Goose ESR1
ESR1 gene sequences of species such as Gallus gallus (NM_205183.2), Anas platyrhynchos (XM_021275842.3), Homo sapiens (NM_000125.4), Mus musculus (NM_001302531.1), Xenopus Laevis (XM_041562111.1), and Danio rerio (NM_152959.1) were downloaded from NCBI. The DNAMAN software package was utilized to convert the sequenced CDS region sequence into an amino acid sequence. The nucleotide sequences of ESR1 amino acids were aligned using the Cluster omega tool (http://www.ebi.ac.uk/Tools/msa/clustalo/, accessed on 29 October 2022), and the DNAMAN software package was employed to generate multiple sequence alignment images. The evolutionary tree was constructed using MEGA X software through the Maximum Likelihood method with a bootstrap value of 1000, and the data visualization was performed using the Figtree software package. The amino acid sequence of ESR1 underwent domain prediction using the CCD online tool (http://www.ncbi.nlm.nih.gov/Structure/cdd/cdd.shtml, accessed on 30 October 2022).
2.5. Isolation and Culture of phGCs and poGCs
The granular layers were collected from follicles and cut into small pieces using scissors in a 5 mL centrifuge tube. The mixture was then centrifuged for 5–10 min until no obvious precipitation was observed. The resulting mixture was transferred to a 15 mL centrifuge tube containing 0.3% type II collagenase for digestion. During digestion, the mixture was placed in a water bath at 37 °C and oscillated for about 3 min until the granulosa cells were completely dispersed. The digestion was terminated with cold PBS, and the scattered cells were filtered through a 200-mesh sieve and separated by centrifugation at 1000 RPM for 10 min. The cell morphology was observed under an Olympus microscope. Finally, the cell density was measured, and the cells were cultured in DMEM/F12 medium supplemented with 10% FBS. To prepare the medium, 50 mL of FBS was filtered using a 0.22 µM disposable filter and added to 450 mL of DMEM/F12. Then, 5 mL of penicillin-streptomycin mixture was added to the medium, mixed, and stored at 4 °C. The cells were inoculated into the culture plate at a density of 4 × 105/mL and transferred to a carbon dioxide constant temperature incubator for culture. After 6 h of culture, the medium was changed to remove the non-adherent cells. The cell morphology was recorded using a microscope at 48 and 96 h.
2.6. Construction of ESR1 Overexpression and Interference Model in GCs
The previously cloned CDS region sequence of ESR1 was sent to Sangong (Shanghai, China) and Gima Biotechnology Co., Ltd. (Shanghai, China) for the design and synthesis of overexpression vector (pcDNA3.1) and siRNA, as shown in Table 1. After transfection for 24 h, cell samples were collected following a media change. Then, the optimal concentration was then used to transfect ESR1-siRNA and siRNA-NC, respectively, and cell samples were collected 24 h after transfection. RNA extraction and cDNA synthesis were performed on the collected cell samples using the aforementioned method. The expression of ESR1 was detected using the previously described method.

Table 1.
The sequences of siRNA-ESR1 oligo.
2.7. Oil Red O Staining
A 0.5% Oil Red O solution was prepared using isopropanol. Subsequently, the 0.5% Oil Red O solution was diluted with PBS in a 2:3 ratio to obtain a 0.3% Oil Red O solution. The 0.3% Oil Red O solution was then filtered using double-layer filter paper. The cells to be stained were washed three times with PBS and fixed at room temperature with 4% paraformaldehyde for 30 min. Following fixation, the cells were stained with the 0.3% Oil Red O solution at room temperature for 1 h. The excess Oil Red O dye was rinsed off with 60% isopropanol for 10 s, followed by a rinse with PBS. Finally, the staining characteristics and morphology of granulosa cells (GCs) were observed under a microscope, and images were captured.
