Biological Fitness Cost, Demographic Growth Characteristics, and Resistance Mechanism in Alpha-Cypermethrin-Resistant Musca domestica (Diptera: Muscidae)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains of M. domestica
2.2. Chemicals
2.3. Concentration–Response Bioassay
2.4. Synergism Experiment
2.5. Assessment of Life Tables of Alpha-RS and Alpha-SS M. domestica
2.6. Data Analysis
3. Results
3.1. Toxicity and Resistance of Alpha-RS M. domestica to Alpha-Cypermethrin
3.2. Effect of Synergists on Alpha-Cypermethrin Toxicity in Alpha-RS and Alpha-SS M. domestica
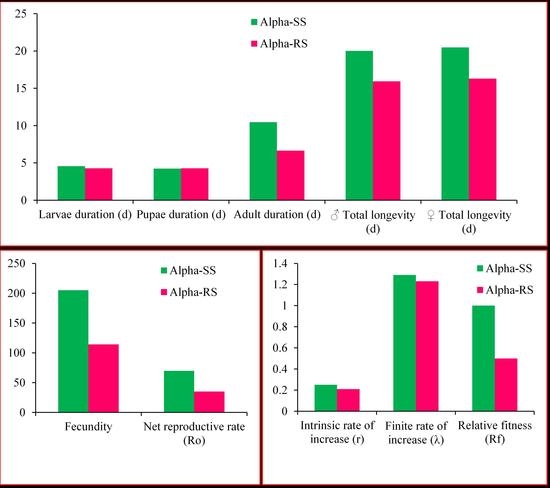
3.3. Biological and Demographic Parameters of Alpha-RS and Alpha-SS M. domestica
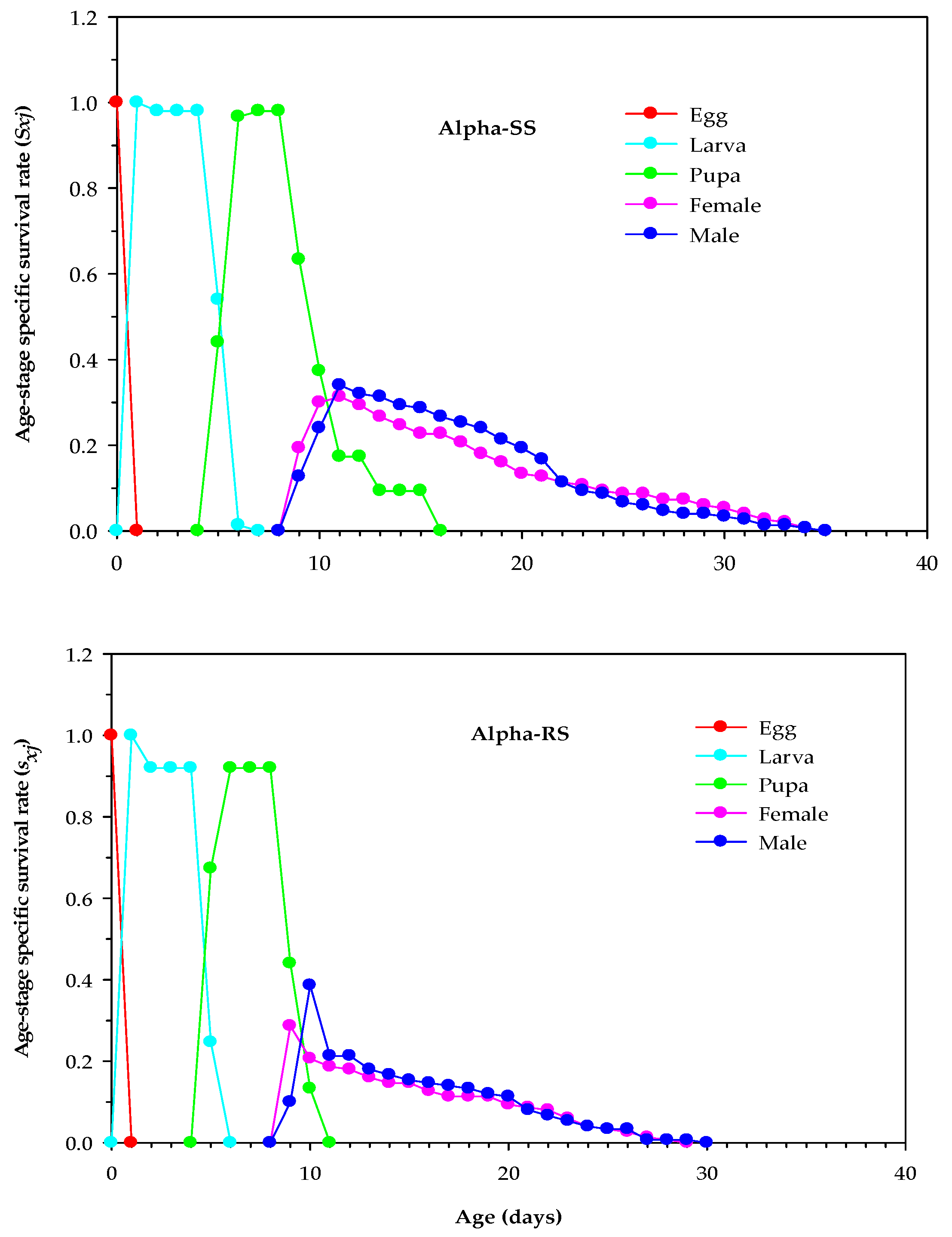
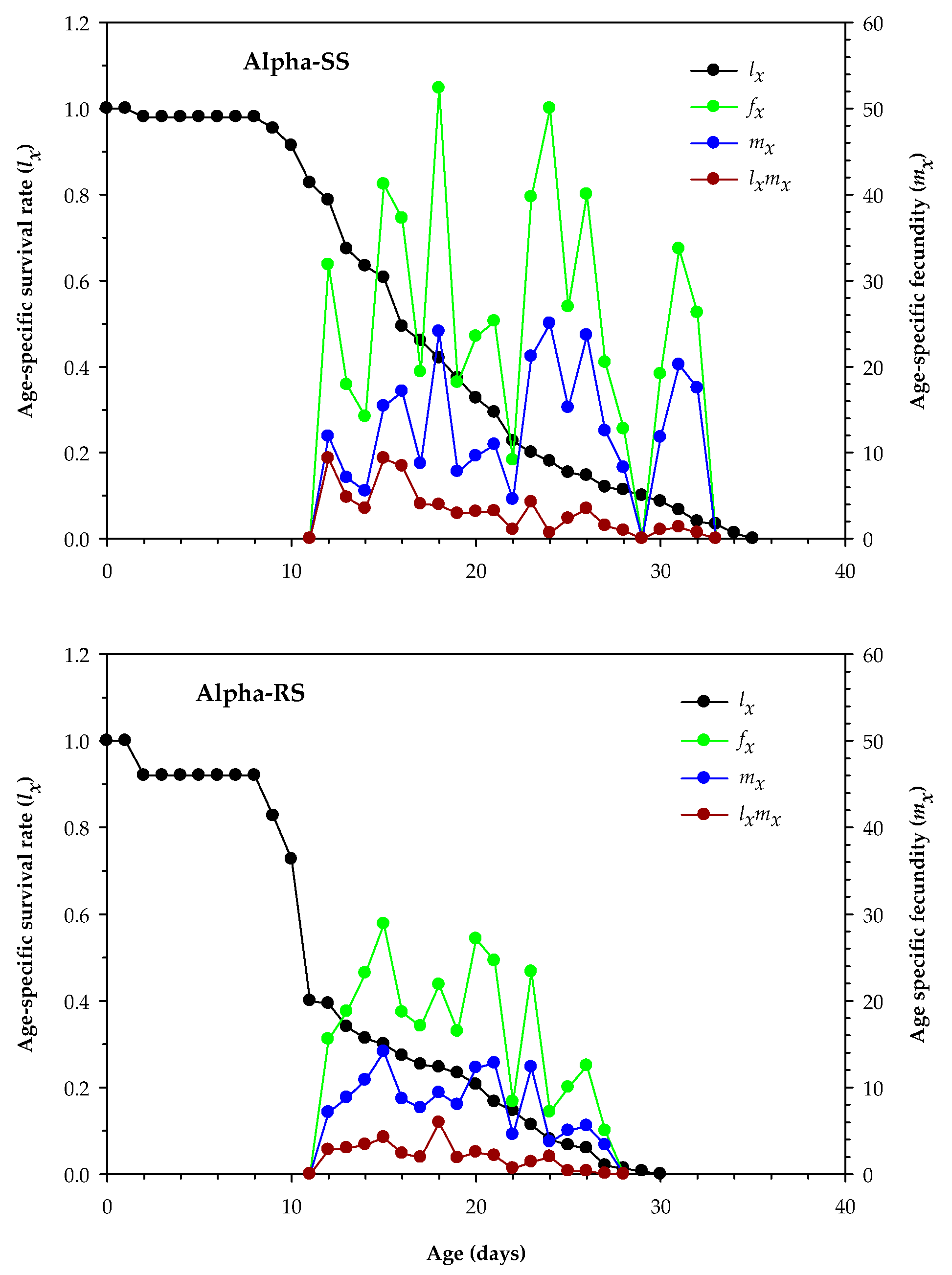
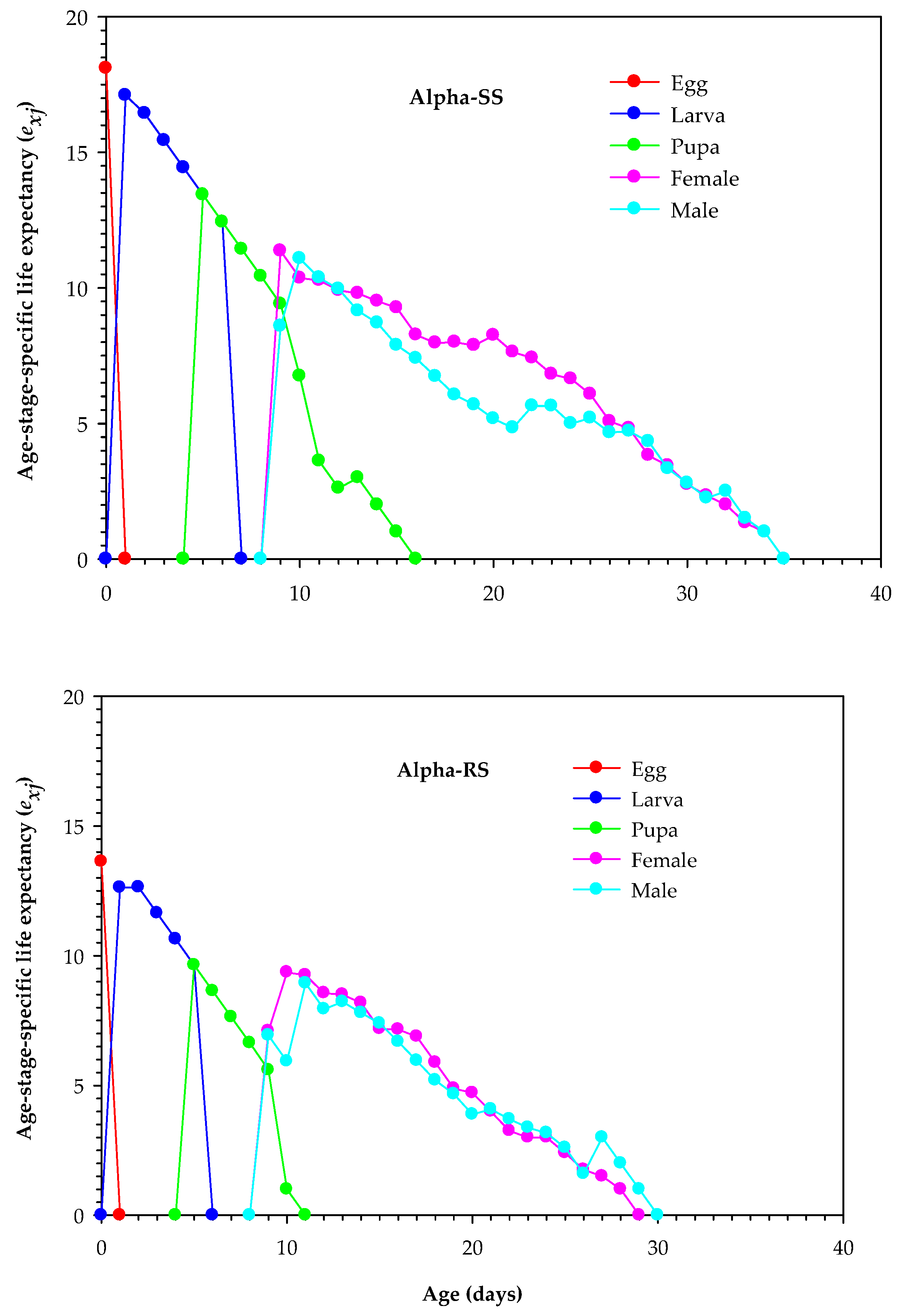
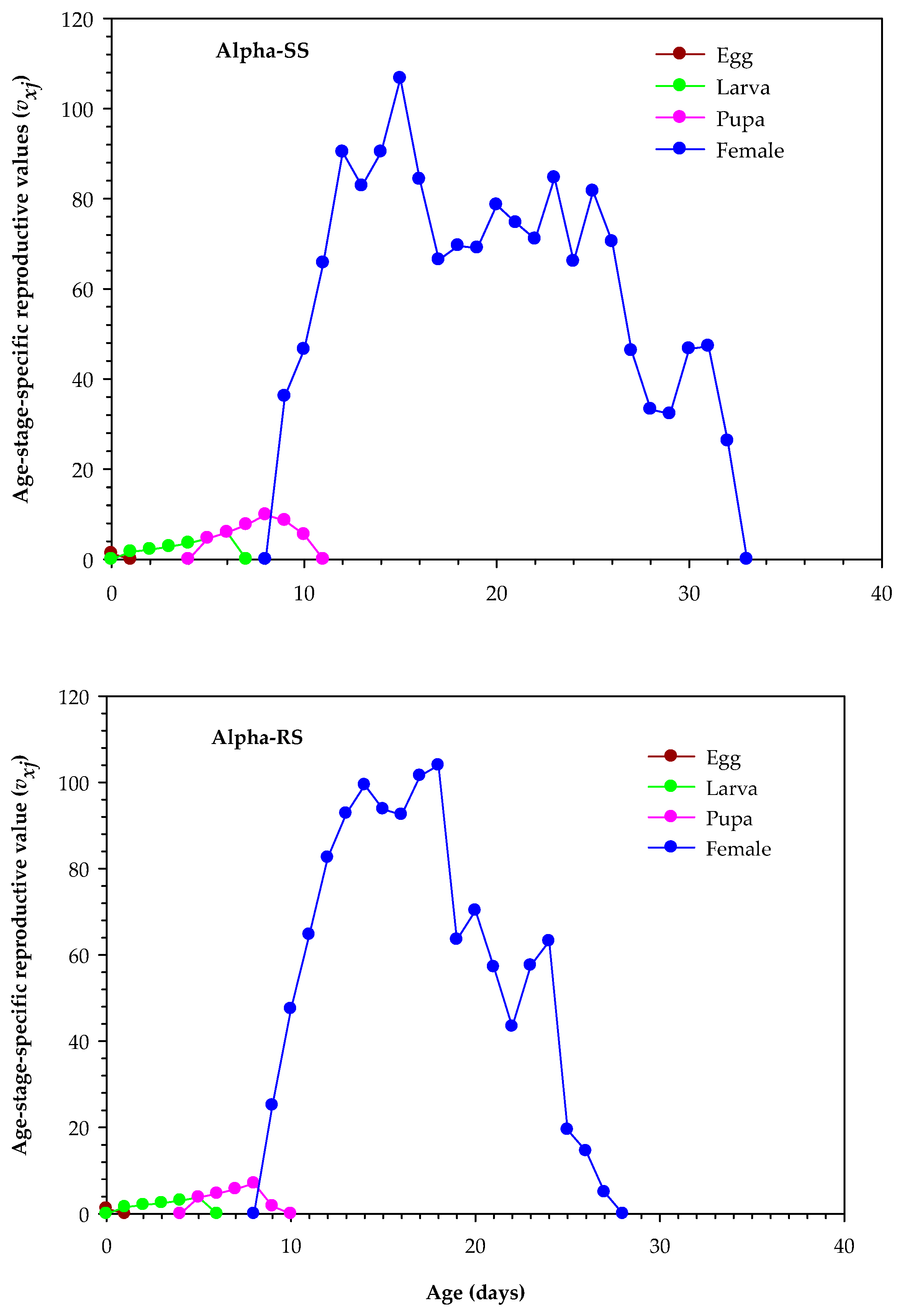
3.4. Age-Stage Survival and Reproduction Parameters of Alpha-RS and Alpha-SS M. domestica
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, N.; Hafez, A.M. Resistance to insect growth regulators and age-stage, two-sex life table in Musca domestica from different dairy facilities. PLoS ONE 2021, 16, e0248693. [Google Scholar] [CrossRef] [PubMed]
- Nayduch, D.; Neupane, S.; Pickens, V.; Purvis, T.; Olds, C. House flies are underappreciated yet important reservoirs and vectors of microbial threats to animal and human health. Microorganisms 2023, 11, 583. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.A.; Skovgard, H.; Stockmarr, A.; Handberg, K.J.; Jorgensen, P.H. Persistence of low-pathogenic avian influenza H5N7 and H7N1 subtypes in house flies (Diptera: Muscidae), J. Med. Entomol. 2011, 48, 608–614. [Google Scholar] [CrossRef]
- Butler, J.F.; Garcia-Maruniak, A.; Meek, F.; Maruniak, J.E. Wild Florida house flies (Musca domestica) as carriers of pathogenic bacteria. Fla. Entomol. 2010, 93, 218–223. [Google Scholar] [CrossRef]
