Substrate Heterogeneity as a Trigger for Species Diversity in Marine Benthic Assemblages
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
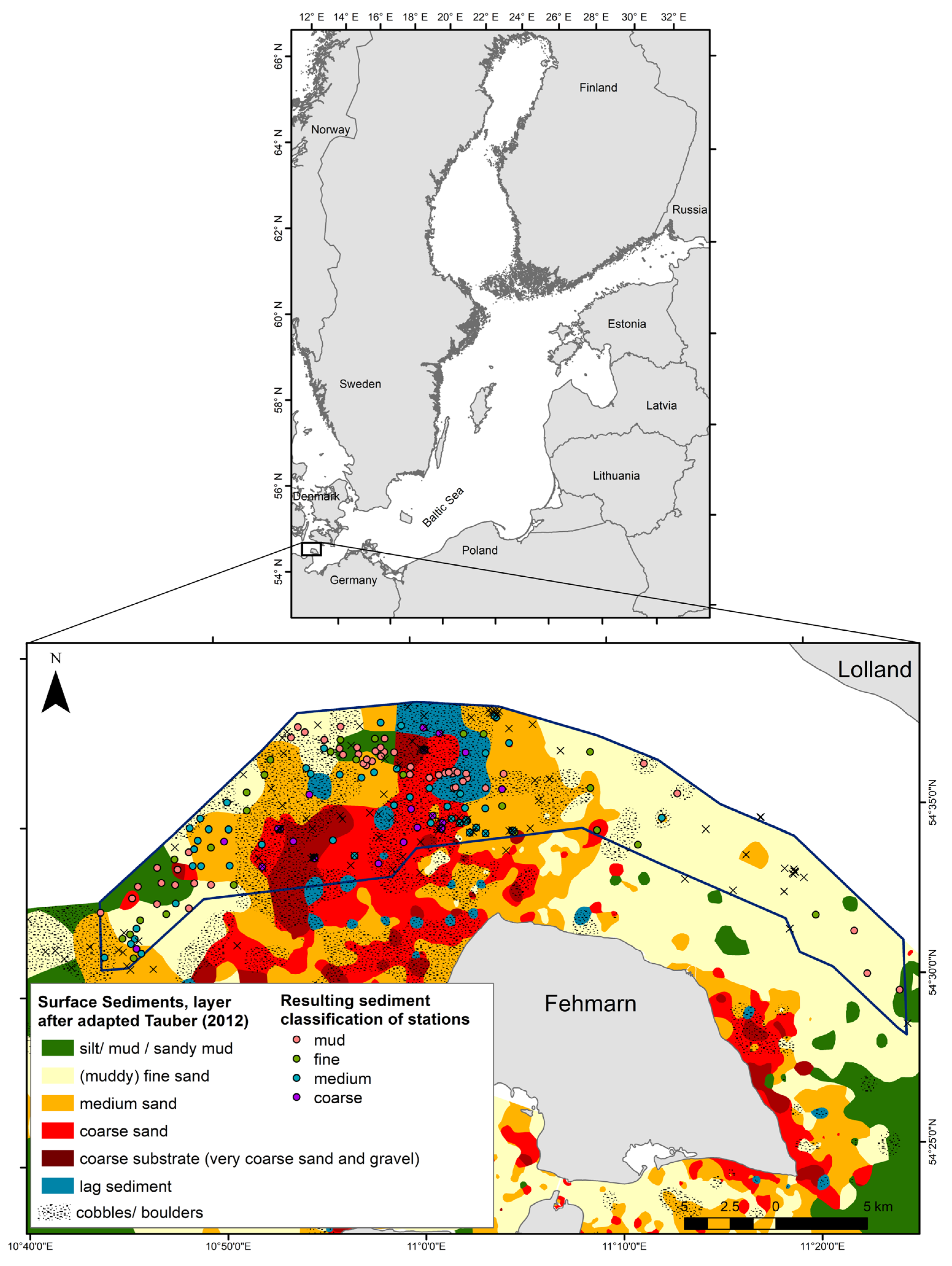
2.2. Infauna and Sediment Data
- No heterogeneity (none): Other than the dominant (very dense) substrate class, at most one additional feature occurs occasionally;
- Low heterogeneity: Other than the dominant (very dense) substrate class, at most three additional features occur occasionally, or at most two additional features occur occasionally or frequently;
- Medium heterogeneity: Other than the dominant (very dense) substrate class, at most five additional features occur occasionally, or at most three additional features occur frequently of which one feature might occur densely;
- High heterogeneity: Any other combination, including at least four feature classes. Often, no single feature exceeds 50% coverage.
