Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor
Abstract
Simple Summary
Abstract
1. Introduction
2. General Features of BDNF
2.1. Expression and Isoforms
2.2. Relevance, Limitations and Therapeutic Potential
3. Rosiglitazone: General Features of PPARγ and Treatment of Type 2 Diabetes Mellitus
3.1. Pharmacokinetics
3.2. Adverse Effects
4. Rosiglitazone and Alzheimer’s Disease
4.1. Rosiligtazone’s Treatment of Alzheimer’s Disease-Related Pathology in Pre-Clinical Models
4.1.1. Cognitive Function
4.1.2. Glycogen Synthase Kinase 3 Beta and Tau
4.1.3. Amyloid-Beta
4.2. Rosiglitazone in Alzheimer’s Disease Clinical Trials
5. BDNF and Alzheimer’s Disease
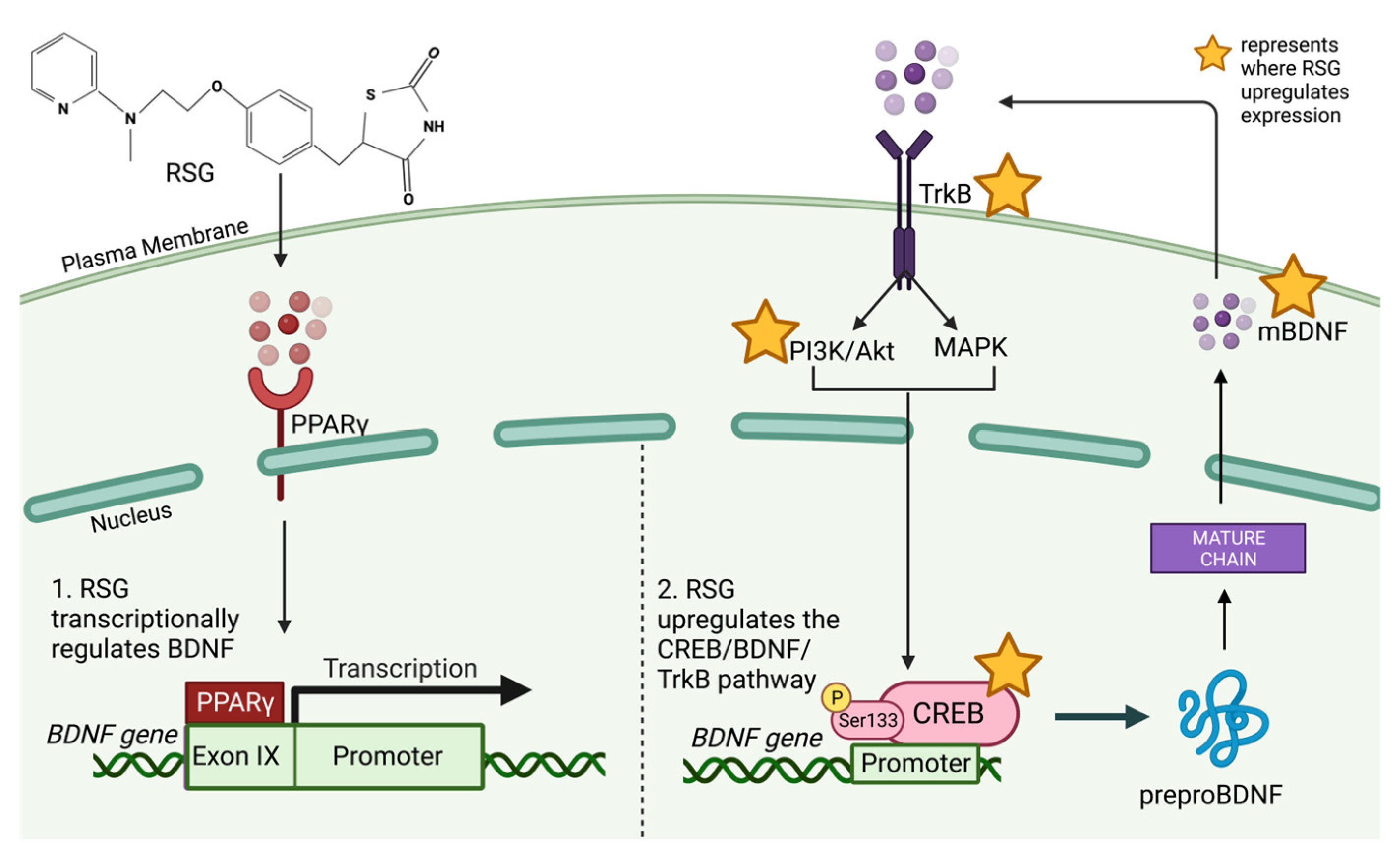
6. Rosiglitazone Modulates CREB, BDNF and TrkB Expression
Rosiglitazone’s Modulation of BDNF for the Treatment of Alzheimer’s Disease
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balfour, J.A.; Plosker, G.L. Rosiglitazone. Drugs 1999, 57, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.; Seely, E.W.; Bekins, S.A.; Williams, G.H.; Simonson, D.C. Rosiglitazone improves insulin sensitivity and lowers blood pressure in hypertensive patients. Diabetes Care 2003, 26, 172–178. [Google Scholar] [CrossRef]
- Lebovitz, H.E.; Dole, J.F.; Patwardhan, R.; Rappaport, E.B.; Freed, M.I. Rosiglitazone Clinical Trials Study Group. Rosiglitazone monotherapy is effective in patients with type 2 diabetes. J. Clin. Endocrinol. Metab. 2001, 86, 280–288. [Google Scholar] [CrossRef]
- Gold, M.; Alderton, C.; Zvartau-Hind, M.; Egginton, S.; Saunders, A.M.; Irizarry, M.; Craft, S.; Landreth, G.; Linnamägi, U.; Sawchak, S. Rosiglitazone monotherapy in mild-to-moderate Alzheimer’s disease: Results from a randomized, double-blind, placebo-controlled phase III study. Demen. Geriatr. Cogn. Disord. 2010, 30, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trials.gov. Brain Imaging Study of Rosiglitazone Efficacy and Safety in Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00265148 (accessed on 8 March 2023).