- Nayduch, D.; Burrus, R.G. Flourishing in filth: House fly–microbe interactions across life history. Ann. Entomol. Soc. Am. 2017, 110, 6–18. [Google Scholar] [CrossRef]
- Geden, C.J.; Nayduch, D.; Scott, J.G.; Burgess IV, E.R.; Gerry, A.; Kaufman, P.E.; Thomson, J.; Pickens, V.; Machtinger, E.T. House fly (Diptera: Muscidae): Biology, pest status, current management prospects, and research needs. J. Integr. Pest Manag. 2021, 12, 39. [Google Scholar] [CrossRef]
- Jacques, B.J.; Bourret, T.J.; Shaffer, J. Role of fly cleaning behavior on carriage of Escherichia coli and Pseudomonas aeruginosa. J. Med. Entomol. 2017, 54, 1712–1717. [Google Scholar] [CrossRef]
- Ngufor, C.; Agbevo, A.; Fagbohoun, J.; Fongnikin, A.; Rowland, M. Efficacy of Royal Guard, a new alpha-cypermethrin and pyriproxyfen treated mosquito net, against pyrethroid-resistant malaria vectors. Sci. Rep. 2020, 10, 12227. [Google Scholar] [CrossRef]
- Pessoa, G.C.D.; Lopes, J.V.; Rocha, M.F.; Pinheiro, L.C.; Rosa, A.C.L.; Michalsky, É.M.; Dias, E.S. Baseline susceptibility to alpha-cypermethrin in Lutzomyia longipalpis (Lutz & Neiva, 1912) from Lapinha Cave (Brazil). Parasit. Vectors 2015, 8, 469. [Google Scholar]
- Hafez, A.M. First evaluation of field evolved resistance to commonly used insecticides in house fly populations from Saudi Arabian dairy farms. Insects 2021, 12, 1120. [Google Scholar] [CrossRef]
- Kampouraki, A.; Stavrakaki, M.; Karataraki, A.; Katsikogiannis, G.; Pitika, E.; Varikou, K.; Vlachaki, A.; Chrysargyris, A.; Malandraki, E.; Sidiropoulos, N. Recent evolution and operational impact of insecticide resistance in olive fruit fly Bactrocera oleae populations from Greece. J. Pest Sci. 2018, 91, 1429–1439. [Google Scholar] [CrossRef]