2.3. Analyses and Statistics
3. Results
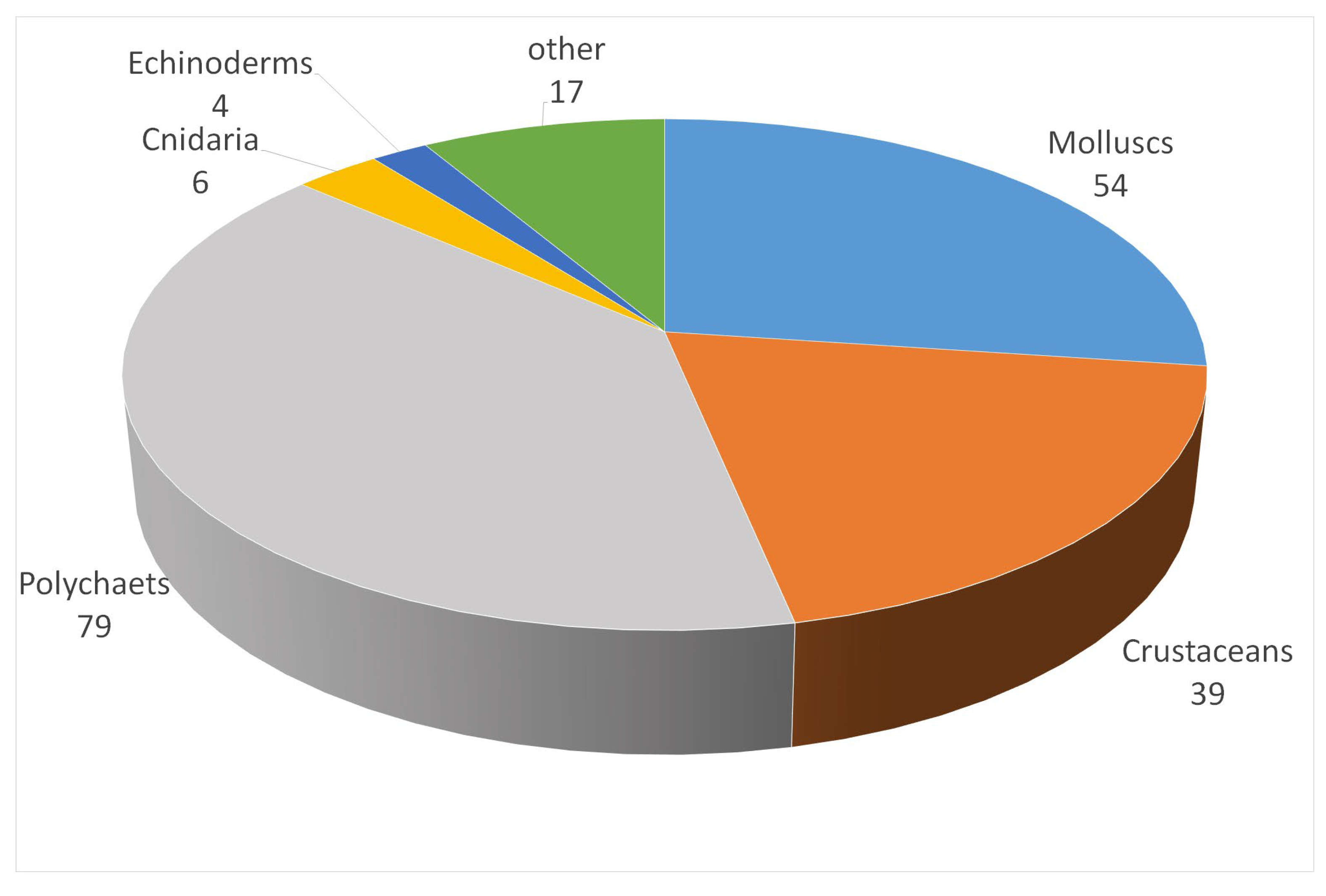
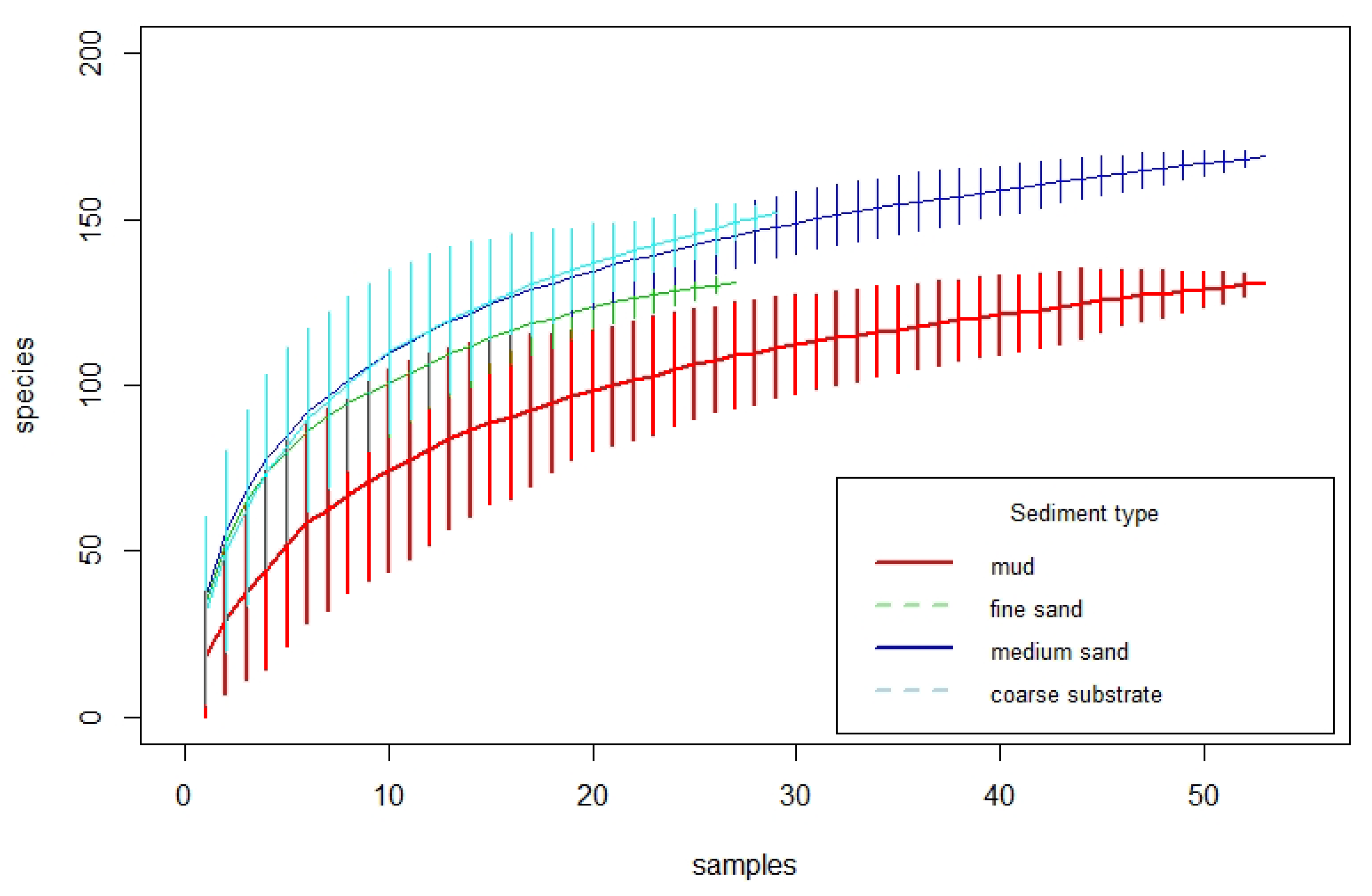
3.1. Overall Species Inventory
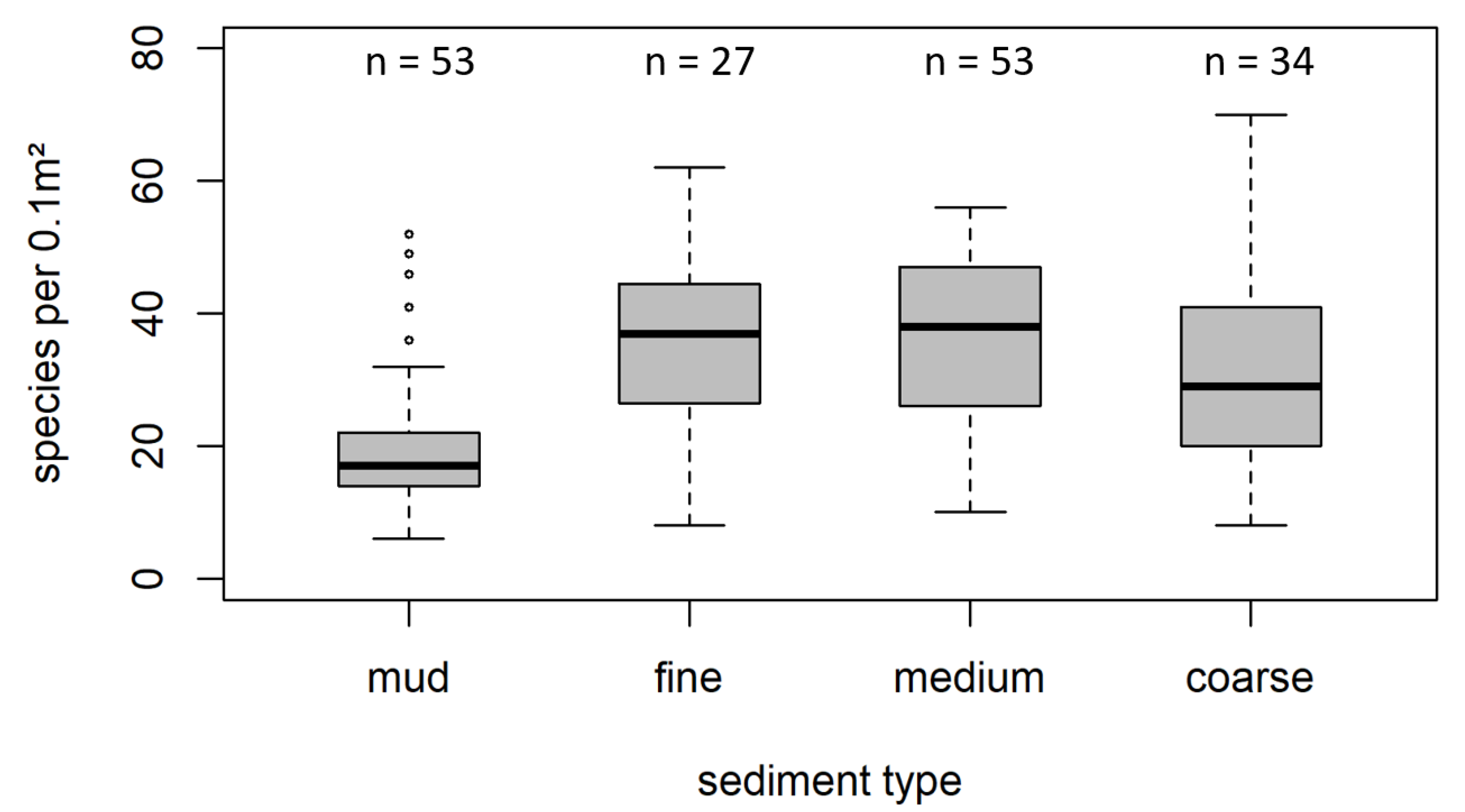
3.2. Species Richness in Different Sediment Types
3.3. Testing the Relevance of Other Environmental Parameters
3.4. Occurrence of Rare Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cochrane, S.K.J.; Andersen, J.H.; Berg, T.; Blanchet, H.; Borja, A.; Carstensen, J.; Elliott, M.; Hummel, H.; Niquil, N.; Renaud, P.E. What Is Marine Biodiversity? Towards Common Concepts and Their Implications for Assessing Biodiversity Status. Front. Mar. Sci. 2016, 3, 248. [Google Scholar] [CrossRef]
- Gogina, M.; Nygård, H.; Blomqvist, M.; Daunys, D.; Josefson, A.B.; Kotta, J.; Maximov, A.; Warzocha, J.; Yermakov, V.; Gräwe, U.; et al. The Baltic Sea Scale Inventory of Benthic Faunal Communities. ICES J. Mar. Sci. 2016, 73, 1196–1213. [Google Scholar] [CrossRef]
- Zettler, M.L.; Friedland, R.; Gogina, M.; Darr, A. Variation in Benthic Long-Term Data of Transitional Waters: Is Interpretation More than Speculation? PLoS ONE 2017, 12, e0175746. [Google Scholar] [CrossRef]
- Glockzin, M.; Zettler, M.L. Spatial Macrozoobenthic Distribution Patterns in Relation to Major Environmental Factors—A Case Study from the Pomeranian Bay (Southern Baltic Sea). J. Sea Res. 2008, 59, 144–161. [Google Scholar] [CrossRef]
- Martins, M.G.; Faria, J.; Rubal, M.; Neto, A.I. Linkages between Rocky Reefs and Soft-Bottom Habitats: Effects of Predation and Granulometry on Sandy Macrofaunal Assemblages. J. Sea Res. 2013, 81, 1–9. [Google Scholar] [CrossRef]