2.8. BODIPY Staining of Lipids in GCs
First, the cell samples were washed 2–3 times with PBS to remove the culture medium. Next, 4% paraformaldehyde solution was added to the cell sample to fix them at room temperature for 20–30 min. After fixation, the cell samples were washed 2–3 times with PBS. Then, the BODIPY staining solution was added, and the cell samples were incubated in the staining solution for 10–30 min. The cell samples were washed with PBS 2–3 times to remove unbound staining solution. Subsequently, the DAPI staining solution was added and was incubated at room temperature for 5 min. Finally, the cell sample was observed under a fluorescence microscope using appropriate fluorescence filters to observe BODIPY and DAPI staining signals. For further analysis and processing of the images, the ImageJ software package was utilized.
2.9. Detection of Triglycerides (TG) and Cholesterol (CH) in GCs
After 48 h of treatment, cells were collected using RIPA buffer to determine TG and CH content. The ELISA kit instructions (Hengyuan, Shanghai, China) were followed by adding the standard and sample to different wells. Subsequently, the kit’s enzyme-labeled antibody and detection antibody were added. To remove the unbound protein and antibody, the wells were washed with the wash buffer provided by the kit or PBS. Following that, the substrate provided by the kit was added to react with the enzyme-labeled antibody. Finally, the chemical reaction was stopped with the stop solution provided by the kit. Using an ELISA reader at 450 nm OD, the absorbance value was measured, and the concentration of TG and CH in the sample was calculated by drawing a standard curve based on the standard sample.
2.10. Lipid-Related Gene Expression Detection
RNA from the cell was extracted according to the instructions using the cell/tissue Total RNA extraction kit (RC101-01 produced by Vazyme Biotechnology Co., Ltd., Nanjing, China). An amount of 2 µL RNA solution was mixed with 0.5 µL 6 × RNA loading buffer and then detected by 1.5% agarose-gel electrophoresis. 1 µL Total RNA was taken, and the concentration and OD (A) values at 280 nm and 260 nm were determined in a nucleic acid protein analyzer. The RNAs with A260/A280 values between 1.8 and 2.0 and the band brightness ratio of 28S: 18S ribosomal RNAs should be about 2:1, and the RNAs with very light 5S bands were used for subsequent reverse transcription tests. The cDNA was synthesized according to the instructions of the reverse transcription kit HiScript III RT SuperMix for qPCR (+gDNA wiper) (R323-01 produced by Vazyme Biotechnology Co., Ltd.). Primer 5.0 was used to design the primers (Table 2). ACTB was used as housekeeping gene. The qPCR reaction systems are as follows: 2 × Taq Pro Universal SYBR qPCR Master Mix (Vazyme, Nanjing, China) 10.0 μL, PCR Forward Primer (10 mM) 0.4 μL, PCR Reverse Primer (10 mM) 10.4 μL, cDNA 2 μL, ddH20 7.2 μL. The qPCR amplification conditions are as follows: 95 °C for 30 s; 40 cycles of 95 °C for 5 s and 60 °C for 30 s. The 2−ΔΔCT method was used for normalization of the qPCR results, after which the normalized data were used for statistical analysis, and p < 0.05 was considered significantly different.

Table 2.
Primer used in this study.
2.11. Statistical Analysis
Experiments for each group were repeated at least three times, and the data are presented as means ± SEM. The statistical differences between groups were analyzed using the T-test analysis method in PRISM. A p-value < 0.05 was considered statistically significant, while a p-value < 0.01 was considered highly significant.
3. Results
3.1. Cloning and Sequence Analysis of Goose ESR1
We cloned and obtained the 1866 bp full-length CDS region of the ESR1 gene in Sichuan white geese. The nucleotide and amino acid homology between different species were shown in Table 3, and the amino acid conservation in poultry is above 98%. Compared with Danio rerio, both nucleic acid and amino acid homology are around 50%. Through domain prediction, it was found that 74–207 of the amino acids encoded by the goose ESR1 gene were estrogen receptor domains (Oest reply), 206–287 were estrogen DNA-binding domains (NR_DBD_ER) composed of two C4 type zinc finger, 336–573 were hormone-activated ligand-binding domains (NR_LBD_ER), and 578–621 were estrogen-type nuclear receptor terminal C-terminal domains (ESR1_C). The results of the evolutionary tree show that the goose ESR1 gene has the closest relationship with the Anas platyrhynchos, which is also a waterfowl, and then converges into the same branch with the Gallus gallus, with the farthest relationship with the Danio rerio.