- Mironidis, G.K.; Kapantaidaki, D.; Bentila, M.; Morou, E.; Savopoulou-Soultani, M.; Vontas, J. Resurgence of the cotton bollworm Helicoverpa armigera in northern Greece associated with insecticide resistance. Insect Sci. 2013, 20, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Lorn, S.; Klakankhai, W.; Nusen, P.; Sumarnrote, A.; Tainchum, K. Pyrethroid susceptibility in Stomoxys calcitrans and Stomoxys indicus (Diptera: Muscidae) collected from cattle farms in Southern Thailand. Insects 2022, 13, 711. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Hafez, A.M. Alpha-cypermethrin resistance in Musca domestica: Resistance instability, realized heritability, risk assessment, and insecticide cross-resistance. Insects 2023, 14, 233. [Google Scholar] [CrossRef]
- Rodríguez-Hidalgo, R.; Pérez-Otáñez, X.; Garcés-Carrera, S.; Vanwambeke, S.O.; Madder, M.; Benítez-Ortiz, W. The current status of resistance to alpha-cypermethrin, ivermectin, and amitraz of the cattle tick (Rhipicephalus microplus) in Ecuador. PLoS ONE 2017, 12, e0174652. [Google Scholar] [CrossRef]
- Scott, J.G. Cytochrome P450 Monooxygenases and Insecticide Resistance: Lessons from CYP6D1 in Biochemical Sites of Insecticide Action and Resistance; Ishaaya, I., Ed.; Springer: New York, NY, USA, 2001; pp. 255–267. [Google Scholar]
- Abbas, N.; Khan, H.A.A.; Shad, S.A. Resistance of the house fly Musca domestica (Diptera: Muscidae) to lambda-cyhalothrin: Mode of inheritance, realized heritability, and cross-resistance to other insecticides. Ecotoxicology 2014, 23, 791–801. [Google Scholar] [CrossRef]
- Tian, L.; Cao, C.; He, L.; Li, M.; Zhang, L.; Zhang, L.; Liu, H.; Liu, N. Autosomal interactions and mechanisms of pyrethroid resistance in house flies, Musca domestica. Int. J. Biol. Sci. 2011, 7, 902. [Google Scholar] [CrossRef]
- Roca-Acevedo, G.; Boscaro, I.; Toloza, A.C. Global pattern of kdr-type alleles in Musca domestica (L.). Curr. Trop. Med. Rep. 2022, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soderlund, D.M.; Knipple, D.C. The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem. Mol. Biol. 2003, 33, 563–577. [Google Scholar] [CrossRef]
