Breaking Bad: Inflammasome Activation by Respiratory Viruses
Abstract
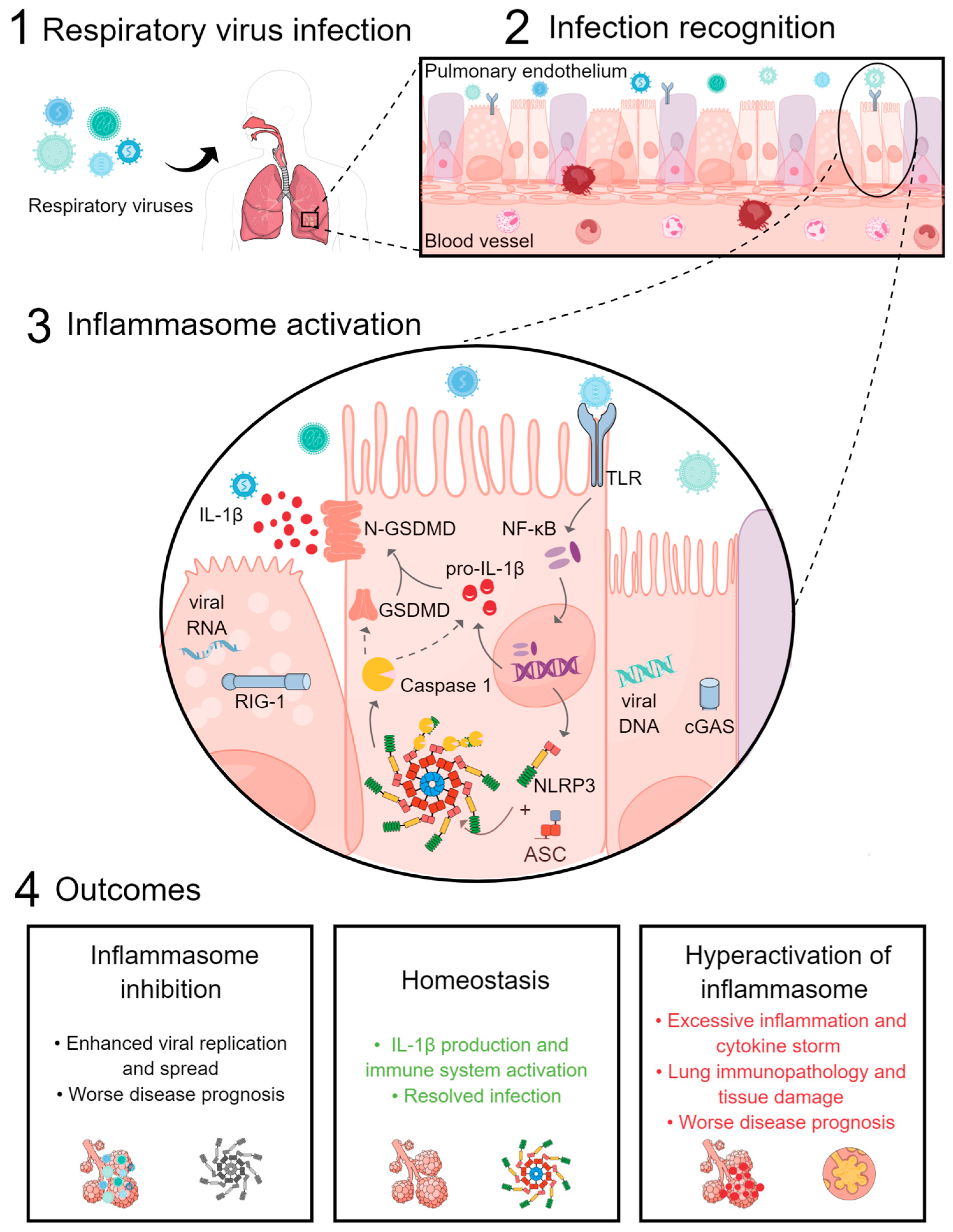
Simple Summary
Abstract
1. Introduction
2. Influenza Virus
3. Parainfluenza Virus
4. Human Metapneumovirus
5. Respiratory Syncytial Virus
6. Coronavirus
7. Rhinovirus
8. Adenovirus
9. Human Bocavirus
10. Non-Coding RNAs and Inflammasome Modulation during Respiratory Virus Infection
11. Conclusions
| Virus | Activator | Inflammasome |
|---|---|---|
| Adenovirus | ||
| Ad5 | Protein VI [21] | AIM2 [21] |
| Ad5 | dsDNA [97,98] | NLRP3 [97,98,102,103] |
| Ad5 | dsDNA [102] | cGAS/STING-NLRP3 [102] |
| Bocavirus | Viral RNA [113] | NLRP3 [113] |
| Influenza | ||
| IAV | dsDNA [20] | AIM2 [20] |
| IAV | M2 protein [27] | NLRP3 [123] |
| Parainfluenza | Viral particle [35] | TLR2/NLRP3 [35] |
| Metapneumovirus | HMPV SH [38] | NLRP3 [38,39] |
| Respiratory syncytial virus | Viroporin SH [12] | NLRP3 [12,13] |
| Coronavirus | ||
| SARS-CoV | Spike [77] | NLRP3 [77] |
| SARS-CoV | ORF3a [70,73] | NLRP3 [72]/RIPK3 [74] |
| SARS-CoV | ORF8b [75] | NLRP3 [75] |
| SARS-CoV-2 | Envelope [52] | NLRP3 [52] |
| SARS-CoV-2 | ORF3a [71] | NLRP3 [71] |
| SARS-CoV-2 | Nucleocapsid [58] | NLRP3 [58] |
| SARS-CoV-2 | NS6 [63] | NLRP3 [63] |
| SARS-CoV-2 | Spike [76,77] | NLRP3 [76,77] |
| SARS-CoV-2 | NS5 [78] | NLRP1 [78] |
| Rhinovirus | ||
| HRV | 2B [19] | NLRP3/NLRC5 [19] |
| HRV | 3C [89] | NLRP1 [89] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pribul, P.K.; Harker, J.; Wang, B.; Wang, H.; Tregoning, J.S.; Schwarze, J.; Openshaw, P.J.M. Alveolar Macrophages Are a Major Determinant of Early Responses to Viral Lung Infection but Do Not Influence Subsequent Disease Development. J. Virol. 2008, 82, 4441–4448. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar Macrophages Are Essential for Protection from Respiratory Failure and Associated Morbidity Following Influenza Virus Infection. PLoS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef]
- Peiró, T.; Patel, D.F.; Akthar, S.; Gregory, L.G.; Pyle, C.J.; Harker, J.A.; Birrell, M.A.; Lloyd, C.M.; Snelgrove, R.J. Neutrophils Drive Alveolar Macrophage IL-1β Release during Respiratory Viral Infection. Thorax 2018, 73, 546–556. [Google Scholar] [CrossRef]
- Tan, X.; Sun, L.; Chen, J.; Chen, Z.J. Detection of Microbial Infections Through Innate Immune Sensing of Nucleic Acids. Annu. Rev. Microbiol. 2018, 72, 447–478. [Google Scholar] [CrossRef]
- Man, S.M.; Kanneganti, T.-D. Converging Roles of Caspases in Inflammasome Activation, Cell Death and Innate Immunity. Nat. Rev. Immunol. 2016, 16, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Mariathasan, S.; Newton, K.; Monack, D.M.; Vucic, D.; French, D.M.; Lee, W.P.; Roose-Girma, M.; Erickson, S.; Dixit, V.M. Differential Activation of the Inflammasome by Caspase-1 Adaptors ASC and Ipaf. Nature 2004, 430, 213–218. [Google Scholar] [CrossRef]
- Agostini, L.; Martinon, F.; Burns, K.; McDermott, M.F.; Hawkins, P.N.; Tschopp, J. NALP3 Forms an IL-1β-Processing Inflammasome with Increased Activity in Muckle-Wells Autoinflammatory Disorder. Immunity 2004, 20, 319–325. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Kuriakose, T.; Kanneganti, T.D. Regulation and Functions of NLRP3 Inflammasome during Influenza Virus Infection. Mol. Immunol. 2017, 86, 56–64. [Google Scholar] [CrossRef]
- Lupfer, C.; Kanneganti, T.-D. The Expanding Role of NLRs in Antiviral Immunity. Immunol. Rev. 2013, 255, 13–24. [Google Scholar] [CrossRef]
- Triantafilou, K.; Kar, S.; Vakakis, E.; Kotecha, S.; Triantafilou, M. Human Respiratory Syncytial Virus Viroporin SH: A Viral Recognition Pathway Used by the Host to Signal Inflammasome Activation. Thorax 2013, 68, 66–75. [Google Scholar] [CrossRef]
- Segovia, J.; Sabbah, A.; Mgbemena, V.; Tsai, S.-Y.; Chang, T.-H.; Berton, M.T.; Morris, I.R.; Allen, I.C.; Ting, J.P.-Y.; Bose, S. TLR2/MyD88/NF-ΚB Pathway, Reactive Oxygen Species, Potassium Efflux Activates NLRP3/ASC Inflammasome during Respiratory Syncytial Virus Infection. PLoS ONE 2012, 7, e29695. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes Are Activated in Response to SARS-CoV-2 Infection and Are Associated with COVID-19 Severity in Patients. J. Exp. Med. 2021, 218, e20201707. [Google Scholar] [CrossRef]
