The Relationship between Lifespan of Marine Bivalves and Their Fatty Acids of Mitochondria Lipids
Abstract
Simple Summary
Abstract
1. Introduction
- -
- to determine the FAs of mitochondrial gill membranes, in bivalves with different lifespans belonging to the same family, and to calculate their peroxidation index;
- -
- to compare the levels of ROS generation, products of oxidative damage to lipids—malondialdehyde (MDA)—and protein carbonyls in the mitochondria of mollusk gills, in vitro, during the initiation of free-radical oxidation in the Fe-ascorbic acid model;
- -
- to investigate whether the FAs of gill mitochondrial membranes affect the degree of their oxidative damage and the MLS of species.
2. Materials and Methods
2.1. Site of Bivalves Collection and Material
2.2. Mitochondria Isolation
2.3. Biochemical Analysis
2.4. Oxidative Stress In Vitro
2.5. Determination of FAs
2.6. Statistical Analysis
3. Results
3.1. FAs in Mitochondrial Membranes of Mollusk Gill Cells
3.2. PI of Mitochondrial Membranes
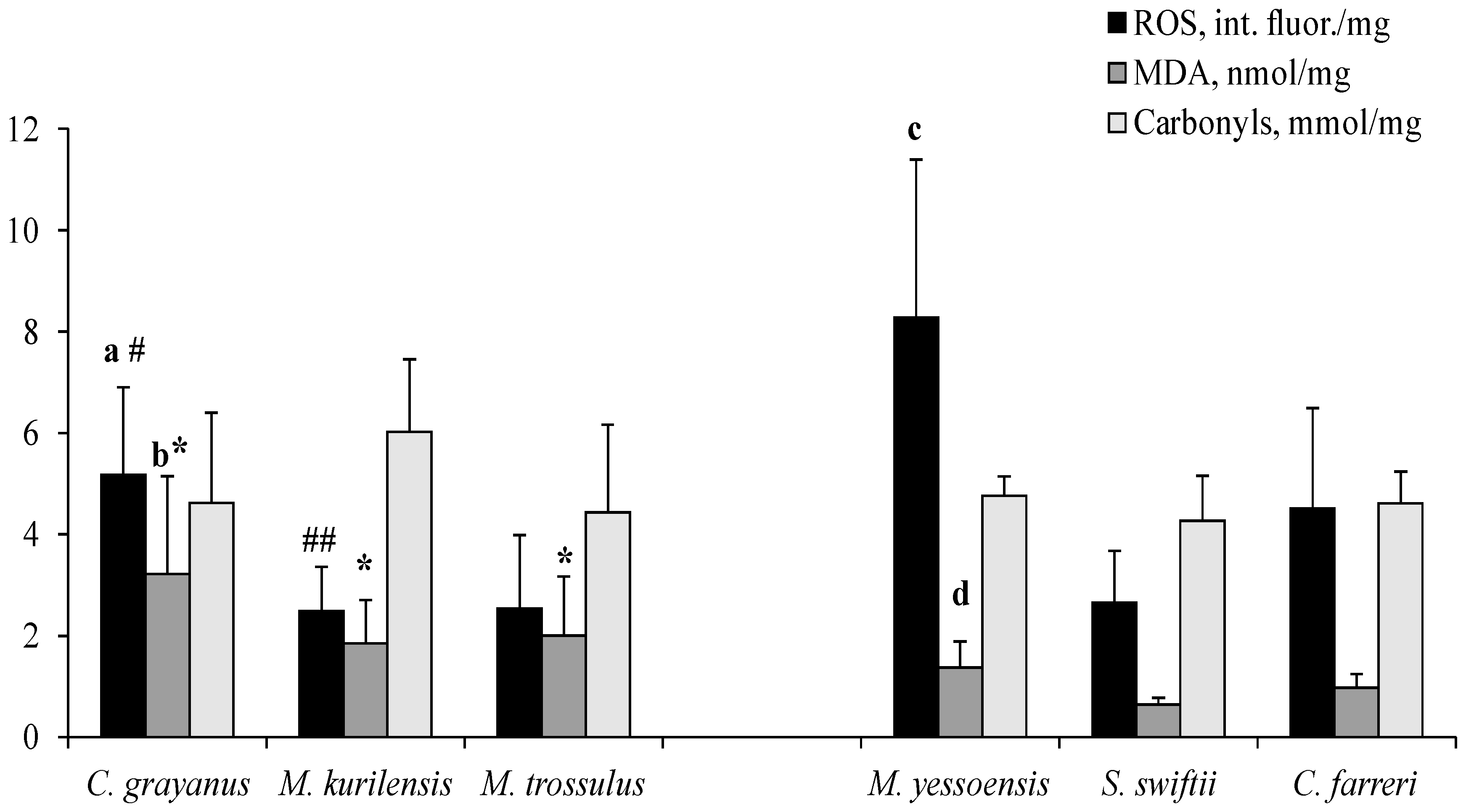
3.3. Constitutive Levels of ROS, MDA and Carbonyls
3.4. Induction of Oxidative Stress In Vitro
4. Discussion
4.1. Specific Features of FAs in Gill Mitochondria Lipids
4.2. Constitutive Levels of ROS, MDA and Carbonyls in Gill Mitochondria
4.3. Response to Induced Oxidative Stress In Vitro
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulbert, A.J.; Pamplona, R.; Buffenstein, R.; Buttemer, W.A. Life and death: Metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007, 87, 1175–1213. [Google Scholar] [CrossRef] [PubMed]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Aging: ROS or TOR. Cell Cycle 2008, 7, 3344–3354. [Google Scholar] [CrossRef]
- Pérez, V.I.; Bokov, A.; Van Remmen, H.; Mele, J.; Ran, Q.; Ikeno, Y.; Richardson, A. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta 2009, 1790, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.B.; Richardson, A.; Pérez, V.I. Update on the oxidative stress theory of aging: Does oxidative stress play a role in aging or healthy aging? Free Radic. Biol. Med. 2010, 48, 642–655. [Google Scholar] [CrossRef]
- Du, C.; Anderson, A.; Lortie, M.; Parsons, R.; Bodnar, A. Oxidative damage and cellular defense mechanisms in sea urchin models of aging. Free Radic. Biol. Med. 2013, 63, 254–263. [Google Scholar] [CrossRef]
