Characterisation of Luvisols Based on Wide-Scale Biological Properties in a Long-Term Organic Matter Experiment
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Test Methods
2.3. Statistical Methods
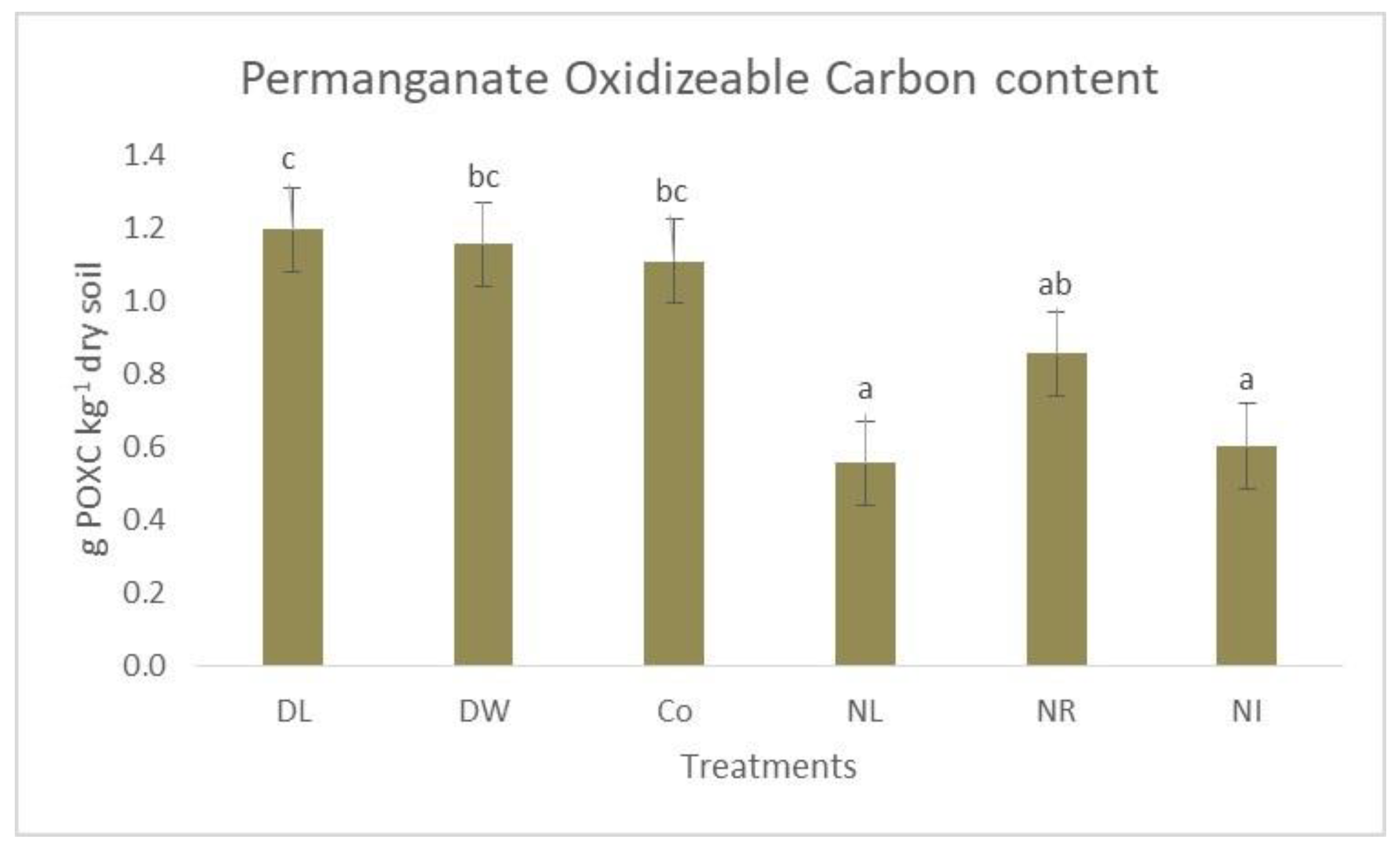
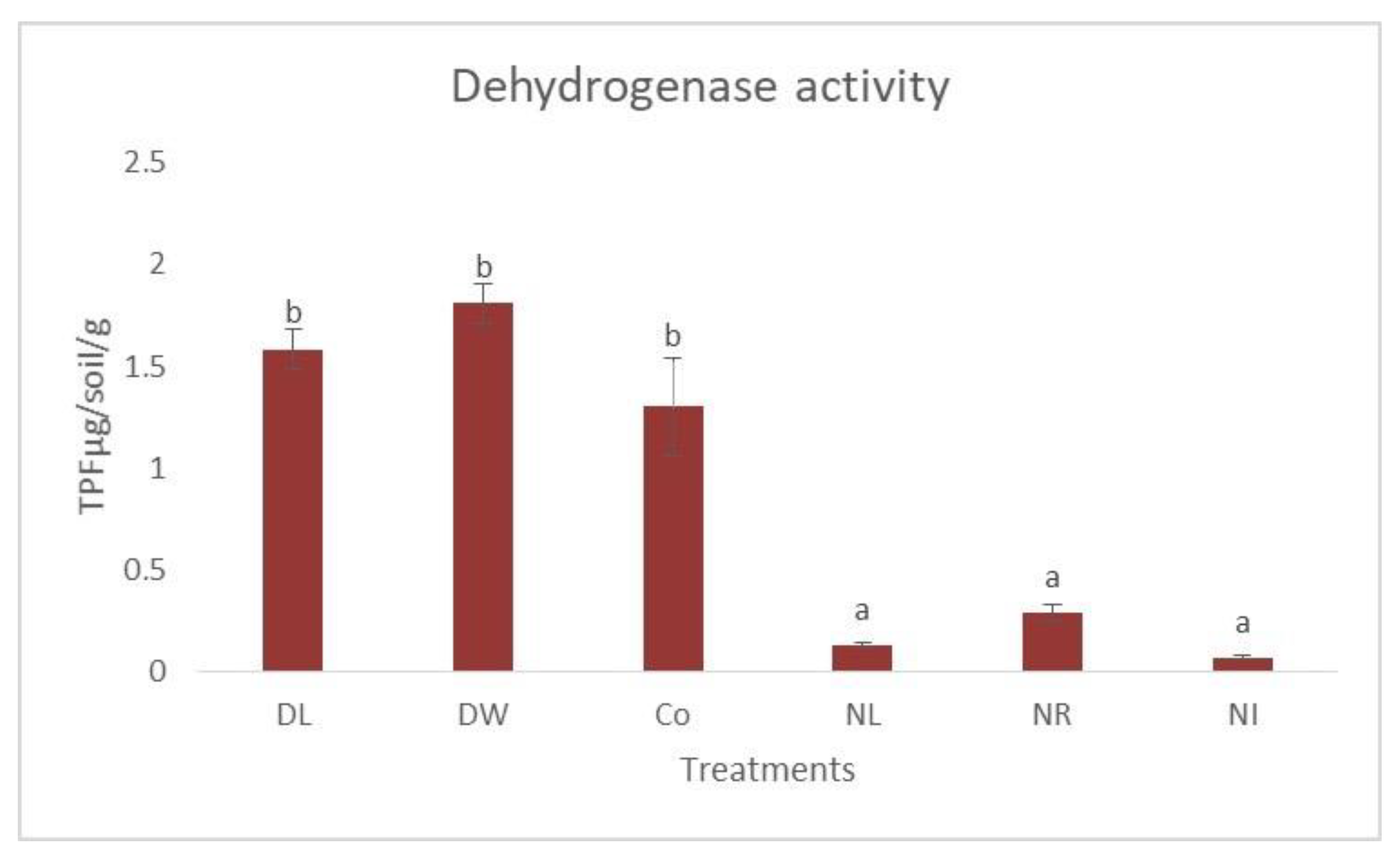
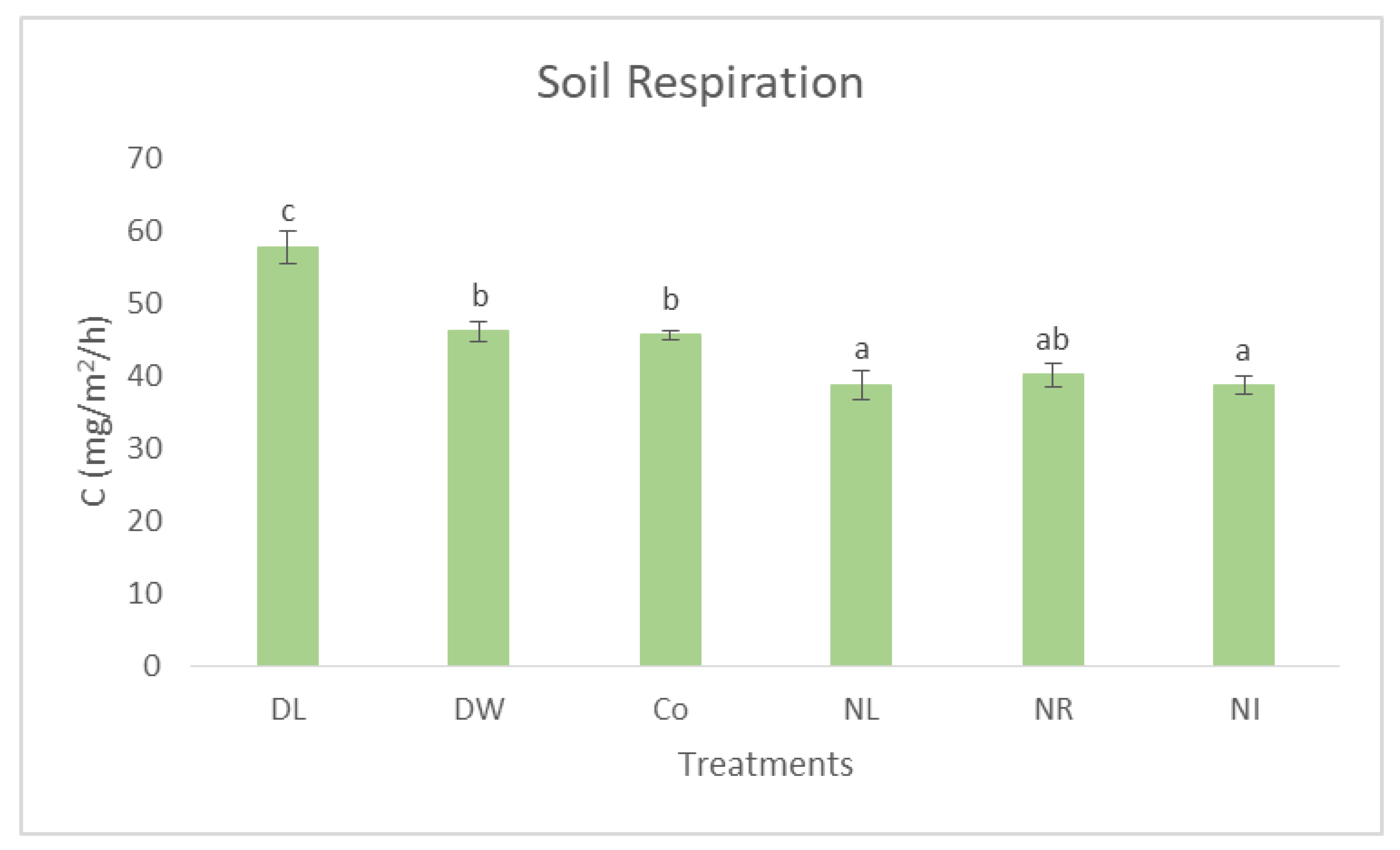
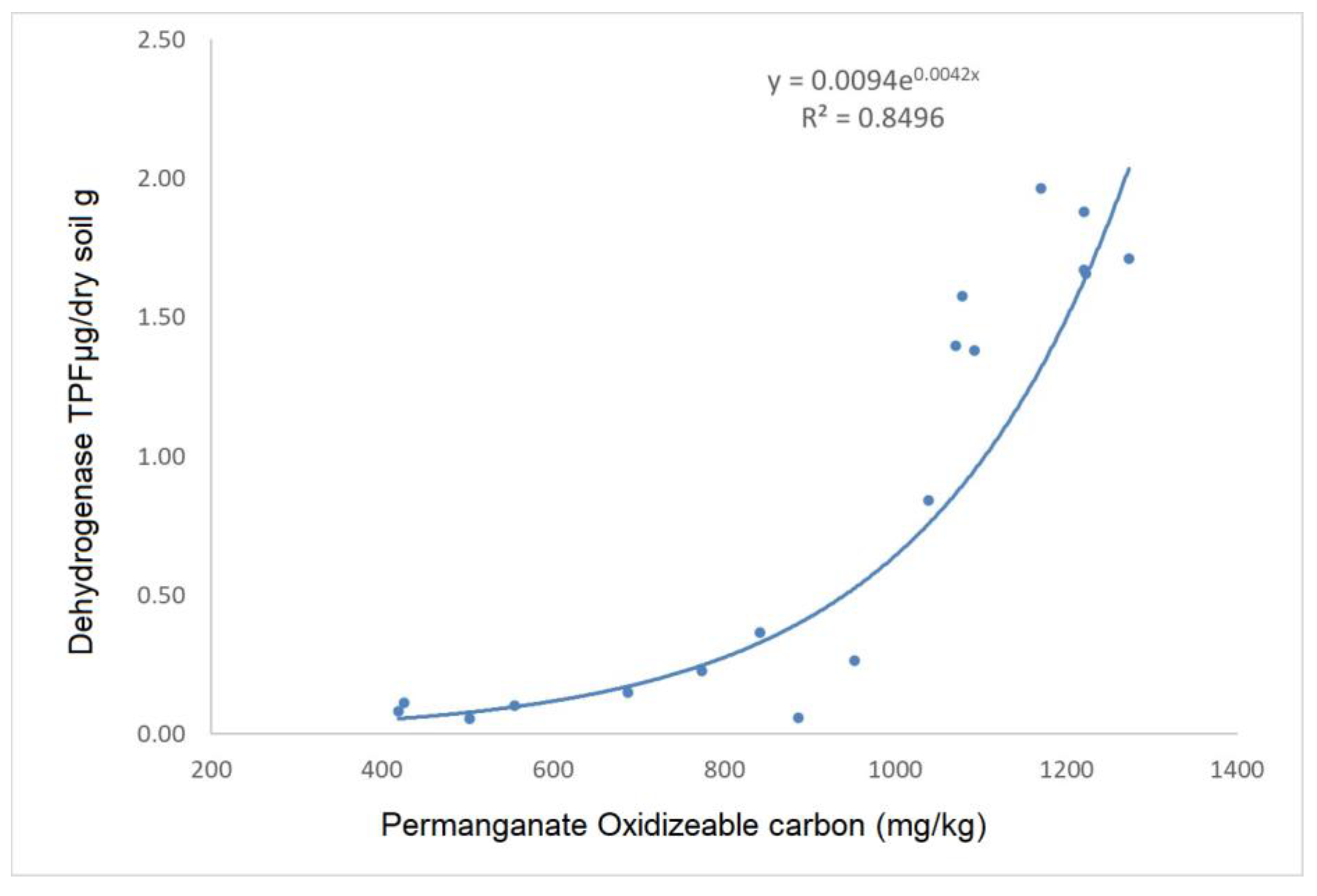
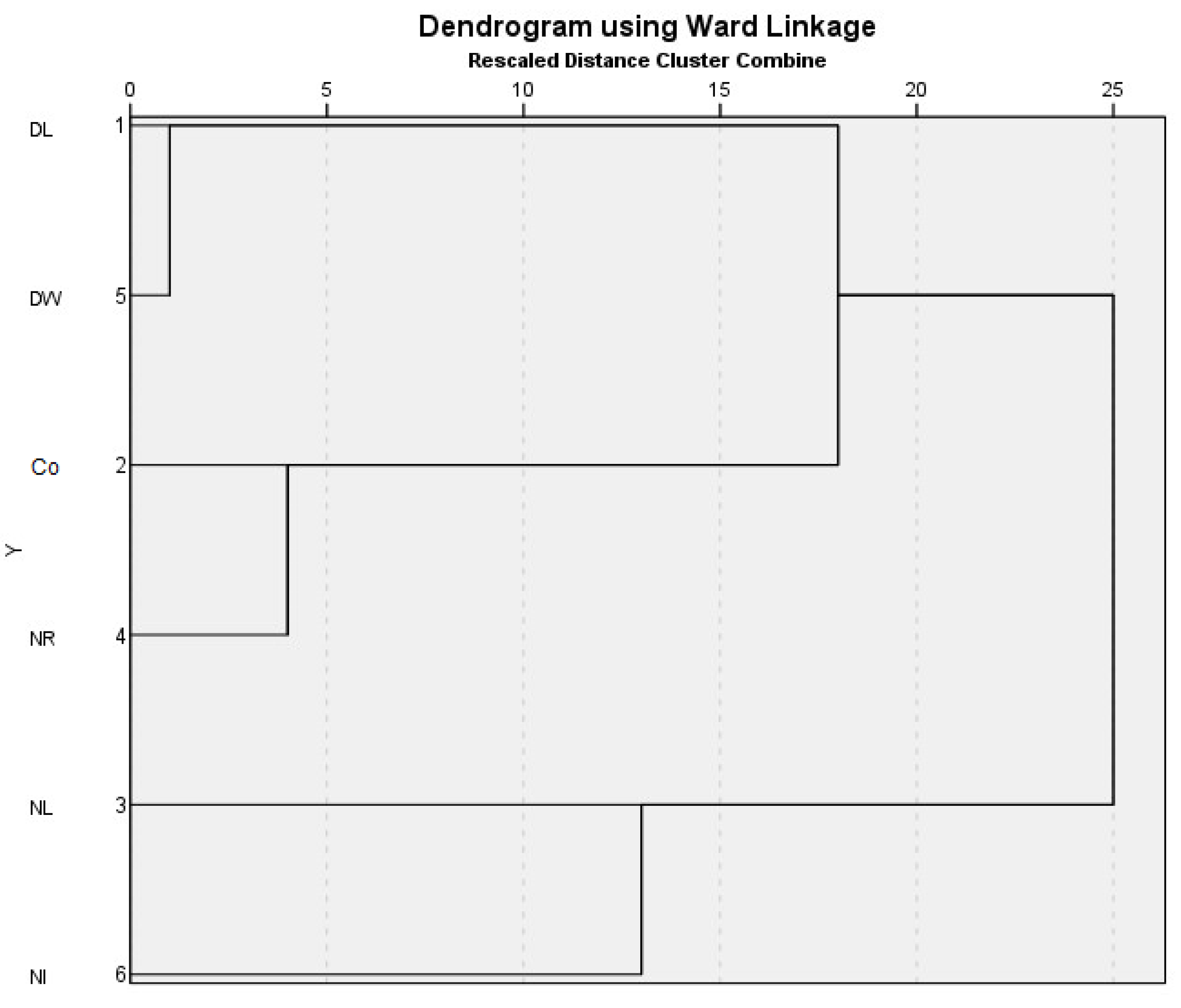
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Misik, T.; Kárász, I. Long-term relationship between oak decline and shrub growth dynamics in an hungarian oak forest, 1972–2017. Agrofor 2020, 5, 47–54. [Google Scholar] [CrossRef]
- Rózsa, P.; Novák, T. Mapping anthropic geomorphological sensitivity on global scale. Z. Fur Geomorphol. Suppl. 2011, 55, 109–117. [Google Scholar] [CrossRef]
- Fekete, I.; Halasz, J.; Kramoperger, Z.; Krausz, E. Study of litter decomposition intensity in litter manipulative trials in Síkfőkút Cambisols. Cereal Res. Commun. 2008, 36, 1779–1782. [Google Scholar]
- Szalai, Z.; Ringer, M.; Németh, T.; Sipos, P.; Perényi, K.; Pekker, P.; Jakab, G. Accelerated soil development due to seasonal water-saturation under hydric conditions. Geoderma 2021, 401, 115328. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Torn, M.S.; Abiven, S.; Dittmar, T.; Guggenberger, G.; Janssens, I.A.; Weiner, S.; Trumbore, S.E. Persistence of soil organic matter as an ecosystem property. Nature 2011, 478, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Krishnaveni, A.; Chinnasamy, S.; Elumalai, J.; Muthaiyan, P. Sugar industry wastes as wealth of organic carbon for soil. Environ. Factors Affect. Hum. Health 2020, Chapter 8, 137–151. [Google Scholar]
- Wei, W.; Weile, C.; Shaopeng, W. Forest soil respiration and its heterotrophic and autotrophic components: Global patterns and responses to temperature and precipitation. Soil Biol. Biochem. 2010, 42, 1236–1244. [Google Scholar] [CrossRef]