- You, C.; Zhang, L.; Song, J.; Zhang, L.; Zhen, C.; Gao, X. The variation of a cytochrome P450 gene, CYP6G4, drives the evolution of Musca domestica L. (Diptera: Muscidae) resistance to insecticides in China. Int. J. Biol. Macromol 2023, 236, 123399. [Google Scholar] [CrossRef]
- Riaz, B.; Kashif Zahoor, M.; Malik, K.; Ahmad, A.; Majeed, H.N.; Jabeen, F.; Zulhussnain, M.; Ranian, K. Frequency of Pyrethroid Insecticide Resistance kdr Gene and Its Associated Enzyme Modulation in Housefly, Musca domestica L. Populations from Jhang, Pakistan. Front. Environ. Sci. 2022, 9, 806456. [Google Scholar] [CrossRef]
- Li, M.; Feng, X.; Reid, W.R.; Tang, F.; Liu, N. Multiple-P450 gene co-up-regulation in the development of permethrin resistance in the house fly, Musca domestica. Int. J. Mol. Sci. 2023, 24, 3170. [Google Scholar] [CrossRef] [PubMed]
- Bass, C.; Field, L.M. Gene amplification and insecticide resistance. Pest Manag. Sci. 2011, 67, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Pridgeon, J.W.J.P.b. Metabolic detoxication and the kdr mutation in pyrethroid resistant house flies, Musca domestica (L.). Pestic. Biochem. Physiol. 2002, 73, 157–163. [Google Scholar] [CrossRef]
- Abbas, N.; Shad, S.A.; Razaq, M. Fitness cost, cross resistance and realized heritability of resistance to imidacloprid in Spodoptera litura (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2012, 103, 181–188. [Google Scholar] [CrossRef]
- Wu, S.; Yang, L.; He, M.; Xia, F.; Shi, Y.; Chen, H.; Liao, X.; Li, R. Inheritance mode and fitness costs of acetamiprid resistance in brown planthopper, Nilaparvata lugens (Stål). Crop Prot. 2022, 156, 105958. [Google Scholar] [CrossRef]
- Gassmann, A.J. Chapter fourteen—Fitness costs of resistance and their potential application for insect resistance management. In Insect Resistance Management, 3rd ed.; Onstad, D.W., Knolhoff, L.M., Eds.; Elsevier: Amsterdam, The Netherlands; Academic Press: Cambridge, MA, USA, 2023; pp. 465–491. [Google Scholar] [CrossRef]
