Targeted Glutamate Supply Boosts Insulin Concentrations, Ovarian Activity, and Ovulation Rate in Yearling Goats during the Anestrous Season
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Location, Environmental Conditions, Animal Selection, and Management
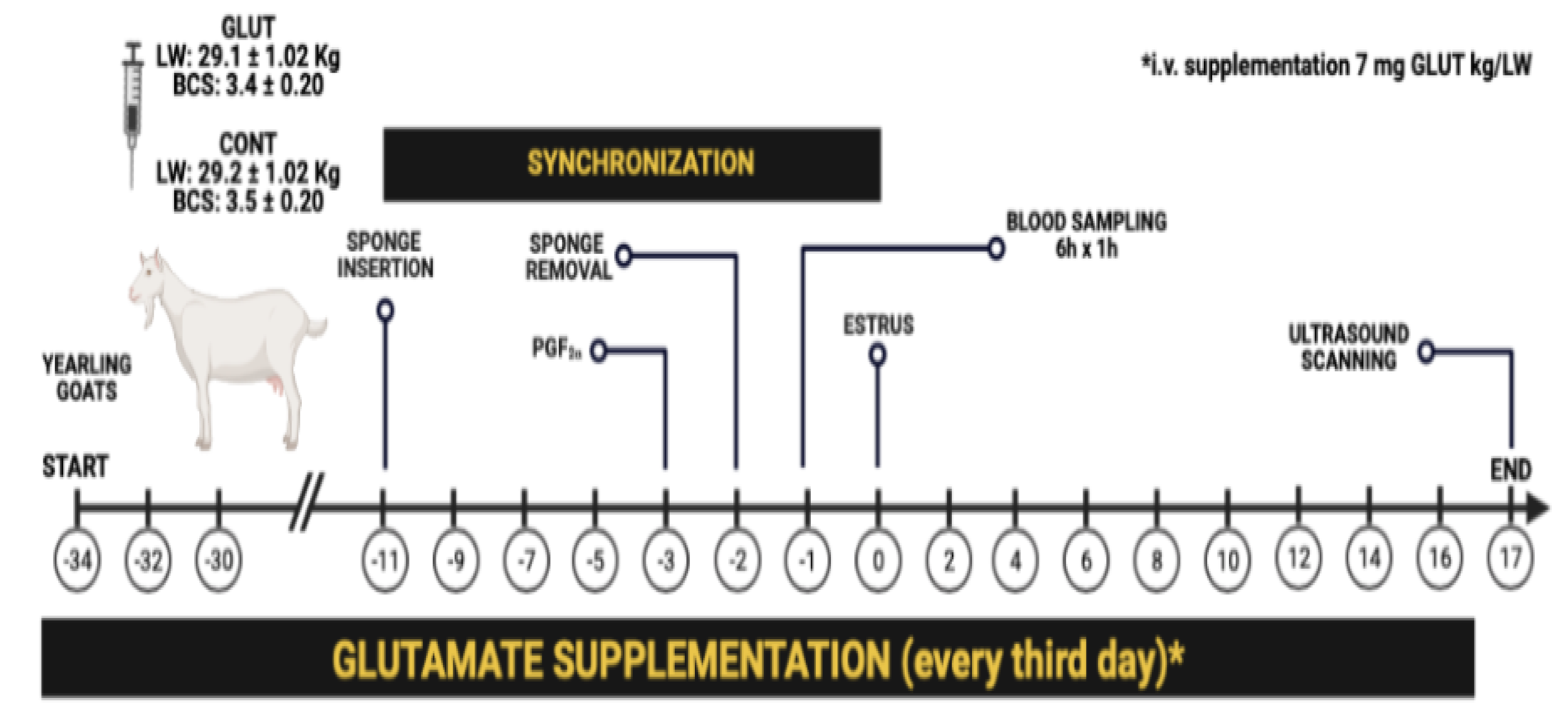
2.2. Experimental Design and Treatments
2.3. Estrus Synchronization, Blood Sampling, and Insulin Measurement
2.4. Ultrasonographic Scanning of Ovarian Activity
2.5. Statistical Analyses
3. Results
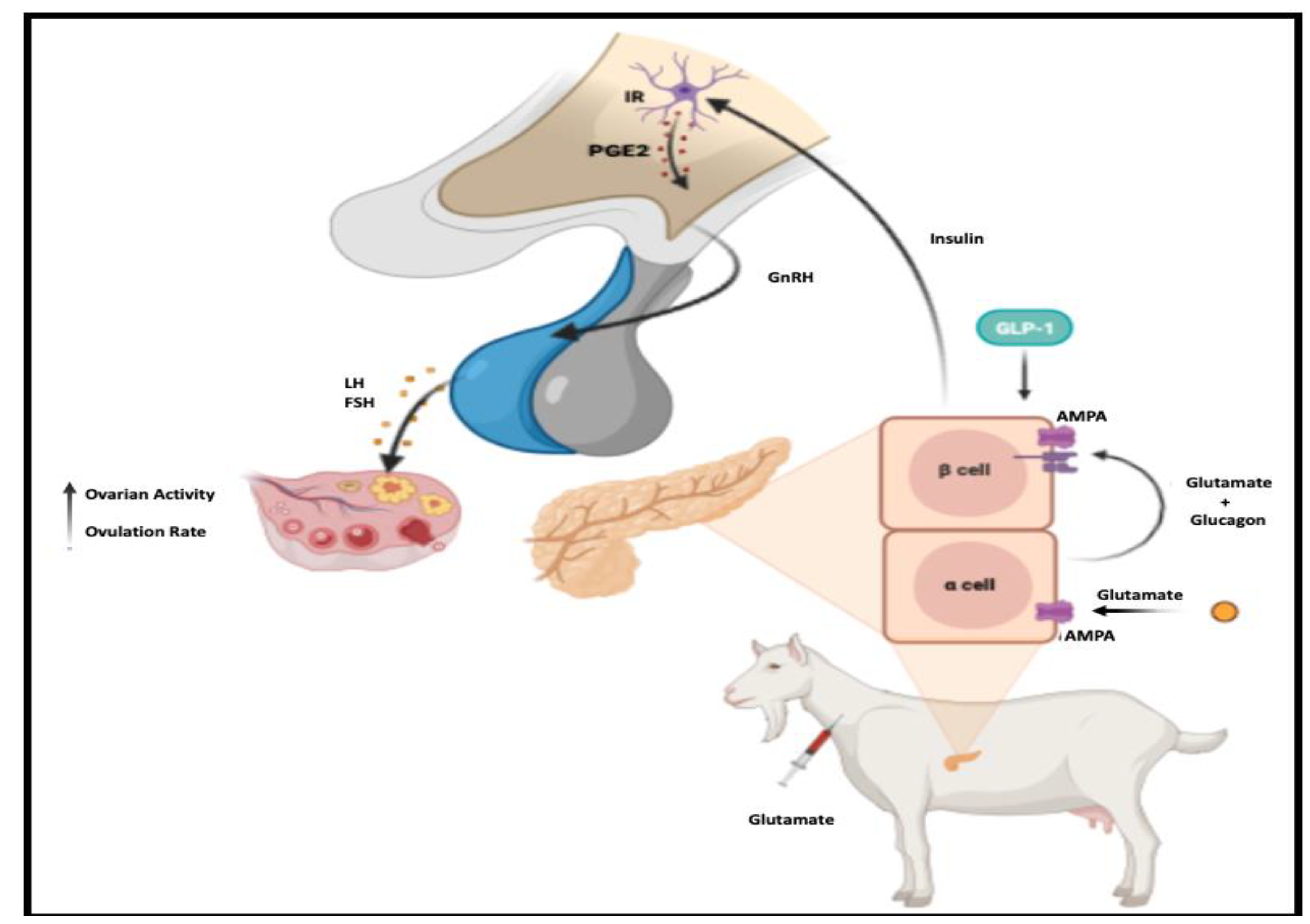
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guh, Y.J.; Tamai, T.K.; Yoshimura, T. The Underlying Mechanisms of Vertebrate Seasonal Reproduction. Proc. Jpn. Acad. Ser. B 2019, 95, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Dardente, H.; Simonneaux, V. GnRH and the Photoperiodic Control of Seasonal Reproduction: Delegating the Task to Kisspeptin and RFRP-3. J. Neuroendocrinol. 2022, 34, e13124. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Barrett, P.; Morgan, P.J. A Unifying Hypothesis for Control of Body Weight and Reproduction in Seasonally Breeding Mammals. J. Neuroendocrinol. 2019, 31, e12680. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Chen, Z.; Li, X.; Cai, E.; Yang, H.; Chen, Y.; Wang, C.; Ju, L.; Deng, W.; Mu, L. Advances in Clinical Applications of Kisspeptin-GnRH Pathway in Female Reproduction. Reprod. Biol. Endocrinol. 2022, 20, 81. [Google Scholar] [CrossRef]
- Szeliga, A.; Meczekalski, B. Kisspeptin Modulation of Reproductive Function. Endocrines 2022, 3, 367–374. [Google Scholar] [CrossRef]
- Starrett, J.R.; Moenter, S.M. Hypothalamic Kisspeptin Neurons as Potential Mediators of Estradiol Negative and Positive Feedback. Peptides 2023, 163, 170963. [Google Scholar] [CrossRef]
- Simonneaux, V. A Kiss to Drive Rhythms in Reproduction. Eur. J. Neurosci. 2020, 51, 509–530. [Google Scholar] [CrossRef]
