Mitochondria Quality Control and Male Fertility
Abstract
Simple Summary
Abstract
1. Introduction
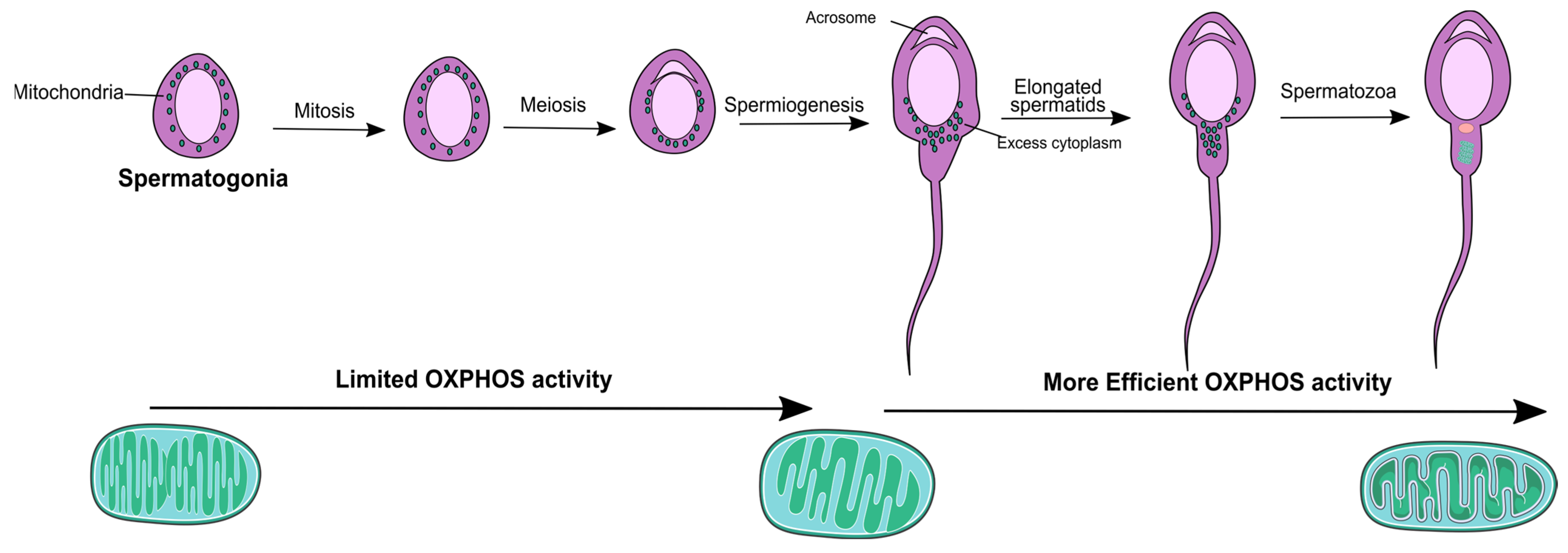
2. Mitochondria Physiology throughout the Male Reproductive Tract
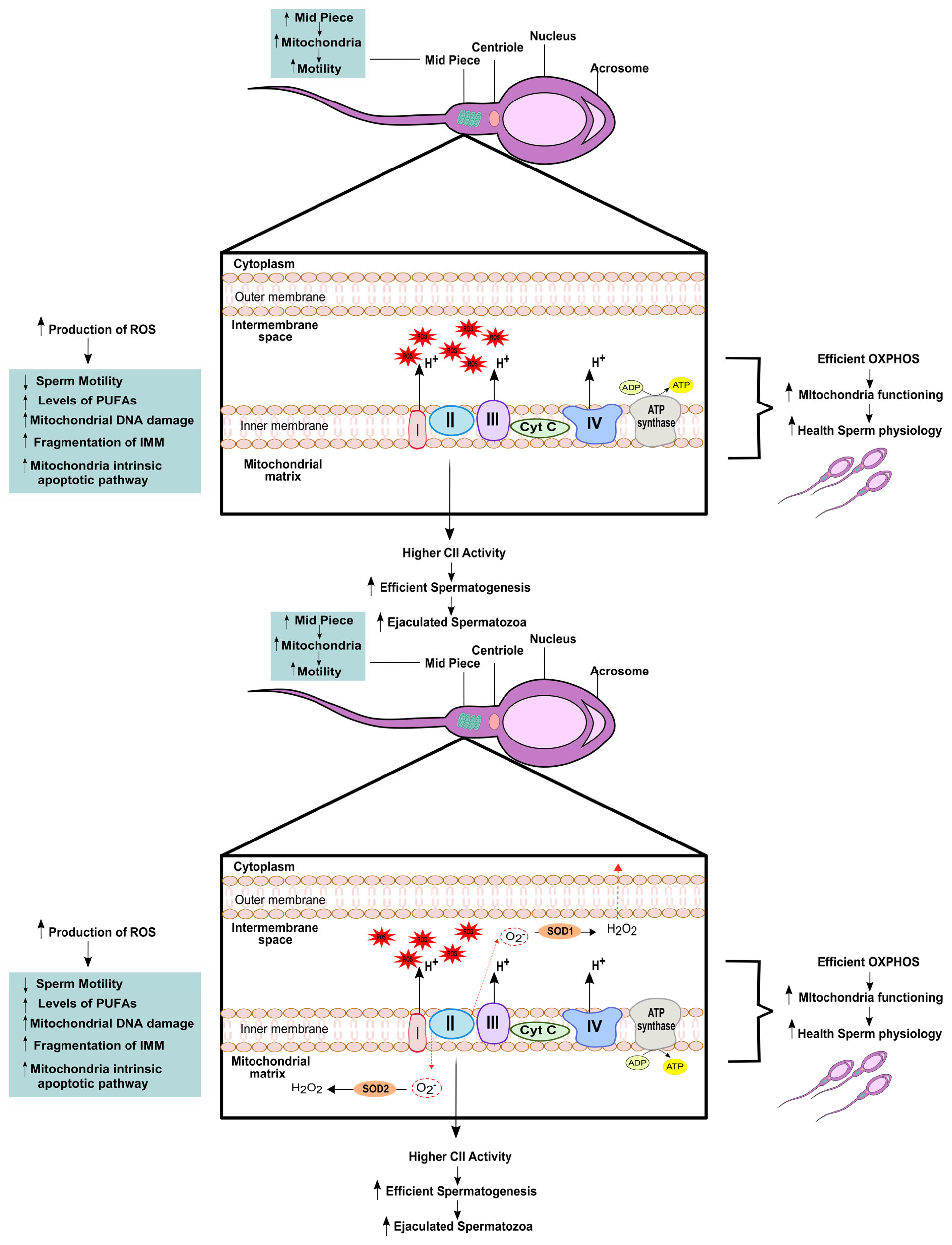
3. Relevance of Mitochondrial (Dys)function to Male Fertility
| Mitochondrial Associated Mechanism | Spermatozoa Outcomes | References |
|---|---|---|
| ↓ MMP | ↓ sperm viability ↓ Acrosome reaction | [57] |
| ↓ ETC | ↓ sperm motility ↓ sperm vitality | [21] |
| ↑ amount of mitochondria in the sheath | ↑ sperm morphology ↑ ATP production | [53] |
| Enrichment of complex II | More efficient spermatogenesis ↑ ejaculated spermatozoa | [21] |
| ↑ Oxidative stress | ↓ sperm motility ↓ fertilization capacity | [59] |
| Damage in IMM | ↑ apoptotic pathway ↓ MMP ↓ sperm motility | [63] |
| ↓ mtDNA integrity | Poor sperm quality | [14,83,85] |
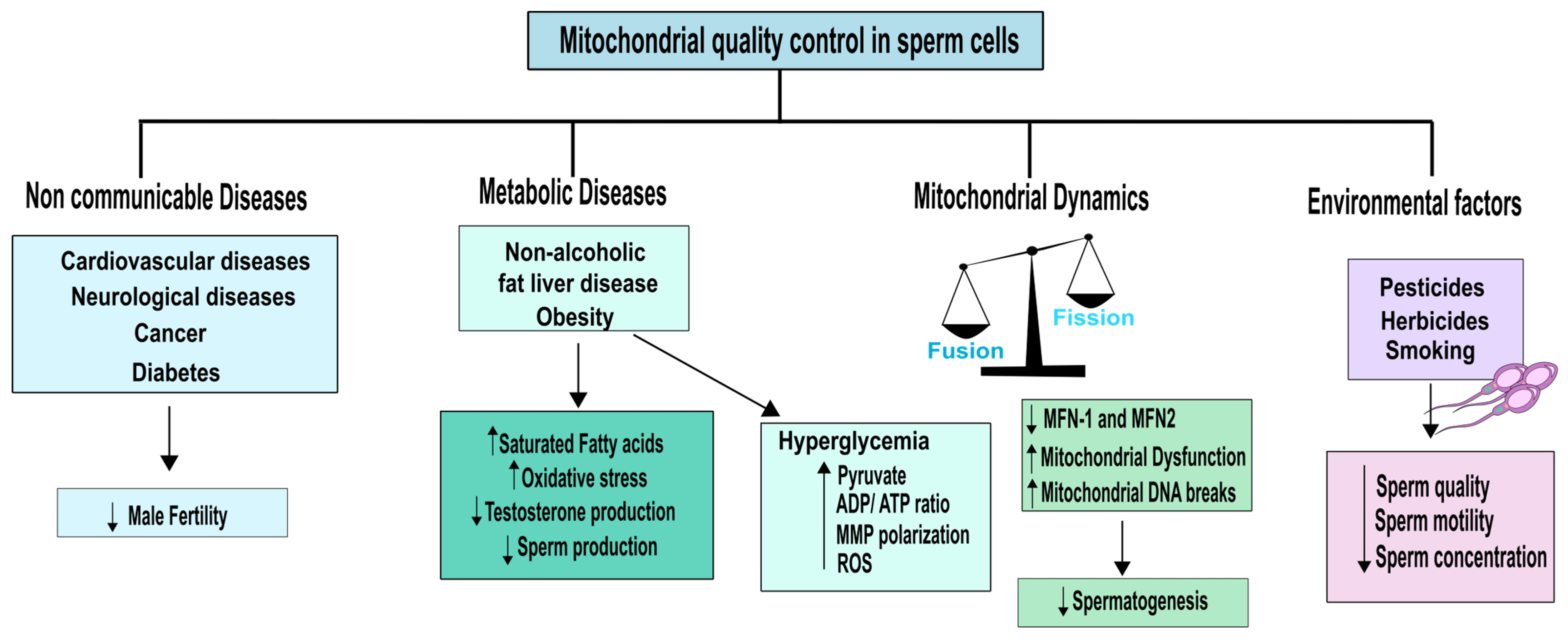
4. Non-Communicable Diseases and Environmental Impact on Mitochondrial Quality of Testicular Cells and Sperm
5. Mitochondria Quality Control Parameters Essential to Evaluate
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turcotte, L.P. Mitochondria: Biogenesis, structure, and function—Symposium introduction. Med. Sci. Sports Exerc. 2003, 35, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Durairajanayagam, D.; Singh, D.; Agarwal, A.; Henkel, R. Causes and consequences of sperm mitochondrial dysfunction. Andrologia 2021, 53, e13666. [Google Scholar] [CrossRef] [PubMed]
- Kuhlbrandt, W. Structure and function of mitochondrial membrane protein complexes. BMC Biol. 2015, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, C.L.; Perevoshchikova, I.V.; Hey-Mogensen, M.; Orr, A.L.; Brand, M.D. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox Biol. 2013, 1, 304–312. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Pellegrino, M.W.; Haynes, C.M. Mitophagy and the mitochondrial unfolded protein response in neurodegeneration and bacterial infection. BMC Biol. 2015, 13, 22. [Google Scholar] [CrossRef]
- Huang, M.L.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The Role of the Antioxidant Response in Mitochondrial Dysfunction in Degenerative Diseases: Cross-Talk between Antioxidant Defense, Autophagy, and Apoptosis. Oxid. Med. Cell. Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef]
- Lv, C.; Wang, X.; Guo, Y.; Yuan, S. Role of Selective Autophagy in Spermatogenesis and Male Fertility. Cells 2020, 9, 2523. [Google Scholar] [CrossRef]
- Staub, C.; Johnson, L. Review: Spermatogenesis in the bull. Animal 2018, 12, s27–s35. [Google Scholar] [CrossRef]
- De Martino, C.; Floridi, A.; Marcante, M.L.; Malorni, W.; Scorza Barcellona, P.; Bellocci, M.; Silvestrini, B. Morphological, histochemical and biochemical studies on germ cell mitochondria of normal rats. Cell Tissue Res. 1979, 196, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Vertika, S.; Singh, K.K.; Rajender, S. Mitochondria, spermatogenesis, and male infertility—An update. Mitochondrion 2020, 54, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.J.; Pang, M.G. Mitochondrial Functionality in Male Fertility: From Spermatogenesis to Fertilization. Antioxidants 2021, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- St John, J.C.; Jokhi, R.P.; Barratt, C.L. The impact of mitochondrial genetics on male infertility. Int. J. Androl. 2005, 28, 65–73. [Google Scholar] [CrossRef]
- Nakada, K.; Sato, A.; Yoshida, K.; Morita, T.; Tanaka, H.; Inoue, S.; Yonekawa, H.; Hayashi, J. Mitochondria-related male infertility. Proc. Natl. Acad. Sci. USA 2006, 103, 15148–15153. [Google Scholar] [CrossRef]
- Ramalho-Santos, J.; Varum, S.; Amaral, S.; Mota, P.C.; Sousa, A.P.; Amaral, A. Mitochondrial functionality in reproduction: From gonads and gametes to embryos and embryonic stem cells. Hum. Reprod. Update 2009, 15, 553–572. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef]
- Aitken, R.J.; Drevet, J.R. The Importance of Oxidative Stress in Determining the Functionality of Mammalian Spermatozoa: A Two-Edged Sword. Antioxidants 2020, 9, 111. [Google Scholar] [CrossRef]