- de la Monte, S.M.; Wands, J.R. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2008, 2, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Movassat, J.; Delangre, E.; Liu, J.; Gu, Y.; Janel, N. Hypothesis and Theory: Circulating Alzheimer’s-Related Biomarkers in Type 2 Diabetes. Insight From the Goto-Kakizaki Rat. Front. Neurol. 2019, 10, 649. [Google Scholar] [CrossRef]
- Caberlotto, C.; Nguyen, T.P.; Lauria, M.; Priami, C.; Rimondini, R.; Maioli, S.; Cedazo-Minguez, A.; Sita, G.; Morroni, F.; Corsi, M.; et al. Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci. Rep. 2019, 9, 3965. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, K.L.; Van Pelt, R.E.; Schwartz, R.S. Obesity, insulin resistance, and Alzheimer’s disease. Obesity 2012, 20, 1549–1557. [Google Scholar] [CrossRef]
- Chen, Q.; Cao, T.; Li, N.; Zeng, C.; Zhang, S.; Wu, X.; Zhang, B.; Cai, H. Repurposing of Anti-Diabetic Agents as a New Opportunity to Alleviate Cognitive Impairment in Neurodegenerative and Neuropsychiatric Disorders. Front. Pharmacol. 2021, 12, 667874. [Google Scholar] [CrossRef]
- Michailidis, M.; Tata, D.A.; Moraitou, D.; Kavvadas, D.; Karachrysafi, S.; Papamitsou, T.; Vareltzis, P.; Papaliagkas, V. Antidiabetic Drugs in the Treatment of Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 4641. [Google Scholar] [CrossRef]
- Sarathlal, K.C.; Kakoty, V.; Marathe, S.; Chitkara, D.; Taliyan, R. Exploring the Neuroprotective Potential of Rosiglitazone Embedded Nanocarrier System on Streptozotocin Induced Mice Model of Alzheimer’s Disease. Neurotox. Res. 2020, 39, 240–255. [Google Scholar] [CrossRef]
- Pedersen, W.A.; McMillan, P.J.; Kulstad, J.J.; Leverenz, J.B.; Craft, S.; Haynatzki, G.R. Rosiglitazone attenuates learning and memory deficits in Tg2576 Alzheimer mice. Exp Neurol. 2006, 199, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Escribano, L.; Simón, A.M.; Gimeno, E.; Cuadrado-Tejedor, M.; López de Maturana, R.; García-Osta, A.; Ricobaraza, A.; Pérez-Mediavilla, A.; Del Río, J.; Frechilla, D. Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: Mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 2010, 35, 1593–1604. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Li, X.; Blanchard, J.; Li, Y.; Iqbal, K.; Liu, F.; Gong, C.X. Insulin sensitizers improve learning and attenuate tau hyperphosphorylation and neuroinflammation in 3xTg-AD mice. J. Neural Transm. 2015, 122, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Toledo, E.M.; Inestrosa, N.C. Activation of Wnt signaling by lithium and rosiglitazone reduced spatial memory impairment and neurodegeneration in brains of an APPswe/PSEN1DeltaE9 mouse model of Alzheimer’s disease. Mol. Psychiatry 2010, 15, 272–285. [Google Scholar] [CrossRef]
- Cortez, I.; Hernandez, C.M.; Dineley, K.T. Enhancement of select cognitive domains with rosiglitazone implicates dorsal hippocampus circuitry sensitive to PPARγ agonism in an Alzheimer’s mouse model. Brain Behav. 2021, 11, e01973. [Google Scholar] [CrossRef]
- Chiang, M.C.; Nicol, C.J.; Cheng, Y.C.; Lin, K.H.; Yen, C.H.; Lin, C.H. Rosiglitazone activation of PPARγ-dependent pathways is neuroprotective in human neural stem cells against amyloid-beta-induced mitochondrial dysfunction and oxidative stress. Neurobiol. Aging 2016, 40, 181–190. [Google Scholar] [CrossRef]
- Yoon, S.Y.; Park, J.S.; Choi, J.E.; Choi, J.M.; Lee, W.J.; Kim, S.W.; Kim, D.H. Rosiglitazone reduces tau phosphorylation via JNK inhibition in the hippocampus of rats with type 2 diabetes and tau transfected SH-SY5Y cells. Neurobiol. Dis. 2010, 40, 449–455. [Google Scholar] [CrossRef]
- Watson, G.S.; Cholerton, B.A.; Reger, M.A.; Baker, L.D.; Plymate, S.R.; Asthana, S.; Fishel, M.A.; Kulstad, J.J.; Green, P.S.; Cook, D.G.; et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. Am. J. Geriatr. Psychiatry 2005, 13, 950–958. [Google Scholar] [CrossRef]
- Risner, M.E.; Saunders, A.M.; Altman, J.F.; Ormandy, G.C.; Craft, S.; Foley, I.M.; Zvartau-Hind, M.E.; Hosford, D.A.; Roses, A.D. Rosiglitazone in Alzheimer’s Disease Study Group. Efficacy of rosiglitazone in a genetically defined population with mild-to-moderate Alzheimer’s disease. Pharmacogenomics J. 2006, 6, 246–254. [Google Scholar] [CrossRef]
- Tseng, C.H. Rosiglitazone has a neutral effect on the risk of dementia in type 2 diabetes patients. Aging 2019, 11, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, H.E. Thiazolidinediones: The Forgotten Diabetes Medications. Curr. Diabetes Rep. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Galindo, D.C.; Banks, W.A.; Rhea, E.M. The impact of acute rosiglitazone on insulin pharmacokinetics at the blood-brain barrier. Endocrinol. Diabetes Metab. 2020, 3, e00149. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Development and Validation of PEG-PCL Based Nanoformulation of Rosiglitazone and Evaluation of Its Brain Selectivity in Mice Model of Alzheimer’s Disease. Available online: https://alz-journals.onlinelibrary.wiley.com/doi/abs/10.1002/alz.058674 (accessed on 18 March 2023).