- Fekete, I.; Berki, I.; Lajtha, K.; Trumbore, S.; Francioso, O.; Gioacchini, P.; Montecchio, D.; Várbíró, G.; Béni, Á.; Makádi, M.; et al. How will a drier climate change carbon sequestration in soils of the deciduous forests of Central Europe? Biogeochemistry 2021, 152, 13–32. [Google Scholar] [CrossRef]
- Chapin, F.S.I.; McFarland, J.; McGuire, A.D.; Euskirchen, E.S.; Ruess, R.W.; Kielland, K. The changing global carbon cycle: Linking plant-soil carbon dynamics to global consequences. J. Ecol. 2009, 97, 840–850. [Google Scholar]
- Süle, G.; Fóti, S.; Körmöczi, L.; Petrás, D.; Kardos, L.; Balogh, J. Co-varying effects of vegetation structure and terrain attributes are responsible for soil respiration spatial patterns in a sandy forest–steppe transition zone. Web Ecol. 2021, 21, 95–107. [Google Scholar] [CrossRef]
- Canadell, J.G.; Raupach, M.R. Managing forests for climate change mitigation. Science 2008, 320, 1456–1457. [Google Scholar] [CrossRef] [PubMed]
- Hisano, M.; Searle, E.B.; Chen, H.Y. Biodiversity as a solution to mitigate climate change impacts on the functioning of forest ecosystems. Biol. Rev. 2018, 93, 439–456. [Google Scholar] [CrossRef]
- Fekete, I.; Lajtha, K.; Kotroczó, Z.S.; Várbíró, G.; Varga, C.; Tóth, J.A.; Demeter, I.; Verpedi, G.; Berki, I. Long-term effects of climate change on carbon storage and tree species composition in a dry deciduous forest. Glob. Change Biol. 2017, 23, 3154–3168. [Google Scholar] [CrossRef] [PubMed]
- Galos, B.; Goettel, H.; Haensler, A.; Preuschmann, S.; Matyas, C.; Jacob, D. Do forest cover changes have any feedback on temperature and precipitation extremes over Hungary? In EGU General Assembly Conference Abstracts; European Geosciences Union: Vienna, Austria, 2009. [Google Scholar]
- Bartholy, J.; Pongrácz, R.; Gelybó, G. Regional climate change expected in Hungary for 2071–2100. Appl. Ecol. Environ. Res. 2007, 5, 1–17. [Google Scholar] [CrossRef]
- Ringer, M.; Jakab, G.; Sipos, P.; Szabó, M.; Perényi, K.; Szalai, Z. Vertical differentiation of pedogenic iron forms–a key of hydromorphic soil profile development. Hung. Geogr. Bull. 2021, 70, 369–380. [Google Scholar] [CrossRef]