- Navarro, V.M. Metabolic Regulation of Kisspeptin—The Link Between Energy Balance and Reproduction. Nat. Rev. Endocrinol. 2020, 16, 407–420. [Google Scholar] [CrossRef]
- Sobrino, V.; Avendano, M.S.; Perdices-Lopez, C.; Jimenez-Puyer, M.; Tena-Sempere, M. Kisspeptins and the Neuroendocrine Control of Reproduction: Recent Progress and New Frontiers in Kisspeptin Research. Front. Neuroendocrinol. 2022, 65, 100977. [Google Scholar] [CrossRef]
- García-Galiano, D.; Pineda, R.; Roa, J.; Ruiz-Pino, F.; Sánchez-Garrido, M.A.; Castellano, J.M.; Aguilar, E.; Navarro, V.M.; Pinilla, L.; Tena-Sempere, M. Differential Modulation of Gonadotropin Responses to Kisspeptin by Aminoacidergic, Peptidergic, and Nitric Oxide Neurotransmission. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1252–E1263. [Google Scholar] [CrossRef]
- Takahashi, H.; Yokoi, N.; Seino, S. Glutamate as Intracellular and Extracellular Signals in Pancreatic Islet Functions. Proc. Jpn. Acad. Ser. B 2019, 95, 246–260. [Google Scholar] [CrossRef] [PubMed]
- Javed, K.; Fairweather, S.J. Amino Acid Transporters in the Regulation of Insulin Secretion and Signaling. Biochem. Soc. Trans. 2019, 47, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Iremonger, K.J.; Constantin, S.; Liu, X.; Herbison, A.E. Glutamate Regulation of GnRH Neuron Excitability. Brain Res. 2010, 1364, 35–43. [Google Scholar] [CrossRef]
- Constantin, S.; Jasoni, C.L.; Wadas, B.; Herbison, A.E. Gamma-aminobutyric acid and glutamate differentially regulate intracellular calcium concentrations in mouse gonadotropin-releasing hormone neurons. Endocrinology 2010, 151, 262–270. [Google Scholar] [CrossRef]