- KC, S.; Kakoty, V.; Krishna, K.V.; Dubey, S.K.; Chitkara, D.; Taliyan, R. Neuroprotective efficacy of co-encapsulated rosiglitazone and vorinostat nanoparticle on streptozotocin induced mice model of Alzheimer disease. ACS Chem. Neurosci. 2021, 12, 1528–1541. [Google Scholar]
- Kariharan, T.; Nanayakkara, G.; Parameshwaran, K.; Bagasrawala, I.; Ahuja, M.; Abdel-Rahman, E.; Amin, A.T.; Dhanasekaran, M.; Suppiramaniam, V.; Amin, R.H. Central activation of PPAR-gamma ameliorates diabetes induced cognitive dysfunction and improves BDNF expression. Neurobiol. Aging 2015, 36, 1451–1461. [Google Scholar] [CrossRef]
- Baghcheghi, Y.; Beheshti, F.; Salmani, H.; Hosseini, M. Brainderived neurotrophic factor and nitric oxide contribute to protective effects of rosiglitazone on learning and memory in hypothyroid rats. Acta Neurobiol. Exp. 2021, 81, 218–232. [Google Scholar] [CrossRef]
- Watson, P.A.; Nesterova, A.; Burant, C.F.; Klemm, D.J.; Reusch, J.E. Diabetes-related changes in cAMP response element-binding protein content enhance smooth muscle cell proliferation and migration. J. Biol. Chem. 2001, 276, 46142–46150. [Google Scholar] [CrossRef]
- Kim, H.S.; Hwang, Y.C.; Koo, S.H.; Park, K.S.; Lee, M.S.; Kim, K.W.; Lee, M.K. PPAR-γ activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic β-cells. PLoS ONE 2013, 8, e50128. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef]
- Rozanska, O.; Uruska, A.; Zozulinska-Ziolkiewicz, D. Brain-derived neurotrophic factor and diabetes. Int. J. Mol. Sci. 2020, 21, 841. [Google Scholar] [CrossRef]
- Ng, T.; Ho, C.; Tam, W.; Kua, E.H.; Ho, R.C. Decreased Serum Brain-Derived Neurotrophic Factor [BDNF] Levels in Patients with Alzheimer’s Disease [AD]: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. [Google Scholar] [CrossRef]
- Laske, C.; Stransky, E.; Lehye, T.; Koehler, N.; Schott, K. P3–340: Decrease of BDNF serum concentration from MCI to early Alzheimer’s disease. Alzheimer’s Dement. 2006, 2, S475. [Google Scholar] [CrossRef]
- Ciaramella, A.; Salani, F.; Bizzoni, F.; Orfei, M.D.; Langella, R.; Angelucci, F.; Spalletta, G.; Taddei, A.R.; Caltagirone, C.; Bossù, P. The stimulation of dendritic cells by amyloid beta 1–42 reduces BDNF production in Alzheimer’s disease patients. Brain Behav. Immun. 2013, 32, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Wuu, J.; Mufson, E.J.; Fahnestock, M. Precursor form of brain-derived neurotrophic factor and mature brain-derived neurotrophic factor are decreased in the pre-clinical stages of Alzheimer’s disease. J. Neurochem. 2005, 93, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Pruunsild, P.; Kazantseva, A.; Aid, T.; Palm, K.; Timmusk, T. Dissecting the human BDNF locus: Bidirectional transcription, complex splicing, and multiple promoters. Genomics 2007, 90, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Bayarsaikhan, D.; Lee, J.; Bayarsaikhan, G.; Lee, B. Brain-Derived Neurotrophic Factor Secreting Human Mesenchymal Stem Cells Improve Outcomes in Rett Syndrome Mouse Models. Front. Neurosci. 2021, 15, 725398. [Google Scholar] [CrossRef]
- Yao, W.; Lin, S.; Su, J.; Cao, Q.; Chen, Y.; Chen, J.; Zhang, Z.; Hashimoto, K.; Qi, Q.; Zhang, J.C. Activation of BDNF by transcription factor Nrf2 contributes to antidepressant-like actions in rodents. Transl. Psychiatry 2021, 11, 140. [Google Scholar] [CrossRef]
- Finkbeiner, S.; Tavazoie, S.F.; Maloratsky, A.; Jacobs, K.M.; Harris, K.M.; Greenberg, M.E. CREB: A major mediator of neuronal neurotrophin responses. Neuron 1997, 19, 1031–1047. [Google Scholar] [CrossRef]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef]
- Esvald, E.E.; Tuvikene, J.; Sirp, A.; Patil, S.; Bramham, C.R.; Timmusk, T. CREB Family Transcription Factors Are Major Mediators of BDNF Transcriptional Autoregulation in Cortical Neurons. J. Neurosci. 2020, 40, 1405–1426. [Google Scholar] [CrossRef]
- Tao, X.; Finkbeiner, S.; Arnold, D.B.; Shaywitz, A.J.; Greenberg, M.E. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998, 20, 709–726. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Ratto, G.M.; Putignano, E.; Maffei, L. Brain-derived neurotrophic factor causes cAMP response element-binding protein phosphorylation in absence of calcium increases in slices and cultured neurons from rat visual cortex. J. Neurosci. 2000, 20, 2809–2816. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wu, Z.Y.; Zhu, B.Q.; Wang, Y.X.; Kan, Y.Q.; Zeng, H.C. The BDNF-TrkB-CREB Signalling Pathway Is Involved in Bisphenol S-Induced Neurotoxicity in Male Mice by Regulating Methylation. Toxics 2022, 10, 413. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Liu, J.; Ye, Z.; Huang, J.; He, F.; Xiao, W.; Hu, X.; Luo, Z. CaMKII-Mediated CREB Phosphorylation Is Involved in Ca2+-Induced BDNF mRNA Transcription and Neurite Outgrowth Promoted by Electrical Stimulation. PLoS ONE 2016, 11, e0162784. [Google Scholar] [CrossRef]
- Yang, B.; Yang, C.; Ren, Q.; Zhang, J.C.; Chen, Q.X.; Shirayama, Y.; Hashimoto, K. Regional differences in the expression of brain-derived neurotrophic factor (BDNF) pro-peptide, proBDNF and preproBDNF in the brain confer stress resilience. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 765–769. [Google Scholar] [CrossRef]
- Lu, B.; Pang, P.T.; Woo, N.H. The yin and yang of neurotrophin action. Nat. Rev. Neurosci. 2005, 6, 603–614. [Google Scholar] [CrossRef]
- Carlino, D.; De Vanna, M.; Tongiorgi, E. Is altered BDNF biosynthesis a general feature in patients with cognitive dysfunctions? Neuroscientist 2013, 19, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. Recent Advances on the role of brain-derived neurotrophic factor (BDNF) in neurodegenerative diseases. Int. J. Mole. Sci. 2022, 23, 6827. [Google Scholar] [CrossRef] [PubMed]
- Mowla, S.J.; Farhadi, H.F.; Pareek, S.; Atwal, J.K.; Morris, S.J.; Seidah, N.G.; Murphy, R.A. Biosynthesis and post-translational processing of the precursor to brain-derived neurotrophic factor. J. Biol. Chem. 2001, 276, 12660–12666. [Google Scholar] [CrossRef]
- Lebmann, V.; Brigadski, T. Mechanisms, locations, and kinetics of synaptic BDNF secretion: An update. Neurosci. Res. 2009, 65, 11–22. [Google Scholar] [CrossRef]
