MCT-Dependent Cryptosporidium parvum-Induced Bovine Monocyte Extracellular Traps (METs) under Physioxia
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statements
2.2. Parasite Strain and Excystation of Cryptosporidium parvum Sporozoites
2.3. Bovine Monocyte Isolation
2.4. Extracellular Acidification Rates (ECAR) and Oxygen Consumption Rates (OCR) in Bovine Monocytes Exposed to Cryptosporidium parvum
2.5. Glycolysis, MCT1, MCT2 and ATP Purinergic Receptor P2X1 Inhibition in Cryptosporidium parvum-Triggered METs
2.6. Notch Signaling Inhibition of Cryptosporidium parvum-Mediated METs under Hyperoxic and Physioxic Circumstances
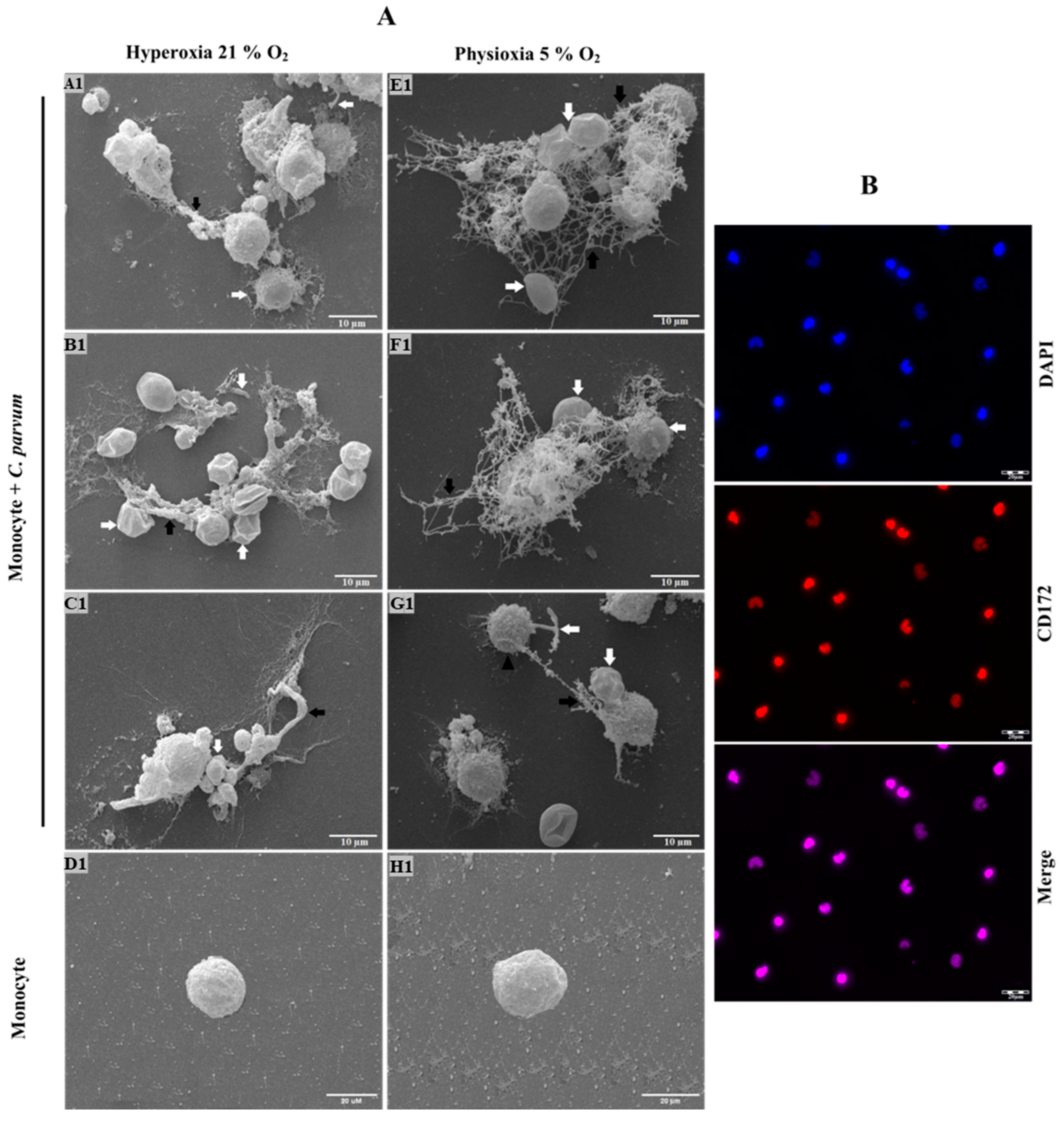
2.7. Scanning Electron Microscopy (SEM) Analysis for Cryptosporidium parvum-Induced METs Investigation
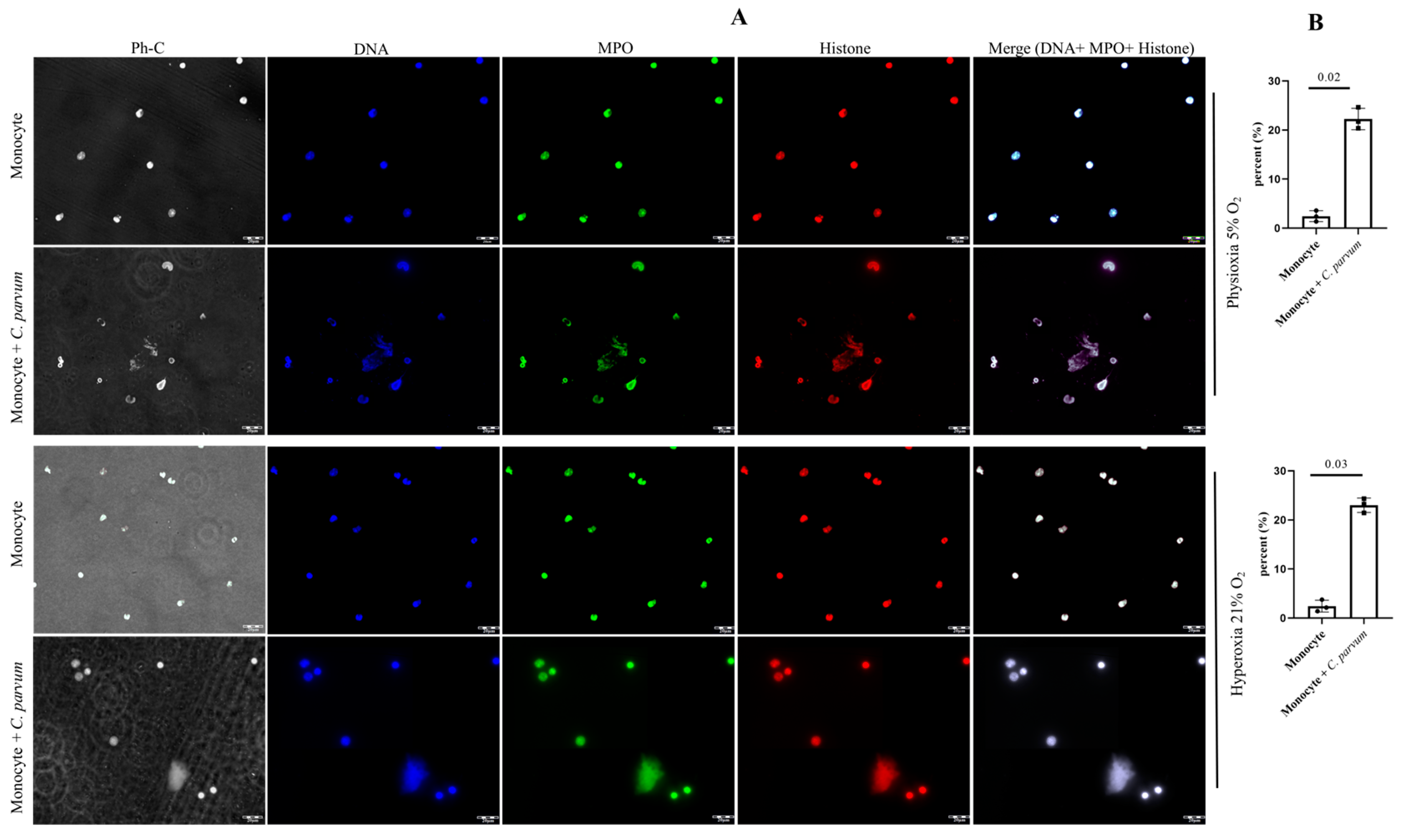
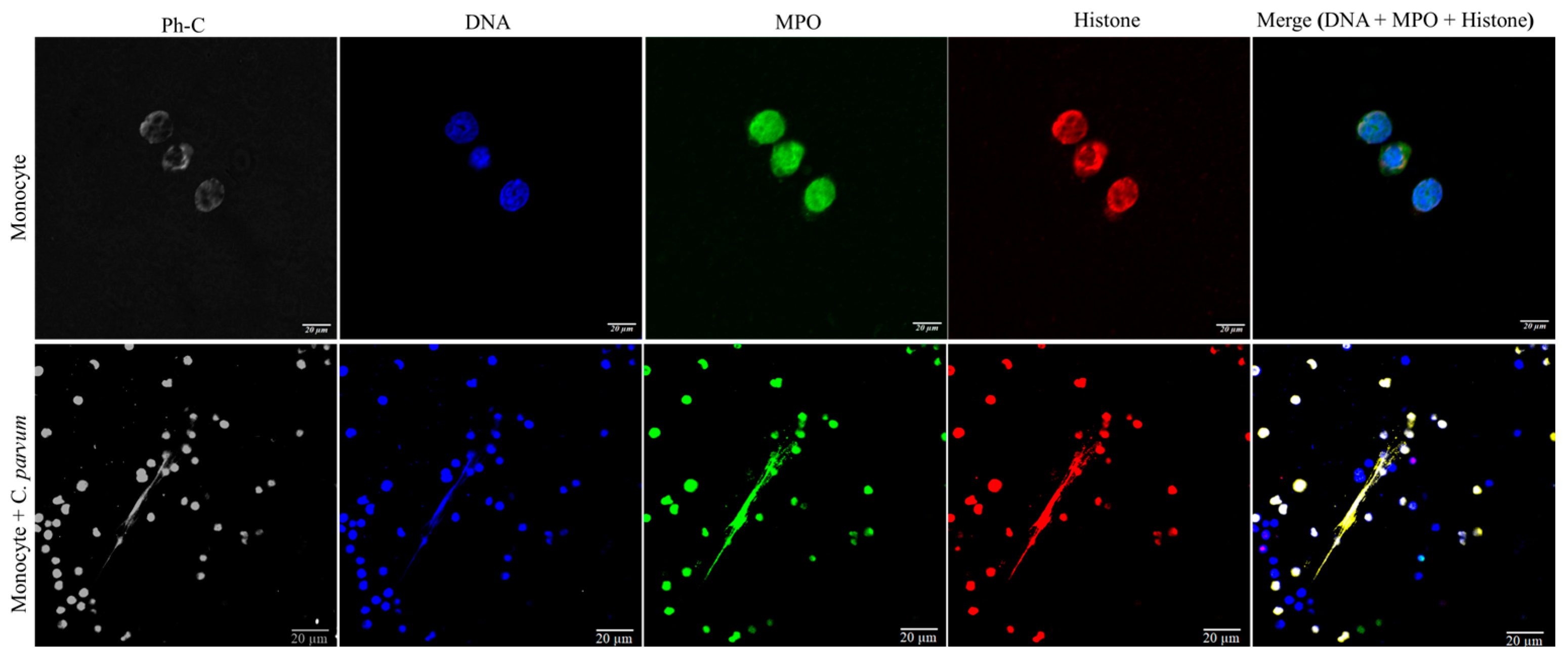
2.8. Visualization of Cryptosporidium parvum-Induced METosis Using Confocal- and Immunofluorescence Microscopy Assays
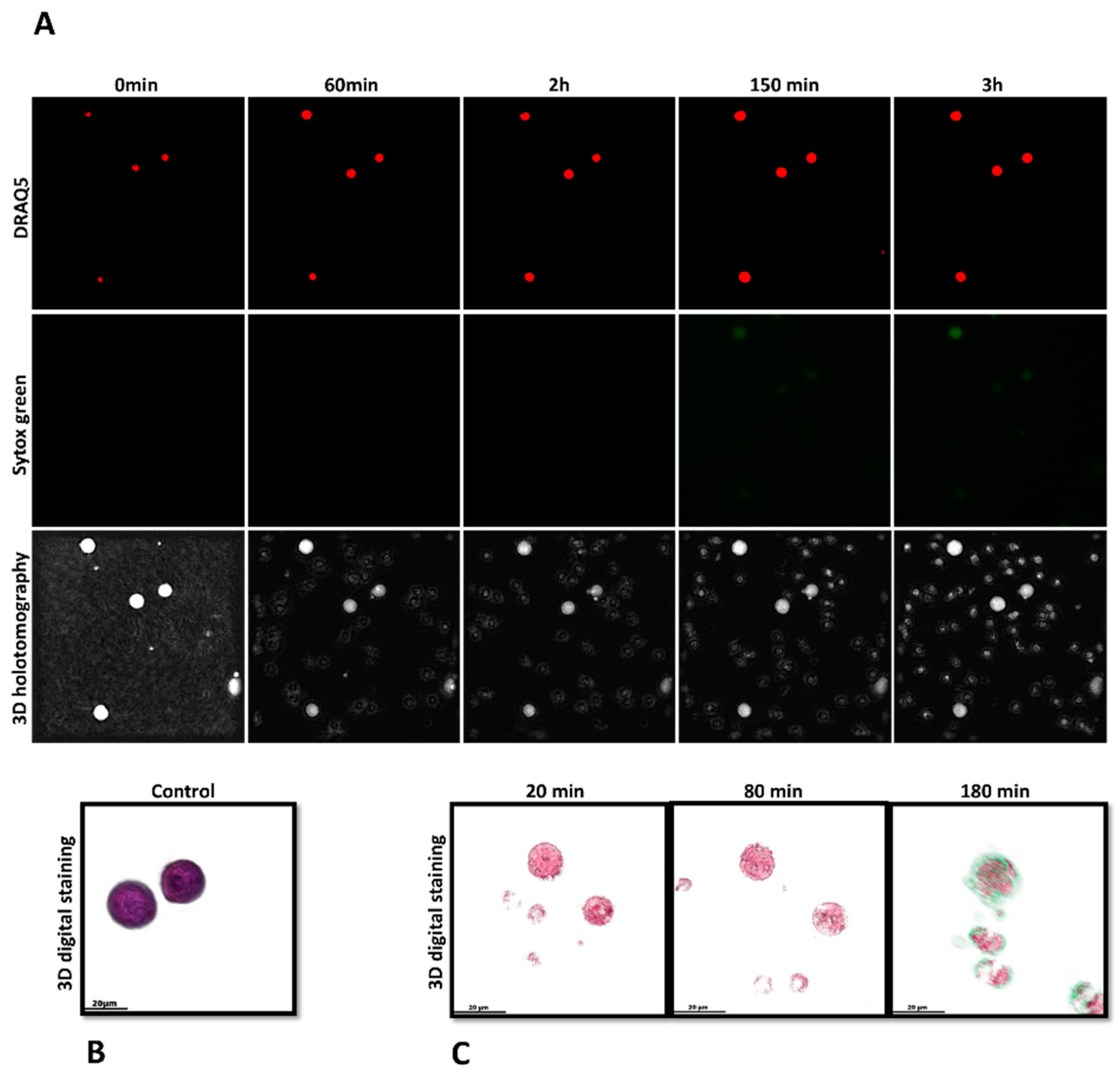
2.9. Investigation of METosis Induced by Cryptosporidium parvum in Live Cells Using 3D Holotomographic Microscopy
2.10. Statistical Methods
3. Results
3.1. Suicidal METosis Induced by Cryptosporidium parvum-Oocysts and Sporozoites
3.2. Imaging Cryptosporidium parvum-Mediated METosis Illustration Using Live Cell 3D-Holotomography
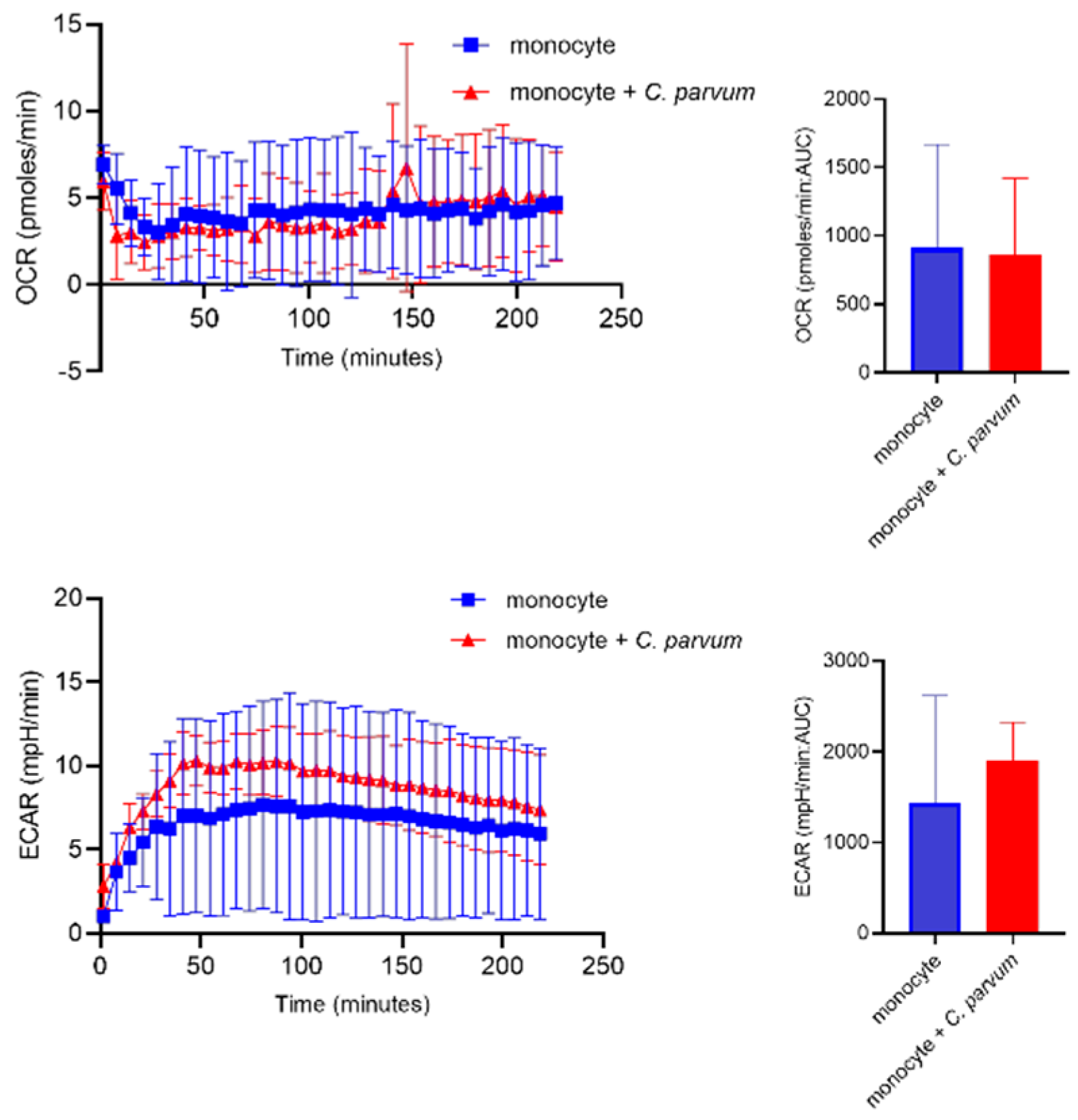
3.3. Monocyte Exposure to Cryptosporidium parvum Neither Affected Oxygen Consumption Rates (OCR) Nor Extracellular Acidification Rates (ECAR)
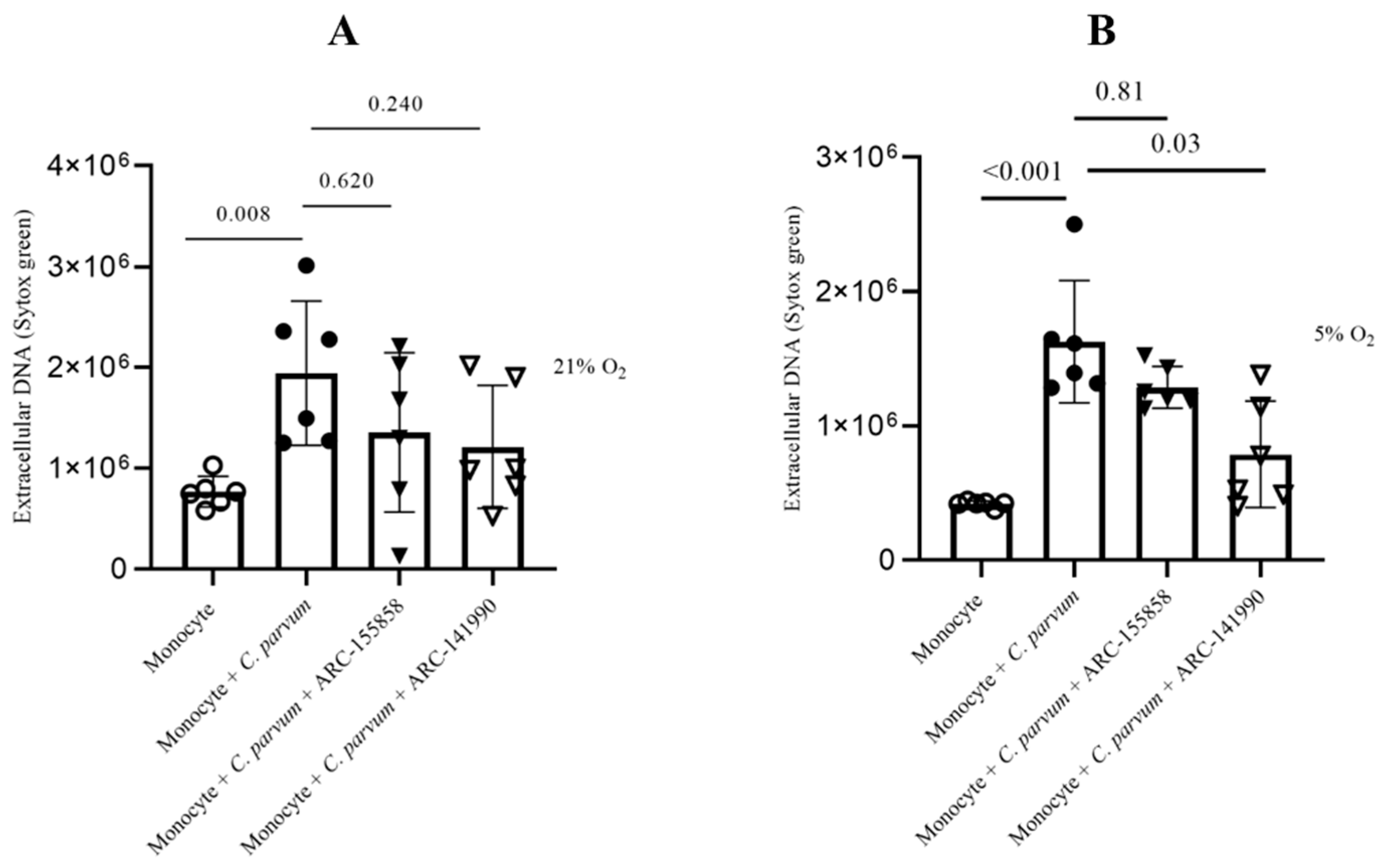
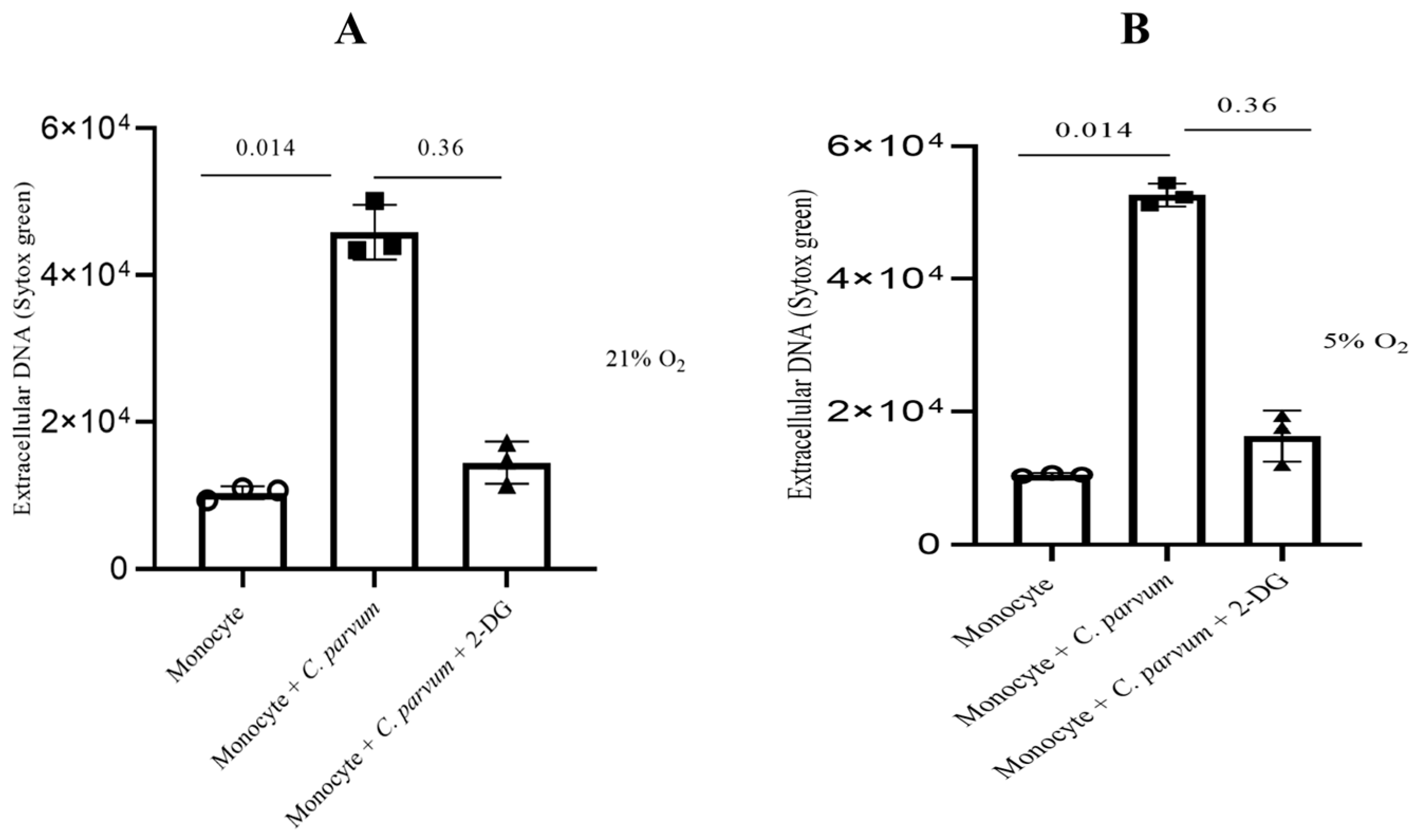
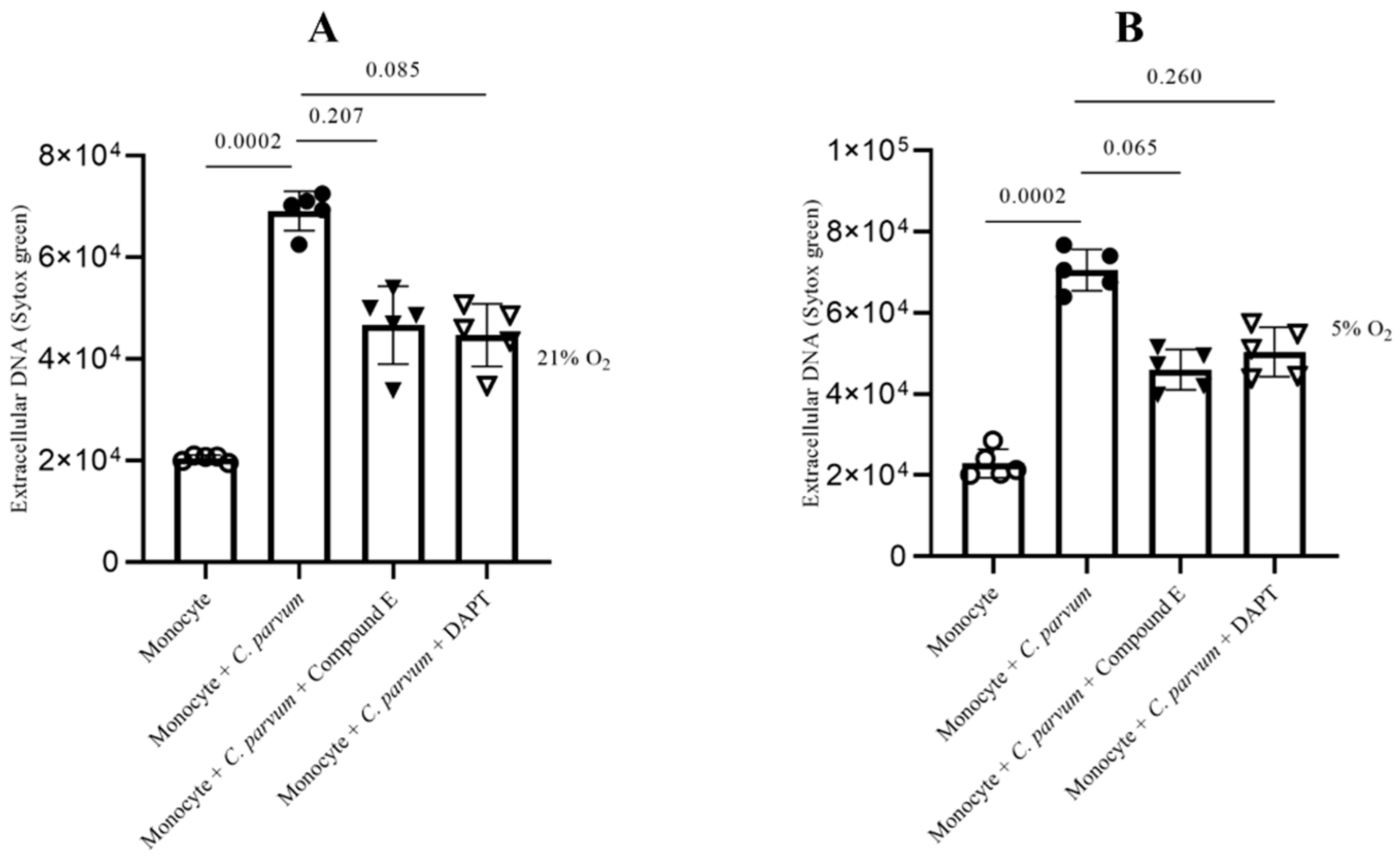
3.4. Cryptosporidium parvum-Mediated METosis Is a Physioxia- and a MCT1/MCT2-Dependent Cell Death Process
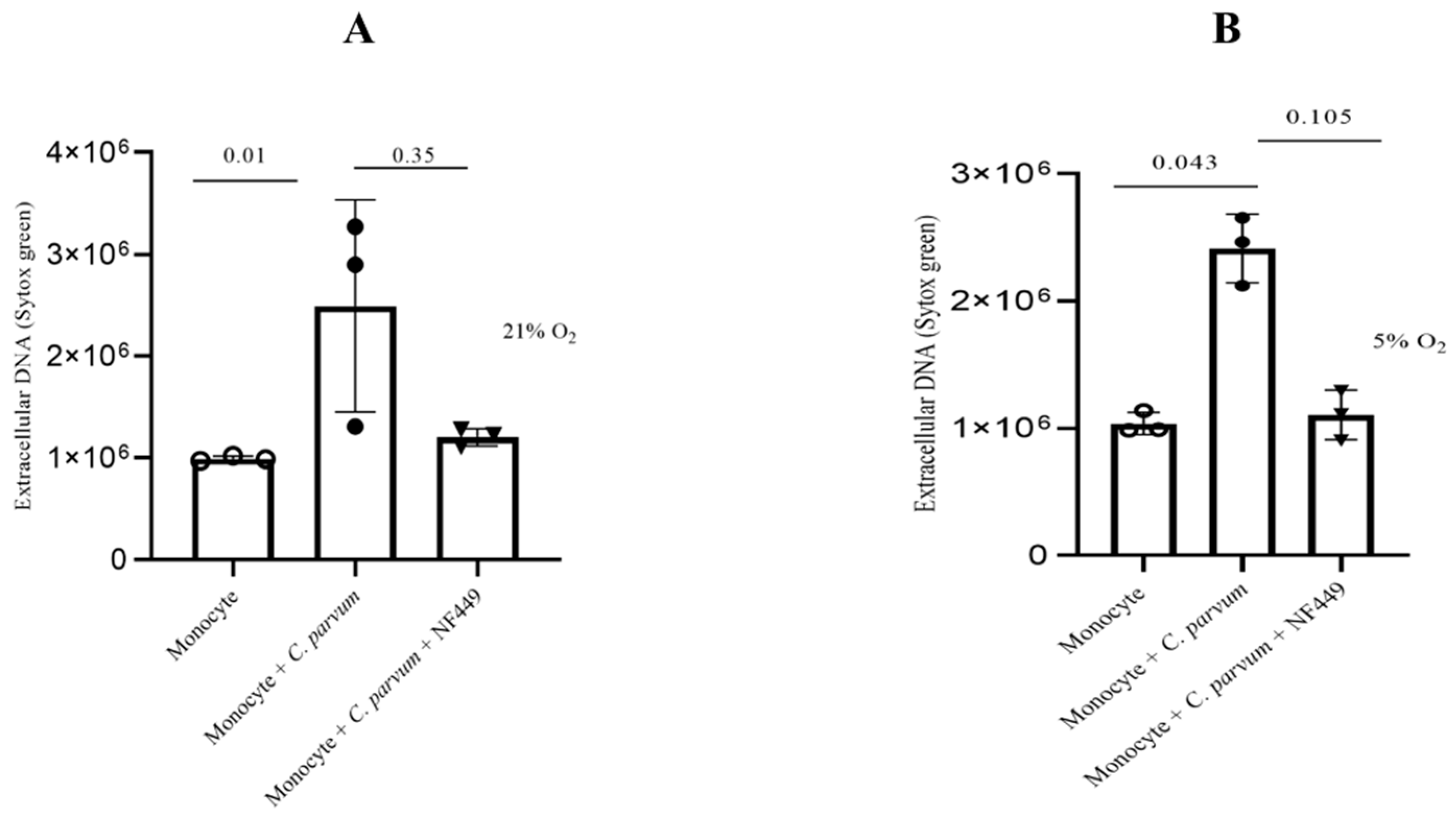
3.5. Cryptosporidium parvum-Induced Suicidal METosis Is Neither a P2X1- Nor a Notch-Dependent Cell Death Process
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Striepen, B. Parasitic infections: Time to tackle cryptosporidiosis. Nature 2013, 503, 189–191. [Google Scholar] [CrossRef]
- De Sablet, T.; Potiron, L.; Marquis, M.; Bussière, F.I.; Lacroix-Lamandé, S.; Laurent, F. Cryptosporidium parvum increases intestinal permeability through interaction with epithelial cells and IL-1β and TNFα released by inflammatory monocytes: C. parvum and intestinal permeability. Cell. Microbiol. 2016, 18, 1871–1880. [Google Scholar] [CrossRef]
- Korich, D.G.; Mead, J.R.; Madore, M.S.; Sinclair, N.A.; Sterling, C.R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 1990, 56, 1423–1428. [Google Scholar] [CrossRef]
- Lendner, M.; Daugschies, A. Cryptosporidium infections: Molecular advances. Parasitology 2014, 141, 1511–1532. [Google Scholar] [CrossRef]
- Thomson, S.; Hamilton, C.A.; Hope, J.C.; Katzer, F.; Mabbott, N.A.; Morrison, L.J.; Innes, E.A. Bovine cryptosporidiosis: Impact, host-parasite interaction and control strategies. Vet. Res. 2017, 48, 42. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.D.; Daugschies, A. Therapy and prevention of cryptosporidiosis in animals. Vet. Parasitol. 2012, 188, 203–214. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Lendner, M.; Daugschies, A.; Hermosilla, C.; Taubert, A. NADPH oxidase, MPO, NE, ERK1/2, p38 MAPK and Ca2+ influx are essential for Cryptosporidium parvum-induced NET formation. Dev. Comp. Immunol. 2015, 52, 245–254. [Google Scholar] [CrossRef]