- Abbas, N.; Shah, R.M.; Shad, S.A.; Iqbal, N.; Razaq, M. Biological trait analysis and stability of lambda-cyhalothrin resistance in the house fly, Musca domestica L. (Diptera: Muscidae). Parasitol. Res. 2016, 115, 2073–2080. [Google Scholar] [CrossRef]
- Kliot, A.; Ghanim, M. Fitness costs associated with insecticide resistance. Pest Manag. Sci. 2012, 68, 1431–1437. [Google Scholar] [CrossRef]
- Khan, H.A.A. Spinosad resistance affects biological parameters of Musca domestica Linnaeus. Sci. Rep. 2018, 8, 14031. [Google Scholar] [CrossRef]
- Saeed, R.; Abbas, N.; Hafez, A.M. Biological fitness costs in emamectin benzoate-resistant strains of Dysdercus koenigii. Entomol. Gen. 2021, 41, 267–278. [Google Scholar] [CrossRef]
- Gonzalez-Santillan, F.J.; Contreras-Perera, Y.; Davila-Barboza, J.A.; Juache-Villagrana, A.E.; Gutierrez-Rodriguez, S.M.; Ponce-Garcia, G.; Lopez-Monroy, B.; Rodriguez-Sanchez, I.P.; Lenhart, A.E.; Mackenzie-Impoinvil, L. Fitness cost of sequential selection with deltamethrin in Aedes aegypti (Diptera: Culicidae). J. Med. Entomol. 2022, 59, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Ran, W.; Zhang, J.S.; Che, W.N.; Wang, J.D.; Chen, L.U.O. Genetics and fitness costs of resistance to flupyradifurone in Bemisia tabaci from China. J. Integr. Agric. 2022, 21, 1436–1443. [Google Scholar]
- Wazir, S.; Shad, S.A. Development of fipronil resistance, fitness cost, cross-resistance to other insecticides, stability, and risk assessment in Oxycarenus hyalinipennis (Costa). Sci. Total Environ. 2022, 803, 150026. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.; Abubakar, M.; Hassan, M.W.; Shad, S.A.; Hafez, A.M. Risk assessment of flonicamid resistance in Musca domestica (Diptera: Muscidae): Resistance monitoring, inheritance, and cross-resistance potential. J. Med. Entomol. 2021, 58, 1779–1787. [Google Scholar] [CrossRef]
- IRAC. IRAC Susceptibility Test Methods Series Method No: 026. 2011. Available online: https://irac-online.org/methods/musca-domestica-adults/ (accessed on 16 April 2019).