Table 3.
Nucleotide and amino acid sequence identity of ESR1 among 7 vertebrate species.
3.2. Differential Expression of ESR1 in the Granulosa Layer of Follicles at Different Developmental Stages
As shown in Figure 1, the expression of ESR1 exhibited a notable increase in the granulosa layers from LYF to F5 follicles development stage in Tianfu meat goose. Similarly, in the transcriptome data of Sichuan white geese, significant changes in ESR1 expression were observed only during the follicular development stage from LYF to F5. Moreover, the expression of ESR1 increased in the granulosa layers from the F5 to F2 follicles development stage, while it decreased from F2 to F1.
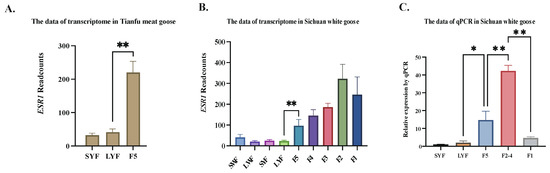
Figure 1.
Expression trend of ESR1 in granulosa layers at different breeds. (A) Read counts of ESR1 in Tianfu meat goose granulosa layers by transcriptome data (N = 3). (B) Read counts of ESR1 in Sichuan white goose granulosa layers by transcriptome data (N = 3). (C) Relative expression of ESR1 in Sichuan white goose granulosa layers by qPCR (N = 3). Note: * represents p < 0.05, ** represents p < 0.01. Data are shown as Mean ± SEM. SWF: small white follicle; LWF: large white follicle.
3.3. Effect of ESR1 on Lipid Metabolism of phGCs
The expression of ESR1 in phGCs was significantly higher in the ESR1ov group than in the ESR1ov-NC group, with a fold change of 142.02. Conversely, the ESR1si group had only 46.8% of the expression level of ESR1 compared to the ESR1si-NC group in phGCs. The staining results of Oil Red O and BODIPY indicated that there we no significant changes in the lipid droplet (LDs) content in the phGCs after treatment with ESR1 (Figure 2A,B). ELISA results showed that the CH content in phGCs of the ESR1ov group was significantly higher than that of the ESR1ov-NC group (Figure 2C). However, there was no significant changes in CH content in phGCs between the ESR1si and ESR1si-NC groups (Figure 2C). Meanwhile, there was no significant change in the content of TG in phGC, whether it was in terms of overexpression or interference of ESR1. (Figure 2D). Additionally, the expression of genes ACCα, FASN, DGAT2, PPARα, PPARγ, APOB, SCD, CPT, and ATGL did not change between the two comparisons (ESR1ov and ESR1ov-NC, ESR1si and ESR1si-NC) (Supplementary Figure S1).
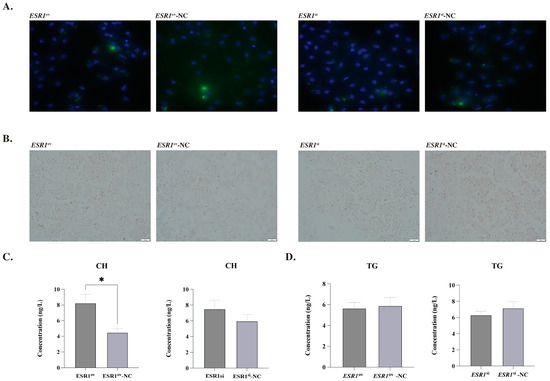
Figure 2.
Effect of ESR1 on lipid metabolism of phGCs. (A) Morphological characteristics of LDs in phGCs detection by BODIPY (green) and DAPI (blue) staining. (B) Morphological characteristics of LDs in phGCs detection by oil-red O staining. (C) Effect of ESR1 on CH secretion of phGCs (N = 4). (D) Effect of ESR1 on TG secretion of phGCs (N = 4). Note: * represents p < 0.05. Data are shown as Mean ± SEM.