- Lu, J.; Yang, M.; Zhou, X. Synthesis, Trafficking and Release of BDNF. In Handbook of Neurotoxicity; Kostrewza, R.M., Ed.; Springer: New York, NY, USA, 2014; Volume 1, pp. 1955–1971. [Google Scholar]
- Zhang, X.Y.; Liu, F.; Chen, Y.; Guo, W.C.; Zhang, Z.H. Proprotein convertase 1/3-mediated down-regulation of brain-derived neurotrophic factor in cortical neurons induced by oxygen-glucose deprivation. Neural Regen. Res. 2020, 15, 1066–1070. [Google Scholar] [CrossRef]
- Lou, H.; Kim, S.K.; Zaitsev, E.; Snell, C.R.; Lu, B.; Loh, Y.P. Sorting and activity-dependent secretion of BDNF require interaction of a specific motif with the sorting receptor carboxypeptidase E. Neuron 2005, 45, 245–255. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Ieraci, A.; Teng, H.; Dall, H.; Meng, C.X.; Herrera, D.G.; Nykjaer, A.; Hempstead, B.L.; Lee, F.S. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. J. Neurosci. 2005, 25, 6156–6166. [Google Scholar] [CrossRef]
- Cunha, C.; Brambilla, R.; Thomas, K.L. A simple role for BDNF in learning and memory? Front. Mol. Neurosci. 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Glombik, M.M.; Gerdes, H.H. Signal-mediated sorting of neuropeptides and prohormones: Secretory granule biogenesis revisited. Biochimie 2000, 82, 315–326. [Google Scholar] [CrossRef]
- Sasi, M.; Vignoli, B.; Canossa, M.; Blum, R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017, 469, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Borodinova, A.A.; Salozhin, S.V. Differences in the Biological Functions of BDNF and proBDNF in the Central Nervous System. Neurosci. Behav. Physiol. 2017, 47, 251–265. [Google Scholar] [CrossRef]
- Yang, J.; Harte-Hargrove, L.C.; Siao, C.J.; Marinic, T.; Clarke, R.; Ma, Q.; Jing, D.; Lafrancois, J.J.; Bath, K.G.; Mark, W.; et al. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014, 7, 796–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Wang, L.; Nie, Y.; Wei, W.; Xiong, W. proBDNF expression induces apoptosis and inhibits synaptic regeneration by regulating the RhoA-JNK pathway in an in vitro post-stroke depression model. Transl. Psychiatry 2021, 11, 578. [Google Scholar] [CrossRef]
- Je, H.S.; Yang, F.; Ji, Y.; Nagappan, G.; Hempstead, B.L.; Lu, B. Role of pro-brain-derived neurotrophic factor (proBDNF) to mature BDNF conversion in activity-dependent competition at developing neuromuscular synapses. Proc. Natl. Acad. Sci. USA 2012, 109, 15924–15929. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Murer, M.G.; Yan, Q.; Raisman-Vozari, R. Brain-derived neurotrophic factor in the control human brain, and in Alzheimer’s disease and Parkinson’s disease. Prog. Neurobiol. 2001, 63, 71–124. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, M.C.; Zimbone, S.; Saab, M.W.; Tomasello, M.F. The Pleiotropic Potential of BDNF beyond Neurons: Implication for a Healthy Mind in a Healthy Body. Life 2021, 11, 1256. [Google Scholar] [CrossRef] [PubMed]
- Amidfar, M.; de Oliveira, J.; Kucharska, E.; Budni, J.; Kim, Y.K. The role of CREB and BDNF in neurobiology and treatment of Alzheimer’s disease. Life Sci. 2020, 257, 118020. [Google Scholar] [CrossRef] [PubMed]
- Nagahara, A.; Tuszynski, M. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef]
- Nagahara, A.H.; Merrill, D.A.; Coppola, G.; Tsukada, S.; Schroeder, B.E.; Shaked, G.M.; Wang, L.; Blesch, A.; Kim, A.; Conner, J.M.; et al. Neuroprotective effects of brain-derived neurotrophic factor in rodent and primate models of Alzheimer’s disease. Nat. Med. 2009, 15, 331–337. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef]
- Clinical Trials.gov. A Clinical Trial of AAV2-BDNF Gene Therapy in Early Alzheimer’s Disease and Mild Cognitive Impairment. Available online: https://clinicaltrials.gov/ct2/show/NCT05040217 (accessed on 16 June 2023).
- Arora, S.; Kanekiyo, T.; Singh, J. Functionalized nanoparticles for brain targeted BDNF gene therapy to rescue Alzheimer’s disease pathology in transgenic mouse model. Int. J. Biol. Macromol. 2022, 208, 901–911. [Google Scholar] [CrossRef]
- Wellington, K. Rosiglitazone/Metformin. Drugs 2005, 65, 1581–1594. [Google Scholar] [CrossRef]
- Malinowski, J.M.; Bolesta, S. Rosiglitazone in the treatment of type 2 diabetes mellitus: A critical review. Clin. Ther. 2000, 22, 1151–1168. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, R.A.; Utreras, E.; Cabezas-Opazo, F.A. Role of PPAR γ in the Differentiation and Function of Neurons. PPAR Res. 2014, 768594. [Google Scholar] [CrossRef]
- Villapol, S. Roles of Peroxisome Proliferator-Activated Receptor Gamma on Brain and Peripheral Inflammation. Cell Mol. Neurobiol. 2018, 38, 121–132. [Google Scholar] [CrossRef]
- Warden, A.; Truitt, J.; Merriman, M.; Ponomareva, O.; Jameson, K.; Ferguson, L.B.; Mayfield, R.D.; Harris, R.A. Localization of PPAR isotypes in the adult mouse and human brain. Sci. Rep. 2016, 6, 27618. [Google Scholar] [CrossRef]
- Festuccia, W.T.; Oztezcan, S.; Laplante, M.; Berthiaume, M.; Michel, C.; Dohgu, S.; Denis, R.G.; Brito, M.N.; Brito, N.A.; Miller, D.S.; et al. Peroxisome proliferator-activated receptor-gamma-mediated positive energy balance in the rat is associated with reduced sympathetic drive to adipose tissues and thyroid status. Endocrinology 2008, 149, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Waksman, J.C. Cardiovascular risk of rosiglitazone: Another perspective. J. Pharm. Pharmacol. 2008, 60, 1573–1582. [Google Scholar] [CrossRef]
- Psaty, B.M.; Furberg, C.D. Rosiglitazone and cardiovascular risk. N. Engl. J. Med. 2007, 356, 2522–2524. [Google Scholar] [CrossRef]
- Nathan, D.M.; Buse, J.B.; Davidson, M.B.; Ferrannini, E.; Holman, R.R.; Sherwin, R.; Zinman, B. Management of hyperglycemia in type 2 diabetes: A consensus algorithm for the initiation and adjustment of therapy: Update regarding thiazolidinediones: A consensus statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2008, 31, 173–175. [Google Scholar] [CrossRef]
- Kaul, S.; Bolger, A.F.; Herrington, D.; Giugliano, R.P.; Eckel, R.H.; American Heart Association; American College of Cardiology Foundation. Thiazolidinedione drugs and cardiovascular risks: A science advisory from the American Heart Association and American College of Cardiology Foundation. J. Am. Coll. Cardiol. 2010, 55, 1885–1894. [Google Scholar] [CrossRef]