- Buttemer, W.A.; Abele, D.; Costantini, D. From bivalves to birds: Oxidative stress and longevity. Funct. Ecol. 2010, 24, 971–983. [Google Scholar] [CrossRef]
- Pamplona, R.; Costantini, D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, 843–863. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Skrha, J. Caloric restriction and oxidative stress. In Oxidative Stress in Vertebrates and Invertebrates: Molecular Aspects of Cell Signaling, 1st ed.; Blackwell: Hoboken, NJ, USA, 2012; p. 424. [Google Scholar]
- Sohal, R.S.; Donato, H.; Biehl, E.R. Effect of age and metabolic rate on lipid peroxidation in the housefly, Musca domestica L. Mech. Ageing. Dev. 1981, 16, 159–167. [Google Scholar] [CrossRef]
- Ku, H.H.; Sohal, R.S. Comparison of mitochondrial pro-oxidant generation and anti-oxidant defenses between rat and pigeon: Possible basis of variation in longevity and metabolic potential. Mech. Ageing. Dev. 1993, 72, 67–76. [Google Scholar] [CrossRef]
- Zielinski, S.; Pörtner, H.O. Oxidative stress and antioxidative defense in cephalopods: A function of metabolic rate or age? Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 147–160. [Google Scholar] [CrossRef]
- Barja, G. Aging in vertebrates, and the effect of caloric restriction: A mitochondrial free radical production DNA damage mechanism? Biol. Rev. Camb. Philos. Soc. 2004, 79, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Couture, P.; Hulbert, A.J. Membrane fatty acid composition is related to body mass in mammals. J. Membr. Biol. 1995, 148, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Turner, N.; Ocloo, A.; Else, P.L.; Hulbert, A.J. Proton conductance and fatty acyl composition of liver mitochondria correlates with body mass in birds. Biochem. J. 2003, 376, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Pamplona, R.; Portero-Otin, M.; Riba, D. Mitochondrial membrane peroxidizability index is inversely related to maximum lifespan in mammals. J. Lipid. Res. 1998, 39, 1989–1994. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Rate of generation of oxidative stress-related damage and animal longevity. Free Radic. Biol. Med. 2002, 33, 1167–1172. [Google Scholar] [CrossRef]
- Pamplona, R.; Barja, G.; Portero-Otin, M. Membrane fatty acid unsaturation, protection against oxidative stress, maximum life span: A homeoviscous-longevity adaptation? Ann. NY Acad. Sci. 2002, 959, 475–490. [Google Scholar] [CrossRef]
