The Effects of Short-Term Exposure to pH Reduction on the Behavioral and Physiological Parameters of Juvenile Black Rockfish (Sebastes schlegelii)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.3. Behavioral Analysis
2.4. Sampling and Physiological Measurement
2.5. Statistical Analysis
3. Results
3.1. Behavior Analysis
3.1.1. Locomotor Activity
3.1.2. Activity State
3.1.3. Proximity
3.2. Physiological Response
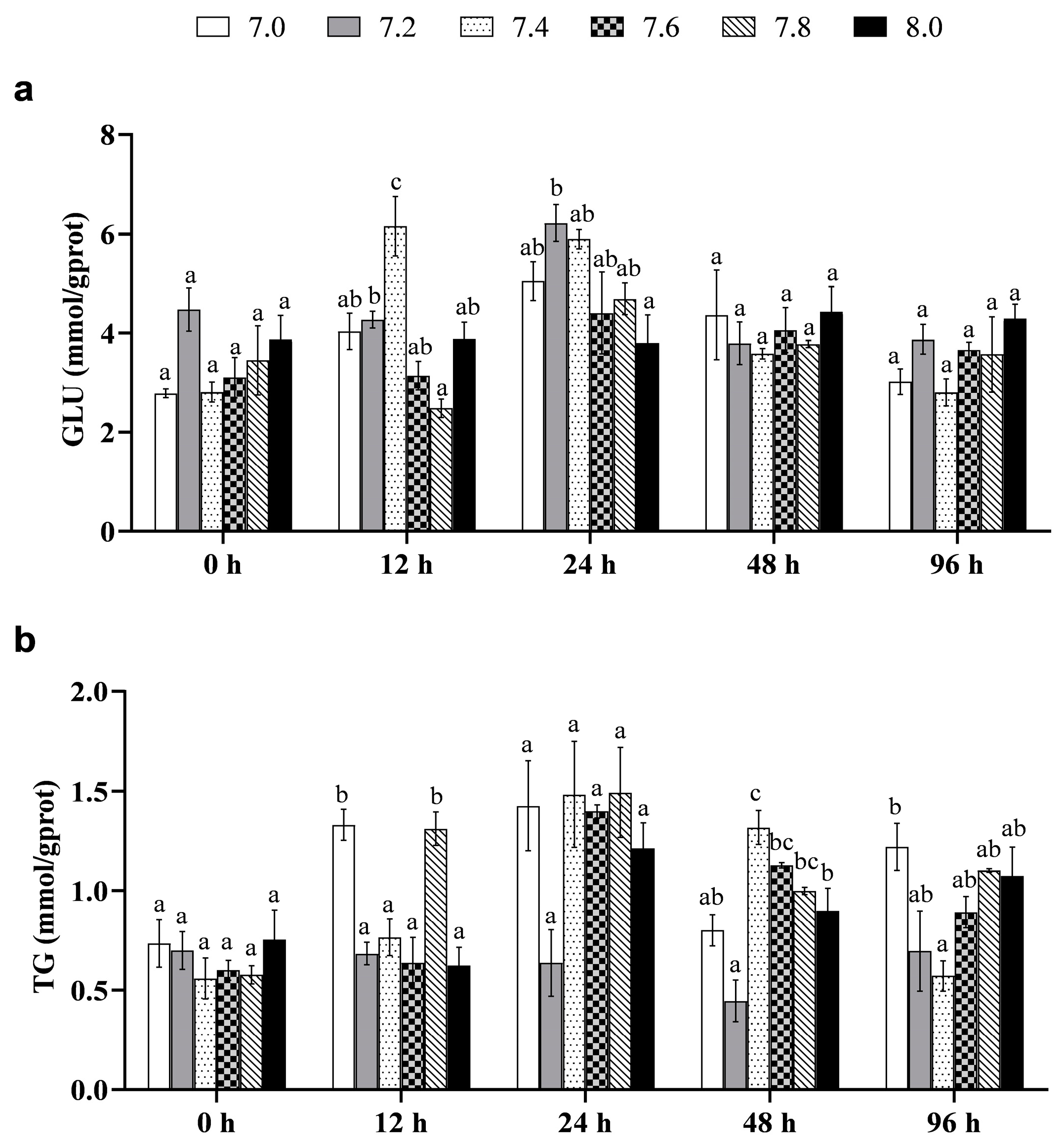
3.2.1. Immune Function
3.2.2. Metabolic Level
3.2.3. Stress Response
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hönisch, B.; Ridgwell, A.; Schmidt, D.N.; Thomas, E.; Gibbs, S.J.; Sluijs, A.; Zeebe, R.; Kump, L.; Martindale, R.C.; Greene, S.E.; et al. The geological record of ocean acidification. Science 2012, 335, 1058–1063. [Google Scholar] [CrossRef]
- Orr, J.C.; Fabry, V.J.; Aumont, O.; Bopp, L.; Doney, S.C.; Feely, R.A.; Gnanadesikan, A.; Gruber, N.; Ishida, A.; Joos, F. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 2005, 437, 681–686. [Google Scholar] [CrossRef]
- Raupach, M.R.; Marland, G.; Ciais, P.; Le Quéré, C.; Canadell, J.G.; Klepper, G.; Field, C.B. Global and regional drivers of accelerating CO2 emissions. Proc. Natl. Acad. Sci. USA 2007, 104, 10288–10293. [Google Scholar] [CrossRef]
- Hendriks, I.; Duarte, C.; Álvarez, M. Vulnerability of marine biodiversity to ocean acidification: A meta-analysis. Estuar. Coast. Shelf Sci. 2010, 86, 157–164. [Google Scholar] [CrossRef]
- Wootton, J.T.; Pfister, C.A.; Forester, J.D. Dynamic patterns and ecological impacts of declining ocean pH in a high-resolution multi-year dataset. Proc. Natl. Acad. Sci. USA 2008, 105, 18848–18853. [Google Scholar] [CrossRef]
- Hofmann, G.E.; Smith, J.E.; Johnson, K.S.; Send, U.; Levin, L.A.; Micheli, F.; Paytan, A.; Price, N.N.; Peterson, B.; Takeshita, Y.; et al. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLoS ONE 2011, 6, e28983. [Google Scholar] [CrossRef]
- Pansch, C.; Nasrolahi, A.; Appelhans, Y.S.; Wahl, M. Impacts of ocean warming and acidification on the larval development of the barnacle Amphibalanus. J. Exp. Mar. Biol. Ecol. 2012, 420, 48–55. [Google Scholar] [CrossRef]
- Baumann, H.; Wallace, R.B.; Tagliaferri, T.; Gobler, C.J. Large natural pH, CO2 and O2 fluctuations in a temperate tidal salt marsh on diel, seasonal, and interannual time scales. Estuaries Coasts 2015, 38, 220–231. [Google Scholar] [CrossRef]
- Boyd, P.W.; Cornwall, C.E.; Davison, A.; Doney, S.C.; Fourquez, M.; Hurd, C.L.; Lima, I.D.; McMinn, A. Biological responses to environmental heterogeneity under future ocean conditions. Glob. Chang. Biol. 2016, 22, 2633–2650. [Google Scholar] [CrossRef]
- Henson, S.A.; Beaulieu, C.; Lampitt, R. Observing climate change trends in ocean biogeochemistry: When and where. Glob. Chang. Biol. 2016, 22, 1561–1571. [Google Scholar] [CrossRef]
- Cornwall, C.E.; Hepburn, C.D.; McGraw, C.M.; Currie, K.I.; Pilditch, C.A.; Hunter, K.A.; Boyd, P.W.; Hurd, C.L. Diurnal fluctuations in seawater pH influence the response of a calcifying macroalga to ocean acidification. Proc. R. Soc. B Biol. Sci. 2013, 280, 20132201. [Google Scholar] [CrossRef] [PubMed]
- Challener, R.C.; Robbins, L.L.; McClintock, J.B. Variability of the carbonate chemistry in a shallow, seagrass-dominated ecosystem: Implications for ocean acidification experiments. Mar. Freshw. Res. 2015, 67, 163. [Google Scholar] [CrossRef]
- Duarte, C.M.; Hendriks, I.E.; Moore, T.S.; Olsen, Y.S.; Steckbauer, A.; Ramajo, L.; Carstensen, J.; Trotter, J.A.; McCulloch, M. Is ocean acidification an open-ocean syndrome? Understanding anthropogenic impacts on seawater pH. Estuaries Coasts 2013, 36, 221–236. [Google Scholar] [CrossRef]