- Schulte, M.; Sorkin, M.; Al-Benna, S.; Stupka, J.; Hirsch, T.; Daigeler, A.; Kesting, M.R.; Steinau, H.-U.; Jacobsen, F.; Steinstraesser, L. Innate Immune Response after Adenoviral Gene Delivery into Skin Is Mediated by AIM2, NALP3, DAI and Mda5. Springerplus 2013, 2, 234. [Google Scholar] [CrossRef]
- Graham, A.C.; Hilmer, K.M.; Zickovich, J.M.; Obar, J.J. Inflammatory Response of Mast Cells during Influenza A Virus Infection Is Mediated by Active Infection and RIG-I Signaling. J. Immunol. 2013, 190, 4676–4684. [Google Scholar] [CrossRef]
- Pothlichet, J.; Meunier, I.; Davis, B.K.; Ting, J.P.Y.; Skamene, E.; von Messling, V.; Vidal, S.M. Type I IFN Triggers RIG-I/TLR3/NLRP3-Dependent Inflammasome Activation in Influenza A Virus Infected Cells. PLoS Pathog. 2013, 9, e1003256. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhou, Y.T.; Wang, L.Q.; Li, L.Y.; Bao, Q.; Tian, S.; Chen, M.X.; Chen, H.X.; Cui, J.; Li, C.W. NOD-like Receptor Family, Pyrin Domain Containing 3 (NLRP3) Contributes to Inflammation, Pyroptosis, and Mucin Production in Human Airway Epithelium on Rhinovirus Infection. J. Allergy Clin. Immunol. 2019, 144, 777–787.e9. [Google Scholar] [CrossRef] [PubMed]
- Triantafilou, K.; Kar, S.; Van Kuppeveld, F.J.M.; Triantafilou, M. Rhinovirus-Induced Calcium Flux Triggers NLRP3 and NLRC5 Activation in Bronchial Cells. Am. J. Respir. Cell Mol. Biol. 2013, 49, 923–934. [Google Scholar] [CrossRef]
- Zhang, H.; Luo, J.; Alcorn, J.F.; Chen, K.; Fan, S.; Pilewski, J.; Liu, A.; Chen, W.; Kolls, J.K.; Wang, J. AIM2 Inflammasome Is Critical for Influenza-Induced Lung Injury and Mortality. J. Immunol. 2017, 198, 4383–4393. [Google Scholar] [CrossRef]
- Eichholz, K.; Bru, T.; Thu, T.; Tran, P.; Fernandes, P.; Welles, H.; Mennechet, F.J.D.; Manel, N.; Alves, P.; Perreau, M.; et al. Immune-Complexed Adenovirus Induce AIM2-Mediated Pyroptosis in Human Dendritic Cells. PLoS Pathog. 2016, 12, e1005871. [Google Scholar] [CrossRef]
- Thomas, P.G.; Dash, P.; Aldridge, J.R.; Ellebedy, A.H.; Reynolds, C.; Funk, A.J.; Martin, W.J.; Lamkanfi, M.; Webby, R.J.; Boyd, K.L.; et al. The Intracellular Sensor NLRP3 Mediates Key Innate and Healing Responses to Influenza A Virus via the Regulation of Caspase-1. Immunity 2009, 30, 566–575. [Google Scholar] [CrossRef]
- Malinczak, C.A.; Schuler, C.F.; Duran, A.J.; Rasky, A.J.; Mire, M.M.; Núñez, G.; Lukacs, N.W.; Fonseca, W. NLRP3-Inflammasome Inhibition during Respiratory Virus Infection Abrogates Lung Immunopathology and Long-Term Airway Disease Development. Viruses 2021, 13, 692. [Google Scholar] [CrossRef]
- Kanneganti, T.-D.; Body-Malapel, M.; Amer, A.; Park, J.-H.; Whitfield, J.; Franchi, L.; Taraporewala, Z.F.; Miller, D.; Patton, J.T.; Inohara, N.; et al. Critical Role for Cryopyrin/Nalp3 in Activation of Caspase-1 in Response to Viral Infection and Double-Stranded RNA. J. Biol. Chem. 2006, 281, 36560–36568. [Google Scholar] [CrossRef]
- Allen, I.C.; Scull, M.A.; Moore, C.B.; Holl, E.K.; McElvania-TeKippe, E.; Taxman, D.J.; Guthrie, E.H.; Pickles, R.J.; Ting, J.P.Y. The NLRP3 Inflammasome Mediates In Vivo Innate Immunity to Influenza A Virus through Recognition of Viral RNA. Immunity 2009, 30, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Tate, M.D.; Ong, J.D.H.; Dowling, J.K.; McAuley, J.L.; Robertson, A.B.; Latz, E.; Drummond, G.R.; Cooper, M.A.; Hertzog, P.J.; Mansell, A. Reassessing the Role of the NLRP3 Inflammasome during Pathogenic Influenza A Virus Infection via Temporal Inhibition. Sci. Rep. 2016, 6, 27912. [Google Scholar] [CrossRef]
- Ichinohe, T.; Pang, I.K.; Iwasaki, A. Influenza Virus Activates Inflammasomes via Its Intracellular M2 Ion Channel. Nat. Immunol. 2010, 11, 404–410. [Google Scholar] [CrossRef]
- Moriyama, M.; Chen, I.-Y.; Kawaguchi, A.; Koshiba, T.; Nagata, K.; Takeyama, H.; Hasegawa, H.; Ichinohe, T. The RNA- and TRIM25-Binding Domains of Influenza Virus NS1 Protein Are Essential for Suppression of NLRP3 Inflammasome-Mediated Interleukin-1β Secretion. J. Virol. 2016, 90, 4105–4114. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Lee, H.K.; Ogura, Y.; Flavell, R.; Iwasaki, A. Inflammasome Recognition of Influenza Virus Is Essential for Adaptive Immune Responses. J. Exp. Med. 2009, 206, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, J.M.; Poon, L.L.; Lee, K.C.; Ng, W.F.; Lai, S.T.; Leung, C.Y.; Chu, C.M.; Hui, P.K.; Mak, K.L.; Lim, W.; et al. Lung Pathology of Fatal Severe Acute Respiratory Syndrome. Lancet 2003, 361, 1773–1778. [Google Scholar] [CrossRef]
