Animal Models of IgE Anaphylaxis
Abstract
Simple Summary
Abstract
1. Introduction
2. Physiopathology
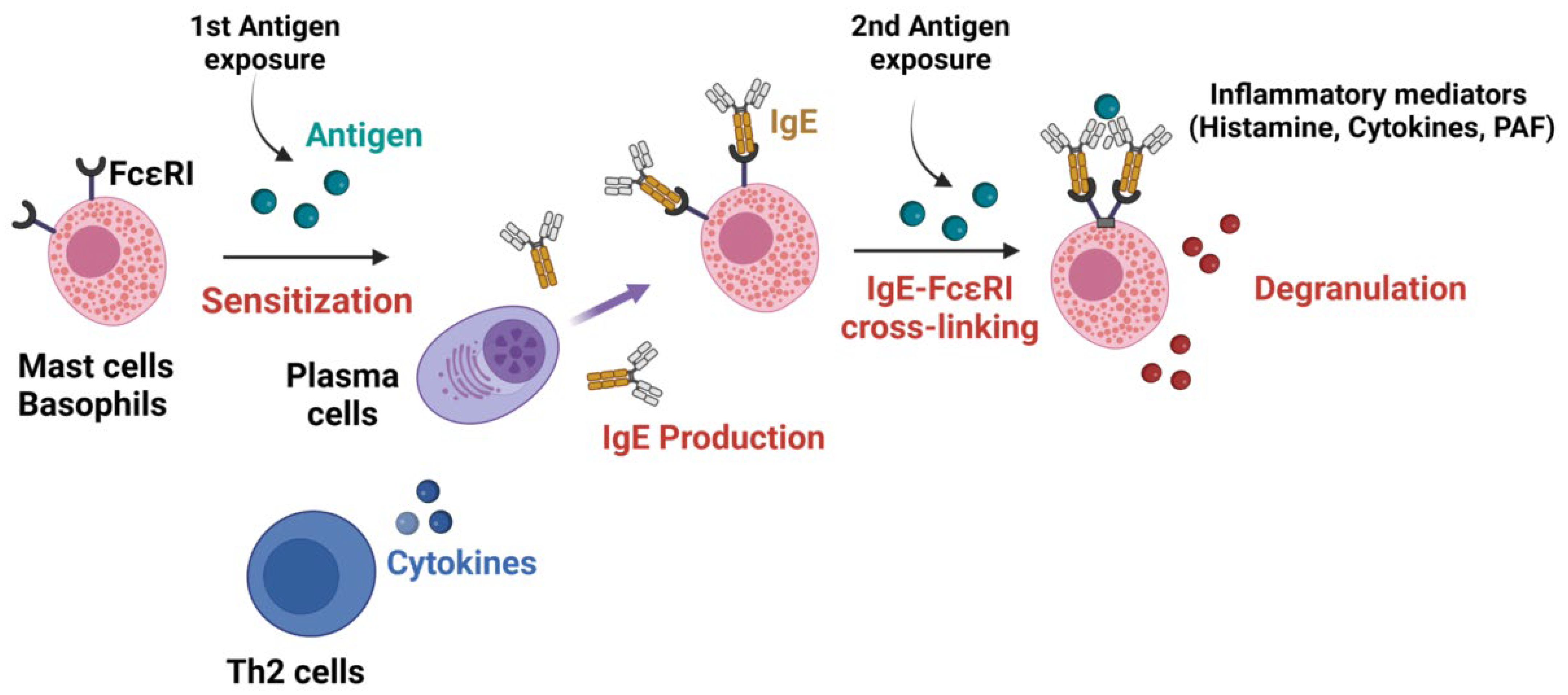
2.1. Antibodies in IgE-Mediated Anaphylaxis
2.2. Mechanisms of IgE-Mediated Anaphylaxis
3. Anaphylaxis Monitoring in Animal Models
4. Animal Models
4.1. Sensitization Routes
4.2. Passive Cutaneous Anaphylaxis
4.3. Passive Systemic Anaphylaxis
4.4. Active Systemic Anaphylaxis
4.5. Intestinal Models of Anaphylaxis
4.6. Transgenic Mice and Humanized Mouse Models
5. Animal Strain Influence
6. Limits of Animal Models in Anaphylaxis
7. Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, R.L.; Shtessel, M.; Robinson, L.B.; Banerji, A. Advances in Drug Allergy, Urticaria, Angioedema and Anaphylaxis in 2018. J. Allergy Clin. Immunol. 2019, 144, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Turner, P.J.; Worm, M.; Ansotegui, I.J.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Tanno, L.K.; et al. Time to revisit the definition and clinical criteria for anaphylaxis? World Allergy Organ. J. 2019, 12, 100066. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A. Anaphylaxis and emergency treatment. Pediatrics 2003, 111, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.; Ansotegui, I.J.; Ebisawa, M.; El-Gamal, Y.; Rivas, M.F.; Fineman, S.; Geller, M.; Gonzalez-Estrada, A.; Greenberger, P.A.; Borges, M.S.; et al. World Allergy Organization Anaphylaxis Guidance 2020. World Allergy Organ. J. 2020, 13, 100472. [Google Scholar] [CrossRef]
- Hanschmann, T.; Francuzik, W.; Dölle-Bierke, S.; Hofmeier, K.S.; Grabenhenrich, L.; Ruëff, F.; Renaudin, J.-M.; Pföhler, C.; Treudler, R.; Bilò, M.B.; et al. Different phenotypes of drug-induced anaphylaxis-Data from the European Anaphylaxis Registry. Allergy 2023, 78, 1615–1627. [Google Scholar] [CrossRef]
- Evora, P.R.; Simon, M.R. Role of nitric oxide production in anaphylaxis and its relevance for the treatment of anaphylactic hypotension with methylene blue. Ann. Allergy Asthma Immunol. 2007, 99, 306–313. [Google Scholar] [CrossRef]
- Dünser, M.W.; Mayr, A.J.; Ulmer, H.; Ritsch, N.; Knotzer, H.; Pajk, W.; Luckner, G.; Mutz, N.J.; Hasibeder, W.R. The effects of vasopressin on systemic hemodynamics in catecholamine-resistant septic and postcardiotomy shock: A retrospective analysis. Anesth Analg 2001, 93, 7–13. [Google Scholar] [CrossRef]
- Guerci, P.; Tacquard, C.; Chenard, L.; Millard, D.; Soufir, L.; Malinovsky, J.-M.; Garot, M.; Lalot, J.-M.; Besch, G.; Louis, G.; et al. Epidemiology and outcome of patients admitted to intensive care after anaphylaxis in France: A retrospective multicentre study. Br. J. Anaesth. 2020, 125, 1025–1033. [Google Scholar] [CrossRef]
- Finkelman, F.D. Anaphylaxis: Lessons from mouse models. J. Allergy Clin. Immunol. 2007, 120, 506–515. [Google Scholar] [CrossRef]
- Strait, R.T.; Morris, S.C.; Yang, M.; Qu, X.-W.; Finkelman, F.D. Pathways of anaphylaxis in the mouse. J. Allergy Clin. Immunol. 2002, 109, 658–668. [Google Scholar] [CrossRef]
- Jönsson, F.; de Chaisemartin, L.; Granger, V.; Gouel-Chéron, A.; Gillis, C.M.; Zhu, Q.; Dib, F.; Nicaise-Roland, P.; Ganneau, C.; Hurtado-Nedelec, M.; et al. An IgG-induced neutrophil activation pathway contributes to human drug-induced anaphylaxis. Sci. Transl. Med. 2019, 11, eaat1479. [Google Scholar] [CrossRef] [PubMed]