- Robertson, J.L.; Savin, N.E.; Preisler, H.K.; Russell, R.M. Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Saeed, R.; Abbas, N.; Hafez, A.M. Fitness cost of imidacloprid resistance in the cotton-staining bug, Dysdercus koenigii. Chemosphere 2021, 265, 129118. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin 1985, 24, 225–240. [Google Scholar]
- Euler, L. Demonstratio theorematis Fermatiani omnem numerum sive integrum sive fractum esse summam quatuor pauciorumve quadratorum. Novi Comment. Acad. Sci. Petropolitanae 1760, 5, 13–58. [Google Scholar]
- Lotka, A.J. Studies on the mode of growth of material aggregates. Am. J. Sci. 1907, 24, 199. [Google Scholar] [CrossRef]
- Goodman, D. Optimal life histories, optimal notation, and the value of reproductive value. Am. Nat. 1982, 119, 803–823. [Google Scholar] [CrossRef]
- Chi, H.; Su, H.-Y. Age-stage, two-sex life tables of Aphidius gifuensis (Ashmead) (Hymenoptera: Braconidae) and its host Myzus persicae (Sulzer) (Homoptera: Aphididae) with mathematical proof of the relationship between female fecundity and the net reproductive rate. Environ. Entomol. 2006, 35, 10–21. [Google Scholar] [CrossRef]
- Huang, Y.-B.; Chi, H. Assessing the application of the jackknife and bootstrap techniques to the estimation of the variability of the net reproductive rate and gross reproductive rate: A case study in Bactrocera cucurbitae (Coquillett) (Diptera: Tephritidae). J. Agric. For. 2012, 61, 37–45. [Google Scholar]
- Software, L. POLO for Windows. LeOra Software: Petaluma, CA, USA, 2005. [Google Scholar]
- Abbott, W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX-MSChart: A Computer Program for the Age-Stage, Two-Sex Life Table Analysis. 2019. Available online: https://140.120.197.173/Ecol./Prod02.Htm (accessed on 9 June 2022).
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H.; Yang, T.-C. Two-sex life table and predation rate of Propylaea japonica Thunberg (Coleoptera: Coccinellidae) fed on Myzus persicae (Sulzer) (Homoptera: Aphididae). Environ. Entomol. 2003, 32, 327–333. [Google Scholar] [CrossRef]
- Afzal, M.B.S.; Ijaz, M.; Farooq, Z.; Shad, S.A.; Abbas, N. Genetics and preliminary mechanism of chlorpyrifos resistance in Phenacoccus solenopsis Tinsley (Homoptera: Pseudococcidae). Pestic. Biochem. Physiol. 2015, 119, 42–47. [Google Scholar] [CrossRef]
- Scott, J.G. Evolution of resistance to pyrethroid insecticides in Musca domestica. Pest Manag. Sci. 2017, 73, 716–722. [Google Scholar] [CrossRef]
- Gao, Q.; Li, M.; Sheng, C.; Scott, J.G.; Qiu, X. Multiple cytochrome P450s overexpressed in pyrethroid resistant house flies (Musca domestica). Pestic. Biochem. Physiol. 2012, 104, 252–260. [Google Scholar] [CrossRef]
- Khan, H.A.A.; Akram, W.; Haider, M.S. Genetics and mechanism of resistance to deltamethrin in the house fly, Musca domestica L., from Pakistan. Ecotoxicology 2015, 24, 1213–1220. [Google Scholar] [CrossRef]
- Zhang, L.; Gao, X.; Liang, P. Beta-cypermethrin resistance associated with high carboxylesterase activities in a strain of house fly, Musca domestica (Diptera: Muscidae). Pestic. Biochem. Physiol. 2007, 89, 65–72. [Google Scholar] [CrossRef]
- Abbas, N.; Khan, H.; Shad, S.A. Cross-resistance, stability, and fitness cost of resistance to imidacloprid in Musca domestica L., (Diptera: Muscidae). Parasitol. Res. 2015, 114, 247–255. [Google Scholar] [CrossRef]
- Shah, R.M.; Shad, S.A. Inheritance, stability, cross-resistance, and life history parameters of a clothianidin-selected strain of house fly, Musca domestica Linnaeus. Environ. Pollut. 2021, 278, 116880. [Google Scholar] [CrossRef] [PubMed]