- Harms, P.W.; Schmidt, L.A.; Smith, L.B.; Newton, D.W.; Pletneva, M.A.; Walters, L.L.; Tomlins, S.A.; Fisher-Hubbard, A.; Napolitano, L.M.; Park, P.K.; et al. Autopsy Findings in Eight Patients with Fatal H1N1 Influenza. Am. J. Clin. Pathol. 2010, 134, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Coates, B.M.; Staricha, K.L.; Koch, C.M.; Cheng, Y.; Shumaker, D.K.; Budinger, G.R.S.; Perlman, H.; Misharin, A.V.; Ridge, K.M. Inflammatory Monocytes Drive Influenza A Virus–Mediated Lung Injury in Juvenile Mice. J. Immunol. 2018, 200, 2391–2404. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Frank, A.L.; Taber, L.H.; Kasel, J.A. Parainfluenza Virus Type 3: Seasonality and Risk of Infection and Reinfection in Young Children. J. Infect. Dis. 1984, 150, 851–857. [Google Scholar] [CrossRef]
- Fox, T.G.; Christenson, J.C. Influenza and Parainfluenza Viral Infections in Children. Pediatr. Rev. 2014, 35, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Shil, N.K.; Pokharel, S.M.; Banerjee, A.K.; Hoffman, M.; Bose, S. Inflammasome Antagonism by Human Parainfluenza Virus Type 3 C Protein. J. Virol. 2018, 92, e01776-17. [Google Scholar] [CrossRef] [PubMed]
- Ohta, K.; Matsumoto, Y.; Nishio, M. Human Parainfluenza Virus Type 2 V Protein Inhibits Caspase-1. J. Gen. Virol. 2018, 99, 501–511. [Google Scholar] [CrossRef]
- Schuster, J.E.; Williams, J.V. Human Metapneumovirus. Microbiol. Spectr. 2014, 2, 237–247. [Google Scholar] [CrossRef]
- Lê, V.B.; Dubois, J.; Couture, C.; Cavanagh, M.-H.; Uyar, O.; Pizzorno, A.; Rosa-Calatrava, M.; Hamelin, M.-È.; Boivin, G. Human Metapneumovirus Activates NOD-like Receptor Protein 3 Inflammasome via Its Small Hydrophobic Protein Which Plays a Detrimental Role during Infection in Mice. PLoS Pathog. 2019, 15, e1007689. [Google Scholar] [CrossRef]
- Malmo, J.; Moe, N.; Krokstad, S.; Ryan, L.; Loevenich, S.; Johnsen, I.B.; Espevik, T.; Nordbø, S.A.; Døllner, H.; Anthonsen, M.W. Cytokine Profiles in Human Metapneumovirus Infected Children: Identification of Genes Involved in the Antiviral Response and Pathogenesis. PLoS ONE 2016, 11, e0155484. [Google Scholar] [CrossRef]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global Burden of Acute Lower Respiratory Infections Due to Respiratory Syncytial Virus in Young Children: A Systematic Review and Meta-Analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef]
- Griffiths, C.; Drews, S.J.; Marchant, D.J. Respiratory Syncytial Virus: Infection, Detection, and New Options for Prevention and Treatment. Clin. Microbiol. Rev. 2017, 30, 277–319. [Google Scholar] [CrossRef]
- Simoes, E.A. Respiratory Syncytial Virus Infection. Lancet 1999, 354, 847–852. [Google Scholar] [CrossRef]
- Shim, Y.R.; Lee, H.K. Caspase-1 Independent Viral Clearance and Adaptive Immunity against Mucosal Respiratory Syncytial Virus Infection. Immune Netw. 2015, 15, 73. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-K.; Kim, T.S.; Hufford, M.M.; Braciale, T.J. Viral Infection of the Lung: Host Response and Sequelae. J. Allergy Clin. Immunol. 2013, 132, 1263–1276. [Google Scholar] [CrossRef]
- Tabarani, C.M.; Bonville, C.A.; Suryadevara, M.; Branigan, P.; Wang, D.; Huang, D.; Rosenberg, H.F.; Domachowske, J.B. Novel Inflammatory Markers, Clinical Risk Factors and Virus Type Associated with Severe Respiratory Syncytial Virus Infection. Pediatr. Infect. Dis. J. 2013, 32, e437–e442. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Troutman, T.D.; Edukulla, R.; Pasare, C. Priming Microenvironments Dictate Cytokine Requirements for T Helper 17 Cell Lineage Commitment. Immunity 2011, 35, 1010–1022. [Google Scholar] [CrossRef]
- Stoppelenburg, A.J.; de Roock, S.; Hennus, M.P.; Bont, L.; Boes, M. Elevated Th17 Response in Infants Undergoing Respiratory Viral Infection. Am. J. Pathol. 2014, 184, 1274–1279. [Google Scholar] [CrossRef]