- Padlan, E.A. Anatomy of the antibody molecule. Mol. Immunol. 1994, 31, 169–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Krémer, V.; Iannascoli, B.; Goff, O.R.-L.; Mancardi, D.A.; Ramke, L.; de Chaisemartin, L.; Bruhns, P.; Jönsson, F. Specificity of mouse and human Fcgamma receptors and their polymorphic variants for IgG subclasses of different species. Eur. J. Immunol. 2022, 52, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.A.; Iannascoli, B.; Hoos, S.; England, P.; Daeron, M.; Bruhns, P. FcgammaRIV is a mouse IgE receptor that resembles macrophage FcepsilonRI in humans and promotes IgE-induced lung inflammation. J. Clin. Investig. 2008, 118, 3738–3750. [Google Scholar] [CrossRef] [PubMed]
- Bruhns, P.; Jönsson, F. Mouse and human FcR effector functions. Immunol. Rev. 2015, 268, 25–51. [Google Scholar] [CrossRef]
- Hakimi, J.; Seals, C.; Kondas, J.A.; Pettine, L.; Danho, W.; Kochan, J. The alpha subunit of the human IgE receptor (FcERI) is sufficient for high affinity IgE binding. J. Biol. Chem. 1990, 265, 22079–22081. [Google Scholar] [CrossRef]
- Dejoux, A.; de Chaisemartin, L.; Bruhns, P.; Longrois, D.; Gouel-Chéron, A. Neuromuscular blocking agent induced hypersensitivity reaction exploration: An update. Eur. J. Anaesthesiol. 2022, 40, 95–104. [Google Scholar] [CrossRef]
- Lieberman, P.; Garvey, L.H. Mast Cells and Anaphylaxis. Curr. Allergy Asthma Rep. 2016, 16, 20. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. IgE and mast cells in allergic disease. Nat. Med. 2012, 18, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Mackay, G.A.; Fernandopulle, N.A.; Ding, J.; McComish, J.; Soeding, P.F. Antibody or Anybody? Considering the Role of MRGPRX2 in Acute Drug-Induced Anaphylaxis and as a Therapeutic Target. Front. Immunol. 2021, 12, 688930. [Google Scholar] [CrossRef]
- Kounis, N.G.; Mazarakis, A.; Bardousis, C.; Patsouras, N. The heart and coronary arteries as primary target in severe allergic reactions: Cardiac troponins and the Kounis hypersensitivity-associated acute coronary syndrome. Int. J. Cardiol. 2015, 198, 83–84. [Google Scholar] [CrossRef]
- Pichler, W.J. Immune pathomechanism and classification of drug hypersensitivity. Allergy 2019, 74, 1457–1471. [Google Scholar] [CrossRef]
- Piacentini, G.L.; Bertolini, A.; Spezia, E.; Piscione, T.; Boner, A.L. Ability of a new infant formula prepared from partially hydrolyzed bovine whey to induce anaphylactic sensitization: Evaluation in a guinea pig model. Allergy 1994, 49, 361–364. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Frick, O.L. The dog as a model for food allergy. Ann. N. Y. Acad. Sci. 2002, 964, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Ladics, G.S.; Knippels, L.M.J.; Penninks, A.H.; Bannon, G.A.; Goodman, R.E.; Herouet-Guicheney, C. Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regul. Toxicol. Pharmacol. 2010, 56, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.M.; Furuta, G.T.; Stanley, J.S.; Ye, J.; Cockrell, G.; Connaughton, C.; Simpson, P.; Bannon, G.A.; Burks, A.W. A neonatal swine model for peanut allergy. J. Allergy Clin. Immunol. 2002, 109, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Helm, R.M.; Ermel, R.W.; Frick, O.L. Nonmurine animal models of food allergy. Environ. Health Perspect. 2003, 111, 239–244. [Google Scholar] [CrossRef] [PubMed]
