Decision-Making in Patients with Vasovagal Syncope: A Preliminary Study
Abstract
Simple Summary
Abstract
1. Introduction
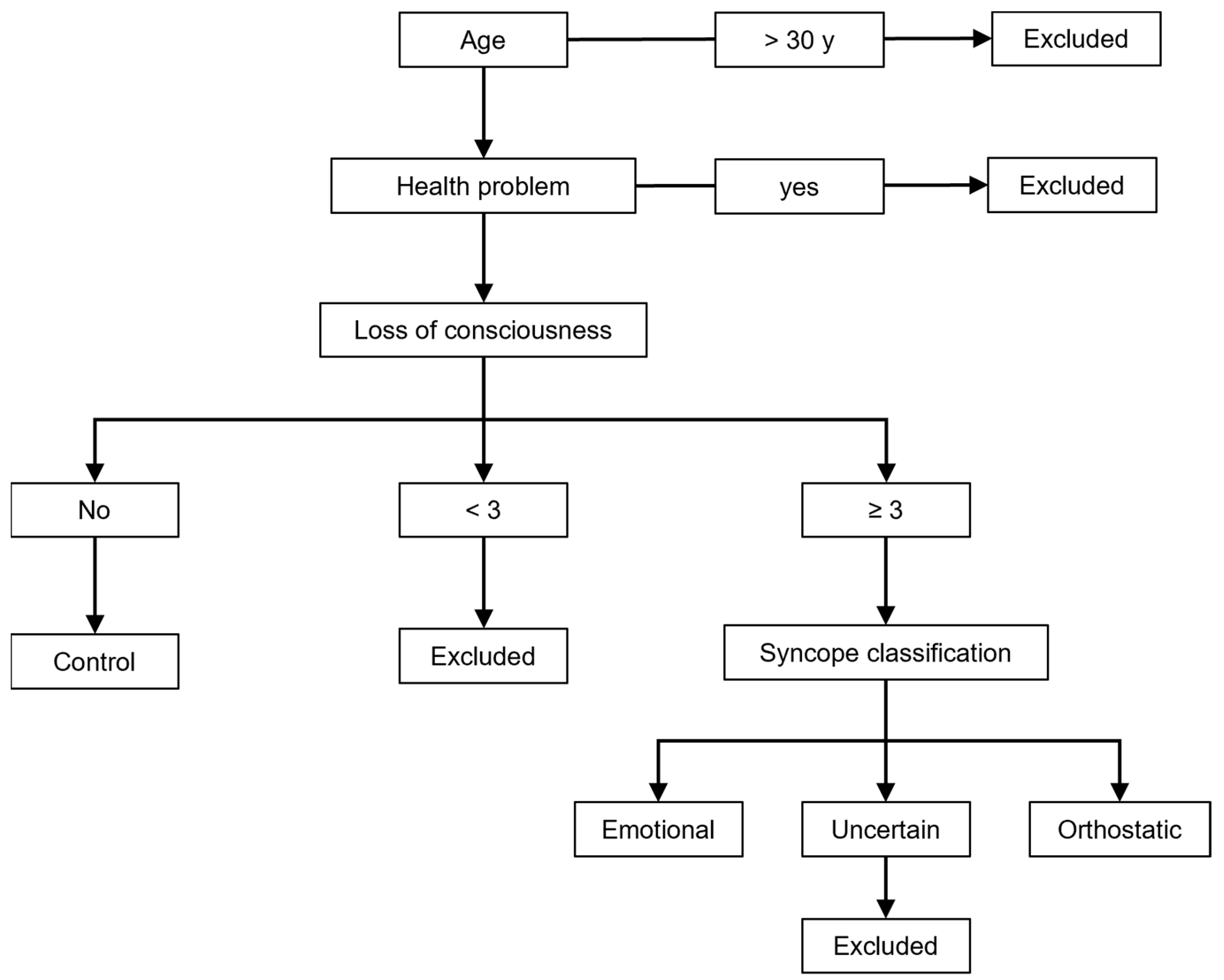
2. Materials and Methods
2.1. Questionnaire
2.2. Decisional Conflict Test
2.3. Statistics
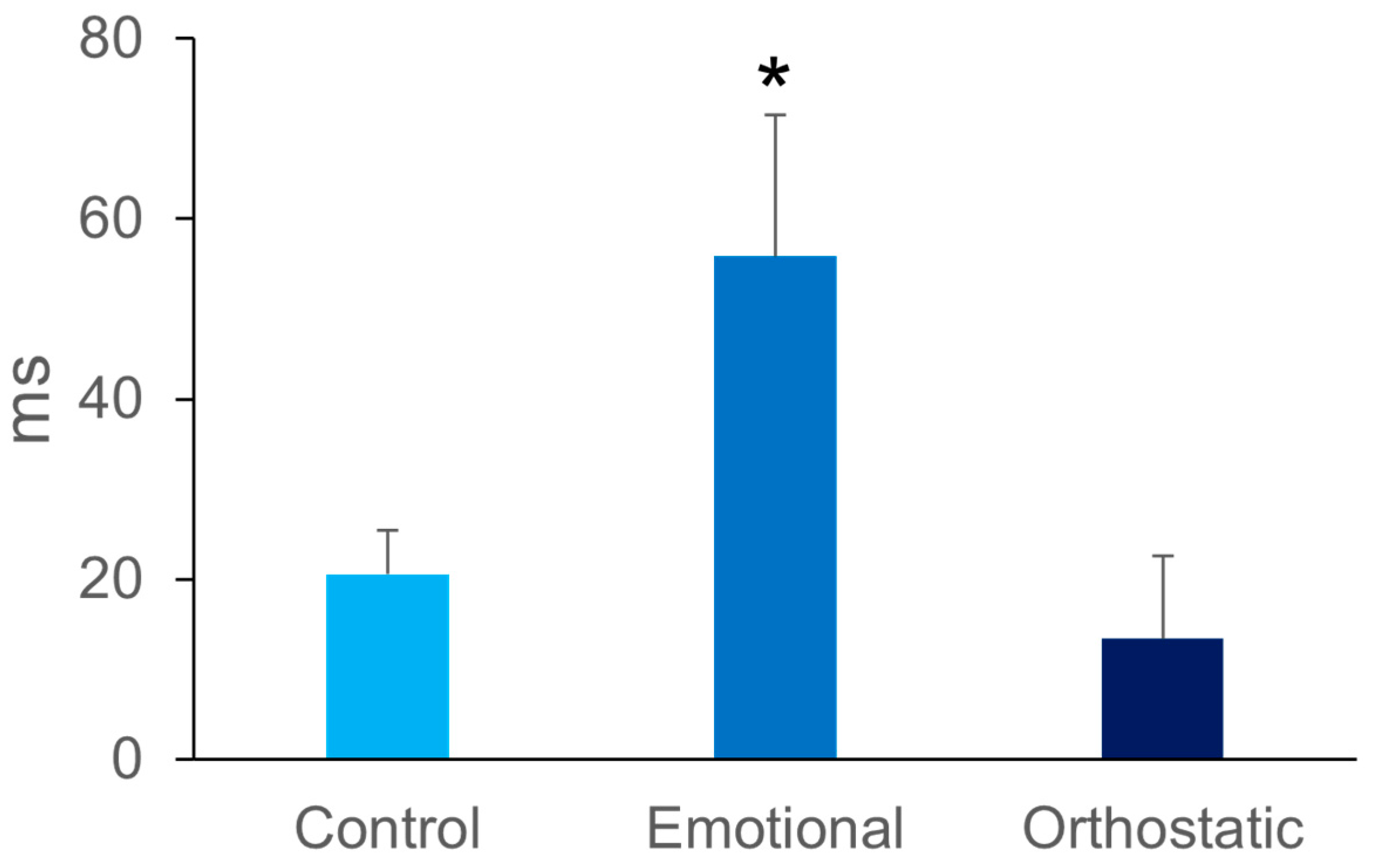
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
References
- Colman, N.; Nahm, K.; Ganzeboom, K.S.; Shen, W.K.; Reitsma, J.; Linzer, M.; Wieling, W.; Kaufmann, H. Epidemiology of reflex syncope. Clin. Auton. Res. 2004, 14 (Suppl. S1), i9–i17. [Google Scholar] [CrossRef]
- Grubb, B.P. Neurocardiogenic syncope and related disorders of orthostatic intolerance. Circulation 2005, 111, 2997–3006. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neutrally mediated syncope and the postural tachycardia syndrome. Auton. Neurosci. 2011, 161, 46–48. [Google Scholar] [CrossRef]
- Coffin, S.T.; Raj, S.R. Non-invasive management of vasovagal syncope. Auton. Neurosci. 2014, 184, 27–32. [Google Scholar] [CrossRef]
- Ayabe, K.; Komiyama, T.; Hasegawa, M.; Sakai, T.; Morise, M.; Sakama, S.; Yagishita, A.; Amino, M.; Ikari, Y.; Yoshioka, K. Clinical Significance of the Head-Up Tilt Test in Improving Prognosis in Patients with Possible Neurally Mediated Syncope. Biology 2021, 10, 919. [Google Scholar] [CrossRef]
- Saal, D.P.; van Dijk, J.G. Classifying syncope. Auton. Neurosci. 2014, 184, 3–9. [Google Scholar] [CrossRef]
- Brignole, M.; Moya, A.; de Lange, F.J.; Deharo, J.C.; Elliott, P.M.; Fanciulli, A.; Fedorowski, A.; Furlan, R.; Kenny, R.A.; Martín, A.; et al. ESC Guidelines for the diagnosis and management of syncope. Eur. Heart J. 2018, 39, 1883–1948. [Google Scholar] [CrossRef]
- Fu, Q.; Levine, B.D. Pathophysiology of neurally mediated syncope: Role of cardiac output and total peripheral resistance. Auton. Neurosci. 2014, 184, 24–26. [Google Scholar] [CrossRef]