- Melzner, F.; Thomsen, J.; Koeve, W.; Oschlies, A.; Gutowska, M.A.; Bange, H.W.; Hansen, H.P.; Körtzinger, A. Future ocean acidification will be amplified by hypoxia in coastal habitats. Mar. Biol. 2013, 160, 1875–1888. [Google Scholar] [CrossRef]
- Waldbusser, G.G.; Voigt, E.; Bergschneider, H.; Green, M.A.; Newell, R.I.E. Biocalcification in the eastern oyster (Crassostrea virginica) in relation to long-term trends in chesapeake bay PH. Estuaries Coasts 2011, 34, 221–231. [Google Scholar] [CrossRef]
- Segman, R.F.; Dubinsky, Z.; Iluz, D. Impacts of ocean acidification on calcifying macroalgae: Padina sp. as a test case–A review. Isr. J. Plant Sci. 2016, 65, 1–8. [Google Scholar] [CrossRef]
- Ishimatsu, A.; Hayashi, M.; Kikkawa, T. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 2008, 373, 295–302. [Google Scholar] [CrossRef]
- Baumann, H.; Talmage, S.C.; Gobler, C.J. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Chang. 2012, 2, 38–41. [Google Scholar] [CrossRef]
- Munday, P.L.; Dixson, D.L.; Donelson, J.M.; Jones, G.P.; Pratchett, M.S.; Devitsina, G.V.; Døving, K.B. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl. Acad. Sci. USA 2009, 106, 1848–1852. [Google Scholar] [CrossRef]
- Simpson, S.D.; Munday, P.L.; Wittenrich, M.L.; Manassa, R.; Dixson, D.L.; Gagliano, M.; Yan, H.Y. Ocean acidification erodes crucial auditory behaviour in a marine fish. Biol. Lett. 2011, 7, 917–920. [Google Scholar] [CrossRef]
- Ferrari, M.C.; McCormick, M.I.; Munday, P.L.; Meekan, M.G.; Dixson, D.L.; Lönnstedt, O.; Chivers, D.P. Effects of ocean acidification on visual risk assessment in coral reef fishes. Funct. Ecol. 2012, 26, 553–558. [Google Scholar] [CrossRef]
- Lönnstedt, O.M.; Munday, P.L.; McCormick, M.I.; Ferrari, M.C.; Chivers, D.P. Ocean acidification and responses to predators: Can sensory redundancy reduce the apparent impacts of elevated CO2 on fish? Ecol. Evol. 2013, 3, 3565–3575. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.; Jutfelt, F.; Nilsson, G.E. Altered neurotransmitter function in CO2-exposed stickleback (Gasterosteus aculeatus): A temperate model species for ocean acidification research. Conserv. Physiol. 2015, 3, cov018. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chon, T.-S.; Park, Y.-S.; Shi, X.; Ren, Z. Application of temporal self-organizing maps to patterning short-time series of fish behavior responding to environmental stress. Ecol. Model. 2020, 433, 109242. [Google Scholar] [CrossRef]
- Chapman, L.J.; Mckenzie, D.J. Behavioral responses and ecological consequences. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 27, pp. 25–77. [Google Scholar] [CrossRef]
- Nilsson, G.E.; Dixson, D.; Domenici, P.; McCormick, M.; Sørensen, C.; Watson, S.-A.; Munday, P. Near-future carbon dioxide levels alter fish behaviour by interfering with neurotransmitter function. Nat. Clim. Chang. 2012, 2, 201–204. [Google Scholar] [CrossRef]
- Lopes, A.F.; Morais, P.; Pimentel, M.; Rosa, R.; Munday, P.L.; Gonçalves, E.J.; Faria, A.M. Behavioural lateralization and shoaling cohesion of fish larvae altered under ocean acidification. Mar. Biol. 2016, 163, 243. [Google Scholar] [CrossRef]
- Tresguerres, M.; Hamilton, T.J. Acid-base physiology, neurobiology and behaviour in relation to CO2-induced ocean acidification. J. Exp. Biol. 2017, 220, 2136–2148. [Google Scholar] [CrossRef]
- McCormick, M.I.; Watson, S.-A.; Simpson, S.D.; Allan, B.J. Effect of elevated CO2 and small boat noise on the kinematics of predator–prey interactions. Proc. R. Soc. B Biol. Sci. 2018, 285, 20172650. [Google Scholar] [CrossRef]
- Scott, G.R.; Sloman, K.A. The effects of environmental pollutants on complex fish behaviour: Integrating behavioural and physiological indicators of toxicity. Aquat. Toxicol. 2004, 68, 369–392. [Google Scholar] [CrossRef]