- Nadelhoffer, K.; Boone, R.; Bowden, R.D.; Canary, J.; Kaye, J.; Micks, P.; Ricca, A.; McDowell, W.; Aitkenhead, J. The DIRT experiment. In Forests in Time; Foster, D.R., Aber, D.J., Eds.; Yale University Press: Michigan, MA, USA, 2014. [Google Scholar]
- Crow, S.E.; Lajtha, K.; Filley, T.R.; Swanston, C.W.; Bowden, R.D.; Caldwell, B.A. Sources of plant-derived carbon and stability of organic matter in soil: Implications for global change. Glob. Change Biol. 2009, 15, 2003–2019. [Google Scholar] [CrossRef]
- Blair, G.J.; Lefroy, R.D.; Lisle, L. Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 1995, 46, 1459–1466. [Google Scholar] [CrossRef]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar]
- Wright, S.F.; Rillig, M.C.; Nichols, K.A. Glomalin: A soil protein important in carbon sequestration. Abstr. Pap. Am. Chem. Soc. 2000, 220, 721–725. [Google Scholar]
- Rillig, M.C.; Wright, S.F.; Eviner, V.T. The role of arbuscular mycorrhizal fungi and glomalin in soil aggregation: Comparing effects of five plant species. Plant Soil 2002, 238, 325–333. [Google Scholar] [CrossRef]
- He, J.D.; Chi, G.G.; Zou, Y.N.; Shu, B.; Wu, Q.S.; Srivastava, A.K.; Kuča, K. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil Ecol. 2020, 154, 103592. [Google Scholar] [CrossRef]
- Gao, W.Q.; Wang, P.; Wu, Q.S. Functions and application of glomalin-related soil proteins: A review. Sains Malays. 2019, 48, 111–119. [Google Scholar] [CrossRef]
- Hurisso, T.T.; Moebius-Clune, D.J.; Culman, S.W.; Moebius-Clune, B.N.; Thies, J.E.; van Es, H.M. Soil protein as a rapid soil health indicator of potentially available organic nitrogen. Agric. Environ. Lett. 2018, 3, 180006. [Google Scholar] [CrossRef]
- Zhu, Y.G.; Miller, R.M. Carbon cycling by arbuscular mycorrhizal fungi in soil–plant systems. Trends Plant Sci. 2003, 8, 407–409. [Google Scholar] [CrossRef]