- Laurent, F.; Lacroix-Lamandé, S. Innate immune responses play a key role in controlling infection of the intestinal epithelium by Cryptosporidium. Int. J. Parasitol. 2017, 47, 711–721. [Google Scholar] [CrossRef]
- Muñoz-Caro, T.; Mena Huertas, S.; Conejeros, I.; Alarcón, P.; Hidalgo, M.A.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Eimeria bovis-triggered neutrophil extracellular trap formation is CD11b-, ERK 1/2-, p38 MAP kinase- and SOCE-dependent. Vet. Res. 2015, 46, 23. [Google Scholar] [CrossRef]
- McDonald, V.; Korbel, D.; Barakat, F.; Choudhry, N.; Petry, F. Innate immune responses against Cryptosporidium parvum infection. Parasite Immunol. 2013, 35, 55–64. [Google Scholar] [CrossRef]
- Blackwelder, W.C.; Biswas, K.; Wu, Y.; Kotloff, K.L.; Farag, T.H.; Nasrin, D.; Graubard, B.I.; Sommerfelt, H.; Levine, M.M. Statistical Methods in the Global Enteric Multicenter Study (GEMS). Clin. Infect. Dis. 2012, 55, S246–S253. [Google Scholar] [CrossRef]
- Pantenburg, B.; Dann, S.M.; Wang, H.-C.; Robinson, P.; Castellanos-Gonzalez, A.; Lewis, D.E.; White, A.C. Intestinal Immune Response to Human Cryptosporidium sp. Infection. Infect. Immun. 2008, 76, 23–29. [Google Scholar] [CrossRef]
- Codices, V.; Martins, C.; Novo, C.; de Sousa, B.; Lopes, Â.; Borrego, M.; Matos, O. Dynamics of cytokines and immunoglobulins serum profiles in primary and secondary Cryptosporidium parvum infection: Usefulness of Luminex® xMAP technology. Exp. Parasitol. 2013, 133, 106–113. [Google Scholar] [CrossRef]
- Lacroix-Lamandé, S.; Mancassola, R.; Naciri, M.; Laurent, F. Role of Gamma Interferon in Chemokine Expression in the Ileum of Mice and in a Murine Intestinal Epithelial Cell Line after Cryptosporidium parvum Infection. Infect. Immun. 2002, 70, 2090–2099. [Google Scholar] [CrossRef]
- Takeuchi, D.; Jones, V.C.; Kobayashi, M.; Suzuki, F. Cooperative Role of Macrophages and Neutrophils in Host Antiprotozoan Resistance in Mice Acutely Infected with Cryptosporidium parvum. Infect. Immun. 2008, 76, 3657–3663. [Google Scholar] [CrossRef]
- Zadrozny, L.M.; Stauffer, S.H.; Armstrong, M.U.; Jones, S.L.; Gookin, J.L. Neutrophils Do Not Mediate the Pathophysiological Sequelae of Cryptosporidium parvum Infection in Neonatal Piglets. Infect. Immun. 2006, 74, 5497–5505. [Google Scholar] [CrossRef]
- Tzipori, S. Cryptosporidiosis in Perspective. In Advances in Parasitology; Elsevier: Amsterdam, The Netherlands, 1988; Volume 27, pp. 63–129. ISBN 978-0-12-031727-1. [Google Scholar]
- Drinkall, E.; Wass, M.J.; Coffey, T.J.; Flynn, R.J. A rapid IL-17 response to Cryptosporidium parvum in the bovine intestine. Vet. Immunol. Immunopathol. 2017, 191, 1–4. [Google Scholar] [CrossRef]
- Niine, T.; Dorbek-Kolin, E.; Lassen, B.; Orro, T. Cryptosporidium outbreak in calves on a large dairy farm: Effect of treatment and the association with the inflammatory response and short-term weight gain. Res. Vet. Sci. 2018, 117, 200–208. [Google Scholar] [CrossRef]