- Hulbert, A.J.; Else, P.L. Membranes as possible pacemakers of metabolism. J. Theor. Biol. 1999, 199, 257–274. [Google Scholar] [CrossRef]
- Munro, D.; Blier, P.U. The extreme longevity of Arctica Islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging Cell 2012, 11, 845–855. [Google Scholar] [CrossRef]
- Pamplona, R. Low fatty acid unsaturation protects against lipid peroxidation in liver mitochondria from long-lived species: The pigeon and human case. Mech. Ageing. Dev. 1996, 86, 53–66. [Google Scholar] [CrossRef]
- Rodríguez, E.; Dégletagne, C.; Hagen, T.M.; Abele, D.; Blier, P.U. Mitochondrial Traits Previously Associated with Species Maximum Lifespan Do Not Correlate with Longevity Across Populations of the Bivalve Arctica islandica. Front. Physiol. 2019, 10, 946. [Google Scholar] [CrossRef] [PubMed]
- Speakman, J.R. Correlations between physiology and lifespan—Two widely ignored problems with comparative studies. Aging Cell 2005, 4, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Abele, D.; Brey, T.; Philipp, E. Bivalve models of aging and the determination of molluscan lifespans. Exp. Gerontol. 2009, 44, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Philipp, E.E.; Abele, D. Masters of longevity: Lessons from long-lived bivalves--a mini-review. Gerontology 2010, 56, 55–65. [Google Scholar] [CrossRef]
- Zakhartsev, M.V.; Naumenko, N.V.; Chelomin, V.P. Non-methylene-interrupted fatty acids in phospholipids of the membranes of the mussel Crenomytilus grayanus. Russ. J. Mar. Biol. 1998, 24, 183–186. [Google Scholar]
- Sadykhova, I.A. Gray mussel growth in the Peter-the-Great Bay (Sea of Japan). Biology of Gray mussel. Nauka 1983, 1, 62–68. [Google Scholar]
- Zolotarev, V.N. Sklerohronologiya morskih dvustvorchatyh mollyuskov. Kiev. Nauk. Dumka 1989, 112. [Google Scholar]
- Selin, N.I. The composition and structure of a mixed population of Crenomytilus grayanus (Dunker, 1853) and Modiolus kurilensis (Bernard, 1983) (bivalvia: Mytilidae) in Peter the Great bay, Sea of Japan. Russ. J. Mar. Bio. 2018, 449, 363–372. [Google Scholar] [CrossRef]
- Leskova, S.E. The growth of the Pacific mussel in the conditions of the Voevoda Bay (O. Russian). Proc. Dalrybvtuz. 2021, 56, 15–19. [Google Scholar]
- Motavkin, P.A. Primorskiy Grebeshok. 1986. Available online: https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/116222.pdf (accessed on 4 May 2023).