- Tian, H.; Liu, X.L.; Gai, J.P.; Zhang, J.L.; Li, X.L. Review of glomalin-related soil protein and its function. Chin. J. Soil Sci. 2009, 40, 1215–1220. [Google Scholar]
- Veres, Z.S.; Kotroczó, Z.S.; Fekete, I.; Tóth, J.A.; Lajtha, K.; Townsend, K.; Tóthmérész, B. Soil extracellular enzyme activities are sensitive indicators of detrital inputs and carbon availability. Appl. Soil Ecol. 2015, 92, 18–23. [Google Scholar] [CrossRef]
- Wolinska, A.; Steäpniewska, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; Canuto, R.A., Ed.; InTech: Bolton, UK, 2012; ISBN 978-953-307-019-3. [Google Scholar] [CrossRef]
- Koncz, G.; Papp, M.; Török, P.; Kotroczó, Z.; Krakomperger, Z.; Matus, G.; Tóthmérész, B. The role of seed bank in the dynamics of understorey in an oak forest in Hungary. ACTA Biol. Hung. 2010, 61, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Misik, T.; Kárász, I. Low understory condition in an oak forest in Hungary, 1972 and 2022–Síkfőkút Project is 50 years old. ACTA Biol. Plant. Agriensis. 2022, 10, 22–35. [Google Scholar] [CrossRef]
- Misik, T.; Kárász, I. Long-Term Dynamics of Subcanopy Layer as New Layer in an Oak Forest of Hungary. Agrofor Int. J. 2022, 7, 85–94. [Google Scholar]
- Kotroczó, Z.S.; Veres, Z.S.; Fekete, I.; Papp, M.; Tóth, J.A. Effects of Climate Change on Litter Production in a Quercetum petraeae-cerris Forest in Hungary. Acta Silv. Et Lignaria Hung. 2012, 8, 31–38. [Google Scholar] [CrossRef]
- Switoniak, M.; Charzyński, P.; Novák, T.J.; Zalewska, K.; Bednarek, R. Forested hilly landscape of Bükkalja Foothill (Hungary). In Soil Sequences Atlas; Nicholaus Copernicus University Press: Torun, Poland, 2014; pp. 169–181. [Google Scholar]
- Juhos, K.; Madarász, B.; Kotroczó, Z.; Béni, Á.; Makádi, M.; Fekete, I. Carbon sequestration of forest soils is reflected by changes in physicochemical soil indicators—A comprehensive discussion of a long-term experiment on a detritus manipulation. Geoderma 2021, 385, 114918. [Google Scholar] [CrossRef]
- Thalmann, A. Dehydrogenase activity. In 1995 Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press Ltd.: New York, NY, USA, 1968; pp. 228–230. [Google Scholar]