- McClain, S.; Bannon, G.A. Animal models of food allergy: Opportunities and barriers. Curr. Allergy Asthma Rep. 2006, 6, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, R.; Osterfeld, H.; Wu, D.; Chen, C.-Y.; Arumugam, M.; Groschwitz, K.; Strait, R.; Wang, Y.-H.; Finkelman, F.D.; Hogan, S.P. Intestinal mast cell levels control severity of oral antigen-induced anaphylaxis in mice. Am. J. Pathol. 2012, 180, 1535–1546. [Google Scholar] [CrossRef]
- Zheng, F.; Copotoiu, R.; Tacquard, C.; Demoulin, B.; Malinovsky, J.M.; Levy, B.; Longrois, D.; Barthel, G.; Mertes, P.M.; Marchal, F.; et al. Epinephrine but not vasopressin attenuates the airway response to anaphylactic shock in rats. Exp. Lung Res. 2017, 43, 158–166. [Google Scholar] [CrossRef]
- Davidson, J.; Zheng, F.; Tajima, K.; Barthel, G.; Alb, I.; Tabarna, A.; Thornton, S.N.; Lambert, M.; Longrois, D.; Audibert, G.; et al. Anaphylactic Shock Decreases Cerebral Blood Flow More Than What Would Be Expected from Severe Arterial Hypotension. Shock 2012, 38, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Barthel, G.; Collange, O.; Montémont, C.; Thornton, S.N.; Longrois, D.; Levy, B.; Audibert, G.; Malinovsky, J.-M.; Mertes, P.-M. Methylene blue and epinephrine: A synergetic association for anaphylactic shock treatment. Crit. Care Med. 2013, 41, 195–204. [Google Scholar] [CrossRef]
- Zheng, F.; Collange, O.; Davidson, J.; Barthel, G.; Oulehri, W.; Thornton, S.N.; Longrois, D.; Levy, B.; Audibert, G.; Malinovsky, J.-M.; et al. Epinephrine, compared with arginine vasopressin, is associated with similar haemodynamic effects but significantly improved brain oxygenation in the early phase of anaphylactic shock in rats: An experimental study. Eur. J. Anaesthesiol. 2015, 32, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Jouan-Hureaux, V.; Franck, P.; Menu, P.; de Talancé, N.; Zannad, F.; Laxenaire, M.-C.; Longrois, D.; Mertes, P.M. Anaphylactic Shock: A Form of Distributive Shock without Inhibition of Oxygen Consumption. Anesthesiology 2005, 103, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tacquard, C.; Oulehri, W.; Collange, O.; Garvey, L.H.; Nicoll, S.; Tuzin, N.; Geny, B.; Mertes, P.M. Treatment with a platelet-activating factor receptor antagonist improves hemodynamics and reduces epinephrine requirements, in a lethal rodent model of anaphylactic shock. Clin. Exp. Allergy 2020, 50, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Lips, D.J.; van der Nagel, T.; Steendijk, P.; Palmen, M.; Janssen, B.J.; van Dantzig, J.-M.; de Windt, L.J.; Doevendans, P.A. Left ventricular pressure-volume measurements in mice: Comparison of closed-chest versus open-chest approach. Basic Res. Cardiol. 2004, 99, 351–359. [Google Scholar] [CrossRef]
- Boura, C.; Caron, A.; Longrois, D.; Mertes, P.M.; Labrude, P.; Menu, P. Volume Expansion with Modified Hemoglobin Solution, Colloids, or Crystalloid After Hemorrhagic Shock in Rabbits: Effects in Skeletal Muscle Oxygen Pressure and Use Versus Arterial Blood Velocity and Resistance. Shock 2003, 19, 176–182. [Google Scholar] [CrossRef]
- Osterfeld, H.; Ahrens, R.; Strait, R.; Finkelman, F.D.; Renauld, J.-C.; Hogan, S.P. Differential roles for the IL-9/IL-9 receptor alpha-chain pathway in systemic and oral antigen-induced anaphylaxis. J. Allergy Clin. Immunol. 2010, 125, 469–476.e2. [Google Scholar] [CrossRef]