- Ponurovsky, S.K.; Silina, A.V. Determination of the age and rate of linear growth of the Swift scallop. Biol. Sea 1983, 1, 20–24. [Google Scholar]
- Karpenko, D.T. Growth rates of the Japanese scallop (Chlamys farreri) in the bays of the coastal zone of Russian Island (Peter-the-Great Bay, Sea of Japan). Complex research in the fisheries industry. In Proceedings of the V International Scientific and Technical Conference of Students, Postgraduates and Young Scientists Vladivostok, Vladivostok, Russia, 29 November 2020; pp. 14–18. [Google Scholar]
- Viarengo, B.; Burlando, M.; Cavaletto, B.; Marchi, E.; Ponzano, J.; Blasco, J. Role of metallothionein against oxidative stress in the mussel Mytilus galloprovincialis. Am. J. Physiol. 1999, 277, 1612–1619. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Markwell, M.; Haas, S.; Bieber, L.; Tolbert, N. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 1978, 82, 206–210. [Google Scholar] [CrossRef]
- Kramer, J.K.G.; Fouchard, R.C.; Jenkins, K.J. Differences in chromatographic properties of fused silica capillary columns, coated, crosslnked, bonded, or crosslincked and bonded with polyethylene glycols (Carbowax 20M) using complex fatty acid methyl ester mixtures. J. Chromatogr. Sci. 1985, 23, 54–56. [Google Scholar] [CrossRef]
- Christie, W.W. Equivalent chain length of methyl ester derivatives of fatty acids on gas chromatography. J. Cromatogr. 1988, 447, 305–314. [Google Scholar] [CrossRef]
- Carrol, K.K. Quantitative estimation of peak areasin gas-liquid chromatography. Nature 1961, 191, 377–378. [Google Scholar] [CrossRef]
- Carreau, J.P.; Dubacq, J.P. Adaptation of macro-scale method to the microscale for faty acid methyl trans esterification of biologicall lipid extracts. J. Chromatogr. Sci. 1985, 23, 54–56. [Google Scholar] [CrossRef]
- Crockett, E.L. The cold but not hard fats in ectotherms: Consequences of lipid restructuring on susceptibility of biological membranes to peroxidation, a review. J. Comp. Physiol. B 2008, 178, 795–809. [Google Scholar] [CrossRef]
- Naudí, A.; Jové, M.; Ayala, V.; Portero-Otín, M.; Barja, G.; Pamplona, R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front. Physiol. 2013, 17, 372. [Google Scholar] [CrossRef]
- Hulbert, A.J. On the importance of fatty acid composition of membranes for aging. J. Theor. Biol. 2005, 234, 277–288. [Google Scholar] [CrossRef]
- Valencak, T.G.; Ruf, T. N-3 polyunsaturated fatty acids impair lifespan but have no role for metabolism. Aging Cell 2007, 6, 15–25. [Google Scholar] [CrossRef]