- Leduc, A.O.H.C.; Munday, P.L.; Brown, G.E.; Ferrari, M.C.O. Effects of acidification on olfactory-mediated behaviour in freshwater and marine ecosystems: A synthesis. Philos. Trans. R. Soc. B Biol. Sci. 2013, 368, 20120447. [Google Scholar] [CrossRef]
- Clements, J.; Hunt, H. Marine animal behaviour in a high CO2 ocean. Mar. Ecol. Prog. Ser. 2015, 536, 259–279. [Google Scholar] [CrossRef]
- Munday, P.L.; Dixson, D.L.; McCormick, M.I.; Meekan, M.; Ferrari, M.C.O.; Chivers, D.P. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl. Acad. Sci. USA 2010, 107, 12930–12934. [Google Scholar] [CrossRef]
- Jarrold, M.D.; Welch, M.J.; McMahon, S.J.; McArley, T.; Allan, B.J.; Watson, S.-A.; Parsons, D.M.; Pether, S.M.; Pope, S.; Nicol, S.; et al. Elevated CO2 affects anxiety but not a range of other behaviours in juvenile yellowtail kingfish. Mar. Environ. Res. 2020, 157, 104863. [Google Scholar] [CrossRef]
- Jarrold, M.D.; Humphrey, C.; McCormick, M.I.; Munday, P.L. Diel CO2 cycles reduce severity of behavioural abnormalities in coral reef fish under ocean acidification. Sci. Rep. 2017, 7, 10153. [Google Scholar] [CrossRef]
- Maulvault, A.L.; Santos, L.H.; Paula, J.R.; Camacho, C.; Pissarra, V.; Fogaça, F.; Barbosa, V.; Alves, R.; Ferreira, P.P.; Barceló, D.; et al. Differential behavioural responses to venlafaxine exposure route, warming and acidification in juvenile fish (Argyrosomus regius). Sci. Total. Environ. 2018, 634, 1136–1147. [Google Scholar] [CrossRef]
- Duteil, M.; Pope, E.C.; Pérez-Escudero, A.; de Polavieja, G.G.; Fürtbauer, I.; Brown, M.R.; King, A.J. European sea bass show behavioural resilience to near-future ocean acidification. R. Soc. Open Sci. 2016, 3, 160656. [Google Scholar] [CrossRef]
- Porteus, C.S.; Hubbard, P.C.; Uren Webster, T.M.; van Aerle, R.; Canário, A.V.M.; Santos, E.M.; Wilson, R.W. Near-future CO2 levels impair the olfactory system of a marine fish. Nat. Clim. Chang. 2018, 8, 737–743. [Google Scholar] [CrossRef]
- Kumai, Y.; Nesan, D.; Vijayan, M.M.; Perry, S.F. Cortisol regulates Na+ uptake in zebrafish, Danio rerio, larvae via the glucocorticoid receptor. Mol. Cell Endocrinol. 2012, 364, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Balasch, J.C.; Tort, L. Netting the stress responses in fish. Front. Endocrinol. 2019, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Korte, S.M.; Koolhaas, J.M.; Wingfield, J.C.; McEwen, B.S. The Darwinian concept of stress: Benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005, 29, 3–38. [Google Scholar] [CrossRef]
- Kwong, R.W.M.; Kumai, Y.; Perry, S.F. The physiology of fish at low pH: The zebrafish as a model system. J. Exp. Biol. 2014, 217, 651–662. [Google Scholar] [CrossRef]
- Zhang, Z.; Bai, Q.; Xu, X.; Guo, H.; Zhang, X. Effects of environmental enrichment on the welfare of juvenile black rockfish Sebastes schlegelii: Growth, behavior and physiology. Aquaculture 2020, 518, 734782. [Google Scholar] [CrossRef]
- Zhang, Z.; Fu, Y.; Guo, H.; Zhang, X. Effect of environmental enrichment on the stress response of juvenile black rockfish Sebastes schlegelii. Aquaculture 2021, 533, 736088. [Google Scholar] [CrossRef]
- Lü, H.; Zhang, X.; Xi, D.; Gao, T. Use of calcein and alizarin red S for immersion marking of black rockfish Sebastes schlegelii juveniles. Chin. J. Oceanol. Limnol. 2014, 32, 88–98. [Google Scholar] [CrossRef]