- Kantari, C.; Pederzoli-Ribeil, M.; Witko-Sarsat, V. The Role of Neutrophils and Monocytes in Innate Immunity. In Contributions to Microbiology; Egesten, A., Schmidt, A., Herwald, H., Eds.; KARGER: Basel, Switzerland, 2008; pp. 118–146. ISBN 978-3-8055-8548-4. [Google Scholar]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y.C. The β-Glucan Receptor, Dectin-1, Is Predominantly Expressed on the Surface of Cells of the Monocyte/Macrophage and Neutrophil Lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [PubMed]
- Sándor, N.; Lukácsi, S.; Ungai-Salánki, R.; Orgován, N.; Szabó, B.; Horváth, R.; Erdei, A.; Bajtay, Z. CD11c/CD18 Dominates Adhesion of Human Monocytes, Macrophages and Dendritic Cells over CD11b/CD18. PLoS ONE 2016, 11, e0163120. [Google Scholar] [CrossRef]
- Thomas, G.; Tacke, R.; Hedrick, C.C.; Hanna, R.N. Nonclassical Patrolling Monocyte Function in the Vasculature. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1306–1316. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.-Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef]
- Jacinto, T.A.; Meireles, G.S.; Dias, A.T.; Aires, R.; Porto, M.L.; Gava, A.L.; Vasquez, E.C.; Pereira, T.M.C.; Campagnaro, B.P.; Meyrelles, S.S. Increased ROS production and DNA damage in monocytes are biomarkers of aging and atherosclerosis. Biol. Res. 2018, 51, 33. [Google Scholar] [CrossRef] [PubMed]
- Himmelfarb, J.; Lazarus, J.M.; Hakim, R. Reactive Oxygen Species Production by Monocytes and Polymorphonuclear Leukocytes During Dialysis. Am. J. Kidney Dis. 1991, 17, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Cline, M.J.; Lehrer, R.I. Phagocytosis by Human Monocytes. Blood 1968, 32, 423–435. [Google Scholar] [CrossRef]
- Zurbrick, B.G.; Czuprynski, C.J. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect. Immun. 1987, 55, 1588–1593. [Google Scholar] [CrossRef]
- Godson, C.; Mitchell, S.; Harvey, K.; Petasis, N.A.; Hogg, N.; Brady, H.R. Cutting Edge: Lipoxins Rapidly Stimulate Nonphlogistic Phagocytosis of Apoptotic Neutrophils by Monocyte-Derived Macrophages. J. Immunol. 2000, 164, 1663–1667. [Google Scholar] [CrossRef]
- Schlesinger, L.S.; Bellinger-Kawahara, C.G.; Payne, N.R.; Horwitz, M.A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 1990, 144, 2771–2780. [Google Scholar] [CrossRef]
- Bounous, D.I.; Enright, F.M.; Gossett, K.A.; Berry, C.M. Phagocytosis, killing, and oxidant production by bovine monocyte-derived macrophages upon exposure to Brucella abortus strain 2308. Vet. Immunol. Immunopathol. 1993, 37, 243–256. [Google Scholar] [CrossRef]
- Ngaira, J.M.; Nantulya, V.M.; Musoke, A.J.; Hirumi, K. Phagocytosis of antibody-sensitized Trypanosoma brucei in vitro by bovine peripheral blood monocytes. Immunology 1983, 49, 393–400. [Google Scholar] [PubMed]
- Muñoz-Caro, T.; Silva, L.M.R.; Ritter, C.; Taubert, A.; Hermosilla, C. Besnoitia besnoiti tachyzoites induce monocyte extracellular trap formation. Parasitol. Res. 2014, 113, 4189–4197. [Google Scholar] [CrossRef]
- Pérez, D.; Muñoz, M.C.; Molina, J.M.; Muñoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae induces NADPH oxidase-dependent monocyte extracellular trap formation and upregulates IL-12 and TNF-α, IL-6 and CCL2 gene transcription. Vet. Parasitol. 2016, 227, 143–150. [Google Scholar] [CrossRef]
- Schulz, M.; Zambrano, F.; Schuppe, H.-C.; Wagenlehner, F.; Taubert, A.; Gaertner, U.; Sánchez, R.; Hermosilla, C. Monocyte-derived extracellular trap (MET) formation induces aggregation and affects motility of human spermatozoa in vitro. Syst. Biol. Reprod. Med. 2019, 65, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rayner, B.S.; Jensen, M.; Hawkins, C.L. In Vitro Stimulation and Visualization of Extracellular Trap Release in Differentiated Human Monocyte-derived Macrophages. J. Vis. Exp. JoVE 2019, 153, e60541. [Google Scholar] [CrossRef]