- Buhl-Mortensen, L.; Vanreusel, A.; Gooday, A.J.; Levin, L.A.; Priede, I.G.; Buhl-Mortensen, P.; Gheerardyn, H.; King, N.J.; Raes, M. Biological Structures as a Source of Habitat Heterogeneity and Biodiversity on the Deep Ocean Margins. Mar. Ecol. 2010, 31, 21–50. [Google Scholar] [CrossRef]
- Kovalenko, K.E.; Thomaz, S.M.; Warfe, D.M. Habitat Complexity: Approaches and Future Directions. Hydrobiologia 2012, 685, 1–17. [Google Scholar] [CrossRef]
- Franz, M.; Ann Von Rönn, G.; Barboza, F.R.; Karez, R.; Reimers, H.-C.; Schwarzer, K.; Wahl, M. How Do Geological Structure and Biological Diversity Relate? Benthic Communities in Boulder Fields of the Southwestern Baltic Sea. Estuaries Coasts 2021, 44, 1994–2009. [Google Scholar] [CrossRef]
- Burnett, M.R.; August, P.V.; Brown, J.H.; Killingbeck, K.T. The Influence of Geomorphological Heterogeneity on Biodiversity I. A Patch-Scale Perspective. Conserv. Biol. 1998, 12, 363–370. [Google Scholar] [CrossRef]
- Burlakova, L.E.; Karatayev, A.Y.; Karatayev, V.A.; May, M.E.; Bennett, D.L.; Cook, M.J. Endemic Species: Contribution to Community Uniqueness, Effect of Habitat Alteration, and Conservation Priorities. Biol. Conserv. 2011, 144, 155–165. [Google Scholar] [CrossRef]
- Alsterberg, C.; Roger, F.; Sundbäck, K.; Juhanson, J.; Hulth, S.; Hallin, S.; Gamfeldt, L. Habitat Diversity and Ecosystem Multifunctionality—The Importance of Direct and Indirect Effects. Sci. Adv. 2017, 3, e1601475. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Flynn, D.F.B.; Prager, C.M.; Hart, G.M.; Devan, C.M.; Ahrestani, F.S.; Palmer, M.I.; Bunker, D.E.; Knops, J.M.H.; Jouseau, C.F.; et al. The Importance of Rare Species: A Trait-Based Assessment of Rare Species Contributions to Functional Diversity and Possible Ecosystem Function