- Veres, Z.; Kotroczó, Z.; Magyaros, K.; Tóth, J.A.; Tóthmérész, B. Dehydrogenase Activity in a Litter Manipulation Experiment in Temperate Forest Soil. Acta Silv. Lign. Hung. 2013, 9, 25–33. [Google Scholar] [CrossRef]
- Chichester, F.W.; Chaison, R.F. Analysis of carbon in calcareous soils using a two temperature dry combustion infrared instrumental procedure. Soil Sci. 1992, 153, 237–241. [Google Scholar] [CrossRef]
- Raich, J.W.; Bowden, R.D.; Steudler, P.A. Comparison of two static chamber techniques for determining carbon dioxide eflux from forest soils. Soil Sci. Soc. Am. J. 1990, 54, 1754–1757. [Google Scholar] [CrossRef]
- Grogan, P. CO2 flux measurement using soda lime: Correction for water formed during CO2 adsorption. Ecology 1998, 79, 1467–1468. [Google Scholar] [CrossRef]
- Monteith, J.L.; Steicz, G.; Yabuki, K. Crop photosynthesis and the flux of carbon dioxide below the canopy. J. Appl. Ecol. 1964, 1, 321–337. [Google Scholar] [CrossRef]
- Kotroczó, Z.; Makádi, M.; Kocsis, T.; Béni, Á.; Várbíró, G.; Fekete, I. Long-Term Changes in Organic Matter Content and Soil Moisture Determine the Degree of Root and Soil Respiration. Plants 2023, 12, 251. [Google Scholar] [CrossRef]
- Khalvati, M.; Bartha, B.; Dupigny, A.; Schröder, P. Arbuscular mycorrhizal association is beneficial for growth and detoxification of xenobiotics of barley under drought stress. J. Soils Sediments 2010, 10, 54–64. [Google Scholar] [CrossRef]
- Fekete, I.; Béni, Á.; Juhos, K.; Kotroczó, Z. The effects of a twenty-year litter manipulation experiment on the carbon content and water retention capacity of the examined Luvisols: Síkfőkút DIRT Project. Agrokémia És Talajt. 2022, 71, 239–253. [Google Scholar] [CrossRef]
- Fekete, I.; Varga, C.; Biró, B.; Tóth, J.A.; Várbíró, G.; Lajtha, K.; Szabó, G.; Kotroczó, Z. The effects of litter production and litter depth on soil microclimate in a central european deciduous forest. Plant Soil 2016, 398, 291–300. [Google Scholar] [CrossRef]