3.4. Effect of ESR1 on Lipid Metabolism of poGCs
The expression of ESR1 in poGCs was significantly higher in the ESR1ov group than in the ESR1ov-NC group, with a fold change of 176.62. Conversely, the ESR1si group had only 74.5% of the expression level of ESR1 compared to the ESR1si-NC group in poGCs. The staining results of BODIPY and DAPI showed that overexpression of ESR1 in poGCs can decrease lipid droplets deposition, while interference of ESR1 can significantly increase LDs deposition (Figure 3A). Consistently with this, the staining results of oil-red O also showed that the overexpression of ESR1 could show less LDs deposition, and the interference of ESR1 showed more LDs (Figure 3B). Moreover, the results of ELISA showed that the interference of ESR1 could significantly increase the content of CH and TG in poGCs, while the overexpression of ESR1 could reduce the content of CH and TG (Figure 3C,D).
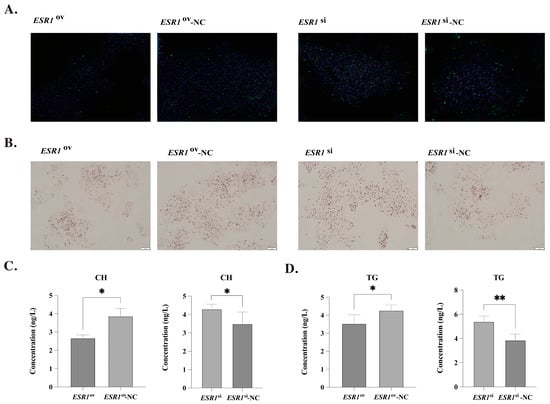
Figure 3.
Effect of ESR1 on lipid metabolism of poGCs. (A) Morphological characteristics of LDs in poGCs detection by BODIPY (green) and DAPI (blue) staining. (B) Morphological characteristics of LDs in poGCs detection by oil-red O staining. (C) Effect of ESR1 on CH secretion of poGCs (N = 4). (D) Effect of ESR1 on TG secretion of poGCs (N = 4). Note: * represents p < 0.05, ** represents p < 0.01. Data are shown as Mean ± SEM.
3.5. Expression Profiles of Lipid Metabolism-Related Marker Genes in poGCs
Regardless of interference or overexpression of ESR1 in poGC, the expression of FASN, DGAT2, and PPARγ did not show significant changes (Figure 4). Compared with the ESR1si-NC group, the expression of ACCα, DGAT1, SCD, CPT1, and ATGL was significantly downregulated in the ESR1si group. Meanwhile, the expression of APOB and PPARα in the ESR1ov group was significantly lower than that of the ESR1ov-NC group.
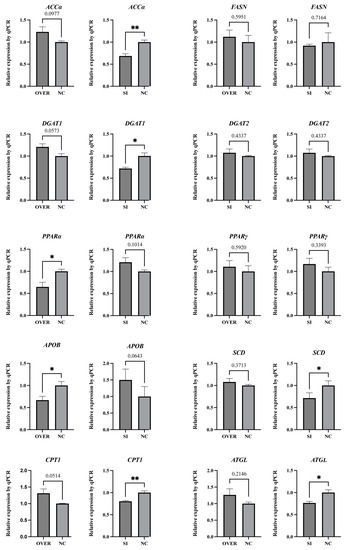
Figure 4.
Expression patterns of genes involved in lipid metabolism in poGCs (N = 4). Note: * represents p < 0.05, ** represents p < 0.01. Data are shown as Mean ± SEM.
4. Discussion
Previous studies have indicated that lipid accumulation in poGCs is higher compared to phGCs [2]. In line with these findings, our study observed significant differences in the expression of ESR1 in the granular layers between SWF and F5, as well as at F5 and F2–4, and F2–4 and F1. These findings suggest the involvement of ESR1 in lipid deposition during follicle development. Moreover, it has been reported that phGCs predominantly express genes associated with lipid synthesis and oxidation, while poGCs predominantly express genes related to lipid migration and deposition [2]. Our experimental results further support the notion that ESR1 primarily influences lipid metabolism in poGCs, indicating its regulatory role in the functions of lipid migration and deposition in these cells.