in Tall-Grass Prairies. Ecol. Evol. 2014, 4, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Bolnick, D.I.; Amarasekare, P.; Araújo, M.S.; Bürger, R.; Levine, J.M.; Novak, M.; Rudolf, V.H.W.; Schreiber, S.J.; Urban, M.C.; Vasseur, D.A. Why Intraspecific Trait Variation Matters in Community Ecology. Trends Ecol. Evol. 2011, 26, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, D.; Pusceddu, A.; Trincardi, F.; Danovaro, R. Seafloor Heterogeneity Influences the Biodiversity-Ecosystem Functioning Relationships in the Deep Sea. Sci. Rep. 2016, 6, 26352. [Google Scholar] [CrossRef] [PubMed]
- Mudroch, A.; McKnight, S.D. Handbook of Techniques for Aquatic Sediments Sampling, 2nd ed.; Taylor & Francis: London, UK, 2019; ISBN 978-0-367-44940-7. [Google Scholar]
- Rumohr, H. Soft-Bottom Macrofauna: Collection, Treatment, and Quality Assurance of Samples; International Council for the Exploration of the Sea (ICES): Copenhagen, Denmark, 2009. [Google Scholar]
- Beisiegel, K.; Darr, A.; Gogina, M.; Zettler, M.L. Benefits and Shortcomings of Non-Destructive Benthic Imagery for Monitoring Hard-Bottom Habitats. Mar. Pollut. Bull. 2017, 121, 5–15. [Google Scholar] [CrossRef]
- Ojaveer, H.; Jaanus, A.; Mackenzie, B.R.; Martin, G.; Olenin, S.; Radziejewska, T.; Telesh, I.; Zettler, M.L.; Zaiko, A. Status of Biodiversity in the Baltic Sea. PLoS ONE 2010, 5, e12467. [Google Scholar] [CrossRef]
- Matthäus, W. The History of Investigation of Salt Water Inflows into the Baltic Sea—From the Early Beginning to Recent Results. In Meereswissenschaftliche Berichte No 65, Marine Science Reports; Leibniz Institute for Baltic Sea Research Warnemünde: Rostock, Germany, 2006. [Google Scholar] [CrossRef]