- Juurlink, D.N. Rosiglitazone and the case for safety over certainty. JAMA 2010, 304, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Nesto, R.W.; Bell, D.; Bonow, R.O.; Fonseca, V.; Grundy, S.M.; Horton, E.S.; Le Winter, M.; Porte, D.; Semenkovich, C.F.; Smith, S.; et al. Thiazolidinedione use, fluid retention, and congestive heart failure: A consensus statement from the American Heart Association and American Diabetes Association. Circulation 2003, 108, 2941–2948. [Google Scholar] [CrossRef] [PubMed]
- Kahn, S.E.; Zinman, B.; Lachin, J.M.; Haffner, S.M.; Herman, W.H.; Holman, R.R.; Kravitz, B.G.; Yu, D.; Heise, M.A.; Aftring, R.P.; et al. Rosiglitazone-associated fractures in type 2 diabetes: An Analysis from A Diabetes Outcome Progression Trial [ADOPT]. Diabetes Care 2008, 31, 845–851. [Google Scholar] [CrossRef]
- Schwartz, A.V.; Chen, H.; Ambrosius, W.T.; Sood, A.; Josse, R.G.; Bonds, D.E.; Schnall, A.M.; Vittinghoff, E.; Bauer, D.C.; Banerji, M.A.; et al. Effects of TZD Use and Discontinuation on Fracture Rates in ACCORD Bone Study. J. Clin. Endocrinol. Metab. 2015, 100, 4059–4066. [Google Scholar] [CrossRef]
- De Felice, F.G.; Ferreira, S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 2014, 63, 2262–2272. [Google Scholar] [CrossRef]
- Hölscher, C. Insulin Signaling Impairment in the Brain as a Risk Factor in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 88. [Google Scholar] [CrossRef]
- Yarchoan, M.; Arnold, S.E. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes 2014, 63, 2253–2261. [Google Scholar] [CrossRef]
- Xu, S.; Guan, Q.; Wang, C.; Wei, X.; Chen, X.; Zheng, B.; An, P.; Zhang, J.; Chang, L.; Zhou, W.; et al. Rosiglitazone prevents the memory deficits induced by amyloid-beta oligomers via inhibition of inflammatory responses. Neurosci. Lett. 2014, 578, 7–11. [Google Scholar] [CrossRef]
- Ma, L.; Shao, Z.; Wang, R.; Zhao, Z.; Dong, W.; Zhang, J.; Zhang, X.; Sheng, S.; Ji, Z.; Zhang, J. Rosiglitazone improves learning and memory ability in rats with type 2 diabetes through the insulin signaling pathway. Am. J. Med. Sci. 2015, 350, 121–128. [Google Scholar] [CrossRef]
- Wang, L.; Liu, W.; Fan, Y.; Liu, T.; Yu, C. Effect of rosiglitazone on amyloid precursor protein processing and Aβ clearance in streptozotocin-induced rat model of Alzheimer’s disease. Iran. J. Basic Med. Sci. 2017, 20, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, J.; Zhu, L.; Sha, L.; Yang, S.; Wei, J.; Ji, L.; Tang, X.; Mao, K.; Cao, L.; et al. Insulin degrading enzyme contributes to the pathology in a mixed model of Type 2 diabetes and Alzheimer’s disease: Possible mechanisms of IDE in T2D and AD. Biosci. Rep. 2018, 38, BSR20170862. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.A.; Tan, Y.; Seale, J.P.; Qu, X. Targeting BuChE-inflammatory pathway by SK0506 to manage type 2 diabetes and Alzheimer disease. Neurochem. Res. 2009, 34, 2163–2169. [Google Scholar] [CrossRef] [PubMed]
- Jahrling, J.B.; Hernandez, C.M.; Denner, L.; Dineley, K.T. PPARγ recruitment to active ERK during memory consolidation is required for Alzheimer’s disease-related cognitive enhancement. J. Neurosci. 2014, 34, 4054–4063. [Google Scholar] [CrossRef]
- Avila, J.; León-Espinosa, G.; García, E.; García-Escudero, V.; Hernández, F.; DeFelipe, J. Tau Phosphorylation by GSK3 in Different Conditions. Int. J. Alzheimers Dis. 2012, 2012, 578373. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Avila, J. New perspectives on the role of tau in Alzheimer’s disease: Implications for therapy. Biochem. Pharmacol. 2014, 88, 540–547. [Google Scholar] [CrossRef]
- Inestrosa, N.C.; Toledo, E.M. The role of Wnt signaling in neuronal dysfunction in Alzheimer’s Disease. Mol. Neurodegener. 2008, 3, 9. [Google Scholar] [CrossRef]
- Wang, T.; Xie, C.; Yu, P.; Fang, F.; Zhu, J.; Cheng, J.; Gu, A.; Wang, J.; Xiao, H. Involvement of Insulin Signaling Disturbances in Bisphenol A-Induced Alzheimer’s Disease-like Neurotoxicity. Sci. Rep. 2017, 7, 7497. [Google Scholar] [CrossRef]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef]
- Bahrami, F.; Asgari, A.; Hosseinmardi, N.; Janahmadi, M. Peroxisome Proliferator-activated Receptor (PPAR)-γ Modifies Aβ Neurotoxin-induced Electrophysiological Alterations in Rat Primary Cultured Hippocampal Neurons. Iran. J. Pharm. Res. 2019, 18, 1403–1418. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, H.J.; Yang, A.H.; Kim, H.M.; Lee, B.W.; Kang, E.S.; Lee, H.C.; Cha, B.S. The effect of rosiglitazone on LRP1 expression and amyloid β uptake in human brain microvascular endothelial cells: A possible role of a low-dose thiazolidinedione for dementia treatment. Int. J. Neuropsychopharmacol. 2012, 15, 135–142. [Google Scholar] [CrossRef]
- Kurochkin, I.V.; Goto, S. Alzheimer’s beta-amyloid peptide specifically interacts with and is degraded by insulin degrading enzyme. FEBS Lett. 1994, 345, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Vekrellis, K.; Ye, Z.; Qiu, W.Q.; Walsh, D.; Hartley, D.; Chesneau, V.; Selkoe, D.J. Neurons regulate extracellular levels of amyloid beta-protein via proteolysis by insulin-degrading enzyme. J. Neurosci. 2000, 20, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Soccio, R.E.; Chen, E.R.; Lazar, M.A. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metab. 2014, 20, 573–591. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, C.J.; Liu, W.; Cheng, L.Y.; Zhang, Y.N. Rosiglitazone protects neuroblastoma cells against advanced glycation end products-induced injury. Acta Pharmacol. Sin. 2011, 32, 991–998. [Google Scholar] [CrossRef]
- Camacho, I.E.; Serneels, L.; Spittaels, K.; Merchiers, P.; Dominguez, D.; De Strooper, B. Peroxisome-proliferator-activated receptor gamma induces a clearance mechanism for the amyloid-beta peptide. J. Neurosci. 2004, 24, 10908–10917. [Google Scholar] [CrossRef]
- Clinical Trials.gov. Open-Label Extension Assessing Long-Term Safety of Rosiglitazone in Subjects with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00381238 (accessed on 8 April 2023).