- Chen, Y.; Shan, X.; Ovando, D.; Yang, T.; Dai, F.; Jin, X. Predicting current and future global distribution of black rockfish (Sebastes schlegelii) under changing climate. Ecol. Indic. 2021, 128, 107799. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, X.; Li, Z.; Zhang, X. Effects of different levels of environmental enrichment on the sheltering behaviors, brain development and cortisol levels of black rockfish Sebastes schlegelii. Appl. Anim. Behav. Sci. 2019, 218, 104825. [Google Scholar] [CrossRef]
- Wang, L.; Wu, Z.; Liu, M.; Liu, W.; Zhao, W.; Liu, H.; Zhang, P.; You, F. Length-weight, length-length relationships, and condition factors of black rockfish Sebastes schlegelii Hilgendorf, 1880 in Lidao Bay, China. Thalass. Int. J. Mar. Sci. 2017, 33, 57–63. [Google Scholar] [CrossRef]
- The People’s Republic of China Ministry of Agriculture, F.B. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Jiahuan, R.; Wenhao, S.; Xiaofan, G.; Wei, S.; Shanjie, Z.; Maolong, H.; Haifeng, W.; Guangxu, L. Ocean Acidification impairs foraging behavior by interfering with olfactory neural signal transduction in black sea bream, Acanthopagrus schlegelii. Front. Physiol. 2018, 9, 1592. [Google Scholar] [CrossRef] [PubMed]
- Sundin, J.; Amcoff, M.; Mateos-González, F.; Raby, G.D.; Jutfelt, F.; Clark, T.D. Long-term exposure to elevated carbon dioxide does not alter activity levels of a coral reef fish in response to predator chemical cues. Behav. Ecol. Sociobiol. 2017, 71, 108. [Google Scholar] [CrossRef] [PubMed]
- Munday, P.L.; Jones, G.P.; Pratchett, M.S.; Williams, A.J. Climate change and the future for coral reef fishes. Fish Fish. 2010, 9, 261–285. [Google Scholar] [CrossRef]
- Carstensen, J.; Duarte, C.M. Drivers of pH variability in coastal ecosystems. Environ. Sci. Technol. 2019, 53, 4020–4029. [Google Scholar] [CrossRef]
- Nakamura, F. Avoidance behavior and swimming activity of fish to detect pH changes. Bull. Environ. Contam. Toxicol. 1986, 37, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, S.L.; Logan, C.A.; Fennie, H.W.; Sogard, S.M.; Barry, J.P.; Makukhov, A.D.; Tobosa, L.R.; Boyer, K.; Lovera, C.F.; Bernardi, G. Species-Specific Responses of Juvenile Rockfish to Elevated pCO2: From Behavior to Genomics. PLoS ONE 2017, 12, e0169670. [Google Scholar] [CrossRef]
- Cripps, I.L.; Munday, P.; McCormick, M. Ocean acidification affects prey detection by a predatory reef fish. PLoS ONE 2011, 6, e22736. [Google Scholar] [CrossRef] [PubMed]
- Schunter, C.; Ravasi, T.; Munday, P.L.; Nilsson, G.E. Neural effects of elevated CO2 in fish may be amplified by a vicious cycle. Conserv. Physiol. 2019, 7, coz100. [Google Scholar] [CrossRef] [PubMed]
- Tembo, R. The mechanism of chemoreception in fish under low pH condition. J. Environ. Anal. Toxicol. 2021, 11, S6. [Google Scholar]
- Domenici, P.; Allan, B.J.M.; Watson, S.-A.; McCormick, M.I.; Munday, P.L. Shifting from right to left: The combined effect of elevated CO2 and temperature on behavioural lateralization in a coral reef fish. PLoS ONE 2014, 9, e87969. [Google Scholar] [CrossRef]
- Sundin, J.; Jutfelt, F. Effects of elevated carbon dioxide on male and female behavioural lateralization in a temperate goby. R. Soc. Open Sci. 2018, 5, 171550. [Google Scholar] [CrossRef]
- Jutfelt, F.; de Souza, K.B.; Vuylsteke, A.; Sturve, J. Behavioural disturbances in a temperate fish exposed to sustained high-CO2 levels. PLoS ONE 2013, 8, e65825. [Google Scholar] [CrossRef]
- Munday, P.L.; Cheal, A.J.; Dixson, D.L.; Rummer, J.L.; Fabricius, K.E. Behavioural impairment in reef fishes caused by ocean acidification at CO2 seeps. Nat. Clim. Chang. 2014, 4, 487–492. [Google Scholar] [CrossRef]
- Biro, P.A.; Post, J.R.; Parkinson, E.A. From individuals to populations: Prey fish risk-taking mediates mortality in whole-system experiments. Ecology 2003, 84, 2419–2431. [Google Scholar] [CrossRef]