- Granger, V.; Faille, D.; Marani, V.; Noël, B.; Gallais, Y.; Szely, N.; Flament, H.; Pallardy, M.; Chollet-Martin, S.; de Chaisemartin, L. Human blood monocytes are able to form extracellular traps. J. Leukoc. Biol. 2017, 102, 775–781. [Google Scholar] [CrossRef]
- Quintin, J.; Saeed, S.; Martens, J.H.A.; Giamarellos-Bourboulis, E.J.; Ifrim, D.C.; Logie, C.; Jacobs, L.; Jansen, T.; Kullberg, B.-J.; Wijmenga, C.; et al. Candida albicans Infection Affords Protection against Reinfection via Functional Reprogramming of Monocytes. Cell Host Microbe 2012, 12, 223–232. [Google Scholar] [CrossRef]
- Netea, M.G.; Quintin, J.; van der Meer, J.W.M. Trained Immunity: A Memory for Innate Host Defense. Cell Host Microbe 2011, 9, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Quintin, J.; Cheng, S.-C.; van der Meer, J.W.M.; Netea, M.G. Innate immune memory: Towards a better understanding of host defense mechanisms. Curr. Opin. Immunol. 2014, 29, 1–7. [Google Scholar] [CrossRef]
- Ifrim, D.C.; Quintin, J.; Joosten, L.A.B.; Jacobs, C.; Jansen, T.; Jacobs, L.; Gow, N.A.R.; Williams, D.L.; van der Meer, J.W.M.; Netea, M.G. Trained Immunity or Tolerance: Opposing Functional Programs Induced in Human Monocytes after Engagement of Various Pattern Recognition Receptors. Clin. Vaccine Immunol. 2014, 21, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.B.; Ifrim, D.C.; Saeed, S.; Jacobs, C.; van Loenhout, J.; de Jong, D.; Stunnenberg, H.G.; et al. Bacille Calmette-Guérin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc. Natl. Acad. Sci. USA 2012, 109, 17537–17542. [Google Scholar] [CrossRef] [PubMed]
- Reichel, M.; Muñoz-Caro, T.; Sanchez Contreras, G.; Rubio García, A.; Magdowski, G.; Gärtner, U.; Taubert, A.; Hermo-silla, C. Harbour seal (Phoca vitulina) PMN and monocytes release extracellular traps to capture the apicomplexan parasite Toxoplasma gondii. Dev. Comp. Immunol. 2015, 50, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, Z.; Hermosilla, C.; Taubert, A.; He, X.; Wang, X.; Gong, P.; Li, J.; Zhang, X. Caprine Monocytes Release Extracellular Traps against Neospora caninum In Vitro. Front. Immunol. 2018, 8, 2016. [Google Scholar] [CrossRef]
- Tammam, J.; Ware, C.; Efferson, C.; O’Neil, J.; Rao, S.; Qu, X.; Gorenstein, J.; Angagaw, M.; Kim, H.; Kenific, C.; et al. Down-regulation of the Notch pathway mediated by a γ-secretase inhibitor induces anti-tumour effects in mouse models of T-cell leukaemia: Anti-tumour effects of GSI in xenograft model. Br. J. Pharmacol. 2009, 158, 1183–1195. [Google Scholar] [CrossRef]
- Wu, W.; Nie, L.; Zhang, L.; Li, Y. The notch pathway promotes NF-κB activation through Asb2 in T cell acute lymphoblastic leukemia cells. Cell. Mol. Biol. Lett. 2018, 23, 37. [Google Scholar] [CrossRef]
- Amsen, D.; Blander, J.M.; Lee, G.R.; Tanigaki, K.; Honjo, T.; Flavell, R.A. Instruction of Distinct CD4 T Helper Cell Fates by Different Notch Ligands on Antigen-Presenting Cells. Cell 2004, 117, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.; Tang, S.-M.T.; Canner, J.P.; Morishige, K.; Arboleda-Velasquez, J.F.; Cardoso, A.A.; Carlesso, N.; Aster, J.C.; Ai-kawa, M. Delta-Like 4 Induces Notch Signaling in Macrophages: Implications for Inflammation. Circulation 2007, 115, 2948–2956. [Google Scholar] [CrossRef]