- Clinical Trials.gov. Rosiglitazone (Extended Release Tablets) as Monotherapy in Subjects with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT00428090 (accessed on 8 April 2023).
- Clinical Trials.gov. Rosiglitazone (Extended Release Tablets) as Adjunctive Therapy in Subjects with Mild to Moderate Alzheimer’s Disease (REFLECT-3). Available online: https://clinicaltrials.gov/ct2/show/NCT00348140 (accessed on 8 April 2023).
- Clinical Trials.gov. Rosiglitazone (Extended Release Tablets) as Adjunctive Therapy for Subjects with Mild to Moderate Alzheimer’s Disease (REFLECT-2). Available online: https://clinicaltrials.gov/ct2/show/NCT00348309 (accessed on 8 April 2023).
- Clinical Trials.gov. Effects of Colesevelam HCl, Rosiglitazone, Sitagliptin on Control of Blood Glucose and Lipids in Type 2 Diabetes Patients Whose Blood Glucose Isn’t Completely Controlled with Metformin. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00484419?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=2&rank=1 (accessed on 18 June 2023).
- Clinical Trials.gov. Rosiglitazone-Metformin Combination versus Metformin-Sulfonylurea Combination on Beta-Cell Function in Type 2 Diabetes. Available online: https://clinicaltrials.gov/ct2/show/study/NCT00367055?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=2&rank=2 (accessed on 18 June 2023).
- Clinical Trials.gov. Safety/Efficacy of Sitagliptin in Patient w/Type 2 Diabetes (0431-801). Available online: https://clinicaltrials.gov/ct2/show/NCT00541775?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=2&rank=4 (accessed on 18 June 2023).
- Clinical Trials.gov. Avandia™ + Amaryl™ or Avandamet™ Compared with Metformin (AVALANCHE™ Study) (AVALANCHE). Available online: https://clinicaltrials.gov/ct2/show/NCT00131664?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=2&rank=5 (accessed on 18 June 2023).
- Clinical Trials.gov. A Study of BRL49653C for the Treatment of Type 2 Diabetes (Combination Therapy with Sulfonyl Urea)—With Placebo Study. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00432679?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=2&rank=8 (accessed on 18 June 2023).
- Clinical Trials.gov. An Evaluation of Exenatide and Rosiglitazone in Subjects with Type 2 Diabetes Mellitus. Available online: https://clinicaltrials.gov/ct2/show/NCT00135330?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=2&rank=10 (accessed on 18 June 2023).
- Clinical Trials.gov. Study in Postmenopausal Women with Type 2 Diabetes Looking at Approved Diabetes Drugs and How They Affect Bone Health. Available online: https://clinicaltrials.gov/ct2/show/NCT00679939?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=3 (accessed on 18 June 2023).
- Clinical Trials.gov. Glycemic Control and Complications in Diabetes Mellitus Type 2 (VADT) (VADT). Available online: https://clinicaltrials.gov/ct2/show/NCT00032487?term=Rosiglitazone&recrs=e&rslt=With&cond=Type+2+diabetes&draw=3&rank=13 (accessed on 18 June 2023).
- Yiannopoulou, K.G.; Anastasiou, A.I.; Zachariou, V.; Pelidou, S.H. Reasons for Failed Trials of Disease-Modifying Treatments for Alzheimer Disease and Their Contribution in Recent Research. Biomedicines 2019, 7, 97. [Google Scholar] [CrossRef]
- Pinheiro, R.G.R.; Coutinho, A.J.; Pinheiro, M.; Neves, A.R. Nanoparticles for targeted brain drug delivery: What do we know? Int. J. Mol. Sci. 2021, 22, 11654. [Google Scholar] [CrossRef]
- Scharfman, H.E.; MacLusky, N.J. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: Complexity of steroid hormone-growth factor interactions in the adult CNS. Front. Neuroendocr. 2006, 27, 415–435. [Google Scholar] [CrossRef]
- Azevedo, K.P.M.; de Oliveira, V.H.; Medeiros, G.C.B.S.; Mata, Á.N.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef]
- Grønli, J.; Bramham, C.; Murison, R.; Kanhema, T.; Fiske, E.; Bjorvatn, B.; Ursin, R.; Portas, C.M. Chronic mild stress inhibits BDNF protein expression and CREB activation in the dentate gyrus but not in the hippocampus proper. Pharmacol. Biochem. Behav. 2006, 85, 842–849. [Google Scholar] [CrossRef]
- Dou, S.H.; Cui, Y.; Huang, S.M.; Zhang, B. The role of brain-derived neurotrophic factor signaling in Central Nervous System Disease Pathogenesis. Front. Hum. Neurosci. 2022, 16, 924155. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.S.; Coughlan, C.; Heyn, P.C.; Tagawa, A.; Carollo, J.J.; Kua, E.H.; Mahendran, R. Increased plasma brain-derived neurotrophic factor (BDNF) as a potential biomarker for and compensatory mechanism in mild cognitive impairment: A case-control study. Aging 2021, 13, 22666–22689. [Google Scholar] [CrossRef] [PubMed]
- Faria, M.C.; Gonçalves, G.S.; Rocha, N.P.; Moraes, E.N.; Bicalho, M.A.; Gualberto Cintra, M.T.; Jardim de Paula, J.; José Ravic de Miranda, L.F.; Clayton de Souza Ferreira, A.; Teixeira, A.L.; et al. Increased plasma levels of BDNF and inflammatory markers in Alzheimer’s disease. J. Psychiatr. Res. 2014, 53, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Durany, N.; Michel, T.; Kurt, J.; Cruz-Sánchez, F.F.; Cervás-Navarro, J.; Riederer, P. Brain-derived neurotrophic factor and neurotrophin-3 levels in Alzheimer’s disease brains. Int. J. Dev. Neurosci. 2000, 18, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Silhol, M.; Moulière, F.; Meffre, J.; Höllinger, I.; Maurice, T.; Tapia-Arancibia, L. Protective effect of BDNF against beta-amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiol. Dis. 2008, 31, 316–326. [Google Scholar] [CrossRef]
- Ferrer, I.; Marín, C.; Rey, M.J.; Ribalta, T.; Goutan, E.; Blanco, R.; Tolosa, E.; Martí, E. BDNF and full-length and truncated TrkB expression in Alzheimer disease. Implications in therapeutic strategies. J. Neuropathol. Exp. Neurol. 1999, 58, 729–739. [Google Scholar] [CrossRef]