- Biro, P.A.; Dingemanse, N.J. Sampling bias resulting from animal personality. Trends Ecol. Evol. 2009, 24, 66–67. [Google Scholar] [CrossRef]
- Brun, N.R.; van Hage, P.; Hunting, E.R.; Haramis, A.-P.G.; Vink, S.C.; Vijver, M.G.; Schaaf, M.J.M.; Tudorache, C. Polystyrene nanoplastics disrupt glucose metabolism and cortisol levels with a possible link to behavioural changes in larval zebrafish. Commun. Biol. 2019, 2, 382. [Google Scholar] [CrossRef]
- Mitchell, A.; Booth, D.J.; Nagelkerken, I. Ocean warming and acidification degrade shoaling performance and lateralization of novel tropical-temperate fish shoals. Glob. Chang. Biol. 2022, 28, 1388–1401. [Google Scholar] [CrossRef]
- Laubenstein, T.; Rummer, J.L.; Nicol, S.; Parsons, D.M.; Pether, S.M.J.; Pope, S.; Smith, N.; Munday, P.L. Correlated effects of ocean acidification and warming on behavioral and metabolic traits of a large pelagic fish. Diversity 2018, 10, 35. [Google Scholar] [CrossRef]
- Sheehan, D.; Meade, G.; Foley, V.M.; Dowd, C.A. Structure, function and evolution of glutathione transferases: Implications for classification of non-mammalian members of an ancient enzyme superfamily. Biochem. J. 2001, 360, 1–16. [Google Scholar] [CrossRef]
- Geret, F.; Bebianno, M.J. Does zinc produce reactive oxygen species in Ruditapes decussatus? Ecotoxicol. Environ. Saf. 2004, 57, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.D.D.; Maltez, L.C.; Rodrigues, R.V.; Planas, M.; Sampaio, L.A. Does acidification lead to impairments on oxidative status and survival of orange clownfish Amphiprion percula juveniles? Fish Physiol. Biochem. 2021, 47, 841–848. [Google Scholar] [CrossRef]
- Noor, N.M.; De, M.; Cob, Z.C.; Das, S.K. Welfare of scaleless fish, Sagor catfish (Hexanematichthys sagor) juveniles under different carbon dioxide concentrations. Aquac. Res. 2021, 52, 2980–2987. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, Z.; Mousavi, S.E. Effect of seawater pH on selected blood biochemical parameters of juvenile turbot Scophthalmus maximus (Linnaeus, 1758). Indian J. Fish. 2019, 66, 78–83. [Google Scholar] [CrossRef]
- Esbaugh, A.J. Physiological implications of ocean acidification for marine fish: Emerging patterns and new insights. J. Comp. Physiol. B 2018, 188, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pan, T.-C.F.; Applebaum, S.L.; Manahan, D.T. Experimental ocean acidification alters the allocation of metabolic energy. Proc. Natl. Acad. Sci. USA 2015, 112, 4696–4701. [Google Scholar] [CrossRef] [PubMed]
- Chance, R.J.; Cameron, G.A.; Fordyce, M.; Noguera, P.; Wang, T.; Collins, C.; Secombes, C.J.; Collet, B. Effects of repeated anaesthesia on gill and general health of Atlantic salmon, Salmo salar. J. Fish Biol. 2018, 93, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Dineshram, R.; Quan, Q.; Sharma, R.; Chandramouli, K.; Yalamanchili, H.K.; Chu, I.; Thiyagarajan, V. Comparative and quantitative proteomics reveal the adaptive strategies of oyster larvae to ocean acidification. Proteomics 2015, 15, 4120–4134. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zheng, X.; Fu, Z.; Lin, S.; Yu, G.; Qin, J.G. Transcriptional analysis reveals physiological response to acute acidification stress of barramundi Lates calcarifer (Bloch) in coastal areas. Fish Physiol. Biochem. 2020, 46, 1729–1741. [Google Scholar] [CrossRef]
- Tsang, H.H.; Welch, M.J.; Munday, P.L.; Ravasi, T.; Schunter, C. Proteomic responses to ocean acidification in the brain of juvenile coral reef fish. Front. Mar. Sci. 2020, 7, 605. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef]
- Moltesen, M.; Laursen, D.C.; Thörnqvist, P.-O.; Andersson, M.; Winberg, S.; Höglund, E. Effects of acute and chronic stress on telencephalic neurochemistry and gene expression in rainbow trout (Oncorhynchus mykiss). J. Exp. Biol. 2016, 219, 3907–3914. [Google Scholar] [CrossRef]