- Ankel-Simons, F.; Cummins, J.M. Misconceptions about mitochondria and mammalian fertilization: Implications for theories on human evolution. Proc. Natl. Acad. Sci. USA 1996, 93, 13859–13863. [Google Scholar] [CrossRef]
- Piomboni, P.; Focarelli, R.; Stendardi, A.; Ferramosca, A.; Zara, V. The role of mitochondria in energy production for human sperm motility. Int. J. Androl. 2012, 35, 109–124. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Lapena, A.C.; Diez-Sanchez, C.; Perez-Martos, A.; Montoya, J.; Alvarez, E.; Diaz, M.; Urries, A.; Montoro, L.; Lopez-Perez, M.J.; et al. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am. J. Hum. Genet. 2000, 67, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Naito, M.; Itoh, M. Patterns of infiltration of lymphocytes into the testis under normal and pathological conditions in mice. Am. J. Reprod. Immunol. 2008, 59, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, A.; Hedger, M.P. Immunological, paracrine and endocrine aspects of testicular immune privilege. Mol. Cell. Endocrinol. 2011, 335, 60–68. [Google Scholar] [CrossRef]
- Boussouar, F.; Benahmed, M. Lactate and energy metabolism in male germ cells. Trends Endocrinol. Metab. 2004, 15, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.M.; Kwon, S.; Pak, Y.K.; Seol, H.W.; Choi, Y.M.; Park, D.J.; Park, K.S.; Lee, H.K. Dynamic changes in mitochondrial biogenesis and antioxidant enzymes during the spontaneous differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 2006, 348, 1472–1478. [Google Scholar] [CrossRef] [PubMed]
- Varuzhanyan, G.; Chan, D.C. Mitochondrial dynamics during spermatogenesis. J. Cell. Sci. 2020, 133, jcs235937. [Google Scholar] [CrossRef]
- Hara, H.; Kuwano, K.; Araya, J. Mitochondrial Quality Control in COPD and IPF. Cells 2018, 7, 86. [Google Scholar] [CrossRef]
- Rato, L.; Alves, M.G.; Socorro, S.; Duarte, A.I.; Cavaco, J.E.; Oliveira, P.F. Metabolic regulation is important for spermatogenesis. Nat. Rev. Urol. 2012, 9, 330–338. [Google Scholar] [CrossRef]
- Bajpai, M.; Gupta, G.; Setty, B.S. Changes in carbohydrate metabolism of testicular germ cells during meiosis in the rat. Eur. J. Endocrinol. 1998, 138, 322–327. [Google Scholar] [CrossRef]
- Handel, M.A.; Schimenti, J.C. Genetics of mammalian meiosis: Regulation, dynamics and impact on fertility. Nat. Rev. Genet. 2010, 11, 124–136. [Google Scholar] [CrossRef]
- Meinhardt, A.; McFarlane, J.R.; Seitz, J.; de Kretser, D.M. Activin maintains the condensed type of mitochondria in germ cells. Mol. Cell. Endocrinol. 2000, 168, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Mannella, C.A. The relevance of mitochondrial membrane topology to mitochondrial function. Biochim. Biophys. Acta 2006, 1762, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Richburg, J.H.; Younkin, S.C.; Boekelheide, K. The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 1997, 138, 2081–2088. [Google Scholar] [CrossRef]
- Xiong, W.; Wang, H.; Wu, H.; Chen, Y.; Han, D. Apoptotic spermatogenic cells can be energy sources for Sertoli cells. Reproduction 2009, 137, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Shen, J.J.; Bournat, J.C.; Huang, L.; Chattopadhyay, A.; Li, Z.; Shaw, C.; Graham, B.H.; Brown, C.W. Activin signaling: Effects on body composition and mitochondrial energy metabolism. Endocrinology 2009, 150, 3521–3529. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, P.J. Hormonal control of germ cell development and spermatogenesis. Semin. Cell Dev. Biol. 2014, 29, 55–65. [Google Scholar] [CrossRef]
- McLachlan, R.I.; O’Donnell, L.; Meachem, S.J.; Stanton, P.G.; de Kretser, D.M.; Pratis, K.; Robertson, D.M. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent. Prog. Horm. Res. 2002, 57, 149–179. [Google Scholar] [CrossRef]
- Flück, C.E.; Miller, W.L.; Auchus, R.J. The 17, 20-lyase activity of cytochrome p450c17 from human fetal testis favors the delta5 steroidogenic pathway. J. Clin. Endocrinol. Metab. 2003, 88, 3762–3766. [Google Scholar] [CrossRef]
- Martin, L.A.; Kennedy, B.E.; Karten, B. Mitochondrial cholesterol: Mechanisms of import and effects on mitochondrial function. J. Bioenerg. Biomembr. 2016, 48, 137–151. [Google Scholar] [CrossRef]