The LDs are dynamic organelles known for their ability to store neutral lipids and participate in a range of physiological processes [20]. LDs are present in nearly all mammalian follicles and are recognized as an important energy source for oocyte maturation [21,22]. Our experimental results demonstrate that ESR1 can inhibitor LDs synthesis in goose poGCs, which could be a pathway for its involvement in the development of goose follicles and regulation of egg production.
To further explore the molecular pathway through which ESR1 participates in lipid metabolism in goose granulosa cells, we conducted qRT-PCR analysis on 10 marker genes associated with lipid metabolism. PPARα and PPARγ are key transcription factors involved in lipid metabolism [23]. PPARα promotes the uptake, esterification, and transportation of cellular fatty acids, and it also regulates lipoprotein metabolism genes [24]. Apart from PPARα, another gene related to lipoprotein tissue related, APOB, showed a significantly downregulation upon ESR1 overexpression of [25]. Polymorphisms in ESR1 have been confirmed to be closely associated with human lipoprotein levels [26,27]. Our results demonstrated that the expression of PPARα and APOB significantly decreased in poGCs upon ESR1 overexpression. Lipoproteins consist of various lipid and protein components, including TG and CH. Their main function is to transport lipids and proteins in the bloodstream and between tissues and cells [28,29]. Based on these findings, we hypothesize that the decreased expression of PPARα and APOB, resulting from ESR1 overexpression, contributes to the reduction in LDs, TG, and CH levels.
ACCα plays a crucial role in catalyzing the conversion of Acetyl-CoA to Malonyl-CoA, which is the initial and rate-limiting step in de novo fatty acid biosynthesis [30]. The de novo lipogenesis function of goose granulosa cells has been confirmed, indicating that ESR1 may enhance de novo lipogenesis in these cells [31]. DGAT1 and DGAT2 are considered to be a key enzyme in TG synthesis [32]. Previous studies have shown that DGAT1 is not as effective as DGAT2 in promoting lipid accumulation in poGCs [33]. Mammalian experiments have confirmed that DGAT1 and DGAT2 can compensate for each other to a large extent in TG storage [34]. These results suggest that, in goose poGCs, ESR1-mediated TG storage is mainly mediated by DGAT1 rather than DGAT2. SCD acts as a pivotal enzyme in catalyzing the rate-limiting step of monounsaturated fatty acid production. Recent studies have indicated that SCD is involved in the development of goose follicles, specifically promoting LDs deposition and CH storage in granulosa cells [35,36].
Despite observing a significant decrease in the expression levels of three genes involved in fatty acid synthesis (ACCα, DGAT1, and SCD) in poGCs following interference of ESR1, we still found a significant increase in phenotypes (LDs, TG, and CH). This observation may be closely associated with the expression levels of two genes involved in fatty acid degradation (ATGL and CPT1). ATGL initiates the hydrolysis of TGs to release free fatty acids [37]. Fatty acid β-oxidation within mitochondria is a crucial pathway for fatty acid catabolism, playing a pivotal role in maintaining energy homeostasis throughout the body. CPT1 is the gene encoding an important enzyme in this process [38]. Our findings demonstrate that ESR1 promotes the expression of both ATGL and CPT1, thus activating the fatty acid decomposition pathway. The process of fatty acid oxidation also generates ATP, providing energy for granulosa cell activities [39]. This mechanism may represent one of the pathways through which ESR1 participates in the development of goose follicles.
5. Conclusions
In conclusion, we successfully cloned and obtained the complete sequence of the CDS region of the goose ESR1 gene. We further investigated its expression pattern in granulosa cells at different stages of follicular development. Our experiment results confirmed that there were no significant changes in LDs deposition in phGCs following either overexpression or interference of ESR1, while LDs deposition in poGCs significantly decreased after the overexpression of ESR1 and increased significantly after the interference of ESR1. Meanwhile, the content of TG and CH in poGCs significantly decreased after the overexpression of ESR1, and the content of TG and CH significantly increased after the interference of ESR1. Moreover, qPCR results suggest that these phenotypic results may relate to changes in the expression of ACCα, DGAT1, SCD, CPT1, ATGL, APOB, and PPARα in poGCs, which are involved in the synthesis and decomposition of fatty acids and TG, as well as the metabolism and assembly of lipoproteins.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biology12070962/s1, Figure S1: Expression patterns of genes involved in lipid metabolism in phGCs.