- Tomar, S.; Ganesan, V.; Sharma, A.; Zeng, C.; Waggoner, L.; Smith, A.; Kim, C.H.; Licona-Limón, P.; Reinhardt, R.L.; Flavell, R.A.; et al. IL-4-BATF signaling directly modulates IL-9 producing mucosal mast cell (MMC9) function in experimental food allergy. J. Allergy Clin. Immunol. 2021, 147, 280–295. [Google Scholar] [CrossRef]
- Dewachter, P.; Jouan-Hureaux, V.; Lartaud, I.; Bello, G.; de Talancé, N.; Longrois, D.; Mertes, P.M. Comparison of Arginine Vasopressin, Terlipressin, or Epinephrine to Correct Hypotension in a Model of Anaphylactic Shock in Anesthetized Brown Norway Rats. Anesthesiology 2006, 104, 734–741. [Google Scholar] [CrossRef]
- Bellou, A.; Lambert, H.; Gillois, P.; Montémont, C.; Gerard, P.; Vauthier, E.; Sainte-Laudy, J.; Longrois, D.; Guéant, J.L.; Mallié, J.P. Constitutive nitric oxide synthase inhibition combined with histamine and serotonin receptor blockade improves the initial ovalbumin-induced arterial hypotension but decreases the survival time in brown norway rats anaphylactic shock. Shock 2003, 19, 71–78. [Google Scholar] [CrossRef]
- Todorova, B.; Godon, O.; Conde, E.; Gillis, C.M.; Iannascoli, B.; Richard-Le Goff, O.; Fiole, D.; Roumenina, L.T.; Leusen, J.H.W.; Murphy, A.J.; et al. IgG Subclass-Dependent Pulmonary Antigen Retention during Acute IgG-Dependent Systemic Anaphylaxis in Mice. J. Immunol. 2022, 209, 1243–1251. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Oyoshi, M.K.; Elkhal, A.; Scott, J.E.; Wurbel, M.-A.; Hornick, J.L.; Campbell, J.J.; Geha, R.S. Epicutaneous challenge of orally immunized mice redirects antigen-specific gut-homing T cells to the skin. J. Clin. Investig. 2011, 121, 2210–2220. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-M.; Serebrisky, D.; Lee, S.-Y.; Huang, C.-K.; Bardina, L.; Schofield, B.H.; Stanley, J.S.; Burks, A.W.; Bannon, G.A.; Sampson, H.A. A murine model of peanut anaphylaxis: T- and B-cell responses to a major peanut allergen mimic human responses. J. Allergy Clin. Immunol. 2000, 106, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Borcard, L.; Walsh, K.P.; Pena Rodriguez, M.; Mueller, C.; Kim, B.S.; Kubo, M.; Artis, D.; Noti, M. Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy. J. Allergy Clin. Immunol. 2018, 141, 223–234.e5. [Google Scholar] [CrossRef] [PubMed]
- Noti, M.; Kim, B.S.; Siracusa, M.C.; Rak, G.D.; Kubo, M.; Moghaddam, A.E.; Sattentau, Q.A.; Comeau, M.R.; Spergel, J.M.; Artis, D. Exposure to food allergens through inflamed skin promotes intestinal food allergy through the thymic stromal lymphopoietin-basophil axis. J. Allergy Clin. Immunol. 2014, 133, 1390–1399.e6. [Google Scholar] [CrossRef] [PubMed]
- Spergel, J.M.; Mizoguchi, E.; Brewer, J.P.; Martin, T.R.; Bhan, A.K.; Geha, R.S. Epicutaneous sensitization with protein antigen induces localized allergic dermatitis and hyperresponsiveness to methacholine after single exposure to aerosolized antigen in mice. J. Clin. Investig. 1998, 101, 1614–1622. [Google Scholar] [CrossRef]
- Bartnikas, L.M.; Gurish, M.F.; Burton, O.T.; Leisten, S.; Janssen, E.; Oettgen, H.C.; Beaupré, J.; Lewis, C.N.; Austen, K.F.; Schulte, S.; et al. Epicutaneous sensitization results in IgE-dependent intestinal mast cell expansion and food-induced anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 451–460.e6. [Google Scholar] [CrossRef]