- Glanowska, K.M.; Moenter, S.M. Endocannabinoids and prostaglandins both contribute to GnRH neuron-GABAergic afferent local feedback circuits. J. Neurophysiol. 2011, 106, 3073–3081. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Torres-Moreno, M.; Lopez-Medrano, J.I.; Gonzalez-Bulnes, A.; Veliz, F.G.; Mellado, M.; Wurzinger, M.; Soto-Sanchez, M.J.; Calderon-Leyva, M.G. Glutamate Supply Positively Affects Serum Release of Triiodothyronine and Insulin Across Time Without Increases of Glucose During the Onset of Puberty in the Female Goat. Anim. Reprod. Sci. 2011, 125, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Leyva, G.; Meza-Herrera, C.A.; Rodríguez-Martínez, R.; Ángel-García, O.; Rivas-Muñoz, R.; Delgado-Bermejo, J.V.; Véliz-Deras, F.G. Effect of Glutamate and/or Testosterone Administration Upon Appetitive and Consummatory Sexual Behaviors in Pubertal Rams and Their Influence Upon the Reproductive Performance of Nulliparous Anovulatory Ewes. J. Vet. Behav. Clin. Appl. Res. 2019, 30, 96–102. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Vergara-Hernández, H.P.; Paleta-Ochoa, A.; Álvarez-Ruíz, A.R.; Véliz-Deras, F.G.; Arellano-Rodríguez, G.; Rosales-Nieto, C.A.; Macías-Cruz, U.; Rodríguez-Martínez, R.; Carrillo, E. Glutamate Supply Reactivates Ovarian Function While Increases Serum Insulin and Triiodothyronine Concentrations in Criollo X Saanen-Alpine Yearlings’ Goats During the Anestrous Season. Animals 2020, 10, 234. [Google Scholar] [CrossRef]
- Luna-García, L.A.; Meza-Herrera, C.A.; Pérez-Marín, C.C.; Corona, R.; Luna-Orozco, J.R.; Véliz-Deras, F.G.; Delgado-Gonzalez, R.; Rodrgiuez-Venegas, R.; Rosales-Nieto, C.A.; Bustamante-Andrade, J.A.; et al. Goats as Valuable Animal Model to Test the Targeted Glutamate Supplementation upon Antral Follicle Number, Ovulation Rate, and LH-Pulsatility. Biology 2022, 11, 1015. [Google Scholar] [CrossRef]
- Simões, J. Recent advances on synchronization of ovulation in goats, out of season, for a more sustainable production. Asian Pac. J. Reprod. 2015, 4, 157–165. [Google Scholar] [CrossRef]