Author Contributions
Conceptualization, J.W., Q.O. and H.X.; methodology, J.W., Q.O. and H.X.; formal analysis, Q.O. and H.X.; investigation, M.R., H.X., X.Z., Y.L., Z.H., J.H., H.H., L.L., H.L. and S.H.; writing—original draft preparation, Q.O.; writing—review and editing, J.W. and H.X. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (31972567), the China Agricultural Research System (CARS-42-4), and The National Natural Science Foundation of China (32272882).
Institutional Review Board Statement
All experiments were conducted according to the institutional ethical guidelines for animal experiments of the National Defense Medical College. These experiments were approved by the Sichuan Agricultural University Animal Welfare Committee (approval number: 20180034, date of approval 4 March 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.; Du, X.; Wang, L.; Shi, K.; Li, Q. TGF-β1 controls porcine granulosa cell states: A miRNA-mRNA network view. Theriogenology 2021, 160, 50–60. [Google Scholar] [CrossRef]
- Gao, S.; Gan, X.; He, H.; Hu, S.; Deng, Y.; Chen, X.; Li, L.; Hu, J.; Li, L.; Wang, J. Dynamic characteristics of lipid metabolism in cultured granulosa cells from geese follicles at different developmental stages. Biosci. Rep. 2019, 39, BSR20192188. [Google Scholar] [CrossRef]
- Chen, X.; Huang, K.; Hu, S.; Lan, G.; Gan, X.; Gao, S.; Deng, Y.; Hu, J.; Li, L.; Hu, B.; et al. Integrated Transcriptome and Metabolome Analysis Reveals the Regulatory Mechanisms of FASN in Geese Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 14717. [Google Scholar] [CrossRef]
- Weickert, C.S.; Miranda-Angulo, A.L.; Wong, J.; Perlman, W.R.; Ward, S.E.; Radhakrishna, V.; Straub, R.E.; Weinberger, D.R.; Kleinman, J.E. Variants in the estrogen receptor alpha gene and its mRNA contribute to risk for schizophrenia. Hum. Mol. Genet. 2012, 21, 5238. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Xu, J.; Reboussin, D.M.; Brosnihan, K.B.; Zheng, S.L.; Hawkins, G.A.; Herrington, D.M.; Howard, T.D.; Meyers, D.A. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N. Engl. J. Med. 2002, 346, 967–974. [Google Scholar]
- Schomberg, D.W.; Couse, J.F.; Abir, M.; Lubahn, D.B.; Sar, M.; Mayo, K.E.; Korach, K.S. Targeted disruption of the estrogen receptor-alpha gene in female mice: Characterization of ovarian responses and phenotype in the adult. Endocrinology 1999, 140, 2733. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Yates, M.M.; Walker, V.R.; Korach, K.S. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) Null mice reveals hypergonadism and endocrine sex reversal in females lacking ERalpha but not ERbeta. Mol. Endocrinol. 2003, 17, 1039–1053. [Google Scholar] [CrossRef]
- Singh, S.P.; Wolfe, A.; Ng, Y.; DiVall, S.A.; Buggs, C.; Levine, J.E.; Wondisford, F.E.; Radovick, S. Impaired estrogen feedback and infertility in female mice with pituitary-specific deletion of estrogen receptor alpha (ESR1). Biol. Reprod. 2009, 81, 488–496. [Google Scholar] [CrossRef]
- Shen, M.M.; Li, T.T.; Chen, F.X.; Wu, P.F.; Wang, Y.; Chen, L.; Xie, K.Z.; Wang, J.Y.; Zhang, G.X. Transcriptomic Analysis of circRNAs and mRNAs Reveals a Complex Regulatory Network That Participate in Follicular Development in Chickens. Front. Genet. 2020, 11, 503. [Google Scholar] [CrossRef]