- Lass, H.U.; Schwabe, R.; Matthäus, W.; Francke, E. On the Dynamics of Water Exchange between Baltic and North Sea. Beitr. Meereskd. 1987, 56, 27–49. [Google Scholar]
- Darr, A.; Heinicke, K.; Meier, F.; Papenmeier, S.; Richter, P.; Schwarzer, K.; Valerius, J.; Boedeker, D. Die Biotope Des Meeresbodens Im Naturschutzgebiet Fehmarnbelt. BfN-Schriften 2022, 636. [Google Scholar] [CrossRef]
- Schwarzer, K.; Diesing, M. Erforschung der FFH-Lebensraumtypen Sandbank und Riff in der AWZ der Deutschen Nord-und Ostsee; Report of the Institute for Geosciences at the University of Kiel on behalf of BfN; University of Kiel: Kiel, Germany, 2006; pp. 6–68. [Google Scholar]
- Feldens, P.; Diesing, M.; Schwarzer, K.; Heinrich, C.; Schlenz, B. Occurrence of Flow Parallel and Flow Transverse Bedforms in Fehmarn Belt (SW Baltic Sea) Related to the Local Palaeomorphology. Geomorphology 2015, 231, 53–62. [Google Scholar] [CrossRef]
- Tauber, F. Meeresbodenrelief in der Deutschen Ostsee/Seabed Relief in the German Baltic Sea; Bundesamt für Seeschifffahrt und Hydrographie: Hamburg, Germany, 2012. [Google Scholar]
- Bobertz, B.; Harff, J.; Wolff, J.-O. Sediment Facies and Hydrodynamic Setting: A Study in the South Western Baltic Sea. Ocean Dyn. 2004, 54, 39–48. [Google Scholar] [CrossRef]
- de Juan, S.; Ospina-Alvarez, A.; Hinz, H.; Moranta, J.; Barberá, C. The Continental Shelf Seascape: A Network of Species and Habitats. Biodivers. Conserv. 2023, 32, 1271–1290. [Google Scholar] [CrossRef]
- Pitacco, V.; Mistri, M.; Granata, T.; Moruzzi, L.; Meloni, M.L.; Massara, F.; Sfriso, A.; Sfriso, A.A.; Munari, C. Habitat Heterogeneity: A Confounding Factor for the Effect of Pollutants on Macrobenthic Community in Coastal Waters. Mar. Environ. Res. 2021, 172, 105499. [Google Scholar] [CrossRef] [PubMed]