- Hainsworth, R. Pathophysiology of syncope. Clin. Auton. Res. 2004, 14 (Suppl. S1), i18–i24. [Google Scholar] [CrossRef]
- Bialystok, E.; Craik, F.I.; Klein, R.; Viswanathan, M. Bilingualism, aging, and cognitive control: Evidence from the Simon task. Psychol. Aging 2004, 19, 290–303. [Google Scholar] [CrossRef]
- Brignole, M.; Rivasi, G.; Sutton, R.; Kenny, R.A.; Morillo, C.A.; Sheldon, R.; Raj, S.R.; Ungar, A.; Furlan, R.; van Dijk, G.; et al. Low-blood pressure phenotype underpins the tendency to reflex syncope. J. Hypertens. 2021, 39, 1319–1325. [Google Scholar] [CrossRef]
- Cevada, T.; Conde, E.; Marques, D.; Deslandes, A.C. Test-retest reliability of the simon task: A short version proposal. Somatosens. Mot. Res. 2019, 36, 275–282. [Google Scholar] [CrossRef]
- Ganzeboom, K.S.; Colman, N.; Reitsma, J.B.; Shen, W.K.; Wieling, W. Prevalence and triggers of syncope in medical students. Am. J. Cardiol. 2003, 91, 1006–1008. [Google Scholar] [CrossRef]
- Fortrat, J.O.; Victor, J. Limitations of studies on vasovagal syncope. Am. J. Cardiol. 2004, 94, 149–150. [Google Scholar]
- Thijs, R.D.; Brignole, M.; Falup-Pecurariu, C.; Fanciulli, A.; Freeman, R.; Guaraldi, P.; Jordan, J.; Habek, M.; Hilz, M.; Pavy-LeTraon, A.; et al. Recommendations for tilt table testing and other provocative cardiovascular autonomic tests in conditions that may cause transient loss of consciousness: Consensus statement of the European Federation of Autonomic Societies (EFAS) endorsed by the American Autonomic Society (AAS) and the European Academy of Neurology (EAN). Auton. Neurosci. 2021, 233, 102792. [Google Scholar]
- Colman, N.; Nahm, K.; van Dijk, J.G.; Reitsma, J.B.; Wieling, W.; Kaufmann, H. Diagnostic value of history taking in reflex syncope. Clin. Auton. Res. 2004, 14 (Suppl. S1), i37–i44. [Google Scholar] [CrossRef]