- Prince, F.P. Lamellar and tubular associations of the mitochondrial cristae: Unique forms of the cristae present in steroid-producing cells. Mitochondrion 2002, 1, 381–389. [Google Scholar] [CrossRef]
- Breton, S.; Ruan, Y.C.; Park, Y.J.; Kim, B. Regulation of epithelial function, differentiation, and remodeling in the epididymis. Asian J. Androl. 2016, 18, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Breton, S. Mitochondria-rich, proton-secreting epithelial cells. J. Exp. Biol. 1996, 199, 2345–2358. [Google Scholar] [CrossRef] [PubMed]
- Leclerc, P.; de Lamirande, E.; Gagnon, C. Cyclic adenosine 3′,5′monophosphate-dependent regulation of protein tyrosine phosphorylation in relation to human sperm capacitation and motility. Biol. Reprod. 1996, 55, 684–692. [Google Scholar] [CrossRef] [PubMed]
- de Lamirande, E.; Gagnon, C. Impact of reactive oxygen species on spermatozoa: A balancing act between beneficial and detrimental effects. Hum. Reprod. 1995, 10 (Suppl. 1), 15–21. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C. Peroxiredoxins: Hidden players in the antioxidant defence of human spermatozoa. Basic. Clin. Androl. 2014, 24, 4. [Google Scholar] [CrossRef]
- O’Flaherty, C. Orchestrating the antioxidant defenses in the epididymis. Andrology 2019, 7, 662–668. [Google Scholar] [CrossRef]
- Vernet, P.; Aitken, R.J.; Drevet, J.R. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef]
- Galantino-Homer, H.L.; Visconti, P.E.; Kopf, G.S. Regulation of protein tyrosine phosphorylation during bovine sperm capacitation by a cyclic adenosine 3′5′-monophosphate-dependent pathway. Biol. Reprod. 1997, 56, 707–719. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Guerra-Carvalho, B.; Sousa, M.; Barros, A.; Oliveira, P.F.; Monteiro, M.P.; Alves, M.G. Mitochondrial Activation and Reactive Oxygen-Species Overproduction during Sperm Capacitation are Independent of Glucose Stimuli. Antioxidants 2020, 9, 750. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, W.; Zou, P.; Jiang, F.; Zeng, Y.; Chen, Q.; Sun, L.; Yang, H.; Zhou, N.; Wang, X.; et al. Mitochondrial functionality modifies human sperm acrosin activity, acrosome reaction capability and chromatin integrity. Hum. Reprod. 2019, 34, 3–11. [Google Scholar] [CrossRef]
- Boguenet, M.; Bouet, P.E.; Spiers, A.; Reynier, P.; May-Panloup, P. Mitochondria: Their role in spermatozoa and in male infertility. Hum. Reprod. Update 2021, 27, 697–719. [Google Scholar] [CrossRef] [PubMed]
- Olson, G.E.; Winfrey, V.P. Mitochondria-cytoskeleton interactions in the sperm midpiece. J. Struct. Biol. 1990, 103, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Carter, A.P. Review: Structure and mechanism of the dynein motor ATPase. Biopolymers 2016, 105, 557–567. [Google Scholar] [CrossRef]
- Gu, N.H.; Zhao, W.L.; Wang, G.S.; Sun, F. Comparative analysis of mammalian sperm ultrastructure reveals relationships between sperm morphology, mitochondrial functions and motility. Reprod. Biol. Endocrinol. 2019, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Baccetti, B.; Capitani, S.; Collodel, G.; Strehler, E.; Piomboni, P. Recent advances in human sperm pathology. Contraception 2002, 65, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, F.; La Vignera, S.; Cannarella, R.; Aversa, A.; Calogero, A.E.; Condorelli, R.A. Evaluation of Sperm Mitochondrial Function: A Key Organelle for Sperm Motility. J. Clin. Med. 2020, 9, 363. [Google Scholar] [CrossRef] [PubMed]
- Gallon, F.; Marchetti, C.; Jouy, N.; Marchetti, P. The functionality of mitochondria differentiates human spermatozoa with high and low fertilizing capability. Fertil. Steril. 2006, 86, 1526–1530. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, P.; Ballot, C.; Jouy, N.; Thomas, P.; Marchetti, C. Influence of mitochondrial membrane potential of spermatozoa on in vitro fertilisation outcome. Andrologia 2012, 44, 136–141. [Google Scholar] [CrossRef]