- Zheng, S.; Ouyang, J.; Liu, S.; Tang, H.; Xiong, Y.; Yan, X.; Chen, H. Genomic signatures reveal selection in Lingxian white goose. Poult. Sci. 2023, 102, 102269. [Google Scholar] [CrossRef]
- Efstathiadou, Z.A.; Sakka, C.; Polyzos, S.A.; Goutou, M.; Stakias, N.; Bargiota, A.; Koukoulis, G.N. Associations of estrogen receptor alpha and Beta gene polymorphisms with lipid levels and insulin resistance in men. Metabolism 2015, 64, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Guclu-Geyik, G. The rs2175898 Polymorphism in theESR1Gene has a Significant Sex-Specific Effect on Obesity. Biochem. Genet. 2020, 58, 935–952. [Google Scholar] [CrossRef] [PubMed]
- Khristi, V.; Ratri, A.; Ghosh, S.; Pathak, D.; Borosha, S.; Dai, E.; Roy, R.; Chakravarthi, V.P.; Wolfe, M.W.; Karim Rumi, M.A. Disruption of ESR1 alters the expression of genes regulating hepatic lipid and carbohydrate metabolism in male rats. Mol. Cell Endocrinol. 2019, 490, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ribas, V.; Drew, B.G.; Zhou, Z.; Phun, J.; Kalajian, N.Y.; Soleymani, T.; Daraei, P.; Widjaja, K.; Wanagat, J.; de Aguiar Vallim, T.Q.; et al. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci. Transl. Med. 2016, 8, 334ra354. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, A.B.; Evans, A.J.; Perry, M.M.; Davidson, M.H. A method for separating the granulosa cells, the basal lamina and the theca of the preovulatory ovarian follicle of the domestic fowl (Gallus domesticus). J. Reprod. Fertil. 1977, 50, 179–181. [Google Scholar] [CrossRef]
- Li, Q.; Hu, S.; Wang, Y.; Deng, Y.; Yang, S.; Hu, J.; Li, L.; Wang, J. mRNA and miRNA Transcriptome Profiling of Granulosa and Theca Layers From Geese Ovarian Follicles Reveals the Crucial Pathways and Interaction Networks for Regulation of Follicle Selection. Front. Genet. 2019, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Hu, S.; Zhang, K.; Wang, H.; Xie, Y.; Zhang, C.; Wu, R.; Zhao, X.; Zhang, H.; Wang, Q. Genome-Wide Gene Expression Profiles Reveal Distinct Molecular Characteristics of the Goose Granulosa Cells. Front. Genet. 2021, 12, 786287. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Walther, T.C.; Farese, R.V., Jr. The life of lipid droplets. Biochim. Biophys. Acta 2009, 1791, 459–466. [Google Scholar] [CrossRef]
- Silva, R.C.; Bao, S.N.; Jivago, J.L.; Lucci, C.M. Ultrastructural characterization of porcine oocytes and adjacent follicular cells during follicle development: Lipid component evolution. Theriogenology 2011, 76, 1647–1657. [Google Scholar] [CrossRef] [PubMed]
- Sturmey, R.G.; O’Toole, P.J.; Leese, H.J. Fluorescence resonance energy transfer analysis of mitochondrial:lipid association in the porcine oocyte. Reproduction 2006, 132, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Montaigne, D.; Butruille, L.; Staels, B. PPAR control of metabolism and cardiovascular functions. Nat. Rev. Cardiol. 2021, 18, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Colin, S.; Briand, O.; Touche, V.; Wouters, K.; Baron, M.; Pattou, F.; Hanf, R.; Tailleux, A.; Chinetti, G.; Staels, B.; et al. Activation of intestinal peroxisome proliferator-activated receptor-alpha increases high-density lipoprotein production. Eur. Heart J. 2013, 34, 2566–2574. [Google Scholar] [CrossRef]
- Sirwi, A.; Hussain, M.M. Lipid transfer proteins in the assembly of apoB-containing lipoproteins. J. Lipid Res. 2018, 59, 1094–1102. [Google Scholar] [CrossRef]