- Yu, R.; Igawa, K.; Handa, Y.; Munetsugu, T.; Satoh, T.; Yokozeki, H. Basophils and mast cells are crucial for reactions due to epicutaneous sensitization to ovalbumin. Exp. Dermatol. 2017, 26, 778–784. [Google Scholar] [CrossRef]
- Muto, T.; Fukuoka, A.; Kabashima, K.; Ziegler, S.F.; Nakanishi, K.; Matsushita, K.; Yoshimoto, T. The role of basophils and proallergic cytokines, TSLP and IL-33, in cutaneously sensitized food allergy. Int. Immunol. 2014, 26, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.T.; Sasieni, P.; Du, T.G.; Syed, H.; Lack, G. Household peanut consumption as a risk factor for the development of peanut allergy. J. Allergy Clin. Immunol. 2009, 123, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Repa, A.; Wild, C.; Hufnagl, K.; Winkler, B.; Bohle, B.; Pollak, A.; Wiedermann, U. Influence of the route of sensitization on local and systemic immune responses in a murine model of type I allergy. Clin. Exp. Immunol. 2004, 137, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Jonsson, F.; Mancardi, D.A.; Kita, Y.; Karasuyama, H.; Iannascoli, B.; Van Rooijen, N.; Shimizu, T.; Daeron, M.; Bruhns, P. Mouse and human neutrophils induce anaphylaxis. J. Clin. Investig. 2011, 121, 1484–1496. [Google Scholar] [CrossRef]
- Liu, C.; Chen, C.; Yan, X.; Gu, S.; Jia, X.; Fu, W.; Meng, X.; Xue, W. Assessment of immune responses and intestinal flora in BALB/c mice model of wheat food allergy via different sensitization methods. Food Sci. Hum. Wellness 2023, 12, 871–881. [Google Scholar] [CrossRef]
- Mine, Y.; Yang, M. Epitope characterization of ovalbumin in BALB/c mice using different entry routes. Biochim. Biophys. Acta 2007, 1774, 200–212. [Google Scholar] [CrossRef]
- Yoo, J.; Manicone, A.M.; McGuire, J.K.; Wang, Y.; Parks, W.C. Systemic sensitization with the protein allergen ovalbumin augments local sensitization in atopic dermatitis. J. Inflamm. Res. 2014, 7, 29–38. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Chang, L.-J.; Li, N.; Li, H.; Yu, Y.; Bryce, P.J.; Grammer, L.C.; Schleimer, R.P.; Zhu, D. Blockade of peanut allergy with a novel Ara h 2-Fcγ fusion protein in mice. J. Allergy Clin. Immunol. 2013, 131, 213–221.e5. [Google Scholar] [CrossRef]
- Dombrowicz, D.; Flamand, V.; Brigman, K.K.; Koller, B.H.; Kinet, J.P. Abolition of anaphylaxis by targeted disruption of the high affinity immunoglobulin E receptor alpha chain gene. Cell 1993, 75, 969–976. [Google Scholar] [CrossRef]
- Jo, S.K.; Ahn, B.-E.; Choi, E.H.; Kang, J.E.; An, H.; Oh, M.; Rhie, G. Evaluation of the protective efficacy of recombinant protective antigen vaccine (GC1109)-immunized human sera using passive immunization in a mouse model. Vaccine 2020, 38, 1586–1588. [Google Scholar] [CrossRef]
- Paolucci, M.; Homère, V.; Waeckerle-Men, Y.; Wuillemin, N.; Bieli, D.; Pengo, N.; Sonati, T.; Kündig, T.M.; Johansen, P. Strain matters in mouse models of peanut-allergic anaphylaxis: Systemic IgE-dependent and Ara h 2-dominant sensitization in C3H mice. Clin. Exp. Allergy 2023, 53, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J. Pathogenesis and management of anaphylaxis: Current status and future challenges. J. Allergy Clin. Immunol. 2005, 115, 571–574. [Google Scholar] [CrossRef]