- Hglund, E.; Korzan, W.; Tland, S.; Haraldstad, T.; Verli, Y. Neuroendocrine indicators of allostatic load reveal impact of environmental acidification in fish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 229, 108679. [Google Scholar] [CrossRef]


| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Velocity (cm/s) | pH 7.8 | −0.624 | 0.375 | −1.662 | 0.098 |
| pH 7.6 | −0.545 | 0.375 | −1.452 | 0.148 | |
| pH 7.4 | −1.243 | 0.375 | −3.314 | 0.001 | |
| pH 7.2 | −0.941 | 0.375 | −2.510 | 0.013 | |
| pH 7.0 | −0.615 | 0.375 | −1.639 | 0.103 | |
| Time | −0.647 | 0.080 | −8.097 | <0.001 | |
| Time * [pH 7.8] | 0.451 | 0.113 | 3.985 | <0.001 | |
| Time * [pH 7.6] | 0.383 | 0.113 | 3.384 | 0.001 | |
| Time * [pH 7.4] | 0.382 | 0.113 | 3.382 | 0.001 | |
| Time * [pH 7.2] | 0.349 | 0.113 | 3.086 | 0.002 | |
| Time * [pH 7.0] | 0.067 | 0.113 | 0.590 | 0.556 |
| Time | pH | ||||||
|---|---|---|---|---|---|---|---|
| 8.0 | 7.8 | 7.6 | 7.4 | 7.2 | 7.0 | ||
| Velocity (cm/s) | 0 h | 0.95 ± 0.14 | 0.74 ± 0.16 | 0.89 ± 0.17 | 0.71 ± 0.11 | 0.62 ± 0.14 | 0.76 ± 0.14 |
| 12 h | 0.73 ± 0.16 a | 0.01 ± 0.06 ab | 0.60 ± 0.11 a | −1.54 ± 0.06 b | −0.66 ± 0.03 b | −0.73 ± 0.02 b | |
| 24 h | −0.08 ± 0.05 a | 2.19 ± 0.11 b | 0.90 ± 0.16 ab | 1.16 ± 0.17 ab | 1.17 ± 0.10 b | −0.04 ± 0.05 a | |
| 48 h | −0.92 ± 0.04 a | 0.31 ± 0.07 e | −0.03 ± 0.03 d | −0.38 ± 0.06 c | −0.36 ± 0.04 c | −0.65 ± 0.07 b | |
| 96 h | −1.46 ± 0.08 b | −0.39 ± 0.04 d | −0.12 ± 0.06 d | −1.20 ± 0.04 bc | −1.02 ± 0.06 c | −2.19 ± 0.11 a | |
| Maximum Acceleration (cm/s2) | 0 h | 0.35 ± 0.15 | 0.49 ± 0.05 | 0.56 ± 0.07 | 0.36 ± 0.08 | 0.42 ± 0.13 | 0.22 ± 0.08 |
| 12 h | −0.66 ± 0.25 a | −0.05 ± 0.17 ab | 0.30 ± 0.20 ab | −2.19 ± 0.11 b | 2.18 ± 0.12 a | −0.70 ± 0.15 b | |
| 24 h | −1.44 ± 0.13 a | 0.97 ± 0.07 c | −0.55 ± 0.27 b | 0.56 ± 0.17 c | 1.66 ± 0.04 d | −0.54 ± 0.12 b | |
| 48 h | −0.56 ± 0.16 | −0.84 ± 0.20 | −0.33 ± 0.14 | −0.69 ± 0.18 | −0.60 ± 0.08 | −0.46 ± 0.11 | |
| 96 h | 0.42 ± 0.18 ab | −0.06 ± 0.22 b | −0.30 ± 0.28 b | −0.96 ± 0.06 b | 1.29 ± 0.05 a | 1.15 ± 0.14 ab | |
| Angular Velocity (º/s) | 0 h | −0.10 ± 0.14 | −0.16 ± 0.14 | −0.19 ± 0.06 | −0.22 ± 0.10 | −0.24 ± 0.21 | −0.05 ± 0.12 |
| 12 h | 0.58 ± 0.14 | 0.23 ± 0.40 | 0.12 ± 0.32 | 0.35 ± 0.22 | 0.17 ± 0.18 | 0.46 ± 0.27 | |
| 24 h | −0.23 ± 0.20 | −0.77 ± 0.17 | −0.09 ± 0.22 | −0.72 ± 0.45 | −0.60 ± 0.28 | 0.03 ± 0.20 | |
| 48 h | −0.83 ± 0.20 | −0.67 ± 0.17 | −0.92 ± 0.23 | −1.17 ± 0.55 | −1.48 ± 0.25 | −1.09 ± 0.19 | |
| 96 h | 1.63 ± 0.18 ab | 0.80 ± 0.07 c | 1.17 ± 0.19 bc | 1.01 ± 0.16 bc | 1.13 ± 0.13 bc | 1.86 ± 0.19 a | |
| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Maximum Acceleration (cm/s2) | pH 7.8 | 1.120 | 0.445 | 2.515 | 0.013 |
| pH 7.6 | 1.091 | 0.445 | 2.449 | 0.015 | |
| pH 7.4 | 0.206 | 0.445 | 0.461 | 0.645 | |
| pH 7.2 | 1.751 | 0.445 | 3.931 | <0.001 | |
| pH 7.0 | −0.243 | 0.445 | −0.545 | 0.587 | |
| Time | 0.025 | 0.095 | 0.259 | 0.796 | |
| Time * [pH 7.8] | −0.214 | 0.134 | −1.597 | 0.112 | |
| Time * [pH 7.6] | −0.260 | 0.134 | −1.934 | 0.054 | |
| Time * [pH 7.4] | −0.137 | 0.134 | −1.023 | 0.307 | |
| Time * [pH 7.2] | −0.128 | 0.134 | −0.953 | 0.342 | |
| Time * [pH 7.0] | 0.186 | 0.134 | 1.382 | 0.168 |
| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Angular Velocity (º/s) | pH 7.8 | 0.008 | 0.299 | 0.028 | 0.978 |
| pH 7.6 | −0.114 | 0.299 | −0.381 | 0.704 | |
| pH 7.4 | −0.007 | 0.299 | −0.024 | 0.981 | |
| pH 7.2 | −0.045 | 0.299 | −0.149 | 0.882 | |
| pH 7.0 | 0.016 | 0.299 | 0.055 | 0.956 | |
| Time | 0.110 | 0.098 | 1.126 | 0.262 | |