- Shahiduzzaman, M.; Dyachenko, V.; Obwaller, A.; Unglaube, S.; Daugschies, A. Combination of cell culture and quantitative PCR for screening of drugs against Cryptosporidium parvum. Vet. Parasitol. 2009, 162, 271–277. [Google Scholar] [CrossRef]
- Vélez, J.; Velasquez, Z.; Silva, L.M.R.; Gärtner, U.; Failing, K.; Daugschies, A.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic Signatures of Cryptosporidium parvum-Infected HCT-8 Cells and Impact of Selected Metabolic Inhibitors on C. parvum Infection under Physioxia and Hyperoxia. Biology 2021, 10, 60. [Google Scholar] [CrossRef]
- Vélez, J.; Lange, M.K.; Zieger, P.; Yoon, I.; Failing, K.; Bauer, C. Long-term use of yeast fermentation products in comparison to halofuginone for the control of cryptosporidiosis in neonatal calves. Vet. Parasitol. 2019, 269, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Plutzer, J.; Lassen, B.; Jokelainen, P.; Djurković-Djaković, O.; Kucsera, I.; Dorbek-Kolin, E.; Šoba, B.; Sréter, T.; Imre, K.; Omeragić, J.; et al. Review of Cryptosporidium and Giardia in the eastern part of Europe, 2016. Eurosurveillance 2018, 23, 16–00825. [Google Scholar] [CrossRef]
- Broglia, A.; Reckinger, S.; Cacció, S.; Nöckler, K. Distribution of Cryptosporidium parvum subtypes in calves in Germany. Vet. Parasitol. 2008, 154, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Najdrowski, M.; Joachim, A.; Daugschies, A. An improved in vitro infection model for viability testing of Cryptosporidium parvum oocysts. Vet. Parasitol. 2007, 150, 150–154. [Google Scholar] [CrossRef]
- Taubert, A.; Behrendt, J.H.; Sühwold, A.; Zahner, H.; Hermosilla, C. Monocyte- and macrophage-mediated immune reactions against Eimeria bovis. Vet. Parasitol. 2009, 164, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Grob, D.; Conejeros, I.; Velásquez, Z.D.; Preußer, C.; Gärtner, U.; Alarcón, P.; Burgos, R.A.; Hermosilla, C.; Taubert, A. Trypanosoma brucei brucei Induces Polymorphonuclear Neutrophil Activation and Neutrophil Extracellular Traps Release. Front. Immunol. 2020, 11, 559561. [Google Scholar] [CrossRef]
- Holzhausen, I.; Lendner, M.; Göhring, F.; Steinhöfel, I.; Daugschies, A. Distribution of Cryptosporidium parvum gp60 subtypes in calf herds of Saxony, Germany. Parasitol. Res. 2019, 118, 1549–1558. [Google Scholar] [CrossRef]
- García-Sánchez, M.; Jiménez-Pelayo, L.; Horcajo, P.; Regidor-Cerrillo, J.; Ólafsson, E.B.; Bhandage, A.K.; Barragan, A.; Werling, D.; Ortega-Mora, L.-M.; Collantes-Fernández, E. Differential Responses of Bovine Monocyte-Derived Macrophages to Infection by Neospora caninum Isolates of High and Low Virulence. Front. Immunol. 2019, 10, 915. [Google Scholar] [CrossRef]
- Borggrefe, T.; Giaimo, B.D. (Eds.) Molecular Mechanisms of Notch Signaling; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2018; pp. 187–204. ISBN 978-3-319-89511-6. [Google Scholar]
- Zhou, E.; Conejeros, I.; Gärtner, U.; Mazurek, S.; Hermosilla, C.; Taubert, A. Metabolic requirements of Besnoitia besnoiti tachyzoite-triggered NETosis. Parasitol. Res. 2020, 119, 545–557. [Google Scholar] [CrossRef]
- Rodríguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.B.; López-Villegas, E.O.; Sánchez-García, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef]
- Zhu, X.; Meyers, A.; Long, D.; Ingram, B.; Liu, T.; Yoza, B.K.; Vachharajani, V.; McCall, C.E. Frontline Science: Monocytes sequentially rewire metabolism and bioenergetics during an acute inflammatory response. J. Leukoc. Biol. 2019, 105, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Conejeros, I.; López-Osorio, S.; Zhou, E.; Velásquez, Z.D.; Del Río, M.C.; Burgos, R.A.; Alarcón, P.; Chaparro-Gutiérrez, J.J.; Hermosilla, C.; Taubert, A. Glycolysis, monocarboxylate transport, and purinergic signaling are key events in Eimeria bovis-induced NETosis. Front. Immunol. 2022, 13, 842482. [Google Scholar] [CrossRef]
- Alarcón, P.; Manosalva, C.; Conejeros, I.; Carretta, M.D.; Muñoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. d(−) Lactic Acid-Induced Adhesion of Bovine Neutrophils onto Endothelial Cells Is Dependent on Neutrophils Extracellular Traps Formation and CD11b Expression. Front. Immunol. 2017, 8, 975. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, M.; Young, S.P. Modulation of iron metabolism in monocyte cell line U937 by inflammatory cytokines: Changes in transferrin uptake, iron handling and ferritin mRNA. Biochem. J. 1993, 296, 175–181. [Google Scholar] [CrossRef]
- O’Brien, E.C.; White, C.A.; Wyse, J.; Leacy, E.; Porter, R.K.; Little, M.A.; Hickey, F.B. Pro-inflammatory Stimulation of Monocytes by ANCA Is Linked to Changes in Cellular Metabolism. Front. Med. 2020, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- McGarry, T.; Hanlon, M.M.; Marzaioli, V.; Cunningham, C.C.; Krishna, V.; Murray, K.; Hurson, C.; Gallagher, P.; Nagpal, S.; Veale, D.J.; et al. Rheumatoid arthritis CD14+ monocytes display metabolic and inflammatory dysfunction, a phenotype that precedes clinical manifestation of disease. Clin. Transl. Immunol. 2021, 10, e1237. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Ahmad, Z.; Iskandar, M.A.; Tuomilehto, J.; Al-Mulla, F.; Ahmad, R. TNF-α Induces a Pro-Inflammatory Phenotypic Shift in Monocytes through ACSL1: Relevance to Metabolic Inflammation. Cell. Physiol. Biochem. 2019, 52, 397–407. [Google Scholar] [CrossRef]
- Gálvez, I.; Martín-Cordero, L.; Hinchado, M.D.; Álvarez-Barrientos, A.; Ortega, E. Anti-inflammatory effect of β2 adrenergic stimulation on circulating monocytes with a pro-inflammatory state in high-fat diet-induced obesity. Brain Behav. Immun. 2019, 80, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Viola, A.; Munari, F.; Sánchez-Rodríguez, R.; Scolaro, T.; Castegna, A. The Metabolic Signature of Macrophage Responses. Front. Immunol. 2019, 10, 1462. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Nicholls, D.G.; Brand, M.D. Determining Maximum Glycolytic Capacity Using Extracellular Flux Measurements. PLoS ONE 2016, 11, e0152016. [Google Scholar] [CrossRef] [PubMed]
- Mookerjee, S.A.; Goncalves, R.L.S.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 2015, 1847, 171–181. [Google Scholar] [CrossRef]