- Smiderle, L.; Fiegenbaum, M.; Hutz, M.H.; Van Der Sand, C.R.; Van Der Sand, L.C.; Ferreira, M.E.; Pires, R.C.; Almeida, S. ESR1 polymorphisms and statin therapy: A sex-specific approach. Pharmacogenomics J. 2016, 16, 507–513. [Google Scholar] [CrossRef]
- Nogueira-de-Souza, N.C.; Guerreiro da Silva, I.D.; Carvalho, C.V.; Pulchinelli, A.; Haidar, M.A.; Baracat, E.C.; Massad-Costa, A.M. Effect of estrogen receptor-alpha (ESR1) gene polymorphism on high density lipoprotein levels in response to hormone replacement therapy. Braz. J. Med. Biol. Res. 2009, 42, 1138–1142. [Google Scholar] [CrossRef]
- Illingworth, D.R. Lipoprotein metabolism. Am. J. Kidney Dis. 1993, 22, 90–97. [Google Scholar] [CrossRef]
- Feingold, K.R. Lipid and Lipoprotein Metabolism. Endocrinol. Metab. Clin. N. Am. 2022, 51, 437–458. [Google Scholar] [CrossRef]
- Kim, K.H. Regulation of mammalian acetyl-coenzyme A carboxylase. Annu. Rev. Nutr. 1997, 17, 77–99. [Google Scholar] [CrossRef]
- Wen, R.; Gan, X.; Hu, S.; Gao, S.; Deng, Y.; Qiu, J.; Sun, W.; Li, L.; Han, C.; Hu, J.; et al. Evidence for the existence of de novo lipogenesis in goose granulosa cells. Poult. Sci. 2019, 98, 1023–1030. [Google Scholar] [CrossRef]
- Hung, Y.H.; Carreiro, A.L.; Buhman, K.K. Dgat1 and Dgat2 regulate enterocyte triacylglycerol distribution and alter proteins associated with cytoplasmic lipid droplets in response to dietary fat. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862, 600–614. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.Q.; Gao, S.Y.; Zhu, J.R.; Gan, X.; Chen, X.; He, H.; Liang, L.; Hu, B.; Hu, J.W.; Liu, H.H.; et al. Differential actions of diacylglycerol acyltransferase (DGAT) 1 and 2 in regulating lipid metabolism and progesterone secretion of goose granulosa cells. J. Steroid. Biochem. 2020, 202, 105721. [Google Scholar] [CrossRef] [PubMed]
- Chitraju, C.; Walther, T.C.; Farese, R.V. The triglyceride synthesis enzymes DGAT1 and DGAT2 have distinct and overlapping functions in adipocytes. J. Lipid Res. 2019, 60, 1112–1120. [Google Scholar] [CrossRef]
- Yuan, X.; Abdul-Rahman, I.; Hu, S.; Li, L.; He, H.; Xia, L.; Hu, J.; Ran, M.; Liu, Y.; Abdulai, M.; et al. Mechanism of SCD Participation in Lipid Droplet-Mediated Steroidogenesis in Goose Granulosa Cells. Genes 2022, 13, 1516. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, S.; Li, L.; Han, C.; Liu, H.; He, H.; Xia, L.; Hu, J.; Hu, B.; Ran, M.; et al. Lipidomics profiling of goose granulosa cell model of stearoyl-CoA desaturase function identifies a pattern of lipid droplets associated with follicle development. Cell Biosci. 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Haemmerle, G.; Lass, A.; Zimmermann, R.; Gorkiewicz, G.; Meyer, C.; Rozman, J.; Heldmaier, G.; Maier, R.; Theussl, C.; Eder, S.; et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 2006, 312, 734–737. [Google Scholar] [CrossRef]
- Schlaepfer, I.R.; Joshi, M. CPT1A-mediated Fat Oxidation, Mechanisms, and Therapeutic Potential. Endocrinology 2020, 161, bqz046. [Google Scholar] [CrossRef]
- Elis, S.; Desmarchais, A.; Maillard, V.; Uzbekova, S.; Monget, P.; Dupont, J. Cell proliferation and progesterone synthesis depend on lipid metabolism in bovine granulosa cells. Theriogenology 2015, 83, 840–853. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).