- Beni, Á.; Lajtha, K.; Kozma, J.; Fekete, I. Application of a Stir Bar Sorptive Extraction sample preparation method with HPLC for soil fungal biomass determination in soils from a detrital manipulation study. J. Microbiol. Methods 2017, 136, 1–5. [Google Scholar] [CrossRef]
- Bongiorno, G.; Bünemann, E.K.; Oguejiofor, C.U.; Meier, J.; Gort, G.; Comans, R.; Brussaard, L.; de Goede, R. Sensitivity of labile carbon fractions to tillage and organic matter management and their potential as comprehensive soil quality indicators across pedoclimatic conditions in Europe. Ecol. Indic. 2019, 99, 38–50. [Google Scholar] [CrossRef]
- Rillig, M.C.; Caldwell, B.A.; Wösten, H.A.; Sollins, P. Role of proteins in soil carbon and nitrogen storage: Controls on persistence. Biogeochemistry 2007, 85, 25–44. [Google Scholar] [CrossRef]
- Knicker, H.; Hatcher, P.G. Survival of protein in an organic-rich sediment. Possible protection by encapsulation in organic matter. Naturwissenschaften 1997, 84, 231–234. [Google Scholar] [CrossRef]
- Zang, X.; Van Heemst, J.; Jasper, D.H.; Dria, K.J.; Hatcher, P.G. Encapsulation of protein in humic acid from Histosols as an explanation for the occurrence of organic nitrogen in soil and sediment. Org. Geochem. 2000, 31, 679–695. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, Y.H.; Liu, T.; Huang, S.P.; Chang, Y.F. Elevated CO2 increases glomalin-related soil protein (GRSP) in the rhizosphere of Robinia pseudoacacia L. seedlings in Pb-and Cd-contaminated soils. Environ. Pollut. 2016, 218, 349–357. [Google Scholar] [CrossRef]
- Zou, Y.N.; Srivastava, A.K.; Wu, Q.S. Glomalin: A potential soil conditioner for perennial fruits. Int. J. Agric. Biol. 2016, 18, 293–297. [Google Scholar] [CrossRef]
- Hossain, M.B. Glomalin and contribution of glomalin to carbon sequestration in soil: A review. Turk. J. Agric. -Food Sci. Technol. 2021, 9, 191–196. [Google Scholar] [CrossRef]