- Graff, B.; Graff, G.; Makowiec, D.; Kaczkowska, A.; Wejer, D.; Budrejko, S.; Kozłowski, D.; Narkiewicz, K. Entropy Measures in the Assessment of Heart Rate Variability in Patients with Cardiodepressive Vasovagal Syncope. Entropy 2015, 17, 1007–1022. [Google Scholar] [CrossRef]
- Fortrat, J.O.; Gharib, C. Self-Organization of Blood Pressure Regulation: Clinical Evidence. Front. Physiol. 2016, 7, 113. [Google Scholar] [CrossRef]
- Olde Nordkamp, L.R.; van Dijk, N.; Ganzeboom, K.S.; Reitsma, J.B.; Luitse, J.S.; Dekker, L.R.; Shen, W.K.; Wieling, W. Syncope prevalence in the ED compared to general practice and population: A strong selection process. Am. J. Emerg. Med. 2009, 27, 271–279. [Google Scholar] [CrossRef]
- Buelow, M.T.; Barnhart, W.R. Test-Retest Reliability of Common Behavioral Decision Making Tasks. Arch. Clin. Neuropsychol. 2018, 33, 125–129. [Google Scholar] [CrossRef]
- Owens, A.P.; Low, D.A.; Critchley, H.D.; Mathias, C.J. Emotional orienting during interoceptive threat in orthostatic intolerance: Dysautonomic contributions to psychological symptomatology in the postural tachycardia syndrome and vasovagal syncope. Auton. Neurosci. 2018, 212, 42–47. [Google Scholar] [CrossRef]
- Beissner, F.; Meissner, K.; Bär, K.J.; Napadow, V. The autonomic brain: An activation likelihood estimation meta-analysis for central processing of autonomic function. J. Neurosci. 2013, 33, 10503–10511. [Google Scholar] [CrossRef]
- Duschek, S.; Werner, N.S.; Reyes Del Paso, G.A. The behavioral impact of baroreflex function: A review. Psychophysiology 2013, 50, 1183–1193. [Google Scholar] [CrossRef]
- McKlveen, J.M.; Myers, B.; Herman, J.P. The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. J. Neuroendocrinol. 2015, 27, 446–456. [Google Scholar] [CrossRef]
- Fortrat, J.O.; Levrard, T.; Courcinous, S.; Victor, J. Self-Organization of Blood Pressure Regulation: Experimental Evidence. Front. Physiol. 2016, 7, 112. [Google Scholar] [CrossRef]