- Hanuš, L.O.; Levitsky, D.O.; Shkrob, I.; Dembitsky, V.M. Plasmalogens, fatty acids and alkyl glyceryl ethers of marine and freshwater clams and mussels. Food Chem. 2009, 116, 491–498. [Google Scholar] [CrossRef]
- Yin, D. Biochemical basis of lipofuscin, ceroid, and age pigment-like fluorophores. Free Radic. Biol. Med. 1996, 21, 871–888. [Google Scholar] [CrossRef] [PubMed]
- Labinskyy, N.; Csiszar, A.; Orosz, Z. Comparison of endothelial function, O2−·and H2O2 production, and vascular oxidative stress resistance between the longest-living rodent, the naked mole rat, and mice. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Istomina, A.; Yelovskaya, O.; Chelomin, V.; Karpenko, A.; Zvyagintsev, A. Antioxidant activity of Far Eastern bivalves in their natural habitat. Mar. Environ. Res. 2021, 169, 105383. [Google Scholar] [CrossRef]
- Ungvari, Z.; Ridgway, I.; Philipp, E.E.R. Extreme Longevity Is Associated with Increased Resistance to Oxidative Stress in Arctica islandica, the Longest-Living Non-Colonial Animal. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2011, 66, 741–750. [Google Scholar] [CrossRef]
- Munro, D.; Pichaud, N.; Paquin, F.; Kemeid, V.; Blier, P.U. Low hydrogen peroxide production in mitochondria of the long-lived Arctica islandica: Underlying mechanisms for slow aging. Aging Cell 2013, 12, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, Z.; Csiszar, A.; Sosnowska, D. Testing Predictions of the Oxidative Stress Hypothesis of Aging Using a Novel Invertebrate Model of Longevity: The Giant Clam (Tridacna derasa). J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012, 68, 359–367. [Google Scholar] [CrossRef]
- Ungvari, Z.; Sosnowska, D.; Mason, J.B. Resistance to Genotoxic Stresses in Arcticai slandica, the Longest Living Noncolonial Animal: Is Extreme Longevity Associated with a Multistress Resistance Phenotype? J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, P.; Boulton, M.E.; Kirkwood, T.B. Positive correlation between mammalian life span and cellular resistance to stress. Free Radic. Biol. Med. 1999, 26, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Perez, V.I.; Buffenstein, R.; Masamsetti, V. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole rat. Proc. Natl. Acad. Sci. USA 2009, 106, 3059–3064. [Google Scholar] [CrossRef] [PubMed]


| Species | Length, mm | Approximate Age, Years | MLS, Years | Reference |
|---|---|---|---|---|
| Mytilidae | ||||
| Crenomytilus grayanus | 116.9 ± 5.0 | 24 | 150 | [28,29] |
| Modiolus kurilensis | 111.7 ± 4.9 | 20 | 61 | [29,30] |
| Mytilus trossulus | 42.2 ± 3.9 | 4 | 6 | [31] |
| Pectinidae | ||||
| Mizuhopecten yessoensis | 132.0 ± 11.2 | 5 | 22 | [32] |