- Stendardi, A.; Focarelli, R.; Piomboni, P.; Palumberi, D.; Serafini, F.; Ferramosca, A.; Zara, V. Evaluation of mitochondrial respiratory efficiency during in vitro capacitation of human spermatozoa. Int. J. Androl. 2011, 34, 247–255. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Nixon, B. Molecular Changes Induced by Oxidative Stress that Impair Human Sperm Motility. Antioxidants 2020, 9, 134. [Google Scholar] [CrossRef]
- Koppers, A.J.; De Iuliis, G.N.; Finnie, J.M.; McLaughlin, E.A.; Aitken, R.J. Significance of mitochondrial reactive oxygen species in the generation of oxidative stress in spermatozoa. J. Clin. Endocrinol. Metab. 2008, 93, 3199–3207. [Google Scholar] [CrossRef] [PubMed]
- O’Flaherty, C.; Matsushita-Fournier, D. Reactive oxygen species and protein modifications in spermatozoa. Biol. Reprod. 2017, 97, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J.; Whiting, S.; De Iuliis, G.N.; McClymont, S.; Mitchell, L.A.; Baker, M.A. Electrophilic aldehydes generated by sperm metabolism activate mitochondrial reactive oxygen species generation and apoptosis by targeting succinate dehydrogenase. J. Biol. Chem. 2012, 287, 33048–33060. [Google Scholar] [CrossRef] [PubMed]
- Aitken, R.J. Reactive oxygen species as mediators of sperm capacitation and pathological damage. Mol. Reprod. Dev. 2017, 84, 1039–1052. [Google Scholar] [CrossRef]
- Aitken, R.J. Not every sperm is sacred; a perspective on male infertility. Mol. Hum. Reprod. 2018, 24, 287–298. [Google Scholar] [CrossRef]
- Espino, J.; Mediero, M.; Lozano, G.M.; Bejarano, I.; Ortiz, A.; Garcia, J.F.; Pariente, J.A.; Rodriguez, A.B. Reduced levels of intracellular calcium releasing in spermatozoa from asthenozoospermic patients. Reprod. Biol. Endocrinol. 2009, 7, 11. [Google Scholar] [CrossRef]
- Treulen, F.; Uribe, P.; Boguen, R.; Villegas, J.V. Mitochondrial permeability transition increases reactive oxygen species production and induces DNA fragmentation in human spermatozoa. Hum. Reprod. 2015, 30, 767–776. [Google Scholar] [CrossRef]
- Barroso, G.; Taylor, S.; Morshedi, M.; Manzur, F.; Gavino, F.; Oehninger, S. Mitochondrial membrane potential integrity and plasma membrane translocation of phosphatidylserine as early apoptotic markers: A comparison of two different sperm subpopulations. Fertil. Steril. 2006, 85, 149–154. [Google Scholar] [CrossRef]
- Espinoza, J.A.; Paasch, U.; Villegas, J.V. Mitochondrial membrane potential disruption pattern in human sperm. Hum. Reprod. 2009, 24, 2079–2085. [Google Scholar] [CrossRef]
- Grunewald, S.; Sharma, R.; Paasch, U.; Glander, H.J.; Agarwal, A. Impact of caspase activation in human spermatozoa. Microsc. Res. Tech. 2009, 72, 878–888. [Google Scholar] [CrossRef]
- Aitken, R.J.; Koppers, A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011, 13, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sharma, R.K.; Sikka, S.C.; Thomas, A.J., Jr.; Falcone, T.; Agarwal, A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003, 80, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, S.; Fitzl, G.; Springsguth, C. Induction of ultra-morphological features of apoptosis in mature and immature sperm. Asian J. Androl. 2017, 19, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Paasch, U.; Grunewald, S.; Dathe, S.; Glander, H.J. Mitochondria of human spermatozoa are preferentially susceptible to apoptosis. Ann. N. Y. Acad. Sci. 2004, 1030, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, D.; Ramalingam, M.; Garrido, N.; Barratt, C.L. Sperm selection in natural conception: What can we learn from Mother Nature to improve assisted reproduction outcomes? Hum. Reprod. Update 2015, 21, 711–726. [Google Scholar] [CrossRef]
- Szymonowicz, K.; Oeck, S.; Malewicz, N.M.; Jendrossek, V. New Insights into Protein Kinase B/Akt Signaling: Role of Localized Akt Activation and Compartment-Specific Target Proteins for the Cellular Radiation Response. Cancers 2018, 10, 78. [Google Scholar] [CrossRef]
- Koppers, A.J.; Mitchell, L.A.; Wang, P.; Lin, M.; Aitken, R.J. Phosphoinositide 3-kinase signalling pathway involvement in a truncated apoptotic cascade associated with motility loss and oxidative DNA damage in human spermatozoa. Biochem. J. 2011, 436, 687–698. [Google Scholar] [CrossRef]