- Lukacs, N.W.; Smit, J.J.; Mukherjee, S.; Morris, S.B.; Nunez, G.; Lindell, D.M. Respiratory Virus-Induced TLR7 Activation Controls IL-17–Associated Increased Mucus via IL-23 Regulation. J. Immunol. 2010, 185, 2231–2239. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Zhang, Z.; Xie, T.; Xu, J.; Yan, J.; Kang, A.; Dai, Q.; Wang, S.; Ji, J.; Shan, J. Jinxin Oral Liquid Inhibits Human Respiratory Syncytial Virus-Induced Excessive Inflammation Associated with Blockade of the NLRP3/ASC/Caspase-1 Pathway. Biomed. Pharmacother. 2018, 103, 1376–1383. [Google Scholar] [CrossRef]
- Shen, C.; Zhang, Z.; Xie, T.; Ji, J.; Xu, J.; Lin, L.; Yan, J.; Kang, A.; Dai, Q.; Dong, Y.; et al. Rhein Suppresses Lung Inflammatory Injury Induced by Human Respiratory Syncytial Virus through Inhibiting NLRP3 Inflammasome Activation via NF-κB Pathway in Mice. Front. Pharmacol. 2020, 10, 1600. [Google Scholar] [CrossRef]
- de Almeida, L.; da Silva, A.L.N.; Rodrigues, T.S.; Oliveira, S.; Ishimoto, A.Y.; Seribelli, A.A.; Becerra, A.; Andrade, W.A.; Ataide, M.A.; Caetano, C.C.S.; et al. Identification of Immunomodulatory Drugs That Inhibit Multiple Inflammasomes and Impair SARS-CoV-2 Infection. Sci. Adv. 2022, 8, 5400. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Dediego, M.L.; Verdiá-Báguena, C.; Jimenez-Guardeñ, O.J.M.; Regla-Nava, J.A. Severe Acute Respiratory Syndrome Coronavirus Envelope Protein Ion Channel Activity Promotes Virus Fitness and Pathogenesis. PLoS Pathog. 2014, 10, 1004077. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, H.T.; Goncalves, J.; Xiao, Y.; Wang, M.; Guo, Y.; Sun, C.; Tang, X.; Jing, L.; Zhang, M.; et al. An Interpretable Mortality Prediction Model for COVID-19 Patients. Nat. Mach. Intell. 2020, 2, 283–288. [Google Scholar] [CrossRef]
- Adamik, B.; Ambrożek-Latecka, M.; Dragan, B.; Jeznach, A.; Śmiechowicz, J.; Gożdzik, W.; Skirecki, T. Inflammasome-Related Markers upon ICU Admission Do Not Correlate with Outcome in Critically Ill COVID-19 Patients. Shock 2022, 57, 672–679. [Google Scholar] [CrossRef]
- Ma, J.; Zhu, F.; Zhao, M.; Shao, F.; Yu, D.; Ma, J.; Zhang, X.; Li, W.; Qian, Y.; Zhang, Y.; et al. SARS-CoV-2 Nucleocapsid Suppresses Host Pyroptosis by Blocking Gasdermin D Cleavage. EMBO J. 2021, 40, e108249. [Google Scholar] [CrossRef] [PubMed]
- Ahn, M.; Anderson, D.E.; Zhang, Q.; Tan, C.W.; Lim, B.L.; Luko, K.; Wen, M.; Chia, W.N.; Mani, S.; Wang, L.C.; et al. Dampened NLRP3-Mediated Inflammation in Bats and Implications for a Special Viral Reservoir Host. Nat. Microbiol. 2019, 4, 789–799. [Google Scholar] [CrossRef]
- Xian, H.; Liu, Y.; Rundberg Nilsson, A.; Gatchalian, R.; Crother, T.R.; Tourtellotte, W.G.; Zhang, Y.; Aleman-Muench, G.R.; Lewis, G.; Chen, W.; et al. Metformin Inhibition of Mitochondrial ATP and DNA Synthesis Abrogates NLRP3 Inflammasome Activation and Pulmonary Inflammation. Immunity 2021, 54, 1463–1477.e11. [Google Scholar] [CrossRef]
- Pan, P.; Shen, M.; Yu, Z.; Ge, W.; Chen, K.; Tian, M.; Xiao, F.; Wang, Z.; Wang, J.; Jia, Y.; et al. SARS-CoV-2 N Protein Promotes NLRP3 Inflammasome Activation to Induce Hyperinflammation. Nat. Commun. 2021, 12, 4664. [Google Scholar] [CrossRef]
- Zeng, J.; Xie, X.; Feng, X.L.; Xu, L.; Han, J.B.; Yu, D.; Zou, Q.C.; Liu, Q.; Li, X.; Ma, G.; et al. Specific Inhibition of the NLRP3 Inflammasome Suppresses Immune Overactivation and Alleviates COVID-19 like Pathology in Mice. EBioMedicine 2022, 75, 103803. [Google Scholar] [CrossRef]
- Courjon, J.; Dufies, O.; Robert, A.; Bailly, L.; Torre, C.; Chirio, D.; Contenti, J.; Vitale, S.; Loubatier, C.; Doye, A.; et al. Heterogeneous NLRP3 Inflammasome Signature in Circulating Myeloid Cells as a Biomarker of COVID-19 Severity. Blood Adv. 2021, 5, 1523–1534. [Google Scholar] [CrossRef]
- Sefik, E.; Qu, R.; Junqueira, C.; Kaffe, E.; Mirza, H.; Zhao, J.; Brewer, J.R.; Han, A.; Steach, H.R.; Israelow, B.; et al. Inflammasome Activation in Infected Macrophages Drives COVID-19 Pathology. Nature 2022, 606, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-Mediated SARS-CoV-2 Infection of Monocytes Activates Inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, Y.; Huang, Z.; Xu, W.; Hu, W.; Yi, L.; Liu, Z.; Chan, H.; Zeng, J.; Liu, X.; et al. SARS-CoV-2 Non-Structural Protein 6 Triggers NLRP3-Dependent Pyroptosis by Targeting ATP6AP1. Cell Death Differ. 2022, 29, 1240–1254. [Google Scholar] [CrossRef]