- Iulita, M.F.; Bistué Millón, M.B.; Pentz, R.; Aguilar, L.F.; Do Carmo, S.; Allard, S.; Michalski, B.; Wilson, E.N.; Ducatenzeiler, A.; Bruno, M.A.; et al. Differential deregulation of NGF and BDNF neurotrophins in a transgenic rat model of Alzheimer’s disease. Neurobiol. Dis. 2017, 108, 307–323. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H.; Suzuki, T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014, 6, 69. [Google Scholar] [CrossRef]
- Pláteník, J.; Fišar, Z.; Buchal, R.; Jirák, R.; Kitzlerová, E.; Zvěřová, M.; Raboch, J. GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 50, 83–93. [Google Scholar] [CrossRef]
- Curto, M.; Martocchia, A.; Ferracuti, S.; Comite, F.; Scaccianoce, S.; Girardi, P.; Nicoletti, F.; Falaschi, P. Increased Total Urinary Cortisol (tUC) and Serum Brain-derived Neurotrophic Factor (BDNF) Ratio in Alzheimer Disease (AD)-affected Patients. Alzheimer Dis. Assoc. Disord. 2017, 31, 173–176. [Google Scholar] [CrossRef]
- Rosa, E.; Mahendram, S.; Ke, Y.D.; Ittner, L.M.; Ginsberg, S.D.; Fahnestock, M. Tau downregulates BDNF expression in animal and cellular models of Alzheimer’s disease. Neurobiol. Aging. 2016, 48, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Bharani, K.L.; Ledreux, A.; Gilmore, A.; Carroll, S.L.; Granholm, A.C. Serum pro-BDNF levels correlate with phospho-tau staining in Alzheimer’s disease. Neurobiol. Aging. 2020, 87, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.C.; Yu, J.; Liu, Y.L.; Hong, Z.Z.; Ling, L.; Li, G.Q.; Zhuo, Y.F.; Wang, W.R.; Zhang, Y. Reduced Serum Levels of Brain-Derived Neurotrophic Factor Are Related to Mild Cognitive Impairment in Chinese Patients with Type 2 Diabetes Mellitus. Ann. Nutr. Metab. 2018, 73, 271–281. [Google Scholar] [CrossRef]
- Murillo Ortíz, B.; Ramírez Emiliano, J.; Ramos-Rodríguez, E.; Martínez-Garza, S.; Macías-Cervantes, H.; Solorio-Meza, S.; Pereyra-Nobara, T.A. Brain-derived neurotrophic factor plasma levels and premature cognitive impairment/dementia in type 2 diabetes. World J. Diabetes 2016, 7, 615–620. [Google Scholar] [CrossRef]
- Passaro, A.; Dalla Nora, E.; Morieri, M.L.; Soavi, C.; Sanz, J.M.; Zurlo, A.; Fellin, R.; Zuliani, G. Brain-derived neurotrophic factor plasma levels: Relationship with dementia and diabetes in the elderly population. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 294–302. [Google Scholar] [CrossRef][Green Version]
- Zhen, Y.F.; Zhang, J.; Liu, X.Y.; Fang, H.; Tian, L.B.; Zhou, D.H.; Kosten, T.R.; Zhang, X.Y. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology 2013, 227, 93–100. [Google Scholar] [CrossRef]
- Naegelin, Y.; Dingsdale, H.; Säuberli, K.; Schädelin, S.; Kappos, L.; Barde, Y.A. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro 2018, 5, ENEURO.0419-17. [Google Scholar] [CrossRef]
- Zuccato, C.; Marullo, M.; Vitali, B.; Tarditi, A.; Mariotti, C.; Valenza, M.; Lahiri, N.; Wild, E.J.; Sassone, J.; Ciammola, A.; et al. Brain-derived neurotrophic factor in patients with Huntington’s disease. PLoS ONE 2011, 6, e22966. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, T.; Jiao, S.; Zhou, X.; Zhong, J.; Wang, Y.; Liu, J.; Deng, J.; Wang, S.; Xu, Z. ProBDNF Accelerates Brain Amyloid-β Deposition and Learning and Memory Impairment in APPswePS1dE9 Transgenic Mice. J. Alzheimer’s Dis. 2017, 59, 941–949. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669. [Google Scholar] [CrossRef]
- Fleitas, C.; Piñol-Ripoll, G.; Marfull, P.; Rocandio, D.; Ferrer, I.; Rampon, C.; Egea, J.; Espinet, C. proBDNF is modified by advanced glycation end products in Alzheimer’s disease and causes neuronal apoptosis by inducing p75 neurotrophin receptor processing. Mol. Brain 2018, 11, 68. [Google Scholar] [CrossRef]
- Sun, W.; Cheng, H.; Yang, Y.; Tang, D.; Li, X.; An, L. Requirements of Postnatal proBDNF in the Hippocampus for Spatial Memory Consolidation and Neural Function. Front. Cell Dev. Biol. 2021, 9, 678182. [Google Scholar] [CrossRef]
- Buhusi, M.; Etheredge, C.; Granholm, A.C.; Buhusi, C.V. Increased Hippocampal ProBDNF Contributes to Memory Impairments in Aged Mice. Front. Aging Neurosci. 2017, 9, 284. [Google Scholar] [CrossRef]
- Pisani, A.; Paciello, F.; Del Vecchio, V.; Malesci, R.; De Corso, E.; Cantone, E.; Fetoni, A.R. The Role of BDNF as a Biomarker in Cognitive and Sensory Neurodegeneration. J. Pers. Med. 2023, 13, 652. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Wang, M.; Pham, S.; Sze, C.; Eckman, C.B. Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol. Neurodegener. 2011, 6, 60. [Google Scholar] [CrossRef]
- Rosa, E.; Fahnestock, M. CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol. Aging 2015, 36, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Bartolotti, N.; Bennett, D.; Lazarov, O. Reduced pCREB in Alzheimer’s disease prefrontal cortex is reflected in peripheral blood mononuclear cells. Mol. Psychiatry 2016, 21, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Tessarollo, L.; Yanpallewar, S. TrkB Truncated Isoform Receptors as Transducers and Determinants of BDNF Functions. Front. Neurosci. 2022, 16, 847572. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, L.; Guo, X.D.; Cao, L.L.; Xue, T.F.; Zhao, X.J.; Yang, D.D.; Yang, J.; Ji, J.; Huang, J.Y.; et al. Rosiglitazone Exerts an Anti-depressive Effect in Unpredictable Chronic Mild-Stress-Induced Depressive Mice by Maintaining Essential Neuron Autophagy and Inhibiting Excessive Astrocytic Apoptosis. Front. Mol. Neurosci. 2017, 10, 293. [Google Scholar] [CrossRef]
- Patel, S.S.; Ray, R.S.; Sharma, A.; Mehta, V.; Katyal, A.; Udayabanu, M. Antidepressant and anxiolytic like effects of Urtica dioica leaves in streptozotocin induced diabetic mice. Metab. Brain Dis. 2018, 33, 1281–1292. [Google Scholar] [CrossRef]