| Time * [pH 7.8] | −0.115 | 0.139 | −0.827 | 0.409 | |
| Time * [pH 7.6] | −0.026 | 0.139 | −0.188 | 0.851 | |
| Time * [pH 7.4] | −0.118 | 0.139 | −0.853 | 0.395 | |
| Time * [pH 7.2] | −0.127 | 0.139 | −0.918 | 0.360 | |
| Time * [pH 7.0] | 0.002 | 0.139 | 0.015 | 0.988 |
| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Highly Mobile (s) | pH 7.8 | 0.103 | 0.373 | 0.276 | 0.783 |
| pH 7.6 | −0.192 | 0.373 | −0.515 | 0.607 | |
| pH 7.4 | −0.772 | 0.373 | −2.068 | 0.040 | |
| pH 7.2 | −1.138 | 0.373 | −3.049 | 0.003 | |
| pH 7.0 | −0.772 | 0.373 | −2.068 | 0.040 | |
| Time | −0.370 | 0.081 | −4.573 | <0.001 | |
| Time * [pH 7.8] | 0.401 | 0.115 | 3.505 | 0.001 | |
| Time * [pH 7.6] | 0.346 | 0.115 | 3.021 | 0.003 | |
| Time * [pH 7.4] | 0.582 | 0.115 | 5.083 | <0.001 | |
| Time * [pH 7.2] | 0.892 | 0.115 | 7.783 | <0.001 | |
| Time * [pH 7.0] | 0.770 | 0.115 | 6.719 | <0.001 |
| Time | pH | ||||||
|---|---|---|---|---|---|---|---|
| 8.0 | 7.8 | 7.6 | 7.4 | 7.2 | 7.0 | ||
| Highly mobile (s) | 0 h | −0.40 ± 0.11 | −0.17 ± 0.14 | −0.42 ± 0.12 | −0.26 ± 0.08 | −0.93 ± 0.30 | −0.65 ± 0.27 |
| 12 h | −0.63 ± 0.11 a | 0.46 ± 0.16 b | −0.34 ± 0.11 b | −1.21 ± 0.14 a | 0.51 ± 0.20 b | 0.68 ± 0.22 b | |
| 24 h | −0.89 ± 0.18 a | 0.75 ± 0.09 b | −0.10 ± 0.24 ab | 1.09 ± 0.16 b | 1.06 ± 0.12 b | 0.38 ± 0.43 ab | |
| 48 h | −1.31 ± 0.18 a | 0.26 ± 0.14 cd | −0.79 ± 0.18 ab | −0.18 ± 0.21 bc | −0.11 ± 0.20 bc | 0.89 ± 0.13 d | |
| 96 h | −1.93 ± 0.24 a | 0.05 ± 0.14 b | 0.03 ± 0.28 b | 0.27 ± 0.26 bc | 1.99 ± 0.16 d | 1.22 ± 0.30 cd | |
| Mobile (s) | 0 h | 0.18 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 | 0.17 ± 0.01 |
| 12 h | 0.16 ± 0.01 a | 0.14 ± 0.01 b | 0.14 ± 0.01 b | 0.12 ± 0.01 c | 0.14 ± 0.01 b | 0.14 ± 0.01 b | |
| 24 h | 0.17 ± 0.01 a | 0.17 ± 0.01 a | 0.18 ± 0.01 a | 0.17 ± 0.01 a | 0.18 ± 0.01 a | 0.14 ± 0.01 b | |
| 48 h | 0.17 ± 0.01 ab | 0.15 ± 0.01 b | 0.20 ± 0.01 a | 0.15 ± 0.01 ab | 0.16 ± 0.01 ab | 0.13 ± 0.01 b | |
| 96 h | 0.13 ± 0.02 a | 0.15 ± 0.01 ab | 0.20 ± 0.01 b | 0.13 ± 0.01 a | 0.14 ± 0.01 ab | 0.12 ± 0.02 a | |
| Immobile (s) | 0 h | 0.08 ± 0.12 | −0.51 ± 0.12 | −0.21 ± 0.04 | −0.19 ± 0.06 | −0.47 ± 0.43 | −0.07 ± 0.20 |
| 12 h | −0.65 ± 0.08 a | −0.68 ± 0.09 a | −0.54 ± 0.19 a | 1.57 ± 0.05 b | 0.73 ± 0.06 ab | 1.19 ± 0.06 b | |
| 24 h | −0.24 ± 0.10 b | −2.01 ± 0.13 d | −1.35 ± 0.08 c | −1.37 ± 0.13 c | −1.03 ± 0.11 c | 0.36 ± 0.05 a | |
| 48 h | 1.27 ± 0.06 a | −1.02 ± 0.05 b | −0.23 ± 0.08 b | 0.28 ± 0.15 ab | 0.39 ± 0.13 ab | 0.14 ± 0.13 ab | |
| 96 h | 2.19 ± 0.11 a | 0.93 ± 0.19 b | 0.47 ± 0.09 b | 0.96 ± 0.06 b | 0.57 ± 0.11 b | −0.56 ± 0.22 c | |
| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Mobile (s) | pH 7.8 | −0.009 | 0.012 | −0.769 | 0.443 |
| pH 7.6 | −0.019 | 0.012 | −1.529 | 0.128 | |
| pH 7.4 | −0.004 | 0.012 | −0.298 | 0.766 | |
| pH 7.2 | −0.009 | 0.012 | −0.768 | 0.444 | |
| pH 7.0 | 0.005 | 0.012 | 0.436 | 0.663 | |
| Time | −0.004 | 0.004 | −1.065 | 0.288 | |
| Time * [pH 7.8] | 0.001 | 0.006 | 0.113 | 0.910 | |
| Time * [pH 7.6] | 0.010 | 0.006 | 1.756 | 0.080 | |
| Time * [pH 7.4] | −0.004 | 0.006 | −0.685 | 0.494 | |
| Time * [pH 7.2] | 0.004 | 0.006 | 0.619 | 0.537 | |
| Time * [pH 7.0] | −0.007 | 0.006 | −1.303 | 0.194 |
| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Immobile (s) | pH 7.8 | −0.102 | 0.431 | −0.237 | 0.813 |
| pH 7.6 | 0.444 | 0.431 | 1.031 | 0.304 | |
| pH 7.4 | 1.267 | 0.431 | 2.939 | 0.004 | |
| pH 7.2 | 0.831 | 0.431 | 1.926 | 0.055 | |
| pH 7.0 | 2.137 | 0.431 | 4.956 | <0.001 | |
| Time | 0.614 | 0.092 | 6.681 | <0.001 | |
| Time * [pH 7.8] | −0.361 | 0.130 | −2.775 | 0.006 | |
| Time * [pH 7.6] | −0.448 | 0.130 | −3.445 | 0.001 | |