- Dumont, B.; González-García, E.; Thomas, M.; Fortun-Lamothe, L.; Ducrot, C.; Dourmad, J.Y.; Tichit, M. Forty research issues for the redesign of animal production systems in the 21st century. Animal 2014, 8, 1382–1393. [Google Scholar] [CrossRef]
- Roger, P.A. Welfare issues in the reproductive management of small ruminants. Anim. Reprod. Sci. 2012, 130, 141–146. [Google Scholar] [CrossRef] [PubMed]
- FASS—Federation Animal Science Society. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaign, IL, USA, 2010; p. 177. [Google Scholar]
- NAM—National Academy of Medicine. Guide for the Care and Use of Laboratory Animals, 1st ed.; Co-Produced by the National Academy of Medicine—Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International: Harlan, KY, USA; Mexico City, Mexico, 2002. [Google Scholar]
- NRC—National Research Council. Nutrient Requirements of Goats: Angora, Dairy and Meat Goats in Temperature and Tropical Countries; National Academy Press: Washington, DC, USA, 1998. [Google Scholar]
- AOAC—Association of Official Analytical Chemists. Official Methods of Analysis, 15th ed.; AOAC: Arlington, VA, USA, 1990. [Google Scholar]
- Hoefler, H.; Hallford, D.M. Influence of Suckling Status and Type of Birth on Serum Hormone Profiles and Return to Estrus Early-Postpartum Spring-Lambing Ewes. Theriogenology 1987, 27, 887–892. [Google Scholar] [CrossRef]
- Martin, G.B.; Oldham, C.M.; Lindsay, D.R. Increased Plasma LH Levels in Seasonally Anovulatory Merino Ewes Following the Introduction of Rams. Anim. Reprod. Sci. 1980, 3, 125–132. [Google Scholar] [CrossRef]
- Martin, G.B.; Scaramuzzi, R.J.; Oldham, C.M.; Lindsay, D.R. Effects of Progesterone on the Responses of Merino Ewes to the Introduction of Rams During Anoestrus. Aust. J. Biol. Sci. 1983, 36, 369–378. [Google Scholar] [CrossRef]