- Chi, G.G.; Wu, Q.S. Effects of mycorrhizal fungi on plant and growth soil properties in trifoliate orange seedlings grown in a root–box. Philipp. Agric. Sci. 2017, 3, 271–277. [Google Scholar]
- Fokom, R.; Teugwa, M.C.; Nana, W.L.; Ngonkeu, M.E.L.; Tchameni, S.; Nwaga, D.; Rillig, C.M.; Amvam, Z.P.H. Glomalin, carbon, nitrogen and soil aggregate stability as affected by land use changes in the humid forest zone in South Cameroon. Appl. Ecol. Environ. Res. 2013, 11, 581–592. [Google Scholar] [CrossRef]
- Atakan, A.; Özkaya, H.Ö. Arbuscular mycorrhizal fungi and glomalin. Turk. J. Agric. Food Sci. Technol. 2021, 9, 2371–2375. [Google Scholar] [CrossRef]
- Lajtha, K.; Peterson, F.; Nadelhoffer, K.; Bowden, R.D. Twenty years of litter and root manipulations: Insights into multi-decadal SOM dynamics. Soil Sci. Soc. Am. J. 2015, 78, 61–69. [Google Scholar]
- Bowden, R.D.; Deem, L.; Plante, A.F.; Peltre, C.; Nadelhoffer, K.; Lajtha, K. Litter input controls on soil carbon in a temperate deciduous forest. Soil Sci. Soc. Am. J. 2014, 78, S66–S75. [Google Scholar] [CrossRef]
- Lajtha, K.; Townsend, K.L.; Kramer, M.G.; Swanston, C.; Bowden, R.D.; Nadelhoffer, K. Changes to particulate versus mineral-associated soil carbon after 50 years of litter manipulation in forest and prairie experimental ecosystems. Biogeochemistry 2014, 119, 341–360. [Google Scholar] [CrossRef]


| Applied Treatments | Description of Treatments |
|---|---|
| Control (Co) | Average litter amount typical of the forest site. |
| No Litter (NL) | Above-ground inputs are excluded from plots. Leaf litter was removed using a rake. This process was repeated continuously every year. |
| No Roots (NR) | The plots were trenched around 40 cm wide and 100 cm deep. The soil dug out was placed outside the plot. Root-proof Delta MS 500 PE foil, which was 0.6 mm thick and 1 m wide and of high density, was put in the trenches. Then, the trenches were filled with soil. So as to eliminate root production, plants were cleared (bushes had been cut out at the establishment). |