- Martínez-Levy, G.A.; Cruz-Fuentes, C.S. Genetic and epigenetic regulation of the brain-derived neurotrophic factor in the central nervous system. Yale J. Biol. Med. 2014, 87, 173–186. [Google Scholar] [PubMed]
- Carvalho, A.L.; Caldeira, M.V.; Santos, S.D.; Duarte, C.B. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Br. J. Pharmacol. 2008, 153, S310–S324. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Young, K.C.; Bai, C.H.; Yu, B.C.; Ma, C.T.; Chien, Y.C.; Chiang, C.L.; Liao, C.S.; Lai, H.W.; Tsao, C.W. Rosiglitazone regulates anti-inflammation and growth inhibition via PTEN. BioMed Res. Int. 2014, 2014, 787924. [Google Scholar] [CrossRef]
- Mohanty, P.; Aljada, A.; Ghanim, H.; Hofmeyer, D.; Tripathy, D.; Syed, T.; Al-Haddad, W.; Dhindsa, S.; Dandona, P. Evidence for a potent antiinflammatory effect of rosiglitazone. J. Clin. Endocrinol. Metab. 2004, 89, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shen, X. Effect of rosiglitazone on inflammatory cytokines and oxidative stress after intensive insulin therapy in patients with newly diagnosed type 2 diabetes. Diabetol. Metab. Syndr. 2019, 11, 35. [Google Scholar] [CrossRef] [PubMed]
- Steinke, I.; Govindarajulu, M.; Pinky, P.D.; Bloemer, J.; Yoo, S.; Ward, T.; Schaedig, T.; Young, T.; Wibowo, F.S.; Suppiramaniam, V.; et al. Selective PPAR-Delta/PPAR-Gamma Activation Improves Cognition in a Model of Alzheimer’s Disease. Cells 2023, 12, 1116. [Google Scholar] [CrossRef]
- Kapadia, R.; Yi, J.H.; Vemuganti, R. Mechanisms of anti-inflammatory and neuroprotective actions of PPAR-gamma agonists. Front. Biosci. 2008, 13, 1813–1826. [Google Scholar] [CrossRef]
- Giacalone, G.; Tsapis, N.; Mousnier, L.; Chacun, H.; Fattal, E. PLA-PEG Nanoparticles Improve the Anti-Inflammatory Effect of Rosiglitazone on Macrophages by Enhancing Drug Uptake Compared to Free Rosiglitazone. Materials 2018, 11, 1845. [Google Scholar] [CrossRef]
- Kim, C.K.; Lee, Y.R.; Ong, L.; Gold, M.; Kalali, A.; Sarkar, J. Alzheimer’s Disease: Key Insights from Two Decades of Clinical Trial Failures. J. Alzheimer’s Dis. 2022, 87, 83–100. [Google Scholar] [CrossRef]

| Participants | Length of RSG Treatment | Primary Outcome Measures | Main Results | In-Text Reference |
|---|---|---|---|---|
| 30 participants with AD or amnestic mild cognitive impairment | 4 mg of RSG or placebo daily for 6 months | To assess cognitive performance and plasma Aβ levels | Participants that received RSG exhibited better delayed recall and selective attentive relative to participants that received placebo. Plasma Aβ levels were unchanged compared to baseline in participants that received RSG | [20] |
| 33 participants with mild to moderate AD | 4 mg of RSG XR orally, once daily for 4 weeks followed by 8 mg of RSG orally, once daily for 44 weeks | To assess the number of participants with adverse events | 2 of 33 participants experienced serious adverse events while 10/33 participants experienced non-serious adverse events | [110] |
| 80 participants with mild to moderate AD | 4 mg of RSG XR once a day for 1 month increasing to 8 mg once a day or placebo for 12 months | Change from baseline in global and regional indices of the cerebral metabolic rate of glucose | Suggests that RSG is associated with an early increase in whole brain glucose metabolism but not any biological or clinical evidence for slowing the progression of AD | [5] |
| 693 participants with mild to moderate AD | Once daily of placebo, 2 mg RSG XR, 8 mg RSG XR or 10 mg donepezil (control) for 24 weeks | To assess the change from baseline to week 24 in the ADAS-Cog score and CIBIC+ global functioning score | No evidence of 2 mg or 8 mg RSG XR monotherapy in cognition or global function | [111] |
| 1496 participants with mild to moderate AD | Once daily of placebo + donepezil, 2 mg RSG XR + donepezil or 8 mg RSG XR + donepezil for 54 weeks | To assess the change from baseline to week 48 in ADAS-Cog and CDR-SB with the use of RSG XR as adjunctive therapy with donepezil treatment in AD | No evidence of statistically or clinically significant efficacy in cognition or global function was detected for 2 mg or 8 mg RSG XR as adjunctive therapy to ongoing AChEIs | [112] |
| 1468 participants with mild to moderate AD | Once daily of placebo, 2 mg RSG XR or 8 mg RSG XR for 54 weeks | To assess the change from baseline to week 48 in ADAS-Cog and CDR-SB with the use of RSG XR as adjunctive therapy with AChEI treatment in AD | No evidence of statistically or clinically significant efficacy in cognition or global function was detected for 2 mg or 8 mg RSG XR as adjunctive therapy to ongoing AChEIs | [113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelson, M.L.; Pfeifer, J.A.; Hickey, J.P.; Collins, A.E.; Kalisch, B.E. Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology 2023, 12, 1042. https://doi.org/10.3390/biology12071042
Nelson ML, Pfeifer JA, Hickey JP, Collins AE, Kalisch BE. Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology. 2023; 12(7):1042. https://doi.org/10.3390/biology12071042
Chicago/Turabian StyleNelson, Mackayla L., Julia A. Pfeifer, Jordan P. Hickey, Andrila E. Collins, and Bettina E. Kalisch. 2023. "Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor" Biology 12, no. 7: 1042. https://doi.org/10.3390/biology12071042
APA StyleNelson, M. L., Pfeifer, J. A., Hickey, J. P., Collins, A. E., & Kalisch, B. E. (2023). Exploring Rosiglitazone’s Potential to Treat Alzheimer’s Disease through the Modulation of Brain-Derived Neurotrophic Factor. Biology, 12(7), 1042. https://doi.org/10.3390/biology12071042