- Gholaminejhad, M.; Forouzesh, M.; Ebrahimi, B.; Mahdavi, S.A.; Mirtorabi, S.D.; Liaghat, A.; Monabati, S.J.; Hamza, M.O.; Hassanzadeh, G. Formation and Activity of NLRP3 Inflammasome and Histopathological Changes in the Lung of Corpses with COVID-19. J. Mol. Histol. 2022, 53, 883–890. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Soares, V.C.; De Azevedo-Quintanilha, I.G.; Da, S.; Gomes Dias, S.; Fintelman-Rodrigues, N.; Sacramento, C.Q.; Mattos, M.; De Freitas, C.S.; Temerozo, J.R.; et al. Cell Death Discovery SARS-CoV-2 Engages Inflammasome and Pyroptosis in Human Primary Monocytes. Cell Death Discov. 2021, 7, 43. [Google Scholar] [CrossRef]
- Silva, C.S.M.; Wagner Wanderley, C.S.; Protasio Veras, F.; Velozo Gonçalves, A.; Haruo Fernandes Lima, M.; Escher Toller-Kawahisa, J.; Freitas Gomes, G.; Carvalho Nascimento, D.; Silva Monteiro, V.V.; Marques Paiva, I.; et al. Gasdermin-D Activation by SARS-CoV-2 Triggers NET and Mediate COVID-19 Immunopathology. Crit. Care 2022, 26, 206. [Google Scholar] [CrossRef]
- Maes, M.; Luiz, W.; Tedesco Junior, D.; Alysson, M.; Lozovoy, B.; Tiemi, M.; Mori, E.; Danelli, T.; Delicato De Almeida, E.R.; Mestre Tejo, A.; et al. In COVID-19, NLRP3 Inflammasome Genetic Variants Are Associated with Critical Disease and These Effects Are Partly Mediated by the Sickness Symptom Complex: A Nomothetic Network Approach. Mol. Psychiatry 2022, 27, 1945–1955. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Verdiá-Báguena, C.; Jimenez-Guardeño, J.M.; Regla-Nava, J.A.; Castaño-Rodriguez, C.; Fernandez-Delgado, R.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe Acute Respiratory Syndrome Coronavirus E Protein Transports Calcium Ions and Activates the NLRP3 Inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef]
- Chen, I.-Y.; Moriyama, M.; Chang, M.-F.; Ichinohe, T. Severe Acute Respiratory Syndrome Coronavirus Viroporin 3a Activates the NLRP3 Inflammasome. Front. Microbiol. 2019, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Akinyemi, I.A.; Chitre, S.A.; Loeb, J.C.; Lednicky, J.A.; Mcintosh, M.T.; Bhaduri-Mcintosh, S. SARS-CoV-2 Viroporin Encoded by ORF3a Triggers the NLRP3 Inflammatory Pathway. Virology 2022, 568, 13–22. [Google Scholar] [CrossRef]
- Chang, Y.-S.; Ko, B.-H.; Ju, J.-C.; Chang, H.-H.; Huang, S.-H.; Lin, C.-W. SARS Unique Domain (SUD) of Severe Acute Respiratory Syndrome Coronavirus Induces NLRP3 Inflammasome-Dependent CXCL10-Mediated Pulmonary Inflammation. Int. J. Mol. Sci. 2020, 21, 3179. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.-L.; Yuen, K.-S.; Castaño-Rodriguez, C.; Ye, Z.-W.; Yeung, M.-L.; Fung, S.-Y.; Yuan, S.; Chan, C.-P.; Yuen, K.-Y.; Enjuanes, L.; et al. Severe Acute Respiratory Syndrome Coronavirus ORF3a Protein Activates the NLRP3 Inflammasome by Promoting TRAF3-Dependent Ubiquitination of ASC. FASEB J. 2019, 8865–8877. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Nabar, N.R.; Shi, C.-S.; Kamenyeva, O.; Xiao, X.; Hwang, I.-Y.; Wang, M.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-3a Drives Multimodal Necrotic Cell Death. Cell Death Dis. 2018, 9, 904. [Google Scholar] [CrossRef]
- Shi, C.S.; Nabar, N.R.; Huang, N.N.; Kehrl, J.H. SARS-Coronavirus Open Reading Frame-8b Triggers Intracellular Stress Pathways and Activates NLRP3 Inflammasomes. Cell Death Discov. 2019, 5, 101. [Google Scholar] [CrossRef]
- Theobald, S.J.; Simonis, A.; Georgomanolis, T.; Kreer, C.; Zehner, M.; Eisfeld, H.S.; Albert, M.-C.; Chhen, J.; Motameny, S.; Erger, F.; et al. Long-Lived Macrophage Reprogramming Drives Spike Protein-Mediated Inflammasome Activation in COVID-19. EMBO Mol. Med. 2021, 13, e14150. [Google Scholar] [CrossRef] [PubMed]
- Eisfeld, H.S.; Simonis, A.; Winter, S.; Chhen, J.; Ströh, L.J.; Krey, T.; Koch, M.; Theobald, S.J.; Rybniker, J. Viral Glycoproteins Induce Nlrp3 Inflammasome Activation and Pyroptosis in Macrophages. Viruses 2021, 13, 2076. [Google Scholar] [CrossRef]