- Abrahamsen, M.S.; Templeton, T.J.; Enomoto, S.; Abrahante, J.E.; Zhu, G.; Lancto, C.A.; Deng, M.; Liu, C.; Widmer, G.; Tzipori, S.; et al. Complete Genome Sequence of the Apicomplexan, Cryptosporidium parvum. Science 2004, 304, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Zambrano, F.; Schulz, M.; Pilatz, A.; Wagenlehner, F.; Schuppe, H.-C.; Conejeros, I.; Uribe, P.; Taubert, A.; Sánchez, R.; Hermosilla, C. Increase of leucocyte-derived extracellular traps (ETs) in semen samples from human acute epididymitis patients—A pilot study. J. Assist. Reprod. Genet. 2020, 37, 2223–2231. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, T.G.; Savochkina, A.Y.; Dolgushin, I.I.; Nikushkina, K.V.; Samuseva, I.V. Changes in Functional Activity of Neutrophils and Monocytes Isolated from the Peripheral Blood of Women at Different Phases of the Menstrual Cycle. Bull. Exp. Biol. Med. 2018, 166, 222–224. [Google Scholar] [CrossRef]
- Silva, L.M.R.; Muñoz Caro, T.; Gerstberger, R.; Vila-Viçosa, M.J.M.; Cortes, H.C.E.; Hermosilla, C.; Taubert, A. The apicomplexan parasite Eimeria arloingi induces caprine neutrophil extracellular traps. Parasitol. Res. 2014, 113, 2797–2807. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef] [PubMed]
- Helander, H.F.; Fändriks, L. Surface area of the digestive tract—Revisited. Scand. J. Gastroenterol. 2014, 49, 681–689. [Google Scholar] [CrossRef]
- Dhup, S.; Kumar Dadhich, R.; Ettore Porporato, P.; Sonveaux, P. Multiple Biological Activities of Lactic Acid in Cancer: Influences on Tumor Growth, Angiogenesis and Metastasis. Curr. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Hasheminasab, S.S.; Conejeros, I.; Velásquez, Z.D.; Borggrefe, T.; Gärtner, U.; Kamena, F.; Taubert, A.; Hermosilla, C. ATP Purinergic Receptor P2X1-Dependent Suicidal NETosis Induced by Cryptosporidium parvum under Physioxia Conditions. Biology 2022, 11, 442. [Google Scholar] [CrossRef]
- Parihar, A.; Eubank, T.D.; Doseff, A.I. Monocytes and Macrophages Regulate Immunity through Dynamic Networks of Survival and Cell Death. J. Innate Immun. 2010, 2, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Morada, M.; Lee, S.; Gunther-Cummins, L.; Weiss, L.M.; Widmer, G.; Tzipori, S.; Yarlett, N. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int. J. Parasitol. 2016, 46, 21–29. [Google Scholar] [CrossRef]
- Halestrap, A.P. The SLC16 gene family—Structure, role and regulation in health and disease. Mol. Asp. Med. 2013, 34, 337–349. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Meredith, D. The SLC16 gene family? From monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch. Eur. J. Physiol. 2004, 447, 619–628. [Google Scholar] [CrossRef]
- Desgrandchamps, D.; Munzinger, J. Infectious gastroenteritis in the immunocompetent child. Significance of Cryptosporidium spp. and Aeromonas ssp. Schweiz. Med. Wochenschr. 1989, 119, 276–281. [Google Scholar] [PubMed]
- Koch, A.; Kaske, M. Clinical Efficacy of Intravenous Hypertonic Saline Solution or Hypertonic Bicarbonate Solution in the Treatment of Inappetent Calves with Neonatal Diarrhea. J. Vet. Intern. Med. 2008, 22, 202–211. [Google Scholar] [CrossRef]
- Aronsen, L.; Orvoll, E.; Lysaa, R.; Ravna, A.W.; Sager, G. Modulation of high affinity ATP-dependent cyclic nucleotide transporters by specific and non-specific cyclic nucleotide phosphodiesterase inhibitors. Eur. J. Pharmacol. 2014, 745, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Cowen, D.S.; Lazarus, H.M.; Shurin, S.B.; Stoll, S.E.; Dubyak, G.R. Extracellular adenosine triphosphate activates calcium mobilization in human phagocytic leukocytes and neutrophil/monocyte progenitor cells. J. Clin. Investig. 1989, 83, 1651–1660. [Google Scholar] [CrossRef] [PubMed]