| Swiftopecten swiftii | 83.9 ± 5.2 | 4 | 15 | [29,33] |
| Chlamys farreri | 92.3 ± 5.3 | 4 | 9 | [34] |
| Fatty Acid | Mytilidae | Pectinidae | ||||
|---|---|---|---|---|---|---|
| C. grayanus | M. kurilensis | M. trossulus | M. yessoensis | S. swiftii | C. farreri | |
| 12:0 | 1.0 ± 0.0 | 0.7 ± 0.2 | 1.1 ± 0.1 | 0.7 ± 0.0 | 0.7 ± 0.2 | 1.0 ± 0.7 |
| 14:0 ai | 0.2 ± 0.0 | 0.7 ± 0.1 | 0.8 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.1 | 0.8 ± 0.2 |
| 14:0 | 0.6 ± 0.0 | 0.3 ± 0.1 | 0.7 ± 0.0 | 0.1 ± 0.0 | 0.4 ± 0.1 | 0.1 ± 0.0 |
| 15:1 n−7 | 1.0 ± 0.0 | 1.8 ± 0.0 | 1.6 ± 0.3 | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.6 ± 0.0 |
| 16:0 | 17.9 ± 0.9 | 15.9 ± 0.3 | 19.0 ± 0.1 | 12.5 ± 0.6 | 12.7 ± 0.8 | 13.7 ± 0.7 |
| 16:1 n−9 | 2.1 ± 0.1 | 2.0 ± 0.5 | 2.7 ± 0.3 | 1.3 ± 0.0 | - | 2.2 ± 0.2 |
| 16:1 n−7 | 2.3 ± 0.1 | 2.3 ± 0.1 | 1.9 ± 0.0 | 1.4 ± 0.0 | 2.3 ± 0.5 | 1.2 ± 0.0 |
| 17:0 i | 0.6 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.3 ± 0.0 | 0.9 ± 0.1 | 0.1 ± 0.0 |
| 17:0 ai | 1.1 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.0 | 1.2 ± 0.0 | 0.3 ± 0.1 | 2.3 ± 0.0 |
| 17:0 | 0.8 ± 0.0 | 1.9 ± 0.1 | 1.7 ± 0.0 | 1.0 ± 0.0 | 0.9 ± 0.2 | - |
| 18:0 i | 2.8 ± 0.1 | 2.5 ± 0.2 | 4.0 ± 0.1 | 0.9 ± 0.0 | 1.2 ± 0.3 | 1.3 ± 0.1 |
| 18:0 | 9.6 ± 0.5 | 10.9 ± 0.3 | 19.8 ± 3.1 | 4.8 ± 0.2 | 3.3 ± 0.6 | 9.7 ± 0.1 |
| 18:1 n−9 | 0.7 ± 0.0 | - | - | - | 0.1 ± 0.1 | 1.0 ± 0.0 |
| 18:1 n−7 | 4.3 ± 0.2 | 5.1 ± 0.3 | 2.3 ± 1.6 | 4.3 ± 0.2 | 4.5 ± 0.4 | 5.4 ± 0.4 |
| 18:2 n−6 | 1.0 ± 0.0 | 2.4 ± 0.5 | 1.6 ± 0.3 | 2.0 ± 0.1 | 2.2 ± 0.2 | 2.5 ± 0.1 |
| 18:2 n−4 | 0.4 ± 0.0 | 1.5 ± 1.5 | 0.5 ± 0.2 | 0.6 ± 0.0 | 0.1 ± 0.0 | 0.3 ± 0.1 |
| 18:3 n−6 | - | - | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | - |
| 18:3 n−3 | 0.8 ± 0.0 | 1.2 ± 0.0 | 0.3 ± 0.0 | 0.3 ± 0.0 | 0.2 ± 0.0 | 0.1 ± 0.0 |
| 20:0-i | 1.8 ± 0.1 | 1.7 ± 0.1 | 0.4 ± 0.1 | - | - | 0.8 ± 0.1 |
| 18:4 n−3 | 0.2 ± 0.0 | - | 0.4 ± 0.0 | - | 0.1 ± 0.0 | - |
| 20:1 n−13 | 2.2 ± 0.1 | 0.6 ± 0.1 | 0.8 ± 0.2 | 2.0 ± 0.1 | 1.8 ± 0.0 | 2.9 ± 0.1 |
| 20:1 n−9 | 5.8 ± 0.3 | 3.4 ± 0.1 | 1.9 ± 0.3 | 5.5 ± 0.3 | 4.2 ± 0.5 | 4.2 ± 0.2 |
| 20:1 n−7 | 2.2 ± 0.1 | 3.9 ± 0.0 | 1.3 ± 0.2 | 1.0 ± 0.1 | 0.6 ± 0.3 | 2.5± 0.1 |
| 20:2 (5,11) | 3.9 ± 0.2 | 1.9 ± 0.0 | 1.8 ± 0.1 | 5.4 ± 0.3 | 5.5 ± 0.6 | 0.9 ± 0.0 |
| 20:2 (5,13) | 1.6 ± 0.1 | 0.6 ± 0.1 | 1.1 ± 0.2 | 1.7 ± 0.1 | 1.0 ± 0.1 | 0.7 ± 0.1 |
| 20:4 n−6 | 5.5 ± 0.3 | 5.7 ± 0.5 | 3.5 ± 0.3 | 6.7 ± 0.3 | 5.8 ± 1.2 | 3.9 ± 0.2 |
| 20:5 n−3 | 4.2 ± 0.2 | 6.2 ± 0.5 | 6.0 ± 0.4 | 3.9 ± 0.2 | 4.7 ± 0.6 | 9.7 ± 0.3 |