- Kujoth, G.C.; Hiona, A.; Pugh, T.D.; Someya, S.; Panzer, K.; Wohlgemuth, S.E.; Hofer, T.; Seo, A.Y.; Sullivan, R.; Jobling, W.A.; et al. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science 2005, 309, 481–484. [Google Scholar] [CrossRef]
- Jiang, M.; Kauppila, T.E.S.; Motori, E.; Li, X.; Atanassov, I.; Folz-Donahue, K.; Bonekamp, N.A.; Albarran-Gutierrez, S.; Stewart, J.B.; Larsson, N.G. Increased Total mtDNA Copy Number Cures Male Infertility Despite Unaltered mtDNA Mutation Load. Cell. Metab. 2017, 26, 429–436.e424. [Google Scholar] [CrossRef]
- Brower, J.V.; Lim, C.H.; Jorgensen, M.; Oh, S.P.; Terada, N. Adenine nucleotide translocase 4 deficiency leads to early meiotic arrest of murine male germ cells. Reproduction 2009, 138, 463–470. [Google Scholar] [CrossRef]
- Ieremiadou, F.; Rodakis, G.C. Correlation of the 4977 bp mitochondrial DNA deletion with human sperm dysfunction. BMC Res. Notes 2009, 2, 18. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, M.; McClure, N.; Lewis, S.E. A comparison of mitochondrial and nuclear DNA status in testicular sperm from fertile men and those with obstructive azoospermia. Hum. Reprod. 2002, 17, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Rajender, S.; Rahul, P.; Mahdi, A.A. Mitochondria, spermatogenesis and male infertility. Mitochondrion 2010, 10, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Faja, F.; Carlini, T.; Coltrinari, G.; Finocchi, F.; Nespoli, M.; Pallotti, F.; Lenzi, A.; Lombardo, F.; Paoli, D. Human sperm motility: A molecular study of mitochondrial DNA, mitochondrial transcription factor A gene and DNA fragmentation. Mol. Biol. Rep. 2019, 46, 4113–4121. [Google Scholar] [CrossRef]
- Song, G.J.; Lewis, V. Mitochondrial DNA integrity and copy number in sperm from infertile men. Fertil. Steril. 2008, 90, 2238–2244. [Google Scholar] [CrossRef]
- Wei, Y.-H.; Kao, S.-H. Mitochondrial DNA mutation and depletion are associated with decline of fertility and motility of human sperm. Zool. Stud. 2000, 39, 1–12. [Google Scholar]
- Martinez-Heredia, J.; de Mateo, S.; Vidal-Taboada, J.M.; Ballesca, J.L.; Oliva, R. Identification of proteomic differences in asthenozoospermic sperm samples. Hum. Reprod. 2008, 23, 783–791. [Google Scholar] [CrossRef]
- Parte, P.P.; Rao, P.; Redij, S.; Lobo, V.; D’Souza, S.J.; Gajbhiye, R.; Kulkarni, V. Sperm phosphoproteome profiling by ultra performance liquid chromatography followed by data independent analysis (LC-MS(E)) reveals altered proteomic signatures in asthenozoospermia. J. Proteomics 2012, 75, 5861–5871. [Google Scholar] [CrossRef]
- Nowicka-Bauer, K.; Lepczynski, A.; Ozgo, M.; Kamieniczna, M.; Fraczek, M.; Stanski, L.; Olszewska, M.; Malcher, A.; Skrzypczak, W.; Kurpisz, M.K. Sperm mitochondrial dysfunction and oxidative stress as possible reasons for isolated asthenozoospermia. J. Physiol. Pharmacol. 2018, 69, 403–417. [Google Scholar] [CrossRef]
- Siva, A.B.; Kameshwari, D.B.; Singh, V.; Pavani, K.; Sundaram, C.S.; Rangaraj, N.; Deenadayal, M.; Shivaji, S. Proteomics-based study on asthenozoospermia: Differential expression of proteasome alpha complex. Mol. Hum. Reprod. 2010, 16, 452–462. [Google Scholar] [CrossRef]
- Camps, J.; Garcia-Heredia, A.; Hernandez-Aguilera, A.; Joven, J. Paraoxonases, mitochondrial dysfunction and non-communicable diseases. Chem. Biol. Interact. 2016, 259, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Cerda, C.; Sanchez, C.; Climent, B.; Vazquez, A.; Iradi, A.; El Amrani, F.; Bediaga, A.; Saez, G.T. Oxidative stress and DNA damage in obesity-related tumorigenesis. Adv. Exp. Med. Biol. 2014, 824, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhai, L.; Liu, Z.; Wu, S.; Xu, L. Leptin level and oxidative stress contribute to obesity-induced low testosterone in murine testicular tissue. Oxid. Med. Cell. Longev. 2014, 2014, 190945. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Zara, V. Diet and Male Fertility: The Impact of Nutrients and Antioxidants on Sperm Energetic Metabolism. Int. J. Mol. Sci. 2022, 23, 2542. [Google Scholar] [CrossRef]