- Miyajima, I.; Dombrowicz, D.; Martin, T.R.; Ravetch, J.V.; Kinet, J.P.; Galli, S.J. Systemic anaphylaxis in the mouse can be mediated largely through IgG1 and Fc gammaRIII. Assessment of the cardiopulmonary changes, mast cell degranulation, and death associated with active or IgE- or IgG1-dependent passive anaphylaxis. J. Clin. Investig. 1997, 99, 901–914. [Google Scholar] [CrossRef]
- Baniyash, M.; Eshhar, Z. Inhibition of IgE binding to mast cells and basophils by monoclonal antibodies to murine IgE. Eur. J. Immunol. 1984, 14, 799–807. [Google Scholar] [CrossRef]
- Ohtsu, H. Histamine synthesis and lessons learned from histidine decarboxylase deficient mice. Adv. Exp. Med. Biol. 2010, 709, 21–31. [Google Scholar] [CrossRef]
- Bruhns, P. Properties of mouse and human IgG receptors and their contribution to disease models. Blood 2012, 119, 5640–5649. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, Y.; Obata, K.; Mukai, K.; Shindou, H.; Yoshida, M.; Nishikado, H.; Kawano, Y.; Minegishi, Y.; Shimizu, T.; Karasuyama, H. Basophils play a pivotal role in immunoglobulin-G-mediated but not immunoglobulin-E-mediated systemic anaphylaxis. Immunity 2008, 28, 581–589. [Google Scholar] [CrossRef]
- Forbes, E.E.; Groschwitz, K.; Abonia, J.P.; Brandt, E.B.; Cohen, E.; Blanchard, C.; Ahrens, R.; Seidu, L.; McKenzie, A.; Strait, R.; et al. IL-9- and mast cell-mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 2008, 205, 897–913. [Google Scholar] [CrossRef] [PubMed]
- Brandt, E.B.; Strait, R.T.; Hershko, D.; Wang, Q.; Muntel, E.E.; Scribner, T.A.; Zimmermann, N.; Finkelman, F.D.; Rothenberg, M.E. Mast cells are required for experimental oral allergen-induced diarrhea. J. Clin. Investig. 2003, 112, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, N.P.; Parvataneni, S.; Hassan, H.M.A.; Harkema, J.; Samineni, S.; Navuluri, L.; Kelly, C.J.; Gangur, V. An adjuvant-free mouse model of tree nut allergy using hazelnut as a model tree nut. Int. Arch. Allergy Immunol. 2007, 144, 203–210. [Google Scholar] [CrossRef]
- Birmingham, N.; Gangur, V.; Samineni, S.; Navuluri, L.; Kelly, C. Hazelnut Allergy: Evidence that hazelnut can directly elicit specific IgE antibody response via activating type 2 cytokines in mice. Int. Arch. Allergy Immunol. 2005, 137, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Fish, S.C.; Donaldson, D.D.; Goldman, S.J.; Williams, C.M.M.; Kasaian, M.T. IgE generation and mast cell effector function in mice deficient in IL-4 and IL-13. J. Immunol. 2005, 174, 7716–7724. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Lee, J.-B.; Liu, B.; Ohta, S.; Wang, P.-Y.; Kartashov, A.V.; Mugge, L.; Abonia, J.P.; Barski, A.; Izuhara, K.; et al. Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy. Immunity 2015, 43, 788–802. [Google Scholar] [CrossRef] [PubMed]
- Komai-Koma, M.; Brombacher, F.; Pushparaj, P.N.; Arendse, B.; McSharry, C.; Alexander, J.; Chaudhuri, R.; Thomson, N.C.; McKenzie, A.N.J.; McInnes, I.; et al. Interleukin-33 amplifies IgE synthesis and triggers mast cell degranulation via interleukin-4 in naïve mice. Allergy 2012, 67, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.-X.; Roberts, D.; Bartley, P.A.; Jackson, D.E. Absence of platelet endothelial cell adhesion molecule-1 (CD31) leads to increased severity of local and systemic IgE-mediated anaphylaxis and modulation of mast cell activation. J. Immunol. 2002, 168, 6455–6462. [Google Scholar] [CrossRef]