- Chacko, B.K.; Zhi, D.; Darley-Usmar, V.M.; Mitchell, T. The Bioenergetic Health Index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 2016, 8, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.-J.; Lee, H.-J.; Kim, J.-H.; Kim, D.-K. Extracellular ATP induces apoptotic signaling in human monocyte leukemic cells, HL-60 and F-36P. Arch. Pharmacal Res. 2006, 29, 1032–1041. [Google Scholar] [CrossRef]
- Jeon, J.-H.; Hong, C.-W.; Kim, E.Y.; Lee, J.M. Current Understanding on the Metabolism of Neutrophils. Immune Netw. 2020, 20, e46. [Google Scholar] [CrossRef]
- Shang, Y.; Smith, S.; Hu, X. Role of Notch signaling in regulating innate immunity and inflammation in health and disease. Protein Cell 2016, 7, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, E.; Pérez, M.A.; Rubio, A.; Ruiz-Hidalgo, M.J.; Baladrón, V.; García-Ramírez, J.J.; Gómez, J.C.; Laborda, J.; Díaz-Guerra, M.J.M. Notch-1 Up-Regulation and Signaling following Macrophage Activation Modulates Gene Expression Patterns Known to Affect Antigen-Presenting Capacity and Cytotoxic Activity. J. Immunol. 2006, 176, 5362–5373. [Google Scholar] [CrossRef]
- Monsalve, E.; Ruiz-García, A.; Baladrón, V.; Ruiz-Hidalgo, M.J.; Sánchez-Solana, B.; Rivero, S.; García-Ramírez, J.J.; Rubio, A.; Laborda, J.; Díaz-Guerra, M.J.M. Notch1 upregulates LPS-induced macrophage activation by increasing NF-κB activity. Eur. J. Immunol. 2009, 39, 2556–2570. [Google Scholar] [CrossRef]
- Palaga, T.; Buranaruk, C.; Rengpipat, S.; Fauq, A.H.; Golde, T.E.; Kaufmann, S.H.E.; Osborne, B.A. Notch signaling is activated by TLR stimulation and regulates macrophage functions. Eur. J. Immunol. 2008, 38, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C.; Liu, Z.; Liu, X.; Han, C.; Cao, X.; Li, N. Notch Signal Suppresses Toll-like Receptor-triggered Inflammatory Responses in Macrophages by Inhibiting Extracellular Signal-regulated Kinase 1/2-mediated Nuclear Factor κB Activation. J. Biol. Chem. 2012, 287, 6208–6217. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasheminasab, S.S.; Conejeros, I.; Gärtner, U.; Kamena, F.; Taubert, A.; Hermosilla, C.R. MCT-Dependent Cryptosporidium parvum-Induced Bovine Monocyte Extracellular Traps (METs) under Physioxia. Biology 2023, 12, 961. https://doi.org/10.3390/biology12070961
Hasheminasab SS, Conejeros I, Gärtner U, Kamena F, Taubert A, Hermosilla CR. MCT-Dependent Cryptosporidium parvum-Induced Bovine Monocyte Extracellular Traps (METs) under Physioxia. Biology. 2023; 12(7):961. https://doi.org/10.3390/biology12070961
Chicago/Turabian StyleHasheminasab, Seyed Sajjad, Iván Conejeros, Ulrich Gärtner, Faustin Kamena, Anja Taubert, and Carlos R. Hermosilla. 2023. "MCT-Dependent Cryptosporidium parvum-Induced Bovine Monocyte Extracellular Traps (METs) under Physioxia" Biology 12, no. 7: 961. https://doi.org/10.3390/biology12070961
APA StyleHasheminasab, S. S., Conejeros, I., Gärtner, U., Kamena, F., Taubert, A., & Hermosilla, C. R. (2023). MCT-Dependent Cryptosporidium parvum-Induced Bovine Monocyte Extracellular Traps (METs) under Physioxia. Biology, 12(7), 961. https://doi.org/10.3390/biology12070961