- Lunetti, P.; Capobianco, L.; Zara, V.; Ferramosca, A. Physical Activity and Male Reproductive Function: A New Role for Gamete Mitochondria. Exerc. Sport. Sci. Rev. 2021, 49, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Siri-Tarino, P.W.; Sun, Q.; Hu, F.B.; Krauss, R.M. Saturated fat, carbohydrate, and cardiovascular disease. Am. J. Clin. Nutr. 2010, 91, 502–509. [Google Scholar] [CrossRef]
- Saez, F.; Drevet, J.R. Dietary Cholesterol and Lipid Overload: Impact on Male Fertility. Oxid. Med. Cell. Longev. 2019, 2019, 4521786. [Google Scholar] [CrossRef]
- Ferramosca, A.; Moscatelli, N.; Di Giacomo, M.; Zara, V. Dietary fatty acids influence sperm quality and function. Andrology 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Ferramosca, A.; Zara, V. Bioenergetics of mammalian sperm capacitation. Biomed. Res. Int. 2014, 2014, 902953. [Google Scholar] [CrossRef]
- Ferramosca, A.; Conte, A.; Moscatelli, N.; Zara, V. A high-fat diet negatively affects rat sperm mitochondrial respiration. Andrology 2016, 4, 520–525. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.; Vicari, E.; D’Agata, R.; Calogero, A.E. Diabetes mellitus and sperm parameters. J. Androl. 2012, 33, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Pinto Provenzano, S.; Montagna, D.D.; Coppola, L.; Zara, V. Oxidative stress negatively affects human sperm mitochondrial respiration. Urology 2013, 82, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, E.; Gerdes, C.; Petersmann, A.; Muller-Wieland, D.; Muller, U.A.; Freckmann, G.; Heinemann, L.; Nauck, M.; Landgraf, R. Definition, Classification and Diagnosis of Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes 2022, 130, S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.G. The Physiological and Biochemical Effects of Diabetes on The Balance between Oxidative Stress and Antioxidant Defense System. Med. J. Islam. World Acad. Sci. 2005, 15, 31–42. [Google Scholar]
- Yu, T.; Robotham, J.L.; Yoon, Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc. Natl. Acad. Sci. USA 2006, 103, 2653–2658. [Google Scholar] [CrossRef]
- Rolo, A.P.; Palmeira, C.M. Diabetes and mitochondrial function: Role of hyperglycemia and oxidative stress. Toxicol. Appl. Pharmacol. 2006, 212, 167–178. [Google Scholar] [CrossRef]
- Amaral, S.; Oliveira, P.J.; Ramalho-Santos, J. Diabetes and the impairment of reproductive function: Possible role of mitochondria and reactive oxygen species. Curr. Diabetes Rev. 2008, 4, 46–54. [Google Scholar] [CrossRef]
- Saez Lancellotti, T.E.; Boarelli, P.V.; Monclus, M.A.; Cabrillana, M.E.; Clementi, M.A.; Espinola, L.S.; Cid Barria, J.L.; Vincenti, A.E.; Santi, A.G.; Fornes, M.W. Hypercholesterolemia impaired sperm functionality in rabbits. PLoS ONE 2010, 5, e13457. [Google Scholar] [CrossRef]
- Carrageta, D.F.; Guerra-Carvalho, B.; Spadella, M.A.; Yeste, M.; Oliveira, P.F.; Alves, M.G. Animal models of male reproductive ageing to study testosterone production and spermatogenesis. Rev. Endocr. Metab. Disord. 2022, 23, 1341–1360. [Google Scholar] [CrossRef]
- Sharma, R.; Agarwal, A.; Rohra, V.K.; Assidi, M.; Abu-Elmagd, M.; Turki, R.F. Effects of increased paternal age on sperm quality, reproductive outcome and associated epigenetic risks to offspring. Reprod. Biol. Endocrinol. 2015, 13, 35. [Google Scholar] [CrossRef]
- Trifunovic, A.; Wredenberg, A.; Falkenberg, M.; Spelbrink, J.N.; Rovio, A.T.; Bruder, C.E.; Bohlooly, Y.M.; Gidlof, S.; Oldfors, A.; Wibom, R.; et al. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature 2004, 429, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Jarak, I.; Almeida, S.; Carvalho, R.A.; Sousa, M.; Barros, A.; Alves, M.G.; Oliveira, P.F. Senescence and declining reproductive potential: Insight into molecular mechanisms through testicular metabolomics. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- Ferramosca, A.; Lorenzetti, S.; Di Giacomo, M.; Murrieri, F.; Coppola, L.; Zara, V. Herbicides glyphosate and glufosinate ammonium negatively affect human sperm mitochondria respiration efficiency. Reprod. Toxicol. 2021, 99, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Chohan, K.R.; Badawy, S.Z. Cigarette smoking impairs sperm bioenergetics. Int. Braz. J. Urol. 2010, 36, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, R.; Altieri, I.; Gandini, L.; Lenzi, A.; Pichini, S.; Rosa, M.; Zuccaro, P.; Dondero, F. Nicotine, cotinine, and trans-3-hydroxycotinine levels in seminal plasma of smokers: Effects on sperm parameters. Ther. Drug. Monit. 1993, 15, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Collin, O.; Kilter, S.; Bergh, A. Tobacco smoke disrupts testicular microcirculation in the rat. Int. J. Androl. 1995, 18, 141–145. [Google Scholar] [CrossRef]