- Rinkevich, F.D.; Leichter, C.A.; Lazo, T.A.; Hardstone, M.C.; Scott, J.G. Variable fitness costs for pyrethroid resistance alleles in the house fly, Musca domestica, in the absence of insecticide pressure. Pestic. Biochem. Physiol. 2013, 105, 161–168. [Google Scholar] [CrossRef]
- Fawaz, E.Y.; Zayed, A.B.; Fahmy, N.T.; Villinski, J.T.; Hoel, D.F.; Diclaro, J.W. Pyrethroid insecticide resistance mechanisms in the adult Phlebotomus papatasi (Diptera: Psychodidae). J. Med. Entomol. 2016, 53, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Saingamsook, J.; Yanola, J.; Lumjuan, N.; Walton, C.; Somboon, P. Investigation of relative development and reproductivity fitness cost in three insecticide-resistant strains of Aedes aegypti from Thailand. Insects 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.B.; Silva, J.J.; Chen, C.; Harrington, L.C.; Scott, J.G. Fitness costs of individual and combined pyrethroid resistance mechanisms, kdr and CYP-mediated detoxification, in Aedes aegypti. PLoS Negl. Trop. Dis. 2021, 15, e0009271. [Google Scholar] [CrossRef] [PubMed]
- Banazeer, A.; Shad, S.A.; Afzal, M.B.S. Laboratory induced bifenthrin resistance selection in Oxycarenus hyalinipennis (Costa) (Hemiptera: Lygaeidae): Stability, cross-resistance, dominance and effects on biological fitness. Crop Prot. 2020, 132, 105107. [Google Scholar] [CrossRef]
- Khan, H.M.U.; Banazeer, A.; Afzal, M.B.S.; Shad, S.A. Evaluation of resistance stability and fitness costs in dimethoate-selected strain of Oxycarenus hyalinipennis Costa (Hemiptera: Lygaeidae). J. Asia-Pacif. Entomol. 2021, 24, 798–804. [Google Scholar] [CrossRef]
- Wang, R.; Qu, C.; Wang, Z.; Yang, G. Cross-resistance, biochemical mechanism and fitness costs of laboratory-selected resistance to pyridalyl in diamondback moth, Plutella xylostella. Pestic. Biochem. Physiol. 2020, 163, 8–13. [Google Scholar] [CrossRef]
- Stacke, R.F.; Godoy, D.N.; Halberstadt, S.A.; Bronzatto, E.S.; Giacomelli, T.; Hettwer, B.L.; Muraro, D.S.; Guedes, J.V.; Bernardi, O. Inheritance of lambda-cyhalothrin resistance, fitness costs and cross-resistance to other pyrethroids in soybean looper, Chrysodeixis includens (Lepidoptera: Noctuidae). Crop. Prot. 2020, 131, 105096. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, J.N.; Bai, J.Y.; Shang, Y.Z.; Zhang, S.Q.; Hou, Y.F.; Chen, M.H.; Han, Z.J. Pyrethroid resistance and fitness cost conferred by the super-kdr mutation M918L in Rhopalosiphum padi (Hemiptera: Aphididae). J. Econ. Entomol. 2021, 114, 1789–1795. [Google Scholar] [CrossRef]
- Castells-Sierra, J.; Guillem-Amat, A.; López-Errasquín, E.; Sánchez, L.; Ortego, F. First detection of resistance to deltamethrin in Spanish populations of the Mediterranean fruit fly, Ceratitis capitata. J. Pest Sci. 2023, 96, 1229–1242. [Google Scholar] [CrossRef]
- Tchouakui, M.; Mugenzi, L.M.J.; Wondji, M.J.; Tchoupo, M.; Njiokou, F.; Wondji, C.S. Combined over-expression of two cytochrome P450 genes exacerbates the fitness cost of pyrethroid resistance in the major African malaria vector Anopheles funestus. Pestic. Biochem. Physiol. 2021, 173, 104772. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.D.; Ebert, T.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Fitness costs associated with thiamethoxam and imidacloprid resistance in three field populations of Diaphorina citri (Hemiptera: Liviidae) from Florida. Bull. Entomol. Res. 2020, 110, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Pekkala, N.; Knott, K.E.; Kotiaho, J.S.; Nissinen, K.; Puurtinen, M. The effect of inbreeding rate on fitness, inbreeding depression and heterosis over a range of inbreeding coefficients. Evol. Appl. 2014, 7, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
| Strain | LC50 (ppm) a | 95% Fiducial Limits | Fit of Probit Line | RR (95% CL) b | |||
|---|---|---|---|---|---|---|---|