- Dickie, A.M.; Paterson, C.; Anderson, L.M.; Boyd, J.S. Determination of Corpora Lutea Numbers in Booroola—Texel Ewes Using Transrectal Ultrasound. Theriogenology 1999, 51, 1209–1224. [Google Scholar] [CrossRef]
- Littell, C.R.; Henry, P.R.; Ammermam, C.B. Statistical Analysis of Repeated Measures Data Using SAS Procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Marquard, J.; Otter, S.; Welters, A.; Stirban, A.; Fischer, A.; Eglinger, J.; Herebian, D.; Kletke, O.; Klemen, M.S.; Stožer, A.; et al. Characterization of Pancreatic NMDA Receptors as Possible Drug Targets for Diabetes Treatment. Nat. Med. 2015, 21, 363–372. [Google Scholar] [CrossRef]
- Inagaki, N.; Kuromi, H.; Gonoi, T.; Okamoto, Y.; Ishida, H.; Seino, Y.; Kaneko, T.; Iwanaga, T.; Seino, S. Expression and Role of Ionotropic Glutamate Receptors in Pancreatic Islet Cells. FASEB J. 1995, 9, 686–691. [Google Scholar] [CrossRef]
- DiVall, S.A.; Herrera, D.; Sklar, B.; Wu, S.; Wondisford, F.; Radovick, S.; Wolfe, A. Insulin Receptor Signaling in the GnRH Neuron Plays a Role in the Abnormal GnRH Pulsatility of Obese Female Mice. PLoS ONE 2015, 10, e0119995. [Google Scholar] [CrossRef]
- Wu, S.; Divall, S.; Nwaopara, A.; Radovick, S.; Wondisford, F.; Ko, C.; Wolfe, A. Obesity-Induced Infertility and Hyperandrogenism are Corrected by Deletion of the Insulin Receptor in the Ovarian Theca Cell. Diabetes 2014, 63, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Scaramuzzi, R.J. Insulin Signalling and Glucose Transport in the Ovary and Ovarian Function During the Ovarian Cycle. Biochem. J. 2016, 473, 1483–1501. [Google Scholar] [CrossRef]
- Evans, M.C.; Rizwan, M.Z.; Anderson, G.M. Insulin Action on GABA Neurons is a Critical Regulator of Energy Balance but not Fertility in Mice. Endocrinology 2014, 155, 4368–4379. [Google Scholar] [CrossRef]