- Evans, H.J.; Fletcher, J.; Torrance, M.; Hargreave, T.B. Sperm abnormalities and cigarette smoking. Lancet 1981, 1, 627–629. [Google Scholar] [CrossRef]
- Vogt, H.J.; Heller, W.D.; Borelli, S. Sperm quality of healthy smokers, ex-smokers, and never-smokers. Fertil. Steril. 1986, 45, 106–110. [Google Scholar] [CrossRef]
- Rantala, M.L.; Koskimies, A.I. Semen quality of infertile couples—Comparison between smokers and non-smokers. Andrologia 1987, 19, 42–46. [Google Scholar] [CrossRef]
- Sofikitis, N.; Takenaka, M.; Kanakas, N.; Papadopoulos, H.; Yamamoto, Y.; Drakakis, P.; Miyagawa, I. Effects of cotinine on sperm motility, membrane function, and fertilizing capacity in vitro. Urol. Res. 2000, 28, 370–375. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, M.; Rolf, C.; Nordhoff, V.; Schrader, G.; Rickert-Fohring, M.; Gassner, P.; Behre, H.M.; Greb, R.R.; Kiesel, L.; Nieschlag, E. Male smokers have a decreased success rate for in vitro fertilization and intracytoplasmic sperm injection. Fertil. Steril. 2003, 79 (Suppl. 3), 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Ko, E.; Barclay, L.; Hoang, T.; Rademaker, A.; Martin, R. Cigarette smoking and aneuploidy in human sperm. Mol. Reprod. Dev. 2001, 59, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Jozkow, P.; Rossato, M. The Impact of Intense Exercise on Semen Quality. Am. J. Mens. Health 2017, 11, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh Maleki, B.; Tartibian, B.; Chehrazi, M. Effects of Aerobic, Resistance, and Combined Exercise on Markers of Male Reproduction in Healthy Human Subjects: A Randomized Controlled Trial. J. Strength Cond. Res. 2019, 33, 1130–1145. [Google Scholar] [CrossRef]
- Finkler, M.; Lichtenberg, D.; Pinchuk, I. The relationship between oxidative stress and exercise. J. Basic Clin. Physiol. Pharmacol. 2014, 25, 1–11. [Google Scholar] [CrossRef]
- Powers, S.K.; Radak, Z.; Ji, L.L. Exercise-induced oxidative stress: Past, present and future. J. Physiol. 2016, 594, 5081–5092. [Google Scholar] [CrossRef]
- Bloomer, R.J.; Goldfarb, A.H.; McKenzie, M.J. Oxidative stress response to aerobic exercise: Comparison of antioxidant supplements. Med. Sci. Sports Exerc. 2006, 38, 1098–1105. [Google Scholar] [CrossRef]
- Finaud, J.; Lac, G.; Filaire, E. Oxidative stress: Relationship with exercise and training. Sports Med. 2006, 36, 327–358. [Google Scholar] [CrossRef]
- Kocabas, R.; Namiduru, E.S.; Bagceci, A.M.; Erenler, A.K.; Karakoc, O.; Orkmez, M.; Akan, M.; Erdemli, H.K.; Taysi, S.; Tarakcioglu, M. The acute effects of interval exercise on oxidative stress and antioxidant status in volleyball players. J. Sports Med. Phys. Fit. 2018, 58, 421–427. [Google Scholar] [CrossRef]
- Sharifi, G.; Najafabadi, A.B.; Ghashghaei, F.E. Oxidative stress and total antioxidant capacity in handball players. Adv. Biomed. Res. 2014, 3, 181. [Google Scholar] [CrossRef] [PubMed]
- Slattery, K.; Bentley, D.; Coutts, A.J. The role of oxidative, inflammatory and neuroendocrinological systems during exercise stress in athletes: Implications of antioxidant supplementation on physiological adaptation during intensified physical training. Sports Med. 2015, 45, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Fragala, M.S.; Watson, G.; Volek, J.S.; Rubin, M.R.; French, D.N.; Maresh, C.M.; Vingren, J.L.; Hatfield, D.L.; Spiering, B.A.; et al. Hormonal responses to a 160-km race across frozen Alaska. Br. J. Sports Med. 2008, 42, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Hooper, D.R.; Kraemer, W.J.; Stearns, R.L.; Kupchak, B.R.; Volk, B.M.; DuPont, W.H.; Maresh, C.M.; Casa, D.J. Evidence of the Exercise-Hypogonadal Male Condition at the 2011 Kona Ironman World Championships. Int. J. Sports Physiol. Perform. 2019, 14, 170–175. [Google Scholar] [CrossRef]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell. 2009, 20, 3525–3532. [Google Scholar] [CrossRef]