- Planès, R.; Pinilla, M.; Santoni, K.; Hessel, A.; Passemar, C.; Lay, K.; Paillette, P.; Valadão, A.L.C.; Robinson, K.S.; Bastard, P.; et al. Human NLRP1 Is a Sensor of Pathogenic Coronavirus 3CL Proteases in Lung Epithelial Cells. Mol. Cell 2022, 82, 2385–2400.e9. [Google Scholar] [CrossRef]
- Yalcinkaya, M.; Liu, W.; Islam, M.N.; Kotini, A.G.; Gusarova, G.A.; Fidler, T.P.; Papapetrou, E.P.; Bhattacharya, J.; Wang, N.; Tall, A.R. Modulation of the NLRP3 Inflammasome by Sars-CoV-2 Envelope Protein. Sci. Rep. 2021, 11, 24432. [Google Scholar] [CrossRef]
- Kim, N.-E.; Kim, D.-K.; Song, Y.-J. Microorganisms SARS-CoV-2 Nonstructural Proteins 1 and 13 Suppress Caspase-1 and the NLRP3 Inflammasome Activation. Microorganisms 2021, 9, 494. [Google Scholar] [CrossRef]
- Mick, E.; Kamm, J.; Pisco, A.O.; Ratnasiri, K.; Babik, J.M.; Castañeda, G.; DeRisi, J.L.; Detweiler, A.M.; Hao, S.L.; Kangelaris, K.N.; et al. Upper Airway Gene Expression Reveals Suppressed Immune Responses to SARS-CoV-2 Compared with Other Respiratory Viruses. Nat. Commun. 2020, 11, 5854. [Google Scholar] [CrossRef] [PubMed]
- Colarusso, C.; Terlizzi, M.; Maglio, A.; Molino, A.; Candia, C.; Vitale, C.; Hansbro, P.M.; Vatrella, A.; Pinto, A.; Sorrentino, R.; et al. Activation of the AIM2 Receptor in Circulating Cells of Post-COVID-19 Patients with Signs of Lung Fibrosis Is Associated with the Release of IL-1a, IFN-a and TGF-b. Front. Immunol. 2022, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Abdulamir, A.S.; Gorial, F.I.; Saadi, S.J.; Maulood, M.F.; Hashim, H.A.; Alnuaimi, A.S.; Abdulrrazaq, M.K. A Randomised Controlled Trial of Effectiveness and Safety of Niclosamide as Add on Therapy to the Standard of Care Measures in COVID-19 Management. Ann. Med. Surg. 2021, 69, 102779. [Google Scholar] [CrossRef] [PubMed]
- Apaydın, Ç.B.; Çınar, G.; Cihan-Üstündağ, G. Small-Molecule Antiviral Agents in Ongoing Clinical Trials for COVID-19. Curr. Drug Targets 2021, 22, 1986–2005. [Google Scholar] [CrossRef]
- Kim, S.R.; Song, J.H.; Ahn, J.H.; Lee, G.S.; Ahn, H.; Yoon, S.i.; Kang, S.G.; Kim, P.H.; Jeon, S.M.; Choi, E.J.; et al. Antiviral and Anti-Inflammatory Activity of Budesonide against Human Rhinovirus Infection Mediated via Autophagy Activation. Antivir. Res. 2018, 151, 87–96. [Google Scholar] [CrossRef]
- Johnston, S.L.; Pattemore, P.K.; Sanderson, G.; Smith, S.; Lampe, F.; Josephs, L.; Symington, P.; Toole, S.O.; Myint, S.H.; Tyrrell, D.A.J.; et al. Community Study of Role of Viral Infections in Exacerbations of Asthma in 9-11 Year Old Children. BMJ 1995, 310, 1225–1229. [Google Scholar] [CrossRef]
- Menzel, M.; Akbarshahi, H.; Mahmutovic Persson, I.; Puthia, M.; Bjermer, L.; Uller, L. Caspase-1 Deficiency Reduces Eosinophilia and Interleukin-33 in an Asthma Exacerbation Model. ERJ Open Res. 2017, 3, 00047-2017. [Google Scholar] [CrossRef]
- Jansen, K.; Wirz, O.F.; van de Veen, W.; Tan, G.; Mirer, D.; Sokolowska, M.; Satitsuksanoa, P.; Message, S.D.; Kebadze, T.; Glanville, N.; et al. Loss of Regulatory Capacity in Treg Cells Following Rhinovirus Infection. J. Allergy Clin. Immunol. 2021, 148, 1016–1029.e16. [Google Scholar] [CrossRef]
- Robinson, K.S.; Teo, D.E.T.; Tan, K.S.; Toh, G.A.; Ong, H.H.; Lim, C.K.; Lay, K.; Au, B.V.; Lew, T.S.; Chu, J.J.H.; et al. Enteroviral 3C Protease Activates the Human NLRP1 Inflammasome in Airway Epithelia. Science 2020, 370, eaay2002. [Google Scholar] [CrossRef]
- Jackson, D.J.; Glanville, N.; Trujillo-Torralbo, M.B.; Shamji, B.W.H.; Del-Rosario, J.; Mallia, P.; Edwards, M.J.; Walton, R.P.; Edwards, M.R.; Johnston, S.L. Interleukin-18 Is Associated with Protection against Rhinovirus-Induced Colds and Asthma Exacerbations. Clin. Infect. Dis. 2015, 60, 1528–1531. [Google Scholar] [CrossRef]
- Han, M.; Ishikawa, T.; Bermick, J.R.; Rajput, C.; Lei, J.; Goldsmith, A.M.; Jarman, C.R.; Lee, J.; Bentley, J.K.; Hershenson, M.B. IL-1β Prevents ILC2 Expansion, Type 2 Cytokine Secretion, and Mucus Metaplasia in Response to Early-Life Rhinovirus Infection in Mice. Allergy 2020, 75, 2001–2015. [Google Scholar] [CrossRef]