| Time * [pH 7.4] | −0.514 | 0.130 | −3.955 | <0.001 | |
| Time * [pH 7.2] | −0.440 | 0.130 | −3.384 | 0.001 | |
| Time * [pH 7.0] | −0.818 | 0.130 | −6.290 | <0.001 |
| Explanatory Variables | Coefficient | SE | t Value | p-Value | |
|---|---|---|---|---|---|
| Proximity (s) | pH 7.8 | −0.002 | 0.382 | −0.005 | 0.996 |
| pH 7.6 | −0.557 | 0.382 | −1.457 | 0.146 | |
| pH 7.4 | 0.297 | 0.382 | 0.776 | 0.438 | |
| pH 7.2 | 0.108 | 0.382 | 0.283 | 0.777 | |
| pH 7.0 | 0.183 | 0.382 | 0.479 | 0.632 | |
| Time | 0.462 | 0.082 | 5.666 | <0.001 | |
| Time * [pH 7.8] | −0.053 | 0.115 | −0.460 | 0.646 | |
| Time * [pH 7.6] | 0.162 | 0.115 | 1.407 | 0.161 | |
| Time * [pH 7.4] | 0.043 | 0.115 | 0.369 | 0.712 | |
| Time * [pH 7.2] | −0.213 | 0.115 | −1.853 | 0.065 | |
| Time * [pH 7.0] | −0.117 | 0.115 | −1.013 | 0.312 |
| Time | pH | ||||||
|---|---|---|---|---|---|---|---|
| 8.0 | 7.8 | 7.6 | 7.4 | 7.2 | 7.0 | ||
| Proximity (s) | 0 h | −1.22 ± 0.16 | −1.63 ± 0.17 | −1.54 ± 0.23 | −1.41 ± 0.14 | −1.12 ± 0.08 | −1.44 ± 0.11 |
| 12 h | −0.13 ± 0.04 a | 0.92 ± 0.08 ab | −0.30 ± 0.11 a | 1.82 ± 0.29 b | −0.31 ± 0.06 a | 0.64 ± 0.13 ab | |
| 24 h | 0.31 ± 0.02 a | −0.65 ± 0.07 c | −0.15 ± 0.06 b | −0.54 ± 0.06 c | −0.55 ± 0.05 c | −0.26 ± 0.10 b | |
| 48 h | 0.85 ± 0.13 ab | 0.23 ± 0.05 ab | 1.29 ± 0.07 a | 1.29 ± 0.20 a | −0.47 ± 0.10 b | 0.08 ± 0.08 b | |
| 96 h | 0.61 ± 0.11 a | 0.76 ± 0.11 ab | 0.78 ± 0.16 ab | 1.38 ± 0.21 b | 0.21 ± 0.11 a | 0.56 ± 0.19 a | |
| Variables | df | MS | F Value | p-Value | |
|---|---|---|---|---|---|
| T-SOD | pH | 5 | 256.100 | 19.179 | <0.001 |
| Time | 4 | 349.213 | 26.152 | <0.001 | |
| Time * pH | 20 | 99.461 | 7.449 | <0.001 | |
| CAT | pH | 5 | 46004.055 | 3.761 | 0.005 |
| Time | 4 | 16250.489 | 1.329 | 0.270 | |
| Time * pH | 20 | 22926.364 | 1.874 | 0.032 | |
| GSH-PX | pH | 5 | 16.125 | 5.195 | 0.001 |
| Time | 4 | 24.356 | 7.847 | <0.001 | |
| Time * pH | 20 | 10.179 | 3.279 | <0.001 | |
| AKP | pH | 5 | 221.015 | 75.589 | <0.001 |
| Time | 4 | 152.130 | 52.030 | <0.001 | |
| Time * pH | 20 | 172.233 | 58.905 | <0.001 |
| Variables | df | MS | F Value | p-Value | |
|---|---|---|---|---|---|
| GLU | pH | 5 | 233.134 | 15.848 | <0.001 |
| Time | 4 | 417.010 | 28.348 | <0.001 | |
| Time * pH | 20 | 87.558 | 5.952 | <0.001 | |
| TG | pH | 5 | 0.438 | 9.659 | <0.001 |
| Time | 4 | 0.883 | 19.480 | <0.001 | |
| Time * pH | 20 | 0.175 | 3.872 | <0.001 |
| Variables | df | MS | F Value | p-Value |
|---|---|---|---|---|
| pH | 5 | 0.283 | 2.526 | 0.039 |
| Time | 4 | 1.682 | 14.996 | <0.001 |
| Time * pH | 20 | 0.553 | 4.927 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zhang, J.; Ge, X.; Chen, S.; Ma, Z. The Effects of Short-Term Exposure to pH Reduction on the Behavioral and Physiological Parameters of Juvenile Black Rockfish (Sebastes schlegelii). Biology 2023, 12, 876. https://doi.org/10.3390/biology12060876
Li H, Zhang J, Ge X, Chen S, Ma Z. The Effects of Short-Term Exposure to pH Reduction on the Behavioral and Physiological Parameters of Juvenile Black Rockfish (Sebastes schlegelii). Biology. 2023; 12(6):876. https://doi.org/10.3390/biology12060876
Chicago/Turabian StyleLi, Haixia, Jia Zhang, Xiaoyu Ge, Songmeng Chen, and Zhen Ma. 2023. "The Effects of Short-Term Exposure to pH Reduction on the Behavioral and Physiological Parameters of Juvenile Black Rockfish (Sebastes schlegelii)" Biology 12, no. 6: 876. https://doi.org/10.3390/biology12060876
APA StyleLi, H., Zhang, J., Ge, X., Chen, S., & Ma, Z. (2023). The Effects of Short-Term Exposure to pH Reduction on the Behavioral and Physiological Parameters of Juvenile Black Rockfish (Sebastes schlegelii). Biology, 12(6), 876. https://doi.org/10.3390/biology12060876