| Slope ± SE | χ2 | Df | p | ||||
| Alpha-SS (G41) | 3.77 | 2.52–5.28 | 1.33 ± 0.18 | 0.66 | 5 | 0.98 | 1 |
| Alpha-RS (G41) | 1530.36 | 1193.24–1992.02 | 1.98 ± 0.23 | 3.42 | 5 | 0.64 | 405.93 (260.73–631.56) |
| Strain | Insecticide | LC50 (ppm) a | 95% Fiducial Limits (ppm) | Fit of Probit Line | SR (95% CL) b | |||
|---|---|---|---|---|---|---|---|---|
| Slope ± SE | χ2 | df | p | |||||
| Alpha-SS (G41) | Alpha-cypermethrin | 3.77 | 2.52–5.28 | 1.33 ± 0.18 | 0.66 | 5 | 0.98 | 1 |
| +PBO | 3.57 | 2.40–4.96 | 1.36 ± 0.19 | 2.47 | 5 | 0.78 | 1.06 (0.64–1.76) | |
| +DEF | 3.09 | 2.16–4.16 | 1.58 ± 0.21 | 2.73 | 5 | 0.74 | 1.22 (0.75–1.99) | |
| +DEM | 3.28 | 2.10–4.66 | 1.27 ± 0.18 | 2.68 | 5 | 0.75 | 1.15 (0.68–1.95) | |
| Alpha-RS (G41) | Alpha-cypermethrin | 1530.36 | 1193.24–1992.02 | 1.98 ± 0.23 | 3.42 | 5 | 0.64 | 1 |
| +PBO | 412.18 | 258.79–559.98 | 2.37 ± 0.43 | 1.04 | 5 | 0.95 | 3.71 (2.39–5.77) * | |
| +DEF | 254.23 | 168.98–354.18 | 1.61 ± 0.22 | 1.32 | 5 | 0.93 | 6.02 (3.87–9.37) * | |
| +DEM | 439.71 | 328.48–585.10 | 1.63 ± 0.20 | 1.19 | 5 | 0.94 | 3.48 (2.37–5.11) * | |
| Parameters | Alpha-SS (Mean ± SE) | Alpha-RS (Mean ± SE) | 95% CI | p |
|---|---|---|---|---|
| Egg duration (d) | 1.00 ± 0.00 a | 1.00 ± 0.00 a | - | >0.05 |
| Larval duration (d) | 4.56 ± 0.04 a | 4.27 ± 0.04 b | 0.18–0.41 | <0.0001 |
| Pupal duration (d) | 4.24 ± 0.04 a | 4.28 ± 0.04 a | −0.08–0.16 | 0.52 |
| Adult duration (d) | 10.47 ± 0.65 a | 6.65 ± 0.60 b | 2.07–5.55 | <0.0001 |
| ♂ Preadult duration (d) | 9.95 ± 0.10 a | 9.74 ± 0.06 a | −0.02–0.44 | 0.08 |
| ♀ Preadult duration (d) | 9.55 ± 0.10 a | 9.07 ± 0.04 b | 0.28–0.69 | <0.0001 |
| ♂ Total longevity (d) | 20.02 ± 0.85 a | 15.93 ± 0.76 b | 1.86–6.31 | <0.0001 |
| ♀ Total longevity (d) | 20.47 ± 1.02 a | 16.30 ± 0.94 b | 1.44–6.88 | 0.003 |
| Overall total longevity (d) | 20.23 ± 0.65 a | 16.10 ± 0.59 b | 2.40–5.86 | 0.00 |
| APOP (d) | 3.54 ± 0.17 a | 3.76 ± 0.17 a | −0.24–0.69 | 0.35 |
| TPOP (d) | 13.08 ± 0.23 a | 12.76 ± 0.17 a | −0.24–0.88 | 0.27 |
| Oviposition period (d) | 4.64 ± 0.47 a | 3.76 ± 0.38 a | −0.31–2.07 | 0.15 |
| Female ratio (%) | 0.47 ± 0.05 a | 0.44 ± 0.05 a | −0.11–0.16 | 0.71 |
| Reproductive female ratio (%) | 0.76 ± 0.06 a | 0.54 ± 0.07 b | 0.03–0.41 | 0.02 |
| Fecundity (eggs produced per female) | 205.18 ± 32.10 a | 114.13 ± 22.11 b | 14.28–167.25 | 0.02 |
| r (d−1) | 0.25 ± 0.01 a | 0.21 ± 0.01 b | 1.08–7.92 | 0.01 |
| λ (d−1) | 1.29 ± 0.01 a | 1.23 ± 0.02 b | 1.38–9.92 | 0.01 |
| T (d) | 16.84 ± 0.35 a | 17.13 ± 0.27 a | −0.58–1.17 | 0.51 |
| Ro (offspring individual−1) | 69.76 ± 13.48 a | 35.00 ± 7.99 b | 3.98–65.40 | 0.03 |
| Rf | 1.00 | 0.50 | / | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hafez, A.M.; Abbas, N. Biological Fitness Cost, Demographic Growth Characteristics, and Resistance Mechanism in Alpha-Cypermethrin-Resistant Musca domestica (Diptera: Muscidae). Biology 2023, 12, 1021. https://doi.org/10.3390/biology12071021
Hafez AM, Abbas N. Biological Fitness Cost, Demographic Growth Characteristics, and Resistance Mechanism in Alpha-Cypermethrin-Resistant Musca domestica (Diptera: Muscidae). Biology. 2023; 12(7):1021. https://doi.org/10.3390/biology12071021
Chicago/Turabian StyleHafez, Abdulwahab M., and Naeem Abbas. 2023. "Biological Fitness Cost, Demographic Growth Characteristics, and Resistance Mechanism in Alpha-Cypermethrin-Resistant Musca domestica (Diptera: Muscidae)" Biology 12, no. 7: 1021. https://doi.org/10.3390/biology12071021
APA StyleHafez, A. M., & Abbas, N. (2023). Biological Fitness Cost, Demographic Growth Characteristics, and Resistance Mechanism in Alpha-Cypermethrin-Resistant Musca domestica (Diptera: Muscidae). Biology, 12(7), 1021. https://doi.org/10.3390/biology12071021