- Han, M.; Bentley, J.K.; Rajput, C.; Lei, J.; Ishikawa, T.; Jarman, C.R.; Lee, J.; Goldsmith, A.M.; Jackson, W.T.; Hoenerhoff, M.J.; et al. Inflammasome Activation Is Required for Human Rhinovirus-Induced Airway Inflammation in Naive and Allergen-Sensitized Mice. Soc. Mucosal Immunol. 2019, 12, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Chen, R.; Zhang, L.; Wu, M.; Wu, J.; Wei, Y.; Dai, W.; Jiang, Y. ESR2 Regulates PINK1-Mediated Mitophagy via Transcriptional Repression of MicroRNA-423 Expression to Promote Asthma Development. Pharmacol. Res. 2021, 174, 1043–6618. [Google Scholar] [CrossRef] [PubMed]
- Hossain, F.M.A.; Park, S.O.; Kim, H.J.; Eo, J.C.; Choi, J.Y.; Tanveer, M.; Uyangaa, E.; Kim, K.; Eo, S.K. Indoleamine 2,3-Dioxygenase in Hematopoietic Stem Cell-Derived Cells Suppresses Rhinovirus-Induced Neutrophilic Airway Inflammation by Regulating Th1- and Th17-Type Responses. Immune Netw. 2021, 21, e26. [Google Scholar] [CrossRef]
- Lynch Iii, J.P.; Kajon, A.E.; Singh, S.K. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef]
- Ma, J.; Ramachandran, M.; Jin, C.; Quijano-Rubio, C.; Martikainen, M.; Yu, D.; Essand, M. Characterization of Virus-Mediated Immunogenic Cancer Cell Death and the Consequences for Oncolytic Virus-Based Immunotherapy of Cancer. Cell Death Dis. 2020, 11, 48. [Google Scholar] [CrossRef]
- Barlan, A.U.; Griffin, T.M.; Mcguire, K.A.; Wiethoff, C.M. Adenovirus Membrane Penetration Activates the NLRP3 Inflammasome. J. Virol. 2011, 85, 146–155. [Google Scholar] [CrossRef]
- Muruve, D.A.; Pétrilli, V.; Zaiss, A.K.; White, L.R.; Clark, S.A.; Ross, P.J.; Parks, R.J.; Tschopp, J. The Inflammasome Recognizes Cytosolic Microbial and Host DNA and Triggers an Innate Immune Response. Nature 2008, 452, 103–107. [Google Scholar] [CrossRef]
- Teigler, J.E.; Kagan, J.C.; Barouch, D.H. Late Endosomal Trafficking of Alternative Serotype Adenovirus Vaccine Vectors Augments Antiviral Innate Immunity. J. Virol. 2014, 88, 10354–10363. [Google Scholar] [CrossRef]
- Barlan, A.U.; Danthi, P.; Wiethoff, C.M. Lysosomal Localization and Mechanism of Membrane Penetration Influence Nonenveloped Virus Activation of the NLRP3 Inflammasome. Virology 2011, 412, 306–314. [Google Scholar] [CrossRef]
- Labzin, L.I.; Bottermann, M.; Rodriguez-Silvestre, P.; Foss, S.; Andersen, J.T.; Vaysburd, M.; Clift, D.; James, L.C. Antibody and DNA Sensing Pathways Converge to Activate the Inflammasome during Primary Human Macrophage Infection. EMBO J. 2019, 38, e101365. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Hwang, D.M.; Palaniyar, N.; Grinstein, S.; Philpott, D.J. Activation of P2X 7 Receptor by ATP Plays an Important Role in Regulating Inflammatory Responses during Acute Viral Infection. PLoS ONE 2012, 7, 35812. [Google Scholar] [CrossRef]
- Darweesh, M.; Kamel, W.; Gavrilin, M.A.; Akusjärvi, G. Adenovirus VA RNAI Blocks ASC Oligomerization and Inhibits NLRP3 Inflammasome Activation. Front. Immunol. 2019, 10, 2791. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Simmonds, P.; Slikas, E.; Li, L.; Bodhidatta, L.; Sethabutr, O.; Triki, H.; Bahri, O.; Oderinde, B.S.; Baba, M.M.; et al. Human Bocaviruses Are Highly Diverse, Dispersed, Recombination Prone, and Prevalent in Enteric Infections. J. Infect. Dis. 2010, 201, 1633–1643. [Google Scholar] [CrossRef]
- Arthur, J.L.; Higgins, G.D.; Davidson, G.P.; Givney, R.C.; Ratcliff, R.M. A Novel Bocavirus Associated with Acute Gastroenteritis in Australian Children. PLoS Pathog. 2009, 5, e1000391. [Google Scholar] [CrossRef]
- Kapoor, A.; Slikas, E.; Simmonds, P.; Chieochansin, T.; Naeem, A.; Shaukat, S.; Alam, M.M.; Sharif, S.; Angez, M.; Zaidi, S.; et al. A Newly Identified Bocavirus Species in Human Stool. J. Infect. Dis. 2009, 199, 196–200. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The Family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef]
- Uršič, T.; Steyer, A.; Kopriva, S.; Kalan, G.; Krivec, U.; Petrovec, M. Human Bocavirus as the Cause of a Life-Threatening Infection. J. Clin. Microbiol. 2011, 49, 1179–1181. [Google Scholar] [CrossRef]
- Bhat, R.; Almajhdi, F.N. Induction of Immune Responses and Immune Evasion by Human Bocavirus. Int. Arch. Allergy Immunol. 2021, 182, 728–735. [Google Scholar] [CrossRef]
- Dijkman, R.; Koekkoek, S.M.; Molenkamp, R.; Schildgen, O.; van der Hoek, L. Human Bocavirus Can Be Cultured in Differentiated Human Airway Epithelial Cells. J. Virol. 2009, 83, 7739–7748. [Google Scholar] [CrossRef]