- Fung-Leung, W.P.; De Sousa-Hitzler, J.; Ishaque, A.; Zhou, L.; Pang, J.; Ngo, K.; Panakos, J.A.; Chourmouzis, E.; Liu, F.T.; Lau, C.Y. Transgenic mice expressing the human high-affinity immunoglobulin (Ig) E receptor alpha chain respond to human IgE in mast cell degranulation and in allergic reactions. J. Exp. Med. 1996, 183, 49–56. [Google Scholar] [CrossRef]
- Ujike, A.; Ishikawa, Y.; Ono, M.; Yuasa, T.; Yoshino, T.; Fukumoto, M.; Ravetch, J.V.; Takai, T. Modulation of immunoglobulin (Ig)E-mediated systemic anaphylaxis by low-affinity Fc receptors for IgG. J. Exp. Med. 1999, 189, 1573–1579. [Google Scholar] [CrossRef]
- Mathias, C.B.; Hobson, S.A.; Garcia-Lloret, M.; Lawson, G.; Poddighe, D.; Freyschmidt, E.-J.; Xing, W.; Gurish, M.F.; Chatila, T.A.; Oettgen, H.C. IgE-mediated systemic anaphylaxis and impaired tolerance to food antigens in mice with enhanced IL-4 receptor signaling. J. Allergy Clin. Immunol. 2011, 127, 795–805.e6. [Google Scholar] [CrossRef]
- Rivas, M.N.; Burton, O.T.; Wise, P.; Zhang, Y.; Hobson, S.A.; Lloret, M.G.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A microbiota signature associated with experimental food allergy promotes allergic sensitization and anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Zhou, J.S.; Sandomenico, A.; Severino, V.; Burton, O.T.; Darling, A.; Oettgen, H.C.; Ruvo, M. An IgE receptor mimetic peptide (PepE) protects mice from IgE mediated anaphylaxis. Mol. Biosyst. 2013, 9, 2853–2859. [Google Scholar] [CrossRef]
- Burton, O.T.; Stranks, A.J.; Tamayo, J.M.; Koleoglou, K.J.; Schwartz, L.B.; Oettgen, H.C. A humanized mouse model of anaphylactic peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 314–322.e9. [Google Scholar] [CrossRef]
- Pagovich, O.E.; Wang, B.; Chiuchiolo, M.J.; Kaminsky, S.M.; Sondhi, D.; Jose, C.L.; Price, C.C.; Brooks, S.F.; Mezey, J.G.; Crystal, R.G. Anti-hIgE gene therapy of peanut-induced anaphylaxis in a humanized murine model of peanut allergy. J. Allergy Clin. Immunol. 2016, 138, 1652–1662. [Google Scholar] [CrossRef]
- Bryce, P.J.; Falahati, R.; Kenney, L.L.; Leung, J.; Bebbington, C.; Tomasevic, N.; Krier, R.A.; Hsu, C.-L.; Shultz, L.D.; Greiner, D.L.; et al. Humanized mouse model of mast cell-mediated passive cutaneous anaphylaxis and passive systemic anaphylaxis. J. Allergy Clin. Immunol. 2016, 138, 769–779. [Google Scholar] [CrossRef]
- Jonsson, F.; Mancardi, D.A.; Zhao, W.; Kita, Y.; Iannascoli, B.; Khun, H.; van Rooijen, N.; Shimizu, T.; Schwartz, L.B.; Daeron, M.; et al. Human FcgammaRIIA induces anaphylactic and allergic reactions. Blood 2012, 119, 2533–2544. [Google Scholar] [CrossRef] [PubMed]
- Mancardi, D.A.; Albanesi, M.; Jonsson, F.; Iannascoli, B.; Van Rooijen, N.; Kang, X.; England, P.; Daeron, M.; Bruhns, P. The high-affinity human IgG receptor FcgammaRI (CD64) promotes IgG-mediated inflammation, anaphylaxis, and antitumor immunotherapy. Blood 2013, 121, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Gillis, C.M.; Jönsson, F.; Mancardi, D.A.; Tu, N.; Beutier, H.; Van Rooijen, N.; Macdonald, L.E.; Murphy, A.J.; Bruhns, P. Mechanisms of anaphylaxis in human low-affinity IgG receptor locus knock-in mice. J. Allergy Clin. Immunol. 2017, 139, 1253–1265.e14. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; DiLillo, D.J.; Bournazos, S.; Li, F.; Ravetch, J.V. Mouse model recapitulating human Fcγ receptor structural and functional diversity. Proc. Natl. Acad. Sci. USA 2012, 109, 6181–6186. [Google Scholar] [CrossRef]