| 22:2 | 8.2 ± 0.4 | 5.2 ± 0.1 | 4.1 ± 0.2 | 11.7 ± 0.6 | 9.0 ± 1.2 | 1.0 ± 0.0 |
| 22:6 n−3 | 8.6 ± 0.4 | 9.7 ± 0.3 | 9.3 ± 0.4 | 22.1 ± 1.1 | 25.8 ± 1.4 | 21.7 ± 1.3 |
| Total | 91.1 ± 4.6 | 90.4 ± 0.9 | 87.4 ± 4.0 | 93.6 ± 4.7 | 89.5 ± 7.2 | 92.0 ± 1.3 |
| SFAs | 36.3 ± 1.8 | 37.0 ± 0.0 | 47.8 ± 0.5 | 22.36 ± 1.1 | 21.0 ± 1.9 | 30.3 ± 1.4 |
| MUFAs | 20.5 ± 1.0 | 19.0 ± 1.0 | 11.4 ± 3.1 | 16.12 ± 0.8 | 13.4 ± 0.4 | 20.4 ± 0.6 |
| PUFAs | 34.3 ± 1.7 | 34.4 ± 0.1 | 28.2 ± 1.4 | 55.13 ± 2.8 | 55.0 ± 4.9 | 43.6 ± 5.7 |
| ∑n−3 | 13.6 ± 0.7 | 17.1 ± 0.8 | 15.4 ± 1.0 | 26.55 ± 1.3 | 30.8 ± 1.9 | 31.6 ± 1.6 |
| ∑n−6 | 6.5 ± 0.3 | 8.1 ± 1.0 | 5.2 ± 0.4 | 9.03 ± 0.5 | 8.3 ± 1.5 | 6.4 ± 0.4 |
| n−3/n−6 | 2.10 | 2.11 | 2.98 | 2.94 | 3.69 | 4.92 |
| SFAs/ PUFAs | 1.05 | 1.08 | 1.70 | 0.41 | 0.38 | 0.70 |
| ∑NMI FAs | 13.7 ± 0.01 | 7.75 ± 0.24 | 6.93 ± 0.15 | 18.9 ± 0.1 | 15.68 ± 1.63 | 2.76 ± 0.22 |
| ∑2n, 3n/ ∑4n, 6n | 1.11 | 0.84 | 0.73 | 0.77 | 0.58 | 0.22 |
| PI | 124.5 ± 6.2 | 146.7 ± 6.6 | 129.7 ± 7.9 | 238.2 ± 11.2 | 267.3 ± 19.5 | 252.7 ± 13.6 |
| MLS | 150 | 61 | 6 | 22 | 15 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Istomina, A.A.; Zhukovskaya, A.F.; Mazeika, A.N.; Barsova, E.A.; Chelomin, V.P.; Mazur, M.A.; Elovskaya, O.A.; Mazur, A.A.; Dovzhenko, N.V.; Fedorets, Y.V.; et al. The Relationship between Lifespan of Marine Bivalves and Their Fatty Acids of Mitochondria Lipids. Biology 2023, 12, 837. https://doi.org/10.3390/biology12060837
Istomina AA, Zhukovskaya AF, Mazeika AN, Barsova EA, Chelomin VP, Mazur MA, Elovskaya OA, Mazur AA, Dovzhenko NV, Fedorets YV, et al. The Relationship between Lifespan of Marine Bivalves and Their Fatty Acids of Mitochondria Lipids. Biology. 2023; 12(6):837. https://doi.org/10.3390/biology12060837
Chicago/Turabian StyleIstomina, Aleksandra Anatolyevna, Avianna Fayazovna Zhukovskaya, Andrey Nikolaevich Mazeika, Ekaterina Andreevna Barsova, Victor Pavlovich Chelomin, Marina Alexandrovna Mazur, Olesya Alexandrovna Elovskaya, Andrey Alexandrovich Mazur, Nadezda Vladimirovna Dovzhenko, Yuliya Vladimirovna Fedorets, and et al. 2023. "The Relationship between Lifespan of Marine Bivalves and Their Fatty Acids of Mitochondria Lipids" Biology 12, no. 6: 837. https://doi.org/10.3390/biology12060837
APA StyleIstomina, A. A., Zhukovskaya, A. F., Mazeika, A. N., Barsova, E. A., Chelomin, V. P., Mazur, M. A., Elovskaya, O. A., Mazur, A. A., Dovzhenko, N. V., Fedorets, Y. V., & Karpenko, A. A. (2023). The Relationship between Lifespan of Marine Bivalves and Their Fatty Acids of Mitochondria Lipids. Biology, 12(6), 837. https://doi.org/10.3390/biology12060837