- Codner, E.; Merino, P.M.; Tena-Sempere, M. Female Reproduction and Type 1 Diabetes: From Mechanisms to Clinical Findings. Hum. Reprod. Update 2012, 18, 568–585. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.M.; Ackerman, K.E.; Berga, S.L.; Kaplan, J.R.; Mastorakos, G.; Misra, M.; Murad, M.H.; Santoro, N.F.; Warren, M.P. Functional Hypothalamic Amenorrhea: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2017, 102, 1413–1439. [Google Scholar] [CrossRef]
- Kim, H.H.; DiVall, S.A.; Deneau, R.M.; Wolfe, A. Insulin Regulation of GnRH Gene Expression Through MAP Kinase Signaling Pathways. Mol. Cell. Endocrinol. 2005, 242, 42–49. [Google Scholar] [CrossRef]
- Evans, M.C.; Hill, J.W.; Anderson, G.M. Role of Insulin in the Neuroendocrine Control of Reproduction. J. Neuroendocrinol. 2021, 33, e12930. [Google Scholar] [CrossRef]
- Clasadonte, J.; Prevot, V. The Special Relationship: Glia–Neuron Interactions in the Neuroendocrine Hypothalamus. Nat. Rev. Endocrinol. 2018, 14, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Clasadonte, J.; Sharif, A.; Baroncini, M.; Prevot, V. Gliotransmission by Prostaglandin E2: A Prerequisite for GnRH Neuronal Function? Front. Endocrinol. 2011, 2, 91. [Google Scholar] [CrossRef]
- Gheni, G.; Ogura, M.; Iwasaki, M.; Yokoi, N.; Minami, K.; Nakayama, Y.; Harada, K.; Hastoy, B.; Wu, X.; Takahashi, H.; et al. Glutamate Acts as a Key Signal Linking Glucose Metabolism to Incretin/cAMP Action to Amplify Insulin Secretion. Cell Rep. 2014, 9, 661–673. [Google Scholar] [CrossRef]
- Chiang, V.S.C.; Park, J.H. Glutamate in Male and Female Sexual Behavior: Receptors, Transporters, and Steroid Independence. Front. Behav. Neurosci. 2020, 14, 589882. [Google Scholar] [CrossRef] [PubMed]
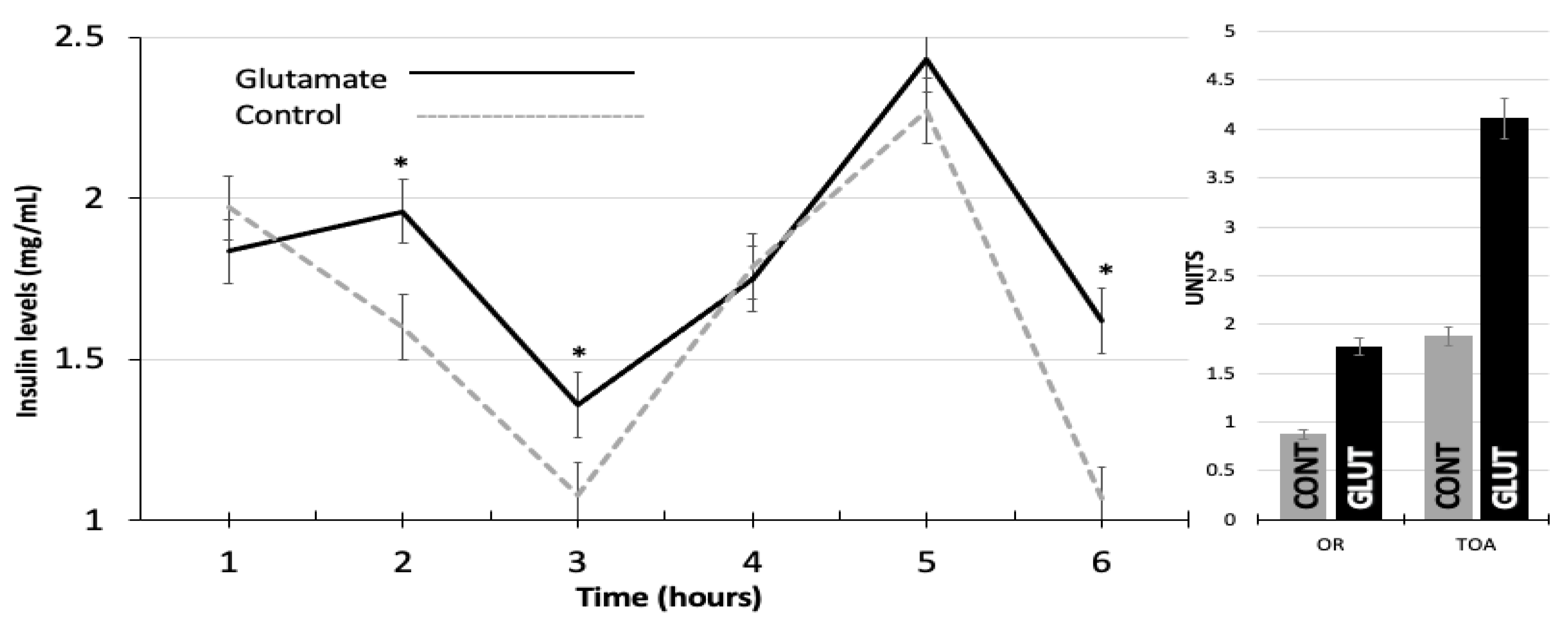
| LW-INI (kg) | BCS-INI (Units) | LW-US (kg) | BCS-US (Units) | OR (Units) | TOA (Units) | |
|---|---|---|---|---|---|---|
| GLUT | 29.60 a | 3.40 a | 35.06 a | 3.50 a | 1.77 a | 4.11 a |