- Sunday, J.M.; Fabricius, K.E.; Kroeker, K.J.; Anderson, K.M.; Brown, N.E.; Barry, J.P.; Connell, S.D.; Dupont, S.; Gaylord, B.; Hall-Spencer, J.M.; et al. Ocean Acidification Can Mediate Biodiversity Shifts by Changing Biogenic Habitat. Nat. Clim. Chang. 2017, 7, 81–85. [Google Scholar] [CrossRef]
- Almond, P.M.; Linse, K.; Dreutter, S.; Grant, S.M.; Griffiths, H.J.; Whittle, R.J.; Mackenzie, M.; Reid, W.D.K. In-Situ Image Analysis of Habitat Heterogeneity and Benthic Biodiversity in the Prince Gustav Channel, Eastern Antarctic Peninsula. Front. Mar. Sci. 2021, 8, 614496. [Google Scholar] [CrossRef]
- Sanvicente-Añorve, L.; Leprêtre, A.; Davoult, D. Diversity of Benthic Macrofauna in the Eastern English Channel: Comparison among and within Communities. Biodivers. Conserv. 2002, 11, 265–282. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing 2019. Available online: https://www.r-project.org/ (accessed on 29 November 2019).
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, H.; Wagner, H. Vegan: Community Ecology Package (Version 2.3-3) 2016. Available online: https://CRAN.R-project.org/package=vegan (accessed on 18 November 2022).
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Proceedings of the 2nd International Symposium on Information Theory, Tsahkadsor, Armenia, 2–8 September 1971; Petrov, B.N., Csák, F., Eds.; Akadémiai Kiadó: Budapest, Hungary, 1973; pp. 267–281. [Google Scholar]
- Hartig, F.; Lohse, L. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models (R Package Version 0.4.6). 2022. Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 26 May 2023).