- Deng, X.; Yan, Z.; Luo, Y.; Xu, J.; Cheng, F.; Li, Y.; Engelhardt, J.F.; Qiu, J. In Vitro Modeling of Human Bocavirus 1 Infection of Polarized Primary Human Airway Epithelia. J. Virol. 2013, 87, 4097–4102. [Google Scholar] [CrossRef]
- Huang, Q.; Deng, X.; Yan, Z.; Cheng, F.; Luo, Y.; Shen, W.; Lei-Butters, D.C.M.; Chen, A.Y.; Li, Y.; Tang, L.; et al. Establishment of a Reverse Genetics System for Studying Human Bocavirus in Human Airway Epithelia. PLoS Pathog. 2012, 8, e1002899. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zou, W.; Xiong, M.; Wang, Z.; Engelhardt, J.F.; Ye, S.Q.; Yan, Z.; Qiu, J. Human Parvovirus Infection of Human Airway Epithelia Induces Pyroptotic Cell Death by Inhibiting Apoptosis. J. Virol. 2017, 91, e01533-17. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.P.; Hua, K.-F. The Long Non-Coding RNAs: Paramount Regulators of the NLRP3 Inflammasome. Front. Immunol. 2020, 11, 569524. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Luo, D.; Wei, G.; Zhan, F.; Hua, F.; Xu, G. Non-Coding RNAs: The Key Regulators in NLRP3 Inflammasome-Mediated Inflammatory Diseases. Int. Immunopharmacol. 2021, 100, 108105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, M.; Du, R.H.; Qiao, C.; Jiang, C.Y.; Zhang, K.Z.; Ding, J.H.; Hu, G. MicroRNA-7 Targets Nod-like Receptor Protein 3 Inflammasome to Modulate Neuroinflammation in the Pathogenesis of Parkinson’s Disease. Mol. Neurodegener. 2016, 11, 1–15. [Google Scholar] [CrossRef]
- Głobińska, A.; Pawełczyk, M.; Kowalski, M.L. MicroRNAs and the Immune Response to Respiratory Virus Infections. Expert Rev. Clin. Immunol. 2014, 10, 963–971. [Google Scholar] [CrossRef]
- Morán, J.; Ramírez-Martínez, G.; Jiménez-Alvarez, L.; Cruz, A.; Pérez-Patrigeon, S.; Hidalgo, A.; Orozco, L.; Martínez, A.; Padilla-Noriega, L.; Avila-Moreno, F.; et al. Circulating Levels of MiR-150 Are Associated with Poorer Outcomes of A/H1N1 Infection. Exp. Mol. Pathol. 2015, 99, 253–261. [Google Scholar] [CrossRef]
- Huang, K.; Wang, C.; Vagts, C.; Raguveer, V.; Finn, P.W.; Perkins, D.L. Long Non-Coding RNAs (LncRNAs) NEAT1 and MALAT1 Are Differentially Expressed in Severe COVID-19 Patients: An Integrated Single-Cell Analysis. PLoS ONE 2022, 17, e0261242. [Google Scholar] [CrossRef]
- Zhang, P.; Cao, L.; Zhou, R.; Yang, X.; Wu, M. The LncRNA Neat1 Promotes Activation of Inflammasomes in Macrophages. Nat. Commun. 2019, 10, 1495. [Google Scholar] [CrossRef]
- Hirose, T.; Virnicchi, G.; Tanigawa, A.; Naganuma, T.; Li, R.; Kimura, H.; Yokoi, T.; Nakagawa, S.; Bénard, M.; Fox, A.H.; et al. NEAT1 Long Noncoding RNA Regulates Transcription via Protein Sequestration within Subnuclear Bodies. Mol. Biol. Cell 2014, 25, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Yang, L.; Guo, R.; Lu, N.; Shi, Y.; Wang, X. Long Noncoding RNA MALAT1 Promotes High Glucose-Induced Human Endothelial Cells Pyroptosis by Affecting NLRP3 Expression through Competitively Binding MiR-22. Biochem. Biophys. Res. Commun. 2019, 509, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Wan, P.; Zhang, S.; Ruan, Z.; Liu, X.; Yang, G.; Jia, Y.; Li, Y.; Pan, P.; Wang, W.; Li, G.; et al. AP-1 Signaling Pathway Promotes pro-IL-1β Transcription to Facilitate NLRP3 Inflammasome Activation upon Influenza A Virus Infection. Virulence 2022, 13, 502–513. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cerato, J.A.; da Silva, E.F.; Porto, B.N. Breaking Bad: Inflammasome Activation by Respiratory Viruses. Biology 2023, 12, 943. https://doi.org/10.3390/biology12070943
Cerato JA, da Silva EF, Porto BN. Breaking Bad: Inflammasome Activation by Respiratory Viruses. Biology. 2023; 12(7):943. https://doi.org/10.3390/biology12070943
Chicago/Turabian StyleCerato, Julia A., Emanuelle F. da Silva, and Barbara N. Porto. 2023. "Breaking Bad: Inflammasome Activation by Respiratory Viruses" Biology 12, no. 7: 943. https://doi.org/10.3390/biology12070943
APA StyleCerato, J. A., da Silva, E. F., & Porto, B. N. (2023). Breaking Bad: Inflammasome Activation by Respiratory Viruses. Biology, 12(7), 943. https://doi.org/10.3390/biology12070943