- Li, X.; Huang, C.K.; Schofield, B.H.; Burks, A.W.; Bannon, G.A.; Kim, K.H.; Huang, S.K.; Sampson, H.A. Strain-dependent induction of allergic sensitization caused by peanut allergen DNA immunization in mice. J. Immunol. 1999, 162, 3045–3052. [Google Scholar] [CrossRef]
- Arumugam, M.; Ahrens, R.; Osterfeld, H.; Kottyan, L.C.; Shang, X.; Maclennan, J.A.; Zimmermann, N.; Zheng, Y.; Finkelman, F.D.; Hogan, S.P. Increased susceptibility of 129SvEvBrd mice to IgE-Mast cell mediated anaphylaxis. BMC Immunol. 2011, 12, 14. [Google Scholar] [CrossRef]
- Kreis, M.E.; Mueller, M.H.; Reber, D.; Glatzle, J.; Enck, P.; Grundy, D. Stress-induced attenuation of brain stem activation following intestinal anaphylaxis in the rat. Neurosci Lett. 2003, 345, 187–191. [Google Scholar] [CrossRef]
- Sun, N.; Zhou, C.; Pu, Q.; Wang, J.; Huang, K.; Che, H. Allergic reactions compared between BN and Wistar rats after oral exposure to ovalbumin. J. Immunotoxicol 2013, 10, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wex, E.; Thaler, E.; Blum, S.; Lamb, D. A novel model of IgE-mediated passive pulmonary anaphylaxis in rats. PLoS ONE 2014, 9, e116166. [Google Scholar] [CrossRef] [PubMed]
- Buras, J.A.; Holzmann, B.; Sitkovsky, M. Animal Models of sepsis: Setting the stage. Nat. Rev. Drug. Discov. 2005, 4, 854–865. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.H.; Dewachter, P.; Hepner, D.L.; Mertes, P.M.; Voltolini, S.; Clarke, R.; Cooke, P.; Garcez, T.; Guttormsen, A.B.; Ebo, D.G.; et al. Management of suspected immediate perioperative allergic reactions: An international overview and consensus recommendations. Br. J. Anaesth. 2019, 123, e50–e64. [Google Scholar] [CrossRef] [PubMed]
| Animal | Physiological Parameter | Probes for Measurement |
|---|---|---|
| Mice/rats | Drop in core body temperature | Rectal temperature |
| Level of intestinal mast cells | Occurrence of diarrhea | |
| Reduced physical activity | Activity scale | |
| Mostly rats | Decreased vascular resistance, fluid loss, and hemodynamic impairment | Tracheal intubation, vein line placement |
| Muscular interstitial lactate concentration | PtiO2 and micro-dialysis probes in the quadriceps muscle | |
| Hemodynamic failure of cerebral oxygenation | Measurements of cerebral cortical blood flow and oxygen partial pressure | |
| Hemodynamic failure | Perivascular ultrasonic flow probes | |
| Left-ventricular function impairment | Catheter insertion | |
| Vascular leakage due to histamine release | Hematocrit measurement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gouel-Chéron, A.; Dejoux, A.; Lamanna, E.; Bruhns, P. Animal Models of IgE Anaphylaxis. Biology 2023, 12, 931. https://doi.org/10.3390/biology12070931
Gouel-Chéron A, Dejoux A, Lamanna E, Bruhns P. Animal Models of IgE Anaphylaxis. Biology. 2023; 12(7):931. https://doi.org/10.3390/biology12070931
Chicago/Turabian StyleGouel-Chéron, Aurélie, Alice Dejoux, Emma Lamanna, and Pierre Bruhns. 2023. "Animal Models of IgE Anaphylaxis" Biology 12, no. 7: 931. https://doi.org/10.3390/biology12070931
APA StyleGouel-Chéron, A., Dejoux, A., Lamanna, E., & Bruhns, P. (2023). Animal Models of IgE Anaphylaxis. Biology, 12(7), 931. https://doi.org/10.3390/biology12070931