- Muñoz, M.A. Colloquium: Criticality and dynamical scaling in living systems. Rev. Mod. Phys. 2018, 90, 031001. [Google Scholar] [CrossRef]
- Nosek, B.A.; Hardwicke, T.E.; Moshontz, H.; Allard, A.; Corker, K.S.; Dreber, A.; Fidler, F.; Hilgard, J.; Kline Struhl, M.; Nuijten, M.B.; et al. Replicability; Robustness; and Reproducibility in Psychological Science. Annu. Rev. Psychol. 2022, 73, 719–748. [Google Scholar] [CrossRef]
- Daitch, C. Cognitive behavioral therapy, mindfulness, and hypnosis as treatment methods for generalized anxiety disorder. Am. J. Clin. Hypn. 2018, 61, 57–69. [Google Scholar] [CrossRef]
| Control | Emotional | Orthostatic | ||
|---|---|---|---|---|
| N | 98 | 10 | 38 | |
| Age (y) | 22 ± 2 | 21 ± 2 | 22 ± 2 | Ns |
| BMI (kg/m²) | 22 ± 2 | 21 ± 2 | 21 ± 2 | Ns |
| Congruence (Non-Confusing Stimulus) | ||||
| Reaction (ms) | 440 ± 8 | 485 ±44 | 460 ± 20 | Ns |
| Accuracy (%) | 97 ± 1 | 95 ± 2 | 98 ± 1 | Ns |
| Incongruence (Confusing Stimulus) | ||||
| Reaction (ms) | 460 ± 8 | 540 ± 53 | 473 ± 16 | p < 0.05 |
| Accuracy (%) | 92 ± 1 | 90 ± 5 | 94 ± 1 | Ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Méchenin, M.; Fortrat, J.-O. Decision-Making in Patients with Vasovagal Syncope: A Preliminary Study. Biology 2023, 12, 930. https://doi.org/10.3390/biology12070930
Méchenin M, Fortrat J-O. Decision-Making in Patients with Vasovagal Syncope: A Preliminary Study. Biology. 2023; 12(7):930. https://doi.org/10.3390/biology12070930
Chicago/Turabian StyleMéchenin, Muriel, and Jacques-Olivier Fortrat. 2023. "Decision-Making in Patients with Vasovagal Syncope: A Preliminary Study" Biology 12, no. 7: 930. https://doi.org/10.3390/biology12070930
APA StyleMéchenin, M., & Fortrat, J.-O. (2023). Decision-Making in Patients with Vasovagal Syncope: A Preliminary Study. Biology, 12(7), 930. https://doi.org/10.3390/biology12070930