| CONT | 29.20 a | 3.50 a | 32.20 a | 3.20 a | 0.87 b | 1.87 b |
| SE 1 | 1.02 | 1.07 | 0.17 | 0.20 | 0.20 | 0.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luna-Garcia, L.A.; Meza-Herrera, C.A.; Perez-Marin, C.C.; De Santiago-Miramontes, A.; Flores-Salas, J.M.; Corona, R.; Calderon-Leyva, G.; Veliz-Deras, F.G.; Navarrete-Molina, C.; Marin-Tinoco, R.I. Targeted Glutamate Supply Boosts Insulin Concentrations, Ovarian Activity, and Ovulation Rate in Yearling Goats during the Anestrous Season. Biology 2023, 12, 1041. https://doi.org/10.3390/biology12071041
Luna-Garcia LA, Meza-Herrera CA, Perez-Marin CC, De Santiago-Miramontes A, Flores-Salas JM, Corona R, Calderon-Leyva G, Veliz-Deras FG, Navarrete-Molina C, Marin-Tinoco RI. Targeted Glutamate Supply Boosts Insulin Concentrations, Ovarian Activity, and Ovulation Rate in Yearling Goats during the Anestrous Season. Biology. 2023; 12(7):1041. https://doi.org/10.3390/biology12071041
Chicago/Turabian StyleLuna-Garcia, Luis A., Cesar A. Meza-Herrera, Carlos C. Perez-Marin, Angeles De Santiago-Miramontes, Jessica M. Flores-Salas, Rebeca Corona, Guadalupe Calderon-Leyva, Francisco G. Veliz-Deras, Cayetano Navarrete-Molina, and Ruben I. Marin-Tinoco. 2023. "Targeted Glutamate Supply Boosts Insulin Concentrations, Ovarian Activity, and Ovulation Rate in Yearling Goats during the Anestrous Season" Biology 12, no. 7: 1041. https://doi.org/10.3390/biology12071041
APA StyleLuna-Garcia, L. A., Meza-Herrera, C. A., Perez-Marin, C. C., De Santiago-Miramontes, A., Flores-Salas, J. M., Corona, R., Calderon-Leyva, G., Veliz-Deras, F. G., Navarrete-Molina, C., & Marin-Tinoco, R. I. (2023). Targeted Glutamate Supply Boosts Insulin Concentrations, Ovarian Activity, and Ovulation Rate in Yearling Goats during the Anestrous Season. Biology, 12(7), 1041. https://doi.org/10.3390/biology12071041