| No Inputs (NI) | Aboveground inputs are excluded from plots; the belowground inputs are provided as in NR plots. This treatment is the combination of NR + NL plots. |
| Double Wood (DW) | Aboveground wood debris inputs are doubled through adding wood to each plot. Annual wood litter amount was measured using boxes placed at the site, and its double amount was applied in the case of every DW plots. |
| Double Litter (DL) | Above-ground leaf inputs are doubled through adding litter removed from No Litter plots. |
| Treatments | Total-C (g SOC kg−1 Dry Soil) | POX-C (g POXC kg−1 Dry Soil) |
|---|---|---|
| Double Litter (DL) | 66.9 | 1.2 |
| Double Wood (DW) | 56.3 | 1.2 |
| Control (Co) | 55.2 | 1.1 |
| No Litter (NL | 32.1 | 0.6 |
| No Roots (NR) | 43.6 | 0.9 |
| No Inputs (NI) | 32.3 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotroczó, Z.; Fekete, I.; Juhos, K.; Prettl, N.; Nugroho, P.A.; Várbíró, G.; Biró, B.; Kocsis, T. Characterisation of Luvisols Based on Wide-Scale Biological Properties in a Long-Term Organic Matter Experiment. Biology 2023, 12, 909. https://doi.org/10.3390/biology12070909
Kotroczó Z, Fekete I, Juhos K, Prettl N, Nugroho PA, Várbíró G, Biró B, Kocsis T. Characterisation of Luvisols Based on Wide-Scale Biological Properties in a Long-Term Organic Matter Experiment. Biology. 2023; 12(7):909. https://doi.org/10.3390/biology12070909
Chicago/Turabian StyleKotroczó, Zsolt, István Fekete, Katalin Juhos, Nándor Prettl, Priyo Adi Nugroho, Gábor Várbíró, Borbála Biró, and Tamás Kocsis. 2023. "Characterisation of Luvisols Based on Wide-Scale Biological Properties in a Long-Term Organic Matter Experiment" Biology 12, no. 7: 909. https://doi.org/10.3390/biology12070909
APA StyleKotroczó, Z., Fekete, I., Juhos, K., Prettl, N., Nugroho, P. A., Várbíró, G., Biró, B., & Kocsis, T. (2023). Characterisation of Luvisols Based on Wide-Scale Biological Properties in a Long-Term Organic Matter Experiment. Biology, 12(7), 909. https://doi.org/10.3390/biology12070909