- Smirnova, E.; Griparic, L.; Shurland, D.L.; van der Bliek, A.M. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol. Biol. Cell. 2001, 12, 2245–2256. [Google Scholar] [CrossRef]
- Varuzhanyan, G.; Rojansky, R.; Sweredoski, M.J.; Graham, R.L.J.; Hess, S.; Ladinsky, M.S.; Chan, D.C. Mitochondrial fusion is required for spermatogonial differentiation and meiosis. eLife 2019, 8, e51601. [Google Scholar] [CrossRef]
- Senos Demarco, R.; Jones, D.L. Mitochondrial fission regulates germ cell differentiation by suppressing ROS-mediated activation of Epidermal Growth Factor Signaling in the Drosophila larval testis. Sci. Rep. 2019, 9, 19695. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, H.; Jia, P.; Zhao, H.; Liu, C.; Liu, W.; Song, Z.; Xu, Z.; Yang, L.; Wang, Y.; et al. Autophagy regulates spermatid differentiation via degradation of PDLIM1. Autophagy 2016, 12, 1575–1592. [Google Scholar] [CrossRef]
- Kausar, S.; Wang, F.; Cui, H. The Role of Mitochondria in Reactive Oxygen Species Generation and Its Implications for Neurodegenerative Diseases. Cells 2018, 7, 274. [Google Scholar] [CrossRef]
- Holecek, M. Why Are Branched-Chain Amino Acids Increased in Starvation and Diabetes? Nutrients 2020, 12, 3087. [Google Scholar] [CrossRef] [PubMed]
- Darr, C.R.; Varner, D.D.; Teague, S.; Cortopassi, G.A.; Datta, S.; Meyers, S.A. Lactate and Pyruvate Are Major Sources of Energy for Stallion Sperm with Dose Effects on Mitochondrial Function, Motility, and ROS Production. Biol. Reprod. 2016, 95, 34. [Google Scholar] [CrossRef] [PubMed]
- Storey, B.T.; Kayne, F.J. Energy metabolism of spermatozoa. VI. Direct intramitochondrial lactate oxidation by rabbit sperm mitochondria. Biol. Reprod. 1977, 16, 549–556. [Google Scholar] [PubMed]
- Storey, B.T. Mammalian sperm metabolism: Oxygen and sugar, friend and foe. Int. J. Dev. Biol. 2008, 52, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.R.; Meyers, S. The sperm mitochondrion: Organelle of many functions. Anim. Reprod. Sci. 2018, 194, 71–80. [Google Scholar] [CrossRef]
- Rodriguez-Gil, J.E.; Bonet, S. Current knowledge on boar sperm metabolism: Comparison with other mammalian species. Theriogenology 2016, 85, 4–11. [Google Scholar] [CrossRef]
- Meyers, S.A. Cryostorage and Oxidative Stress in Mammalian Spermatozoa. In Studies on Men’s Health and Fertility; Agarwal, A., Aitken, R.J., Alvarez, J.G., Eds.; Humana Press: Totowa, NJ, USA, 2012; pp. 41–56. [Google Scholar] [CrossRef]
- Gibb, Z.; Lambourne, S.R.; Aitken, R.J. The paradoxical relationship between stallion fertility and oxidative stress. Biol. Reprod. 2014, 91, 77. [Google Scholar] [CrossRef]
- Aitken, R.J.; Jones, K.T.; Robertson, S.A. Reactive oxygen species and sperm function—In sickness and in health. J. Androl. 2012, 33, 1096–1106. [Google Scholar] [CrossRef]
- Yeste, M.; Estrada, E.; Rocha, L.G.; Marin, H.; Rodriguez-Gil, J.E.; Miro, J. Cryotolerance of stallion spermatozoa is related to ROS production and mitochondrial membrane potential rather than to the integrity of sperm nucleus. Andrology 2015, 3, 395–407. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology 2023, 12, 827. https://doi.org/10.3390/biology12060827
Costa J, Braga PC, Rebelo I, Oliveira PF, Alves MG. Mitochondria Quality Control and Male Fertility. Biology. 2023; 12(6):827. https://doi.org/10.3390/biology12060827
Chicago/Turabian StyleCosta, José, Patrícia C. Braga, Irene Rebelo, Pedro F. Oliveira, and Marco G. Alves. 2023. "Mitochondria Quality Control and Male Fertility" Biology 12, no. 6: 827. https://doi.org/10.3390/biology12060827
APA StyleCosta, J., Braga, P. C., Rebelo, I., Oliveira, P. F., & Alves, M. G. (2023). Mitochondria Quality Control and Male Fertility. Biology, 12(6), 827. https://doi.org/10.3390/biology12060827