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- BSH Bathymetry-Grid Coverage German EEZ Baltic Sea 2018. Available online: https://www.geoseaportal.de (accessed on 26 May 2023).
- Walbridge, S.; Slocum, N.; Pobuda, M.; Esri, D.J.W. Unified Geomorphological Analysis Workflows with Benthic Terrain Modeler. Geosciences 2018, 8, 94. [Google Scholar] [CrossRef]
- Fox, J.; Monette, G. Generalized Collinearity Diagnostics. J. Am. Stat. Assoc. 1992, 87, 178–183. [Google Scholar] [CrossRef]
- Lenth, R. Emmeans: Estimated Marginal Means, Aka Least-Squares Means (R Package Version 1.7.2) 2022. Available online: https://CRAN.R-project.org/package=emmeans (accessed on 26 May 2023).
- Feldens, P.; Schwarzer, K.; Hübscher, C.; Diesing, M. Genesis and Sediment Dynamics of a Subaqueous Dune Field in Fehmarn Belt (South-Western Baltic Sea). Marbg. Geogr. Schr. 2009, 145, 80–97. [Google Scholar]
- Gogina, M.; Glockzin, M.; Zettler, M.L. Distribution of Benthic Macrofaunal Communities in the Western Baltic Sea with Regard to Near-Bottom Environmental Parameters. 1. Causal Analysis. J. Mar. Syst. 2010, 79, 112–123. [Google Scholar] [CrossRef]
- Darr, A.; Gogina, M.; Zettler, M.L. Detecting Hot-Spots of Bivalve Biomass in the South-Western Baltic Sea. J. Mar. Syst. 2014, 134, 69–80. [Google Scholar] [CrossRef]
- Beisiegel, K.; Darr, A.; Zettler, M.L.; Friedland, R.; Gräwe, U.; Gogina, M. Understanding the Spatial Distribution of Subtidal Reef Assemblages in the Southern Baltic Sea Using Towed Camera Platform Imagery. Estuar. Coast. Shelf Sci. 2018, 207, 82–92. [Google Scholar] [CrossRef]
- Sciberras, M.; Parker, R.; Powell, C.; Robertson, C.; Kröger, S.; Bolam, S.; Geert Hiddink, J. Impacts of Bottom Fishing on the Sediment Infaunal Community and Biogeochemistry of Cohesive and Non-Cohesive Sediments. Limnol. Oceanogr. 2016, 61, 2076–2089. [Google Scholar] [CrossRef]
- Transparency for a Sustainable Ocean. Available online: https://globalfishingwatch.org/ (accessed on 15 March 2023).
- Schönke, M.; Clemens, D.; Feldens, P. Quantifying the Physical Impact of Bottom Trawling Based on High-Resolution Bathymetric Data. Remote Sens. 2022, 14, 2782. [Google Scholar] [CrossRef]
- St. Pierre, J.I.; Kovalenko, K.E. Effect of Habitat Complexity Attributes on Species Richness. Ecosphere 2014, 5. [Google Scholar] [CrossRef]
- Michaelis, R.; Hass, H.C.; Mielck, F.; Papenmeier, S.; Sander, L.; Gutow, L.; Wiltshire, K.H. Epibenthic Assemblages of Hard-Substrate Habitats in the German Bight (South-Eastern North Sea) Described Using Drift Videos. Cont. Shelf Res. 2019, 175, 30–41. [Google Scholar] [CrossRef]
- Hewitt, J.E.; Thrush, S.F.; Halliday, J.; Duffy, C. The Importance of Small-Scale Habitat Structure for Maintaining Beta Diversity. Ecology 2005, 86, 1619–1626. [Google Scholar] [CrossRef]
- Matias, M.G.; Underwood, A.J.; Hochuli, D.F.; Coleman, R.A. Independent Effects of Patch Size and Structural Complexity on Diversity of Benthic Macroinvertebrates. Ecology 2010, 91, 1908–1915. [Google Scholar] [CrossRef] [PubMed]
- Menge, B.A.; Lubchenco, J.; Ashkenas, L.R. Diversity, Heterogeneity and Consumer Pressure in a Tropical Rocky Intertidal Community. Oecologia 1985, 65, 394–405. [Google Scholar] [CrossRef]
- Tokeshi, M.; Arakaki, S. Habitat Complexity in Aquatic Systems: Fractals and Beyond. Hydrobiologia 2012, 685, 27–47. [Google Scholar] [CrossRef]


| Substrate | Number of Features Occurring | |||
|---|---|---|---|---|
| Heterogeneity Class | Occasionally | Frequently | Densely | Very Densely |
| (≤1%) | (>1–10%) | (>10–50%) | (>50%) | |
| none | ≤1 | 0 | 0 | 1 |
| low | ≤3 | 0 | 0 | 1 |
| or | ≤2 | 0 | 1 | |
| medium | ≤ 5 | 0 | 0 | 1 |
| or | ≤2 | 1 | 1 | |
| or | ≤3 | 1 | ||
| high | >5 | 0 | 0 | 1 |
| or | >3 | ≤1 | ||
| Model (AIC: 1246) | |||||
|---|---|---|---|---|---|
| Estimate | Std. Error | t-Value | p | Significance | |
| (Intercept) | 307.1 | 93.2 | 3.30 | 0.001 | *** |
| Factor (GeoClass)—low | −0.37 | 0.09 | −4.23 | 0.000 | *** |
| Factor (GeoClass)—medium | −0.05 | 0.09 | −0.60 | 0.550 | |
| Factor (GeoClass)—none | −0.62 | 0.15 | −4.04 | 0.000 | *** |
| Depth | −0.03 | 0.01 | −2.69 | 0.007 | ** |
| Salinity | 0.04 | 0.01 | 2.83 | 0.005 | ** |
| Year | −0.15 | 0.05 | −3.26 | 0.001 | ** |
| Factor (sediment)—fine | −144.7 | 174.5 | −0.83 | 0.407 | |
| Factor (sediment)—medium | −325.2 | 120.0 | −2.71 | 0.007 | ** |
| Factor (sediment)—mud | −374.7 | 139.6 | −2.68 | 0.007 | ** |
| Factor (Season)—summer | 0.29 | 0.17 | 1.74 | 0.082 | . |
| Year: (sediment)—fine | 0.07 | 0.09 | 0.83 | 0.405 | |
| Year: (sediment)—medium | 0.16 | 0.06 | 2.71 | 0.007 | ** |
| Year: (sediment)—mud | 0.19 | 0.07 | 2.69 | 0.007 | ** |
| Summer: (sediment)—fine | −0.26 | 0.28 | −0.92 | 0.356 | |
| Summer: (sediment)—medium | −0.15 | 0.22 | −0.67 | 0.502 | |
| Summer: (sediment)—mud | −0.55 | 0.23 | −2.44 | 0.015 | * |
| Substrate Heterogeneity | Mud | Fine Sand | Medium Sand | Coarse Substrate | Overall |
|---|---|---|---|---|---|
| None | 0.23 (22) | NA | 0 (1) | NA | 0.22 (23) |
| Low | 0.11 (18) | 0 (7) | 0.42 (12) | 0.83 (6) | 0.28 (43) |
| Medium | 0.25 (8) | 0.21 (14) | 0.72 (25) | 0.50 (2) | 0.49 (49) |
| High | 0.60 (5) | 0 (6) | 0.53 (15) | 1.33 (21) | 0.83 (47) |
| Overall | 0.23 (53) | 0.11 (27) | 0.58 (53) | 1.17 (29) | 0.49 (162) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romoth, K.; Darr, A.; Papenmeier, S.; Zettler, M.L.; Gogina, M. Substrate Heterogeneity as a Trigger for Species Diversity in Marine Benthic Assemblages. Biology 2023, 12, 825. https://doi.org/10.3390/biology12060825
Romoth K, Darr A, Papenmeier S, Zettler ML, Gogina M. Substrate Heterogeneity as a Trigger for Species Diversity in Marine Benthic Assemblages. Biology. 2023; 12(6):825. https://doi.org/10.3390/biology12060825
Chicago/Turabian StyleRomoth, Katharina, Alexander Darr, Svenja Papenmeier, Michael L. Zettler, and Mayya Gogina. 2023. "Substrate Heterogeneity as a Trigger for Species Diversity in Marine Benthic Assemblages" Biology 12, no. 6: 825. https://doi.org/10.3390/biology12060825
APA StyleRomoth, K., Darr, A., Papenmeier, S., Zettler, M. L., & Gogina, M. (2023). Substrate Heterogeneity as a Trigger for Species Diversity in Marine Benthic Assemblages. Biology, 12(6), 825. https://doi.org/10.3390/biology12060825







